Abstract
Mutations that influence the sensitivity of an organism to a volatile general anesthetic can be divided into two classes. In one, sensitivity to all other volatile agents is affected to a similar degree. Although this class may contain mutations of interest for understanding anesthesia, it is also likely to contain mutations that merely alter general health. In the second class, mutations confer non-uniform effects on potency (NEP), i.e., larger effects for some volatile anesthetics than for others. Members of this class are of special interest for studies of arousal and its pharmacological suppression because they not only avoid the pitfall of effects on global health, they imply the existence of drug targets that are preferentially affected by particular agents. In this work we provide the first systematic investigation of the relative frequency and diversity of NEP mutations in Drosophila. As a first step we isolated and characterized a set of P element insertion mutations that confer altered sensitivity of the fruit fly to the clinical anesthetic halothane. Then we tested the members of this collection for their effect on the sensitivity of flies to five other volatile agents. Not only do we find that most of the mutations show non-uniform effects, they share a characteristic arrangement of altered potencies (halothane >>desflurane ≥ enflurane ∼ isoflurane ∼ methoxyflurane > sevoflurane). From this result, although we do not know how direct or indirect are the effects of the mutations, we infer the existence of a biologically relevant target for anesthetic action that has a distinct preference for halothane over other agents. Intriguingly, P element insertions that co-map with several NEP loci have been shown to alter the fly’s response to cocaine and ethanol, suggesting that common genetic elements are involved in the response to all three drugs.
Keywords: arousal, locomotion, volatile anesthetics, cocaine, ethanol
INTRODUCTION
When given to patients or laboratory animals, volatile anesthetics alter the state of arousal of the nervous system and thereby produce sedation, immobility, and amnesia (Rudolph and Antkowiak, 2004). Defining the mechanisms by which anesthetics produce these effects is an important goal, and toward this end mutational analysis of anesthesia has been recruited to complement traditional physiological and biochemical studies (Nash, 2002). Volatile agents of diverse chemical structure and physical properties function as anesthetics (Bovill, 2008). Thus, an issue of general concern is the degree to which these inhaled compounds differ from each other in the way they produce the anesthetized state. When acting on an isolated neural component, such as an ion channel, different volatile agents often produce distinct effects or have potencies relative to one another that are distinct from those determined with a different assay (Urban et al., 2006); the same lack of uniformity is true for anesthetic action on isolated neural circuits (Pittson et al., 2004). However, whether these biochemical and physiological distinctions are relevant to the anesthetic effects of these agents in whole animals is hard to discern. Accordingly, the possibility that all inhaled anesthetics produce their clinically relevant effects by acting in a unitary way at a yet-to-be-defined target remains under serious discussion (Eger et al., 2008). This is a question for which genetic studies can be particularly incisive. For example, if the significant in vivo targets of volatile anesthetics were affected equally by all agents, one would never isolate a mutation that conferred a phenotype for one anesthetic that differed from any other agent. Conversely, by the same logic, the isolation of a mutation that confers non-uniform effects on potency (NEP) proves that there exists a biologically important target that varies in its susceptibility to different volatiles. Compared to other mutations that affect anesthesia, a focus on NEP mutations also provides an operational advantage to the experimenter. This is because, if a mutation that alters sensitivity to all anesthetics equally, it is hard to decide whether it does so merely because it has altered the global health of the organism. Such a gloomy scenario cannot apply to NEP mutations, so the effort of studying them is more likely to be rewarding. Thus, even though the pathway that connects the genetic change to the anesthesia phenotype may be complex and unknown, NEP mutations are of practical and theoretical value.
The importance of NEP mutations prompts two questions. What is their frequency relative to the complementary class of anesthesia mutation? And, how many different patterns of altered sensitivity can be observed? The answers should provide further insight into the basis of anesthesia. For example, if the NEP class of mutation is rare (or even non-existent), one would be inclined to believe that most anesthetics use a common target. Conversely, if each mutation that affected sensitivity to one drug altered sensitivity to others in an idiosyncratic way, one would favor the idea of multiple targets with partially overlapping specificity. In previous work with genetic model organisms, a few cases have been found in which a mutation has in vivo effects that are clearly more prominent for some anesthetics than others (Gamo et al., 2003; Humphrey et al., 2007; Quinlan et al., 2002; Quinlan et al., 1998). Since these cases are isolated instances, although they tell us that NEP mutations occur, they cannot indicate the generality of this class. One study, carried out with C. elegans, examined 7 anesthesia mutations with four volatile agents (Morgan and Sedensky, 1994). Four of the mutations conferred uniform alterations of potency but three could be classified as NEP. To our knowledge there are no other systematic studies that provide information about the relative frequency and diversity of NEP mutations. Here, we describe a collection of mutations of Drosophila melanogaster isolated on the basis of altered sensitivity to the volatile anesthetic halothane, and we use this collection to provide an orderly inquiry into non-uniform genetic effects in this organism.
METHODS
Isolation of Mutants, Initial Characterization, and Outcrossing to Canton-S
Lines bearing new X-linked insertions of the enhancer detector P element PZ[ry+] were generated as described by Heberlein and her colleagues (Moore et al., 1998), using genetic reagents provided by those authors. From each of ∼1000 such lines, halothane sensitivity was assessed by the distribution test (Campbell and Nash, 2001; Guan et al., 2000). Briefly, two groups of 10 male flies (2-5 days old) were collected under carbon dioxide anesthesia, allowed to recover for at least 16 hours, and transferred to testing tubes. The latter were 50 ml polypropylene Falcon tubes (Becton Dickinson, Franklin Lakes, NJ) that had been perforated with a grid of tiny holes to permit gas exchange. The tubes were placed in a glove box along with the tubes for 10 to 50 other insert lines, and the entire ensemble of 20 to 100 tubes was equilibrated for at least 90 min with a particular concentration of anesthetic. Testing consisted of tapping down a single tube of flies three times and then determining the minimum number of flies that remained in the conical bottom of the tube over the subsequent 60 sec. The concentration of halothane chosen for this screening step was one that induced ∼50% failure to climb in the parental lines. When this initial test suggested that a particular line was hypersensitive or resistant (i.e,, substantially more or less than half of the flies were found in the bottom of the tube), two or four more groups of fresh males from the this line were retested at a similar concentration. This procedure yielded 78 candidate insertions, each of which was introgressed into a Canton-S background.
As described in the next paragraph, the Cantonization scheme relied on a derivative of our laboratory’s stock of wild-type flies into which we introduced the ry506 mutation. To make this strain, the first step was to cross males from a Canton-S line (de Belle and Heisenberg, 1996; Guan et al., 2000) to virgins from a stock bearing ry506 (provided by Ulrike Heberlein). In the second step, male and female offspring from this cross were mated, and in the third step, male offspring of this cross that had rosy-colored eyes were mated to Canton-S females. The second and third steps were then repeated ten times. In a final round, the second step was followed by a cross of rosy-eyed males to rosy-eyed females to create a line designated ry506(CS).
To Cantonize a particular mutant line, the first step was to cross males bearing the insertion to virgin females from ry506(CS). In the second step, virgin female offspring from this cross were crossed to ry506(CS) males. This step was repeated five times, each time using females carrying a ry+ marker from the transposon insert. Although the final stock was not balanced, ry+ males could be collected from it; these were tested for halothane sensitivity as described above. This indicated that 14 of the 78 backcrossed P-elements insertions conferred a reproducible change in sensitivity to halothane. The location of each insertion was deduced, following amplification of the relevant segment by inverse PCR, from the sequence of the chromosomal DNA that immediately flanked the P-element (Huang et al., 2000). Subsequently, the backcrossed lines were balanced by crossing ry+ males to virgins from FM7a(CS), a line in which the first chromosome balancer had been introduced into an otherwise Canton-S background (de Belle and Heisenberg, 1996). Non-balancer male offspring from these lines were used in the later stages of the work.
Concentration-response Curves
The potency with which volatile anesthetics depress the righting/climbing reflex of flies was determined by evaluating the distribution test as described above, but using multiple drug concentrations (Alone et al., 2009). In this paradigm, the starting anesthetic concentration was chosen to be low enough that almost no flies failed to escape the bottom of the testing tube. After all the tubes had been subjected to this procedure, the concentration of anesthetic was raised and, after an additional equilibration period of 30-70 min, the flies were retested. In a typical experiment, the glove box held 5 to 12 mutant lines, each with three tubes apiece, and six tubes of the control line; all tubes in the ensemble were tested at a total of 3-4 concentrations. After 2 to 24 hours a second set of flies of the same genotypes were tested at 3-4 higher concentrations, and the whole process was repeated with fresh flies about one week later. The data from all the assays were pooled and fit by a logit analysis (Waud, 1972) to a standard concentration-response equation (Motulsky and Christopoulos, 2004). Since no flies failed to escape the bottom of the tube at the lowest concentration tested and all flies failed to escape the bottom of the tube at the highest concentration tested, the equation was:
where fx down is the fraction of flies that fail to escape the bottom of the tube after exposure to concentration C. The fitting program (SPSS Inc., Chicago, IL) uses a maximum likelihood method to find the best value for EC50, the midpoint of the concentration-response curve, and n, a parameter that determines the steepness of the sigmoidal curve. So that all the EC50 values for each strain can be fairly compared to one another, the program fits all the curves simultaneously and determines a single value for n that applies to all the curves (Waud, 1972). The curves are steep; although a distinct n value was computed for each anesthetic, they all fell in the range of 3 to 7. While this indicates that the experimental and genetic variances are generally low (Sonner, 2002; Woodbury, 1975), little else can be deduced from this parameter. More informative are the EC50 values, which provide a metric for the potency with which an anesthetic affects the performance of each line. An equivalent way to fit the concentration-response data, but one that sharpens the focus on genetic alterations in anesthetic sensitivity, is to calculate the EC50 for the control line and the factor by which this value differs from that of each mutant line (Waud, 1972); such “potency ratios” are also determined by the fitting program from the concentration-response data and are used throughout this work. In addition to providing the maximum likelihood estimate for each EC50 value and potency ratio, the fitting program provides 95% confidence limits for these estimates, i.e., the range of values in which one can be 95% certain that the true value will fall. Accordingly, two EC50 values can be considered statistically different from one another if their 95% confidence limits do not overlap (Conover, 1999). In the same way, the sensitivity of a mutant line is considered significantly different from that of the control line if the 95% confidence interval of the corresponding potency ratio is entirely above or below the value 1.0 (Waud, 1972).
Assaying Anesthetic Sensitivity in the Dark
In the past, the influence of illumination on anesthetic sensitivity was assessed by carrying out the distribution test under ambient lighting and in dim red light (Cheng and Nash, 2008). The latter requires the observer to have very sharp night vision, and not every experimenter could score the outcome with confidence. To circumvent this issue but retain a low-tech approach, we modified a countercurrent device (Benzer, 1967) so that the effect of anesthetics on the righting/climbing reflex could be assessed regardless of illumination. The modification was merely to drill tiny holes in the sidewall of each of the tubes used in the apparatus so as to permit gas exchange. Typically, a group of ∼30 flies was loaded into the start tube (labeled base tube 0), the device was placed upright in a glove box, and the flies were equilibrated with a fixed concentration of anesthetic for 40 min. Then, following the traditional countercurrent protocol (Connolly and Tully, 1998), the flies were given 5 opportunities to climb up out of a base tube into a transfer tube within a fixed period of time (60 sec); following each opportunity, the flies in each transfer tube were shifted into the next base tube. At the end, the flies in each base tube were counted under convenient illumination.
The experiment was performed two to five times on each line, all the data for each line was pooled, and a transfer probability, Pt, was calculated as
where the summation is over all the base tubes and 5 = the number of transfer tubes. An algebraic rationale for this heuristic formula has been published (Singh, 1993). Confidence limits (95%) for Pt were calculated as described (Conover, 1999) with the following formula
where tm is the number of transfers made and tp is the number of transfers possible. Significant differences between the Pt value for the control strain and a mutant strain were assigned if their confidence limits did not overlap.
RESULTS
Isolation of Mutants with Altered Sensitivity to a Volatile Anesthetic
A hallmark of healthy, robust adult flies is their ability to recover promptly from a mechanical shock by assuming an upright posture and vigorously climbing upwards. This ability is undermined by many drugs that influence neural function, including volatile anesthetics. Several years ago we devised a simple assay that permits precise and objective measurement of anesthetic-induced loss of the righting/climbing reflex to mechanical agitation (Guan et al., 2000). In the present work we have used this assay, also known as the distribution test, to screen flies bearing X-linked insertions of a P element for their sensitivity to halothane. As described in Materials and Methods, approximately 1000 lines were tested in the presence of a modest concentration of halothane. Promising candidates, i.e., those in which the proportion of flies that failed to climb differed from control, were outcrossed for several generations to the Canton-S line and then retested. Those lines in which the anesthesia phenotype appeared to be tightly linked to the transposon form the basis of the present study. Table 1 presents the genomic position of the insert in each line, deduced by DNA sequencing following inverse PCR using primers from the end of the transposon. These positions range over most of the X chromosome. Not surprisingly, given the known preference of P element to land at “hotspots” (Spradling et al., 1999), several of the inserts are clustered within 100 bp of each other. On the other hand, several genomic regions have only one insert each, indicating that our screen of 1000 random hops has not saturated the X chromosome with anesthesia mutations. Nevertheless, this collection of 14 insertion lines provides enough diversity for an initial overview of the genetic control of sensitivity to halothane.
Table 1.
A collection of X-linked insertion mutations associated with altered sensitivity to halothane
| Strain name | Location of insert |
Putatively affected gene(s) |
Potency ratio (95% CL) |
% Shift in EC50 |
|---|---|---|---|---|
| JC01 | 48,375 | CG17707 | 2.03 (1.83-2.27) | -50.8 |
| JC02 | 1,762,806 | CG14815 & CG14803 | 1.55 (1.41-1.72) | -35.7 |
| JC03 | 1,767,558 | CG14816 & CG14804 | 1.55 (1.40-1.71) | -35.3 |
| JC04 | 2,411,672 | trol | 1.89 (1.70-2.09) | -47.0 |
| JC05 | 2,536,688 | sgg | 1.55 (1.40-1.71) | -35.3 |
| JC06 | 10,184,177 | alpha-Man-I | 1.62 (1.47-1.79) | -38.3 |
| JC07 | 10,184,200 | alpha-Man-I | 1.37 (1.25-1.49) | -26.8 |
| JC08 | 10,184,223 | alpha-Man-I | 1.66 (1.50-1.84) | -39.8 |
| JC09 | 11,623,218 | inaF | 0.88 (0.80-0.96) | +14.2 |
| JC10 | 13,890,439 | ben | 1.98 (1.79-2.21) | -49.6 |
| JC11 | 18,428,614 | Bx | 2.20 (1.97-2.46) | -54.5 |
| JC12 | 18,428,636 | Bx | 1.89 (1.71-2.10) | -47.1 |
| JC13 | 18,428,677 | Bx | 1.41 (1.29-1.54) | -29.0 |
| JC14 | 19,781,155 | amn & CG32529 | 1.11 (1.03-1.22) | -10.6 |
The position of each insert is given using the coordinates of the complete sequence of the Drosophila melanogaster X chromosome (Accession NC_004354.3). The annotation of the Drosophila genome (Release 5.13), which is is available through FlyBase < http://flybase.org/cgi-bin/gbrowse/dmel/>, was used to identify genes that are likely to be affected by the listed insertions because the latter either disrupt the transcription unit of the named gene or lie within a few kb of its 5′-end. The final two columns present related metrics for the halothane sensitivity of each strain relative to that of the Canton-S control strain. The potency ratio is defined as EC50 (control strain)/EC50 (mutant strain). This ratio is calculated from a logistic fit of concentration-response curves and is presented together with 95% confidence limits. The shift in EC50 is defined as 100 * [EC50 (mutant strain)-EC50 (control strain)]/ EC50 (control strain)]; it is calculated from the potency ratio R by the formula: 100* [(1/R)-1]. The absolute value of the EC50 for each mutant line can be calculated from these metrics and the halothane EC50 value determined from the concentration-response curve for the Canton-S control strain: 0.26 (v/v%).
Characterization of the Halothane Phenotype
Insertions that conferred an anesthesia phenotype were identified, as described above, by tests conducted at a single concentration of halothane. While this procedure proved to be adequately sensitive and reliable for mutant isolation, it did not provide a clear assessment of the degree to which the mutant lines differ from control in sensitivity to the drug. Toward this end, the control line and every mutant line was tested at multiple halothane concentrations, the lowest of which had little or no effect on climbing ability and the highest of which rendered all flies unable to climb. Examples of these concentration-response curves are shown in Figure 1. Each such sigmoidal curve can be characterized by a steepness parameter and a location parameter (Motulsky and Christopoulos, 2004). As described in Material and Methods, the entire set of curves is simultaneously fit by a logit analysis (Waud, 1972) to yield a single steepness value that applies to all the curves and a series of location parameters that quantify halothane potency. One such parameter is the midpoint of each curve (EC50 value). To facilitate comparison of mutant and control lines, the fitting program also computes the ratio between the EC50 for the control to the EC50 for a mutant. These “potency ratios”, which are >1 for hypersensitive lines and <1 for lines that are relatively resistant to halothane, are reported together with their 95% confidence limits in Table 1. Another way to gauge the size of the mutant phenotype is to convert the potency ratios into the percentage by which the mutant EC50 differs from that of the control line (Alone et al., 2009); these values are also reported in Table 1.
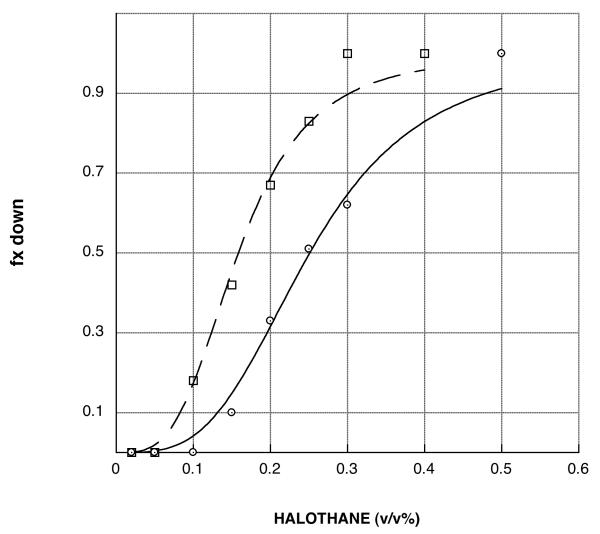
Figure 1.
Representative halothane concentration-response curves. The fraction of flies that are unable to climb out of the bottom of the testing tube during a one minute period are plotted as a function of the concentration of halothane with which they were equilibrated. The curves are fit as described in Materials and Methods to a sigmoidal function. The potency with which halothane interferes with the climbing response is deduced from the midpoint of each curve, i.e, the concentration (EC50) of halothane which is expected to prevent 50% of the flies from climbing. The EC50 value for the Canton-S control strain (open circles, solid line) is 0.26 (v/v%). The other curve shown is for strain JC08 (open boxes, dashed line); it is left-shifted and thus hypersensitive to halothane. The magnitude of this hypersensitivity can be assessed by computing the ratio of EC50 values for the control/mutant strains; for JC08 the potency ratio is 1.66.
From these metrics, it can be seen that the effects of the mutations on halothane sensitivity range from mild resistance (line JC09) to substantial hypersensitivity (lines JC01 & JC10). Indeed, the magnitude of the hypersensitive phenotype of some of these insertions rivals that seen with the mutations of narrow abdomen (na), the locus of mutations with striking effects on anesthesia sensitivity in diverse organisms (Guan et al., 2000; Humphrey et al., 2007). It should be noted that, when tested in the absence of halothane, all insertion lines were uniformly successful in climbing out of the bottom of the testing tube. Moreover, for a representative subset of the lines, locomotor performance in the absence of drug was evaluated quantitatively with a countercurrent device and found to be indistinguishable from control (Table 2). Thus, although it is impossible to rule out this hypothesis definitively from such experiments, altered baseline performance does not appear to be the basis for the substantial hypersensitivity of these lines.
Table 2.
Climbing ability in the absence or presence of halothane with and without illumination
| no anesthetic | 0.2% Halothane | ||||
|---|---|---|---|---|---|
| Strain name | ambient light | dark | ambient light | dark | |
| Canton-S |
0.789 (0.756-0.821) |
0.526 (0.486-0.565) |
0.375 (0.336-0.414) |
0.418 (0.379-0.458) |
|
| JC02 |
0.787 (0.741-0.833) |
0.582 (0.537-0.628) |
0.033* (0.013-0.053) |
0.058* (0.036-0.079) |
|
| JC05 |
0.767 (0.719-0.815) |
0.627 (0.572-0.681) |
0.068* (0.039-0.097) |
0.080* (0.049-0.111) |
|
| JC07 |
0.824 (0.780-0.867) |
0.618 (0.561-0.674) |
0.080* (0.049-0.111) |
0.105* (0.070-0.140) |
|
| JC11 |
0.737 (0.687-0.787) |
0.680* (0.627-0.733) |
0.072* (0.043-0.101) |
0.123* (0.086-0.161) |
|
| JC13 |
0.790 (0.744-0.836) |
0.650* (0.596-0.704) |
0.040* (0.018-0.062) |
0.050* (0.025-0.075) |
|
| inaF[P106x] |
0.813 (0.769-0.857) |
0.471 (0.409-0.532) |
0.673* (0.620-0.726) |
0.477 (0.420-0.533) |
|
Shown in bold is the estimate of Pt, the probability of an upward transfer, and in parentheses its 95% confidence interval. These values were derived from tests that were carried out, using a modified countercurrent device, under the indicated lighting conditions in the presence or absence of 0.2% halothane. Within each column, comparisons are made to judge whether any of the mutant lines significantly differs from the Canton-S control under the indicated conditions of lighting and anesthetic; such differences are marked with an asterisk. In the absence of anesthetic, no mutant line performs worse than control, so the poor performance of the JC lines in the presence of anesthetic does not appear to be due to weakened baseline locomotion. Note that the values for the inaF mutant confirm our previous observation that the relative resistance of this strain to halothane is dependent on ambient lighting (Cheng and Nash, 2008). See Methods for a description of the countercurrent test, the formulas used to calculate Pt and its confidence limits, and the basis for assignment of significant differences
Another important question about the halothane phenotype is its dependence on visual input. In recent work, we have concluded that ambient illumination has both positive and negative effects on arousal, and mutations can influence halothane sensitivity by changing the balance between these effects (Cheng and Nash, 2008). The most dramatic example of this phenomenon involved a null allele of inaF, the gene mutated in line JC09 (Cheng and Nash, 2007); in this case the mutant phenotype disappeared when the line was tested in the dark (Cheng and Nash, 2008). To see if this phenomenon was common in our mutant collection, Canton-S and the mutant lines JC02, JC05, JC07, JC11, and JC13 were tested both in ambient light and in the dark for climbing ability in the presence of a modest concentration of halothane. As described in Methods, to simplify scoring of locomotion in the dark, rather than the distribution test, we used a countercurrent device to quantify climbing ability. Table 2 shows that all these lines were distinguishable from the Canton-S control line in their sensitivity to halothane both in light and in dark. We conclude that the mechanism underlying their halothane hypersensitivity is distinct from that for the inaF mutant, which again showed light-dependence of its phenotype.
Phenotype with Other Volatile Agents
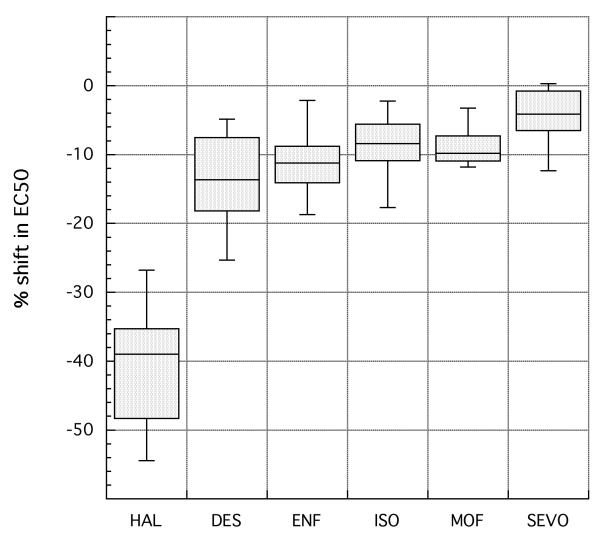
In order to gauge the uniformity of effects of the 14 P-element inserts on sensitivity to various volatile agents, the distribution test was carried out with five additional clinical anesthetics. The particular compounds chosen have potencies that bracket the value for halothane. That is, in order to interfere with the climbing reflex of wild-type flies, some of these agents are required at similar gaseous concentrations to halothane while others are required at much lower or much higher concentrations. The resulting potency ratios (and 95% confidence limits) for each line and each of the five new anesthetics are reported in Table 3. To simplify the comparison of these drugs with halothane, the values from Table 1 are presented again. Note that the strains are listed not in the order of their position on the X chromosome (as in Table 1) but in order of increasing potency ratio for halothane. For the weakly resistant line (JC09) and the weakly hypersensitive lines (JC14), examination of other anesthetics was not very informative because the effects of the mutation on any agent were so modest that it was hard to assign a significant pattern. In contrast, interpretation was straightforward for the 12 mutant lines that display moderately to strongly increased sensitivity to halothane (potency ratios >1.3). In every case, the mutation conferred a weaker effect on sensitivity to all the other agents than to halothane. For example, the halothane potency ratio determined for line JC13 and its Canton-S control was 1.41, but the ratios for the other anesthetics range between 1.00 and 1.10. Similarly, halothane potency ratio for line JC08 is 1.66 but those for the other volatiles vary from 1.03 to 1.17. An overview of the data for these twelve lines is provided in Figure 2. Here we have converted potency ratios into percentage shift in EC50 values and indicated the spread in the data with boxplots. This diagram not only emphasizes the existence of agent-specific effects in these twelve lines, it suggests that they share a common pattern. This pattern is one in which the mutations have, in addition to the very strong effects on halothane sensitivity, moderate effects on sensitivity to desflurane and somewhat weaker effects on sensitivity to sevoflurane, with the other agents falling between these two cases. Examination of the data in Table 3 provides only a few exceptions to this generalization, and most of these cannot be considered significant because of overlap in confidence limits.
Table 3.
Sensitivity of the insertion lines to five additional anesthetics
| Strain name |
Halothane | Desflurane | Enflurane | Isoflurane | Methoxyflurane | Sevoflurane |
|---|---|---|---|---|---|---|
| JC09 |
0.88 (0.80-0.96) |
1.15 (1.08-1.22) |
1.11 (1.02-1.21) |
0.95 (0.89-1.02) |
1.11 (1.03-1.20) |
0.93 (0.90-0.97) |
| JC14 |
1.11 (1.03-1.22) |
1.24 (1.16-1.33) |
1.14 (1.05-1.24) |
1.15 (1.07-1.23) |
1.06 (0.991.15) |
1.10 (1.06-1.15) |
| JC07 |
1.37 (1.25-1.49) |
1.07 (1.01-1.14) |
1.12 (1.03-1.22) |
1.02 (0.96-1.10) |
1.03 (0.96-1.11) |
0.98 (0.96-1.04) |
| JC13 |
1.41 (1.29-1.54) |
1.28 (1.19-1.37) |
1.13 (1.04-1.23) |
1.22 (1.13-1.31) |
1.11 (1.03-1.20) |
1.12 (1.07-1.16) |
| JC05 |
1.55 (1.40-1.71) |
1.16 (1.09-1.24) |
1.23 (1.13-1.34) |
1.10 (1.03-1.18) |
1.10 (1.02-1.19) |
1.06 (1.02-1.10) |
| JC03 |
1.55 (1.40-1.71) |
1.06 (0.99-1.12) |
1.02 (0.94-1.11) |
1.08 (1.01-1.16) |
1.05 (0.97-1.13) |
1.01 (0.97-1.05) |
| JC02 |
1.55 (1.41-1.72) |
1.23 (1.15-1.31) |
1.11 (1.02-1.20) |
1.14 (1.06-1.23) |
1.06 (0.98-1.14) |
1.07 (1.03-1.11) |
| JC06 |
1.62 (1.47-1.79) |
1.34 (1.25-1.44) |
1.10 (1.01-1.19) |
1.05 (0.97-1.12) |
1.13 (1.04-1.22) |
1.14 (1.10-1.19) |
| JC08 |
1.66 (1.50-1.84) |
1.17 (1.10-1.26) |
1.09 (1.00-1.18) |
1.16 (1.08-1.25) |
1.11 (1.03-1.19) |
1.03 (0.99-1.07) |
| JC04 |
1.89 (1.70-2.09) |
1.16 (1.09-1.24) |
1.15 (1.06-1.25) |
1.07 (1.00-1.15) |
1.13 (1.04-1.22) |
1.01 (0.97-1.05) |
| JC12 |
1.89 (1.71-2.10) |
1.09 (1.03-1.16) |
1.17 (1.07-1.27) |
1.10 (1.03-1.18) |
1.11 (1.03-1.20) |
1.01 (0.97-1.05) |
| JC10 |
1.98 (1.79-2.21) |
1.22 (1.14-1.31) |
1.16 (1.07-1.26) |
1.10 (1.02-1.18) |
1.12 (1.04-1.21) |
1.07 (1.03-1.11) |
| JC01 |
2.03 (1.83-2.27) |
1.13 (1.07-1.21) |
1.20 (1.10-1.30) |
1.09 (1.01-1.16) |
1.13 (1.05-1.23) |
1.07 (1.03-1.11) |
| JC11 |
2.20 (1.97-2.46) |
1.05 (0.99-1.12) |
1.09 (1.00-1.18) |
1.04 (0.97-1.11) |
1.10 (1.02-1.19) |
1.00 (0.96-1.04) |
The sensitivity of each strain (relative to that of the control strain) to the indicated anesthetic is given by the potency ratio (and 95% confidence limits). The values for halothane are taken from Table I; note that the order of strains has been rearranged so as to present them in order of increasing halothane potency ratio. The potency ratios for the five additional anesthetics, presented in alphabetical order, are calculated from sets of concentration-response curves. The absolute value of the EC50 for any anesthetic and any mutant line can be calculated from the appropriate potency ratio of the line and the EC50 value for the particular anesthetic, determined from concentration-response curves for the Canton-S control strain. For desflurane, enflurane, isoflurane, methoxyflurane, and sevoflurane, these control EC50 values are, respectively: 1.88, 0.24, 0.32, 0.08, and 0.64 (v/v%).
Figure 2.
Altered sensitivity to halothane and five other agents for twelve mutant lines. The mutant lines were those that show moderate to strong effects with halothane (see Table 1). For any given anesthetic, the twelve values for % shift in potency from that of the control line (calculated as in Table I) are arrayed as a boxplot (Sokal and Rohlf, 1995). In such a plot, the shift values are ordered numerically and the box encompasses those values that are ranked from the 25th to the 75th percentile, with the position of the median value marked by a line. Those % shift values that lie outside the box but within an additional 1.5-fold of its range are encompassed by whiskers; although none were found, any outliers beyond this range would have been plotted as individual points.
Because the NEP phenotype was so surprising, we took pains to insure its correctness. Specifically, we designed a test to confront the possibility of a systematic error in our experiments that might lead to artifactual differences in mutant effects on sensitivity to different drugs. The test used a representative subset of mutant lines: JC02, JC05, JC07, JC11, and JC13. Together with the CS control, these lines were assayed with the distribution test for sensitivity to halothane and sevoflurane. The critical difference from earlier procedures is that sensitivity to both drugs was assessed on the same day by one individual. In every case, the mutant lines showed little or no significant difference from control in sensitivity to sevoflurane (the potency ratios varied from 0.9 to 1.1). In contrast, all the mutant lines showed a clear difference from control in sensitivity to halothane (potency ratios >1.3). That is, when tested under conditions that should remove time-sensitive variations, such as the health of the stocks, or subtle differences in the technique used to assay them, the mutants had a substantial effect on halothane potency but had an insignificant effect on sevoflurane potency. We conclude that NEP phenotype accurately reflects a genetic alteration in these strains.
DISCUSSION
Mutations that influence sensitivity to anesthetics can identify genes involved in the control of arousal and/or genes whose products are targeted by these clinically important compounds. However, many mutations that alter anesthetic sensitivity will not be particularly informative. This is because the endpoints used to judge anesthesia in whole animals are typically the loss of complex behaviors, like the righting reflex of a mouse after being turned on its back or the tendency of a fly to climb after being aroused by mechanical agitation. Achievement of these endpoints relies on global strength and/or coordination, so mutations that affect these attributes can be expected to change drug sensitivity even if they have nothing to do with anesthesia-sensitive circuits (Nash, 2002). Confounding the picture is the possibility that such changes in baseline performance might be too subtle to be detected by routine assays performed in the absence of drugs. On the other hand, mutations that influence sensitivity to one anesthetic more than another (i.e., NEP mutations) cannot by definition act through such a global mechanism and are thus more likely to be informative about mechanisms of anesthesia and/or arousal. It is for this reason that we deemed it important to ask about the relative frequency of such mutations. The answer we got for halothane sensitivity was quite remarkable: at least 12 of 14 random mutations selected for altered sensitivity to this anesthetic have a much different effect on other volatiles. The genes affected by these mutations are thus likely to be of value for exploring the way anesthetics work and/or the way arousal is encoded in the nervous system of the fly.
The definitive characterization of such genes will be a substantial undertaking, involving study of expression patterns and determination of temporal/spatial requirements for rescue of the anesthesia phenotype. Nevertheless, in almost every case, without additional effort a probable identity of the relevant gene can be deduced. This is because serial out-crossing indicates that the anesthesia phenotype of each line is tightly linked to a particular insertion. The validity of this argument is supported by our isolation of independent mutations that map less than 100 bp apart from each other. We thus believe that the precise genomic location of each insert can be used to infer the likely identification of the affected gene. This is particularly the case for insertions that are positioned deep within the body of a single gene. For example, the three inserts that fall around X chromosome coordinate 10.18 Mb disrupt an intron of the gene (alpha-Man-I) that encodes an α-mannosidase; no other gene or non-coding RNA is annotated within 20 kb of these three insertions. Similarly, the insert that maps to X chromosome coordinate 2.54 Mb disrupts an intron of the gene (sgg) that encodes the Drosophila ortholog of glycogen synthase kinase 3B; no other gene or non-coding RNA is annotated within 10 kb of this insertion. Table 1 lists the genes associated with each of the insertions, including a few cases in which it is hard to distinguish between two equally plausible candidates.
Despite the tentative nature of the assignment of these genes, several features are worth noting about them. First, from a microarray analysis provided in a public database (Chintapalli et al., 2007) it appears that, although all of these genes are expressed at some level in all tissues, several (e.g., sgg, Bx, and ben) enjoy preferential and strong expression in the adult brain and thoracoabdominal ganglion. In contrast, expression of none of the genes in Table 1 is strong or highly preferential in the adult carcass, the body part in which muscle predominates. Although one cannot rule out the possibility that some of these mutations affect anesthesia sensitivity through their influence on muscle performance, this survey leads us to believe that alteration of neural functioning underlies the phenotype of our mutants. The second feature of the set of candidate genes is that none of them appear to be involved in the structure or function of the fly’s blood-brain barrier. That is, our collection does not include genes that encode known components of this barrier (e.g., GPCRs (Bainton et al., 2005), molecules involved in formation of septate junctions (Stork et al., 2008), ABC transporters (Mayer et al., 2009), or even genes whose expression is dependent on a glial-specific transcription factor (Altenhein et al., 2006). Nor do any of the genes encode molecules thought to be involved in degradation of xenobiotics (Yang et al., 2007). The important implication of these facts is that the mutations we isolated do not alter anesthetic sensitivity by influencing the way the drugs gain access to or survive in the nervous system. If they do not affect pharmacokinetics, these mutations must confer their phenotype by affecting pharmacodynamics, i.e., the drug-target interaction and/or the consequences of that interaction to the functioning of the organism (Ross and Kenakin, 2001). Of course, the mutant effect may be many steps removed from the binding of the drug to its target. Nevertheless, to the extent that pharmacodynamic action implies alteration of normal neural function, the relevant genes are likely to control some aspect of such function and thus be of interest to neurobiologists regardless of their effect on anesthesia. Of course, some of the genes listed in Table 1 are already well-known for their role(s) in the nervous system, e.g., amn (Keene et al., 2006) and ben (Uthaman et al., 2008); it is our hope that the evidence presented here will stimulate investigation of some of the other entries. The final thing to note about the candidate genes listed in Table 1 is that several of them have also been implicated in sensitivity to drugs of abuse. Specifically, P element insertions that map less than 100 bp from some of those in Table 1 have been found to alter the sensitivity of Drosophila to cocaine and ethanol. The list of genes affected in both kinds of study includes amn (Moore et al., 1998) but, since the mutation we isolated does not confer an obvious NEP phenotype (Table 3), we cannot rule out the possibility that amn affects drug sensitivity merely by influencing baseline coordination and/or strength of the fly. However, that cannot be the case for the genes that govern sensitivity to halothane more strongly than sensitivity to other volatiles, and at least three such genes — Bx (Tsai et al., 2004), sgg (Wolf et al., 2007), and alpha Man-I (U. Heberlein, personal communication) — have also been found to alter the fly’s response to cocaine and/or ethanol. We infer that these genes are quite likely to alter the functioning of neural pathways specifically affected by all three drugs. If so, this is to our knowledge the first evidence suggesting that halothane, cocaine, and ethanol influence common elements.
As noted in the Introduction, if the biologically significant targets for one volatile agent were equally affected by all volatile agents, one could never isolate a mutation that distinguishes among these anesthetics. Thus, just as in previous work (Humphrey et al., 2007), the NEP mutations reported here imply the existence of some degree of agent-specificity in one or more anesthetic target. Since, all 12 such mutations seem to confer a similar phenotypic profile (Figure 2), although we cannot rule out more complex scenarios, we infer the existence of a single target that is strongly affected by halothane, modestly affected by desflurane, weakly affected by sevoflurane, etc. There is no strong reason to favor the hypothesis that one of the genes affected by the NEP mutations reported here encodes such a target. Instead, it seems more likely that these genes participate in a network of interacting loci that ultimately controls its expression, activity, and/or physiological significance. Thus, an important goal for future work is to isolate a more complete set of mutations that confer a pattern identical to that of Figure 2 and then to examine epistatic relationships between these mutations. In a favorable case (see (Anholt et al., 2003) for an example), a prominent node in this network of interacting genes may point the way toward identifying the critical component.
It should be noted that in the work presented above, a single anesthetic endpoint - loss of the fly’s righting and climbing reflex in response to mechanical agitation - was used to isolate and characterize mutations. We thus do not know the universality of the anethestic phenotype, i.e, whether the potency with which volatile agents alter the functioning of other circuits would be affected in the same way as reported in Figure 2. This could be tested by examining flies for other anesthetic endpoints such as movement in response to an irritant (Gamo et al., 2003; Tinklenberg et al., 1991) or transmission of impulses through microcircuits (Rajaram et al., 2005; Walcourt et al., 2001). The only additional observations we have made on the mutations of Table 1 concerns their effect of on loss of postural control as measured by elution from an inebriometer in response to halothane, enflurane and sevoflurane (Campbell and Nash, 1994; Moore et al., 1998; Weber, 1988). Consistent with the presence of an agent-specific target in this pathway, the mutant effects on halothane-promoted elution are not well correlated with mutant effects on elution promoted by exposure to the other two anesthetics (JQG and HAN, unpublished). Although it is true that the distinction between agents conferred by the mutations is less clear-cut with the inebriometer assay than with the distribution test, the former was carried out with only a single concentration of each agent and thus cannot be easily compared to experiments that measured changes in potency. All in all, we believe the NEP mutations are likely to lead to important insights into the regulation of excitability in a significant portion of the fly’s nervous system as well as its perturbation by volatiles and drugs of abuse. Furthermore, because of the high degree of conservation of genes and drug actions between flies and higher organisms, it is also likely that study of the genes affected by these mutations will inform these issues in the human population.
ACKNOWLEDGEMENTS
We are most grateful to Barry Chestnut and Jessica Keys for their invaluable assistance with the initial genetic screen and mutant characterization. Thanks are also due to Robert Scott, Bruno van Swinderen, and Benjamin White for their comments on the manuscript. Ulrike Heberlein is acknowledged for provision of fly stocks and for permission to cite unpublished data. This work was supported by the Intramural Program of the National Institute of Mental Health.
REFERENCES
- Alone DP, Rodriguez JC, Noland CL, Nash HA. Impact of gene copy number variation on anesthesia in Drosophila melanogaster. Anesthesiology. 2009;111:15–24. doi: 10.1097/ALN.0b013e3181a3276c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altenhein B, Becker A, Busold C, Beckmann B, Hoheisel JD, Technau GM. Expression profiling of glial genes during Drosophila embryogenesis. Dev Biol. 2006;296:545–560. doi: 10.1016/j.ydbio.2006.04.460. [DOI] [PubMed] [Google Scholar]
- Anholt RR, Dilda CL, Chang S, Fanara JJ, Kulkarni NH, Ganguly I, et al. The genetic architecture of odor-guided behavior in Drosophila: epistasis and the transcriptome. Nat Genet. 2003;35:180–184. doi: 10.1038/ng1240. [DOI] [PubMed] [Google Scholar]
- Bainton RJ, Tsai LT, Schwabe T, DeSalvo M, Gaul U, Heberlein U. moody encodes two GPCRs that regulate cocaine behaviors and blood-brain barrier permeability in Drosophila. Cell. 2005;123:145–156. doi: 10.1016/j.cell.2005.07.029. [DOI] [PubMed] [Google Scholar]
- Benzer S. Behavioral mutants of Drosophila isolated by countercurrent distribution. Genetics. 1967;58:1112–1119. doi: 10.1073/pnas.58.3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovill JG. Inhalation anaesthesia: from diethyl ether to xenon. Handb Exp Pharmacol. 2008:121–142. doi: 10.1007/978-3-540-74806-9_6. [DOI] [PubMed] [Google Scholar]
- Campbell DB, Nash HA. Use of Drosophila mutants to distinguish among volatile general anesthetics. Proc. Natl. Acad. Sci. USA. 1994;91:2135–2139. doi: 10.1073/pnas.91.6.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JL, Nash HA. Volatile general anesthetics reveal a neurobiological role for the white and brown genes of Drosophila melanogaster. J Neurobiol. 2001;49:339–349. doi: 10.1002/neu.10009. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Nash HA. Drosophila TRP channels require a protein with a distinctive motif encoded by the inaF locus. Proc Natl Acad Sci U S A. 2007;104:17730–17734. doi: 10.1073/pnas.0708368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Nash HA. Visual mutations reveal opposing effects of illumination on arousal in Drosophila. Genetics. 2008;178:2413–2416. doi: 10.1534/genetics.107.085324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintapalli VR, Wang J, Dow JA. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet. 2007;39:715–720. doi: 10.1038/ng2049. [DOI] [PubMed] [Google Scholar]
- Connolly JB, Tully T. Behaviour, learning, and memory. In: Roberts DB, editor. Drosophila: A Practical Approach. Oxford University Press; Oxford: 1998. [Google Scholar]
- Conover WJ. Practical Nonparametric Statstics. John Wiley & Sons; New York: 1999. [Google Scholar]
- de Belle JS, Heisenberg M. Expression of Drosophila mushroom body mutations in alternative genetic backgrounds: A case study of the mushroom body miniature gene (mbm) Proc. Natl. Acad. Sci. USA. 1996;93:9875–9880. doi: 10.1073/pnas.93.18.9875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eger EI, 2nd, Tang M, Liao M, Laster MJ, Solt K, Flood P, et al. Inhaled anesthetics do not combine to produce synergistic effects regarding minimum alveolar anesthetic concentration in rats. Anesth Analg. 2008;107:479–485. doi: 10.1213/01.ane.0000295805.70887.65. [DOI] [PubMed] [Google Scholar]
- Gamo S, Tomida J, Dodo K, Keyakidani D, Matakatsu H, Yamamoto D, et al. Calreticulin Mediates Anesthetic Sensitivity in Drosophila melanogaster. Anesthesiology. 2003;99:867–875. doi: 10.1097/00000542-200310000-00019. [DOI] [PubMed] [Google Scholar]
- Guan Z, Scott RL, Nash HA. A New Assay for the Genetic Study of General Anesthesia in Drosophila melanogaster: Use in Analysis of Mutations in the 12E Region. J. neurogenet. 2000;14:25–42. doi: 10.3109/01677060009083475. [DOI] [PubMed] [Google Scholar]
- Huang AM, Rehm EJ, Rubin GM. Recovery of DNA Sequences Flanking P-element Insertions: Inverse PCR and Plasmid Rescue. In: Sullivan W, Ashburner M, Hawley RS, editors. Drosophila Protocols. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, N. Y.: 2000. [DOI] [PubMed] [Google Scholar]
- Humphrey JA, Hamming KS, Thacker CM, Scott RL, Sedensky MM, Snutch TP, et al. A putative cation channel and its novel regulator: cross-species conservation of effects on general anesthesia. Curr Biol. 2007;17:624–629. doi: 10.1016/j.cub.2007.02.037. [DOI] [PubMed] [Google Scholar]
- Keene AC, Krashes MJ, Leung B, Bernard JA, Waddell S. Drosophila dorsal paired medial neurons provide a general mechanism for memory consolidation. Curr Biol. 2006;16:1524–1530. doi: 10.1016/j.cub.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Mayer F, Mayer N, Chinn L, Pinsonneault RL, Kroetz D, Bainton RJ. Evolutionary conservation of vertebrate blood-brain barrier chemoprotective mechanisms in Drosophila. J Neurosci. 2009;29:3538–3550. doi: 10.1523/JNEUROSCI.5564-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MS, DeZazzo J, Luk AY, Tully T, Singh CM, Heberlein U. Ethanol Intoxication in Drosophila: Genetic and Pharmacological Evidence for Regulation by the cAMP Signaling Pathway. Cell. 1998;93:997–1007. doi: 10.1016/s0092-8674(00)81205-2. [DOI] [PubMed] [Google Scholar]
- Morgan PG, Sedensky MM. Mutations conferring new patterns of sensitivity to volatile anesthetics in Caenorhabditis elegans. Anesthesiology. 1994;81:888–898. doi: 10.1097/00000542-199410000-00016. [DOI] [PubMed] [Google Scholar]
- Motulsky M, Christopoulos A. Fitting Models to Biological Data Using Linear and Nonlinear Regression. Oxford University Press; New York: 2004. [Google Scholar]
- Nash HA. In Vivo Genetics of Anesthetic Action. Brit. J. Anesth. 2002;89:143–155. doi: 10.1093/bja/aef159. [DOI] [PubMed] [Google Scholar]
- Pittson S, Himmel AM, MacIver MB. Multiple synaptic and membrane sites of anesthetic action in the CA1 region of rat hippocampal slices. BMC Neurosci. 2004;5:52. doi: 10.1186/1471-2202-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan JJ, Ferguson C, Jester K, Firestone LL, Homanics GE. Mice with glycine receptor subunit mutations are both sensitive and resistant to volatile anesthetics. Anesth Analg. 2002;95:578–582. doi: 10.1097/00000539-200209000-00016. table of contents. [DOI] [PubMed] [Google Scholar]
- Quinlan JJ, Homanics GE, Firestone LL. Anesthesia sensitivity in mice that lack the beta3 subunit of the gamma-aminobutyric acid type A receptor. Anesthesiology. 1998;88:775–780. doi: 10.1097/00000542-199803000-00030. [DOI] [PubMed] [Google Scholar]
- Rajaram S, Scott RL, Nash HA. Retrograde signaling from the brain to the retina modulates the termination of the light response in Drosophila. Proc Natl Acad Sci U S A. 2005;102:17840–17845. doi: 10.1073/pnas.0508858102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross EM, Kenakin TP. Pharmacodnamics: Mechanisms of Drug Action and the Relationship Between Drug Concentration and Effect. In: Hardman JG, Limbird LE, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. McGraw Hill; New York: 2001. pp. 31–43. [Google Scholar]
- Rudolph U, Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nat Rev Neurosci. 2004;5:709–720. doi: 10.1038/nrn1496. [DOI] [PubMed] [Google Scholar]
- Singh S. Quantification of countercurrent distribution: from molecular partition to animal behavior. Biochem Biophys Res Commun. 1993;196:430–434. doi: 10.1006/bbrc.1993.2267. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry. 3rd edn W. H. Freeman; New York: 1995. [Google Scholar]
- Sonner JM. Issues in the design and interpretation of minimum alveolar anesthetic concentration (MAC) studies. Anesth Analg. 2002;95:609–614. doi: 10.1097/00000539-200209000-00021. [DOI] [PubMed] [Google Scholar]
- Spradling AC, Stern D, Beaton A, Rhem EJ, Laverty T, Mozden N, et al. The Berkeley Drosophila Genome Project gene disruption project: Single P-element insertions mutating 25% of vital Drosophila genes. Genetics. 1999;153:135–177. doi: 10.1093/genetics/153.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork T, Engelen D, Krudewig A, Silies M, Bainton RJ, Klambt C. Organization and function of the blood-brain barrier in Drosophila. J Neurosci. 2008;28:587–597. doi: 10.1523/JNEUROSCI.4367-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinklenberg JA, Segal IS, Tianzhi G, Maze M. Analysis of Anesthetic Action on the Potassium Channels of the Shaker Mutant of Drosophila. Annals of the New York Academy of Sciences. 1991;625:532–539. doi: 10.1111/j.1749-6632.1991.tb33884.x. [DOI] [PubMed] [Google Scholar]
- Tsai LT, Bainton RJ, Blau J, Heberlein U. Lmo mutants reveal a novel role for circadian pacemaker neurons in cocaine-induced behaviors. PLoS Biol. 2004;2:e408. doi: 10.1371/journal.pbio.0020408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban BW, Bleckwenn M, Barann M. Interactions of anesthetics with their targets: non-specific, specific or both? Pharmacol Ther. 2006;111:729–770. doi: 10.1016/j.pharmthera.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Uthaman SB, Godenschwege TA, Murphey RK. A mechanism distinct from highwire for the Drosophila ubiquitin conjugase bendless in synaptic growth and maturation. J Neurosci. 2008;28:8615–8623. doi: 10.1523/JNEUROSCI.2990-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walcourt A, Scott RL, Nash HA. Blockage of one class of potassium channel alters the effectiveness of halothane in a brain circuit of Drosophila. Anesth Analg. 2001;92:535–541. doi: 10.1097/00000539-200102000-00047. [DOI] [PubMed] [Google Scholar]
- Waud DR. On biological assays involving quantal responses. The Journal of Pharmacology and Experimental Therapeutics. 1972;183:577–607. [PubMed] [Google Scholar]
- Weber KE. An apparatus for measurement of resistance to gas-phase agents. Drosophila Information Service. 1988;67:91–93. [Google Scholar]
- Wolf FW, Eddison M, Lee S, Cho W, Heberlein U. GSK-3/Shaggy regulates olfactory habituation in Drosophila. Proc Natl Acad Sci U S A. 2007;104:4653–4657. doi: 10.1073/pnas.0700493104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury JW, D’Arrigo JS, Eyring H. Physiological mechanism of general anestheia: synaptic blockade. In: Fink BR, editor. Molecular mechanisms of anesthesia. 1975. pp. 53–91. [Google Scholar]
- Yang J, McCart C, Woods DJ, Terhzaz S, Greenwood KG, ffrench-Constant RH, et al. A Drosophila systems approach to xenobiotic metabolism. Physiol Genomics. 2007;30:223–231. doi: 10.1152/physiolgenomics.00018.2007. [DOI] [PubMed] [Google Scholar]