Abstract
There is a current controversy regarding whether non-human animals have a capacity for episodic memory, defined by the ability to remember what happened and where and when it occurred. It is also unclear which brain structures support the “what,” “where,” and “when” aspects of episodic memory. Here we addressed these issues by testing episodic memory in mice, using an object recognition task that has previously been employed to assess the “what,” “where,” and “when” components of recognition memory. Whereas intact mice remembered which objects they had explored, as well as when and where they were experienced, mice with damage to the hippocampus were impaired on all three components of the task. In contrast, animals with medial prefrontal cortical lesions were selectively impaired on the “where” component of the task, but had intact memory for “what” and “when.” These results are consistent with the hypothesis that the hippocampus integrates the “what,” “where,” and “when” features of unique experiences, whereas the prefrontal cortex makes a more selective contribution to retrieving source information about where events occurred.
Keywords: recognition, temporal order, spatial memory, object memory, lesions, mice
Introduction
Episodic memory is defined by the integration of all the distinct features of experiences that supports the conscious recollection of those events. The specific components of those memories consist of a particular object or person (memory for “what” happened), the context or environment in which the experience occurred (memory for “where” it happened), and the time at which the event occurred (“when” it happened). The ability to integrate these “what,” “where,” and “when” features of an event is considered fundamental to the subjective experience of human episodic memory (Tulving & Markowitsch, 1998; Dere et al., 2006). However, many studies have now demonstrated these features of memory in non-human animals; for example, food-caching scrub jays can remember a series of food storage sites, the particular places where specific items were stored, and the particular times they were stored (Clayton & Dickinson, 1998). The demonstration of these features of memory across species has raised important questions pertaining to the fundamental mechanisms associated with episodic memory. It has further been suggested that true episodic memory depends on the simultaneous integration of these features pertaining to a unique experience (Griffiths et al., 1999). Here we employed an object recognition task developed by Dere et al. (2005a) that distinguishes “what,” “where,” and “when” information about a series of unique experiences to determine which specific brain areas support these features of memory.
Regarding the anatomical substrates of “what-where-when” memory, previous studies have suggested a role for the hippocampus and medial prefrontal cortex in object recognition (Mitchell & Laiacona, 1998; Mumby et al., 2002; Hammond et al., 2004; Hannesson et al., 2004a). The hippocampus and medial prefrontal cortex are directly connected and their interactions have been implicated in working memory and retrieval processes (Nyberg et al., 1996; Verwer et al, 1997; Simons & Spiers, 2003). Furthermore, recent studies using signal detection analyses to examine recognition memory performance indicate that the hippocampus and prefrontal cortex both play important and complementary roles in the recollection component of recognition memory (Fortin et al., 2004; Farovik et al., 2008). Here, we extend our exploration of hippocampal and prefrontal contributions to episodic memory by evaluating their roles in the “what,” “where,” and “when” components of episodic-like memory in mice.
Materials and Methods
Animals
Male C57 black mice were purchased from the Charles River Lab. All animals were maintained on a reverse 12-hour light/dark cycle [09:00 off; 21:00 on]. Animals were given ad libitum access to food and water, unless otherwise specified in behavioral methods. Forty-five animals were used in this study: 8 sham-operated hippocampus controls, 8 sham-operated medial prefrontal controls, 14 medial prefrontal lesions, and 15 hippocampal lesions. The IACUC of Boston University approved the treatment and use of the animals in these experiments.
Surgery
Bilateral lesions of the hippocampus were made using NMDA, or sterile PBS for sham operations (Sigma, 10 mg/mL), delivered via a microinfusion pump connected to a 5-uL Hamilton syringe. Animals were anesthetized with a ketamine/xylazine cocktail (0.01mL/g), and diazepam (0.02 mL) was administered pre-operatively in order to prevent seizures. After the animal had been placed into a stereotaxic head frame, the skull was exposed and the coordinates of bregma were measured. The skull overlying the four coordinates was drilled and dura was removed. Before infusions were made, the syringe was lowered 0.2 mm for the first two coordinates (dorsal hippocampus) and 0.5 mm for the last two coordinates (ventral hippocampus) past the injection site and kept a lower depth for one minute in order to increase spread of drug diffusion. The syringe was then raised to the injection site and the drug was infused over a 2-minute period (3-minute infusion for the last coordinate). The needle was left in place for another 5 minutes before being slowly withdrawn. The complete dorsal and ventral hippocampus was targeted (including the CA fields, dentate gyrus, and subiculum) at four stereotaxic coordinates: AP + 1.7, ML +/− 1.2, DV − 1.5; AP + 2.3, ML +/− 1.75, DV − 1.75; AP + 2.8, ML +/− 3, DV − 3; AP +3.1, ML +/− 2.85, DV − 3.75; 50 nL was infused into the first three sites and 75 nL was infused into the fourth site.
Bilateral lesions of the medial prefrontal cortex were made using ibotenic acid, or sterile saline for sham operations (Tocris Cookson, 0.06 m), delivered via a microinfusion pump connected to a 10-uL Hamilton syringe attached to a pulled microglass pipette tip. Animals were anesthetized with a ketamine/xylazine cocktail (0.01mL/g), and diazepam (0.02 mL) was administered pre-operatively in order to prevent seizures. After the animal had been placed into a stereotaxic head frame, the skull was exposed and the coordinates of bregma were measured (the medial-lateral values were taken at the level of the mid-sagittal vein, and not at bregma). The skull overlying the two coordinates was drilled and dura was removed. The syringe was lowered to the injection site and the drug was infused over a 5-minute period. The needle was left in place for another 5 minutes before being slowly withdrawn. The infralimbic and prelimbic cortices were targeted at two stereotaxic coordinates: AP −2.1, ML +/− .25, DV − 2.3; 150 nL was infused into both sites.
After all infusions, the scalp was sutured, the animal was given 0.4 mL of Lactated Ringer’s solution to hydrate, and the animal was placed next to a hot water bottle to return body temperature to normal. After surgery, the animal received Children’s Tylenol in its water and was provided with soft food and Nutrical. Each animal was allowed two weeks to recover before behavioral testing.
Object recognition: “What-where-when” memory
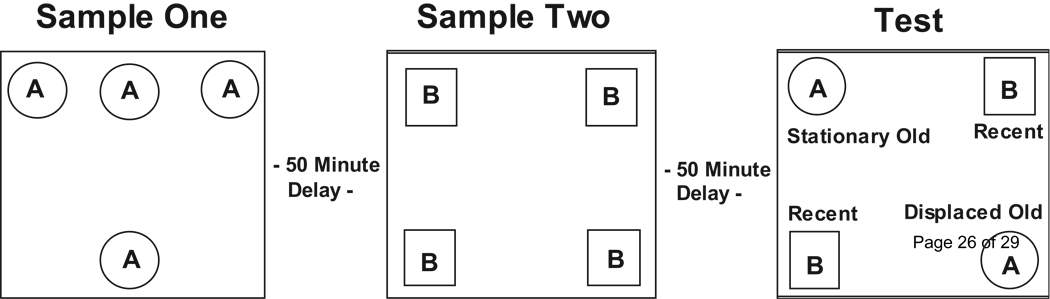
The animals were tested on an episodic memory task that assesses memory for objects (“what”), their locations (“where”), and the order in which they were experienced (“when”; Dere et al., 2005a). Animals were first habituated to a rectangular testing apparatus (40 cm length × 20 cm width × 20 cm height) with visual cues on the wall for a 5-minute period. On the second day, they were placed in the testing apparatus and presented with two different objects, used only on this preliminary exposure phase, for another 5-minute period. The following day, the animals were given two “Sample” sessions and one “Test” trial with Objects “A” and Objects “B” (Figure 1). Pilot studies revealed that the amount of total object exploration significantly increased across the two sample and test sessions. These data confirm previous findings by Dere et al. (2005b) and suggest that the increase in exploration is due to the recognition of novel object and spatial configurations. It also suggests that differences in object exploration in the test session reflect the animal’s memory for the objects and does not reflect innate preferences.
Figure 1.
Object recognition: “What-where-when” memory. Schematic of testing arena used to assess object recognition.
In Sample 1, the animal was allowed 5 minutes to explore 4 identical novel objects (Object A) arranged in a triangular shape. After a 50-minute delay, in Sample 2, the animal explored 4 new identical objects (Object B) arranged in a rectangular shape. After another 50-minute delay, the animal was given the Test trial in which two copies of Object B were placed in the Northeast and Southwest corners, as in Sample 2 (“recent objects”), one copy of Object A was placed in the Northwest corner as it had been in Sample 1 (“stationary old object”), and one copy of Object A was newly located to the Southeast corner (“displaced old object”). Exploratory behavior was recorded via a WebCam (Gigaware®, UK) positioned above the testing arena and later scored for investigation time of each object in both Sample sessions and the Test session. Two researchers blind to the subject’s group coded each video. Any animal that had a significant innate spatial bias, defined by greater exploration at one location compared to all other locations in both Sample and Test trials, was removed from analysis.
Discrimination ratios based on exploration of particular combinations of objects in the Test session were used for measures of each type of memory. The discrimination ratio for recognition of the distinct objects (“what” memory) was calculated as the difference between the average exploration times for both Objects A minus that for the recent Objects B, divided by the sum of those times. This measure reflects the animal’s ability to differentiate the two groups of objects, exploiting the inherent tendency of mice to spend more time exploring a group of less recently experienced objects compared to a group of more recently experienced objects. “What” memory is indicated by a greater than zero “what” discrimination ratio, which reflects greater exploration time for Objects A compared to Objects B.
The discrimination ratio for “where” memory was calculated as the difference between the exploration time for the “displaced” Object A and that for the “stationary” Object A, divided by the total time exploring both objects. This measure exploits the inherent tendency of mice to spend more time exploring an object that has changed location. “Where” memory is indicated by a greater than zero “where” discrimination ratio, which reflects greater exploration time for the “displaced” Object A compared to the “stationary” Object A.
The discrimination ratio for “when” memory was calculated as the difference between the exploration time for the “stationary” Object A (“old”) and that for the average exploration times for Objects B (“recent”), divided by the sum of those times. This measure exploits the inherent tendency of mice to spend more time exploring less recently experienced objects compared to more recently experienced objects, which were all experienced in the same location at different times. “When” memory is indicated by a greater than zero “when” discrimination ratio, which reflects greater exploration time for the “stationary” Object A compared to Objects B.
This variant of the novel object recognition task allows us to assess the ability of animals to integrate identity, temporal order, and spatial arrangement of the objects. It is acknowledged that the “what” memory ratio also reflects differences in exploration of more recently versus less recently experienced objects. However, the discrimination ratio for “what” memory compares exploration times for all more recently versus all less recently experienced groups of objects, whereas that for “when” memory compares exploration times for objects appearing in the same locations where they were presented in Samples 1 and 2 (Dere et al., 2005a). Although the “what” and “when” measures are not completely segregated, they represent different measures of the contribution of time as well as object information into an integrated episode, and help us assess all of the relative components that, when bound together in memory, allow for the retrieval of all of the features that comprise a complete event.
Histology
After behavioral testing, all animals were given an overdose of sodium pentobarbital and perfused transcardially with 4% formalin. The brains were removed and post-fixed for an hour in formalin, and then cryoprotected in 30% sucrose solution (in 7.4 pH PBS). Coronal sections were cut (40 um) using a freezing microtome. Every section was mounted on gelatin-coated slides and dried overnight. Slides were soaked in xylenes and then run through a series of ethanol dehydrations, stained with cresyl violet, and then rehydrated.
Four representative sections along the anterior-posterior axis of the hippocampus (AP: +1.7, 2.3, 2.8, 3.08) were selected from the mouse brain atlas in order to determine tissue damage (Franklin & Paxinos, 1997). Canvas 5.0 (Deneba Software, ACD Systems International Inc.) was used to calculate the area of the hippocampus in each section. For each animal, the four brain slices most closely corresponding to the representative sections in the brain atlas were used. Percentage of tissue damage was calculated as the amount of total damage divided by the total area of the hippocampus in that section X 100; the average of those four values represented the lesion extent. Additional sections were studied under the light microscope in order to identify incidental damage outside the targeted regions and were reported in the results section.
Three representative sections along the anterior-posterior axis of the medial prefrontal cortex (AP: −2.58, 2.1, 1.7) were selected from the mouse brain atlas in order to determine tissue damage (Franklin & Paxinos, 1997). Canvas 5.0 (Deneba Software, ACD Systems International Inc.) was used to calculate the area of the infralimbic and prelimbic cortices in each section. For each animal, the three sections most closely corresponding to the representative sections in the brain atlas were used. Percent damage was calculated as the amount of total damage divided by the total area of the prefrontal cortex in that section X 100; the average of those three values represented the lesion extent. Additional sections were studied under the light microscope in order to determine if any incidental damage occurred outside the targeted regions and were reported in the results section.
Results
Histology
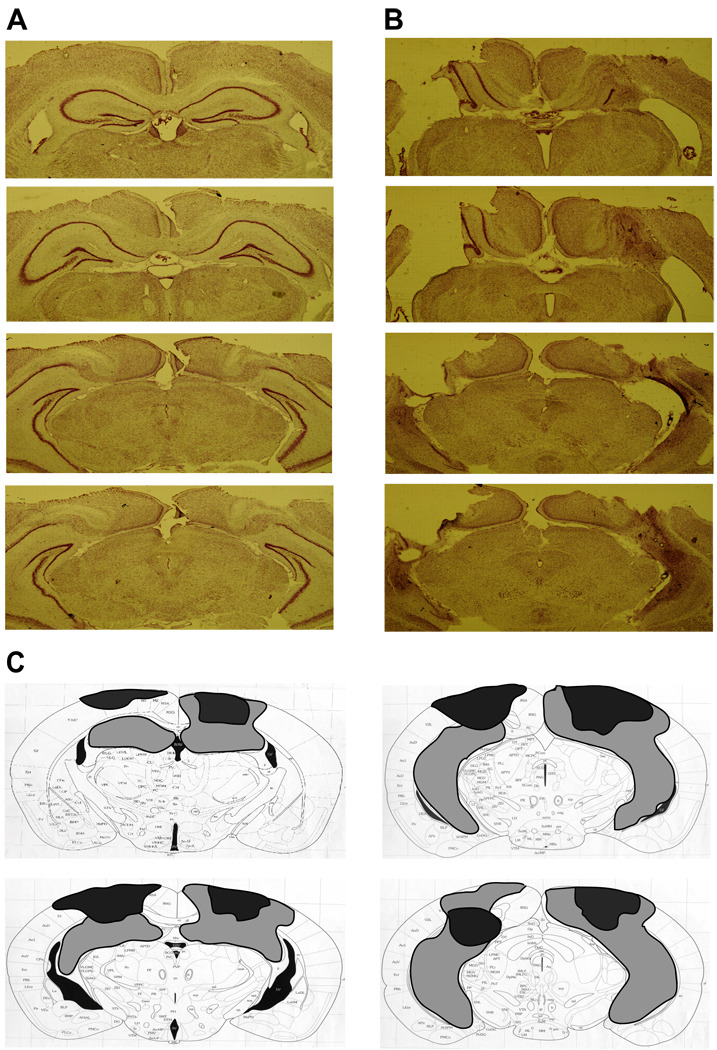
NMDA infusions resulted in a substantial loss of cells within the hippocampal formation, including Ammon’s horn, the dentate gyrus, and the subiculum (Figure 2A, B). An average of 54% of the hippocampus was damaged across animals, ranging from 9%–100% total ablation (Figure 2C). Some animals had partial damage to the thalamus, and three had unilateral medial entorhinal damage. The size of the lesion was not correlated with the extent of the behavioral deficit found (“what” memory: r=−0.19, p=0.491; “where” memory: r=0.26, p=0.338; “when” memory: r=−0.34, p=0.205). One animal with an intended hippocampal lesion was excluded due to lack of significant damage.
Figure 2.
Histological verification of the extent of hippocampal damage. A. Representative sections from four coordinates along the anterior-posterior axis of the mouse hippocampus in a sham-operated animal. B. Corresponding sections from an animal given NMDA infusions into the hippocampus. C. A diagram shows the extent of the largest (light gray) and smallest (black) lesion across the fifteen animals.
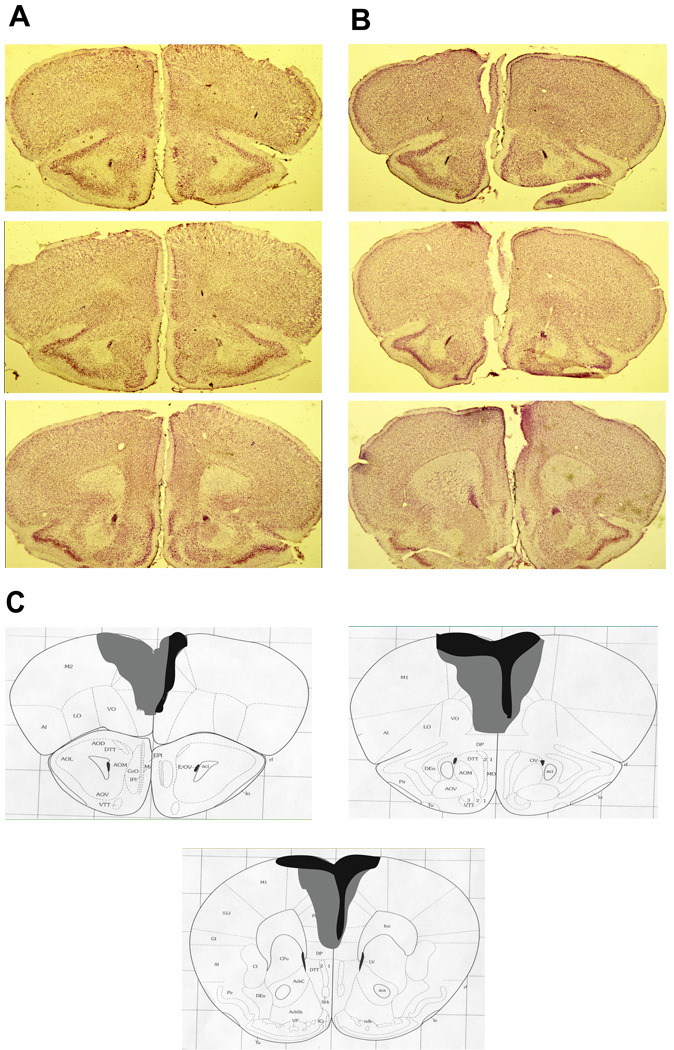
Ibotenic acid infusions resulted in a substantial loss of cells within the medial wall of the prefrontal cortex, including the prelimbic and infralimbic cortices and dorsal cingulate gyrus (Figure 3A, B). An average of 41% of the medial prefrontal cortex was damaged across all animals, ranging from 12%–81% (Figure 3C). Two animals had additional damage to the medial orbital cortex, and one animal had unilateral damage to motor cortex. The size of the lesion was not correlated with the extent of the behavioral deficit found (“where” memory: r=−0.15, p=0.591). One animal with an intended medial prefrontal lesion was excluded due to lack of significant damage.
Figure 3.
Histological verification of the extent of medial prefrontal cortex damage. A. Representative sections from three coordinates along the anterior-posterior axis of the mouse prefrontal cortex in a sham-operated animal. B. Corresponding sections from an animal given ibotenic acid infusions into the medial prefrontal cortex. C. A diagram shows the extent of the largest (light gray) and smallest (black) lesion across the fourteen animals.
There were no significant differences in performance between sham-operated groups where the infusions targeted the hippocampus or the prefrontal cortex (“what” memory: t(1,15)=0.14, p=0.713; “where” memory: t(1,15)=0.01, p=0.917; “when” memory: t(1,15)=0.19, p=0.664); therefore, these two groups were combined and are collectively described as the “sham” group.
Object recognition memory
“What” memory
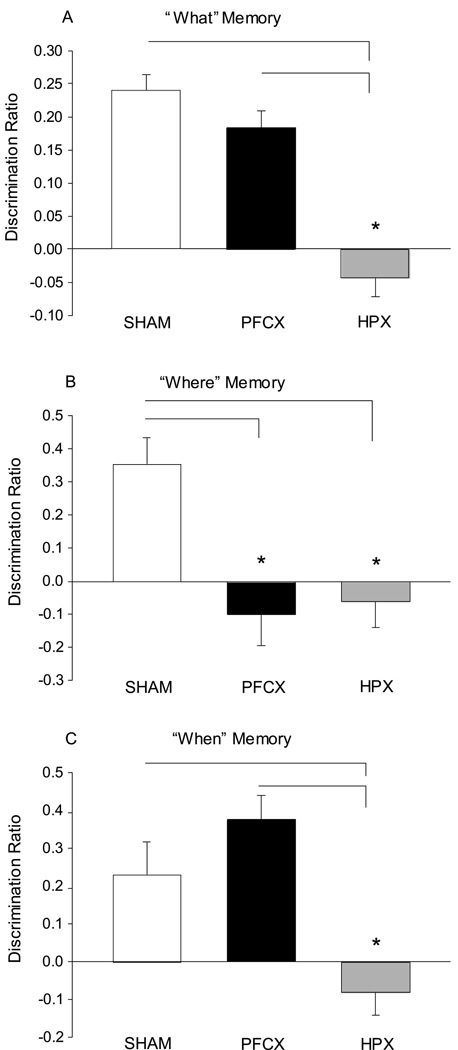
“What” memory is reflected by greater exploration time for all less recently experienced objects over all more recently experienced objects. The strength of the “what” memory discrimination ratio differed significantly between the three groups (1-Way ANOVA: F(2,44)=36.48, p<0.001; Figure 4A). Post hoc comparisons between groups indicated that the animals with hippocampal damage were impaired compared to sham-operated animals (p<0.001) and animals with prefrontal damage (p<0.001). Furthermore, the average discrimination ratios for both the sham-operated mice (t(1,31)=112.20, p<0.001) and mice with prefrontal lesions (t(1,27)=52.01, p<0.001) were significantly greater than zero; however, the ratio for mice with hippocampal lesions did not indicate greater than zero “what” memory (t(1,29)=2.63, p=0.116).
Figure 4.
“What-where-when” memory. A. A discrimination ratio was used to assess preference for the older versus recent objects (“what” memory). Both sham-operated and medial prefrontal lesion animals show a significant preference for the older objects, while the hippocampal lesion animals do not. B. A discrimination ratio was also used to assess preference for the displaced versus the stationary object (“where” memory). Both hippocampal and medial prefrontal lesion animals fail to show significant “where” memory. C. In addition, a discrimination ratio was used to assess preference for the older stationary object versus the more recent objects (“when” memory). Hippocampal lesion animals do not show memory for the temporal order of object presentation. SHAM: sham-operated animals; PFCX: medial prefrontal lesions; HPX: hippocampal lesions. * p<.05.
“Where” memory
“Where” memory is reflected by greater exploration time for objects in novel places. The strength of the “where” memory discrimination ratio differed significantly between the three groups (1-Way ANOVA: F(2,44)=9.50, p<0.001; Figure 4B). Post hoc comparisons between groups indicated that animals with damage to the hippocampus were impaired compared to sham-operated animals (p=0.001). Animals with damage to the prefrontal cortex were also impaired compared to sham controls (p<0.001). The average discrimination ratio for the sham-operated animals was significantly greater than zero (t(1,31)=20.31, p<0.001); however, the ratios for mice with prefrontal lesions (t(1,27)=1.07, p=0.309) and those with hippocampal lesions (t(1,29)=0.66, p=0.423) were not significantly above zero.
“When” memory
“When” memory is reflected by greater exploration times for objects that were experienced earlier in the same location. Discrimination ratios for “when” memory differed significantly between the three groups (1-Way ANOVA: F(2,44)=18.218, p<0.001; Figure 4C). Post hoc comparisons between groups revealed that animals with damage to the hippocampus were impaired relative to sham-operated controls (p=0.003) and animals with damage to the prefrontal cortex (p<0.001). Furthermore, the discrimination ratio for sham-operated mice (t(1,31)=7.42; p=0.011) and mice with prefrontal lesions (t(1,27)=36.11; p<0.001) were significantly above zero, whereas mice with hippocampal lesions did not show above zero “when” memory (t(1,29)=0.66; p=0.423).
Discussion
In previous studies, we have shown that rats can remember the order of a unique sequence of events and the particular places in which they occurred (Fortin et al., 2002; Ergorul & Eichenbaum, 2004). Other studies have used variants of novel object recognition tasks to show that animals can remember what objects have been experienced and when and where they were experienced (Mumby, 2001; Dere et al., 2005a,b). Here we extend these findings by demonstrating that mice can recall the temporal order and locations in which equally familiar objects were presented, and that damage to the hippocampus and medial prefrontal cortex disrupt specific features of this kind of episodic memory.
The hippocampus integrates the “what-where-when” features of episodic memory
Previous studies have suggested that the hippocampus plays a critical role in integrating the “what,” “where,” and “when” components of memories. Eacott & Norman (2004) demonstrated that normal rats could differentiate objects based on their location and context, but that damage to the fornix eliminates memory for the objects, their spatial position, and the context in which they were experienced. Rats can also learn a unique flavor-location association in a single trial and this capability is dependent upon the functional integrity of the hippocampus (Day et al., 2003). In our own previous work, we trained rats on a task that assesses memory for events from single episodes involving a combination of odors (“what”) presented in unique places (“where”) in a specific order (“when”; Ergorul & Eichenbaum, 2004). On each trial, rats sequentially sampled a unique series of four rewarded odor stimulus cups, each presented in a different place along the periphery of a large open field. Then, memory for the order of those events was tested by presenting the animal with a choice between an arbitrarily selected pair of the odor cups in their original locations. Since rats can employ memory for the locations of the cups (“where”) without using odor information (“what”), we also measured responses based purely on location information in two ways: first, we recorded the initial odor cup the animal approached (we separately determined that rats can’t tell which odor is inside until they approach the cup); second, we presented probe memory tests in which the odors were omitted and the rats had to use the locations only to identify which odor was presented earlier.
Normal animals performed well on the standard “what-where-when” memory tests. Furthermore, intact rats performed above chance but less well in first approaching the correct cup than on the standard test. Therefore, it appears that normal rats make an initial good guess about which item occurred first (“when”) based on location information (“where”) and then confirm or disconfirm their choice based on the odor in the cup (“what”). Furthermore, normal rats fell to chance performance on the probe tests in which odors were omitted, providing significant evidence that normal rats form strongly integrated representations of what happened when and where, such that they considered items that lacked the correct “what” component distinct from either correct item. Rats with selective hippocampal damage were severely impaired on the standard “what-where-when” memory judgments, performing no better than chance. Interestingly, animals with hippocampal damage tended to first approach the most recently reinforced cup, in opposition to their training to approach the cup presented earlier, suggesting that their performance was driven by an intact system guided by recent reinforcement. These observations indicate that normal rats can remember single episodes of what happened where and when, and that this ability is based on highly integrated “what-where-when” representations that are supported by the hippocampus.
Confirming this conclusion, in a recent study we recorded from hippocampal neurons in rats performing a task in which they had to learn what happens where. We found that hippocampal neurons develop representations of particular events in specific places. Furthermore, the appearance of these representations, and not representations of individual items or places, parallels learning and predicts performance accuracy (Komorowski et al., 2009). These findings complement the results from the present study suggesting that a fundamental aspect of hippocampal memory processing is the integration of the “what-where-when” features of distinct experiences.
Other studies on the role of the hippocampus in object recognition memory have reported modest effects of hippocampal damage (Hammond et al., 2004; Rossato et al., 2007). In comparing our results to previous studies, it is important to note that our animals were exposed to equally familiar objects in the test phase; thus, we were not testing the ability to detect object novelty. The hippocampus itself might not be necessary to discriminate individual items on the basis of familiarity, but instead may play a role if the memory judgment requires the integration of distinct features (Jenkins et al., 2004). Therefore, it is possible that regions outside of the hippocampus, such as the perirhinal cortex, may be sufficient to support novelty judgments for individual stimuli (Aggleton & Brown, 2005; Mumby et al., 2002). Conversely, when spatial and temporal context are associated with item memories, the hippocampus becomes critically engaged.
The contribution of the prefrontal cortex to episodic memory
Although animals with damage to the prefrontal cortex were able to express memory for “what” (object identity) and “when” (temporal order of object presentation), they were significantly impaired in expressing memory for “where” (object displacement). Studies of patients with damage to the frontal lobes have reported significant impairment in source memory, or memory for the context in which an event occurred (Duarte et al., 2005). In animal studies, prefrontal damage produces deficits in learning object-context associations. For example, Browning et al. (2005) trained monkeys to differentiate objects based on the location of those objects embedded in complex scenes, and the results suggested that the prefrontal cortex is essential for remembering the integration of objects in relation to their spatial context. Additionally, imaging studies have reported engagement of the prefrontal cortex during source memory as compared to item memory judgments. For example, participants are presented with a series of abstract visual shapes at different locations and asked to remember each shape (“item memory”) and the location in which it was presented (“source memory”). Source memory judgments are associated with increased activation of the dorsolateral prefrontal cortex (Rugg et al., 1999; Dobbins et al., 2002; Slotnick et al., 2003).
Data from single neuron recordings suggest that cells in the prefrontal cortex encode both item and place information. Rao et al. (1997) recorded from single cells in the dorsolateral prefrontal cortex while monkeys were trained to remember an object or an object’s spatial location. During the delay period of the task, different cells were associated with the identity of the object (“what” cells), the location of an object (“where” cells), or both features (“what-where” cells). These “what-where” cells in particular may play a key role in integrating object-location information (Rainer et al., 1998).
The findings from the present study are consistent with these reports. Although animals with prefrontal lesions demonstrated intact “what” memory, their ability to integrate object-location (“where”) memory was impaired. Notably, these results conflict with previous studies that have explored the contribution of the prefrontal cortex to object recognition. Medial prefrontal cortex lesions impaired the ability to recognize older as compared to more recent objects (temporal order memory) but left intact the ability to make a novelty judgment (Mitchell & Laiacona, 1998; Hannesson et al., 2004a). Hannesson et al. (2004b) also found that, after damage to the medial prefrontal cortex, spatial temporal order memory, but not spatial recognition memory, was impaired.
Interestingly, a study that used separate novel object, temporal order, object-in-place, and object location tests demonstrated that damage to the prefrontal cortex impairs performance selectively in the temporal order and object-in-place tasks (Barker et al., 2007). As previously mentioned, the task used in our study does not test for novelty judgments or spatial memory per se but instead tests memory for associations between the familiar objects based on their previous temporal and spatial arrangements. Thus, the results from the Barker et al. (2007) fit very well with our findings in terms of the spatial memory impairment found. In their object-in-place task, four different objects are first presented in a square shape. After a delay period, the animal is then presented with the same objects but two of them have switched locations. In their object location task, the animal is first presented with 2 identical objects and then, in the test session, one of the objects is relocated to a different position. In order to successfully perform the object-in-place task, the animal must remember the locations of both objects in relation to all of the others; however, the object location task requires only a detection of spatial novelty. Animals with damage to the prefrontal cortex were impaired selectively on the object-in-place task, suggesting that their deficit was not due simply to an inability to process spatial information but is instead due to a more specific impairment reflecting the role of the prefrontal cortex in integrating object-spatial relationships in context.
These considerations suggest that differences between the current findings and previous studies may be explained by the way in which object recognition was tested. Thus, our protocol emphasized the demand to retrieve spatial and temporal information about the objects in relation to one another. It is possible that results in this study would have differed if we had tested each type of memory independently.
Implications for understanding hippocampal-cortical interactions in episodic memory
Our results indicate that the hippocampus is essential for the recall of all of the features that comprise the “what-where-when” components of object recognition, and that the hippocampal-prefrontal circuitry specifically supports remembering where events occurred. There is substantial evidence demonstrating that these two regions interact in support of spatial learning. For example, Jo et al. (2007) trained animals on the standard Morris water maze task in which they learned to locate a hidden platform based on extra-maze visual cues. Lesions, as well as inactivation of either the hippocampus or prefrontal cortex, resulted in impaired memory for the platform location when some of the cues had been removed. Additionally, levels of the immediate early gene c-fos were elevated in the hippocampus and prefrontal cortex in intact animals performing the partial cue version of the task, compared to the version in which all cues were available. Also, damage to the prefrontal cortex results in an alteration of firing properties of hippocampal cells that code for place information, indicating that the prefrontal cortex normally modulates the spatial properties of cells in the hippocampus (Kyd & Bilkey, 2003). Collectively these findings suggest that interactions between the hippocampus and prefrontal cortex play a role in retrieving memories about what happened where.
Recent work in our laboratory using a different behavioral paradigm has suggested that the hippocampus and prefrontal cortex also play complementary roles in non-spatial recognition memory (Fortin et al., 2004; Farovik et al., 2008). Human recognition memory is commonly assessed using receiver operating characteristic (ROC) curves, which employ signal detection analyses for the dissociation of recollection and familiarity processes. A recognition memory task was developed for rats, taking advantage of their superb ability to perform the standard delayed non-match to sample task using odors as the memory cues. Each day, the animals were presented with a list of odors and then, following a 30-minute delay, were tested for their ability to differentiate old items (odors on the study list of that session) versus new items (odors not on the study list that day). By manipulating the difficulty level and reward payoffs to vary the animals’ response criterion, Fortin et al. (2004) characterized an ROC curve for recognition memory in rats similar to those generated in humans. These findings indicated that, like humans, rats use a combination of both recollection and familiarity to recognize recently experienced stimuli (Eichenbaum et al., 2007). Furthermore, damage to the hippocampus resulted in a selective impairment in recollection, whereas familiarity remained intact. This deficit in recollection was attributed to a decrease in hit rate (correct identification of old items) with no effect on the false alarm rate (incorrect selection of a new item as old), indicating an abnormally high forgetting rate following hippocampal damage.
In a follow-up study, Farovik et al. (2008) used the same behavioral paradigm to assess recognition in rats with damage to the medial prefrontal cortex. They found that prefrontal lesions also result in a deficit specific to recollection while sparing familiarity. However, the recollection deficit in animals with prefrontal lesions was due to a substantial increase in false alarm rates, whereas there was no effect on the hit rate, the converse of the pattern observed in animals with hippocampal damage. These observations suggest that animals with prefrontal lesions failed to distinguish memories of recently studied (“old”) odors from previous experiences with “new” items studied on earlier days, that is, a deficit in source memory. Collectively, these results and the present findings support the idea that the hippocampus and prefrontal cortex play complementary roles in episodic memory.
Acknowledgements
Thanks to Rachael Konigsberg and Christine Lykken for assistance with behavioral coding. Also, thanks to Christine Lykken for assistance with processing histology. This work was supported by grants from the Conte Center for Schizophrenia Research (NIH MH60450).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggleton J, Brown M. Contrasting hippocampal and perirhinal cortex function using immediate early gene imaging. Quarterly Journal of Experimental Psych. 2005;58(3–4):218–233. doi: 10.1080/02724990444000131. [DOI] [PubMed] [Google Scholar]
- Barker G, Bird F, Alexander V, Warburton E. Recognition memory for objects, place, and temporal order: A disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. J Neurosci. 2007;27(11):2948–2957. doi: 10.1523/JNEUROSCI.5289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning P, Easton A, Buckley M, Gaffan D. The role of prefrontal cortex in object-in-place learning in monkeys. Euro J Neurosci. 2005;22:3281–3291. doi: 10.1111/j.1460-9568.2005.04477.x. [DOI] [PubMed] [Google Scholar]
- Clayton N, Dickinson A. Episodic-like memory during cache recovery by scrub jays. Nature. 1998;395:272–274. doi: 10.1038/26216. [DOI] [PubMed] [Google Scholar]
- Day M, Langston R, Morris G. Glutamate-receptor-mediated encoding and retrieval of paired-associate learning. Nature. 2003;424:205–209. doi: 10.1038/nature01769. [DOI] [PubMed] [Google Scholar]
- Dere E, Huston J, De Souza Silva M. Episodic-like memory in mice: Simultaneous assessment of object, place and temporal order memory. Brain Res Prot. 2005a;16(1–3):10–19. doi: 10.1016/j.brainresprot.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Dere E, Huston J, De Souza Silva M. Integrated memory for objects, places, and temporal order: Evidence for episodic-like memory in mice. Neurobio of Learning & Mem. 2005b;84:214–221. doi: 10.1016/j.nlm.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Dere E, Kart-Teke E, Huston J, de Souza Silva. The case for episodic memory in animals. Neurosci & Biobehav Rev. 2006;30:1206–1224. doi: 10.1016/j.neubiorev.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Dobbins I, Foley H, Schacter D, Wagner A. Executive control during episodic retrieval: Multiple prefrontal processes subserve source memory. Neuron. 2002;35:989–996. doi: 10.1016/s0896-6273(02)00858-9. [DOI] [PubMed] [Google Scholar]
- Duarte A, Ranganath C, Knight R. Effects of unilateral prefrontal lesions on familiarity, recollection, and source memory. J Neurosci. 2005;25(36):8333–8337. doi: 10.1523/JNEUROSCI.1392-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eacott M, Norman G. Integrated memory for object, place, and context in rats: A possible model of episodic-like memory? J Neurosci. 2004;24(8):1948–1953. doi: 10.1523/JNEUROSCI.2975-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas A, Ranganath C. The medial temporal lobe and recognition memory. Annual Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergorul C, Eichenbaum H. The hippocampus and memory for "what," "where," and "when.”. Learning & Mem. 2004;11(4):397–405. doi: 10.1101/lm.73304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farovik A, DuPont L, Arce M, Eichenbaum H. Medial prefrontal cortex supports recollection, but not familiarity, in the rat. J Neurosci. 2008;28(50):13428–13434. doi: 10.1523/JNEUROSCI.3662-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin N, Agster K, Eichenbaum H. Critical role of the hippocampus in memory for sequence of events. Nat Neurosci. 2002;5(5):458–462. doi: 10.1038/nn834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin N, Wright S, Eichenbaum H. Recollection-like memory retrieval in rats is dependent on the hippocampus. Nature. 2004;431:188–191. doi: 10.1038/nature02853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin B, Paxinos G. The mouse brain in stereotaxic coordinates. San Diego, CA: Academic Press; 1997. [Google Scholar]
- Griffiths D, Dickinson A, Clayton N. Episodic memory: What can animals remember about their past? Trends in Cog Sci. 1999;3(2):74–80. doi: 10.1016/s1364-6613(98)01272-8. [DOI] [PubMed] [Google Scholar]
- Hammond R, Tull L, Stackman R. On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobio of Learning & Mem. 2004;82:26–34. doi: 10.1016/j.nlm.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Hannesson D, Howland J, Phillips A. Interaction between perirhinal and medial prefrontal cortex is required for temporal order but not recognition memory for objects in rats. J Neurosci. 2004a;24(19):4596–4604. doi: 10.1523/JNEUROSCI.5517-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannesson D, Vacca G, Howland J, Phillips A. Medial prefrontal cortex is involved in spatial temporal order memory but not spatial recognition memory in tests relying on spontaneous exploration in rats. Behav Brain Res. 2004b;153:273–285. doi: 10.1016/j.bbr.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Jenkins T, Amin E, Pearce J, Brown M, Aggleton J. Novel spatial arrangements of familiar visual stimuli promote activity in the rat hippocampal formation but not the parahippocampal cortices: A c-fos expression study. Neurosci. 2004;124:43–52. doi: 10.1016/j.neuroscience.2003.11.024. [DOI] [PubMed] [Google Scholar]
- Jo Y, Park E, Kim I, Park S, Kim H, Kim H, Choi J. The medial prefrontal cortex is involved in spatial memory retrieval under partial-cue conditions. J Neurosci. 2007;27(49):13567–13578. doi: 10.1523/JNEUROSCI.3589-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komorowski R, Manns J, Eichenbaum H. Robust conjunctive item-place coding by hippocampal neurons parallels learning what happens where. J Neurosci. 2009;29:9918–9929. doi: 10.1523/JNEUROSCI.1378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyd R, Bilkey D. Prefrontal cortex lesions modify the spatial properties of hippocampal place cells. Cerebral Cortex. 2003;13:444–451. doi: 10.1093/cercor/13.5.444. [DOI] [PubMed] [Google Scholar]
- Mitchell J, Laiacona J. The medial frontal cortex and temporal memory: Tests using spontaneous exploratory behaviour in the rat. Behav Brain Res. 1998;97:107–113. doi: 10.1016/s0166-4328(98)00032-1. [DOI] [PubMed] [Google Scholar]
- Mumby D. Perspectives on object-recognition memory following hippocampal damage: Lessons from studies in rats. Behav Brain Res. 2001;127:159–181. doi: 10.1016/s0166-4328(01)00367-9. [DOI] [PubMed] [Google Scholar]
- Mumby D, Gaskin S, Glenn M, Schramek T, Lehmann H. Hippocampal damage and exploratory preferences in rats: Memory for objects, places, and contexts. Learn Mem. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L, McIntosh A, Cabeza R, Habib R, Houle S, Tulving E. General and specific brain regions involved in encoding and retrieval of events: What, where, and when. PNAS USA. 1996;93:11280–11285. doi: 10.1073/pnas.93.20.11280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainer G, Asaad W, Miller E. Memory fields of neurons in the primate prefrontal cortex. PNAS USA. 1998;95:15008–15013. doi: 10.1073/pnas.95.25.15008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S, Rainer G, Miller E. Integration of what and where in the primate prefrontal cortex. Science. 1997;276:821–824. doi: 10.1126/science.276.5313.821. [DOI] [PubMed] [Google Scholar]
- Rossato J, Bevilaqua L, Myskiw J, Medina J, Izquierdo I, Cammarota On the role of hippocampal protein synthesis in the consolidation and reconsolidation of object recognition memory. Learn Mem. 2007;14:36–46. doi: 10.1101/lm.422607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg M, Fletcher P, Chua P, Dolan R. The role of the prefrontal cortex in recognition memory and memory for source: An fMRI study. NeuroImage. 1999;10:520–529. doi: 10.1006/nimg.1999.0488. [DOI] [PubMed] [Google Scholar]
- Simons J, Spiers H. Prefrontal and medial temporal lobe interactions in long-term memory. Nature Rev Neurosci. 2003;4:637–648. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- Slotnick S, Moo L, Segal J, Hart J., Jr Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Cog Brain Res. 2003;17:75–82. doi: 10.1016/s0926-6410(03)00082-x. [DOI] [PubMed] [Google Scholar]
- Tulving E, Markowitsch H. Episodic and declarative memory: Role of the hippocampus. Hippocampus. 1998;8:198–204. doi: 10.1002/(SICI)1098-1063(1998)8:3<198::AID-HIPO2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Verwer R, Meijer R, Van Uum H, Witter M. Collateral projections from the rat hippocampal formation to the lateral and medial prefrontal cortex. Hippocampus. 1997;7(4):397–402. doi: 10.1002/(SICI)1098-1063(1997)7:4<397::AID-HIPO5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]