Abstract
Executive functions (EF) evidence significant age-related declines, but the mechanisms underpinning those changes are unclear. In this study, we focus on two potential sources of variation: a physiological indicator of vascular health, and genetic variants related to vascular functions. In a sample of healthy adults (n = 158, ages 18–81), we examine the effects of age, pulse pressure, and two polymorphisms (comt val158met and ace insertion/deletion) on working memory and cognitive flexibility. Results indicate that in addition to often-replicated age differences, the alleles of two polymorphisms that promote vasoconstriction (comt val and ace D) and reduced availability of dopamine in neocortical synapses (comt val), negatively impact virtually all aspects of EF tasks that involve working memory. In some cases, suppression of cognitive performance is limited to men or necessitates a combination of both risk-associated alleles. After accounting for genetic and age-related variation, pulse pressure had no additional effect on EF. These findings suggest that in healthy adults, the effects of genetic risk factors significantly modulate the course of cognitive aging.
Keywords: aging, working memory, ACE, COMT, vascular risk, polymorphism
1. Introduction
Many cognitive skills decline with age, but the magnitude of the age differences varies across functional domains (Horn, 1986). Executive functions (EF) that comprise goal setting, planning, and coordinating multiple tasks while storing information, shifting between stimulus and response sets, and deployment of attentional resources are among the most age-sensitive cognitive skills (Hultsch et al., 1992; West, 1996). Although the mechanisms that underpin such sensitivity are unclear, predilection of EF for age-related declines is likely to reflect heightened vulnerability of their neural substrates to diverse physiological, neurochemical, and genetic factors.
One of the important contributors to age-related vulnerability of EF is vascular risk. Vascular health declines with age (Fleg, 1986) and clinical, epidemiological, and experimental findings indicate that increasing vascular risk and overt cardiovascular disease have a significant negative impact on EF (Apter et al., 1951; Elias et al., 2003; Elias et al., 2004; Waldstein et al., 2008). Although EF is not a homogenous construct (Miyake et al., 2000), many of the executive component processes depend on structural integrity of the tertiary association cortices (especially the prefrontal cortex, PFC) and the associated white matter (Demakis, 2003; Gunning-Dixon and Raz, 2003; Raz et al., 1998). Age-related increase in vascular risk and vascular disease seem to exert an especially significant influence on prefrontal structures (e.g., DeCarli et al., 1995; Kuczynski et al., 2009; Raz et al., 2003, Raz et al., 2007a; Raz et al., 2007b; see Román et al., 2002 for a review). Moreover, even in healthy adults, high-normal values of vascular health indicators may be associated with reduced EF (e.g., Dahle et al., 2009).
Essential hypertension, one of the most common vascular risk factors, occurs in otherwise healthy adults and its prevalence increases with age (Franklin et al, 1997). Among multiple mechanisms proposed for the development of hypertension, an important role belongs to alterations in renin-angiotensin system (RAS) and specifically in activity of a vasoactive peptide angiotensin II (Safar, 2005). Availability of angiotensin II, a vasoconstrictor, is controlled by the angiotensin converting enzyme (ACE), which in addition to enabling its synthesis from angiotensin I, inhibits a vasodilator bradykinin (Vauquelin et al., 2002; von Bohlen und Halbach and Albrecht, 2006). Through its vasoconstrictive effects, ACE plays an important role in regulation of blood pressure, and ACE inhibitors are effective in the treatment of hypertension (Wong et al., 2004). Although it is abundant in the peripheral nervous, renal, and pulmonary systems, angiotensin II has a significant presence in the brain, where its receptors reside in the blood brain barrier and endothelial cells of cerebral vasculature (Saavedra, 2005).
Recent studies show that ACE may influence age-sensitive cognitive processes (Vauquelin et al., 2002; von Bohlen und Halbach and Albrecht, 2006) and promote cognitive impairment (He et al., 2006). Alternately, administration of ACE inhibitors reverses cognitive deficits in hypertensive rats (Srinivasan et al., 2005), reduces incidence of cognitive impairment in older adults (Yasar et al., 2008), slows progression to dementia in persons at risk (Rozzini et al., 2006), and ameliorates cognitive declines in patients with a diagnosis of Alzheimer’s disease (AD; He et al., 2006). In mice, chronically elevated angiotensin II reduces cerebral blood flow and impairs learning (Inaba et al. 2009). However, in some samples, variation of ACE activity had no effect on cognitive functions (Harrap et al., 2003; Visscher et al., 2003), suggesting that the effect of ACE may materialize only in conjunction with other physiological, neurochemical, or genetic factors.
One such established neurochemical modifier of EF is dopamine (DA), and specifically, activity at its D1 receptors located in the prefrontal synapses (see Arnsten and Li, 2005; Floresco and Magyar, 2006; Goldman-Rakic, 1996 for reviews). As aging is associated with a significant decline in dopaminergic functions (Bäckman et al., 2006), the DA system is a highly plausible candidate for explanation of age-related deficits in EF. Because the availability of DA in the PFC depends predominantly on the enzymatic activity of catechol-O-methyl-transferase (COMT; Slifstein et al., 2008; Tunbridge et al., 2004), factors that affect COMT levels may be crucial for maintaining normal EF through the life span.
Although the effects of angiotensin II and DA on cognitive performance can be studied by pharmacological manipulations, an alternative approach is to observe the effects of naturally occuring variations therein. Common mutations in the genes that affect enzymatic control of angiotensin II and DA availability in fact randomly assign individuals to high or low levels of the target compounds. As a substantial share of observed differences in EF stems from genetic factors (Anokhin et al., 2003; Friedman et al., 2008; but see Kremen et al., 2007), examination of specific polymorphisms is a plausible way to clarify the specifics of the genetic influence on EF.
Indeed, recent studies revealed several specific associations between behavioral phenotypes of EF and genetic polymorphisms. Although the extant literature is still relatively scarce, probably no polymorphism has received more attention in the study of EF genetics than comt val158met, a single nucleotide variant in a gene that controls the activity of COMT. In comparison to the double dose of the wild val allele, a double dose of the mutant met allele of that polymorphism is associated with an almost fourfold reduction in COMT activity.
Since publication of the seminal paper by Egan and colleagues (2001), the investigations of genetic associations between selected EF and comt val158met genotypes have thus far delivered mixed results. In multiple studies of healthy adults, carriers of the met allele outperformed the val carriers on many executive tasks, including measures of working memory (WM) capacity and resistance to perseveration (Barnett et al., 2008; Caldú et al., 2007; Diaz-Asper et al., 2008; Goldberg et al., 2003; Rosa et al., 2004; Tan et al., 2007). In a longitudinal study, met carriers showed less decline in EF performance in comparison to val homozygotes across the adult age range (de Frias et al., 2005), and in at least one sample, met homozygocity was associated with improved WM and reduced perseveration in older participants (Nagel et al., 2008). However, no effect of the comt val158met genotype has been observed on other EF, such as inhibition of prepotent response (color Stroop; Raz et al., 2009), or task switching (Erickson et al., 2008). Furthermore, in accord with the inverted-U model of DA effect on cognition that holds optimization rather than maximization of cortical DA as a predictor of better EF performance (Bäckman et al., 2006; Williams and Castner, 2006), a study of two independent samples of children and young adults found a positive heterosis effect of comt val158met on WM (Gosso et al., 2008). To complicate things even further, in several samples, comt val158met showed significant epistasis with other genes (Caldú et al., 2007; Gosso et al., 2008; Nagel et al., 2008; Tan et al., 2007; Xu et al., 2007). Thus, the effect of comt val158met may depend on complex interactions with age, type of task, and the action of other genes. Therefore, the extant literature on associations between comt val158met and various EF could benefit from further clarification.
One of the genetic variants that may modify the effects of DA on EF is an insertion/deletion (I/D) polymorphism in the ace gene that affects vasoconstriction through control of ACE availability. Insertion (I) or deletion (D) of an Alu repetitive element in the intron of ace produces three genotypes: ace II, ace ID, and ace DD, and presence of ace D allele accounts for almost 50% of the phenotypic variation in ACE plasma concentration (Rigat et al., 1990). The dose of ace I allele is associated with proportionate reduction in ACE activity, and as a consequence, limitation of vasoconstriction. Carriers of ace D (high activity) allele evidence increased vascular risk and higher prevalence of vascular disease (Bautista et al., 2008; Bonnet et al., 2008; Castellano et al., 1995; Hassan et al., 2002; Hosoi et al., 1996; Julve et al., 2001; Lao et al., 2005; Morris et al., 1994; Niemiec et al., 2007; Saidi et al., 2007). In contrast, ace I (low activity) allele is associated with reduced vascular risk, e.g., better arterial compliance (Benetos et al., 1996; Mattace-Raso et al., 2004; Taniwaki et al., 1999).
The investigations of the effects of ace I/D polymorphism on cognition are still limited and their results are contradictory. Older carriers of the I allele show less memory decline (Bartrés-Faz et al., 2000; Richard et al., 2000) than D homozygotes, and D homozygocity is excessively frequent among persons with age-related cognitive impairment (Amouyel et al., 1996). However, in some population-based studies, the ace I allele increased risk for dementia (Sleegers et al., 2005; Wang et al., 2006), a finding born out of a recent meta-analysis (Lehmann et al., 2005). As with comt val158met, the discrepancy in findings may reflect variability in vascular risk within specific samples and inclusion of persons with overt vascular disease as well as possible epistasis. In addition, reliance on relatively crude cognitive indices (e.g., Mini-Mental State Examination, MMSE, Folstein et al, 1975) and restriction of the age range to older adults could attenuate the observed effects. Thus, the effects of ace on normal cognitive aging may be overlooked because of failure to take into account synergistic effects of the genotype and vascular risk.
Because vascular risk plays a significant role in cognitive and neural declines associated with aging, it is important to evaluate the potential synergistic effects of vascular health indicators and genetic factors on the aspects of performance that are vulnerable to both. Unfortunately, the number of studies investigating interaction between vascular risk factors and the genetic variant is too small even to allow quantitative comparisons (Wisdom et al., in press). Studies that examined such interactions found that the effect of genetic polymorphisms on cognition is exacerbated by heightened vascular risks (de Frias et al., 2004; Deshmukh et al., 2009; Peila et al., 2001; Raz et al., 2009; see Zeng et al., 2004 for a review).
Measures of arterial blood pressure - systolic, diastolic, mean arterial, and pulse pressure - provide assessment of vascular health and vascular risk (Franklin et al., 1997). However, those indices are too numerous and mutually dependent to be taken into account simultaneously, and in a limited scale study it is prudent to limit their number. Pulse pressure (PP), a surrogate of arterial compliance (Mattace-Raso et al., 2006), appears an optimal candidate, as it is a convenient summary index of systolic and diastolic pressure, two distinct but highly correlated measures. Population-based studies of vascular risk in older adults reveal steep age-related increase in PP, which rises at a greater rate than systolic blood pressure and does not follow an inverted-U curve characteristic of diastolic blood pressure (Franklin et al., 1997). In contrast to PP, another composite vascular measure, mean arterial pressure (MAP) evidences a shallow increase with age reaching its asymptote at the sixth decade (Franklin et al., 1997). Daytime PP is a significantly better predictor of cardiovascular morbidity and mortality than are MAP or systolic blood pressure (Blacher et al., 2000; Boutouyrie et al., 1999; Chae et al., 1999; Glynn et al. 2000; Khattar et al., 2001; Mitchell et al., 2007). Moreover, in young adults, elevated PP is an independent predictor of structural changes such as intima-media thickness in the common carotid artery (Oren et al., 2006), a major vascular risk factor (Bots et al., 1997). Finally, PP is a significant predictor of age-related cognitive differences in healthy adults (Waldstein et al., 2008).
The aim of this study was to examine the joint effects of two polymorphisms, comt val158met and ace I/D, and a vascular risk indicator (PP), on age-sensitive measures of EF: working memory and cognitive flexibility. We hypothesized that a combined influence of older age, increased arterial stiffness (PP), and genetic factors (high-activity ace D and comt val alleles) that affect PFC function and structure of the PFC would have a negative impact on EF performance. We predicted that persons who carry both genotypes deemed detrimental to cognition would show incrementally lower performance, and we expected that the effects of genes would be amplified by high–normal PP.
2. Method
2.1. Participants
The participants were recruited through advertisements in the local media as part of an ongoing longitudinal study of healthy aging, and were screened via a telephone interview and health questionnaire. The reasons for exclusion were history of cardiovascular, neurological and psychiatric conditions, head trauma with a loss of consciousness for more than 5 minutes, history of alcohol and drug abuse, thyroid problems, hypertension, and diabetes. The items used to screen for cardiovascular disease included any sort of “heart troubles” and cardiovascular complaints as well as taking specific medications prescribed for treatment of cardiovascular symptoms. The participants had corrected visual acuity of 50/20 or better (Optic 2000, Stereo Optic) and hearing of 40 dB or better for frequencies of 500–4000 Hz (Maico, MA27). To screen for dementia and depression we used the MMSE (Folstein et al., 1975) and the Center for Epidemiologic Studies Depression Scale (CES-D; Radloff, 1977). Only persons who scored 26 or above on MMSE and 15 or below on CES-D were invited to participate. All participants provided written informed consent in accord with university and hospital review board guidelines. Because of geographic and ethnic differences in the distribution of comt and ace alleles, we used only the data from North American Caucasian participants.
A full set of data were available for 158 participants (113 women and 45 men), age range 18–81 (mean ± SD, 52.20 ± 15.50). Education as assessed by years of formal schooling corresponded to a typical duration required for obtaining a college degree (16.02 ± 2.27). The participants were normotensive (systolic blood pressure range 91–140 mm Hg, mean 119.14 ± 10.63 mm Hg; diastolic blood pressure range 57–89 mm Hg, 73.46 ± 6.77 mm Hg), and those for whom fasting glucose data were available (n = 113) were normoglycemic (fasting blood glucose range 65–113, 86.23 ± 9.55 mg/dl). None of the participants were taking anti-hypertensive, antidepressant, antipsychotic, or anxiolytic medications. The MMSE scores ranged from 26 to 30 (28.87 ± 1.05) out of a possible 30. With an MMSE score ≥ 26, the likelihood ratio for dementia ranges from 0.06 to 0.10 (Siu, 1991), and the only person who scored at MMSE = 26 was a 53-year old, an age with extremely low incidence of dementia. Men and women did not differ on any demographic or clinical parameters (see Table 1) except for a small but significant advantage of men in formal schooling (about 10 months on average). The findings on additional SNPs and cognitive measures on part of this sample have been reported elsewhere (65% of participants in Raz et al., 2008; 91% of Raz et al., 2009).
Table 1.
Description of the sample: men and women compared.
| Variable | Men | Women | t | p |
|---|---|---|---|---|
| Age | 54.40 ± 17.42 | 51.33 ± 14.66 | 1.13 | 0.26 |
| Education | 16.58 ± 2.15 | 15.80 ± 2.29 | 1.97 | 0.05 |
| Systolic blood pressure (mm Hg) | 121.16 ± 9.02 | 118.33 ± 11.14 | 1.51 | 0.13 |
| Diastolic blood pressure (mm Hg) | 75.79 ± 6.64 | 73.92 ± 6.77 | 1.57 | 0.12 |
| Pulse Pressure (mm Hg) | 45.37 ± 7.63 | 44.41 ± 7.81 | 0.71 | 0.48 |
| MMSE | 28.71 ± 1.12 | 28.93 ± 1.01 | −1.23 | 0.21 |
Abbreviations. MMSE: Mini-Mental State Examination; t; t-test statistic; p; probability value.
Data are presented as means ±standard deviations.
2.2. Measures of blood pressure
We measured blood pressure on three separate days by a mercury sphygmomanometer (BMS 12-S25) with a standard blood pressure cuff (Omron Professional) on the left arm, with participants seated in a comfortable chair with feet flat on the floor. The systolic and diastolic measures were averaged for each individual across sessions. Only participants with a mean systolic pressure not exceeding 140 mm Hg and a mean diastolic pressure at or below 90 mm Hg entered the study. Given a relatively small sample size and a relatively high correlation between systolic and diastolic blood pressure (see Table 2 below), we used a derived index of vascular health: pulse pressure (PP = systolic - diastolic pressure).
Table 2.
Zero-order correlations among age, vascular and cognitive variables.
| Variable | Age | PP | Sys | Dias | P Err | NP Err |
Total Err |
RT 1n | RT 2n | RT 3n | RT 1v | RT 2v | RT 3v | Err 3v |
Err 3n |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PP | 0.45 | ||||||||||||||
| Systolic | 0.45 | 0.77 | |||||||||||||
| Diastolic | 0.18 | 0.07 | 0.69 | ||||||||||||
| P Err | 0.40 | 0.14 | 0.11 | −0.00 | |||||||||||
| NP Err | 0.50 | 0.21 | 0.17 | 0.03 | 0.82 | ||||||||||
| Total Err | 0.47 | 0.19 | 0.15 | 0.02 | 0.95 | 0.96 | |||||||||
| RT 1n | 0.65 | 0.28 | 0.23 | 0.04 | 0.39 | 0.43 | 0.43 | ||||||||
| RT 2n | 0.63 | 0.33 | 0.29 | 0.08 | 0.33 | 0.43 | 0.40 | 0.74 | |||||||
| RT 3n | 0.29 | 0.19 | 0.21 | 0.12 | 0.18 | 0.16 | 0.17 | 0.34 | 0.27 | ||||||
| RT 1v | 0.50 | 0.24 | 0.19 | 0.03 | 0.30 | 0.41 | 0.37 | 0.64 | 0.54 | 0.19 | |||||
| RT 2v | 0.37 | 0.17 | 0.17 | 0.07 | 0.27 | 0.34 | 0.31 | 0.54 | 0.53 | 0.27 | 0.69 | ||||
| RT 3v | 0.20 | 0.05 | 0.10 | 0.11 | 0.08 | 0.09 | 0.09 | 0.31 | 0.23 | 0.33 | 0.29 | 0.47 | |||
| Err 3v | 0.17 | 0.11 | 0.13 | 0.08 | 0.13 | 0.13 | 0.13 | 0.06 | 0.14 | 0.09 | 0.06 | 0.04 | 0.07 | ||
| Err 3n | 0.41 | 0.18 | 0.15 | 0.02 | 0.36 | 0.34 | 0.37 | 0.23 | 0.28 | 0.09 | 0.16 | 0.18 | 0.22 | 0.45 | |
| SJ Span | −0.42 | −0.26 | −0.22 | −0.06 | −0.26 | −0.26 | −0.27 | −0.38 | −0.45 | −0.14 | −0.22 | −0.28 | −0.18 | −0.46 | −0.44 |
Note. N = 158, critical r-values are: r = .16 for p = .05, r = .21 for p =.01, r = .26, for p =.001, and r = .30 for p =.0001.
Abbreviations:PP – pulse pressure; Sys – systolic blood pressure; Dias – diastolic blood pressure; P Err and NP Err – perseverative and nonperseverative errors on Wisconsin Card Sorting Test; RT – response time and Err – number of errors on the n-back tasks, with indices 1–3 indicating number of back steps, v – verbal stimuli, n – nonverbal stimuli; SJ – size judgment.
2.3. Genomic analysis
DNA was isolated from buccal cultures obtained in mouthwash samples. We used a Gentra Autopure LS under the standard buccal cell protocol. For genotyping quality control, we performed 10% direct repeats and DNA sequencing for verification, using both control DNA and no-template controls and beta-globin as an amplification control. All 5’- nuclease assays were adapted from a quantitative PCR method (Lo et al., 2000) and implemented on an Applied Biosystems 7900. We amplified DNA through either ACE-1721F (insertion) or ACE-1428F (deletion) and ACE-1826R, and interrogated it with the TaqMan probe 1745T. Polymorphism for COMT (rs4680) was interrogated using Taqman SNP Genotyping assay under the 0.5X protocol for ABI PRISM BigDye Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems). The sequencing extension products were purified utilizing Sephadex, and the purified products were analyzed on an ABI PRISM 3700 DNA Analyzer using a 50 cm capillary array.
The insertion/deletion heterozygotes constituted approximately half the sample (56%, 88 individuals), and 22% of the participants fell into each homozygotic group (D/D = 35, I/I = 35). The distribution of ace I/D alleles conformed to Hardy-Weinberg equilibrium: χ2 = 2.05, p = 0.15. For comt val158met polymorphisms, the distribution included 37 (23%) homozygotes for met, 84 (53%) heterozygotes, and 37 (23%) homozygotes for val. The distribution of comt val158met alleles did not deviate from Hardy-Weinberg equilibrium: χ2 = 0.63, p = 0.43. For both polymorphisms, the genotype frequencies were equal between the sexes (χ2(2) = 1.33 and 0.75 for comt val158met and ace I/D, both ns), and were unrelated to age: both F < 1.
2.4. Cognitive measures
2.4.1. Wisconsin Card Sorting Test (WCST)
We administered a computerized version of the WCST (WCST: Computer Version 4 – Research Edition, Psychological Assessment Resources, Inc., Lutz, FL) on a 17-inch high-resolution color monitor. Participants were to match a card that appeared in the lower center of the computer screen with one of the four key cards displayed at the top of the screen according to the color, shape, or number of geometric designs on the target card. To respond they used four designated keys on the keyboard. Categories appeared in two repeated sets of blocks ordered as color, shape, and number, with category switch occurring after 10 successive correct trials. The participants, who were informed whether each response was correct or incorrect, continued until achieving six categories or completing 128 trials.
The indices of performance were the total number of errors (incorrect matches) and number of perseverative errors, as well as the number of non-perseverative errors. A perseverative error was a perseverative response that was also incorrect. A perseverative response was a response that was incorrect according to the current rule, but would have been correct using the rule for the previous sorting principle. An example of a perseverative response that was also a perseverative error was matching solely on number when the correct response was color. If among the incorrect responses that were matched solely on number, the participant matched according to number and color (an ambiguous correct response), but then continued to incorrectly match on number, then the correct response was counted as a perseverative response that happens to be correct.
2.4.2. Working memory: n-back task
Participants performed two computerized versions of the n-back task modeled after Dobbs and Rule (1989). In the verbal version, digits of variable length appeared in sequence. After the presentation, participants named the digit shown 1-, 2-, or 3-back in blocked trials of the same n-back. Participants were aware of the condition being tested and were familiarized with each condition before the test trials. In the nonverbal version, the stimuli were abstract drawings. The test-retest reliability for the verbal version of the task is .68, .88, and .91, for 1-, 2-, and 3-back conditions respectively (Salthouse et al., 1996).
2.4.3. Working memory: Size Judgment Span task
We used a modified Size Judgment Span task (Cherry and Park, 1993) as a measure of nonverbal working memory. In this task, the examiner read a list of objects and animals, and the participants’ task was to re-order the items from smallest to largest. The first block contained three trials with two items in each trial. For each successive block, the number of items per trial was increased by one. Testing stopped when the participant missed two of the three trials. The sum of the number of correct trials was the index of performance. The estimated test-retest reliability of this task is .79 (Cherry and Park, 1993).
2.5. Statistical analyses
The data were analyzed within a General Linear Model (GLM) framework, with separate models fitted to each of the following indices of EF: errors on WCST (perseverative and non-perseverative), n-back working memory speed of processing (RT), n-back accuracy (number of errors), and Size Judgment Span (number of correct trials). Altogether, we fitted four general linear models. Multiple within-subject indices (error type on WCST) or experimental conditions (1-, 2-, and 3-back load or verbal vs. nonverbal task) were repeated measures. In every model, age and PP (centered at their sample means) served as continuous independent variables. Sex, as well as comt val158met and ace I/D genotypes were also categorical predictors, with two levels for sex, and three levels for each genotype. We also tested if the quadratic age component and its interactions with other predictors made significant contributions in each model.
Altogether, each full GLM contained five independent variables and their interactions. Limiting the tested effects to the linear term of PP and adding a quadratic term for age plus second-order interactions among age terms and other independent variables produced 18 independent variables, i.e. less than 10 subjects per variable. We decided therefore to forego testing higher-order interactions and additional nonlinear contributions.
Because of a considerable skew in their distributions, WM speed and accuracy indices as well as the error counts on WCST were transformed using the natural log function. In each analysis, a full model that included all second-order interactions was tested first. All interactions that did not reach statistical significance (p > 0.10) were removed from the model and the data were fitted to a reduced model. The GLM analyses were followed by univariate analyses of simple effects, and testing the differences among the levels of the categorical variables with Fisher’s Least Significant Difference (LSD) test, or slopes of regressions on the continuous variables by comparing the 95% confidence intervals around them.
3. Results
With age, sex, both genotypes and interactions among them taken into account, the vascular health indicator (PP) was unrelated to the genotypes (F < 1). However, PP increased with age: F(1, 150) = 41.76, p < 0.00001. Moreover, in accord with the reported normative data (Franklin et al., 1997), it exhibited a significant quadratic trend (F(1, 150) = 4.73, p = 0.031), which indicated that the increase in PP accelerated with age: blin = 0.27253 ± 0.04041, p < 0.00001, bquad = 0.00466 ± 0.00204, p = 0.024, R2 = 0.27. Correlations between age and vascular indicators were 0.45 for pulse and systolic, (both p < 0.0001), and 0.18 (p = 0.021) for diastolic pressure (see Table 2).
3.1. Wisconsin Card Sorting Test
The analyses of errors on WCST (Table 3) revealed main effects of age and ace I/D genotype. The main effect of age was modified by a significant Age × Error Type interaction. The analysis of simple effects revealed that although errors increased with age, the magnitude of age-related increase in perseverative errors (F(1, 147) = 20.08) was smaller than in non-perseverative errors (F(1, 147) = 32.80), both p < 0.00001. For non-perseverative errors the slope of regression on age was b = 0.02370 ± 0.00331, r = 0.50, p < 0.0001, whereas for perseverative errors the slope was b = 0.01636 ± 0.00302, r = 0.40. The 95% confidence limits around the slopes did not overlap and the difference between correlated r’s was significant: Steiger’s Z* = 2.39, p = 0.0017.
Table 3.
Summary of results for Wisconsin Cart Sorting Test (WCST) errors.
| Effect | df | F-ratio | Partial η2 | p-value |
|---|---|---|---|---|
| Between Subjects Error df = 147 | ||||
| Age | 1 | 29.56 | 0.172 | 0.00000 |
| Age2 | 1 | 0.06 | 0.001 | 0.804 |
| Sex | 1 | 0.24 | 0.002 | 0.624 |
| Pulse Pressure | 1 | 0.12 | 0.001 | 0.726 |
| comt | 2 | 2.17 | 0.030 | 0.117 |
| ace | 2 | 3.51 | 0.047 | 0.032 |
| comt × Sex | 2 | 3.28 | 0.044 | 0.040 |
| Within Subjects Error df = 147 | ||||
| Error Type | 1 | 0.21 | 0.001 | 0.648 |
| Error Type × Age | 1 | 7.12 | 0.046 | 0.009 |
Note. Non-significant interactions (p > 0.10) were removed from the models.
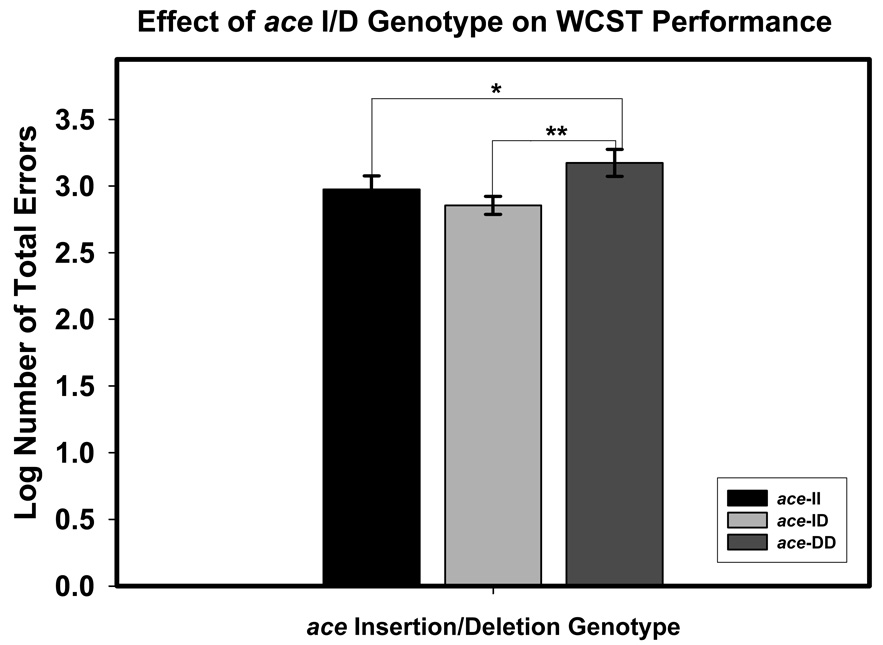
The main effect of ace I/D genotype is illustrated in Figure 1. Post-hoc comparisons with Fisher’s LSD test indicated that ace D homozygotes made more errors than the rest of the sample, with no differences between ace I homozygotes and heterozygotes: p = 0.082 for DD vs. II, and p = 0.003 for DD vs. ID, and p = 0.352 for ID vs. II.
Figure 1.
The effect of ace Insertion-Deletion genotype on the number of Wisconsin Card Sorting Test (WCST) total errors. The ace genotypes are: II – insertion homozygotes; I/D – insertion-deletion heterozygotes; DD – deletion homozygotes. Significance levels for comparisons (two-tailed): *p < .05; **p < .01. The scores are least-square adjusted means from the model; the bars represent standard errors of those means.
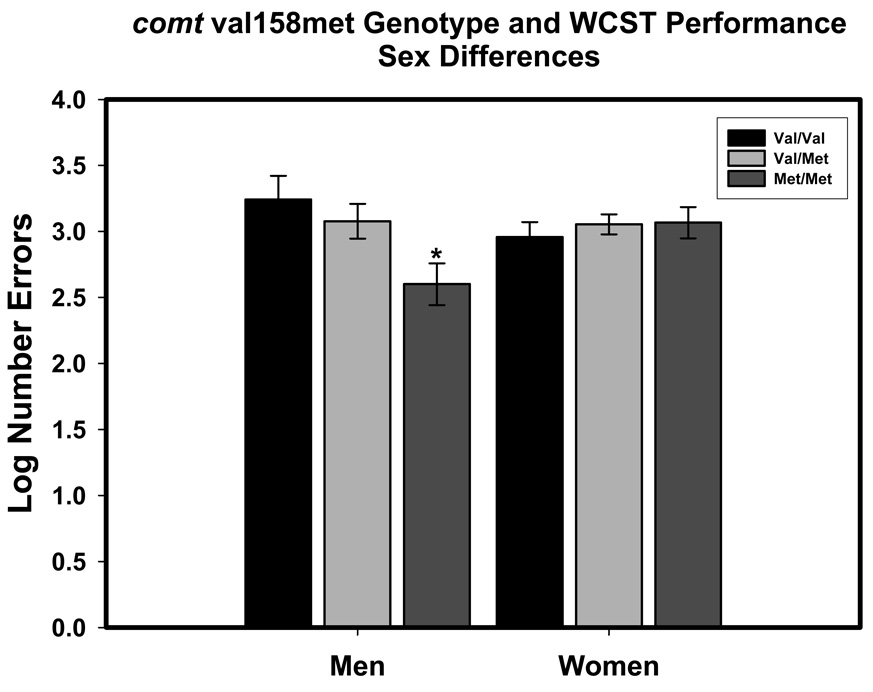
A significant comt × Sex interaction reflected an effect of comt genotype on WCST performance among men but not among women (see Figure 2). Post-hoc comparison showed that male met homozygotes committed fewer errors than all other participants (Fisher’s LSD p = 0.005 to 0.029, median p = 0.001).
Figure 2.
Sex difference in the effect of comt val158met genotype on the Wisconsin Card Sorting Test (WCST) errors. The effect of genotype is significant among men but not among women. Significance level for comparisons: *p < .05. The scores are least-square adjusted means from the model; the bars represent standard errors of those means.
3.2. Working memory: n-back task
3.2.1. Speed of working memory processing
The results of analyses of that task (Table 4) revealed significant main effects of age, sex, stimulus type (task), and working memory load. Slower response times were associated with older age, male sex, and greater n-back load.
Table 4.
Speed of processing in working memory (n-back tasks): Summary of results.
| Effect | df | F-Ratio | Partial η2 | p-Value |
|---|---|---|---|---|
| Between Subjects Error df = 143 | ||||
| Age | 1 | 59.76 | 0.295 | 0.00000 |
| Age2 | 1 | 3.56 | 0.050 | 0.061 |
| Sex | 1 | 9.79 | 0.064 | 0.002 |
| comt | 2 | 1.73 | 0.024 | 0.181 |
| ace | 2 | 1.30 | 0.018 | 0.276 |
| Pulse Pressure | 1 | 0.25 | 0.002 | 0.618 |
| ace × comt | 4 | 1.20 | 0.008 | 0.314 |
| Sex × Age | 1 | 5.79 | 0.039 | 0.017 |
| Sex × Age2 | 1 | 6.89 | 0.046 | 0.010 |
| Within Subjects Error df = 143 | ||||
| Task | 1 | 795.63 | 0.847 | 0.00000 |
| Task × Age | 1 | 3.46 | 0.023 | 0.065 |
| Within Subjects Error df = 286 | ||||
| Load | 2 | 236.36 | 0.623 | 0.00000 |
| Load × Age | 2 | 5.27 | 0.035 | 0.010 |
| Load × Sex | 2 | 3.99 | 0.042 | 0.028 |
| Load × ace | 4 | 2.25 | 0.030 | 0.079 |
| Load × ace × comt | 8 | 3.86 | 0. 097 | 0.0008 |
| Within Subjects Error df = 286 | ||||
| Task × Load | 2 | 16.17 | 0.101 | 0.00000 |
| Task × Load × Age | 2 | 4.11 | 0.028 | 0.026 |
| Task × Load × ace × comt | 8 | 1.90 | 0.050 | 0.078 |
Note. Non-significant interactions (p > 0.12) were removed from the models.
Response time increased with age: b = 0.00627 ± 0.000720 log-ms/year, r = 0.56, p < 0.00001. The main effect of task indicated that the nonverbal stimuli elicited significantly longer response times (3,596.59 ± 49.37 ms) than did the verbal ones (1,903.43 ± 35.62 ms). The main effect of load was due to the progressive load-dependent slowing from 1-back to 3-back tasks across verbal and nonverbal stimuli: 2,104.78 ± 32.81 ms for 1-back, 2,473.62 ± 38.23 ms for 2-back, and 3,671.63 ± 65.84 ms for 3-back tasks.
Decomposition of a significant Task × Load × Age interaction revealed that although age-related slowing was noted across all task and load combinations, the slopes of age-related increase in the response time varied according to the stimulus type and WM loads. For nonverbal stimuli, the slopes were equally steep at 1-back (b = 0.00923 ± 0.00086, r = 0.65, p < 0.00001) and 2-back (b = 0.00876 ± 0.00086, r = 0.63, p < 0.00001) loads, with lesser decline rate evident at 3-back (b = 0.00432 ± 0.00115, r = 0.29, p = 0.0003). For verbal stimuli, the slopes became progressively shallower from 1-back (b = 0.00603 ± 0.00083, r = 0.50, p < 0.00001), to 2-back (b = 0.00530 ± 0.00107, r = 0.37, p < 0.00001), to 3-back (b = 0.00483 ± 0.00186, r = 0.20, p = 0.010).
A significant main effect of sex indicated that women responded faster than men did. However, as indicated by a significant Sex × Load interaction, the advantage was evident only at the easier WM loads: 1-back (F(1, 142) = 20.43, p = 0.00001) and 2-back (F(1, 142) = 19.28, p = 0.00002) task, but not at 3-back (F < 1). Significant Age × Sex and Age2 × Sex interactions reflected the difference in the shape of age-RT relationship between men and women. For men, linear slowing was observed: blin = 0.00487 ± 0.00139, p = 0.001, bquad = −0.00001 ± 0.00007, p = 0.886. For women, the age-related increase in the rate of slowing was noted: blin = 0.00850 ± 0.00102, p < 0.000001, bquad = 0.00016 ± 0.00005, p = 0.003, R2 = 0.40. After removal of two outliers (age 80 and 73), the effect increased to blin = 0.00996 ± 0.00105, p < 0.00001, bquad = 0.00023 ± 0.00005, p = 0.00003, R2 = 0.46.
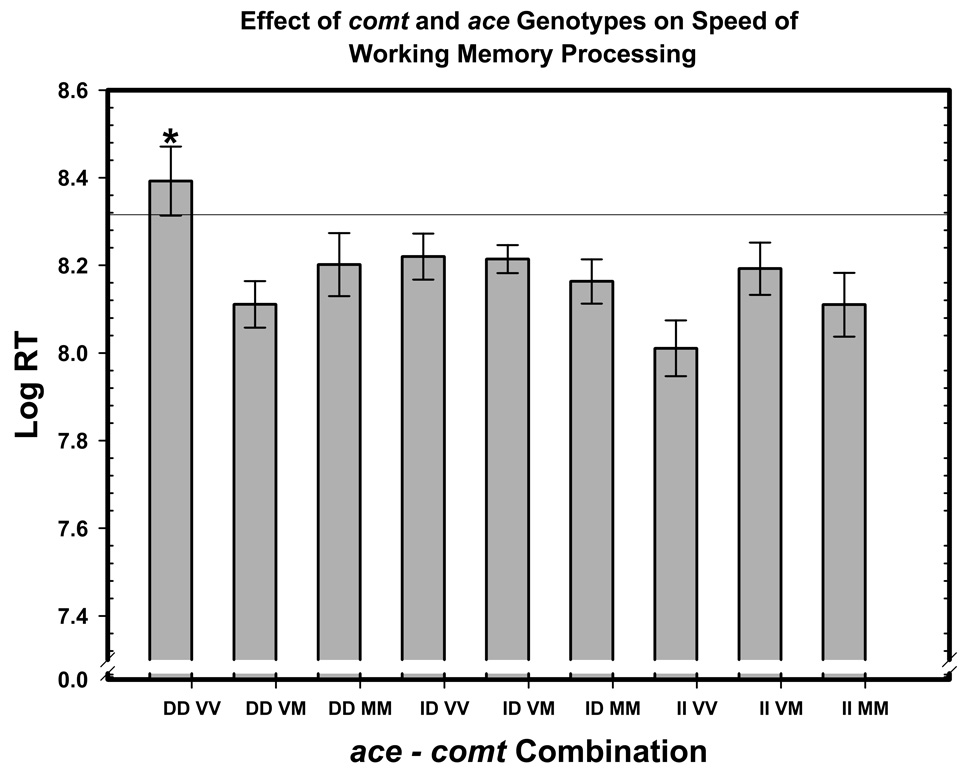
Although neither of the two examined genetic variants alone influenced speed of working memory processing, the combination of the two affected the response time, as indicated by a significant ace × comt × Load interaction. Decomposition of that interaction showed that the effect of the two-genotype combination was significant only for the highest load (3-back): F(4, 142) = 3.48, p = 0.010, F(4, 142) = 1.97, p = 0.110 for 1-back, and F < 1 for 2-back condition. The post-hoc comparisons of 3-back response times for nonverbal stimuli among genotype combinations revealed that homozygotes for both ace D and comt val alleles were consistently slower than the rest of the sample: Fisher’s LSD p’s ranging from 0.0002 for val/val-II to 0.078 for the met/met-DD combination, median p = 0.025 (Figure 3).
Figure 3.
The epistastic effect of ace I/D and comt val158met genotypes on speed of working memory processing. Response times are averaged across verbal and nonverbal n-back tasks (3-back conditions). The ace genotypes are: II – insertion homozygotes; I/D – insertion-deletion heterozygotes; DD – deletion homozygotes; the comt val158met genotypes are: VV – val homozygotes, VM – val/met heterozygotes, MM – met homozygotes. The scores are least-square adjusted means from the model; the bars represent standard errors of those means.
The analyses were conducted under the assumption that responses on the n-back task were valid. However, on any given trial, each participant had a 0.11 probability to answer correctly by chance, thereby making 13.5 errors out of 20 trials equivalent to chance level of performance. Thus, response times for participants whose accuracy was at that level (14 errors or more) could be questionable indicators of their speed of processing. A substantial part of the sample (29 participants) showed chance-level accuracy on the nonverbal 3-back task. On average, participants who performed above chance level were slightly (1.6 years) younger than the whole sample but they covered the same age range of 18 through 81 years. We therefore analyzed response times in 3-back condition without those participants. All main effects and interactions remained unchanged.
3.2.2. Accuracy of working memory processing
With almost no errors on the 1-back task and very few errors on the 2-back tasks, we could use only 3-back errors (log-transformed to reduce the skew) for accuracy analyses. One observation (a 65-year old woman, MMSE = 30) exercised undue influence on the model with a Studentized residual of −4.18. That data point was removed from the analyses of n-back accuracy that are summarized in Table 5.
Table 5.
Accuracy of working memory processing (3-back task): Summary of results.
| Effect | df | F-ratio | Partial η2 | p-value |
|---|---|---|---|---|
| Between Subjects Error df = 142 | ||||
| Age | 1 | 11.03 | 0.072 | 0.001 |
| Age2 | 1 | 0.04 | 0.001 | 0.834 |
| Sex | 1 | 0.12 | 0.001 | 0.734 |
| Pulse Pressure | 1 | 0.28 | 0.002 | 0.590 |
| comt | 2 | 3.85 | 0.051 | 0.024 |
| ace | 2 | 1.74 | 0.024 | 0.180 |
| ace × comt | 4 | 0.57 | 0.016 | 0.684 |
| Sex × comt | 2 | 4.69 | 0.062 | 0.011 |
| Within Subjects Error df = 142 | ||||
| Task | 1 | 55.24 | 0.280 | 0.00000 |
| Task × Age2 | 2 | 3.88 | 0.027 | 0.050 |
| Task × ace × comt | 4 | 2.19 | 0.058 | 0.074 |
Note. Non-significant interactions (p > 0.15) were removed from the models. One outlier was removed from the analyses (see text).
The main effect of age reflected greater number of errors committed by older participants: b = 0.01322 ± 0.00254, r = 0.39, p < 0.0001. For both nonverbal and verbal stimuli, errors increased with age. The main effect of task stemmed from a substantial difference in the number of verbal (4.75 ± 0.27) vs. nonverbal (8.39 ± 0.37) errors.
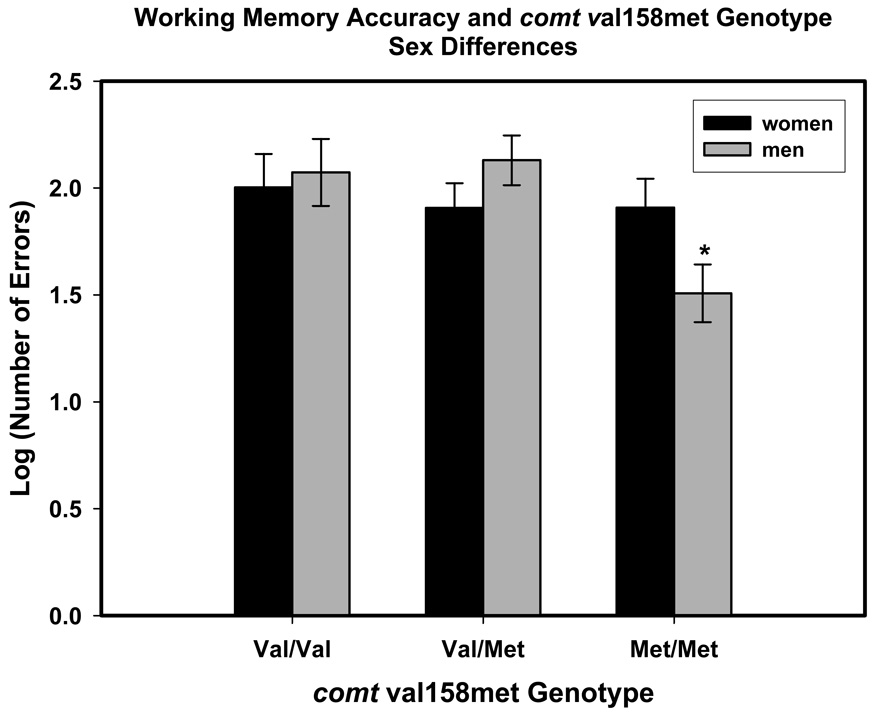
Although the main effect of comt val158met genotype fell short of significance, decomposition of a significant comt × Sex interaction indicated that men with met/met genotype committed fewer errors than the rest of the sample: Fisher’s LSD p’s ranging from 0.002 to 0.044 for all paired comparisons. The effect is illustrated in Figure 4 below.
Figure 4.
Sex differences in the effect of comt val158met genotype on working memory accuracy (n-back tasks, 3-back condition). Significance level for comparisons: *p < .05, two-tailed. The scores are least-square adjusted means from the model; the bars represent standard errors of those means.
Task × Age2 interaction reflected a significantly steeper increase in errors with age for nonverbal than for verbal stimuli: b = 0.01724 ± 0.00281, r = 0.44, p < 0.00001 vs. b = 0.00843 ± 0.00369, r = 0.18, p = 0.024. Moreover, as indicated by a significant Task × ace × Age interaction, the rate of age-related declines in accuracy varied among ace genotypes. For verbal errors, age-related increase was small and significant only among the ace I/D heterozygotes: b = 0.01282 ± 0.00493, r = 0.27, p = 0.011. For nonverbal errors, age-related increases were significant for all ace genotypes, but the slope was steeper for ace II homozygotes (b = 0.03248 ± 0.00649, r = 0.66, p = 0.00002) than for the other genotypes (b = 0.01372 ± 0.00377, r = 0.37, p = 0.00046 for ID, and b = 0.01380 ± 0.00514, r = 0.42, p = 0.011 for DD).
3.3. Working Memory: Size Judgment Span task
There were no significant interactions in the full model. One outlier, an observation with a Studentized residual of −3.89 (a 54 y.o. woman with MMSE = 29) was removed from the analysis. In the reduced model, the main effects of age (linear) and comt val158met genotype were significant (Table 6). The effects of age reflected better performance by younger participants: b = −0.05156 ± 0.00860, r = −0.43, p < 0.0001. Although there was a trend for age-related acceleration of declines in SJ Span, the quadratic component of age did not reach significance.
Table 6.
Summary of the analyses of working memory span (Size Judgment task) scores
| Effect | df | F-ratio | Partial η2 | p-value |
|---|---|---|---|---|
| Between Subjects Error df = 148 | ||||
| Age | 1 | 26.82 | 0.153 | 0.00000 |
| Age2 | 1 | 3.42 | 0.027 | 0.066 |
| Sex | 1 | 0.27 | 0.000 | 0.867 |
| Pulse Pressure | 1 | 0.94 | 0.006 | 0.334 |
| comt | 2 | 4.79 | 0.061 | 0.010 |
| ace | 2 | 0.57 | 0.008 | 0.565 |
Note. Non-significant interactions (p > 0.15) are not shown.
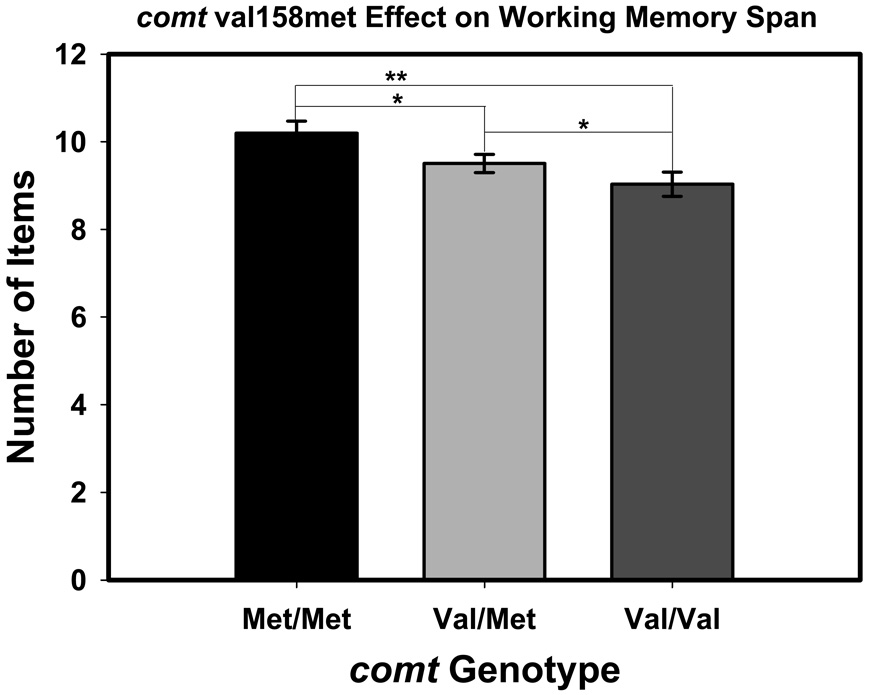
A significant main effect was observed for comt genotype. Post-hoc comparisons revealed that met homozygocity on the comt val158met polymorphism was beneficial to performance: met homozygotes performed better than val homozygotes (p = 0.003), and heterozygotes (p = 0.035), who did not differ from val homozygotes (p = 0.153), Fisher’s LSD test. This effect is illustrated in Figure 5.
Figure 5.
The effect of the comt val158met genotype on working memory (Size Judgment Span). Significance levels for comparisons (two-tailed): *p < .05; **p < .01. The scores are least-square adjusted means from the model; the bars represent standard errors of those means.
4. Discussion
The results of this study demonstrate that in healthy adults, age-sensitive cognitive functions are affected by common genetic variants. The genetic variant associated with increased vasoconstriction (ace D allele) has a negative impact, whereas the polymorphism associated with increased DA availability (comt met allele) conveys an advantage in EF performance. The advantage of comt 158met homozygotes was apparent on all EF tasks, although on some tasks the positive effect was observed only among men. On some EF measures, e.g., speed of WM processing, an epistatic effect of two polymorphisms was evident. The disadvantage conveyed by homozygocity for ace D allele that is associated with increased vasoconstriction was observed only in combination with a double dose of another allele linked to reduced cognitive performance – comt val, and only in the most difficult task condition.
The finding of comt 158met advantage across disparate measures is consistent with the previous studies (e.g., de Frias et al., 2004), including the one conducted on an overlapping sample (Raz et al., 2009), in which we observed the benefits of comt 158met homozygocity on nonverbal reasoning and episodic memory (Raz et al., 2009). The common feature of those indices of cognition is their dependence on working memory (Fristoe et al., 1997; Hartman et al., 2001; Jarrold and Towse, 2006; Kane and Engel, 2002). Thus, it is highly plausible that any task, inasmuch as it relies on WM, would be affected by the genes linked to DA regulation that has been proposed as a key factor in EF (Bäckman et al., 2006; Savitz et al., 2006).
However, the locus of the DA effect on cognition is unclear. According to the most common interpretation, comt val158met influences cognition through its effect on DA availability in the synapses of the prefrontal cortex that are populated with D1 receptors (e.g., Egan et al, 2001). The key role of PFC D1 receptors in WM has been outlined in several theoretical models (Nieoullon, 2002; Williams and Castner, 2006). However, DA, via the same receptors, affects vasodilation and regulation of blood pressure (Jose et al., 2003; Zeng et al. 2004), and the low-activity val allele of comt val158met is linked to increased prevalence of hypertension (Hagen et al., 2007). Therefore, in addition to synaptic effects of comt val158met polymorphism, its vascular effects merit further consideration, especially in the context of aging. A gene × gene interaction (ace DD × comt val/val) observed in this sample may represent such synergy of vasomotor effects. Low availability of dopamine in comt val homozygotes may result in an insufficient vasodilatory response, whereas concomitant high angiotensin II activity brought by ace DD genotype adds excessive vasoconstriction. A combination of these two alleles may create hypoperfusion even at ostensibly normal levels of arterial pressure and result in sluggish processing, especially on a difficult WM task. Other factors, such as sex steroids, may modify the effects of comt val158met on cognition and sex differences therein. The observed sex differences in COMT 158met could reflect the attenuation of COMT variations in women, who have 20 to 30% lower COMT activity compared to men (Fähndrich et al., 1980; Floderous et al., 1981). There is evidence that estrogen inhibits COMT gene transcription (Xie et al., 1999; Weinshilboum, 2006), whereas testosterone levels are associated with vasoconstriction and elevated vascular risk (Kienitz, and Quinkler, 2008).
The hypothesized negative effect of PP on EF performance did not materialize in this sample. Although simple correlations showed the association between elevated pulse pressure and WM (slowing of processing and shorter span), the effects disappeared once the influence of age, sex and genetic variants were taken into account. In past studies on partially overlapping samples, we found that hypertension and vascular risk alleles acted synergistically to suppress cognitive performance (Deshmukh et al., 2009; Raz et al., 2009) and without accounting for genetic variants, pulse pressure had a negative impact on some EF measures (Dahle et al., 2009). The findings in this sample indicate that although high-normal blood pressure may be a risk factor for vascular disease in healthy adults (Vasan et al., 2001; Knecht et al. 2008), the impact of such relatively mild elevations is uncertain. It may be offset by genetic effects and may depend on which part of the life-span is measured (Kennelly et al, 2009). For example, comt val158met heterozygocity and intermediate levels of DA availability that it brings may be beneficial for EF performance in cshildren and adolescents (Gosso et al., 2008) but not in adults (this sample) who can take advantage of higher DA levels. Notably, the genetic effects were adjusted for age that was present in all models, and we found no gene × age interactions. Thus, the neurochemical and vasomotor mechanisms that are controlled by the polymorphisms examined in this study operate in young as well as in older adults. The observed impact of genes associated with vascular function in young normotensive adults suggests that the negative effects of the genes on cognition may be present at a relatively early age without overt cardiovascular risk increase. Early developmental factors predict adult vascular disease (Barker and Fall, 1993) and could have interacted with the polymorphisms examined in this sample. However, lack of relevant developmental data precludes testing of this hypothesis.
Interpretation of the presented results depends on several limitations of the study design. First, in a cross-sectional quasi-experimental study, we could not assess the true age-related change in cognitive performance. As this study represents a first wave of an ongoing longitudinal investigation, we hope that follow-up will permit a more detailed analysis of the effects of age and individual variability therein. Second, the vascular health/vascular risk indices based on measurements of the arterial blood pressure (e.g., PP in this study) are only convenient surrogates of the important vascular characteristics. For example, although PP is a valid surrogate of arterial stiffness, its sensitivity in a healthier-than average sample of a wide age range could have been limited. A more precise evaluation of carotid-femoral pulse wave velocity (PWV) could have shown a more clear exacerbation of age-related cognitive declines by increased arterial stiffness (Elias et al., 2009). However, no genetic effects were assessed in the latter study and it is unclear, therefore, if PWV effects would not be offset by genetic influence as well.
Third, multiple genes, some of which are linked to variations in cognitive performance (Corella and Ordovas, 2004; Lao et al., 2005; McDonald, 2002) affect vascular health. At least in one study, the combined effect of the ApoE ε4 allele and ace deletion-homozygocity was associated with the lowest cognitive scores at baseline and the largest declines at follow-up (Richard et al., 2000). Our sample was too small for investigation of simultaneous effects of additional genes and interactions among them. The same limitation also affected the number of vascular risk indicators that we could investigate. In this sample, only two polymorphisms and one vascular health indicator were assessed. Even for the evaluated polymorphisms, the consequences of genetic variation are broader than would be predicted from their specific action on the enzymes. For example, whereas ace I/D mutation affects availability of angiotensin II, negative effects of that vasoconstrictor on blood pressure, cerebral blood flow, and learning, are mediated by angiotensin II type 1 receptors and alleviated by AT1-receptor blockers (Benicky et al., in press; Inaba et al., 2009). Taking these variables into account may clarify the effects of angiotensin II on cognitive performance. In addition to its vasoconstrictive effects, ACE also promotes inflammation that may have an additional negative impact on cognitive performance (Alley et al., 2008; Benicky et al., in press). Thus, it is imperative to replicate these findings in a study that takes into account other genetic variants, especially those that regulate response to chronic inflammation and variation in the genes controlling AT1 receptors.
Finally, the key limitation of many genetic association studies is statistical power. In this sample, we had enough power to observe medium and large effects that are on par with the values reported in the literature (Barnett et al., 2008; Heinz and Smolka, 2006). The influence of genetic variants (ace I/D and comt val158met) amounted to medium-size effects (partial η2 ~ 0.05) across tasks, and the ace × comt interaction produced a relatively large effect of partial η2 = 0.10 on n-back speed of processing. Those effect sizes fall within a roughly delineated category of medium effects (Cohen, 1988). Several interactions hovered on the border of the conventional p < 0.05 limit and with a larger sample, they could have reached statistical significance. That consideration is important in planning future studies.
In sum, in healthy adults, a significant proportion of variance in performance on age-sensitive cognitive tasks depends on genetic factors linked to vascular health. The effects are complex and at times synergistic and their magnitude may vary between the sexes; they are more apparent on tasks of greater difficulty. Future investigations should pay greater attention to the synergistic interactions among clinical and genetic factors, as the combination thereof may place healthy individuals at increased risk of cognitive decline.
Acknowledgments
This study was supported in part by grant R37-AG-011230-14 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
The authors report no actual or potential conflict of interest.
References
- Alley DE, Crimmins EM, Karlamangla A, Hu P, Seeman TE. Inflammation and rate of cognitive change in high-functioning older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2008;63:50–55. doi: 10.1093/gerona/63.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amouyel P, Richard F, Cottel D, Amant C, Codron V, Helbecque N. The deletion allele of the angiotensin I converting enzyme gene as a genetic susceptibility factor for cognitive impairment. Neurosci. Lett. 1996;217:203–205. [PubMed] [Google Scholar]
- Anokhin AP, Heath AC, Ralano A. Genetic influences on frontal brain function: WCST performance in twins. Neuroreport. 2003;14:1975–1978. doi: 10.1097/00001756-200310270-00019. [DOI] [PubMed] [Google Scholar]
- Apter NS, Halstead WC, Heimburger RF. Impaired cerebral functions in essential hypertension. Am. J. Psychiatry. 1951;107:808–813. doi: 10.1176/ajp.107.11.808. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol. Psychiatry. 2005;57:1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Nyberg L, Lindenberger U, Li S-C, Farde L. The correlative triad among aging, dopamine, and cognition: current status and future prospects. Neurosci. Biobehav. Rev. 2006;30:791–807. doi: 10.1016/j.neubiorev.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Fall CH. Fetal and infant origins of cardiovascular disease. Arch. Dis. Child. 1993;68:797–799. doi: 10.1136/adc.68.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett JH, Scoriels L, Munafò MR. Meta-analysis of the cognitive effects of the catechol-O-methyltransferase gene Val158/108Met polymorphism. Biol. Psychiatry. 2008;64:137–144. doi: 10.1016/j.biopsych.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Bartrés-Faz D, Junqué C, Clemente IC, López-Alomar A, Valveny N, López-Guillén A, López T, Cubells MJ, Moral P. Angiotensin I converting enzyme polymorphism in humans with age-associated memory impairment: relationship with cognitive performance. Neurosci. Lett. 2000;290:177–180. doi: 10.1016/s0304-3940(00)01349-5. [DOI] [PubMed] [Google Scholar]
- Bautista LE, Vargas CI, Oróstegui M, Gamarra G. Population-based case-control study of renin-angiotensin system genes polymorphisms and hypertension among Hispanics. Hypertens. Res. 2008;31:401–408. doi: 10.1291/hypres.31.401. [DOI] [PubMed] [Google Scholar]
- Benetos A, Gautier S, Ricard S, Topouchian J, Asmar R, Poirier O, Larosa E, Guize L, Safar M, Soubrier F, Cambien F. Influence of angiotensin-converting enzyme and angiotensin II type 1 receptor gene polymorphisms on aortic stiffness in normotensive and hypertensive patients. Circulation. 1996;94:698–703. doi: 10.1161/01.cir.94.4.698. [DOI] [PubMed] [Google Scholar]
- Benicky J, Sánchez-Lemus E, Pavel J, Saavedra JM. Anti-inflammatory effects of angiotensin receptor blockers in the brain and the periphery. Cell. Mol. Neurobiol. doi: 10.1007/s10571-009-9368-4. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacher J, Staessen JA, Girerd X, Gasowski J, Thijs L, Liu L, Wang JG, Fagard RH, Safar ME. Pulse pressure not mean pressure determines cardiovascular risk in older hypertensive patients. Arch. Intern. Med. 2000;160:1085–1089. doi: 10.1001/archinte.160.8.1085. [DOI] [PubMed] [Google Scholar]
- Bonnet F, Patel S, Laville M, Balkau B, Favuzzi A, Monti LD, Lalic N, Walker M on behalf of the European Group for the Study of Insulin Resistance Relationship Between Insulin Sensitivity and Cardiovascular Disease Risk Study Group. Influence of the ACE gene insertion/deletion polymorphism on insulin sensitivity and impaired glucose tolerance in healthy subjects. Diabetes Care. 2008;31:789–794. doi: 10.2337/dc07-1788. [DOI] [PubMed] [Google Scholar]
- Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common carotid intima-media thickness and risk of stroke and myocardial infarction: The Rotterdam Study. Circulation. 1997;96:1432–1437. doi: 10.1161/01.cir.96.5.1432. [DOI] [PubMed] [Google Scholar]
- Boutouyrie P, Bussy C, Lacolley P, Girerd X, Laloux B, Laurent S. Association between local pulse pressure, mean blood pressure, and large-artery remodeling. Circulation. 1999;100:1387–1393. doi: 10.1161/01.cir.100.13.1387. [DOI] [PubMed] [Google Scholar]
- Caldú X, Vendrell P, Bartrés-Faz D, Clemente I, Bargalló N, Jurado MA, Serra-Grabulosa JM, Junqué C. Impact of COMT VAL108/158 Met and DAT genotypes on prefrontal function in healthy subjects. Neuroimage. 2007;37:1437–1444. doi: 10.1016/j.neuroimage.2007.06.021. [DOI] [PubMed] [Google Scholar]
- Castellano M, Muiesan ML, Rizzoni D, Beschi M, Pasini G, Cinelli A, Salvetti M, Porteri E, Bettoni G, Kreutz R, Lindpainter K, Rosei EA. Angiotensin-converting enzyme I/D polymorphism and arterial wall thickness in a general population. The Vobarno Study. Circulation. 1995;91:2721–2724. doi: 10.1161/01.cir.91.11.2721. [DOI] [PubMed] [Google Scholar]
- Chae CU, Pfeffer MA, Glynn RJ, Mitchell GF, Taylor JO, Hennekens CH. Increased pulse pressure and risk of heart failure in the elderly. JAMA. 1999;281:634–639. doi: 10.1001/jama.281.7.634. [DOI] [PubMed] [Google Scholar]
- Cherry KE, Park DC. Individual difference and contextual variables influence spatial memory in younger and older adults. Psychol. Aging. 1993;8:517–526. doi: 10.1037//0882-7974.8.4.517. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. second ed. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Corella D, Ordovas JM. The metabolic syndrome: a crossroad for genotype-phenotype associations in atherosclerosis. Curr. Atheroscler. Rep. 2004;6:186–196. doi: 10.1007/s11883-004-0031-8. [DOI] [PubMed] [Google Scholar]
- Dahle CL, Jacobs BS, Raz N. Aging, vascular risk, and cognition: blood glucose, pulse pressure, and cognitive performance in healthy adults. Psychol. Aging. 2009;24:154–162. doi: 10.1037/a0014283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarli C, Murphy DG, Tranh M, Grady CL, Haxby JV, Gillette JA, Salerno JA, Gonzales-Aviles A, Horwitz B, Rapoport SI. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology. 1995;45:2077–2084. doi: 10.1212/wnl.45.11.2077. [DOI] [PubMed] [Google Scholar]
- de Frias CM, Annerbrink K, Westberg L, Eriksson E, Adolfsson R, Nilsson LG. COMT gene polymorphism is associated with declarative memory in adulthood and old age. Behav. Genet. 2004;34:533–539. doi: 10.1023/B:BEGE.0000038491.06972.8c. [DOI] [PubMed] [Google Scholar]
- de Frias CM, Annerbrink K, Westberg L, Eriksson E, Adolfsson R, Nilsson LG. Catechol-O-methyltransferase Val158Met polymorphism is associated with cognitive performance in nondemented adults. J. Cogn. Neurosci. 2005;17:1018–1025. doi: 10.1162/0898929054475136. [DOI] [PubMed] [Google Scholar]
- Demakis GJ. A meta-analytic review of the sensitivity of the Wisconsin Card Sorting Test to frontal and lateralized frontal brain damage. Neuropsychology. 2003;17:255–264. doi: 10.1037/0894-4105.17.2.255. [DOI] [PubMed] [Google Scholar]
- Deshmukh A, Rodrigue KM, Kennedy KM, Land S, Jacobs BS, Raz N. Synergistic effects of the MTHFR C677T polymorphism and hypertension on spatial navigation. Biol. Psychol. 2009;80:240–245. doi: 10.1016/j.biopsycho.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Asper CM, Goldberg TE, Kolachana BS, Straub RE, Egan MF, Weinberger DR. Genetic variation in catechol-O-methyltransferase: effects on working memory in schizophrenic patients, their siblings, and healthy controls. Biol. Psychiatry. 2008;63:72–79. doi: 10.1016/j.biopsych.2007.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs AR, Rule BG. Adult age differences in working memory. Psychol. Aging. 1989;4:500–503. doi: 10.1037//0882-7974.4.4.500. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158Met genotype on frontal lobe function and risk for schizophrenia. Proc. Natl. Acad. Sci. U S A. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias MF, Elias PK, Sullivan LM, Wolf PA, D’Agnostino RB. Lower cognitive function in the presence of obesity and hypertension: The Framingham Heart Study. Int. J. of Obes. Relat. Metab. Disord. 2003;27:260–268. doi: 10.1038/sj.ijo.802225. [DOI] [PubMed] [Google Scholar]
- Elias MF, Robbins MA, Budge MM, Abhayaratna WP, Dore GA, Elias PK. Arterial pulse wave velocity and cognition with advancing age. Hypertension. 2009;53:668–673. doi: 10.1161/HYPERTENSIONAHA.108.126342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias PK, Elias MF, Robbins MA, Budge MM. Blood pressure-related cognitive decline: does age make a difference? Hypertension. 2004;44:631–636. doi: 10.1161/01.HYP.0000145858.07252.99. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Kim JS, Suever BL, Voss MW, Francis BM, Kramer AF. Genetic contributions to age-related decline in executive function: a 10-year longitudinal study of COMT and BDNF polymorphisms. Front. Hum. Neurosci. 2008;2:1–9. doi: 10.3389/neuro.09.011.2008. article 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fähndrich E, Coper H, Christ W, Helmchen H, Müller-Oerlinghausen B, Pietzcker A. Erythrocyte COMT-activity in patients with affective disorders. Acta Psychiatr Scand. 1980;61:427–437. doi: 10.1111/j.1600-0447.1980.tb00881.x. [DOI] [PubMed] [Google Scholar]
- Fleg JL. Alterations in cardiovascular structure and function with advancing age. Am. J. Cardiol. 1986;57:33C–44C. doi: 10.1016/0002-9149(86)91025-8. [DOI] [PubMed] [Google Scholar]
- Floderus Y, Ross SB, Wetterberg L. Erythrocyte catechol-O-methyltransferase activity in a Swedish population. Clin Genet. 1981;19:389–392. doi: 10.1111/j.1399-0004.1981.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Magyar O. Mesocortical dopamine modulation of executive functions: beyond working memory. Psychopharmacology (Berl) 2006;188:567–585. doi: 10.1007/s00213-006-0404-5. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Franklin SS, Gustin W, 4th, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96:308–315. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Young SE, Defries JC, Corley RP, Hewitt JK. Individual differences in executive functions are almost entirely genetic in origin. J. Exp. Psychol. Gen. 2008;137:201–225. doi: 10.1037/0096-3445.137.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fristoe NM, Salthouse TA, Woodard JL. Examination of age-related deficits on the Wisconsin Card Sorting Test. Neuropsychology. 1997;11:428–436. doi: 10.1037//0894-4105.11.3.428. [DOI] [PubMed] [Google Scholar]
- Glynn RJ, Chae CU, Guralnik JM, Taylor JO, Hennekens CH. Pulse pressure and mortality in older people. Arch. Intern. Med. 2000;160:2765–2772. doi: 10.1001/archinte.160.18.2765. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS, Goldman D, Weinberger DR. Executive subprocesses in working memory: relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Arch. Gen. Psychiatry. 2003;60:889–896. doi: 10.1001/archpsyc.60.9.889. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Regional and cellular fractionation of working memory. Proc. Natl. Acad. Sci. U S A. 1996;93:13473–13480. doi: 10.1073/pnas.93.24.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosso MF, de Geus EJC, Polderman TJC, Boomsma DI, Heutink P, Posthuma D. Catechol O-methyl transferase and dopamine D2 receptor gene polymorphisms: evidence of positive heterosis and gene–gene interaction on working memory functioning. Eur. J. Hum. Genet. 2008;16:1075–1082. doi: 10.1038/ejhg.2008.57. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. Neuroanatomical correlates of selected executive functions in middle-aged and older adults: a prospective MRI study. Neuropsychologia. 2003;41:1929–1941. doi: 10.1016/s0028-3932(03)00129-5. [DOI] [PubMed] [Google Scholar]
- Hagen K, Pettersen E, Stovner LJ, Skorpen F, Holmen J, Zwart JA. High systolic blood pressure is associated with Val/Val genotype in the catechol-o-methyltransferase gene. The Nord-Trøndelag Health Study (HUNT) Am. J. Hypertens. 2007;20:21–26. doi: 10.1016/j.amjhyper.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Harrap SB, Tzourio C, Cambien F, Poirier O, Raoux S, Chalmers J, Chapman N, Colman S, Leguennec S, MacMahon S, Neal B, Ohkubo T, Woodward M. The ACE gene I/D polymorphism is not associated with the blood pressure and cardiovascular benefits of ACE inhibition. Hypertension. 2003;42:297–303. doi: 10.1161/01.HYP.0000088322.85804.96. [DOI] [PubMed] [Google Scholar]
- Hartman M, Bolton E, Fehnel SE. Accounting for age differences on the Wisconsin Card Sorting Test: decreased working memory, not inflexibility. Psychol. Aging. 2001;16:385–399. [PubMed] [Google Scholar]
- Hassan A, Lansbury A, Catto AJ, Guthrie A, Spencer J, Craven C, Grant PJ, Bamford JM. Angiotensin converting enzyme insertion/deletion genotype is associated with leukoaraiosis in lacunar syndromes. J. Neurol. Neurosurg. Psychiatry. 2002;72:343–346. doi: 10.1136/jnnp.72.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Ohrui T, Maruyama M, Tomita N, Nakayama K, Higuchi M, Furukawa K, Arai H. ACE activity in CSF of patients with mild cognitive impairment and Alzheimer disease. Neurology. 2006;67:1309–1310. doi: 10.1212/01.wnl.0000238102.04582.ec. [DOI] [PubMed] [Google Scholar]
- Heinz A, Smolka MN. The effects of catechol O-methyltransferase genotype on brain activation elicited by affective stimuli and cognitive tasks. Rev. Neurosci. 2006;17:359–367. doi: 10.1515/revneuro.2006.17.3.359. [DOI] [PubMed] [Google Scholar]
- Horn JL. Intellectual stability concepts. In: Steinberg RJ, editor. Advances in the psychology of human intelligence. Hillsdale, NJ: Erlbaum; 1986. pp. 35–77. [Google Scholar]
- Hosoi M, Nishizawa Y, Kogawa K, Kawagishi T, Konishi T, Maekawa K, Emoto M, Fukumoto S, Shioi A, Shoji T, Inaba M, Okuno Y, Morii H. Angiotensin-converting enzyme gene polymorphism is associated with carotid arterial wall thickness in non-insulin-dependent diabetic patients. Circulation. 1996;94:704–707. doi: 10.1161/01.cir.94.4.704. [DOI] [PubMed] [Google Scholar]
- Hultsch DF, Hertzog C, Small BJ, McDonald-Miszczak L, Dixon RA. Short-term longitudinal change in cognitive performance in later life. Psychol. Aging. 1992;7:571–584. doi: 10.1037//0882-7974.7.4.571. [DOI] [PubMed] [Google Scholar]
- Inaba S, Iwai M, Furuno M, Tomono Y, Kanno H, Senba I, Okayama H, Mogi M, Higaki J, Horiuchi M. Continuous activation of renin-angiotensin system impairs cognitive function in renin/angiotensinogen transgenic mice. Hypertension. 2009;53:356–362. doi: 10.1161/HYPERTENSIONAHA.108.123612. [DOI] [PubMed] [Google Scholar]
- Jarrold C, Towse JN. Individual differences in working memory. Neuroscience. 2006;139:39–50. doi: 10.1016/j.neuroscience.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Jose PA, Eisner GM, Felder RA. Regulation of blood pressure by dopamine receptors. Nephron. Physiol. 2003;95:19–27. doi: 10.1159/000073676. [DOI] [PubMed] [Google Scholar]
- Julve R, Chaves FJ, Rovira E, Pascual JM, Miralles A, Armengod ME, Redon J. Polymorphism insertion/deletion of the ACE gene and ambulatory blood pressure circadian variability in essential hypertension. Blood Press. Monit. 2001;6:27–32. doi: 10.1097/00126097-200102000-00005. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: an individual-differences perspective. Psychon. Bull. Rev. 2002;9:637–671. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- Kennelly SP, Lawlor BA, Kenny RA. Blood pressure and the risk for dementia: a double edged sword. Ageing Res. Rev. 2009;8:61–70. doi: 10.1016/j.arr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Khattar RS, Swales JD, Dore C, Senior R, Lahiri A. Effect of aging on the prognostic significance of ambulatory systolic, diastolic, and pulse pressure in essential hypertension. Circulation. 2001;104:783–789. doi: 10.1161/hc3201.094227. [DOI] [PubMed] [Google Scholar]
- Kienitz T, Quinkler M. Testosterone and blood pressure regulation. Kidney Blood Press. Res. 2008;31:71–79. doi: 10.1159/000119417. [DOI] [PubMed] [Google Scholar]
- Knecht S, Wersching H, Lohmann H, Bruchmann M, Duning T, Dziewas R, Berger K, Ringelstein EB. High-normal blood pressure is associated with poor cognitive performance. Hypertension. 2008;51:663–668. doi: 10.1161/HYPERTENSIONAHA.107.105577. [DOI] [PubMed] [Google Scholar]
- Kremen WS, Eisen SA, Tsuang MT, Lyon MJ. Is the Wisconsin Card Sorting Test a useful, neurocognitive endophenotype? Am. J. Med. Genet. B Neuropsychiatr. Genet. 2007;144:403–406. doi: 10.1002/ajmg.b.30527. [DOI] [PubMed] [Google Scholar]
- Kuczynski B, Jagust W, Chui HC, Reed B. An inverse association of cardiovascular risk and frontal lobe glucose metabolism. Neurology. 2009;72:738–743. doi: 10.1212/01.wnl.0000343005.35498.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao JI, Montoriol C, Morer I, Beyer K. Genetic contribution to aging: deleterious and helpful genes define life expectancy. Ann. N Y Acad. Sci. 2005;1057:50–63. doi: 10.1196/annals.1356.003. [DOI] [PubMed] [Google Scholar]
- Lehmann DJ, Cortina-Borja M, Warden DR, Smith AD, Sleegers K, Prince JA, van Duijn CM, Kehoe PG. Large meta-analysis establishes the ACE insertion-deletion polymorphism as a marker of Alzheimer's disease. Am. J. Epidemiol. 2005;162:305–317. doi: 10.1093/aje/kwi202. [DOI] [PubMed] [Google Scholar]
- Lo YMD, Lau TK, Chan LYS, Leung TN, Chang AMZ. Quantitative analysis of the bi-directional fetomaternal transfer of nucleated cells and plasma DNA. Clin. Chem. 2000;46:1301–1309. [PubMed] [Google Scholar]
- Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, Witteman JC. Arterial stiffness and risk of coronary heart disease and stroke: The Rotterdam Study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- Mattace-Raso FU, van der Cammen TJ, Sayed-Tabatabaei FA, van Popele NM, Asmar R, Schalekamp MA, Hofman A, van Duijn CM, Witteman JC. Angiotensin-converting enzyme gene polymorphism and common carotid stiffness. The Rotterdam Study. Atherosclerosis. 2004;174:121–126. doi: 10.1016/j.atherosclerosis.2004.01.012. [DOI] [PubMed] [Google Scholar]
- McDonald RJ. Multiple combinations of co-factors produce variants of age-related cognitive decline: a theory. Can. J. Exp. Psychol. 2002;56:221–239. doi: 10.1037/h0087399. [DOI] [PubMed] [Google Scholar]
- Mitchell GF, Vasan RS, Keyes MJ, Parise H, Wang TJ, Larson MG, D'Agostino RB, Sr, Kannel WB, Levy D, Benjamin EJ. Pulse pressure and risk of new-onset atrial fibrillation. JAMA. 2007;297:709–715. doi: 10.1001/jama.297.7.709. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cognit. Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Morris BJ, Zee RY, Schrader AP. Different frequencies of angiotensin-converting enzyme genotypes in older hypertensive individuals. J. Clin. Invest. 1994;94:1085–1089. doi: 10.1172/JCI117423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel IE, Chicherio C, Li SC, von Oertzen T, Sander T, Villringer A, Heekeren HR, Bäckman L, Lindenberger U. Human aging magnifies genetic effects on executive functioning and working memory. Front. Hum. Neurosci. 2008;2:1–8. doi: 10.3389/neuro.09.001.2008. article 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemiec P, Zak I, Wita K. Modification of the coronary artery disease risk associated with the presence of traditional risk factors by insertion/deletion polymorphism of the ACE gene. Genet. Test. 2007;11:353–359. doi: 10.1089/gte.2007.0005. [DOI] [PubMed] [Google Scholar]
- Nieoullon A. Dopamine and the regulation of cognition and attention. Prog. Neurobiol. 2002;67:53–83. doi: 10.1016/s0301-0082(02)00011-4. [DOI] [PubMed] [Google Scholar]
- Oren A, Vos LE, Uiterwaal CS, Grobbee DE, Bots ML. Cardiovascular risk factors and increased carotid intima-media thickness in healthy young adults: the Atherosclerosis Risk in Young Adults (ARYA) Study. Arch. Intern. Med. 2003;163:1787–1792. doi: 10.1001/archinte.163.15.1787. [DOI] [PubMed] [Google Scholar]
- Peila R, White LR, Petrovich H, Masaki K, Ross GW, Havlik RJ, Launer LJ. Joint effect of the APOE gene and midlife systolic blood pressure on late-life cognitive impairment: the Honolulu-Asia aging study. Stroke. 2001;32:2882–2889. doi: 10.1161/hs1201.100392. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Raz N, Dahle CL, Rodrigue KM, Kennedy KM, Land SJ, Jacobs BS. Brain-derived neurotrophic factor Val66Met and blood glucose: a synergistic effect on memory. Front. Hum. Neurosci. 2008;2:1–6. doi: 10.3389/neuro.09.012.2008. article 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon FM, Head D, Dupuis JH, Acker JD. Neuroanatomical correlates of cognitive aging: evidence from structural magnetic resonance imaging. Neuropsychology. 1998;12:95–114. doi: 10.1037//0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Acker JD. Hypertension and the brain: vulnerability of the prefrontal regions and executive functions. Behav. Neurosci. 2003;117:1169–1180. doi: 10.1037/0735-7044.117.6.1169. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Haacke EM. Brain aging and its modifiers: insights from in vivo neuromorphometry and susceptibility weighted imaging. Ann. N Y Acad. Sci. 2007a;1097:84–93. doi: 10.1196/annals.1379.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Acker JD. Vascular health and longitudinal changes in brain and cognition in middle-aged and older adults. Neuropsychology. 2007b;21:149–157. doi: 10.1037/0894-4105.21.2.149. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Land S. Genetic and vascular modifiers of age-sensitive cognitive skills: effects of COMT, BDNF, ApoE and hypertension. Neuropsychology. 2009;23:105–116. doi: 10.1037/a0013487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard F, Berr C, Amant C, Helbecque N, Amouyel P, Alpérovitch A The EVA Study Group. Effect of the angiotensin I-converting enzyme I/D polymorphism on cognitive decline. Neurobiol. Aging. 2000;21:75–80. doi: 10.1016/s0197-4580(99)00102-5. [DOI] [PubMed] [Google Scholar]
- Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, Soubrier F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J. Clin. Invest. 1990;86:1343–1346. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Román GC, Erkinjuntti T, Wallin A, Pantoni L, Chui HC. Subcortical ischaemic vascular dementia. Lancet Neurol. 2002;1:426–436. doi: 10.1016/s1474-4422(02)00190-4. [DOI] [PubMed] [Google Scholar]
- Rosa A, Peralta V, Cuesta MJ, Zarzuela A, Serrano F, Martínez-Larrea A, Fañanás L. New evidence of association between COMT gene and prefrontal neurocognitive function in healthy individuals from sibling pairs discordant for psychosis. Am. J. Psychiatry. 2004;161:1110–1112. doi: 10.1176/appi.ajp.161.6.1110. [DOI] [PubMed] [Google Scholar]
- Rozzini L, Chilovi BV, Bertoletti E, Conti M, Del Rio I, Trabucchi M, Padovani A. Angiotensin converting enzyme (ACE) inhibitors modulate the rate of progression of amnestic mild cognitive impairment. Int. J. Geriatr. Psychiatry. 2006;21:550–555. doi: 10.1002/gps.1523. [DOI] [PubMed] [Google Scholar]
- Saavedra JM. Brain angiotensin II: new developments, unanswered questions and therapeutic opportunities. Cell Mol. Neurobiol. 2005;25:485–512. doi: 10.1007/s10571-005-4011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safar ME. Systolic hypertension in the elderly: arterial wall mechanical properties and the renin-angiotensin-aldosterone system. J. Hypertens. 2005;23:673–681. doi: 10.1097/01.hjh.0000163130.39149.fe. [DOI] [PubMed] [Google Scholar]
- Saidi S, Zammiti W, Slamia LB, Ammou SB, Almawi WY, Mahjoub T. Interaction of angiotensin-converting enzyme and apolipoprotein E gene polymorphisms in ischemic stroke involving large-vessel disease. J. Thromb. Thrombolysis. 2009;27:68–74. doi: 10.1007/s11239-007-0165-y. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Hancock HE, Meinz EJ, Hambrick DZ. Interrelations of age, visual acuity, and cognitive functioning. J. Gerontol. B Psychol. Sci. Soc. Sci. 1996;51:P317–P330. doi: 10.1093/geronb/51b.6.p317. [DOI] [PubMed] [Google Scholar]
- Savitz J, Solms M, Ramesar R. The molecular genetics of cognition: dopamine, COMT and BDNF. Genes Brain Behav. 2006;5:311–328. doi: 10.1111/j.1601-183X.2005.00163.x. [DOI] [PubMed] [Google Scholar]
- Siu AL. Screening for dementia and investigating its causes. Ann. Intern. Med. 1991;115:122–132. doi: 10.7326/0003-4819-115-2-122. [DOI] [PubMed] [Google Scholar]
- Sleegers K, den Heijer T, van Dijk EJ, Hofman A, Bertoli-Avella AM, Koudstaal PJ, Breteler MM, van Duijn CM. ACE gene is associated with Alzheimer's disease and atrophy of hippocampus and amygdala. Neurobiol. Aging. 2005;26:1153–1159. doi: 10.1016/j.neurobiolaging.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Slifstein M, Kolachana B, Simpson EH, Tabares P, Cheng B, Duvall M, Frankle WG, Weinberger DR, Laruelle M, Abi-Dargham A. COMT genotype predicts cortical-limbic D1 receptor availability measured with [11C]NNC112 and PET. Mol. Psychiatry. 2008;13:821–827. doi: 10.1038/mp.2008.19. [DOI] [PubMed] [Google Scholar]
- Srinivasan J, Jayadev S, Kumaran D, Ahamed KF, Suresh B, Ramanathan M. Effect of losartan and enalapril on cognitive deficit caused by Goldblatt induced hypertension. Indian J. Exp. Biol. 2005;43:241–246. [PubMed] [Google Scholar]
- Tan HY, Chen Q, Goldberg TE, Mattay VS, Meyer-Lindenberg A, Weinberger DR, Callicott JH. Catechol-O-methyltransferase Val158Met modulation of prefrontal-parietal-striatal brain systems during arithmetic and temporal transformations in working memory. J. Neurosci. 2007;27:13393–13401. doi: 10.1523/JNEUROSCI.4041-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniwaki H, Kawagishi T, Emoto M, Shoji T, Hosoi M, Kogawa K, Nishizawa Y, Morii H. Association of ACE gene polymorphism with arterial stiffness in patients with type 2 diabetes. Diabetes Care. 1999;22:1858–1864. doi: 10.2337/diacare.22.11.1858. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Bannerman DM, Sharp T, Harrison PJ. Catechol-o-methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. J. Neurosci. 2004;24:5331–5335. doi: 10.1523/JNEUROSCI.1124-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasan RS, Larson MG, Leip EP, Evans JC, O’Donnell CJ, Kannel WB, Levy D. Impact of high-normal blood pressure on the risk of cardiovascular disease. N. Engl. J. Med. 2001;345:1291–1297. doi: 10.1056/NEJMoa003417. [DOI] [PubMed] [Google Scholar]
- Vauquelin G, Michotte Y, Smolders I, Sarre S, Ebinger G, Dupont A, Vanderheyden P. Cellular targets for angiotensin II fragments: pharmacological and molecular evidence. J. Renin Angiotensin Aldosterone Syst. 2002;3:195–204. doi: 10.3317/jraas.2002.041. [DOI] [PubMed] [Google Scholar]
- Visscher PM, Tynan M, Whiteman MC, Pattie A, White I, Hayward C, Wright AF, Starr JM, Whalley LJ, Deary IJ. Lack of association between polymorphisms in angiotensin-converting-enzyme and methylenetetrahydrofolate reductase genes and normal cognitive ageing in humans. Neurosci. Lett. 2003;347:175–178. doi: 10.1016/s0304-3940(03)00691-8. [DOI] [PubMed] [Google Scholar]
- von Bohlen und Halbach O, Albrecht D. The CNS renin-angiotensin system. Cell Tissue Res. 2006;326:599–616. doi: 10.1007/s00441-006-0190-8. [DOI] [PubMed] [Google Scholar]
- Waldstein SR, Rice SC, Thayer JF, Najjar SS, Scuteri A, Zonderman AB. Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension. 2008;51:99–104. doi: 10.1161/HYPERTENSIONAHA.107.093674. [DOI] [PubMed] [Google Scholar]
- Wang B, Jin F, Yang Z, Lu Z, Kan R, Li S, Zheng C, Wang L. The insertion polymorphism in angiotensin-converting enzyme gene associated with the APOE epsilon 4 allele increases the risk of late-onset Alzheimer disease. J. Mol. Neurosci. 2006;30:267–271. doi: 10.1385/JMN:30:3:267. [DOI] [PubMed] [Google Scholar]
- Weinshilboum RM. Pharmacogenomics: catechol O-methyltransferase to thiopurine S-methyltransferase. Cell Mol Neurobiol. 2006;26:539–561. doi: 10.1007/s10571-006-9095-z. Epub 2006 Jun 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychol. Bull. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Williams GV, Castner SA. Under the curve: critical issues for elucidating D1 receptor function in working memory. Neuroscience. 2006;139:263–276. doi: 10.1016/j.neuroscience.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Wisdom NM, Callahan JL, Hawkins KA. The effects of apolipoprotein E on non-impaired cognitive functioning: A meta-analysis. Neurobiology of Aging. doi: 10.1016/j.neurobiolaging.2009.02.003. (in press) [DOI] [PubMed] [Google Scholar]
- Wong J, Patel RA, Kowey PR. The clinical use of angiotensin-converting enzyme inhibitors. Prog. Cardiovasc. Dis. 2004;47:116–130. doi: 10.1016/j.pcad.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Xie T, Ho SL, Ramsden D. Characterization and implications of estrogenic down-regulation of human catechol-O-methyltransferase gene transcription. Mol Pharmacol. 1999;56:31–38. doi: 10.1124/mol.56.1.31. [DOI] [PubMed] [Google Scholar]
- Xu H, Kellendonk CB, Simpson EH, Keilp JG, Bruder GE, Polan HJ, Kandel ER, Gilliam TC. DRD2 C957T polymorphism interacts with the COMT Val158Met polymorphism in human working memory ability. Schizophr. Res. 2007;90:104–107. doi: 10.1016/j.schres.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Yasar S, Zhou J, Varadhan R, Carlson MC. The use of angiotensin-converting enzyme inhibitors and diuretics is associated with a reduced incidence of impairment on cognition in elderly women. Clin. Pharmacol. Ther. 2008;84:119–126. doi: 10.1038/sj.clpt.6100483. [DOI] [PubMed] [Google Scholar]
- Zeng C, Wang D, Yang Z, Wang Z, Asico LD, Wilcox CS, Eisner GM, Welch WJ, Felder RA, Jose PA. Dopamine D1 receptor augmentation of D3 receptor action in rat aortic or mesenteric vascular smooth muscles. Hypertension. 2004;43:673–679. doi: 10.1161/01.HYP.0000118958.27649.6f. [DOI] [PubMed] [Google Scholar]