Abstract
Candida species are associated with invasive fungal infections, and C. parapsilosis has become increasingly prevalent. As key antifungal effector cells, the function of human neutrophils confronting C. parapsilosis was investigated. We hypothesized that interaction between neutrophils and Candida species may not be uniform. Opsonins were omitted from these studies to understand the antifungal mechanisms at their most basic level. Human neutrophils underwent phagocytosis of C. parapsilosis with much higher efficiency than C. albicans. Immunofluorescence assays with β-glucan specific antibody detected more surface exposed β-glucan on C. parapsilosis than C. albicans. However, blockade of the β-glucan receptor, Dectin-1, reduced phagocytosis of C. albicans but not C. parapsilosis. Inclusion of excess β-glucan, mannan, or chitin also had no effect on phagocytosis of C. parapsilosis. Consistent with the difference in phagocytosis, neutrophils mediated damage to C. parapsilosis but not C. albicans in assays of residual metabolic activity. C. parapsilosis was more sensitive to oxidative stress, and inclusion of antioxidant in toxicity assays decreased neutrophil mediated damage, suggesting that generation of reactive oxygen species contributes to the mechanism of toxicity. These data suggest that the interaction between neutrophils and Candida species is not uniform and may partially account for differences observed in the epidemiology and natural history of infections caused by these species.
Keywords: Neutrophil, Phagocytosis, Candida, Dectin-1, β-glucan
INTRODUCTION
Candida species are a prevalent cause of invasive fungal infections, currently the fourth leading organism in hospital acquired bloodstream infections in the United States [1]. Candida albicans is associated with the majority of these infections and possesses a number of specific virulence attributes that have been the subject of intensive study [2]. Candida parapsilosis has been considered a less virulent species and historically has been associated with a small proportion of bloodstream infections. However, this organism has increased in prevalence in recent years. C. parapsilosis is a common cause of bloodstream infection in neonates [3-5], and has overtaken C. albicans in frequency of hospital-acquired infection in some hospitals worldwide [4, 6, 7]. Recent studies have identified the importance of secreted lipase in the virulence of this organism and have documented its ability to damage tissue in vitro [8, 9].
The neutrophil is well-recognized for its important role in host defense against invasive fungal infections, and various neutrophil functions have been studied using C. albicans and other fungi as targets [10, 11]. The interaction between neutrophils and C. parapsilosis has received considerably less attention. Because of the increasing prevalence of C. parapsilosis in invasive candidiasis and the central role of neutrophils in host defense, we sought to more clearly understand the specific attributes of human neutrophils confronting this species. In this report, we focus on phagocytosis as an important initial step in this interaction and on the mechanisms of neutrophil-induced toxicity to each species. Opsonization of pathogenic organisms with specific antibody or complement plays a key role in this process in vivo, and is well known to alter the dynamics of phagocytosis under in vitro conditions [12-14]. In an effort to understand the interaction between neutrophils and yeast with the least number of confounding variables, we conducted these studies in the absence of opsonins. We also investigated the role of carbohydrates present in the fungal cell wall in the phagocytosis process.
MATERIALS AND METHODS
Organisms and media
C. albicans strains used in this study include a laboratory strain, Ca3153A [15, 16], and a clinical isolate from an infant with urinary candidiasis, Ca-4 [17]. C. parapsilosis strains included three independent clinical isolates colonizing premature infants in a previously reported study, Ro18, Ro29 and Ro75 [18]. Starter cultures for phagocytosis and toxicity assays were grown 16 h at 37°C with vigorous agitation in YEPD medium (1% yeast extract, 2% peptone, 2% dextrose). Cultures were predominantly (>99%) yeast forms following this incubation.
Preparation and pretreatment of neutrophils
Following review and approval by the Institutional Review Board, human neutrophils were isolated by density gradient centrifugation from peripheral blood of healthy adult volunteers. Briefly, leukocytes were separated from whole blood on Histopaque-1077 (Sigma) by density gradient centrifugation. Neutrophils were further purified by dextran sedimentation and hypotonic lysis of contaminating erythrocytes. Cells were adjusted to 5 × 106 cells/mL in HBSS + Ca/Mg, and were >95% neutrophils as determined by Wright-Giemsa stain. In selected experiments, neutrophils were pretreated with various reagents to study the specifics of the yeast-neutrophil interaction. To evaluate the requirement for actin polymerization, neutrophils were preincubated with cytochalasin D (10 μg/mL) at 4°C for 30 min with constant mixing. The role of β-glucan was studied by pretreating neutrophils with excess β-glucan or with blocking monoclonal antibodies to Dectin- 1. A β-glucan solution (10 mg/ml, Sigma, from barley) was prepared using described methods to maximize solubility [19]. Briefly, β-glucan was dissolved in 1N NaOH by heating to 60°C, diluted to 0.5 mg/ml in HBSS, and corrected to neutral pH with HCl. A vehicle control was used for comparison in these experiments, prepared identically to the β-glucan solution but omitting the β-glucan. Neutrophils were incubated with β-glucan or vehicle control for 30 min at 4°C with constant mixing prior to inclusion in the phagocytosis assay. Dectin-1 receptor was blocked with specific antibody (GE2, generously provided by Gordon Brown) [20] by incubation with 10 μg/mL of antibody for 20 min at 4°C. To investigate the role of other carbohydrate receptors in phagocytosis, neutrophils were pretreated with mannan (Sigma, from Saccharomyces cerevisiae) or chitin (Sigma, from crab shells). Mannan was solubilized in HBSS and preincubated with neutrophils (10 mg/ml or 1 mg/ml) for 30 min at 4°C with constant mixing. Solubility of chitin is limited in aqueous solution, so solutions were prepared according to the described method [21]. Briefly, chitin was suspended in HBSS with 8% NaOH and 4% Urea (w/v) and sonicated for 30 min. The solution was incubated at 4°C overnight with rotation and incubated at −20°C for 1 hour. A 1:100 dilution was performed into HBSS to achieve 0.1 mg/ml, and the pH was adjusted using HCl. A vehicle control was used for comparison in these experiments, prepared identically to the chitin solution but omitting the chitin. Neutrophils were incubated with chitin or vehicle control for 30 min at 4°C with constant mixing prior to inclusion in the phagocytosis assay.
Phagocytosis assay
Candida species were washed in Hank’s Balanced Salt Solution (HBSS), enumerated on a hemacytometer, and adjusted to a final concentration appropriate to provide the desired multiplicity of infection (MOI). In selected experiments, yeast were heat killed at 65°C for 90 min. Yeast were labeled with FITC at 10 μg/mL in the dark with rotation for 30 minutes at 37°C followed by extensive washes with HBSS. Once neutrophils were prepared and pretreated when indicated, equal volumes of Candida yeast and neutrophils were combined at ratios appropriate to provide a MOI ranging from 10 to 100 (10 to 100 yeast per neutrophil). Cells were pelleted at 500 × g for 2 minutes, incubated on ice for 30 min to give a pool of cells with surface-bound Candida, then at 37°C for 30, 60 or 90 min to allow phagocytosis. Cells were pelleted at 500 × g for 2 min and resuspended in 20 μL HBSS. 2.5 μL of ethidium bromide (100 μg/mL) was mixed with 2.5 μL of the sample on the surface of a microscope slide and examined by fluorescence microscopy. Intracellular Candida were differentiated from extracellular by retention of green fluorescence. A minimum of 100 neutrophils were counted, and the percent that had undergone phagocytosis of yeast was calculated. In some experiments, the number of intracellular yeast within neutrophils undergoing phagocytosis was calculated and reported as 1, 2, 3, or 4+ yeast per cell.
Indirect immunofluorescence assay and flow cytometry for β-glucan
C. albicans and C. parapsilosis yeast were harvested from overnight culture and washed with PBS. Yeast cells were blocked with 3% bovine serum albumin (BSA) at 22°C for 30 min, then incubated with 15 μg/ml BF-Div, a mouse IgM monoclonal antibody specific for both β-(1-3)- and β-(1-6)-linked glucan [22] for 30 min at 22°C. Cells were washed with PBS and incubated with an appropriate FITC-conjugated secondary antibody for 30 min at 22°C. Controls included yeast cells incubated in 3% BSA only or with secondary antibody only. Cells were washed and examined by fluorescence microscopy or by flow cytometry with a BD Biosciences FACSCanto instrument.
Neutrophil-induced toxicity assay
Candida species were washed in HBSS + Ca/Mg with 0.1% (w/v) glucose. Human adult neutrophils were prepared as described above. In selected experiments, neutrophils were preincubated with cytochalasin D (10 μg/mL) at 4°C for 30 min with constant mixing. Cells were enumerated on a hemacytometer and combined at varied effector to target (E: T) ratios in a 96 well dish in 60 μl total volume with appropriate controls. After a 60 min incubation at 37°C, plates were centrifuged at 1600 × g and the supernatant was carefully aspirated from the wells. Neutrophils were lysed by agitation in 0.02% (v/v) Tween-20 in water, pH 11.0 [23] and incubated at 37°C for 15 min. The centrifugation and lysis steps were repeated, and lysis confirmed by light microscopy. Aspirated supernatants were plated on YEPD, confirming that only trivial numbers of yeast cells (< 2% of inoculum) were lost in this procedure. Toxicity to Candida was measured by residual metabolic activity, using (2,3)-bis-(2-methoxy-4-nitro-5-sulphenyl)-(2H)-tetrazolium-5-carboxanilide (XTT, Sigma) assay as described [24]. Briefly, XTT was freshly prepared in Dulbecco’s PBS, heated at 60°C for 30 min and filtered. Coenzyme Q (Sigma) was added, and the solution was added to cells at concentrations of 0.5 mg/ml XTT and 40 mg/ml Coenzyme Q. Plates were incubated at 37°C for 1 h and the intensity of the colorimetric reaction, reflecting metabolic activity, was measured by optical density at 450 nm (OD450) using an automated plate reader. Individual experiments were performed in triplicate at minimum, and a minimum of 5 individual neutrophil donors were studied in independent experiments. Percent residual XTT activity was calculated as Mean OD450 (Yeast + neutrophils) / Mean OD450 (Yeast alone). To study the contribution of reactive oxygen species (ROS) in neutrophil-mediated toxicity to Candida yeast, toxicity assays were conducted in the presence of the ROS scavenger, N-acetylcysteine, at concentrations of 1, 2, and 4 mM in HBSS. Percent residual XTT activity was calculated as above for each concentration of N-acetylcysteine.
Hydrogen peroxide (H2O2) toxicity assay
Candida species were washed 3 times in HBSS and enumerated on a hemacytometer. 1×106 cells/well were inoculated into 96 well plates containing HBSS+ 0.1% glucose with and without 1mM H202 in triplicate. Plates were incubated at 37°C for 1 hour. Hydrogen peroxide induced toxicity was measured by residual XTT activity as described above. Percent residual XTT activity was calculated as Mean OD450 (Yeast + H202) / Mean OD450 (Yeast alone).
Statistical analysis
Comparisons of phagocytosis and induced toxicity among different Candida species and strains and among various neutrophil treatments were made by analysis of variance (ANOVA). Between group comparisons were made by Newman-Keuls test, and p values < 0.05 were considered significant.
RESULTS
Phagocytosis of Candida species by human neutrophils
To compare the relative phagocytosis efficiency of C. albicans and C. parapsilosis yeast forms by human neutrophils, phagocytosis assays were conducted using FITC-labeled C. albicans and C. parapsilosis yeast and neutrophils isolated from healthy adult volunteers in the absence of opsonins. Heat killed organisms gave more uniform and reliable labeling with FITC and thus were used preferentially in these experiments. Live organisms behaved similarly (see below). To determine the optimal incubation time to allow phagocytosis to occur, yeast and neutrophils were coincubated for 30, 60, and 90 minutes. No increase in phagocytosis of any strain was detected at incubations longer than 30 minutes (data not shown), so the 30 minute time point was used in all subsequent experiments. Phagocytosis assays were conducted at multiplicity of infection (MOI) ranging from 10 to 100. Two strains of C. albicans (laboratory strain, Ca3153A and a clinical isolate, Ca-4) and 3 strains of C. parapsilosis (clinical isolates) were studied. In all cases and at all MOIs, phagocytosis was much more efficient for neutrophils incubated with C. parapsilosis than C. albicans (Fig. 1A, p < 0.001). Increasing MOI above 10 increased phagocytosis efficiency for C. albicans strain Ca3153A (p < 0.02) but not for Ca-4 (p > 0.16). Increasing MOI above 10 increased phagocytosis efficiency for C. parapsilosis strains Ro18 (p < 0.006) and Ro29 (p < 0.002) but not Ro75 (p > 0.46).
Fig. 1. Phagocytosis of C. albicans and C. parapsilosis by human neutrophils.
C. albicans and C. parapsilosis yeast were labeled with FITC and combined with human neutrophils at the indicated MOI. After allowing phagocytosis to occur, cells were counterstained with ethidium bromide and examined by fluorescence microscopy. Neutrophils containing intracellular (green) yeast were scored as a percentage of total neutrophils. Error bars represent standard deviation. Each experiment was performed in triplicate at minimum, and data represent a minimum of three individual neutrophil donors. (A) Phagocytosis of heat-killed yeast at MOI = 10, 50, or 100. Phagocytosis of C. parapsilosis was significantly higher than C. albicans for all strains and at all MOIs (p < 0.001). (B) Phagocytosis of live vs. heat-killed (HK) yeast at MOI = 100. Phagocytosis of live C. parapsilosis was significantly higher than live C. albicans for all strains (p < 0.001). (C) Percentage of neutrophils undergoing phagocytosis that internalized 1, 2, 3, or 4+ yeast per cell. A single, representative strain of C. albicans (Ca3153a) and C. parapsilosis (Ro18) is depicted in the figure. The percentage of neutrophils that had phagocytosed 4+ yeast/cell was significantly higher for C. parapsilosis than C. albicans at MOI = 50 or 100 (p = 0.0001), and trended toward a higher percentage at MOI = 10 (p = 0.056). (D) Effect of cytochalasin D on phagocytosis. Neutrophils were pretreated with cytochalasin D and phagocytosis assays were conducted at MOI = 100 to maximize phagocytosis efficiency. As expected, cytochalsin D inhibits phagocytosis (p < 0.03). Because of the low baseline phagocytosis rate of Ca-4, statistical significance was not achieved.
To confirm that the difference in phagocytosis efficiency between C. albicans and C. parapsilosis was not an artifact related to the heat-killing process, phagocytosis assays were conducted with live C. albicans and C. parapsilosis yeast at an MOI of 100. Again, significantly higher rates of phagocytosis were seen with all C. parapsilosis strains relative to C. albicans (Fig. 1B). C. albicans strain Ca3153A showed somewhat higher phagocytosis efficiency when using heat-killed vs. live yeast (p=0.001), whereas phagocytosis rates between heat-killed and live yeast of strain Ca-4 were no different (p=0.99). Heat-killed yeast also improved phagocytosis rates for C. parapsilosis strains Ro18 (p=0.006) and Ro29 (p=0.005), but not Ro75 (p=0.39). Although some minor differences in phagocytosis efficiency between live and heat-killed yeast were observed in some strains, in all cases C. parapsilosis maintained higher phagocytosis rates with both live and heat-killed yeast than C. albicans (p < 0.001).
In addition to the higher rates of phagocytosis noted with C. parapsilosis, neutrophils with intracellular yeast were qualitatively noted to have a higher yeast burden with C. parapsilosis than with C. albicans. To quantify this observation, the number of yeast per neutrophil undergoing phagocytosis was counted for each strain. A single, representative strain of C. albicans (Ca3153a) and C. parapsilosis (Ro18) is depicted in Fig. 1C. The percentage of neutrophils undergoing phagocytosis that had 4 or more yeast per cell was significantly higher for C. parapsilosis than for C. albicans at a MOI of 100 or 50 (p=0.0001 for each comparison). There was a trend toward higher yeast burden with C. parapsilosis at a MOI of 10 as well (p=0.056).
Finally, because actin polymerization is known to be required for phagocytosis, we wished to demonstrate this requirement in our assays. Neutrophils were preincubated with cytochalasin D prior to exposure to yeast. Cytochalasin D treatment reduced phagocytosis to 8% or less in all strains (Fig. 1D).
Role of β-glucan/Dectin-1, mannan and chitin in phagocytosis of C. albicans and C. parapsilosis
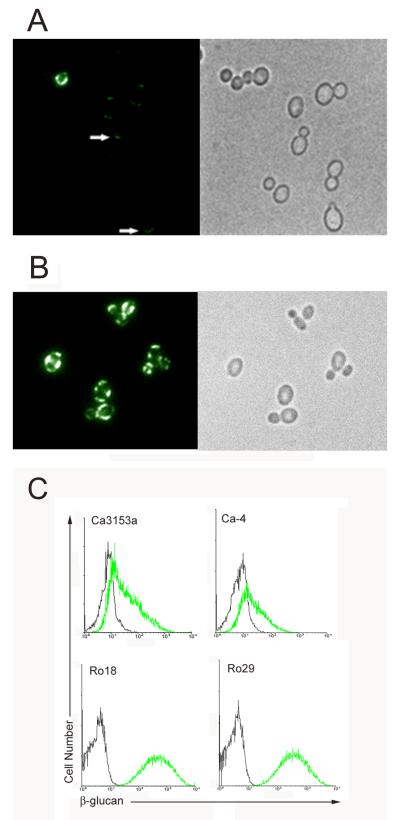
The β-glucan receptor, Dectin-1, has recently been shown to be expressed on neutrophils and to have a role in phagocytosis of C. albicans [20]. To assess whether this receptor was involved in the enhanced phagocytosis activity of C. parapsilosis, exposure of β-glucan on the cell surface of C. albicans and C. parapsilosis yeast was assessed by indirect immunofluorescence assay (IFA). Additionally, Dectin-1 blocking antibody and soluble glucan were used to determine the role of this receptor in phagocytosis. The β-glucan specific monoclonal antibody, BFDiv [22], was used to label β-glucan in live C. albicans and C. parapsilosis yeast cells by IFA (Fig. 2). This antibody binds to both β-(1-3)- and β-(1-6)-linked glucan. Exposure of β-glucan as detected by antibody binding was not uniform for C. albicans. Only occasional yeast showed detectable fluorescence, and in the majority of cells where fluorescence was detected, it was faint and localized to punctate regions of the cell (arrows in Fig. 2A). Labeling of C. parapsilosis yeast with the antibody was much more consistent and brighter, however binding was also not uniform, with some regions of the cell surface brighter than others (Fig. 2B). To quantify the differential binding of β-glucan specific antibody, yeast cells stained in an identical fashion were analyzed by flow cytometry (Fig. 2C). Two C. parapsilosis (Ro18 and Ro29) strains were analyzed. The third strain (Ro75) had a propensity to clump into cellular aggregates and was therefore less amenable to flow cytometric analysis. Consistent with the IFA results, C. parapsilosis strains exhibited much increased fluorescence relative to C. albicans.
Fig. 2. Indirect immunofluorescence assay and flow cytometric analysis of surface exposed β-glucan.
C. albicans (Ca3153a, Panel A) and C. parapsilosis (Ro18, Panel B) yeast incubated with the β-glucan specific monoclonal antibody, BF-Div. Antibody binding was detected with an appropriate FITC-labeled secondary antibody and viewed by fluorescence microscopy. All strains were tested and showed similar patterns. Representative strains are included in the figure. C. albicans yeast were not labeled uniformly by the antibody, with the majority of cells being either entirely negative or showing faint, localized fluorescence (arrows). In contrast, C. parapsilosis yeast were labeled more consistently and intensely by the antibody, but also with a nonuniform distribution. A phase contrast photomicrograph of the same microscopic field is included. To quantitate antibody binding, C. albicans (Ca3153a, Ca-4) and C. parapsilosis (Ro18, Ro29) yeast cells were labeled with β-glucan specific monoclonal antibody as described above and analyzed by flow cytometry (Panel C). The green line represents staining with BF-Div and the black line represents control yeast incubated with the secondary antibody only. Consistent with the IFA results, increased fluorescence intensity was seen with C. parapsilosis relative to C. albicans.
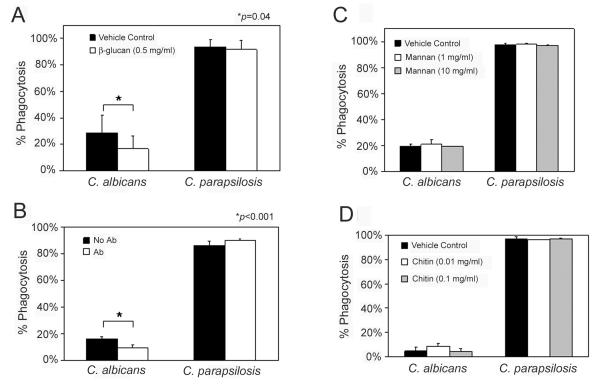
To determine whether the apparently increased surface exposure of β-glucan in C. parapsilosis relative to C. albicans contributed to the increased phagocytosis efficiency of the former, phagocytosis assays were conducted in the presence of excess β-glucan (Fig. 3A). Although β-glucan reduced phagocytosis of C. albicans (p = 0.04), excess β-glucan had no effect on C. parapsilosis phagocytosis. To investigate the role of the β-glucan receptor, Dectin-1, blocking antibody GE2 was coincubated with neutrophils prior to phagocytosis assays. Consistent with previous studies [20], blocking of Dectin-1 decreased the phagocytosis of C. albicans by 57% (p< 0.001) (Fig. 3B). In contrast, treatment of neutrophils with this antibody resulted in a small, but statistically significant increase in phagocytosis of C. parapsilosis (p= 0.02).
Fig. 3. Effect of excess carbohydrate and Dectin-1 blocking antibody on phagocytosis of C. albicans and C. parapsilosis.
Neutrophils were preincubated with excess β-glucan (Panel A); blocking antibody, GE2 (Ab), to the β-glucan receptor, Dectin-1 (Panel B); excess mannan (Panel C); or excess chitin (Panel D) prior to incubation with C. albicans (Ca3153a) or C. parapsilosis (Ro75) yeast in phagocytosis assays. Percent phagocytosis was calculated as described in Fig. 1. Data are from independent experiments, each performed in duplicate at minimum, from a minimum of 2 individual donors. Error bars represent standard deviation. Excess β-glucan and treatment with GE2 resulted in a significant decrease in phagocytosis of C. albicans, (p = 0.04 for glucan, p < 0.001 for GE2), but not C. parapsilosis. Treatment with excess mannan or chitin had no effect on phagocytosis efficiency of either species (p > 0.3 for mannan, p > 0.1 for chitin).
To investigate the possible contribution of other fungal polysaccharides to the difference in phagocytosis efficiency between C. albicans and C. parapsilosis, assays were conducted in the presence of excess mannan (Fig. 3C) or chitin (Fig. 3D). Neither of these polysaccharides resulted in any detectable inhibition of phagocytosis in either species.
Toxicity to Candida species mediated by human neutrophils
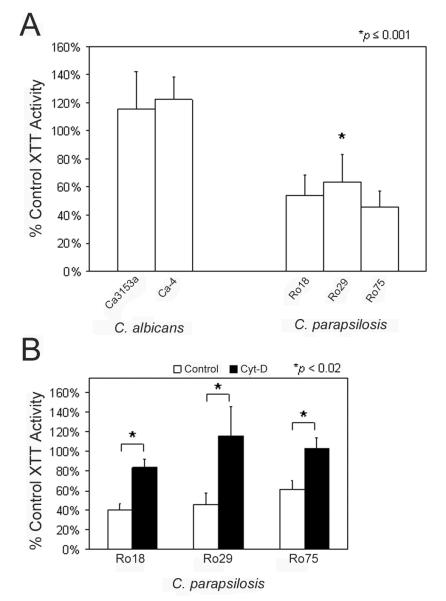
To assess the capacity of human neutrophils to damage unopsonized Candida yeast, metabolic activity of the yeast following coincubation was measured using the colorimetric XTT metabolism assay. Traditional, plate-based methods to measure fungal killing by neutrophils were found to provide inconsistent results and to be unduly affected by the tendency of yeast cells to adhere to plastic or each other (data not shown). This inaccuracy has also been noted by others [25]. To circumvent these issues, we opted to use metabolic activity as a surrogate for toxicity to the yeast cells. In these assays, neutrophils and Candida yeast were coincubated, the neutrophils lysed, and residual metabolic activity of the yeast was measured by XTT assay. After preliminary experiments using varied effector: target (E:T) ratios, a ratio of 2 neutrophils to 1 yeast was selected for subsequent analyses, as it allowed maximal discrimination between species and strains. Mean residual metabolic activity of each strain, expressed as a percent of total metabolic activity of the same strain of Candida incubated in the absence of neutrophils, is shown in Fig. 4A. All strains of C. parapsilosis were more susceptible to neutrophil-induced toxicity than C. albicans (p ≤ 0.001), and no statistically significant differences among strains of the same species were observed.
Fig. 4. Toxicity to C. albicans and C. parapsilosis mediated by neutrophils and effect of cytochalasin D.
(A) C. albicans and C. parapsilosis yeast were combined with neutrophils. After an incubation period, neutrophils were lysed, and residual metabolic activity of the yeast was measured by XTT. Data are expressed as the percentage of XTT activity of yeast of the same strain incubated in the absence of neutrophils. Data are from 5 independent experiments with 5 individual donors, and each was performed in triplicate. Error bars represent standard deviation. Neutrophils induced significant toxicity to each C. parapsilosis strain but no detectable toxicity to C. albicans strains (p ≤ 0.001 comparing each C. parapsilosis strain to each C. albicans strain). There were no significant differences among strains of the same species. (B) Neutrophils were pretreated with either cytochalsin D (Cyt-D) or buffer alone (Control) prior to inclusion in the toxicity assay with the 3 strains of C. parapsilosis. Data are expressed as the percentage of XTT activity of yeast of the same strain incubated in the absence of neutrophils and are from triplicate experiments. Error bars represent standard deviation. In each case, pretreatment of neutrophils with cytochalasin D resulted in significantly less toxicity than control (p < 0.02 for each strain).
The efficient phagocytosis of C. parapsilosis described above required actin polymerization, as pretreatment of neutrophils with cytochalasin D disrupted phagocytosis activity. To determine the requirement for actin polymerization in neutrophil-induced toxicity to C. parapsilosis, cytochalasin D treated neutrophils were used in the XTT assay. The capacity of neutrophils to induce toxicity to C. parapsilosis was significantly inhibited by cytochalasin D (Fig. 4B), suggesting that the induced toxicity was an active process that likely requires phagocytosis.
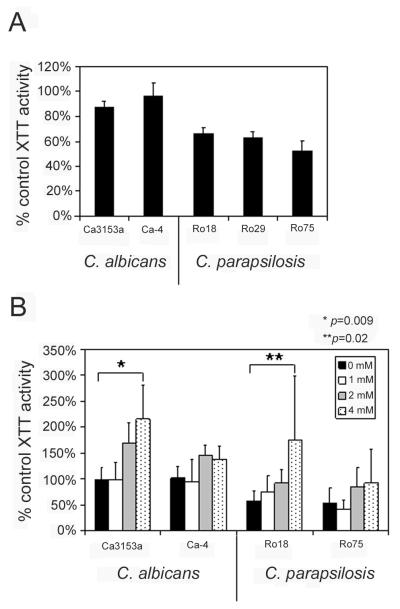
Neutrophils can generate a number of antimicrobial effects, including reactive oxygen species (ROS) as well as non-oxidative mechanisms. The observation that C. albicans is more resistant to neutrophil-induced toxicity may reflect this species’ resistance to phagocytosis. However, there may also be differences between these species in their intrinsic resistance to oxidative stress. To test this hypothesis, yeast forms of both species were treated with 1 mM hydrogen peroxide (H2O2), followed by XTT assay to determine residual metabolic activity (Fig. 5A). C. albicans yeast were more resistant than C. parapsilosis yeast to this form of oxidative stress (p < 0.01 for all strains). To investigate the contribution of oxidative mechanisms to the susceptibility of C. parapsilosis to neutrophil-mediated toxicity, assays were conducted in the presence of the ROS scavenger, N-acetylcysteine (Fig 5B). Although rates of phagocytosis were unaffected (data not shown), N-acetylcysteine decreased the toxicity of neutrophils in an apparently dose-dependent manner, achieving statistical significance in the case of C. albicans strain Ca3153A (p = 0.009) and C. parapsilosis strain Ro18 (p = 0.02). Similar trends were seen with the other strains, although statistical significance was not achieved. These data suggest that ROS play a role in neutrophil mediated toxicity, and the extent to which ROS contribute may vary among strains. Taken together, these data suggest that the resistance of C. albicans to neutrophil-mediated toxicity may be a combination of resistance to phagocytosis and resistance to oxidative stress, while C. parapsilosis is more susceptible to both. Generation of ROS is likely to contribute to neutrophil-mediated toxicity of C. parapsilosis.
Fig. 5. Contribution of oxidative mechanisms to neutrophil-mediated toxicity.
(A) To determine the inherent sensitivity of each species to oxidative stress, all strains were incubated in the presence of 1 mM H2O2, followed by XTT assay. Data are expressed as the percentage of XTT activity of yeast of the same strain incubated in the absence of H2O2. Error bars represent standard deviation. C. parapsilosis strains were significantly more sensitive to H2O2 than C. albicans (p < 0.01 for all strains). (B) Toxicity assays were conducted as described in Fig. 4 in the presence of varied concentrations of the ROS scavenger, N-acetylcysteine. Data are expressed as the percentage of XTT activity of yeast of the same strain incubated in the absence of neutrophils. Data are from 5 individual donors in a minimum of 4 individual experiments, and each was performed in triplicate. Error bars represent standard deviation. This agent led to a dose-dependent inhibition of toxicity, and statistical significance was achieved for C. albicans strain Ca3153A and C. parapsilosis strain Ro75 (* p = 0.009; ** p = 0.02). Because the quantity of neutrophils that could be obtained from an individual donor was limited, only two strains of each species were tested.
DISCUSSION
The importance of opsonins in efficient phagocytosis of C. albicans yeast has long been recognized. Lehrer and Cline reported greater than 90% phagocytosis of C. albicans yeast by neutrophils with human serum present, but when the serum was replaced by albumin or buffered salt solution, phagocytosis was “virtually absent” [26]. Likewise, Diamond et al. observed 5-8% ingestion of C. albicans by neutrophils when serum was absent [13]. Similar trends were observed more recently with the human monocytic cell line, THP-1 [12, 14]. Another study comparing phagocytosis rates among several fungal pathogens found similar rates of phagocytosis between C. albicans and C. parapsilosis, but all samples were opsonized with pooled human serum [27]. The two main sources of opsonins, antibody and complement, have the potential to create variability in assay systems. Antibody properties such as isotype, antigen specificity and binding affinity, as well as properties of the yeast cell target including abundance of cognate antigens and variability in their expression, may all impact the nature of phagocytosis. Likewise, the extent and efficiency of complement deposition on the yeast cell surface and the binding to complement receptors may not be uniform. Because opsonization inherently introduces additional complexities to the interaction between yeast and phagocyte, we conducted these experiments in the absence of opsonins. These conditions may also be relevant for premature infants, a group in which C. parapsilosis is particularly prevalent [3], and in whom low concentrations of antibody and key complement components have been reported [28]. Surprisingly, we detected a previously undescribed efficiency of phagocytosis with C. parapsilosis that is far greater than C. albicans under these conditions, and was associated with increased neutrophil-induced toxicity. Generation of ROS by the neutrophils is likely an important mechanism for the antifugal effect. The differences observed between these species may contribute to the increased virulence of C. albicans over C. parapsilosis in disseminated candidiasis.
We found ample, but nonuniform binding of the β-glucan antibody, BF-Div, to C. parapsilosis, but considerably less binding to yeast forms of C. albicans. These patterns are consistent with previously reported surface exposure of β-glucan in C. albicans as detected by a soluble form of the β-glucan receptor, Dectin-1, as probe [29]. The contribution of β-glucan and its receptor, Dectin-1, in interaction between human neutrophils and C. albicans has been the subject of recent study [20]. These authors demonstrated that treatment of neutrophils with the Dectin-1 blocking antibody, GE2, reduces phagocytosis of C. albicans yeast. Our data are consistent with this observation; however, despite apparently increased exposure of β-glucan on C. parapsilosis, receptor blockade with GE2 caused no reduction in phagocytosis nor did pretreatment with excess β-glucan. These data suggest that Dectin-1 is not required for the increased phagocytosis efficiency of C. parapsilosis. The binding of fungi by phagocytes is complex and is mediated primarily by a host of pattern-recognition receptors (PRRs), which recognize characteristic pathogen-associated molecular patterns (PAMPs) found on a wide range of microorganisms but absent in the host [11]. Individual fungal species can be recognized by several PRRs, and the outcomes of these interactions vary considerably [30]. The nature of these specific interactions likely explains the differences in phagocytosis between these two species. The receptor(s) important for the enhanced phagocytosis of C. parapsilosis is the subject of ongoing investigation.
Our finding of enhanced neutrophil-induced toxicity of C. parapsilosis relative to C. albicans is in agreement with others that found C. parapsilosis more susceptible to neutrophil killing by human [31] or murine [32] neutrophils. The latter study found that killing (based on colony forming unit counts) correlated with increases in toxicity, based on 51Cr release from labeled fungi. In contrast, Roilides et al. reported that C. parapsilosis was less susceptible to human neutrophil induced damage than C. albicans [33]. However, the neutrophils in this study were maintained in the absence of calcium and magnesium, which others have shown to be important for interaction of neutrophils with C. albicans [13]. In a study of murine macrophages, C. parapsilosis was again more efficiently killed than C. albicans [34]. The authors attributed the increased killing of C. parapsilosis to a relative decrease in stimulation of oxygen metabolism in the macrophages by C. albicans. Similar differences in macrophage killing of these two species were also reported by Brummer and Stevens [35]. Interestingly, macrophages were efficient at phagocytosis of both species, with C. parapsilosis rates only slightly higher than C. albicans. Phagocytosis of the two species was only modestly different whether serum was present or absent. This finding implies that the mechanism of phagocytosis by murine macrophages is intrinsically different from that of human neutrophils, perhaps through the use of differing receptors. Alternatively, murine macrophages may behave differently than human cells. Unlike the murine studies, the human monocytic cell line, THP-1, did not phagocytose C. albicans yeast efficiently in the absence of opsonins [12, 14].
The interaction between human neutrophils and C. parapsilosis is strikingly different than the interaction with C. albicans. Understanding these differences may help to explain the difference in virulence between these two species and provide insight in the molecular mechanisms of neutrophils which are varied and tailored toward the specific microbe faced. As Candida infections with non-albicans species increase, insights into the specifics at the host-fungus interface may aid in the design of novel therapeutic strategies in patients at risk.
ACKNOWLEDGEMENTS
We thank Jonathan Reichner and Liz Lavigne for providing mAb BF-Div and for helpful discussions. We thank Gordon Brown for providing mAb GE2, and Sunil Shaw for assistance with FACS analysis.
This work was supported in part by Basil O’Connor Starter Scholar Research Award Grant No. 5-FY05-1211 from the March of Dimes Foundation, a National Institute of Health grant (K08 AI064919), and a NIH COBRE grant (P20 RR018728).
Footnotes
DISCLOSURE OF CONFLICT OF INTEREST None.
REFERENCES
- 1.Wisplinghoff H, Bischoff T, Tallent SM, et al. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 2.Calderone RA, editor. Candida and Candidiasis. ASM Press; Washington D.C.: 2002. [Google Scholar]
- 3.Benjamin DK, Jr., Stoll BJ, Fanaroff AA, et al. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics. 2006;117:84–92. doi: 10.1542/peds.2004-2292. [DOI] [PubMed] [Google Scholar]
- 4.Fridkin SK, Kaufman D, Edwards JR, Shetty S, Horan T. Changing incidence of Candida bloodstream infections among NICU patients in the United States: 1995-2004. Pediatrics. 2006;117:1680–1687. doi: 10.1542/peds.2005-1996. [DOI] [PubMed] [Google Scholar]
- 5.Krcmery V, Fric M, Pisarcikova M, et al. Fungemia in neonates: report of 80 cases from seven university hospitals. Pediatrics. 2000;105:913–914. doi: 10.1542/peds.105.4.913. [DOI] [PubMed] [Google Scholar]
- 6.Pagano L, Antinori A, Ammassari A, et al. Retrospective study of candidemia in patients with hematological malignancies. Clinical features, risk factors and outcome of 76 episodes. Eur J Haematol. 1999;63:77–85. doi: 10.1111/j.1600-0609.1999.tb01120.x. [DOI] [PubMed] [Google Scholar]
- 7.Pfaller MA, Diekema DJ, Jones RN, et al. International surveillance of bloodstream infections due to Candida species: frequency of occurrence and in vitro susceptibilities to fluconazole, ravuconazole, and voriconazole of isolates collected from 1997 through 1999 in the SENTRY antimicrobial surveillance program. J Clin Microbiol. 2001;39:3254–3259. doi: 10.1128/JCM.39.9.3254-3259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gacser A, Schafer W, Nosanchuk JS, Salomon S, Nosanchuk JD. Virulence of Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis in reconstituted human tissue models. Fungal Genet Biol. 2007;44:1336–1341. doi: 10.1016/j.fgb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Gacser A, Trofa D, Schafer W, Nosanchuk JD. Targeted gene deletion in Candida parapsilosis demonstrates the role of secreted lipase in virulence. J Clin Invest. 2007;117:3049–3058. doi: 10.1172/JCI32294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mansour MK, Levitz SM. Interactions of fungi with phagocytes. Curr Opin Microbiol. 2002;5:359–365. doi: 10.1016/s1369-5274(02)00342-9. [DOI] [PubMed] [Google Scholar]
- 11.Nicola AM, Casadevall A, Goldman DL. Fungal killing by mammalian phagocytic cells. Curr Opin Microbiol. 2008;11:313–317. doi: 10.1016/j.mib.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wellington M, Bliss JM, Haidaris CG. Enhanced phagocytosis of Candida species mediated by opsonization with a recombinant human antibody single-chain variable fragment. Infect Immun. 2003;71:7228–7231. doi: 10.1128/IAI.71.12.7228-7231.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diamond RD, Krzesicki R, Jao W. Damage to pseudohyphal forms of Candida albicans by neutrophils in the absence of serum in vitro. J Clin Invest. 1978;61:349–359. doi: 10.1172/JCI108945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wellington M, Dolan K, Haidaris CG. Monocyte responses to Candida albicans are enhanced by antibody in cooperation with antibody-independent pathogen recognition. FEMS Immunol Med Microbiol. 2007;51:70–83. doi: 10.1111/j.1574-695X.2007.00278.x. [DOI] [PubMed] [Google Scholar]
- 15.Morrow B, Ramsey H, Soll DR. Regulation of phase-specific genes in the more general switching system of Candida albicans strain 3153A. J Med Vet Mycol. 1994;32:287–294. doi: 10.1080/02681219480000361. [DOI] [PubMed] [Google Scholar]
- 16.Vargas K, Wertz PW, Drake D, Morrow B, Soll DR. Differences in adhesion of Candida albicans 3153A cells exhibiting switch phenotypes to buccal epithelium and stratum corneum. Infect Immun. 1994;62:1328–1335. doi: 10.1128/iai.62.4.1328-1335.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bliss JM, Sullivan MA, Malone J, Haidaris CG. Differentiation of Candida albicans and Candida dubliniensis by using recombinant human antibody single-chain variable fragments specific for hyphae. J Clin Microbiol. 2003;41:1152–1160. doi: 10.1128/JCM.41.3.1152-1160.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bliss JM, Basavegowda KP, Watson WJ, Sheikh AU, Ryan RM. Vertical and horizontal transmission of Candida albicans in very low birth weight infants using DNA fingerprinting techniques. Pediatr Infect Dis J. 2008;27:231–235. doi: 10.1097/INF.0b013e31815bb69d. [DOI] [PubMed] [Google Scholar]
- 19.Bhatty RS. Laboratory and pilot plant extraction and purification of β-glucans from hullless barley and oat brans. J Cereal Sci. 1995;22:163–170. [Google Scholar]
- 20.Kennedy AD, Willment JA, Dorward DW, et al. Dectin-1 promotes fungicidal activity of human neutrophils. Eur J Immunol. 2007;37:467–478. doi: 10.1002/eji.200636653. [DOI] [PubMed] [Google Scholar]
- 21.Hu X, Du Y, Tang Y, et al. Solubility and property of chitin in NaOH/urea aqueous solution. Carbohydr Polym. 2007;70:451–458. [Google Scholar]
- 22.Lavigne LM, Albina JE, Reichner JS. Beta-glucan is a fungal determinant for adhesion-dependent human neutrophil functions. J Immunol. 2006;177:8667–8675. doi: 10.4049/jimmunol.177.12.8667. [DOI] [PubMed] [Google Scholar]
- 23.Decleva E, Menegazzi R, Busetto S, Patriarca P, Dri P. Common methodology is inadequate for studies on the microbicidal activity of neutrophils. J Leukoc Biol. 2006;79:87–94. doi: 10.1189/jlb.0605338. [DOI] [PubMed] [Google Scholar]
- 24.Meshulam T, Levitz SM, Christin L, Diamond RD. A simplified new assay for assessment of fungal cell damage with the tetrazolium dye, (2,3)-bis-(2-methoxy-4-nitro-5-sulphenyl)-(2H)-tetrazolium-5-carboxanilide (XTT) J Infect Dis. 1995;172:1153–1156. doi: 10.1093/infdis/172.4.1153. [DOI] [PubMed] [Google Scholar]
- 25.Vonk AG, Wieland CW, Netea MG, Kullberg BJ. Phagocytosis and intracellular killing of Candida albicans blastoconidia by neutrophils and macrophages: a comparison of different microbiological test systems. J Microbiol Methods. 2002;49:55–62. doi: 10.1016/s0167-7012(01)00348-7. [DOI] [PubMed] [Google Scholar]
- 26.Lehrer RI, Cline MJ. Interaction of Candida albicans with human leukocytes and serum. J Bacteriol. 1969;98:996–1004. doi: 10.1128/jb.98.3.996-1004.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyman CA, Walsh TJ. Phagocytosis of medically important yeasts by polymorphonuclear leukocytes. Infect Immun. 1994;62:1489–1493. doi: 10.1128/iai.62.4.1489-1493.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carr R. Neutrophil production and function in newborn infants. Br J Haematol. 2000;110:18–28. doi: 10.1046/j.1365-2141.2000.01992.x. [DOI] [PubMed] [Google Scholar]
- 29.Gantner BN, Simmons RM, Underhill DM. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J. 2005;24:1277–1286. doi: 10.1038/sj.emboj.7600594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Netea MG, Brown GD, Kullberg BJ, Gow NA. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol. 2008;6:67–78. doi: 10.1038/nrmicro1815. [DOI] [PubMed] [Google Scholar]
- 31.Borg-von Zepelin M, Schuff-Werner P. Chemiluminescence of polymorphonuclear granulocytes in the presence of selected Candida species. Mycoses. 1992;35:121–129. doi: 10.1111/j.1439-0507.1992.tb00832.x. [DOI] [PubMed] [Google Scholar]
- 32.Vecchiarelli A, Bistoni F, Cenci E, Perito S, Cassone A. In-vitro killing of Candida species by murine immunoeffectors and its relationship to the experimental pathogenicity. Sabouraudia. 1985;23:377–387. doi: 10.1080/00362178585380541. [DOI] [PubMed] [Google Scholar]
- 33.Roilides E, Holmes A, Blake C, Pizzo PA, Walsh TJ. Effects of granulocyte colony-stimulating factor and interferon-gamma on antifungal activity of human polymorphonuclear neutrophils against pseudohyphae of different medically important Candida species. J Leukoc Biol. 1995;57:651–656. doi: 10.1002/jlb.57.4.651. [DOI] [PubMed] [Google Scholar]
- 34.Sasada M, Johnston RB., Jr. Macrophage microbicidal activity. Correlation between phagocytosis-associated oxidative metabolism and the killing of Candida by macrophages. J Exp Med. 1980;152:85–98. doi: 10.1084/jem.152.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brummer E, Stevens DA. Candidacidal mechanisms of peritoneal macrophages activated with lymphokines or gamma-interferon. J Med Microbiol. 1989;28:173–181. doi: 10.1099/00222615-28-3-173. [DOI] [PubMed] [Google Scholar]