Abstract
It has been suggested that low levels of estradiol and testosterone increase risk for dementia. However, results of the existing observational studies examining associations of endogenous sex hormones with cognition and dementia are conflicting. A possible explanation for these inconsistent findings could be the involvement of sex hormone-binding globulin (SHBG) in regulating sex hormone levels. In the present study, we examined whether SHBG levels were associated with development of AD and overall dementia in a cohort of elderly men and women free of dementia at baseline. We observed that in both men and women higher levels of SHBG were associated with an increased risk for AD and overall dementia. These results were independent of vascular risk factors and bioactive hormone levels. Whether SHBG is causally related to dementia or whether it is a surrogate marker for rate of biological aging and increased risk or for preclinical stage of dementia has to be elucidated.
Keywords: SHBG, estradiol, testosterone, Alzheimer’s disease, dementia
1. Introduction
The actions of testosterone and estradiol in the brain of older people are not well understood, but experimental studies suggest these hormones have multiple neuroprotective effects (Veiga et al. 2004). However, results of the existing observational studies examining associations of endogenous sex hormones with cognition and dementia are conflicting (Barrett-Connor et al. 1999; Barrett-Connor and Goodman-Gruen 1999; Geerlings et al. 2006; Geerlings et al. 2003; Moffat et al. 2004; Muller et al. 2005a; Schupf et al. 2006; Yaffe et al. 2000; Yaffe et al. 2002). A possible explanation for these inconsistent findings could be the involvement of sex hormone-binding globulin (SHBG) in regulating sex hormone levels. SHBG is a hepatically secreted protein that is the major binding protein for sex hormones in plasma, thereby preventing hormone binding to the intracellular androgen or estrogen receptors (Hammond 1995). The non-SHBG-bound fraction of hormone is, therefore, considered to be bioactive and it is recognized that SHBG is a major controlling factor in the balance between biologically active testosterone and estradiol in both men and women (de Ronde et al. 2005). Differences in levels of SHBG, resulting in changes in the balance between bound and free hormones may be the reason why we find inconsistent results for testosterone and estradiol in relation to dementia. It has, therefore, been hypothesized that SHBG modulates dementia risk.
This hypothesis is supported by findings from cross-sectional observational studies showing that patients with Alzheimer’s disease (AD) have higher SHBG levels (Hogervorst et al. 2004; Hoskin et al. 2004; Paoletti et al. 2004), and that subjects with higher SHBG levels had poorer cognitive test scores (Yaffe et al. 2002). A longitudinal cohort study in women with Down syndrome showed that risk for incident AD was associated with high levels of SHBG (Schupf et al. 2006). In addition, in a cohort of elderly men baseline SHBG levels were related to incident AD (Moffat et al. 2004). However, after adjustment for potential confounders the effect estimates became non-significant.
In the present study, we examined whether SHBG levels were associated with development of AD and overall dementia. Data are from the prospectively studied population-based multi-ethnic cohort of elderly men and women who participated in the Washington Heights-Inwood Columbia Aging Project (WHICAP).
2. Methods
2.1. Subjects and setting
This longitudinal cohort study included participants recruited in 1992 who were identified from a probability sample, stratified for age and ethnicity, of Medicare recipients 65 years or older residing in an area of 3 contiguous census tracts within a geographically defined area of northern Manhattan (Tang et al. 1998). Each participant underwent an in-person interview of general health and function at the time of study entry followed by a standard assessment, including medical history, physical and neurological examination, as well as a neuropsychological battery (Stern et al. 1992). Baseline data were collected from 1992 through 1994 and follow-up data were collected during evaluations at sequential intervals of approximately 18 months. This study was approved by the institutional review board of the Columbia-University Medical Center.
The sample for this study was restricted to individuals who had a blood draw for assessment of SHBG and hormone levels. We conducted SHBG assays in a sample of 1122 subjects from the 1st follow-up examination, chosen at random from among the 2,126 original subjects. This sample included 221 cases of prevalent dementia at baseline (20%), 170 of the non-demented subjects (19%) did not have follow-up measurements because they were dead, unable to be located, had moved or refused toparticipate further. Thus we restricted the sample for these analyses to 731 individualswithout dementia and with follow-up measurements. Data on bioactive hormone levels were available in 573 of the 731 subjects.
2.2. Diagnosis of dementia
At every visit all participantsunderwent a standardized neuropsychological test battery, validated in this community, thatexamined multiple cognitive domains in either English or Spanish (Stern et al. 1992).
Diagnosis of dementia and assignment of specific cause was madeby consensus of two neurologists, one psychiatrist, and two neuropsychologists based on baseline and follow-up information. The diagnosis of dementia was based on Diagnostic and StatisticalManual of Mental Disorders (DSM)–IV criteria and requiredevidence of cognitive deficit on the neuropsychological testbattery as well as evidence of impairment in social or occupational function (Clinical Dementia Rating of 1 or more) (Hughes et al. 1982). AD diagnosis was based on NINCDS-ADRDA criteria (McKhann et al. 1984). A diagnosis of probable AD was made when dementia could not be explained by other disorders. We conducted analyses with two outcomes, all-cause-dementia, and probable AD. Cognitive impairment without dementia was diagnosed in participants who had abnormal results in cognitive tests, but did not meet the full criteria for dementia (CDR = 0.5) (Stern et al. 1992).
2.3. SHBG and hormones
Blood samples were taken during the first follow-up visit (1994) and were collected from each subject between 10 AM and 3 PM. Blood samples were centrifuged in a refrigerated centrifuge and, after separation, sera were frozen at −20 °C untilassayed. Solid-phase, chemiluminescent enzyme immunoassays were performed to measure levels of sex hormone-binding globulin (SHBG) and total testosterone (T)(Diagnostic Products Co., Immulite). Sensitivity or minimum detection limit for SHBG was calculated at 0.2 nmol/L. The intra-assay coefficient of variation was 2.3%, and the interassay coefficient of variation was 5.5%. Sensitivity or minimum detection limit for total T was calculated at 20 pg/mL (=0.7 nmol/L). The intra-assay coefficient of variationwas 7.4%, and the interassay coefficient of variation was 9.8%. Serum levels of estradiol (E2) were measured by radioimmunoassay in duplicate using a commercial kit (Diagnostics Systems Labs, Webster, TX). Sensitivity or minimum detection limit for estradiol was calculated at 4 pg/mL (= 14 pmol/L). The intra-assay coefficient of variation for the E2 assay was 3.5%, and the interassay coefficient of variationwas 5.8%. The antiserum was specific for E2, with cross-reactivityfor estrone of 6.9%.
The non-SHBG-bound fractions (i.e., the bioavailable fractions) of T and E2 consist of the free and albumin-bound hormones, and is thought to better reflect the active component of the hormone (van den Beld et al. 2000). Bioavailable and free fractions (i.e., not SHBG- and not albumin-bound) of T and E2 were calculated using total T, total E2, SHBG, and albumin (Sodergard et al. 1982; van den Beld et al. 2000). This method is based on the knowledge of the total concentration of all steroids competing for the same binding site on SHBG, the concentration of albumin (using a fixed concentration of 40 gr/L), the binding capacity of SHBG, and the association constant of E2 to the binding proteins.
The proportion of samples that fell below the detection limit for estradiol and testosterone was 1.1% and 3.0% in men, and 6.7% and 45.5% in women. Each hormone measurement that fell below the detection limit was recoded, using the detection limit, in order to analyze the variable as continuous. Analyses were conducted with and without these cases, as well as categorical analyses, and results remained the same.
2.4. Other variables
Fasting plasma total cholesterol levels weredetermined using standard enzymatictechniques. APOE genotypes were determined as described by Hixson and Vernier (Hixson and Vernier 1990)with slight modification (Maestre et al. 1995). We classified persons as homozygousor heterozygous for the APOE-ε4 allele or not having any ε4 allele. Body mass index (BMI) was calculated as weight (kg)/height (m) squared. Fasting insulin levels were conducted and were measured in μIU/mL from serumcollected at baseline andfrozen at −70 °C (Luchsinger et al. 2004). Insulin levels were measured usinga solid-phase chemiluminescent enzyme immunoassay (Immulite, Diagnostic Products, Los Angeles, CA). The intra-assay coefficientof variation was 4.7% and the interassay coefficient of variationwas 8.2%. The normal insulin range for this assay is 6 – 27μIU/mL. Insulin levels were log transformed. Diabetes mellitus, hypertension, thyroid disease, chronic liver disease, HRT use, hysterectomy, and cancer were ascertained by self-report and were defined as a history at any time during life. If participants reported a history of any condition, they were asked whether they were under treatment and the specific medication or treatment. Heart disease was defined as a history of myocardial infarction, congestive heart failure, or angina pectoris at any time in life. Smoking and alcohol use were defined as none, past, or current use. Ethnic group was classified by self-report using the formatof the 1990 US Census (STF 1A database 1991). Individuals were asked whether they were of Hispanic origin. Using this information, individualswere separated into 3 ethnic groups: African-American (non-Hispanic), Hispanic, or white (non-Hispanic).
2.5. Data analysis
The relation between SHBG and incident probable AD and all-cause dementia was studiedby calculating cumulative incidence since baselineby the Kaplan-Meier method. Kaplan-Meier curves (one-minus survival plots) were constructedfor tertiles of age-adjusted SHBG levels. Cox proportional hazard models were used to estimate the adjusted hazard ratio (HR) and 95% CI for the association between SHBG levels and the risk of incident dementia within all subjects. Because there are differences between the two sexes in factors regulating SHBG levels, we repeated all analyses in men and women separately (Lecomte et al. 1998). The time-to-event variable was the age at onset of dementia (Korn et al. 1997). Individuals who did not develop the outcome of interest or who died or were lost to follow-up were censored at the time of their last evaluation. Participants with dementia other than AD were censored at the time of onset of that dementia. The proportional hazards assumption was checked using a log minus log plot. In the Cox proportional hazard models, SHBG levels were examined in two ways: as a continuous variable, expressed as increase per standard deviation, and as tertiles. In the first model, we adjusted for age (timescale). In the second model we added sex, years of education (continuous), ethnic group (Caucasian, African-American, Hispanic), APOE genotype (one or two ε4 alleles vs. no ε4 allele), smoking habits (current and ever smoker), BMI (continuous), total cholesterol (continuous), hypertension (yes vs. no), diabetes (yes vs. no), and insulin (continuous) as covariates. These covariates were considered as potential confounders because SHBG levels are associated with life style factors, vascular risk factors, and especially weight, diabetes and insulin levels (Lecomte et al. 1998; Muller et al. 2005b; Muller et al. 2003; Selby 1990). Finally, adjustments were made for thyroid disease (yes vs. no), liver disease (yes vs. no), cancer (yes vs. no), HRT-use (yes vs. no), and hysterectomy (yes vs. no).
Since increased SHBG levels may be strongly associated with increased age and a decrease in body weight we repeated analyses within strata of age (< 77 yrs vs. ≥ 77 yrs) and BMI (lowest quartile (< 23 kg/m2) vs. highest three quartiles (≥ 23 kg/m2)). Furthermore, since SHBG levels are associated with testosterone and estradiol levels we repeated analyses within strata of bioactive hormone levels (tertiles). Given that SHBG could be a marker for preclinical dementia, we repeated the analyses after excluding subjects with cognitive impairment (CDR=0.5). Data-analysis was performed using SPSS version 12.0 and SAS for windows version 9.1.
3. Results
There were 731 individuals at baseline, with 3,775 person-years of follow-up (mean (SD) is 5.2 (3.1) person-years). The mean age of our study sample was 77.4 years. One hundred forty six subjects developed dementia (20%): 91 (12%) with probable AD. Mean (SD) SHBG in men was 48.2 (21.1) nmol/L, in women was 59.0 (32.3) nmol/L, and in the total group was 55.8 (29.8) nmol/L. No differences in demographic characteristics (age, sex, ethnicity, education) were found between the total cohort (N=2,126) and our sample (N=731).
Tables 1 and 2 present the baseline characteristics for women and men separately. Women with the highest SHBG levels (highest tertile) were older, were better educated, had lower BMI, had lower prevalence of hypertension, diabetes, HRT-use/hysterectomy, had lower insulin levels, and a higher frequency of the APOE ε4 allele. They also had lower bioactive estradiol and testosterone levels (Table 1). Men with the highest SHBG levels (highest tertile) were older, were better educated, had lower BMI, had lower prevalence of hypertension, diabetes, heart disease, and cancer, had lower insulin levels, and a higher APOE ε4 allele presence. They also had lower bioactive estradiol levels (Table 2).
Table 1.
Baseline characteristics (mean (SD) unless stated otherwise) of the female population without prevalent dementia according to tertiles of SHBG
| Tertiles of SHBG |
Total | ||||
|---|---|---|---|---|---|
| 1st | 2nd | 3rd | |||
| N | 7–42 nmol/L | 42 – 64 nmol/L | 64 – 180 nmol/L | ||
| Age (yr) | 514 | 76 (5) | 77 (6) | 79 (6) | 78 (6) |
| Ethnic group, % | 514 | ||||
| Caucasian | 13 | 19 | 28 | 20 | |
| African American | 26 | 34 | 35 | 31 | |
| Hispanic | 61 | 47 | 38 | 49 | |
| Education (yrs) | 514 | 8.2 (4.6) | 8.8 (4.1) | 9.5 (4.5) | 8.8 (4.4) |
| APOE – ε4, % | 514 | 27 | 29 | 31 | 29 |
| Smoking, % | 514 | ||||
| Current | 7 | 10 | 11 | 9 | |
| Ever | 23 | 20 | 13 | 19 | |
| BMI (kg/m2) | 514 | 30 (5) | 28 (6) | 25 (5) | 28 (6) |
| Cholesterol (mg/dL) | 514 | 216 (39) | 210 (36) | 207 (37) | 211 (38) |
| Insulin, median (25th–75th percentile) | 514 | 25 (15–47) | 22 (13–43) | 14 (8–31) | 21 (11–39) |
| Hypertension, % | 514 | 77 | 77 | 69 | 74 |
| Diabetes, % | 514 | 33 | 22 | 11 | 23 |
| Heart disease, % | 514 | 31 | 38 | 30 | 33 |
| Thyroid disease, % | 514 | 13 | 16 | 13 | 13 |
| Liver disease, % | 514 | 1 | 4 | 3 | 3 |
| Cancer, % | 514 | 13 | 8 | 9 | 10 |
| HRT-use/hysterectomy | 514 | 22 | 18 | 14 | 18 |
| Bioactive E2 (pmol/L) | 399 | 43 (31) | 29 (19) | 25 (19) | 33 (25) |
| Bioactive T (nmol/L) | 399 | 0.7 (0.6) | 0.6 (0.5) | 0.5 (0.7) | 0.6 (0.6) |
APOE – apolipoprotein E, BMI – body mass index, E2 – estradiol, T – testosterone.
Table 2.
Baseline characteristics (mean (SD) unless stated otherwise) of the male population without prevalent dementia to tertiles of SHBG
| Tertiles of SHBG | Total | ||||
|---|---|---|---|---|---|
| 1st | 2nd | 3rd | |||
| N | 7 – 37 nmol/L | 37 – 55 nmol/L | 55 – 180 nmol/L | ||
| Age (yr) | 217 | 76 (5) | 78 (6) | 77 (6) | 77 (6) |
| Ethnic group, % | 217 | ||||
| Caucasian | 25 | 27 | 28 | 27 | |
| African American | 27 | 24 | 31 | 27 | |
| Hispanic | 48 | 49 | 41 | 46 | |
| Education (yrs) | 217 | 8.5 (4.5) | 8.9 (5.6) | 9.3 (4.7) | 8.9 (5.0) |
| APOE – ε4, % | 217 | 27 | 28 | 31 | 29 |
| Smoking, % | 217 | ||||
| Current | 14 | 17 | 16 | 16 | |
| Ever | 27 | 38 | 42 | 36 | |
| BMI (kg/m2) | 217 | 28 (5) | 26 (4) | 25 (4) | 26 (5) |
| Cholesterol (mg/dL) | 217 | 195 (38) | 190 (39) | 191 (38) | 192 (38) |
| Insulin, median (25th–75th percentile) | 217 | 26 (13–44) | 19 (12–32) | 15 (9–28) | 19 (11–35) |
| Hypertension, % | 217 | 70 | 57 | 47 | 59 |
| Diabetes, % | 217 | 38 | 16 | 20 | 24 |
| Heart disease, % | 217 | 39 | 31 | 27 | 32 |
| Thyroid disease, % | 217 | 2 | 7 | 6 | 5 |
| Liver disease, % | 217 | 0 | 2 | 2 | 1 |
| Cancer, % | 217 | 11 | 9 | 5 | 8 |
| Bioactive E2 (pmol/L) | 174 | 67 (34) | 59 (23) | 51 (26) | 59 (28) |
| Bioactive T (nmol/L) | 174 | 5.9 (2.6) | 6.9 (2.2) | 6.9 (2.5) | 6.6 (2.5) |
APOE – apolipoprotein E, BMI – body mass index, E2 – estradiol, SHBG – sex hormone-binding globulin, T – testosterone.
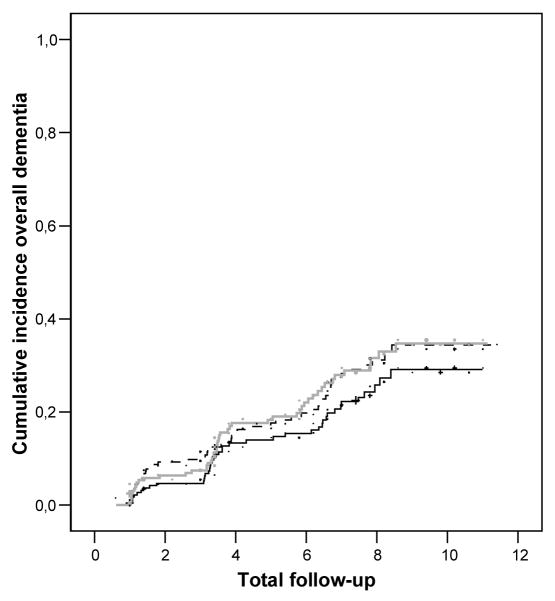
The Kaplan-Meier estimates of the occurrence of overall dementia according to tertiles of age-adjusted SHBG levels are depicted in the figure. Subjects with SHBG levels in the second and third tertile had an increased risk for developing overall dementia compared with the lowest tertile (Figure). Similar results were found for probable AD (data not shown)
Figure.
Cumulative incidence of overall dementia during follow-up according to tertiles of age adjusted SHBG levels (1st tertile —, 2nd tertile ---, 3rd tertile  ).
).
Multivariate Cox proportional hazard analysis in the total group showed that SHBG (per SD increase) was associated with a 20–30% increased risk for developing dementia (Table 3), independent of age, sex, education, ethnicity, APOE genotype, lifestyle factors, and vascular risk factors. Stratification by sex did not materially change the effect estimates (Table 3). Additional adjustment for thyroid disease, liver disease, history of cancer, hysterectomy and HRT-use did not change the effect estimates mentioned in table 3 (data not shown), however, due to incomplete data the confidence intervals widened.
Table 3.
Hazard ratios (HR) with corresponding 95% confidence intervals (95% CI) of the association of SHBG levels per SD increase (nmol/L) with risk of overall dementia and its subtypes according to strata of sex in subjects free of dementia at baseline
| Probable AD | Overall dementia | ||||
|---|---|---|---|---|---|
| N (cases/total) | HR (95% CI) | N (cases/total) | HR (95% CI) | ||
| Total | |||||
|
| |||||
| SHBG per SD | model 1 a | 91/731 | 1.2 (1.0–1.5) | 146/731 | 1.2 (1.0–1.4) |
| model 2 b | 1.3 (1.1–1.6) | 1.3 (1.1–1.5) | |||
|
| |||||
| Women | |||||
|
| |||||
| SHBG per SD | model 1 a | 58/514 | 1.2 (1.0–1.5) | 96/514 | 1.1 (0.9–1.4) |
| model 2 b | 1.4 (1.1–1.9) | 1.3 (1.1–1.7) | |||
|
| |||||
| Men | |||||
|
| |||||
| SHBG per SD | model 1 a | 33/217 | 1.3 (1.0–1.9) | 50/217 | 1.3 (0.9–1.7) |
| model 2 b | 1.3 (1.0–2.0) | 1.2 (0.9–1.8) | |||
SHBG standard deviation for the total group: 29.8 nmol/L; SHBG standard deviation for women: 31.6 nmol/L; SHBG standard deviation for men: 22.9 nmol/L
AD – Alzheimer’s disease; CI – confidence interval; SD – standard deviation; SHBG – sex hormone-binding globulin
model 1 – HR adjusted for age (timescale)
model 2 – HR adjusted for age (timescale), ethnic group, education, APOE genotype, smoking, BMI, cholesterol, insulin, hypertension, diabetes
Table 4 presents the relation between SHBG and dementia stratified for age. In both younger (<77 yrs) and older (≥77 yrs) subjects higher SHBG levels were associated with an increased risk for probable AD and overall dementia, indicating that this relation was not mediated or modified by age.
Table 4.
Hazard ratios (HR) with corresponding 95% confidence intervals (95% CI) of the association of SHBG levels per SD increase (nmol/L) with risk of overall dementia and its subtypes according to strata of age in subjects free of dementia at baseline
| Probable AD | Overall dementia | ||||
|---|---|---|---|---|---|
| N (cases/total) | HR (95% CI) | N (cases/total) | HR (95% CI) | ||
| Age <77 yrs | |||||
|
| |||||
| SHBG per SD | model 1 a | 25/399 | 1.3 (0.9–1.8) | 46/399 | 1.2 (0.9–1.6) |
| model 2 b | 1.3 (0.9–2.0) | 1.3 (1.0–1.8) | |||
|
| |||||
| Age ≥ 77 yrs | |||||
|
| |||||
| SHBG per SD | model 1 a | 66/332 | 1.2 (1.0–1.5) | 100/332 | 1.2 (1.1–1.4) |
| model 2 b | 1.2 (1.0–1.6) | 1.2 (1.0–1.5) | |||
SHBG standard deviation for the total group: 29.8 nmol/L.
AD – Alzheimer’s disease; CI – confidence interval; SD – standard deviation; SHBG – sex hormone-binding globulin
model 1 – HR adjusted for age (timescale)
model 2 – HR adjusted for age (timescale), sex, ethnic group, education, APOE genotype, smoking, BMI, cholesterol, insulin, hypertension, diabetes
Stratification by BMI did not change the effect estimates relating SHBG to dementia and therefore did not indicate that this relation was modulated by BMI (data not shown). Stratification by bioactive hormone levels did not change the effect estimates presented in table 3, indicating that the relation between SHBG and dementia was not mediated or modified by bioactive hormone levels (data not shown).
Secondary analyses excluding subjects with prevalent cognitive impairment without dementia (CDR=0.5) at baseline (N=94) did not materially change the effect estimates relating SHBG to probable AD and overall dementia (data not shown).
4. Discussion
This study examined risk for dementia with endogenous levels of SHBG in a cohort of elderly men and women free of dementia at baseline. We observed that higher levels of SHBG were associated with an increased risk for AD and overall dementia.
To appreciate these findings, some issues need to be addressed. To our knowledge this is the first study relating endogenous SHBG levels with incident dementia in both elderly men and women. Furthermore, this is a prospective cohortstudy designed for the diagnosis of dementia and withcomplete clinical and neuropsychological evaluation at eachinterval. In addition, we had the ability to diagnose incident dementia among participants who were not demented at baseline, thus allowing us to follow anunbiased sample. And finally, we were able to correct for a large number of potential confounding factors.
Our interpretation of the results may be limited by several factors. In this study we used a randomly selected sample of the total cohort. However, no differences were found in baseline characteristics between our sample and the total cohort (data not shown), which makes it unlikely that our results are due to sampling variability issues. Some error could have occurred in the measurement of sex hormone levels resulting from the assays and time of blood sampling. The testosterone assay used in this study is not sensitive enough for women, which has resulted in a large proportion of samples that fell below the detection limit. In addition, because of the circadian variation, the wide range of time of blood sampling could have led to misclassification and limited precision of testosterone levels. These errors most likely have led to an underestimation of the possible mediating or modifying role of bioactive hormone levels in the relation between SHBG and dementia.
Our results are in agreement with cross-sectional studies describing higher levels of SHBG in association with prevalence of Alzheimer’s disease (AD) (Hogervorst et al. 2004; Hoskin et al. 2004; Paoletti et al. 2004), and cognitive dysfunction (Yaffe et al. 2002). However, a large-scaled longitudinal study in men aged 32–87 years showed that baseline SHBG levels were not independently associated with incident AD 19 yrs of follow-up (Moffat et al. 2004).
The finding of an increased risk for incident dementia, especially probable AD, associated with increased levels of SHBG is of interest. It is recognized that SHBG is a major controlling factor in the balance between biologically active androgens and estrogens in both men and women (Rosner 1990). Higher SHBG levels have been associated with lower levels of bioactive testosterone and estradiol (de Ronde et al. 2005; Rosner 1990). It could, therefore, be hypothesized that our findings reflect a possible relation between low levels of endogenous sex hormones and risk of dementia. However, the effect estimates did not change when repeating the analyses within strata of bioactive hormone levels to explore mediation or modification by these factors.
An alternative hypothesis to explain our findings could be that increased SHBG is a surrogate marker for another risk factor for dementia. High SHBG levels have been associated with increasing age (Muller et al. 2003), which could indicate that SHBG is a marker for accelerated aging. Furthermore, high SHBG levels have been associated with weight loss, smoking, and with thyroid and liver dysfunction (Lecomte et al. 1998; Muller et al. 2003; Selby 1990), whereas low SHBG levels have been associated with diabetes, hyperinsulinemia, hypertension, dyslipidemia, and obesity (Lecomte et al. 1998; Muller et al. 2003; Muller et al. 2005b). Many of these conditions could have led to both a change in SHBG levels as to the development of dementia. We have tried to adjust for these factors in our analyses and repeated the analyses within strata of age and BMI, and our findings did not change. Although we cannot rule out residual confounding, our findings suggest that endogenous SHBG levels are independently associated with incident dementia.
Finally, it could be hypothesized that increase SHBG levels is a preclinical marker for dementia. The presence of SHBG in the human brain has suggested its involvement in neurophysiology and possibly neuropathology (Caldwell et al. 2006), and it is possible that in early stages of dementia the regulation and production of SHBG is disrupted. To account for preclinical disease we excluded subjects with cognitive impairment without dementia at baseline, and found a similar relation of SHBG to risk of dementia.
In conclusion, this population-based study in elderly men and women shows that high levels of SHBG are independently related to an increased risk of AD and overall dementia. Whether SHBG is causally related to dementia or whether it is a surrogate marker for increased risk or for preclinical stage of dementia has to be elucidated in further studies.
Acknowledgments
Support for this work was provided by grants from the National Institutes of Health AG07232, RR00645 from the Charles S. Robertson Memorial Gift for research on Alzheimer’s disease, from the Blanchette Hooker Rockefeller Foundation, from the New York City Council Speaker’s fund for Public Health Research, and from the Netherlands Organization for Scientific Research (NWO) S92-254.
Footnotes
Conflict of interest
All authors confirm that they have no actual or potential conflicts of interest to disclose.
References
- Barrett-Connor E, Goodman-Gruen D. Cognitive function and endogenous sex hormones in older women. J Am Geriatr Soc. 1999;47:1289–1293. doi: 10.1111/j.1532-5415.1999.tb07427.x. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Goodman-Gruen D, Patay B. Endogenous sex hormones and cognitive function in older men. J Clin Endocrinol Metab. 1999;84:3681–3685. doi: 10.1210/jcem.84.10.6086. [DOI] [PubMed] [Google Scholar]
- Caldwell JD, Suleman F, Chou SH, Shapiro RA, Herbert Z, Jirikowski GF. Emerging roles of steroid-binding globulins. Horm Metab Res. 2006;38:206–218. doi: 10.1055/s-2006-925328. [DOI] [PubMed] [Google Scholar]
- De Ronde W, van der Schouw YT, Muller M, Grobbee DE, Gooren LJ, Pols HA, de Jong FH. Associations of sex-hormone-binding globulin (SHBG) with non-SHBG-bound levels of testosterone and estradiol in independently living men. J Clin Endocrinol Metab. 2005;90:157–162. doi: 10.1210/jc.2004-0422. [DOI] [PubMed] [Google Scholar]
- Geerlings MI, Launer LJ, de Jong FH, Ruitenberg A, Stijnen T, Van Swieten JC, Hofman A, Witteman J, Pols HA, Breteler MM. Endogenous estradiol and risk of dementia in women and men: The Rotterdam Study. Ann Neurol. 2003;53:607–615. doi: 10.1002/ana.10521. [DOI] [PubMed] [Google Scholar]
- Geerlings MI, Strozyk D, Masaki K, Remaley AT, Petrovitch H, Webster Ross G, White LR, Launer LJ. Endogenous sex hormones, cognitive decline, and future dementia in old men. Ann Neurol. 2006;60:346–355. doi: 10.1002/ana.20918. [DOI] [PubMed] [Google Scholar]
- Hammond GL. Potential functions of plasma steroid-binding proteins. Trends Endocrinol Metab. 1995;6:298–304. doi: 10.1016/1043-2760(95)00162-x. [DOI] [PubMed] [Google Scholar]
- Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31:545–548. [PubMed] [Google Scholar]
- Hogervorst E, Bandelow S, Combrinck M, Smith AD. Low free testosterone is an independent risk factor for Alzheimer’s disease. Exp Gerontol. 2004;39:1633–1639. doi: 10.1016/j.exger.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Hoskin EK, Tang MX, Manly JJ, Mayeux R. Elevated sex-hormone binding globulin in elderly women with Alzheimer’s disease. Neurobiol Aging. 2004;25:141–147. doi: 10.1016/s0197-4580(03)00046-0. [DOI] [PubMed] [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Korn EL, Graubard BI, Midthune D. Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol. 1997;145:72–80. doi: 10.1093/oxfordjournals.aje.a009034. [DOI] [PubMed] [Google Scholar]
- Lecomte P, Lecureuil N, Lecureuil M, Lemonnier Y, Mariotte N, Valat C, Garrigue MA. Sex differences in the control of sex-hormone-binding globulin in the elderly: role of insulin-like growth factor-I and insulin. Eur J Endocrinol. 1998;139:178–183. doi: 10.1530/eje.0.1390178. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Tang MX, Shea S, Mayeux R. Hyperinsulinemia and risk of Alzheimer disease. Neurology. 2004;63:1187–1192. doi: 10.1212/01.wnl.0000140292.04932.87. [DOI] [PubMed] [Google Scholar]
- Maestre G, Ottman R, Stern Y, Gurland B, Chun M, Tang MX, Shelanski M, Tycko B, Mayeux R. Apolipoprotein E and Alzheimer’s disease: ethnic variation in genotypic risks. Ann Neurol. 1995;37:254–259. doi: 10.1002/ana.410370217. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Zonderman AB, Metter EJ, Kawas C, Blackman MR, Harman SM, Resnick SM. Free testosterone and risk for Alzheimer disease in older men. Neurology. 2004;62:188–193. doi: 10.1212/wnl.62.2.188. [DOI] [PubMed] [Google Scholar]
- Muller M, Aleman A, Grobbee DE, de Haan EH, van der Schouw YT. Endogenous sex hormone levels and cognitive function in aging men. Is there an optimal level? Neurology. 2005a:866–871. doi: 10.1212/01.WNL.0000153072.54068.E3. [DOI] [PubMed] [Google Scholar]
- Muller M, den Tonkelaar I, Thijssen JHH, Grobbee DE, van der Schouw YT. Endogenous sex hormones in men aged 40–80 years. Eur J Endocrinol. 2003;149:583–589. doi: 10.1530/eje.0.1490583. [DOI] [PubMed] [Google Scholar]
- Muller M, Grobbee DE, den TI, Lamberts SW, van der Schouw YT. Endogenous sex hormones and metabolic syndrome in aging men. J Clin Endocrinol Metab. 2005b;90:2618–2623. doi: 10.1210/jc.2004-1158. [DOI] [PubMed] [Google Scholar]
- Paoletti AM, Congia S, Lello S, Tedde D, Orru M, Pistis M, Pilloni M, Zedda P, Loddo A, Melis GB. Low androgenization index in elderly women and elderly men with Alzheimer’s disease. Neurology. 2004;62:301–303. doi: 10.1212/01.wnl.0000094199.60829.f5. [DOI] [PubMed] [Google Scholar]
- Rosner W. The functions of corticosteroid-binding globulin and sex hormone-binding globulin: recent advances. Endocr Rev. 1990;11:80–91. doi: 10.1210/edrv-11-1-80. [DOI] [PubMed] [Google Scholar]
- Schupf N, Winsten S, Patel B, Pang D, Ferin M, Zigman WB, Silverman W, Mayeux R. Bioavailable estradiol and age at onset of Alzheimer’s disease in postmenopausal women with Down syndrome. Neurosci Lett. 2006;406:298–302. doi: 10.1016/j.neulet.2006.07.062. [DOI] [PubMed] [Google Scholar]
- Selby C. Sex hormone binding globulin: origin, function and clinical significance. Ann Clin Biochem. 1990;27 ( Pt 6):532–541. doi: 10.1177/000456329002700603. [DOI] [PubMed] [Google Scholar]
- Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16:801–810. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- Stern Y, Andrews H, Pittman J, Sano M, Tatemichi T, Lantigua R, Mayeux R. Diagnosis of dementia in a heterogeneous population. Development of a neuropsychological paradigm-based diagnosis of dementia and quantified correction for the effects of education. Arch Neurol. 1992;49:453–460. doi: 10.1001/archneur.1992.00530290035009. [DOI] [PubMed] [Google Scholar]
- STF 1A database. 1990 Census of population and housing:summary tape file 1, technical documentation (computer program/diskette). STF 1A database [computer program] 1991.
- Tang MX, Stern Y, Marder K, Bell K, Gurland B, Lantigua R, Andrews H, Feng L, Tycko B, Mayeux R. The APOE-epsilon4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. JAMA. 1998;279:751–755. doi: 10.1001/jama.279.10.751. [DOI] [PubMed] [Google Scholar]
- Van den Beld AW, de Jong FH, Grobbee DE, Pols HAP, Lamberts SWJ. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle strength, bone density, and body composition in elderly men. J Clin Endocrinol Metab. 2000;85:3276–3282. doi: 10.1210/jcem.85.9.6825. [DOI] [PubMed] [Google Scholar]
- Veiga S, Melcangi RC, Doncarlos LL, Garcia-Segura LM, Azcoitia I. Sex hormones and brain aging. Exp Gerontol. 2004;39:1623–1631. doi: 10.1016/j.exger.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Lui LY, Grady D, Cauley J, Kramer J, Cummings SR. Cognitive decline in women in relation to non-protein-bound oestradiol concentrations. Lancet. 2000;356:708–712. doi: 10.1016/S0140-6736(00)02628-3. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Lui LY, Zmuda J, Cauley J. Sex hormones and cognitive function in older men. J Am Geriatr Soc. 2002;50:707–712. doi: 10.1046/j.1532-5415.2002.50166.x. [DOI] [PubMed] [Google Scholar]