Abstract
The objective of this study was to determine the potential long-term impact of dietary habits on age-related decline among 4,809 elderly women (born between 1925 and 1930) in the E3N study, a French longitudinal cohort. In 1993, an extensive diet history self-administered questionnaire was sent to all participants, and in 2006 another questionnaire on instrumental activities of daily living (IADL) and recent cognitive change was sent to a close relative/friend of each woman. Logistic models adjusted for sociodemographic, lifestyle and health factors were performed to evaluate associations between habitual dietary intakes and two outcomes of interest based on the informant response: recent cognitive decline and IADL impairment. Recent cognitive decline was associated with lower intakes of poultry, fish, and animal fats, as well as higher intakes of dairy dessert and ice-cream. IADL impairment was associated with lower intake of vegetables. The odds of recent cognitive decline increased significantly with decreasing intake of soluble dietary fibre and n-3 fatty acids but with increasing intake of retinol. The odds of IADL impairment increased significantly with decreasing intake of vitamins B2, B6, and B12. These results are consistent with a possible long-term neuroprotective effect of dietary fibre, n-3 polyunsaturated fats, and B-group vitamins, and support dietary intervention to prevent cognitive decline.
Keywords: Activities of Daily Living; Aged; Aged, 80 and over; Aging; physiology; Cognition Disorders; prevention & control; psychology; Diet; Diet Surveys; Dietary Fiber; administration & dosage; Educational Status; Energy Intake; Fatty Acids, Omega-3; administration & dosage; Female; Follow-Up Studies; Food Habits; France; Humans; Logistic Models; Marriage; Vegetables; Vitamin B Complex; administration & dosage; Vitamins; administration & dosage
Keywords: Ageing, Cognition, Dietary habits, Function, Longitudinal study, Nutrition, Women
INTRODUCTION
Age-related cognitive decline is an important public health concern, with a prevalence that is rapidly increasing with population ageing. Cognitive impairment leads to significant functional loss and is a major component of total age-related deterioration 1. In the absence of curative treatments, prevention has become a real challenge. Several risk factors are known to be involved in the aetiology of cognitive impairment 2, but few are modifiable, among them nutrition. Longitudinal studies investigating dietary influence on cognitive outcomes in the elderly have suggested that increased intake of specific vitamins or minerals could be associated with reduced incidence of cognitive impairment 3. To explain these results, different hypotheses involving oxidative stress, or inflammatory or vascular pathways have been put forward. However, the long-term relationship between diet and cognitive decline remains unclear and even conflicting. A better understanding of dietary factors that contribute to the maintenance of cognitive ability is thus of high priority 4. The importance of prospective studies of specifically long duration, which would include subjects whose diet was monitored long before cognitive assessment, has previously been stressed 5. Using longitudinal data from the “Etude Epidémiologique de Femmes de la Mutuelle Générale de l’Education Nationale” (E3N) study, we examined the associations between previous usual diet and age-related cognitive impairment.
SUBJECTS and METHODS
The E3N cohort
The E3N cohort includes 98,995 French women within the National Education System, born between 1925 and 1950. This ongoing prospective study primarily investigates cancer risk factors 6 in women, with particular focus on diet and hormones. The research program was approved by the Bicêtre Hospital Review Board and the French National Commission for Data Protection and Privacy. All study participants gave informed consent, and since June 1990, they have been asked at approximately 24-month intervals to complete self-administered questionnaires on medical events and a variety of lifestyle characteristics.
The ageing sub-cohort of the E3N study
In 2006, a specific ageing survey was carried out. Of the 98,995 women in the cohort, those who where born between 1925 and 1930 (n=10,040) represented the target population, because of the higher prevalence of cognitive impairment in this stratum than in middle-aged women. Among these 10,040 elderly women, 1,095 were deceased or had dropped out. Therefore, the ageing survey involved 8,945 cohort participants. A questionnaire intended to a close relative/friend of the E3N participant was designed to obtain indirect data on cognitive and functional problems faced by the elderly women. The questionnaire was sent in January 2006. It included eight Instrumental Activities of Daily Living (IADL 7) and the “DEtérioration Cognitive Observée” (observed cognitive deterioration) (DECO) scale 8. While IADL items provide a comprehensive view of the functional consequences of cognitive decline in everyday life (telephone use, shopping, mode of transportation, ability to handle personal medication, finance handling, food preparation, housekeeping, laundry), DECO provides more specific information on cognitive functioning observed by informants over the past year. This 19-item Likert scale allows to evaluate recent cognitive decline through alterations of the capacity to perform specific tasks related to memory, attention, and visuospatial and language skills.
A questionnaire from a relative was obtained for 5,941 participants (66.4%). Functional and cognitive data were complete for a total of 5,839 women. Because of missing or non-physiologically plausible dietary data — the exposition of interest, assessed in 1993 — the analysis sample finally included 4,809 elderly women. Dietary data was considered as non-physiologically plausible when the calculated ratio of energy intake to energy requirement fell in the highest or lowest percentile for the entire cohort 9.
Outcomes of interest (2006)
In order to explore the nutrition-cognition relationship, we examined two aspects of cognitive functioning, based on informant report: cognitive decline over a period of one year and cognitive status as reflected in current impact on everyday functioning.
Recent cognitive decline was defined on the basis of the DECO score (range 0–38), according to a threshold of 33. This cut-off point has been previously shown to distinguish subjects with a high risk of progressive pathological decline in a French general population sample, with a sensitivity of 89% and a specificity of 67% 10. The individuals with a DECO score under 33 constituted the group of recent cognitive decliners.
The functional dimension of age-related decline was based on a simplified IADL scale, which has been previously validated 11: for each woman, we calculated the 4-IADL score by summing up the number of limitations to the subject’s ability to use the telephone, take her medications, use public transport, and manage her own budget. The women with a non-null 4-IADL score constituted the group of functional decliners (sensitivity of 62% and specificity of 80% for cognitive troubles).
Dietary assessment (1993)
Dietary data was collected in 1993 with an extensive diet history questionnaire covering daily consumption of 208 foods and beverages. The dietary questionnaire was sent with a booklet of photographs to facilitate estimation of portion sizes. Both the questionnaire and the illustrated booklet had been previously validated 12,13 on a sample of 115 women, taking the average of twelve 24-h dietary recalls obtained at monthly intervals over a 1-year period as reference. Average daily dietary intakes of macro- and micronutrients were estimated based on dietary questionnaire data, and using a food composition table derived from the French national database 14. A high proportion of subjects (76% for foods and 72% for nutrients on average) were classified in the same or adjacent quintiles for the dietary questionnaire and 24-h recalls. Among the analysis sample, mean age at dietary assessment was 65.5 years (SD=1.8).
Potential confounders
Socioeconomic factors, lifestyle, and medical background have been associated with cognitive ageing 15. To limit confounding effect in analyses, adjustment variables included socio-demographic characteristics (age in 2006 as 76–79 years vs. 80–82 years; education level as < 12 years vs. ≥ 12 years), BMI in kg/m2 (< 18.5; 18.5–24.9; 25–29.9; ≥ 30), an indicator of average physical activity based on the median value in metabolic equivalents per week (≤ 50; > 50), the quartiles of average dietary energy intake in kJ/day, as well as the smoking status (current/past smoker; non-smoker). Supplement consumption was taken into account (use of vitamin D and/or calcium, use of other vitamins or minerals). Other self-reported variables related to medical follow-up: use of post-menopausal hormones (ever; never); diabetes mellitus (ever; never); hypertension (ever; never); and hypercholesterolemia (ever; never). Adjustment variables also included self-reported history of coronary heart disease (myocardial infarction or angina pectoris), stroke, cancer, and depression. Data on cancer were validated through pathological reports, as the main outcome of the E3N study.
Missing values
Missing values for educational level, BMI, supplement consumption, physical activity and smoking status represented less than 5% of subjects for each of these covariates. Thus, we replaced them by the modal value. This procedure is routinely used when analysing E3N data for adjustment categorical variables, in order to include all subjects with non-missing data for the main outcomes/exposures, so as to limit bias and power loss due to too many excluded cases. However, we verified that results of statistical models were globally unchanged when subjects with one or more missing values for any of these variables were excluded from the analyses (restricted sample: n=4,515).
Statistical analyses
To illustrate the link between cognitive and functional status as appraised by the informant, we performed kernel density estimation 16 of the DECO score according to the 4-IADL score. This analysis was conducted among the 4,758 women for whom both scores were computable. We studied bivariate associations between potential confounders and the two outcomes of interest: DECO score <33 and 4-IADL score > 0. All variables listed above as potential confounders were then included in models assessing nutritional factors.
Dietary habits were approached by considering successively daily intakes of food groups (e.g., vegetables, fish, eggs), macronutrients (e.g., carbohydrates, proteins), and micronutrients (e.g., vitamins and minerals). Raw intakes of food groups as well as residuals on energy 17 of nutrient intakes were categorized into tertiles according to the distribution in our elderly population, except when food groups were consumed by less than 10% of the sample. In those cases (i.e., legumes; pizza, sandwiches and snacks; beef, pork and lamb; poultry; offal; animal fats; dairy desserts and ice-cream; sugar and confectionary; pastries and cakes; coffee; tea; soups; wine; beer; other alcoholic drinks), we isolated a category of non-consumers, and considered consumers in two groups according to the median consumption. Although alcohol is a nutrient, it was categorized as described above for rarely consumed food groups, since 13.8% of women in our sample showed null intake.
We used multivariable logistic regression to compute the odds ratios (OR) and 95% confidence intervals [95% CI]. Each nutritional factor considered was entered into separate logistic regression models. Tests for linear trend were performed using the ordinal score on categories of nutritional intake.
Except for the kernel density estimation, which was computed with R software version 2.3.0 (http://www.r-project.org), all analyses were performed using SAS software, version 9.1 (SAS Institute, Inc., Cary, NC). All results were considered significant at the 5 % level. All statistical tests were two-sided.
RESULTS
Sample selection
Compared with women for whom no relative responded, women for whom informant data were obtained in 2006 were somewhat younger and more educated. They were also more likely to be married and to have responded to the dietary questionnaire sent in 1993. In addition, women excluded from the analysis sample because of missing dietary data were less educated than the investigated population.
Characteristics of women according to their cognitive and functional status
Among the 4,758 women included in the analysis sample, 518 women had a DECO score < 33 (12.4%), 716 had a 4-IADL score > 0 (14.9%), and 268 presented both declines (5.6%). Although non-interchangeable, functional status and recent cognitive decline were closely related in our sample, as illustrated by Figure 1, which simultaneously represents DECO score distributions in subjects with the 4-IADL score respectively equal to 0, 1, 2, 3 or 4. While the maximum score on the DECO scale is 38 and the minimum is 0 (with lower scores indicating a sharper decrease in cognitive performance), DECO cut-off points for the first, second and third quartiles were, respectively, 36-38-38 for women without disability, and 32-35-37, 28-33-36, 23-27-34, and 10-17-28 for women who had one, two, three or four IADL impairments.
Figure 1.
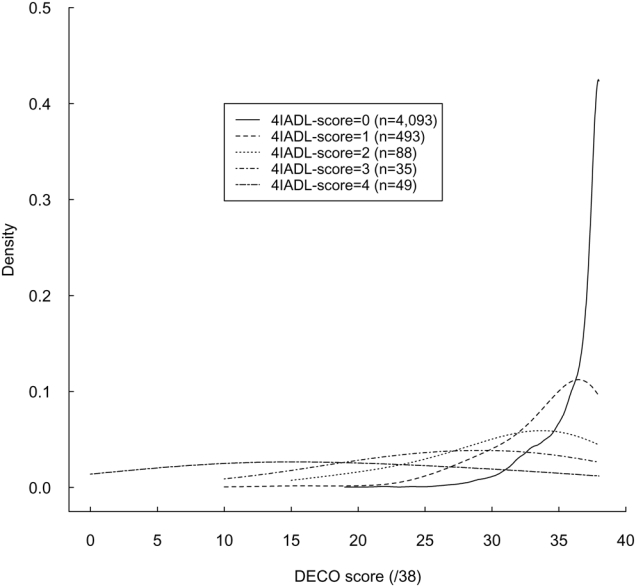
Cognitive decline and functional impairment: Kernel density estimation of the DECO score according to the 4-IADL score1, E3N Study2, France, 2006
DECO, questionnaire “Détérioration Cognitive Observée”; IADL, Instrumental Activities of Daily Living; E3N, “Etude Epidémiologique de Femmes de la Mutuelle Générale de l’Education Nationale”;
1 4-IADL score defined as number of limitations to the subject’s ability to use the telephone, take medication, use public transport and manage own budget
2 The analysis was conducted among the 4,758 subjects for whom both scores were computable
Characteristics of women as a function of their cognitive and functional status were described in Table 1. Recent cognitive decline was positively associated with age, history of depression, cancer, coronary heart disease, stroke, and diabetes mellitus, but inversely associated with education level and physical activity level. IADL impairment was associated with higher age, lower education level, BMI ≥ 25, lower energy intake, never-smoker status, lower level of physical activity, non-use of supplement and non-use of post-menopausal hormones. IADL impairment was also positively associated with history of depression, coronary heart disease, stroke, diabetes mellitus and hypertension.
Table 1.
Comparison of elderly women depending of their cognitive and functional status, n=4,809 elderly women of the E3N study, France, 1993–2006
| Recent cognitive decline DECO score < 33 | Functional impairment 4 IADL score > 0 | ||||||
|---|---|---|---|---|---|---|---|
| Covariate | Modality | Non-case n=4,211 (%) | Case n=598 (%) | P value1 | Non-case n=4,093 (%) | Case n=716 (%) | P value1 |
| Age at the time of cognitive assessment (2006) | 76–79 years | 78.8 | 75.1 | 79.1 | 74.2 | ||
| 80–82 years | 21.2 | 24.9 | .037 | 20.9 | 25.8 | .003 | |
| Education level2 | < 12 years | 15.6 | 21.9 | 15.1 | 23.6 | ||
| ≥ 12 years | 84.4 | 78.1 | .000 | 84.9 | 76.4 | .000 | |
| Body mass index2 (kg/m2) | < 18.5 | 2.8 | 4.0 | .114 | 2.9 | 3.4 | .312 |
| 18.5–24.9 | 72.5 | 71.1 | 73.3 | 66.5 | |||
| 25.0–29.9 | 21.2 | 22.2 | .523 | 21.0 | 23.2 | .044 | |
| ≥ 30.0 | 3.5 | 2.7 | .345 | 2.8 | 7.0 | .000 | |
| Indicator of average physical activity2 (Metabolic equivalents per day) | ≤ 50 | 49.3 | 53.8 | 48.6 | 57.1 | ||
| > 50 | 50.7 | 46.2 | .039 | 51.4 | 42.9 | . 000 | |
| Average daily energy intake3 (kJ/day) | Energy ≤ 6,276 | 23.1 | 24.6 | .370 | 22.5 | 27.7 | .007 |
| 6,276< energy ≤ 7,950 | 24.1 | 22.9 | 24.3 | 21.8 | |||
| 7,950< energy ≤ 9,623 | 26.9 | 24.2 | .675 | 26.9 | 24.7 | .825 | |
| Energy > 9,623 | 25.9 | 28.3 | .263 | 26.3 | 25.8 | .422 | |
| Smoking status2 | Never-smoker | 66.5 | 69.7 | 66.3 | 70.3 | ||
| Smoker or past smoker | 33.5 | 30.3 | .118 | 33.7 | 29.7 | .040 | |
| Supplement of vitamin D and/or calcium2 | Non-use | 83.8 | 84.6 | 83.8 | 84.4 | ||
| Use | 16.2 | 15.4 | .614 | 16.2 | 15.6 | .724 | |
| Supplement of other vitamins or minerals2 | Non-use | 80.0 | 77.3 | 79.1 | 83.0 | ||
| Use | 20.0 | 22.7 | .119 | 20.9 | 17.0 | .018 | |
| Use of post-menopausal hormones | Never | 50.4 | 51.5 | 49.5 | 56.4 | ||
| Ever | 49.6 | 48.5 | .618 | 50.5 | 43.6 | .001 | |
| History of depression | Never | 80.2 | 69.6 | 80.2 | 71.4 | ||
| Ever | 19.8 | 30.4 | .000 | 19.8 | 28.6 | .000 | |
| History of cancer | Never | 84.2 | 80.8 | 83.9 | 83.0 | ||
| Ever | 15.8 | 19.2 | .036 | 16.1 | 17.0 | .541 | |
| History of coronary heart disease4 | Never | 94.2 | 90.3 | 94.2 | 90.6 | ||
| Ever | 5.8 | 9.7 | .000 | 5.8 | 9.4 | .000 | |
| History of stroke | Never | 96.3 | 93.0 | 96.6 | 91.8 | ||
| Ever | 3.7 | 7.0 | .000 | 3.4 | 8.2 | .000 | |
| History of diabetes mellitus | Never | 94.2 | 90.6 | 94.5 | 89.5 | ||
| Ever | 5.8 | 9.4 | .001 | 5.5 | 10.5 | .000 | |
| History of hypertension | Never | 56.5 | 57.9 | 57.4 | 52.7 | ||
| Ever | 43.5 | 42.1 | .543 | 42.6 | 47.3 | .018 | |
| History of hypercholesterolemia | Never | 57.6 | 56.5 | 57.5 | 57.4 | ||
| Ever | 42.4 | 43.5 | .621 | 42.5 | 42.6 | .975 | |
P values obtained through univariate logistic regression analyses
Missing values replaced by most frequent modality (variables with less than 5 % missing values)
Except energy from alcohol
Myocardial infarction or angina pectoris
Dietary intakes and age-related decline
Results of multi-adjusted logistic models that tested associations between age-related decline and habitual dietary intakes of 21 food-groups are shown in Table 2. After controlling for various potential factors, women with recent cognitive decline consumed in the past significantly lower amounts of poultry, fish, and animal fats. They also consumed higher amounts of dairy desserts and ice-cream. The association between higher odds of cognitive decline and higher intake of pastries and cakes was borderline significant. Women with IADL impairment had lower intake of vegetables.
Table 2.
Multi-adjusted OR of habitual dietary habits (assessed in 1993) associated with cognitive decline or IADL impairment (assessed in 2006), n=4,809 elderly women of the E3N study, France, 1993–2006
| Recent cognitive decline DECO score < 33 (n=598) | Functional impairment 4 IADL score > 0 (n=716) | |||||||
|---|---|---|---|---|---|---|---|---|
| Group 2 vs. group 12 | Group 3 vs. group 12 | Group 2 vs. group 12 | Group 3 vs. group 12 | |||||
| Food group intake (g/day) | Mean3 | SD3 | OR (95% CI) | OR (95% CI) | P for trend | OR (95% CI) | OR (95% CI) | P for trend |
| Potatoes | 65.82 | 55.01 | 1.01 (0.81,1.25) | 0.90 (0.72,1.13) | .377 | 1.07 (0.87,1.31) | 0.95 (0.77,1.18) | .646 |
| Vegetables | 231.7 | 118.7 | 1.12 (0.90,1.39) | 1.10 (0.89,1.37) | .389 | 0.83 (0.68,1.01) | 0.80 (0.65,0.98) | .029 |
| Legumes* | 14.89 | 19.89 | 0.89 (0.71,1.11) | 1.03 (0.82,1.29) | .700 | 0.92 (0.75,1.14) | 0.91 (0.74,1.13) | .405 |
| Fruits and fruit juice | 354.5 | 199.4 | 0.89 (0.72,1.11) | 0.88 (0.70,1.09) | .240 | 0.94 (0.77,1.15) | 0.86 (0.70,1.06) | .164 |
| Milk & yoghurt | 234.8 | 191.9 | 1.21 (0.97,1.50) | 1.17 (0.93,1.46) | .182 | 1.04 (0.85,1.26) | 0.97 (0.79,1.20) | .799 |
| Cheese | 48.78 | 39.16 | 1.11 (0.89,1.39) | 1.15 (0.92,1.44) | .223 | 1.04 (0.85,1.28) | 1.00 (0.82,1.24) | .974 |
| Bread and cereal products | 131.6 | 85.59 | 0.91 (0.72,1.14) | 1.00 (0.78,1.27) | .997 | 0.91 (0.74,1.13) | 1.02 (0.81,1.28) | .902 |
| Pizza, sandwiches and snacks* | 19.98 | 22.56 | 0.91 (0.69,1.22) | 0.95 (0.71,1.27) | .947 | 0.81 (0.62,1.04) | 0.85 (0.66,1.11) | .523 |
| Beef, pork and lamb* | 45.45 | 35.53 | 0.99 (0.76,1.30) | 0.87 (0.66,1.15) | .206 | 0.93 (0.73,1.20) | 0.96 (0.74,1.23) | .873 |
| Poultry* | 16.93 | 17.89 | 0.88 (0.71,1.09) | 0.73 (0.58,0.91) | .004 | 0.82 (0.67,1.00) | 0.85 (0.70,1.04) | .136 |
| Processed meat | 22.12 | 19.26 | 1.13 (0.91,1.41) | 1.16 (0.92,1.46) | .205 | 0.96 (0.79,1.18) | 1.06 (0.86,1.31) | .596 |
| Offal* | 6.343 | 9.964 | 1.07 (0.87,1.32) | 1.01 (0.81,1.25) | .899 | 1.06 (0.87,1.28) | 0.90 (0.74,1.10) | .376 |
| Fish | 38.03 | 28.45 | 0.88 (0.71,1.08) | 0.80 (0.64,0.99) | .043 | 0.87 (0.71,1.06) | 0.99 (0.81,1.21) | .939 |
| Eggs | 22.20 | 21.48 | 0.98 (0.79,1.22) | 0.99 (0.79,1.23) | .897 | 0.99 (0.81,1.21) | 1.06 (0.86,1.30) | .584 |
| Vegetable fats | 20.37 | 10.76 | 0.95 (0.76,1.18) | 1.02 (0.82,1.27) | .842 | 1.03 (0.84,1.26) | 1.02 (0.83,1.26) | .860 |
| Animal fats* | 8.665 | 9.969 | 0.94 (0.75,1.17) | 0.71 (0.56,0.90) | .003 | 0.82 (0.66,1.01) | 0.81 (0.65,1.01) | .083 |
| Dairy desserts and ice-cream* | 22.89 | 35.78 | 1.02 (0.82,1.28) | 1.33 (1.07,1.65) | .010 | 0.97 (0.80,1.19) | 1.06 (0.86,1.29) | .612 |
| Sugar and confectionary* | 34.03 | 29.75 | 0.95 (0.70,1.28) | 0.96 (0.70,1.31) | .885 | 0.92 (0.70,1.21) | 0.95 (0.72,1.27) | .924 |
| Pastries and cakes* | 33.56 | 32.38 | 1.10 (0.79,1.54) | 1.29 (0.92,1.81) | .056 | 0.94 (0.70,1.25) | 0.99 (0.74,1.34) | .764 |
| Coffee* | 237.2 | 212.5 | 0.95 (0.71,1.27) | 0.95 (0.71,1.28) | .804 | 1.19 (0.89,1.59) | 1.12 (0.84,1.50) | .837 |
| Tea* | 169.1 | 270.0 | 1.08 (0.87,1.33) | 0.96 (0.78,1.19) | .781 | 0.89 (0.73,1.08) | 0.90 (0.74,1.09) | .248 |
| Soups* | 151.4 | 125.0 | 1.17 (0.87,1.57) | 1.07 (0.79,1.45) | .938 | 0.98 (0.75,1.27) | 0.93 (0.71,1.22) | .548 |
| Beer* | 11.31 | 47.67 | 1.04 (0.78,1.38) | 0.86 (0.63,1.18) | .459 | 1.08 (0.82,1.42) | 1.19 (0.91,1.56) | .175 |
| Wine* | 91.44 | 124.3 | 1.01 (0.80,1.26) | 0.94 (0.75,1.18) | .556 | 0.90 (0.73,1.11) | 0.85 (0.68,1.04) | .123 |
| Alcoholic drink* (other than beer and wine) | 15.25 | 42.11 | 0.87 (0.70,1.08) | 0.96 (0.77,1.20) | .774 | 0.85 (0.69,1.04) | 0.82 (0.66,1.01) | .061 |
OR, Odds Ratio; SD, Standard Deviation
Through multi-adjusted logistic regression models, taking healthy elderly as reference group. Adjustment on all covariates listed in Table 1. Each food group was considered separately
* Group 1, group 2 and group 3 were defined as tertile 1, tertile 2 and tertile 3 of food intake, or, for food marked by *, as no consumption, consumption ≤ median and consumption > median
Mean(SD) calculated among whole sample
Results of multi-adjusted logistic models that tested associations between age-related decline and habitual dietary intakes of 30 macro- or micronutrients are shown in Table 3. The odds of recent cognitive decline increased with decreasing intake of total/soluble dietary fibre and of n-3 fatty acids, while they increased with increasing intake of retinol. Furthermore, recent cognitive decliners showed higher n-6/n-3 fatty acids ratio than women with a DECO score ≥ 33. Concerning functional status, the odds of IADL impairment increased significantly with decreasing intake of vitamin B2, B6, and B12.
Table 3.
Multi-adjusted OR of habitual nutrient intakes (assessed in 1993) associated with cognitive decline or IADL impairment (assessed in 2006), n=4,809 elderly women of the E3N study, France, 1993–2006
| Recent cognitive decline DECO score < 33 (n=598) | Functional impairment 4 IADL score > 0 (n=716) | |||||||
|---|---|---|---|---|---|---|---|---|
| T2 vs. T12 | T3 vs. T12 | T2 vs. T12 | T3 vs. T12 | |||||
| Nutrient intake (per day) | Mean3 | SD3 | OR (95% CI) | OR (95% CI) | P for trend | OR (95% CI) | OR (95% CI) | P for trend |
| Energy4 (kJ) | 8343 | 2232 | 0.93 (0.75,1.15) | 1.00 (0.81,1.24) | .957 | 0.87 (0.71,1.06) | 0.91 (0.75,1.11) | .367 |
| Alcohol* (g) | 10.43 | 12.96 | 0.86 (0.66,1.11) | 0.84 (0.65,1.09) | .264 | 0.78 (0.61,0.98) | 0.76 (0.59,0.96) | .051 |
| Total carbohydrates (g) | 228.5 | 74.47 | 1.15 (0.92,1.42) | 1.05 (0.84,1.30) | .696 | 0.93 (0.75,1.14) | 1.12 (0.92,1.37) | .266 |
| Mono- and disaccharides (g) | 108.2 | 38.80 | 1.11 (0.89,1.37) | 0.94 (0.75,1.17) | .579 | 0.91 (0.74,1.11) | 0.84 (0.69,1.03) | .092 |
| Starch (g) | 120.3 | 52.75 | 1.06 (0.85,1.32) | 1.11 (0.90,1.38) | .327 | 0.99 (0.81,1.22) | 1.18 (0.96,1.44) | .106 |
| Soluble dietary fibre (g) | 5.233 | 1.685 | 0.90 (0.73,1.11) | 0.74 (0.60,0.92) | .006 | 0.79 (0.65,0.97) | 0.86 (0.70,1.05) | .126 |
| Total dietary fibre (g) | 24.80 | 8.029 | 0.83 (0.67,1.03) | 0.79 (0.64,0.98) | .033 | 0.93 (0.76,1.13) | 0.89 (0.72,1.08) | .236 |
| Proteins (g) | 87.70 | 24.55 | 0.99 (0.80,1.22) | 0.92 (0.74,1.14) | .429 | 1.02 (0.84,1.25) | 0.84 (0.68,1.03) | .095 |
| Total lipids (g) | 81.20 | 25.83 | 1.17 (0.94,1.45) | 1.03 (0.83,1.28) | .818 | 1.00 (0.82,1.22) | 0.98 (0.80,1.20) | .824 |
| Saturated fatty acids (g) | 32.28 | 12.02 | 0.99 (0.80,1.23) | 1.02 (0.82,1.26) | .886 | 0.93 (0.76,1.14) | 0.89 (0.73,1.09) | .269 |
| Mono-unsaturated fatty acids (g) | 28.54 | 9.740 | 1.16 (0.93,1.44) | 1.16 (0.93,1.44) | .199 | 1.06 (0.87,1.30) | 1.03 (0.84,1.26) | .798 |
| Polyunsaturated fatty acids (g) | 13.99 | 5.920 | 1.14 (0.92,1.42) | 1.04 (0.84,1.30) | .703 | 1.04 (0.85,1.27) | 1.06 (0.87,1.30) | .565 |
| n-6 fatty acids (g) | 12.55 | 5.614 | 1.11 (0.90,1.37) | 1.03 (0.83,1.28) | .794 | 1.04 (0.85,1.28) | 1.03 (0.84,1.26) | .768 |
| n-3 fatty acids (g) | 1.419 | 0.558 | 0.91 (0.74,1.13) | 0.79 (0.63,0.98) | .029 | 0.84 (0.69,1.03) | 0.94 (0.77,1.15) | .573 |
| α-linolenic fatty acids (g) | 0.926 | 0.354 | 0.95 (0.77,1.18) | 0.90 (0.73,1.12) | .341 | 0.99 (0.80,1.21) | 0.97 (0.79,1.18) | .756 |
| Long-chain n-3 fatty acids (g) | 0.488 | 0.345 | 0.95 (0.77,1.17) | 0.85 (0.68,1.05) | .134 | 0.85 (0.70,1.04) | 0.95 (0.78,1.16) | .596 |
| n-6/n-3 fatty acids ratio | 9.383 | 4.113 | 1.08 (0.87,1.34) | 1.25 (1.01,1.55) | .041 | 1.10 (0.90,1.35) | 1.09 (0.89,1.33) | .429 |
| β-carotene (μg) | 4188 | 1762 | 0.94 (0.76,1.16) | 1.02 (0.82,1.26) | .882 | 0.87 (0.71,1.06) | 0.86 (0.71,1.05) | .138 |
| Retinol (μg) | 1061 | 1061 | 1.46 (1.16,1.83) | 1.38 (1.10,1.72) | .007 | 0.94 (0.77,1.16) | 0.91 (0.74,1.11) | .349 |
| Vitamin B1 (mg) | 1.256 | 0.379 | 0.87 (0.70,1.08) | 0.93 (0.75,1.15) | .477 | 0.82 (0.67,1.00) | 0.96 (0.79,1.17) | .680 |
| Vitamin B2 (mg) | 2.142 | 0.706 | 1.02 (0.82,1.27) | 1.05 (0.85,1.30) | .649 | 0.86 (0.70,1.05) | 0.80 (0.65,0.98) | .028 |
| Vitamin B6 (mg) | 1.762 | 0.481 | 0.84 (0.68,1.04) | 0.82 (0.66,1.02) | .066 | 0.91 (0.75,1.11) | 0.80 (0.65,0.98) | .032 |
| Folic Acid (μg) | 401.4 | 117.0 | 1.18 (0.95,1.46) | 1.12 (0.90,1.39) | .305 | 0.87 (0.71,1.06) | 0.86 (0.71,1.05) | .140 |
| Vitamin B12 (μg) | 7.713 | 4.813 | 1.02 (0.82,1.26) | 1.05 (0.85,1.30) | .643 | 0.71 (0.58,0.87) | 0.79 (0.65,0.97) | .022 |
| Vitamin C (mg) | 144.3 | 61.76 | 0.98 (0.79,1.21) | 0.81 (0.66,1.01) | .065 | 0.85 (0.69,1.03) | 0.86 (0.71,1.05) | .134 |
| Vitamin D (μg) | 2.466 | 1.306 | 0.88 (0.71,1.09) | 0.89 (0.72,1.10) | .280 | 1.10 (0.90,1.35) | 1.02 (0.83,1.25) | .848 |
| Vitamin E (mg) | 13.61 | 5.663 | 1.11 (0.89,1.37) | 1.03 (0.83,1.28) | .806 | 1.18 (0.97,1.44) | 1.04 (0.85,1.28) | .709 |
| Calcium (mg) | 1026 | 403.6 | 1.26 (1.01,1.57) | 1.17 (0.94,1.46) | .174 | 0.97 (0.80,1.19) | 0.90 (0.74,1.11) | .320 |
| Iron (mg) | 13.41 | 3.701 | 0.97 (0.79,1.20) | 0.90 (0.72,1.12) | .338 | 1.06 (0.87,1.29) | 0.95 (0.78,1.17) | .652 |
| Magnesium (mg) | 392.2 | 122.7 | 0.89 (0.72,1.09) | 0.84 (0.68,1.04) | .105 | 1.04 (0.85,1.26) | 0.88 (0.72,1.08) | .228 |
| Phosphorus (mg) | 1360 | 406.5 | 0.99 (0.80,1.22) | 0.97 (0.78,1.21) | .793 | 0.95 (0.77,1.15) | 0.83 (0.68,1.02) | .077 |
| Manganese (mg) | 3.863 | 6.531 | 0.98 (0.77,1.24) | 1.15 (0.92,1.44) | .176 | 0.88 (0.71,1.09) | 1.02 (0.83,1.26) | .701 |
| Iodine (μg) | 145.5 | 46.87 | 1.17 (0.94,1.45) | 0.98 (0.78,1.21) | .819 | 1.04 (0.85,1.27) | 0.95 (0.78,1.17) | .632 |
OR, Odds Ratio; SD, Standard Deviation
Through multi-adjusted logistic regression models, taking healthy elderly as reference group. Adjustment on all covariates listed in Table 1. Each nutrient was considered separately
T1, T2, T3 for tertile 1, tertile 2 and tertile 3 of intake residuals on energy (except for alcohol: see *)
Mean(SD) calculated among whole sample
Except energy from alcohol
Because of the non-negligible percentage of abstainers and the specificity of this group, the population was split into abstainers (13.8%); 0 < mean intake ≤ 8.3 g/day (43.5%); mean intake > 8.3 g/day (42.7%)
Interactions, sensitivity analysis and alternate analysis
We verified that modification of cut-off points for adjustment variables had no effect on the final results. For example, the 80-year cut-off point was chosen for age for easier comparison, as it is a symbolic threshold, but use of 78 years (the median value), as the cut-off did not substantially modify the overall findings, nor did any sensitivity analysis using different cut-off values.
We tested potential interactions between dietary intakes and age or education level, but none yielded significant differential effects.
In order to test the stability of our results, we used a more restrictive definition of cognitive decline using a cut-off point of 31 rather than 33 for the DECO score, which resulted in 341 women with recent cognitive decline vs. 4,468 control women. Associations between informant-appraised cognitive status and dietary intake remained quite stable for most nutrients. However, the association with n-3 fatty acids was no longer statistically significant, even though this nutrient still displayed a similar inverse association with cognitive decline (OR [95% CI] = 0.93 [0.70–1.22] and 0.88 [0.67–1.15] for tertiles 2 and 3 respectively, taking the first tertile of intake as reference).
To rule out a possible confounding effect of dietary supplement use, we conducted our analyses in a restricted sample (n=3,347), excluding all women who declared taking at least one type of supplement. Associations involving lower intakes of vitamin C became statistically significant with higher odds of both cognitive decline and functional impairment. No other difference was found in this sub-sample compared to those obtained for the whole sample (data not tabulated).
Furthermore, we tested the stability of our results when including all women with available dietary data: women with extreme values for the energy intake/energy requirement ratio were reintegrated in the sample analysis (n=4,922). The set of significant associations remained unchanged in this larger sample. For example: regarding soluble dietary fibre and DECO score < 33 (n=622 cases in this larger sample): OR [95% CI] for the second and third tertiles of intake (versus the first tertile) were respectively 0.92 [0.75–1.13] and 0.76 [0.61–0.94], as compared to women with high DECO score.
DISCUSSION
In 2006, elderly women participating in the E3N cohort that were reported by informants to undergo recent cognitive decline had, 13 years previously, lower intake of poultry, fish, and animal fats, as well as higher intake of dairy desserts and ice-cream. They had lower habitual intake of dietary fibre and n-3 fatty acids, but higher intake of retinol. Furthermore, elderly women that were reported by informants to be functionally impaired had, in the past, lower intake of vegetables, vitamins B2, B6, and B12.
The main interest of our study lies in the time interval (more than a decade) between dietary and cognitive/functional assessment, which enabled us to explore the long-term effect of dietary habits in ageing. Although diet is likely to vary throughout life, our hypothesis is that it remains quite stable after menopause and retirement, but before advanced state of ageing processes. Because these women were 62–68 years old at the time of dietary assessment, nutritional data considered in the present study seem to be informative when relating long-term individual dietary habits to late life outcome. Moreover, the nutritional data of the E3N cohort have provided meaningful results when studying associations between usual dietary intake and cancer occurrence, a disease that may also have a latency period of many years before being diagnosed 6,18. Finally, the life-course approach to age-related disorders provided opportunities for identifying the nature and timing of environmental contributions 19, which is not possible in a short-term design study.
Another strength of our study lies in the availability of extensive adjustment data. Careful control for potential confounders is important in an observational setting so as to limit biases that may arise, since socioeconomic status and other behavioural and medical characteristics may influence both dietary intake and cognitive function. In this study, we were able to implement fully adjusted models. In particular, the association between dietary intake and cognitive decline remained significant after controlling for various vascular factors (hypertension, hypercholesterolemia, diabetes mellitus, coronary heart disease, and stroke), suggesting that diet may influence cognitive ageing through pathways that are partly separate from cardiovascular processes.
In this large epidemiological study, cognitive assessment was based on informant-self response to validated questionnaires (IADL and DECO) rather than face-to-face interviews. Substantial data support the validity of using both functional and informant measures in ageing studies 20. More precisely, DECO, which is independent of socioeconomic background 10, has been recently reported to be a good screening device for evaluating cognitive decline due to multiple causes 21. In addition, the 4-IADL score has proven to be a valid indicator of cognitive loss and is highly predictive of early dementia 11. Descriptive analyses showed relationships between our outcomes of interest and characteristics usually associated with cognitive/functional impairment (age, education level, history of stroke, etc.), thus supporting our definitions for testing hypotheses in the research field on age-related decline.
Outcome information analyzed in the present study was informant-reported, perhaps leading to some misclassification, but there is no reason to suspect differential errors.
Missing values in dietary data or in cognitive information may lead to a selection of the study sample. Indeed, exclusion of women with non-available dietary data resulted in overrepresentation of younger and more educated women. These observations are consistent with the hypothesis that non-responders are more likely to undergo adverse outcomes or to be less health-conscious 22. Such a selection may influence study results in two ways: by decreasing the statistical power and by masking some of the effects of nutritional intake, since the cognitive impact of some nutritional deficiencies may have been underestimated because of an insufficient range of exposure in the selected analysis sample. In particular, several epidemiologic studies found an association with fruit consumption, vitamin C, vitamin E or β-carotene intake, in agreement with the antioxidant hypothesis. In our sample, we observed only a borderline association between cognitive decline and lower vitamin C intake. The limited range of intake in our population, with few women having low consumption levels in foods rich in antioxidant components may explain the absence of certain associations in our study. For example, over 95 % of the elderly E3N participants consumed more than one 80g portion of fruit daily, with a median intake of 350 g/day. As a comparison, the fruit consumption level was quite lower in another French cohort showing a significant protective association involving fruit and dementia: in the “three-City cohort” study, more than 20% of the participants ate less than one daily serving of fruit 23.
Another limitation is that our sample includes only women, which precludes generalizing our results to men, who have on the whole different food intake and nutritional status 24. Our findings of significant associations between age-related decline and lower long-term intake of certain specific nutrients — namely n-3 fatty acids, dietary fibre and vitamin B6 — has already been described, generally in studies with shorter prospective design 25. However, our study is not in complete agreement with previous ones in that it shows that higher intake of retinol and lower intake of animal fats were both associated with cognitive decline. Potential explanations for these unexpected results include confounding effects and chance resulting from multiple analyses. Regarding residual confounding, a null intake of animal fats could be, for instance, an indicator of poor general health (similar to alcohol abstinence), thus explaining that a low intake of animal fats was related to an increased OR of cognitive decline. Moreover, in the studied sample, animal fats was 70% butter and intake level was generally low, with 95% of the studied women consuming less than 20g/day (median intake: 6g/day). As a comparison, 90% of the population included in the Chicago Health and Aging Project consumed more than 16g/day of animal fat 26. This latter study concluded that a diet high in saturated fat might be associated with cognitive decline among older persons. The intake range in the E3N population could thus be below the level where saturated fat becomes deleterious.
Regarding chance due to multiple analyses, we performed nearly 60 models for each outcome of interest. Nutritional epidemiology, especially when dealing with large scale studies such as ours, is restricted by both the fact that multiple analyses are performed (often leading to separate reports for each category of foods or nutrients), and that misclassification of the diet results in a decreased power of the studies 27. Thus very few, if any, use the Bonferroni correction, which would most often lead to no significant association at all. Restricting analyses to variables already described in the literature, such as vitamins and fatty acids, would limit the opportunity to explore novel hypotheses, although more emphasis should be made on findings consistent with the literature. New findings, such as the association with retinol, need to be further confirmed.
Several neuropathogenic mechanisms can be evoked as underlying biological processes for the observed associations. N-3 fatty acids, in which fish is rich, act on heart and brain not only through the vascular pathway but also through different cellular mechanisms: heart rhythm, neurotransmission, neuroprotection, neurogenesis. Similarly, some B vitamins and homocysteine can act directly on brain cell functioning. Anti-inflammatory and antioxidant properties are suggested to account for the inverse association with dietary fibre and vegetables. On the whole, a number of experimental works, and a variety of partly common mechanisms, support a favourable effect of these compounds.
Although underlying biological pathways are not yet fully elucidated, our study nonetheless has public health implications along with previous studies 28. Our results suggest that prevention of age-related impairment may be reached through a balanced diet rich in vegetables, fish and poultry, and limited in sweet dairy products, not only in later life but starting in middle age. These recommendations, also suggested for cancer 29 and cardiovascular protection 30, may enhance the quality of life 31.
Our large-scale longitudinal study with validated dietary data may represent a valuable contribution to a better understanding of the link between long-term nutrition and late-life cognition. Our results support the hypothesis that high intakes of n-3 fatty acids, dietary fibre and some B-group vitamins may contribute to a reduction in age-related impairment.
Acknowledgments
The authors report no conflict of interest. The E3N cohort is supported by the French League against Cancer, the European Community, the 3M Company, the “Mutuelle Générale de l’Education Nationale”, the French Institute of Health and Medical Research, the Gustave Roussy Institute and several general councils in France. MN Vercambre is on a grant from the Statlife Company and the “Association Nationale de la Recherche Technique”. None of the funding agencies played a role in the design or conducting of the study, analysis or interpretation of data, nor in preparation and approval of the manuscript. F Clavel-Chapelon is the principal investigator of the E3N Cohort Study and takes responsibility for the integrity of the data. MN Vercambre conducted statistical analysis, interpreted data and drafted the manuscript. MC Boutron Ruault, K Ritchie, F Clavel-Chapelon and C Berr contributed to the writing and revisions. C Berr supervised statistical analyses. All authors critically reviewed the manuscript.
Abbreviations used
- DECO
questionnaire “Détérioration Cognitive Observée”
- E3N
“Etude Epidémiologique de Femmes de la Mutuelle Générale de l’Education Nationale”
- IADL
Instrumental Activities of Daily Living
References
- 1.McGuire LC, Ford ES, Ajani UA. Cognitive functioning as a predictor of functional disability in later life. Am J Geriatr Psychiatry. 2006;14:36–42. doi: 10.1097/01.JGP.0000192502.10692.d6. [DOI] [PubMed] [Google Scholar]
- 2.Blennow K, de Leon MJ, Zetterberg H. Alzheimer’s disease. Lancet. 2006;368:387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 3.Gillette-Guyonnet S, AbellanVan Kan G, Andrieu S, et al. IANA task force on nutrition and cognitive decline with aging. J Nutr Health Aging. 2007;11:132–152. [PubMed] [Google Scholar]
- 4.Ortega RM, Requejo AM, Andres P, Lopez-Sobaler AM, Quintas ME, Redondo MR, Navia B, Rivas T. Dietary intake and cognitive function in a group of elderly people. Am J Clin Nutr. 1997;66:803–809. doi: 10.1093/ajcn/66.4.803. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez-Gross M, Marcos A, Pietrzik K. Nutrition and cognitive impairment in the elderly. Br J Nutr. 2001;86:313–321. doi: 10.1079/bjn2001388. [DOI] [PubMed] [Google Scholar]
- 6.Touillaud MS, Thiebaut AC, Fournier A, Niravong M, Boutron-Ruault MC, Clavel-Chapelon F. Dietary lignan intake and postmenopausal breast cancer risk by estrogen and progesterone receptor status. J Natl Cancer Inst. 2007;99:475–486. doi: 10.1093/jnci/djk096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katz S. Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31:721–727. doi: 10.1111/j.1532-5415.1983.tb03391.x. [DOI] [PubMed] [Google Scholar]
- 8.Ritchie K, Fuhrer R. A comparative study of the performance of screening tests for senile dementia using receiver operating characteristics analysis. J Clin Epidemiol. 1992;45:627–637. doi: 10.1016/0895-4356(92)90135-a. [DOI] [PubMed] [Google Scholar]
- 9.Ferrari P, Slimani N, Ciampi A, et al. Evaluation of under- and overreporting of energy intake in the 24-hour diet recalls in the European Prospective Investigation into Cancer and Nutrition (EPIC) Public Health Nutr. 2002;5:1329–1345. doi: 10.1079/PHN2002409. [DOI] [PubMed] [Google Scholar]
- 10.Ritchie K, Fuhrer R. The validation of an informant screening test for irreversible cognitive decline in the elderly: performance characteristics within a general population sample. Int J Geriatr Psychiatry. 1996;11:149–156. [Google Scholar]
- 11.Barberger-Gateau P, Fabrigoule C, Helmer C, Rouch I, Dartigues JF. Functional impairment in instrumental activities of daily living: an early clinical sign of dementia? J Am Geriatr Soc. 1999;47:456–462. doi: 10.1111/j.1532-5415.1999.tb07239.x. [DOI] [PubMed] [Google Scholar]
- 12.van Liere MJ, Lucas F, Clavel F, Slimani N, Villeminot S. Relative validity and reproducibility of a French dietary history questionnaire. Int J Epidemiol. 1997;26 (Suppl 1):S128–S136. doi: 10.1093/ije/26.suppl_1.s128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lucas F, Niravong M, Villeminot S, Kaaks R, Clavel-Chapelon F. Estimation of food portion size using photographs: validity, strength, weaknesses and recommendations. J Human Nutr Dietetics. 1995;8:65–74. [Google Scholar]
- 14.Favier JC, Ireland-Ripert J, Toque C, Feinberg M. Table de composition (Food composition table) CIQUAL-REGAL (French) INRA; AFSSA; CIQUAL; TEC & DOC; 1995. Répertoire général des aliments. [Google Scholar]
- 15.Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3:343–353. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- 16.Silverman BW. Density estimation. Chapman and Hall; London: 1986. [Google Scholar]
- 17.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S–1228S. doi: 10.1093/ajcn/65.4.1220S. [DOI] [PubMed] [Google Scholar]
- 18.Touvier M, Kesse E, Clavel-Chapelon F, Boutron-Ruault MC. Dual Association of beta-carotene with risk of tobacco-related cancers in a cohort of French women. J Natl Cancer Inst. 2005;97:1338–1344. doi: 10.1093/jnci/dji276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whalley LJ, Dick FD, McNeill G. A life-course approach to the aetiology of late-onset dementias. Lancet Neurol. 2006;5:87–96. doi: 10.1016/S1474-4422(05)70286-6. [DOI] [PubMed] [Google Scholar]
- 20.Tierney MC, Herrmann N, Geslani DM, Szalai JP. Contribution of informant and patient ratings to the accuracy of the mini-mental state examination in predicting probable Alzheimer’s disease. J Am Geriatr Soc. 2003;51:813–818. doi: 10.1046/j.1365-2389.2003.51262.x. [DOI] [PubMed] [Google Scholar]
- 21.Cullen B, O’Neill B, Evans JJ, Coen RF, Lawlor BA. A review of screening tests for cognitive impairment. J Neurol Neurosurg Psychiatry. 2007;78:790–799. doi: 10.1136/jnnp.2006.095414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldberg M, Chastang JF, Zins M, Niedhammer I, Leclerc A. Health problems were the strongest predictors of attrition during follow-up of the GAZEL cohort. J Clin Epidemiol. 2006;59:1213–1221. doi: 10.1016/j.jclinepi.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 23.Barberger-Gateau P, Raffaitin C, Letenneur L, Berr C, Tzourio C, Dartigues JF, Alperovitch A. Dietary patterns and risk of dementia: the Three-City cohort study. Neurology. 2007;69:1921–1930. doi: 10.1212/01.wnl.0000278116.37320.52. [DOI] [PubMed] [Google Scholar]
- 24.Hercberg S, Czernichow S, Galan P. Antioxidant vitamins and minerals in prevention of cancers: lessons from the SU. VI.MAX study. Br J Nutr. 2006;96 (Suppl 1):S28–S30. doi: 10.1079/bjn20061695. [DOI] [PubMed] [Google Scholar]
- 25.Luchsinger JA, Mayeux R. Dietary factors and Alzheimer’s disease. Lancet Neurol. 2004;3:579–587. doi: 10.1016/S1474-4422(04)00878-6. [DOI] [PubMed] [Google Scholar]
- 26.Morris MC, Evans DA, Bienias JL, Tangney CC, Wilson RS. Dietary fat intake and 6-year cognitive change in an older biracial community population. Neurology. 2004;62:1573–1579. doi: 10.1212/01.wnl.0000123250.82849.b6. [DOI] [PubMed] [Google Scholar]
- 27.Willett W. Nutritional Epidemiology. Oxford University Press; New York, Oxford: 1998. [Google Scholar]
- 28.Luchsinger JA, Noble JM, Scarmeas N. Diet and Alzheimer’s disease. Curr Neurol Neurosci Rep. 2007;7:366–372. doi: 10.1007/s11910-007-0057-8. [DOI] [PubMed] [Google Scholar]
- 29.World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity and the Prevention of Cancer: a Global Perspective. AICR; Washington DC: 2007. [Google Scholar]
- 30.De Caterina R, Zampolli A, Del Turco S, Madonna R, Massaro M. Nutritional mechanisms that influence cardiovascular disease. Am J Clin Nutr. 2006;83:421S–426S. doi: 10.1093/ajcn/83.2.421S. [DOI] [PubMed] [Google Scholar]
- 31.Kennedy ET. Evidence for nutritional benefits in prolonging wellness. Am J Clin Nutr. 2006;83:410S–414S. doi: 10.1093/ajcn/83.2.410S. [DOI] [PubMed] [Google Scholar]


