Figure 4.
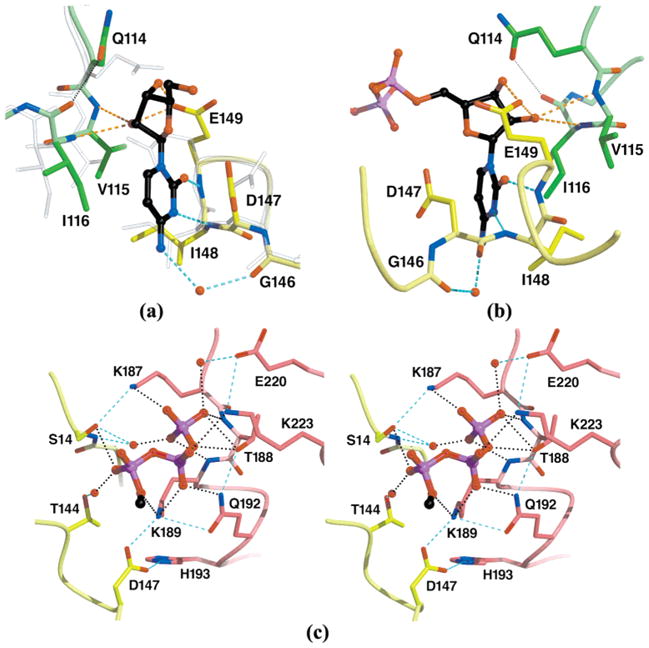
Atomic details of CTP recognition by EcCTPS. The CTP binding site at the model 2AD5 A–B–B′ subunit interface is shown. The bound CTP is indicated with black carbon atoms. Residue subunit identities are indicated by color (see Figure 2). (a) Cytidine recognition by the B and B′ subunits. The triphosphate moiety has been removed for clarity. Subunit B′ rotated 1.1°, and its center of mass shifted 0.3 Å closer to subunit B; side chains D147, E149, and Q114(B′) rotate to accommodate CTP binding (compare CTP complex opaque colored atoms to translucent apo-EcCTPS 1S1M atoms). The cytosine ring is bound via two protein-mediated hydrogen bond with N147 and N148 and one water-mediated hydrogen bond to O146. The ring is sandwiched in a pocket formed by D147, I148, V115(B′), and I116(B′). The identities of Q114, G146, D147, I148, and E149 are conserved in >95% of the CTPS sequences. (b) Ribose recognition. This view is rotated approximately 150° about the vertical axis from the view in panel a. D149 packs against the ribose ring accepting two hydrogen bonds from the 2′- and 3′-hydroxyls. The 2′-hydroxyl is additionally recognized by main chain hydrogen bonding with I116(B′). (c) A–B cross-subunit recognition of the CTP triphosphate. The cytidine nucleoside has been removed for clarity. The triphosphate moiety cements a bridge between the A and B subunits, suggesting a basis for CTP-induced oligomerization. Extensive γ-phosphate interactions are mediated by a P-loop-like structure formed by residues 187–189(A). Note the extensive network of ligand-dependent side chain–side chain hydrogen bonds that would amplify binding specificity. The rotation of D147 induced by cytosine ring binding (see panel a) promotes intersubunit contacts with K189(A) and H193(A). S14, T144, D147, K187, T188, K189, Q192, K223, are conserved in 41 of 43 CTPS sequences (4; http://www.ncbi.nlm.nih.gov/COG/old/aln/COG0504.aln), while H193 is conserved in 37 of 43.
