Abstract
Transgenic (TG) female mice expressing bLHβ-CTP (a chimeric protein derived from the β-subunit of bovine luteinizing hormone [LH] and a fragment of the β-subunit of human chorionic gonadotropin [hCG]) exhibit elevated serum LH, infertility, polycystic ovaries, and ovarian tumors. In humans, increased LH secretion also occurs in infertility and polycystic ovarian syndrome, often concomitant with adrenocortical dysfunction. We therefore investigated adrenal function in LH overexpressing bLHβ-CTP female mice. The size of their adrenals was increased by 80% with histological signs of cortical stimulation. Furthermore, adrenal steroid production was increased, with up to 14-fold elevated serum corticosterone. Primary adrenal cells from TG and control females responded similarly to ACTH stimulation, but, surprisingly, the TG adrenals responded to hCG with significantly increased cAMP, progesterone, and corticosterone production. LH receptor (LHR) expression and activity were also elevated in adrenals from female TG mice, but gonadectomized TG females showed no increase in corticosterone, suggesting that the dysfunctional ovaries of the intact TG females promote adrenocortical hyperfunction. We suggest that, in intact TG females, enhanced ovarian estrogen synthesis causes increased secretion of prolactin (PRL), which elevates LHR expression. Chronically elevated serum LH, augmented by enhanced PRL production, induces functional LHR expression in mouse adrenal cortex, leading to elevated, LH-dependent, corticosterone production. Thus, besides polycystic ovaries, the bLHβ-CTP mice provide a useful model for studying human disorders related to elevated LH secretion and adrenocortical hyperfunction.
Introduction
Women with polycystic ovary syndrome (PCOS) typically present with hirsutism, chronic anovulation, infertility, polycystic ovaries, and elevated serum concentrations of luteinizing hormone (LH) and androgens (1). Although the majority of androgens in this syndrome are secreted from the ovaries, in approximately half of the cases there is also excessive production of adrenal androgens (2). Interestingly, several investigators have demonstrated an increased adrenal response to adrenocorticotropin (ACTH) stimulation in PCOS (3–5), despite normal serum ACTH levels (6). However, the underlying cause of adrenocortical disturbances in PCOS still remains unclear.
The primary tropic regulator of glucocorticoid production in the adrenal cortex is ACTH (7). Unlike the human adrenal gland, which produces both cortisol and corticosterone, the latter steroid is the only glucocorticoid produced in the mouse (8), and the mouse adrenal gland does not produce androgen precursors such as dehydroepiandrosterone (DHEA), its sulfate (DHEA-S), or androstenedione (9). Besides ACTH, other hormones, including LH, prolactin (PRL), and IGF-1, have also been implicated in the regulation of adrenal androgen production (7). Whether the adrenal gland expresses the LH receptor (LHR) remains controversial. Recently, it was shown that human adrenals express the LHR gene in the zona fasciculata and zona reticularis (10). There is also circumstantial evidence of LH effects on adrenal function. For example, the adrenal androgen production that begins to increase during adrenarche, reaches adult levels during puberty without a concomitant increase in ACTH (11), and very recently, a case of postmenopausal Cushing’s syndrome was described, in which the adrenocortical hyperfunction was found to be LH dependent and responsive to treatment with gonadotropin-releasing hormone agonist (12). Likewise, a significant proportion of women with chronic anovulation have elevated serum levels of LH and DHEA-S, but normal levels of ACTH (13). The LH agonist, human chorionic gonadotropin (hCG) has been shown to stimulate DHEA-S secretion in the human fetal adrenal gland during early pregnancy (14), although the results of other studies suggest that gonadotropins do not have a direct effect on adrenal androgen secretion (15). Further studies are therefore needed to elucidate the role of LH in adrenal function.
In the present study, we evaluated adrenal gland function and LHR expression using a TG mouse model with chronically elevated serum LH concentrations, namely, bLHβ-CTP mice (16). These mice have previously been found to develop polycystic ovaries, with elevated serum androgen and estrogen levels, as well as ovarian cysts and tumors. In the present study, we present evidence of a novel mechanism behind the altered adrenocortical function in bLHβ-CTP mice that results surprisingly in elevated glucocorticoid production.
Methods
Experimental animals and treatments.
The TG mice used harbor a bovine LH α-subunit gene, fused to DNA encoding the COOH-terminal peptide extension of the human chorionic gonadotropin β-subunit (bLH β-CTP). The transgene is expressed under control of the bovine LH α-subunit promoter (16). Male bLH β-CTP mice with a CD-1 genetic background were bred with C57BL/6 female mice, and the experiments were carried out using F1 to F3 generations of these crosses. Three to 9 mice were used in each experiment, with non-TG, sex-matched littermates as controls. The mice were sacrificed in the morning (0730–0830 hours) by cervical dislocation within 30 seconds of touching the cage. Genotyping was accomplished using a PCR method of tail DNA, as described previously (16). Gonadectomies of male and female mice were carried out at 3–4 weeks of age. Adrenalectomy was carried out with 5-month-old mice, and after the operation, 0.9% NaCl was given as drinking solution. One day before adrenalectomy, a capillary blood sample was taken by puncturing the lateral vein of a hind leg within 1 minute of touching the cage. Three days after adrenalectomy, the mice were sacrificed as already described here, and a blood sample was taken at the same time of day as the previous capillary blood sample. The University of Turku Ethics Committee on Use and Care of Animals approved all procedures using mice.
Assay of LH, PRL, steroids, and cAMP.
Serum LH concentrations in non-TG mice were measured using an immunofluorometric assay (IFMA; Wallac Oy, Turku, Finland), as described previously (17). Serum LH concentrations in bLHβ-CTP mice, measured using a different RIA method (18), were taken from earlier reports (16, 19, 20). PRL concentrations were measured by RIA, as described earlier (21), using a rat PRL antibody and rat PRL reference preparation (RP-3) provided by the National Institute of Diabetes, Digestive and Kidney Diseases (National Institutes of Health, Bethesda, Maryland, USA). Corticosterone concentrations were measured, following dichloromethane extraction, using an RIA with a polyclonal rabbit antiserum against corticosterone (kindly donated by R. Hampl, University of Prague, Czech Republic) and [1,2,6,7-3H]-corticosterone (Amersham International plc, Aylesbury, Bucks, United Kingdom) as tracer. Progesterone and extracellular cAMP concentrations in cell culture media were measured by RIAs as described previously (22, 23).
LH receptor binding assay.
Highly purified human chorionic gonadotropin (hCG; NIH CR-121; 11500 IU/mg; National Institutes of Health) was iodinated with Na-[125I]iodide (Amersham International plc) using a solid-phase lactoperoxidase method to a specific activity of about 20 Ci/g (24). LH radioreceptor assay was performed according to the method of Catt et al. (25). Briefly, for each specimen, 2 adrenal glands were homogenized in 700 μL of Dulbecco’s PBS + 0.1% BSA (D-PBS). Thereafter, 100-μL aliquots of the crude adrenal gland homogenate were incubated with [125I]iodo-hCG (100,000 cpm/tube) in the presence (nonspecific binding) or absence (total binding) of 50 IU of nonradioactive hCG (Pregnyl; Organon, Oss, The Netherlands). After overnight incubation at room temperature, the homogenates were washed with 4 mL ice-cold D-PBS and centrifuged at 2,000 g for 20 minutes. Supernatants were discarded, and the pellets were counted in a γ-spectrometer. Protein concentrations in homogenates were measured using the Bradford method (26), and specific hCG binding was expressed as counts per minute per milligram of protein.
Primary cell culture.
Adrenal capsules were removed, and the parenchymal tissue was cut into small pieces in culture medium (DMEM/F12 [1:1]; Life Technologies, GIBCO BRL, Glasgow, Scotland), supplemented with 10% heat-inactivated FCS (Bioclear, Berks, United Kingdom; glucose 4.5 g/L, HEPES 20 mM, and gentamycin 0.1 g/L [Biological Industries, Bet-HaEmek, Israel]). The tissues were incubated in a shaking water bath at 37°C for 1 hour with collagenase 2 g/L (Sigma Chemical Co., St. Louis, Missouri, USA). The cells were then centrifuged at 200 g for 10 minutes, washed with PBS, and plated on 24-well plates at a density of 100,000 cells per well in the culture medium. After incubation for 24 hours, the cells were washed with PBS and the medium was replaced with culture medium containing 0.2 mM 3-isobutyl-1-methylxanthine (IBMX; Aldrich-Chemie, Steinheim, Germany) and 10 nM ACTH (Sigma Chemical Co.) and/or hCG (NIH CR-121; 1 or 100 μg/L). For cAMP measurements, media were collected after 1 hour, and for steroid measurements (progesterone and corticosterone) after 3 hours of incubation.
For ovarian cell culture, the ovaries were dissected out and minced with a razor blade. The cells were then dissociated as already described here for adrenal cells, except that after collagenase treatment, the dispersed tissue was filtered through a 60-μm nylon mesh. The cells were plated on 24-well plates at a density of approximately 200,000 cells per well and cultured overnight in the culture medium (see earlier here). The cells were then hormone stimulated as already described here.
RT-PCR and Southern hybridization.
One microgram of total RNA was reverse-transcribed, using the avian myeloma virus (AMV) RT (Promega Corp., Madison, Wisconsin, USA), and the resulting cDNAs were PCR-amplified as described earlier (27). The RT and PCR reactions were carried out sequentially in the same assay tube. First the RT reaction was carried out (50°C for 10 minutes), followed by a denaturation period of 3 minutes at 97°C. Thereafter, a PCR reaction with 40 cycles (96°C for 1.5 minutes, 57°C for 1.5 minutes, and 72°C for 3 minutes, with a final extension period of 10 minutes at 72°C) was performed. The sense primer corresponded to nucleotides 176-195 (5′-CTCTCACCTATCTCCCTGTC-3′), and the antisense primer, to nucleotides 878–858 (5′-TCTTTCTTCGGCAAATTCCTG-3′) of mouse LHR cDNA. As control for RNA quality, a 395-bp fragment of the L19 ribosomal protein gene was coamplified with each sample (sense primer, 5′-GAAATCGCCAATGCCAACTC-3′, antisense primer, 5′-TCTTAGACCTGCGAGCCTCA-3′). As a negative control, kidney RNA was used. After RT-PCR, 20-μL aliquots of the reaction mixtures were loaded on 1% agarose gel containing ethidium bromide (0.4 mg/L), to identify the amplified DNA fragments. Southern hybridization was used, according to standard techniques, to confirm the specificity of these PCR products. Hybridization was carried out with the 5′-end labeled oligonucleotide 5′-TGGAGAAGATGCACAGTGGA-3′, corresponding to nucleotides 641–660 of LH receptor (LHR) cDNA. The membrane was washed according to the manufacturer’s instructions, then exposed to Kodak X-ray film (XAR 5; Eastman Kodak, Rochester, New York, USA).
Northern hybridization.
Total RNA was isolated from tissues using a single-step acid guanidinium thiocyanate-phenol-chloroform extraction method, as described previously (28). Twenty micrograms of total RNA were resolved on a 1% denaturing agarose gel and transferred onto Hybond-N nylon membranes (Amersham International). The membranes were hybridized with [32P]-labeled cRNA, corresponding to nucleotides 441–881 of rat LHR cDNA, using standard techniques. Hybridization was viewed by autoradiography using Kodak film.
Histological staining and in situ hybridization.
Tissues were isolated and snap-frozen in liquid nitrogen or were immediately fixed in 4% paraformaldehyde (PFA). For histological evaluation, tissues were dehydrated, embedded in paraffin, and sectioned. Sections were dewaxed by incubating in xylene and used for in situ hybridization or cytochemistry. For in situ hybridization, PFA-fixed sections (5 μm) of adrenal glands were used. The sequence of the LHR-probe was the same as used in Northern blot analysis, and it was labeled with [35S]-UTP (>1,000 Ci/mmol; Amersham International). Sections were hybridized with the probe overnight at 50°C. Thereafter, hybridized sections were washed first in 2× SSC, 50% formamide, and 10 mM DTT (Boehringer Mannheim, Ingelheim, Germany) at 50°C for 30 minutes, and then in 0.2× SSC, 50% formamide, 10 mM DTT at 50°C for 10 minutes. Sections were rinsed in PBS and treated with Ribonuclease A 10 g/L (Boehringer Mannheim) in 10 mM Tris-5mM EDTA, 0.5 M NaCl (pH 8), at 37°C for 30 minutes. After RNase treatment, the sections were further washed in 2× SSC, 50% formamide, 10 mM DTT, rinsed in 2× SSC, and finally dehydrated in ethanol. The slides were then dipped into liquid autoradiography emulsion (NTB-3; Eastman Kodak) and exposed at 4°C for 2 weeks. After developing at 12°C with D-19 solution (Eastman Kodak) for 2.5 minutes, the slides were fixed for 5 minutes in Unifix (Eastman Kodak) and finally rinsed for 5 minutes in distilled water. Nuclei were then fluorescently stained with Hoechst 33258 (Sigma Chemical Co.), after which the slides were mounted with glycergel (DAKO A/S, Glostrup, Denmark).
Statistical analysis.
The Statview program (Windows version 4.57; Abacus Concepts Inc., Berkeley, California, USA) was used for ANOVA and for Fisher’s Protected LSD post hoc tests. Significance was set at P < 0.05. The values are presented as mean ± SEM.
Results
Adrenal gland morphology is altered in bLHβ-CTP female mice.
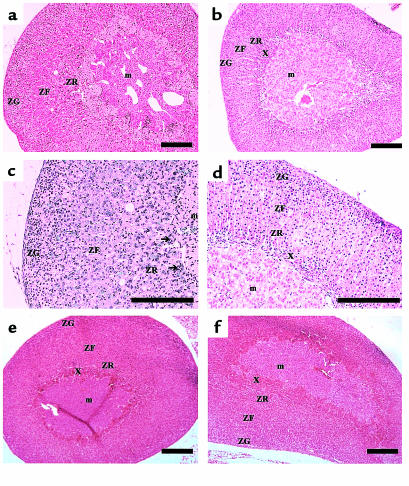
To assess the potential impact of chronically elevated LH on adrenal physiology, we first examined adrenal gland morphology in the bLHβ-CTP mice. Adrenal glands of the TG females weighed significantly more than those of non-TG female littermates used as controls (6.0 ± 0.86 mg [n = 6] vs. 3.3 ± 0.63 mg [n = 6]; P < 0.001). Histologically, the adrenal cortex of the TG females showed signs of stimulation, with widening and centripetal extension of lipid-depleted cells into the zona reticularis (Figure 1). Between the medulla and cortex, there were significant numbers of multinucleated cells, possibly macrophages, some showing nuclear degeneration. There were also foci of acute and chronic inflammatory cells (Figure 1). Such changes were not found in the bLHβ-CTP males, with no elevation of serum LH concentrations (data not shown). At the age of 1 month, a typical area of the X-zone was clearly visible in both bLHβ-CTP female and control female adrenals (Figure 1, e and f). However, in 5-month-old bLHβ-CTP female mice, the typical X-zone had disappeared, whereas, as expected, a narrow X-zone was still visible in non-TG littermate controls (Figure 1, a–d).
Figure 1.
Adrenal gland morphology is altered in bLHβ-CTP female mice. Histological appearance of the adrenal glands of 5-month-old female bLHβ-CTP (a, c) and non-TG littermate (b, d) mice. In a and c, signs of cortical stimulation, with increased cortical width, and centripetal extension of lipid-depleted cells widening the zona reticularis can be seen. The arrows indicate multinucleated cells. (e) Adrenal gland of a 1-month-old bLHβ-CTP female mouse, and (f) of a non-TG littermate control. In panels b, d, e, and f, the X-zone can be seen between the medulla and cortex. ZG, zona glomerulosa; ZF, zona fasciculata; ZR, zona reticularis; X, X-zone; m, medulla. Bars in a–f, 20 μm.
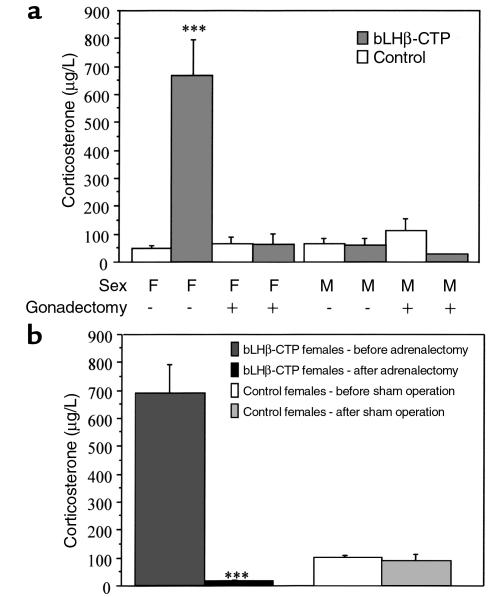
bLHβ-CTP female mice have elevated serum corticosterone levels.
To determine whether the altered morphology of the adrenal gland is related to changes in steroidogenesis, serum corticosterone levels were measured. Highly elevated (on average, 14-fold) morning concentrations of serum corticosterone were detected in the bLHβ-CTP female mice (Figure 2a). In contrast, the values measured in TG males were identical to those of the non-TG controls, suggesting that increased adrenal corticosterone production was associated with the high serum LH. To study whether high LH alone was able to increase corticosterone secretion, we analyzed serum corticosterone concentrations in intact and gonadectomized, non-TG, and TG bLHβ-CTP mice (Figure 2a). Interestingly, elevated corticosterone concentrations were only detected in the intact bLHβ-CTP females, and if the females were gonadectomized, serum corticosterone concentrations decreased to levels similar to those in non-TG controls. Furthermore, gonadectomy of non-TG mice, leading to highly elevated circulating concentrations of LH (Table 1), did not increase corticosterone levels in either sex. Thus, the data suggest that the polycystic ovaries present in bLHβ-CTP female mice have a central role in adrenal hyperfunction and that the adrenal phenotype is not alone due to chronically elevated LH.
Figure 2.
bLHβ-CTP female mice have elevated serum corticosterone levels, and the main source of corticosterone is the adrenal gland. (a) Morning levels of serum corticosterone in gonadectomized (+) and nongonadectomized (–) 5-month-old bLHβ-CTP and non-TG control male (M) and female (F) mice. In bLHβ-CTP female mice, serum corticosterone concentrations were 14-fold higher than in non-TG controls (***P < 0.001; n = 3–6 mice per group). (b) After adrenalectomy, serum corticosterone concentrations in 5-month-old bLHβ-CTP females decreased to nearly undetectable levels, being dramatically lower than those found before adrenalectomy (***P < 0.001). In non-TG sham-operated female mice, no difference was seen (n = 3–5 mice per group).
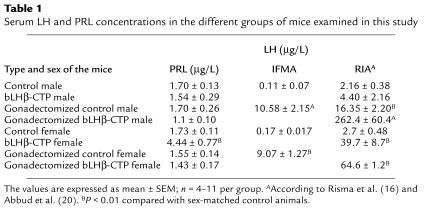
Table 1.
Serum LH and PRL concentrations in the different groups of mice examined in this study
The elevated concentration of serum corticosterone in bLHβ-CTP females is produced by the adrenal gland.
To identify the source of elevated serum corticosterone, we adrenalectomized a group of bLHβ-CTP female mice (Figure 2b). After adrenalectomy, corticosterone rapidly decreased to a nearly undetectable level, suggesting that the adrenal gland was responsible for the elevated corticosterone production. This was further confirmed by showing that in primary cultures of ovarian cells from bLHβ-CTP mice, no corticosterone production was found after stimulation with either hCG or ACTH. In addition, in the ovarian cells there was no increase in the cAMP response to ACTH, indicating the absence of functional ACTH receptors in these cells. As a positive control, cultured ovarian cells stimulated with hCG displayed clear cAMP and progesterone responses (data not shown). The results suggest that the adrenal gland is the major source of elevated corticosterone concentrations in bLHβ-CTP female mice.
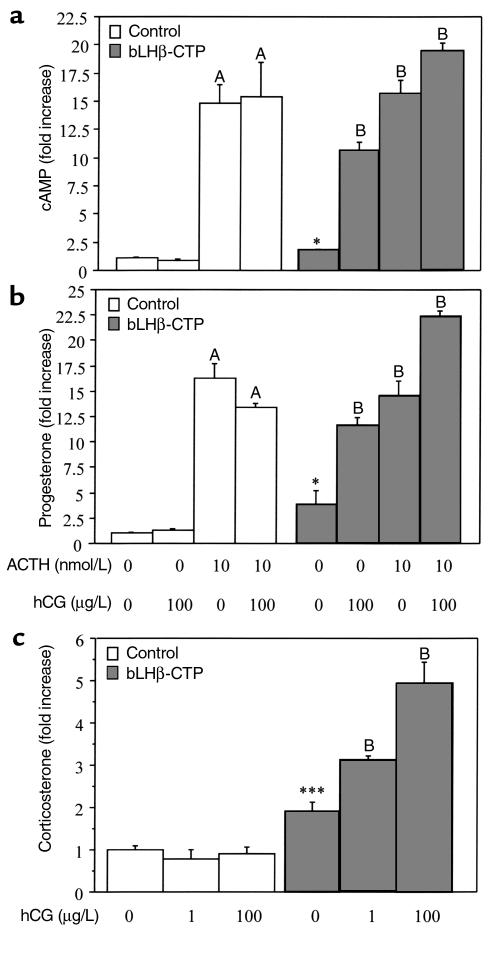
Cultured adrenal cells of bLHβ-CTP female mice have elevated steroidogenic capacity.
To assess further the steroidogenic capacity of adrenal glands of 3-month-old bLHβ-CTP females, dispersed adrenal cells were cultured and compared with those obtained from age-matched non-TG mice. Responsiveness to a high dose of ACTH (10 nM) was similar in cells of bLHβ-CTP females and controls (Figure 3, a and b). However, in basal culture conditions, the cells derived from bLHβ-CTP female adrenals showed 1.8-, 3.8-, and 1.8-fold higher production of cAMP, progesterone, and corticosterone, respectively, compared with cells from non-TG female littermates (Figure 3, a–c). In addition, treating the cells with LH agonist (hCG 100 μg/L) resulted in 5.9-, 3-, and 2.6-fold increases in cAMP, progesterone, and corticosterone production, respectively (Figure 3, a–c), whereas no change was seen with cells from the control mice. Furthermore, combined stimulation with ACTH and hCG resulted in greater responses than with either hormone alone. These data therefore indicate that in addition to ACTH, hCG stimulates adrenal corticosterone production in bLHβ-CTP female mice. The hCG-dependent corticosterone production was further shown to be dose dependent (Figure 3c).
Figure 3.
Adrenal cells of bLHβ-CTP females respond to hCG and ACTH stimulation in vitro, whereas cells from control mice respond to ACTH only. The data show the effects of ACTH (10 nM) and hCG (1 and 100 μg/L) on cAMP (a), progesterone (b), and corticosterone (c) production in primary cultured adrenal cells, isolated from 3-month-old control and bLHβ-CTP female mice. Samples for cAMP assay were taken after 1 hour, and samples for steroid assays after 3 hours of stimulation with ACTH and/or hCG. *P < 0.05 and ***P < 0.001 compared with basal production in non-TG control cells. A, P < 0.001 compared with basal production in non-TG control cells; B, P < 0.001 compared with basal production in bLHβ-CTP cells.
We also analyzed the effect of hCG on adrenal cells from intact male bLHβ-CTP mice, as well as on cells from gonadectomized bLHβ-CTP and non-TG males and females. As expected, cAMP, progesterone, and corticosterone production did not respond to hCG treatment in any of these cells (data not shown). These results are consistent with the normal serum corticosterone concentrations found in these groups of mice (Figure 2). Hence, the elevated serum corticosterone concentrations observed in vivo correlated with the hCG induced corticosterone production by adrenal cells in vitro. No androstenedione or testosterone production was found in any of the adrenal cell incubations (data not shown).
Increased steroidogenic capacity is associated with induction of LHR in the adrenal gland.
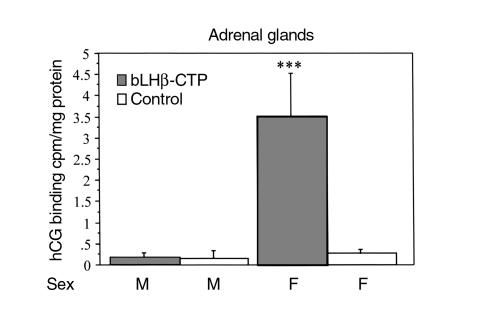
To determine whether hCG-stimulated corticosterone production involved ectopic expression of LHR, we examined receptor binding and mRNA expression in the adrenals of bLHβ-CTP and control mice. In the 3-month-old female bLHβ-CTP mice, hCG-induced corticosterone production of the adrenal cells was associated with very clear specific hCG binding. In the non-TG females, as well as in all male adrenals, no specific hCG binding was observed (Figure 4). The specific hCG binding of adrenal homogenates from the bLHβ-CTP female mice was similar in amount and affinity to that observed in mouse testes, used as positive control (data not shown).
Figure 4.
Adrenal gland homogenates from 3-month-old female bLHβ-CTP mice specifically bind [125I]iodo-hCG (n = 3 or 4 mice per group), whereas no specific binding was detected in TG male (M), non-TG control male or female (F) mice. ***P < 0.001 versus other groups.
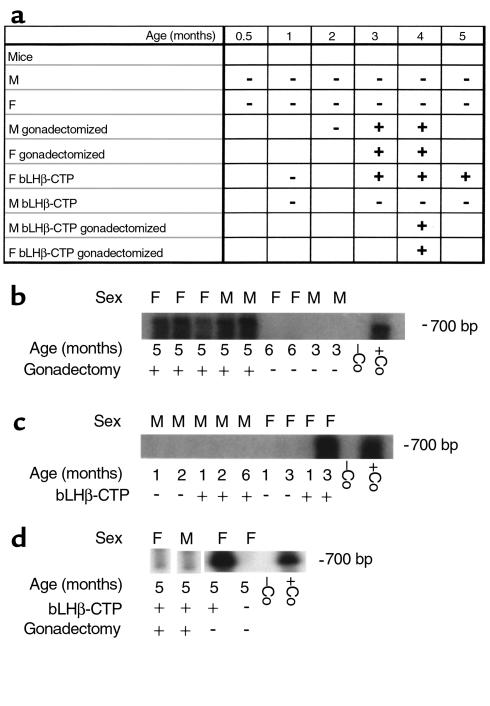
To assess further LHR expression in mouse adrenal glands, RT-PCR analysis of LHR mRNA was performed in several mouse strains at different ages. However, none of the mouse strains studied (C57BL/6, DBA, FVB/N) displayed any LHR specific amplicons at any age, ranging from prepuberty (19 days) to 10 months. This indicates the absence of significant LHR gene expression in normal mouse adrenal glands and the fact that there is no difference between genetic backgrounds in this respect. However, long-term elevation of serum LH concentrations was associated with the presence of LHR mRNA in the adrenal glands, as was confirmed in both intact bLHβ-CTP females and in gonadectomized non-TG mice (Figure 5a). Gonadectomy in non-TG male and female mice resulted in increased serum LH concentration (Table 1), and consequently, in adrenal LHR mRNA expression between 1 and 4 months after gonadectomy (Figure 5, a and b). One month after gonadectomy, no LHR mRNA was detected, but at 5 months of age, expression was detected in all specimens analyzed. Similarly, in the female bLHβ-CTP mice, with high constitutive LH production (Table 1), LHR mRNA was absent at 1 month of age, but expressed at the age of 3 months (Figure 5, c and d).
Figure 5.
LHR mRNA expression in mouse adrenal glands. (a) Table of RT-PCR results in gonadectomized and nongonadectomized, non-TG, and bLHβ-CTP TG female (F) and male (M) mice at different ages. A plus sign indicates amplification of LHR mRNA detected; a minus sign indicates no amplification detected. (b) Representative RT-PCR/Southern blot results from gonadectomized and nongonadectomized C57BL/6 mice of different ages and sex, (c) from female bLHβ-CTP and control mice at different ages, and (d) from 5-month-old gonadectomized male and female bLHβ-CTP mice, intact bLHβ-CTP female mice and control females. In panels b–d, kidney tissue was used as a negative control (–Co) and testis as a positive control (+Co).
Because of a sex difference in regulation of the transgene, TG males do not overexpress LH (16) (Table 1), and consequently LHR mRNA was not detected by RT-PCR in any of the TG male adrenal glands analyzed (1–6 months of age; Figure 5, a and c). However, in gonadectomized bLHβ-CTP males, amplification of adrenal LHR mRNA was detected by RT-PCR at a very low level, in contrast to intact bLHβ-CTP males (Figure 5d). This suggests that although a high LH concentration alone can stimulate the appearance of a low level of LHR expression in the adrenal gland, functionally significant LHR expression, with clearly detectable hCG binding and hCG-stimulated corticosterone production (Figure 2), is dependent on ovarian function in bLHβ-CTP mice.
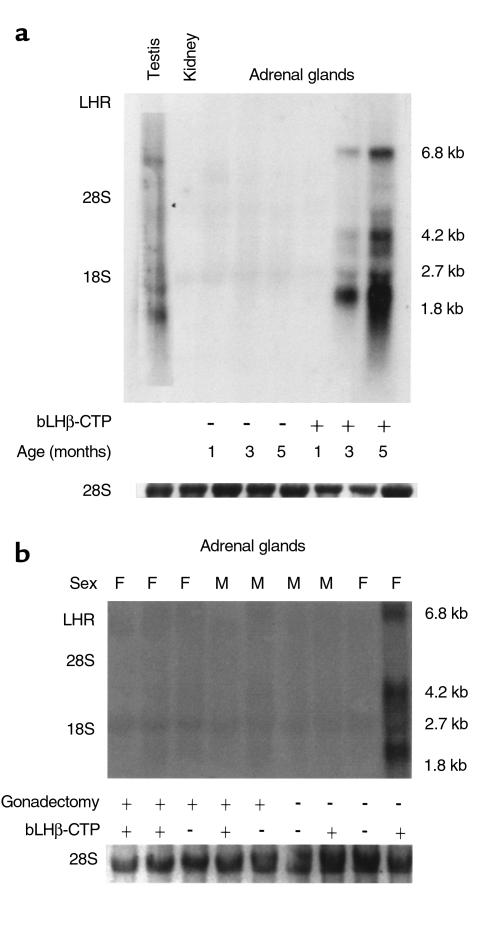
A more quantitative picture of LHR mRNA expression in mouse adrenal glands was obtained by Northern hybridization (Figure 6). Only a very weak hybridization signal was found in gonadectomized mice, compared with intact bLHβ-CTP females. This is consistent with data showing that only the cells obtained from intact bLHβ-CTP females responded to hCG in vitro. The time course of appearance of the LHR mRNA splice variants in mouse adrenal glands was also assessed by Northern hybridization (Figure 6a). As in RT-PCR, upregulation of LHR mRNA expression occurred between the ages of 1 and 3 months in intact female bLHβ-CTP mice. Three abundant (1.8, 4.2, and 6.8 kb) and 2 less-abundant (2.7 and 3.5 kb) LHR transcripts were identified in the adrenal specimens. Thus, the sizes of the main transcripts were similar to those expressed in mouse testis.
Figure 6.
Age-dependent upregulation of LHR mRNA expression occurs in bLHβ-CTP female mice, but not in control or gonadectomized TG or non-TG mice. Northern hybridization analysis for LHR mRNA was carried out using total RNA (20 μg/lane) isolated from adrenal glands of nontransgenic and bLHβ-CTP female mice, at ages 1, 3, and 5 months. RNAs from kidney and testis were used as negative and positive controls, respectively. The molecular sizes of the major LHR mRNA transcripts, as well as the location of the 28 S and 18 S ribosomal RNAs, are indicated. (a) In 3- and 5-month-old bLHβ-CTP female adrenals, a clear hybridization signal with LHR cRNA probe can be seen. (b) Hybridization between LHR cRNA probe and adrenal gland RNA from 5-month-old gonadectomized and nongonadectomized bLHβ-CTP and non-TG mice. (Below a and b, ethidium bromide staining of 28 S ribosomal RNA, used as loading control, is shown.)
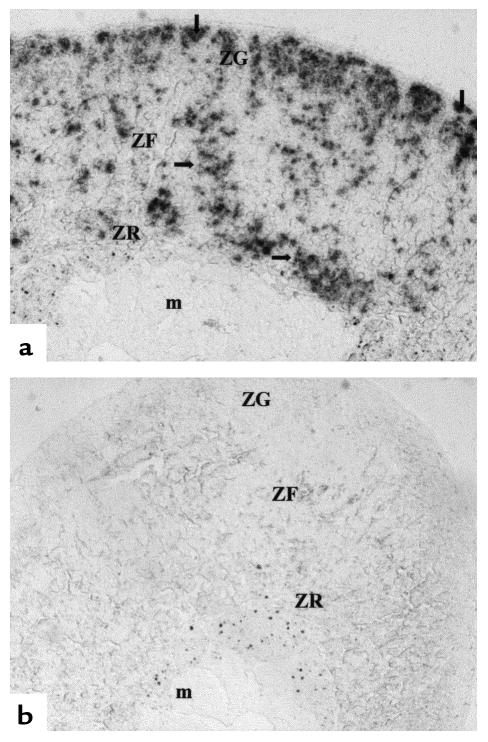
To localize LHR mRNA in the adrenal glands of bLHβ-CTP females, in situ hybridization was performed. An intense hybridization signal with LHR antisense probe was observed across the whole cortex, including the zona glomerulosa, zona fasciculata, and zona reticularis (Figure 7).
Figure 7.
Localization of LHR mRNA in a microscopic section of the adrenal gland of a bLHβ-CTP female mouse, using in situ hybridization. (a) Section hybridized with LHR antisense probe. (b) Section hybridized with LHR sense probe as a control. The arrows indicate areas of specific hybridization over the whole cortex. m, medulla; ZG, zona glomerulosa; ZF, zona fasciculata; ZR, zona reticularis.
bLHβ-CTP females have elevated serum PRL levels.
Previous studies have shown that bLHβ-CTP females have 2- to 3-fold elevated levels of circulating estradiol (16). As estrogens have been shown to increase PRL secretion, and this pituitary hormone plays an important role in induction of LHR expression in rodents (29, 30), we compared serum PRL levels in the different mouse groups of this study. Interestingly, female bLHβ-CTP mice had elevated PRL levels, and after gonadectomy, these were suppressed to those of the non-TG controls (Table 1).
Serum LH concentrations.
Serum LH concentrations in the mice used in this study are presented in Table 1. Those of the bLHβ-CTP mice, measured by RIA (see Methods), were taken, for the sake of comparison, from earlier reports on this TG mouse model (16, 20). In general, the bLHβ-CTP females had highly elevated (up to 15-fold) serum LH concentrations, which were even higher after gonadectomy, whereas intact TG males had normal LH levels. As shown previously (17), the basal LH levels measured by IFMA were clearly lower than those revealed by RIA, and thus the relative fold-increase after gonadectomy is greater when measured by IFMA than by RIA.
Discussion
Our data show that female bLHβ-CTP TG mice, overexpressing LH, develop aberrant function of the adrenal gland, entailing ectopic LHR expression and increased corticosterone production. The latter response was found to be due to the chronic adrenal gland stimulation caused by high serum LH concentrations in these mice. Accordingly, adrenal gland steroidogenesis responded to hCG stimulation in vitro. In addition, we showed that if bLHβ-CTP female mice were gonadectomized, their adrenal corticosterone production was reduced, and the adrenal cells did not respond to hCG in vitro. This suggests, in addition to elevated LH secretion, a role for the polycystic ovaries in the hyperactive adrenal function of these TG mice.
Besides serving as a model for polycystic ovaries (16), our present finding of increased corticosterone production in bLHβ-CTP females suggests that these mice are a useful model for Cushing-like adrenocortical hyperfunction. Hence, we are currently using these animals to explore manifestations of Cushing’s syndrome, such as obesity. Interestingly, in rare cases of ACTH-independent Cushing’s syndrome, the disease becomes more prominent during pregnancy and may improve or remit spontaneously after delivery (31–33). Thus hCG might have a role in worsening of symptoms in these cases. Most of these patients also have adrenal adenomas (31), and our preliminary RT-PCR results suggest that these tumors also express LHR (data not shown). Hence, the present data are of special interest clinically, and it will be intriguing to study whether enhanced adrenocortical expression of the LHR gene is a consistent finding in humans in connection with chronically elevated LH levels, e.g., postmenopausally and in PCOS. In this respect, a very recent report on a postmenopausal patient with Cushing’s syndrome and LH/hCG responsive cortisol production is of particular interest (12).
The mechanisms involved in the induction of LHR expression and corticosterone production in bLHβ-CTP mouse adrenals remain to be studied. Although chronically elevated LH concentrations seem to be essential for the appearance of LHR expression in the adrenal gland, the presence of ovaries is also required for the high LHR expression that leads to hCG responsiveness in vitro and to increased corticosterone production in vivo. It is possible that the ovaries produce a factor that synergies with LH in the induction of LHR and corticosterone production. Conversely, LHR may be normally expressed in the adrenal gland at a very low level, even below that detectable by RT-PCR. Chronic LH stimulation, together with the ovarian factor(s), could then gradually upregulate LHR expression, over some months, to a level above the threshold of detection by less sensitive Northern hybridization and binding assays. This type of homologous upregulation of the LHR gene has been demonstrated in fetal-type Leydig cells (34). The same mechanism of LHR mRNA induction may prevail in the ovaries of bLHβ-CTP mice, where increased LHR mRNA expression was also found (data not shown). We have previously shown in rats, treated with the cytotoxic drug, ethylene dimethane sulfonate (EDS), which specifically destroy mature Leydig cells, that expression of LHR in the new population of Leydig cells is dependent on LH induction (35).
Although we do not yet have direct evidence of the gonadal factor contributing to the induction of adrenal LH responsiveness of female bLHβ-CTP mice, we have data that provide a plausible explanation. These mice have 2- to 3-fold elevated levels of circulating estradiol (16). This is known to upregulate PRL secretion, which was also demonstrated by our current findings. PRL is an important factor that upregulates LHR expression both at the level of transcription and translation (29, 30, 34), and this hormone may be the ovary-dependent factor that enhances the induction of adrenal LHR expression by elevated LH. The latter hormone alone is insufficient, as was found in the gonadectomized animals. It will be interesting to explore whether human conditions with concomitant elevation of circulating PRL and LH concentrations could bring about similar alterations in adrenocortical function (e.g., neuroleptic treatment of postmenopausal women).
The histological changes in the bLHβ-CTP female mouse adrenals were intriguing. The reason for the presence of the inflammatory infiltrate at the corticomedullary junction remains unclear, and further characterization of its exact nature is necessary. Lymphocyte infiltration of the adrenal gland has been noted in some patients with Cushing’s syndrome (36). In addition, a single high dose of hCG can induce inflammation in the human testis (37). Whether the inflammatory changes in bLHβ-CTP female adrenals reflect interactions between the high serum LH levels and locally produced cytokines remains to be elucidated.
One intriguing feature of the mouse adrenal gland is that, in addition to the zona glomerulosa, zona fasciculata, and zona reticularis, it has a fourth zone named the X-zone (38). The function of this juxtamedullary area of the adrenal cortex is not clearly understood. Loss of the X-zone in the adrenal cortex of bLHβ-CTP female mice is interesting and may be related to the elevated ovarian and/or adrenal steroidogenesis. As shown previously (16), the TG females have about 5-fold increased levels of serum testosterone, and their androstenedione levels were over 10-fold elevated (TG mice 11.0 ± 3.2, controls 0.80 ± 0.23 nmol/L [J. Kero, unpublished observation]). This elevation is of ovarian origin, as neither normal mouse adrenals (9) nor those of the bLHβ-CTP females (results not shown) produce androgens. In normal male mice, the X-zone disappears after puberty, possibly due to testosterone (39), and if the mice are gonadectomized, the X-zone persists. In female mice, the X-zone normally disappears after the first pregnancy. However, in 5-month-old virgin bLHβ-CTP females, the adrenal X-zone could not be seen, possibly due to effect of the elevated ovarian androgen production. It has been suggested earlier that the X-zone may be the LH-responsive area of the mouse adrenal cortex (40). Our data on LHR expression do not support this, as there was no correlation between LHR expression and the presence of the X-zone. Expression of LHR was not found by RT-PCR in the adrenal glands of male prepubertal mice (X-zone present) or in normal adult males (X-zone absent) and females (X-zone present). In contrast, in bLHβ-CTP female adrenal glands (X-zone absent), LHR was strongly expressed.
In summary, high circulating LH concentrations, together with a factor(s) associated with the dysfunctional TG ovaries with a polycystic phenotype (possibly estrogen-stimulated PRL secretion), induce LHR expression in female bLHβ-CTP mouse adrenals, causing stimulation of corticosterone production. The mice consequently have a phenotype reminiscent of the obesity associated with Cushing’s syndrome. These mice can therefore be used as a model of human disorders such as polycystic ovaries and adrenocortical hyperfunction. Therefore, further studies on adrenocortical function are warranted in humans with chronically elevated gonadotropin levels.
Acknowledgments
The skillful technical assistance of N. Messner, J. Vesa, and T. Laiho is gratefully acknowledged. We also thank T. Nett, and we thank R. Abbud for her assistance in measuring the LH values. This study was supported by grants from The Sigrid Jusélius Foundation, The Finnish Cancer Societies, and The Academy of Finland to I.T. Huhtaniemi, and a grant from the National Institutes of Health (HD34032) to J.H. Nilson.
References
- 1.Franks S. Polycystic ovary syndrome. N Engl J Med. 1995;333:853–861. doi: 10.1056/NEJM199509283331307. [DOI] [PubMed] [Google Scholar]
- 2.Azziz, R. 1996. The adrenal connection. In Reproductive endocrinology, surgery, and technology. Vol. 1. E. Adashi, J. Rock, and Z. Rosenwaks, editors. Lippincott-Raven Publishers. Philadelphia, PA. 1161–1180.
- 3.Givens JR, Andersen RN, Ragland JB, Wiser WL, Umstot ES. Adrenal function in hirsutism I. Diurnal change and response of plasma androstenedione, testosterone, 17-hydroxyprogesterone, cortisol, LH and FSH to dexamethasone and 1/2 unit of ACTH. J Clin Endocrinol Metab. 1975;40:988–1000. doi: 10.1210/jcem-40-6-988. [DOI] [PubMed] [Google Scholar]
- 4.Lachelin GC, Barnett M, Hopper BR, Brink G, Yen SS. Adrenal function in normal women and women with the polycystic ovary syndrome. J Clin Endocrinol Metab. 1979;49:892–898. doi: 10.1210/jcem-49-6-892. [DOI] [PubMed] [Google Scholar]
- 5.Buffington CK, Givens JR, Kitabchi AE. Enhanced adrenocortical activity as a contributing factor to diabetes in hyperandrogenic women. Metabolism. 1994;43:584–590. doi: 10.1016/0026-0495(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 6.Chang RJ, Mandel FP, Wolfsen AR, Judd HL. Circulating levels of plasma adrenocorticotropin in polycystic ovary disease. J Clin Endocrinol Metab. 1982;54:1265–1267. doi: 10.1210/jcem-54-6-1265. [DOI] [PubMed] [Google Scholar]
- 7.Orth, D.N., and Kovacs, W. 1998. The adrenal cortex. In Williams textbook of endocrinology. W.B. Saunders Company. Philadelphia, PA. 517–664.
- 8.Spackman DH, Riley V. Corticosterone concentrations in the mouse. Science. 1978;200:87. doi: 10.1126/science.635580. [DOI] [PubMed] [Google Scholar]
- 9.van Weerden WM, Bierings HG, van Steenbrugge GJ, de Jong FH, Schroeder FH. Adrenal glands of mouse and rat do not synthesize androgens. Life Sci. 1992;50:857–861. doi: 10.1016/0024-3205(92)90204-3. [DOI] [PubMed] [Google Scholar]
- 10.Pabon JE, et al. Novel presence of luteinizing hormone/chorionic gonadotropin receptors in human adrenal glands. J Clin Endocrinol Metab. 1996;81:2397–2400. doi: 10.1210/jcem.81.6.8964884. [DOI] [PubMed] [Google Scholar]
- 11.Apter D, Pakarinen A, Hammond GL, Vihko R. Adrenocortical function in puberty. Serum ACTH, cortisol and dehydroepiandrosterone in girls and boys. Acta Paediatr Scand. 1979;68:599–604. doi: 10.1111/j.1651-2227.1979.tb05062.x. [DOI] [PubMed] [Google Scholar]
- 12.Lacroix A, Hamet P, Boutin J-M. Leuprolide acetate therapy in luteinizing-hormone-dependent Cushing’s syndrome. N Engl J Med. 1999;341:1577–1581. doi: 10.1056/NEJM199911183412104. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman DI, Klove K, Lobo RA. The prevalence and significance of elevated dehydroepiandrosterone sulfate levels in anovulatory women. Fertil Steril. 1984;42:76–81. doi: 10.1016/s0015-0282(16)47961-6. [DOI] [PubMed] [Google Scholar]
- 14.Seron-Ferre M, Lawrence CC, Jaffe RB. Role of hCG in regulation of the fetal zone of the human fetal adrenal gland. J Clin Endocrinol Metab. 1978;46:834–837. doi: 10.1210/jcem-46-5-834. [DOI] [PubMed] [Google Scholar]
- 15.Parker LN, Odell WD. Control of adrenal androgen secretion. Endocr Rev. 1980;1:392–410. doi: 10.1210/edrv-1-4-392. [DOI] [PubMed] [Google Scholar]
- 16.Risma KA, et al. Targeted overexpression of luteinizing hormone in transgenic mice leads to infertility, polycystic ovaries, and ovarian tumors. Proc Natl Acad Sci USA. 1995;92:1322–1326. doi: 10.1073/pnas.92.5.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haavisto A-M, et al. A Supersensitive immunofluorometric assay for rat luteinizing hormone. Endocrinology. 1993;132:1687–1691. doi: 10.1210/endo.132.4.8462469. [DOI] [PubMed] [Google Scholar]
- 18.Niswender GD, Midgley AR, Jr, Monroe SE, Reichert LE., Jr Radioimmunoassay for rat luteinizing hormone with antiovine LH serum and ovine LH-131-I. Proc Soc Exp Biol Med. 1968;128:807–811. doi: 10.3181/00379727-128-33129. [DOI] [PubMed] [Google Scholar]
- 19.Risma KA, Hirshfield AN, Nilson JH. Elevated luteinizing hormone in prepubertal transgenic mice causes hyperandrogenemia, precocious puberty, and substantial ovarian pathology. Endocrinology. 1997;138:3540–3547. doi: 10.1210/endo.138.8.5313. [DOI] [PubMed] [Google Scholar]
- 20.Abbud RA, Ameduri RK, Rao JS, Nett TM, Nilson JH. Chronic hypersecretion of luteinizing hormone in transgenic mice selectively alters responsiveness of the alpha subunit gene to gonadotropin-releasing hormone and estrogens. Mol Endocrinol. 1999;13:1449–1459. doi: 10.1210/mend.13.9.0348. [DOI] [PubMed] [Google Scholar]
- 21.Bergendahl M, Perheentupa A, Huhtaniemi I. Effect of short-term starvation on reproductive hormone gene expression, secretion and receptor levels in male rats. J Endocrinol. 1989;121:409–417. doi: 10.1677/joe.0.1210409. [DOI] [PubMed] [Google Scholar]
- 22.Vuorento T, Lahti A, Hovatta O, Huhtaniemi I. Daily measurements of salivary progesterone reveal a high rate of anovulation in healthy students. Scand J Clin Lab Invest. 1989;49:395–401. doi: 10.3109/00365518909089113. [DOI] [PubMed] [Google Scholar]
- 23.Harper JF, Brooker G. Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2’0 acetylation by acetic anhydride in aqueous solution. J Cyclic Nucleotide Res. 1975;1:207–218. [PubMed] [Google Scholar]
- 24.Karonen SL, Mörsky P, Sirén M, Seuderling U. An enzymatic solid-phase method for trace iodination of proteins and peptides with 125-iodine. Anal Biochem. 1975;67:1–10. doi: 10.1016/0003-2697(75)90266-3. [DOI] [PubMed] [Google Scholar]
- 25.Catt KJ, Ketelslegers J-M, Dufau ML. Receptors for gonadotropic hormones. In Methods in receptor research. M Blecher, editor Marcel Dekker Inc New York, NY. 1976;1:175–250. [Google Scholar]
- 26.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 27.Zhang FP, Hämäläinen T, Kaipia A, Pakarinen P, Huhtaniemi I. Ontogeny of luteinizing hormone receptor gene expression in the rat testis. Endocrinology. 1994;134:2206–2213. doi: 10.1210/endo.134.5.8156923. [DOI] [PubMed] [Google Scholar]
- 28.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 29.Huhtaniemi IT, Catt KJ. Induction and maintenance of gonadotropin and lactogen receptors in hypoprolactinemic rats. Endocrinology. 1981;109:483–490. doi: 10.1210/endo-109-2-483. [DOI] [PubMed] [Google Scholar]
- 30.Gafvels M, Bjurulf E, Selstam G. Prolactin stimulates the expression of luteinizing hormone/chorionic gonadotropin receptor messenger ribonucleic acid in the rat corpus luteum and rescues early pregnancy from bromocriptine-induced abortion. Biol Reprod. 1992;47:534–540. doi: 10.1095/biolreprod47.4.534. [DOI] [PubMed] [Google Scholar]
- 31.Buescher MA, McClamrock HD, Adashi EY. Cushing syndrome in pregnancy. Obstet Gynecol. 1992;79:130–137. [PubMed] [Google Scholar]
- 32.Da Motta LA, Motta LD, Barbosa AM, Ferreira MA, Gagliardi AR. Two pregnancies in a Cushing’s syndrome. Case report. Panminerva Med. 1991;33:44–47. [PubMed] [Google Scholar]
- 33.Close CF, Mann MC, Watts JF, Taylor KG. ACTH-independent Cushing’s syndrome in pregnancy with spontaneous resolution after delivery: control of the hypercortisolism with metyrapone. Clin Endocrinol (Oxf) 1993;39:375–379. doi: 10.1111/j.1365-2265.1993.tb02380.x. [DOI] [PubMed] [Google Scholar]
- 34.Pakarinen P, Niemimaa T, Huhtaniemi IT, Warren DW. Transcriptional and translational regulation of LH, prolactin and their testicular receptors by hCG and bromocriptine treatments in adult and neonatal rats. Mol Cell Endocrinol. 1994;101:37–47. doi: 10.1016/0303-7207(94)90217-8. [DOI] [PubMed] [Google Scholar]
- 35.Tena-Sempere M, Rannikko A, Kero J, Zhang FP, Huhtaniemi IT. Molecular mechanisms of reappearance of luteinizing hormone receptor expression and function in rat testis after selective Leydig cell destruction by ethylene dimethane sulfonate. Endocrinology. 1997;138:3340–3348. doi: 10.1210/endo.138.8.5325. [DOI] [PubMed] [Google Scholar]
- 36.Willenberg HS, et al. Aberrant interleukin-1 receptors in a cortisol-secreting adrenal adenoma causing Cushing’s syndrome. N Engl J Med. 1998;339:27–31. doi: 10.1056/NEJM199807023390105. [DOI] [PubMed] [Google Scholar]
- 37.Demirbilek S, Atayurt HF, Celik N, Aydin G. Does treatment with human chorionic gonadotropin induce reversible changes in undescended testes in boys? Pediatr Surg Int. 1997;12:591–594. doi: 10.1007/BF01371906. [DOI] [PubMed] [Google Scholar]
- 38.Holmes PV, Dickson AD. X-zone degeneration in the adrenal glands of adult and immature female mice. J Anat. 1971;108:159–168. [PMC free article] [PubMed] [Google Scholar]
- 39.Asari M, Fukaya K, Eguchi Y, Nishida S, Kano Y. Effect of testosterone and progesterone on the adrenal X-zone in female mice. Nippon Juigaku Zasshi. 1979;41:61–67. doi: 10.1292/jvms1939.41.61. [DOI] [PubMed] [Google Scholar]
- 40.Deacon CF, Mosley W, Jones IC. The X zone of the mouse adrenal cortex of the Swiss albino strain. Gen Comp Endocrinol. 1986;61:87–99. doi: 10.1016/0016-6480(86)90253-4. [DOI] [PubMed] [Google Scholar]