Abstract
Resveratrol, a polyphenolic compound rich in grapes and red wine, has been reported to protect cells against oxidative damage and cell death by increasing cellular antioxidant/detoxification capacity. Cigarette smoking is a major risk factor for respiratory diseases and oxidative damage is implicated in its pathogenesis. Here we investigated the enhancement of antioxidant capacity by resveratrol and its potential protection against cell death caused by cigarette smoke in human bronchial epithelial cells (HBE1). At concentrations that did not affect cell growth, resveratrol activated Nrf2 signaling and increased the expression of NAD(P)H:quinone reductase-1, heme oxygenase-1, and the catalytic subunit of glutamate cysteine ligase. Surprisingly, instead of protecting against cell death, resveratrol significantly enhanced cigarette smoke extract-induced apoptosis. To define the underlying mechanism, the effect of resveratrol on caspase activity was examined and it was found that resveratrol significantly enhanced cigarette smoke-stimulated caspase activity. In conclusion, results from this study suggest that although resveratrol increased antioxidant and detoxification capacity, it increased rather than protected against cigarette smoke-induced apoptosis.
Keywords: caspase activity, oxidative stress, antioxidant, lung disease
Introduction
Resveratrol, a polyphenolic compound found in grapes, wine, berries, and herbal medicines, such as Polygonum cuspidatum, has been shown to exhibit various biochemical activities, such as regulation of the cell cycle (Hsieh and Wu, 1999), stimulation of endothelial nitric oxide synthase (Taubert and Berkels, 2003), and inhibition of platelet aggregation (Pace-Asciak et al., 1995). Recent studies have found that resveratrol increased antioxidant capability and protected against oxidative damage by enhancing the expression of antioxidant genes, such as heme oxygenase-1 (Chen et al., 2005; Das et al., 2006; Juan et al., 2005), thioredoxin reductase (Hu et al., 2007), and glutathione (GSH) (Savaskan et al., 2003; Vieira de Almeida et al., 2007).
Apoptosis or programmed cell death is a tightly regulated process that consists of complex biochemical cascades involving the activation of caspases (Zimmermann et al., 2001). Caspases are a class of cysteine proteases involved in the initiation and execution of apoptosis and are activated through either extrinsic or intrinsic apoptosis pathways. In the extrinsic pathway, activation of membrane death receptors such as Fas receptor results in the auto-activation of caspase 8 and the subsequent cleavage of procaspase 3 into its active form. The intrinsic pathway, on the other hand, is triggered by signals that cause the release of cytochrome c, Apaf-1, and other proteins from mitochondria. These proteins then form an apoptosome with procaspase 9, resulting in formation of caspase 9. Caspase 9 then cleaves procaspase 3, resulting in caspase 3, the major apoptosis executor (Zimmermann et al., 2001).
Cigarette smoke is the major environmental hazard causing pulmonary diseases such as chronic obstructive pulmonary disease (COPD) and lung cancers. Cigarette smoke is a mixture of more than 4000 chemicals that include significant amounts of free radicals, peroxides, and electrophiles (Pryor and Stone, 1993). Oxidative damage and cell death caused by these oxidants have been implicated in the pathogenesis of COPD and lung cancers (MacNee, 2000; Traber et al., 2000). Therefore, increasing antioxidant capacity has been proposed as a promising strategy to prevent cigarette smoke-induced lung diseases.
Considering its potential in inducing antioxidant defenses, we hypothesized that resveratrol would alleviate cigarette smoke-caused apoptosis. In this study, we tested this hypothesis and unexpectedly found that, although resveratrol increased the expression of some antioxidant genes, it actually enhanced apoptosis, which was apparently through protection of caspase activity.
Materials and methods
Reagents
Unless otherwise noted, all chemicals were from Sigma (St. Louis, MO). Antibodies and small interfering RNAs were from Santa Cruz (Santa Cruz, CA). Annexin V-FITC Apoptosis Detection kit, Acetyl-Asp-Glu-Val-Asp-7-amino-4-(trifluoromethyl)-coumarin (Ac-DEVD-AFC) and acetyl-Leu-Glu-His-Asp-7-amino-4-(trifluoromethyl)-coumarin (Ac-LEHD-AFC) were bought from EMD Biosciences (La Jolla, CA). TRIzol Reagent was from Life Technologies (Grand Island, NY). DNA-free reagent was from Ambion (Austin, TX). TaqMan Reverse Transcription Reagent and SYBR Green PCR Master Mix were from Applied Biosystems (Foster City, CA). FuGENE 6 transfection reagent was from Roche (Indianapolis, IN).
Cell culture and treatment
A human bronchial epithelial cell line (HBE1 cell) was cultured in collagen-coated dishes in 5% CO2 at 37°C as described by Harper et al. (Harper et al., 2001). The HBE1 cell line, which has been demonstrated to share many properties with primary human bronchial cells (Yankaskas et al., 1993), has been used to study the response of human bronchial epithelial cells to various insults including cigarette smoke. Indeed, Lee and coworkers have shown that cigarette smoke exposure caused similar injury in HBE1 cells as in primary human airway epithelium (Lee et al., 2008). Resveratrol was freshly dissolved in ethanol and the final concentration of ethanol in medium was 0.05%.
Preparation of cigarette smoke extracts (CSE)
CSE was an extract of mainstream cigarette smoke. Briefly, the smoke from one filtered cigarette (Camel regular) containing 1.2 mg of nicotine and 18 mg of tar, according to the manufacturer’s report, was drawn through an experimental apparatus with a constant airflow driven by vacuum. The smoke was bubbled through 25 ml of cell medium in 2 min and the solution was used as the stock (100%) for further dilutions. After adjusting the pH to 7.2, the obtained CSE was filtered through a 0.22-μm filter (Millipore, Bedford, MA) for sterilization and diluted for use in 20 min after the preparation.
Measurement of mRNA content
The content of GCLC, NQO-1, and HO-1 mRNAs was determined with real-time PCR method using the protocol described previously (Zhang et al., 2005a). NQO-1 primers, sense 5’- TCTCGGCTCACTGCAACCTCT, antisense, 5’-GCACTTTGGGAGGCTGAGGTA; HO-1 sense 5’- TCTCTTGGCTGGCTTCCTTAC, antisense 5’- GGCTTTTGGAGGTTTGAGACA. GCL and GAPDH primers are same as described previously (Dickinson et al., 2004).
Western Blotting
Western blotting was performed as described previously (Dickinson et al., 2002). Briefly, protein was extracted and 25 μg protein was heated for 15 min at 95°C in loading buffer containing SDS (Tris base, pH 6.5, glycerol, dithiothreitol (DTT), and pyronin Y), electrophoresed under denaturing conditions on a 10% Tris-glycine acrylamide gel (Invitrogen, Carlsbad, CA), and then electroblotted onto a polyvinylidene difluoride (PVDF) membrane (Immobilon P; Millipore, Bedford, MA). Membranes were blocked with 5% fat-free milk at room temperature for 1 h, and then incubated overnight at 4°C with appropriate primary antibody in 5% milk in Tris-buffered saline (TBS). After being washed with Tris-buffered saline containing 0.05% Tween 20 (TTBS), the membrane was incubated with appropriate secondary antibody at room temperature for 2 h. After TTBS washing, the membrane was treated with chemiluminescence reagent (ECL Plus; Amersham, Arlington Heights, IL) for 5 min. The target bands were then imaged on a Kodak Image Station 2000R.
Cell growth assay
Cell growth was assayed with direct cell counting. Briefly, cells at 60-70% confluence were treated with different concentrations of resveratrol for 24 hours. Cell numbers at 0h and 24h after treatment were counted with hemocytometer.
Assay of caspase activity
Activity of caspase 3 and 9 was determined by following the protocol described previously (Watanabe et al., 2002). Briefly cells in 6-well plate were collected and lysed in 200 μl of 0.1% Triton X-100/NaPi (0.1 M sodium phosphate buffer, pH 7.4) and centrifuged at 10,000 g for 10 min at 4°C to obtain the supernatant. Assays of caspase activity were carried out in 200 μl of reaction containing the following: cell lysate equivalent to the original 6 × 104 cells, 10 mM dithiothreitol, 0.05% (vol/vol) Triton X-100, and 50 μM of either Ac-DEVD-AFC (caspase 3 substrate) or Ac-LEHD-AFC (caspase 9 substrate) in NaPi. After incubation at 37°C for 1 h, the fluorescence intensity was measured in a fluorescence microplate reader (SpectraMax GeminiXS, Molecular Devices) with excitation and emission wavelengths of 400 and 500 nm, respectively. The values were converted to AFC concentrations using an external AFC standard.
Apoptosis assay
Apoptosis was determined with a FITC-conjugated annexin V-propidium iodide (PI) kit in a Guava EasyCyte Mini System (Guava Technologies Inc., Hayward, CA). Briefly, cells in 12-well plate were collected with trypsin treatment and combined with cells in the medium. Cell pellet was rinsed with 1 X PBS for 2 times and incubated with annexin V-FITC in binding buffer for 15 min. After adding PI, samples were incubated in ice for 10 min and then analyzed in the Guava flow cytometer.
Statistical Analysis
A comparative ΔΔCT method was used for the relative mRNA quantification as described before (Zhang et al., 2005b). All data were expressed as the mean ± standard error. Sigma Stat software was used for statistical analysis and statistical significance was accepted when p < 0.05. The one-way ANOVA and Tukey test were used for comparison of mRNA level and relative caspase activity.
Results
Resveratrol increases expression of antioxidant genes
Antioxidant enzymes, such as glutamate cysteine ligase (GCL), NAD(P)H quinone oxidoreductase-1 (NQO-1), and heme oxygenase-1 (HO-1), play crucial roles in the detoxification of oxidants and the maintenance of redox homeostasis. To examine the potential protective effects of resveratrol against oxidative stress, we first determined the effects of resveratrol on the expression of NQO-1, HO-1, and the catalytic unit of GCL (GCLC). As shown in Figure 1A, resveratrol (2 and 5 μM) significantly increased the mRNA levels of GCLC, NQO-1, and HO-1 in HBE1 cells. In addition, the nuclear content of Nrf2, a critical transcription factor involved in the induction of a variety of antioxidant/detoxifying genes, was also increased by resveratrol (Figure 1B). These data demonstrated that resveratrol increased the expression levels of a variety of antioxidant and detoxifying genes in HBE1 cells.
Figure 1.
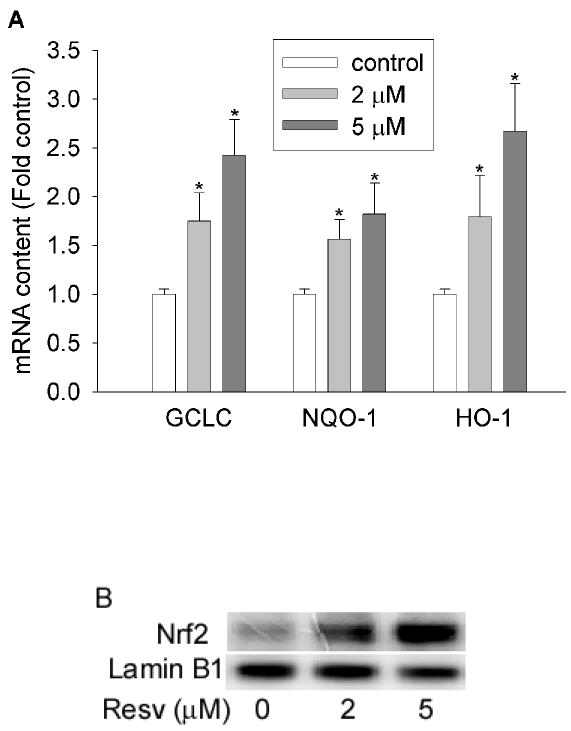
Resveratrol increased antioxidant gene expression. (A) Resveratrol increased mRNA levels of GCLC, NQO-1, and HO-1. HBE1 cells were treated with 0, 2 and 5 μM of resveratrol for 12h and mRNA level of specific gene was determined using RT-real-time PCR assay. N=3, * P<0.05 compared with vehicle control. (B) Nuclear content of Nrf2 is increased by resveratrol. HBE1 cells were treated with 5 μM resveratrol for 1 h and the nucleus was extracted and then nuclear Nrf2 level was determined with Western blotting. Lamin B1 was used as internal control.
Resveratrol increased CSE-triggered apoptosis
As shown in Figure 2A, resveratrol itself did not cause apoptosis. Even when cells were exposed to a concentration as high as 50 μM resveratrol, only 6.84% of the cells were apoptotic (Annexin V +/PI-), similar to vehicle control. These data suggest that resveratrol alone does not cause apoptosis in HBE1 cells at concentrations less than 50 μM. Since resveratrol increased antioxidant gene expression (Figure 1), we initially hypothesized that resveratrol would protect cells from cytoxicity caused by cigarette smoke. To test this, we investigated the protection of resveratrol against CSE-induced cell death. Exposure to CSE for 4 h caused apoptosis in a concentration-dependent manner (Figure 2B); pre-exposure to 2 and 5 μM of resveratrol for 24 h, contrary to our initial hypothesis, further increased CSE-induced apoptosis. Although 5% CSE did not induce apoptosis, with resveratrol pretreatment, it markedly increased early apoptotic cells indicated by higher percentages of Annexin+/PI- cells (represented by high green fluorescence and low red fluorescence). With 10% and 20% CSE, pretreatment with resveratrol caused a similar pattern of increased percentage of early apoptotic cells. With 20% CSE, we also observed a marked increase in late apoptosis in respect to increasing dose of resveratrol as indicated by the higher percentage of Annexin V+/PI+ cells (Figure 2B).
Figure 2.
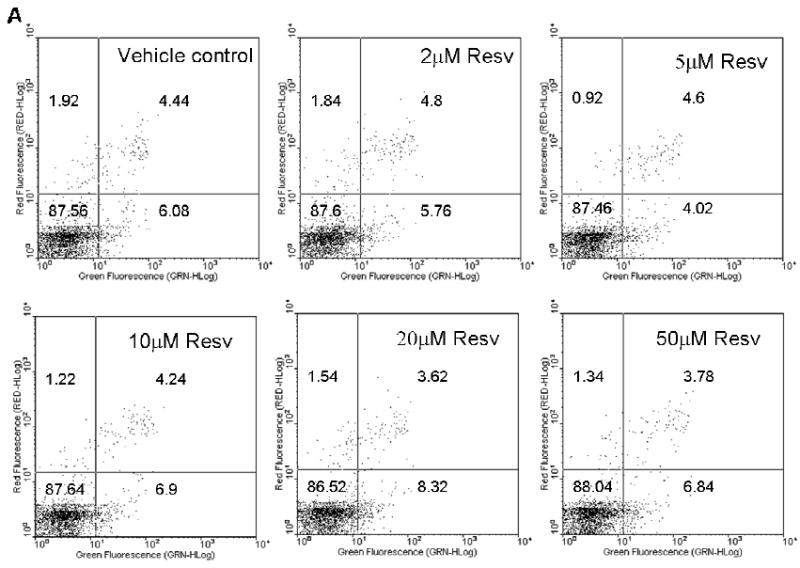
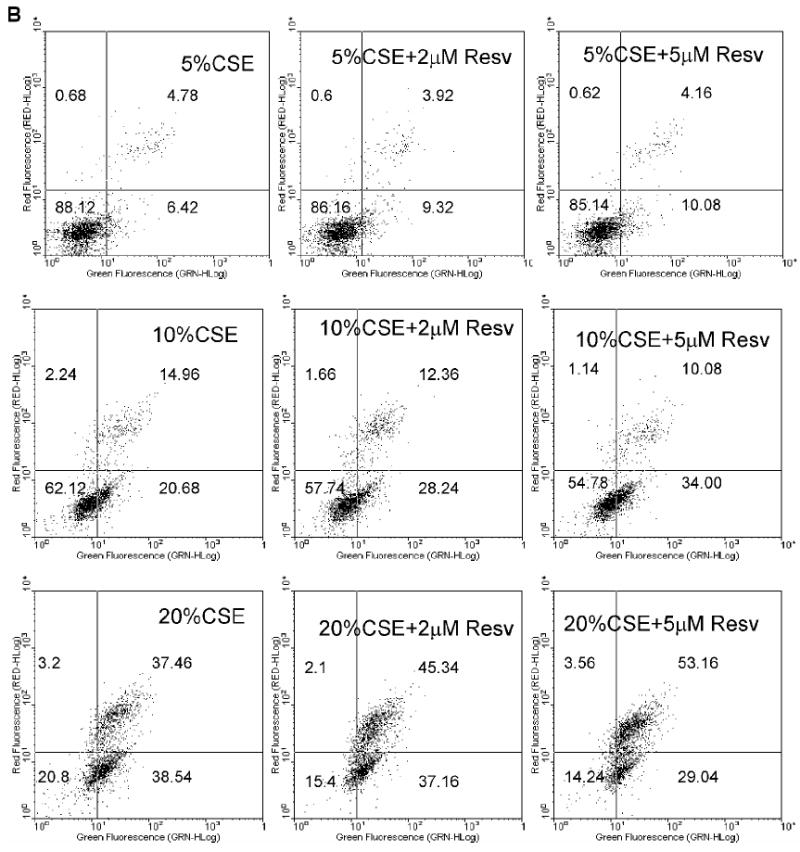
Resveratrol enhanced CSE-induced apoptosis. (A) Resveratrol did not induce apoptosis itself. (B) Pretreatment of resveratrol increased apoptosis induced by CSE. HBE1 cells were pretreated with or without 5 μM resveratrol for 24h before being exposed to CSE for 4h. Apoptosis was then determined with annexin V-FITC method. Experiments were performed 4 times and data from one experiment was shown. The number in low-left, low-right, up-left, up-right phase shows the percentage of cells in normal condition, in the early apoptotic stage, in necrotic stage, and in late apoptotic/necrotic stage, respectively.
Resveratrol mediated inhibition of cell growth
It was reported previously that resveratrol might sensitize cells to apoptosis by inhibiting cell growth (Ahmad et al., 2001; Ferry-Dumazet et al., 2002; Fulda and Debatin, 2004). To examine whether this effect was responsible for the enhancing effect of resveratrol on CSE-triggered apoptosis, we measured the effect of resveratrol on cell growth. As shown in Figure 3, when cells were exposed to less than 5 μM resveratrol, the cells proliferated at the same rate as that of vehicle control with the cell number increasing by 2 fold in 24h. When cells were exposed to 20 μM resveratrol however, cell number increased only by 14% in 24h. Thus, while 20 μM resveratrol could inhibit cell growth as previously reported, inhibition of cell growth was not involved in the increased CSE-triggered apoptosis by 5 μM resveratrol.
Figure 3.
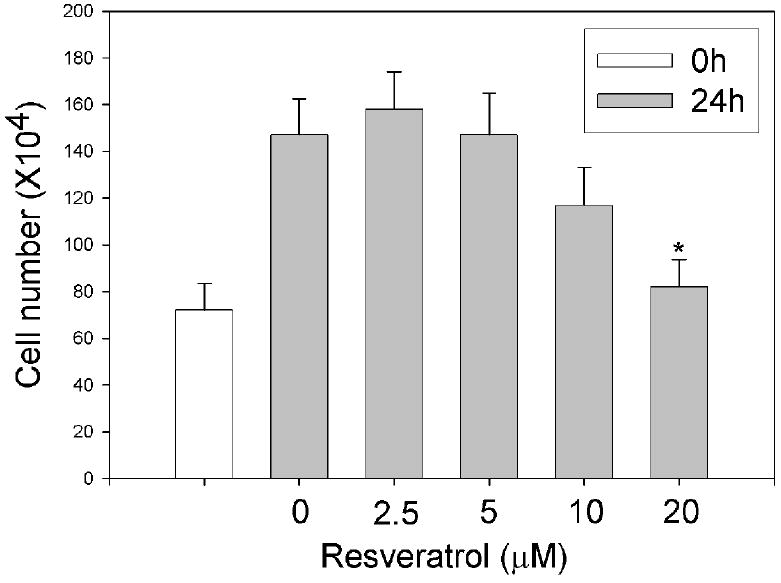
Effect of resveratrol on cell growth. HBE1 cells were treated with different concentrations of resveratrol for 0 and 24 h and the cell number was counted. N=3, * P<0.05 compared with vehicle control.
Resveratrol protected caspase activity
Caspase 9 and caspase 3 play critical roles in the initiation and execution of the apoptotic process. To elucidate how resveratrol increased CSE-induced apoptosis, we investigated the potential effect of resveratrol on caspase activity. CSE alone significantly increased the activities of both caspase 9 and 3 (Table 1). The activities of both caspases were further increased with the pretreatment of cells with 5 μM of resveratrol for 24 h, indicating that resveratrol increased caspase activity. However, resveratrol alone did not elevate the activity of caspase 9 and 3 (Table 1), suggesting that resveratrol enhanced CSE-activated caspase activity through mechanisms other than directly activating the caspases.
Table 1.
Effects of resveratrol on CSE-stimulated caspase activity
| Cas 9 | Without DTT |
With DTT |
||
|---|---|---|---|---|
| 4h | 24h | 4h | 24h | |
| 0 | 1±0.05 | 1.23±0.19 | 1.55±0.08 | 1.55±0.23 |
| R | 0.82±0.13 | 1.46±0.21 | 1.46±0.09 | 2.24±0.33 |
| 10% | 1.77±0.15* | 3.23±0.32* | 4.1±0.33* | 5.04±0.42* |
| 10%+R | 2.31±0.25*# | 4.26±0.28*# | 4.99±0.29*# | 6.1±0.35*# |
| 20% | 3.87±0.42* | 5.74±0.42* | 6.29±0.52* | 8.33±0.31* |
| 20%+R | 5.21±0.38*# | 7.25±0.58*# | 8.64±0.46*# | 9.51±0.49*# |
| Cas 3 | ||||
| 0 | 1±0.05 | 1.04±0.23 | 1.74±0.09 | 2.25±0.31 |
| R | 0.81±0.07 | 1.72±0.37 | 1.61±0.06 | 2.42±0.16 |
| 10% | 1.88±0.21* | 23.13±3.6* | 5.76±0.8* | 44.21±5.18* |
| 10%+R | 2.61±0.3*# | 41.39±5.71*# | 8.12±0.71*# | 58.81±4.32*# |
| 20% | 4.23±0.59* | 34.63±3.42* | 7.26±0.77* | 52.88±6.71* |
| 20%+R | 5.69±0.42*# | 53.24±8.69*# | 9.97±1.26*# | 78.17±5.25*# |
HBE1 cells were pretreated with 5 μM resveratrol for 24 h before being treated with/without CSE for 4 and 24 h; and the caspase activity was measured as described in Methods. Values are mean ± SE of three experiments.
P<0.05 compared with vehicle control (ethanol) of 0% CSE treatment;
P<0.05 compared with vehicle control (ethanol) of same CSE exposure. Cas 3, caspase 3; cas 9, caspase 9; R, resveratrol; 0, vehicle control (ethanol).
Caspase3 and 9 were reversibly oxidized during cigarette smoke exposure in the absence of resveratrol
To support the hypothesis that a fraction of caspase activated by CSE was in an inactive state as a result of oxidation, we examined the post-exposure recovery of caspase inhibition with the dithiothreitol (DTT), a reductant frequently used in biochemical assays to maintain the reduced state of proteins. The caspase activity of cells exposed to CSE was determined with/without DTT (2 mM) in assay mixture. Compared with non-DTT condition, DTT incubation significantly increased activities of both caspase 9 and caspase 3, which are activated by CSE (Table 1), suggesting that reduction could increase caspase activity after CSE exposure.
Discussion
The original purpose of this study was to examine the potential increase in antioxidant defense by resveratrol and its protection against cigarette smoke-induced cell death. To do this, we measured the expression levels of some antioxidant genes and examined the effect of resveratrol pre-treatment on CSE-caused apoptosis in human bronchial epithelial cells (HBE1 cells). The results suggest that although resveratrol increased antioxidant and detoxification capacity, it did not alleviate CSE-triggered apoptosis. Instead, resveratrol exacerbated CSE-induced apoptosis. Furthermore, we demonstrated that resveratrol appeared to potentiate apoptosis by protecting CSE-stimulated caspase activity rather than by activating caspases.
Resveratrol has been shown to exhibit antioxidant properties, particularly through the induction of antioxidant genes (Chen et al., 2005; Das et al., 2006; Hu et al., 2007; Juan et al., 2005; Savaskan et al., 2003; Vieira de Almeida et al., 2007) and the alleviation of oxidative damage (Ara et al., 2005; Cadenas and Barja, 1999; de Almeida et al., 2007; Kasdallah-Grissa et al., 2007; Mizutani et al., 2001). In agreement with this, we found that resveratrol increased the mRNA contents of NQO-1, HO-1, and GCLC, which are critical in defense against oxidative stress. The resveratrol-mediated activation of Nrf2, a key transcription factor involved in the induction of many antioxidant and detoxifying genes, provides one mechanism through which resveratrol increases cellular antioxidant and detoxification capacity.
Significant amounts of free radicals and oxidants are present in cigarette smoke and produce oxidative stress-related damage, such as cell death in the lung of a smoker, which eventually leads to cigarette smoke-induced lung disease (MacNee, 2000; Pryor and Stone, 1993; Traber et al., 2000). Antioxidant treatment is thus usually considered an effective strategy to reduce smoke-induced damage (Kinnula, 2005). This study however, showed that although resveratrol increased the antioxidant and detoxification capacity, it exacerbated rather than alleviated cigarette smoke-induced apoptosis. This unexpected result demonstrates that an increase in antioxidant capacity by resveratrol did not translate into an alleviation of cell death from oxidative stress. Similar phenomena with other phase II enzyme inducers have been reported previously. For example, D’Agostini et al. showed that N-acetylcysteine alone or in combination with oltipraz significantly decreased cigarette smoke-induced apoptosis, while phenylethyl isothiocyanate enhanced apoptosis caused by cigarette smoke (D’Agostini et al., 2001). Like resveratrol, phenylethyl isothiocyanate is a potent inducer of antioxidant genes and considered a chemopreventive agent (Hu et al., 2006; Xu et al., 2006). Martin et al. also found that resveratrol decreased oxidative damage but enhanced apoptosis caused by 2, 4, 6-trinitrobenzene sulfonic acid, an inducer of oxidative stress (Martin et al., 2004). Indeed, resveratrol has been reported to enhance the apoptosis caused by a diversity of apoptosis triggers including cytokines and chemotherapeutic agents (Duraj et al., 2006; Fulda and Debatin, 2004; Jazirehi and Bonavida, 2004; Shankar et al., 2007). The difference here is in the proposed mechanism. Previously, it was proposed that cell growth arrest by resveratrol might be involved in its apoptosis sensitization effect (Ahmad et al., 2001; Ferry-Dumazet et al., 2002; Fulda and Debatin, 2004). In the current study however, we did not see cell growth inhibition by resveratrol at the concentrations that enhanced apoptosis (Figure 3). Instead, we found that the CSE-induced caspase activity was protected (and thus further increased) by resveratrol, suggesting that increased caspase activity rather than cell growth inhibition was involved in the enhancement of cigarette smoke-induced apoptosis by resveratrol.
Caspases play critical roles in the apoptotic process and their activities are highly regulated. In this study we found that resveratrol apparently increased the activities of both caspases 3 and 9 that were induced by CSE although resveratrol itself did not activate caspases (Table 1). This suggests that the resveratrol treatment resulted in prevention of the loss of CSE-induced caspase activity and that this is responsible for at least part of the apoptosis exacerbating effect of resveratrol. Activation of caspase 9 is through the intrinsic apoptosis pathway or mitochondrial pathway and involves a balance between proapoptotic and antiapoptotic members of the Bcl-2 family. Previously, resveratrol was shown to increase caspase 9 activity by up regulating Bcl-2 family members (Benitez et al., 2007; Jazirehi and Bonavida, 2004; Jo et al., 2004). The increased caspase 9 activity would consequently activate more caspase 3. In addition, it was reported that resveratrol could cause redistribution of death receptor (CD95) and thus increase the caspase 3 activity (Delmas et al., 2004). In this study however, 5 μM resveratrol did not cause apoptosis (Figure 2A), nor did it increase caspase activity alone (Table 1), suggesting that resveratrol instead protected caspases that were activated by cigarette smoke.
Caspases are cysteine proteinases that are redox sensitive and could be inhibited upon oxidative modification (Borutaite and Brown, 2001; Hampton and Orrenius, 1997). For instance, Zech et al. found that the caspase 3 activity was inhibited by nitric oxide and peroxynitrite (Mohr et al., 1997). Little is known about the modification of caspases by cigarette smoke. A recent study by Stringer et al. demonstrated that caspase 3 was inhibited by CSE dose-dependently and the inhibited caspase activity could be restored with DTT incubation (Stringer et al., 2007). In the current study, DTT addition to cell extracts post exposure to CSE markedly increased the activities of both caspases 9 and 3 after activation by CSE (Table 1). DTT is a powerful reductant that would reduce the disulfide bonds in caspases and then recover the oxidative modification of caspases, if there were any. These data imply that a fraction of the caspases (caspase 9 or caspase 3) could be recovered from reversibly oxidized inactive form. Based on this, it may be inferred that caspase (3 and 9) activity was affected in two phases by cigarette smoke; caspases were initially activated by CSE and then a fraction of these active caspases were oxidatively inhibited by CSE. By increasing antioxidant levels, resveratrol apparently decreased oxidative modification of caspases and protected their activity.
In summary, results from this study showed that while resveratrol induced antioxidant/detoxifying genes expression, it did not protect cells from apoptosis caused by cigarette smoke but rather exacerbated it, partially through protection of the activities of caspase 9 and caspase 3. Excessive apoptosis is involved in the pathogenesis of lung diseases such as emphysema while dysregulated apoptosis is implicated in the development of lung cancer. Therefore, enhancement of apoptosis can be both beneficial and detrimental within the lung parenchyma depending on the pathogenic changes. Obviously, further studies are required to define the potential beneficial health effects of resveratrol.
Acknowledgments
This work was supported by grant 14RT-0059 from the California Tobacco Related Diseases Research Program and ES05511 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad N, Adhami VM, Afaq F, Feyes DK, Mukhtar H. Resveratrol causes WAF-1/p21-mediated G(1)-phase arrest of cell cycle and induction of apoptosis in human epidermoid carcinoma A431 cells. Clin Cancer Res. 2001;7:1466–73. [PubMed] [Google Scholar]
- Ara C, Kirimlioglu H, Karabulut AB, Coban S, Ay S, Harputluoglu M, Kirimlioglu V, Yilmaz S. Protective effect of resveratrol against oxidative stress in cholestasis. J Surg Res. 2005;127:112–7. doi: 10.1016/j.jss.2005.01.024. [DOI] [PubMed] [Google Scholar]
- Benitez DA, Pozo-Guisado E, Alvarez-Barrientos A, Fernandez-Salguero PM, Castellon EA. Mechanisms involved in resveratrol-induced apoptosis and cell cycle arrest in prostate cancer-derived cell lines. Journal of andrology. 2007;28:282–93. doi: 10.2164/jandrol.106.000968. [DOI] [PubMed] [Google Scholar]
- Borutaite V, Brown GC. Caspases are reversibly inactivated by hydrogen peroxide. FEBS Lett. 2001;500:114–8. doi: 10.1016/s0014-5793(01)02593-5. [DOI] [PubMed] [Google Scholar]
- Cadenas S, Barja G. Resveratrol, melatonin, vitamin E, and PBN protect against renal oxidative DNA damage induced by the kidney carcinogen KBrO3. Free Radic Biol Med. 1999;26:1531–7. doi: 10.1016/s0891-5849(99)00019-2. [DOI] [PubMed] [Google Scholar]
- Chen CY, Jang JH, Li MH, Surh YJ. Resveratrol upregulates heme oxygenase-1 expression via activation of NF-E2-related factor 2 in PC12 cells. Biochem Biophys Res Commun. 2005;331:993–1000. doi: 10.1016/j.bbrc.2005.03.237. [DOI] [PubMed] [Google Scholar]
- D’Agostini F, Balansky RM, Izzotti A, Lubet RA, Kelloff GJ, De Flora S. Modulation of apoptosis by cigarette smoke and cancer chemopreventive agents in the respiratory tract of rats. Carcinogenesis. 2001;22:375–80. doi: 10.1093/carcin/22.3.375. [DOI] [PubMed] [Google Scholar]
- Das S, Fraga CG, Das DK. Cardioprotective effect of resveratrol via HO-1 expression involves p38 map kinase and PI-3-kinase signaling, but does not involve NFkappaB. Free Radic Res. 2006;40:1066–75. doi: 10.1080/10715760600833085. [DOI] [PubMed] [Google Scholar]
- de Almeida LM, Pineiro CC, Leite MC, Brolese G, Leal RB, Gottfried C, Goncalves CA. Protective Effects of Resveratrol on Hydrogen Peroxide Induced Toxicity in Primary Cortical Astrocyte Cultures. Neurochem Res. 2007 doi: 10.1007/s11064-007-9399-5. [DOI] [PubMed] [Google Scholar]
- Delmas D, Rebe C, Micheau O, Athias A, Gambert P, Grazide S, Laurent G, Latruffe N, Solary E. Redistribution of CD95, DR4 and DR5 in rafts accounts for the synergistic toxicity of resveratrol and death receptor ligands in colon carcinoma cells. Oncogene. 2004;23:8979–86. doi: 10.1038/sj.onc.1208086. [DOI] [PubMed] [Google Scholar]
- Dickinson DA, Iles KE, Watanabe N, Iwamoto T, Zhang H, Krzywanski DM, Forman HJ. 4-hydroxynonenal induces glutamate cysteine ligase through JNK in HBE1 cells. Free Radic Biol Med. 2002;33:974. doi: 10.1016/s0891-5849(02)00991-7. [DOI] [PubMed] [Google Scholar]
- Dickinson DA, Iles KE, Wigley AF, Forman HJ. Analysis of transcription factor remodeling in phase II gene expression with curcumin. Methods Enzymol. 2004;378:302–18. doi: 10.1016/S0076-6879(04)78023-4. [DOI] [PubMed] [Google Scholar]
- Duraj J, Bodo J, Sulikova M, Rauko P, Sedlak J. Diverse resveratrol sensitization to apoptosis induced by anticancer drugs in sensitive and resistant leukemia cells. Neoplasma. 2006;53:384–92. [PubMed] [Google Scholar]
- Ferry-Dumazet H, Garnier O, Mamani-Matsuda M, Vercauteren J, Belloc F, Billiard C, Dupouy M, Thiolat D, Kolb JP, Marit G, et al. Resveratrol inhibits the growth and induces the apoptosis of both normal and leukemic hematopoietic cells. Carcinogenesis. 2002;23:1327–33. doi: 10.1093/carcin/23.8.1327. [DOI] [PubMed] [Google Scholar]
- Fulda S, Debatin KM. Sensitization for anticancer drug-induced apoptosis by the chemopreventive agent resveratrol. Oncogene. 2004;23:6702–11. doi: 10.1038/sj.onc.1207630. [DOI] [PubMed] [Google Scholar]
- Hampton MB, Orrenius S. Dual regulation of caspase activity by hydrogen peroxide: implications for apoptosis. FEBS Lett. 1997;414:552–6. doi: 10.1016/s0014-5793(97)01068-5. [DOI] [PubMed] [Google Scholar]
- Harper R, Wu K, Chang MM, Yoneda K, Pan R, Reddy SP, Wu R. Activation of nuclear factor-kappa b transcriptional activity in airway epithelial cells by thioredoxin but not by N-acetyl-cysteine and glutathione. Am J Respir Cell Mol Biol. 2001;25:178–85. doi: 10.1165/ajrcmb.25.2.4471. [DOI] [PubMed] [Google Scholar]
- Hsieh TC, Wu JM. Differential effects on growth, cell cycle arrest, and induction of apoptosis by resveratrol in human prostate cancer cell lines. Exp Cell Res. 1999;249:109–15. doi: 10.1006/excr.1999.4471. [DOI] [PubMed] [Google Scholar]
- Hu R, Xu C, Shen G, Jain MR, Khor TO, Gopalkrishnan A, Lin W, Reddy B, Chan JY, Kong AN. Identification of Nrf2-regulated genes induced by chemopreventive isothiocyanate PEITC by oligonucleotide microarray. Life Sci. 2006;79:1944–55. doi: 10.1016/j.lfs.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Hu Y, Rahlfs S, Mersch-Sundermann V, Becker K. Resveratrol modulates mRNA transcripts of genes related to redox metabolism and cell proliferation in non-small-cell lung carcinoma cells. Biological chemistry. 2007;388:207–19. doi: 10.1515/BC.2007.023. [DOI] [PubMed] [Google Scholar]
- Jazirehi AR, Bonavida B. Resveratrol modifies the expression of apoptotic regulatory proteins and sensitizes non-Hodgkin’s lymphoma and multiple myeloma cell lines to paclitaxel-induced apoptosis. Molecular cancer therapeutics. 2004;3:71–84. [PubMed] [Google Scholar]
- Jo EH, Hong HD, Ahn NC, Jung JW, Yang SR, Park JS, Kim SH, Lee YS, Kang KS. Modulations of the Bcl-2/Bax family were involved in the chemopreventive effects of licorice root (Glycyrrhiza uralensis Fisch) in MCF-7 human breast cancer cell. Journal of agricultural and food chemistry. 2004;52:1715–9. doi: 10.1021/jf035012t. [DOI] [PubMed] [Google Scholar]
- Juan SH, Cheng TH, Lin HC, Chu YL, Lee WS. Mechanism of concentration-dependent induction of heme oxygenase-1 by resveratrol in human aortic smooth muscle cells. Biochem Pharmacol. 2005;69:41–8. doi: 10.1016/j.bcp.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Kasdallah-Grissa A, Mornagui B, Aouani E, Hammami M, El May M, Gharbi N, Kamoun A, El-Fazaa S. Resveratrol, a red wine polyphenol, attenuates ethanol-induced oxidative stress in rat liver. Life Sci. 2007;80:1033–9. doi: 10.1016/j.lfs.2006.11.044. [DOI] [PubMed] [Google Scholar]
- Kinnula VL. Focus on antioxidant enzymes and antioxidant strategies in smoking related airway diseases. Thorax. 2005;60:693–700. doi: 10.1136/thx.2004.037473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YC, Chuang CY, Lee PK, Lee JS, Harper RW, Buckpitt AB, Wu R, Oslund K. TRX-ASK1-JNK signaling regulation of cell density-dependent cytotoxicity in cigarette smoke-exposed human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2008;294:L921–31. doi: 10.1152/ajplung.00250.2007. [DOI] [PubMed] [Google Scholar]
- MacNee W. Oxidants/antioxidants and COPD. Chest. 2000;117:303S–17S. doi: 10.1378/chest.117.5_suppl_1.303s-a. [DOI] [PubMed] [Google Scholar]
- Martin AR, Villegas I, La Casa C, de la Lastra CA. Resveratrol, a polyphenol found in grapes, suppresses oxidative damage and stimulates apoptosis during early colonic inflammation in rats. Biochem Pharmacol. 2004;67:1399–410. doi: 10.1016/j.bcp.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Mizutani K, Ikeda K, Kawai Y, Yamori Y. Protective effect of resveratrol on oxidative damage in male and female stroke-prone spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 2001;28:55–9. doi: 10.1046/j.1440-1681.2001.03415.x. [DOI] [PubMed] [Google Scholar]
- Mohr S, Zech B, Lapetina EG, Brune B. Inhibition of caspase-3 by S-nitrosation and oxidation caused by nitric oxide. Biochem Biophys Res Commun. 1997;238:387–91. doi: 10.1006/bbrc.1997.7304. [DOI] [PubMed] [Google Scholar]
- Pace-Asciak CR, Hahn S, Diamandis EP, Soleas G, Goldberg DM. The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: implications for protection against coronary heart disease. Clin Chim Acta. 1995;235:207–19. doi: 10.1016/0009-8981(95)06045-1. [DOI] [PubMed] [Google Scholar]
- Pryor WA, Stone K. Oxidants in cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann N Y Acad Sci. 1993;686:12–27. doi: 10.1111/j.1749-6632.1993.tb39148.x. discussion -8. [DOI] [PubMed] [Google Scholar]
- Savaskan E, Olivieri G, Meier F, Seifritz E, Wirz-Justice A, Muller-Spahn F. Red wine ingredient resveratrol protects from beta-amyloid neurotoxicity. Gerontology. 2003;49:380–3. doi: 10.1159/000073766. [DOI] [PubMed] [Google Scholar]
- Shankar S, Singh G, Srivastava RK. Chemoprevention by resveratrol: molecular mechanisms and therapeutic potential. Front Biosci. 2007;12:4839–54. doi: 10.2741/2432. [DOI] [PubMed] [Google Scholar]
- Stringer KA, Tobias M, O’Neill HC, Franklin CC. Cigarette smoke extract-induced suppression of caspase-3-like activity impairs human neutrophil phagocytosis. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1572–9. doi: 10.1152/ajplung.00325.2006. [DOI] [PubMed] [Google Scholar]
- Taubert D, Berkels R. Upregulation and activation of eNOS by resveratrol. Circulation. 2003;107:e78–9. doi: 10.1161/01.cir.0000060819.46705.ee. author reply e-9. [DOI] [PubMed] [Google Scholar]
- Traber MG, van der Vliet A, Reznick AZ, Cross CE. Tobacco-related diseases. Is there a role for antioxidant micronutrient supplementation? Clin Chest Med. 2000;21:173–87. x. doi: 10.1016/s0272-5231(05)70016-2. [DOI] [PubMed] [Google Scholar]
- Vieira de Almeida LM, Pineiro CC, Leite MC, Brolese G, Tramontina F, Feoli AM, Gottfried C, Goncalves CA. Resveratrol Increases Glutamate Uptake, Glutathione Content, and S100B Secretion in Cortical Astrocyte Cultures. Cell Mol Neurobiol. 2007;27:661–8. doi: 10.1007/s10571-007-9152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, Dickinson DA, Krzywanski DM, Iles KE, Zhang H, Venglarik CJ, Forman HJ. A549 subclones demonstrate heterogeneity in toxicological sensitivity and antioxidant profile. Am J Physiol Lung Cell Mol Physiol. 2002;283:L726–36. doi: 10.1152/ajplung.00025.2002. [DOI] [PubMed] [Google Scholar]
- Xu C, Yuan X, Pan Z, Shen G, Kim JH, Yu S, Khor TO, Li W, Ma J, Kong AN. Mechanism of action of isothiocyanates: the induction of ARE-regulated genes is associated with activation of ERK and JNK and the phosphorylation and nuclear translocation of Nrf2. Molecular cancer therapeutics. 2006;5:1918–26. doi: 10.1158/1535-7163.MCT-05-0497. [DOI] [PubMed] [Google Scholar]
- Yankaskas JR, Haizlip JE, Conrad M, Koval D, Lazarowski E, Paradiso AM, Rinehart CA, Sarkadi B, Jr, Schlegel R, Boucher RC. Papilloma virus immortalized tracheal epithelial cells retain a well-differentiated phenotype. Am J Physiol. 1993;264:C1219–30. doi: 10.1152/ajpcell.1993.264.5.C1219. [DOI] [PubMed] [Google Scholar]
- Zhang H, Dickinson DA, Liu RM, Forman HJ. 4-Hydroxynonenal increases gamma-glutamyl transpeptidase gene expression through mitogen-activated protein kinase pathways. Free Radic Biol Med. 2005a;38:463–71. doi: 10.1016/j.freeradbiomed.2004.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Liu H, Iles KE, Liu RM, Postlethwait EM, Laperche Y, Forman HJ. 4-Hydroxynonenal Induces Rat Gamma-glutamyl Transpeptidase through MAPK Mediated EpRE/Nrf2 Signaling. Am J Respir Cell Mol Biol. 2005b doi: 10.1165/rcmb.2005-0280OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann KC, Bonzon C, Green DR. The machinery of programmed cell death. Pharmacol Ther. 2001;92:57–70. doi: 10.1016/s0163-7258(01)00159-0. [DOI] [PubMed] [Google Scholar]


