Abstract
Background. Equations for estimating glomerular filtration rate (GFR) have not been validated in Sub-Saharan African populations, and data on GFR are few.
Methods. GFR by creatinine clearance (Ccr) using 24-hour urine collections and estimated GFR (eGFR) using the four-variable Modification of Diet in Renal Disease (MDRD-4)[creatinine calibrated to isotope dilution mass spectrometry (IDMS) standard], Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) and Cockcroft–Gault equations were obtained in Ghanaians aged 40–75. The population comprised 1013 inhabitants in 12 villages; 944 provided a serum creatinine and two 24-hour urines. The mean weight was 54.4 kg; mean body mass index was 21.1 kg/m2.
Results. Mean GFR by Ccr was 84.1 ml/min/1.73 m2; 86.8% of participants had a GFR of ≥60 ml/min/1.73 m2. Mean MDRD-4 eGFR was 102.3 ml/min/1.73 m2 (difference vs. Ccr, 18.2: 95% CI: 16.8–19.5); when the factor for black race was omitted, the value (mean 84.6 ml/min/1.73 m2) was close to Ccr. Mean CKD-EPI eGFR was 103.1 ml/min/1.73 m2, and 89.4 ml/min/1.73 m2 when the factor for race was omitted. The Cockcroft–Gault equation underestimated GFR compared with Ccr by 9.4 ml/min/1.73 m2 (CI: 8.3–10.6); particularly in older age groups. GFR by Ccr, and eGFR by MDRD-4, CKD-EPI and Cockcroft–Gault showed falls with age: MDRD-4 5.5, Ccr 7.7, CKD-EPI 8.8 and Cockcroft–Gault 11.0 ml/min/1.73 m2/10 years. The percentage of individuals identified with CKD stages 3–5 depended on the method used: MDRD-4 1.6% (7.2 % without factor for black race; CKD-EPI 1.7% (4.7% without factor for black race), Ccr 13.2% and Cockcroft–Gault 21.0%.
Conclusions. Mean eGFR by both MDRD-4 and CKD-EPI was considerably higher than GFR by Ccr and Cockcroft–Gault, a difference that may be attributable to leanness. MDRD-4 appeared to underestimate the fall in GFR with age compared with the three other measurements; the fall with CKD-EPI without the adjustment for race was the closest to that of Ccr. An equation tailored specifically to the needs of the lean populations of Africa is urgently needed. For the present, the CKD-EPI equation without the adjustment for black race appears to be the most useful.
Keywords: CKD-EPI eGFR, Cockcroft–Gault eGFR, creatinine clearance measurement, Ghana, MDRD-4 eGFR
Introduction
Chronic kidney disease (CKD) is a leading cause of morbidity and mortality in both developed [1,2] and developing [2–4] countries. While primary kidney disease is believed to account for 1.2% of all deaths worldwide [5], many more renal deaths are a consequence of hypertension and type II diabetes mellitus [6,7]. In the countries of Sub-Saharan Africa, facilities for the treatment of CKD are scanty, and chronic dialysis is available in less than half of them. In those countries that do offer transplantation and dialysis, only a small proportion of the population can afford the treatment [8,9]. Hence, in developing countries, just as in developed countries, detection of kidney disease and prevention of its progression are of paramount importance.
For the people of Sub-Saharan Africa, early detection of CKD, especially in high-risk groups such as hypertensives and diabetics, is likely to be the most cost-effective strategy [10]. An important first step in the assessment of CKD is determination of glomerular filtration rate (GFR). Serum creatinine as a surrogate marker is unreliable, and accurate measurements using inulin, iothalamate or other radioisotopes are too expensive to be used for screening. Creatinine clearance (Ccr) is sufficiently accurate but the required 24-hour urine collection too cumbersome and potentially beset by pitfalls for studies in the community; hence, the popularity of estimating GFR from equations using serum creatinine, in particular the Cockcroft–Gault [11] and four-variable Modification of Diet in Renal Disease (MDRD-4) [12] equation. There is now a new equation [13]—the CKD-EPI—which is claimed to be as accurate as the MDRD-4 for estimated GFR (eGFR) <60 ml/min/1.73 m2, and ‘considerably more accurate’ than MDRD-4 in the group with eGFR >60 ml/min/1.73 m2.
The 1013 individuals described in this paper are healthy 40–75 year old inhabitants of 12 communities in the Ashanti region of Ghana. We have published demographic details of the population [14] and data on the prevalence, detection, treatment and control of hypertension [15]. We have now studied the relationship between Ccr and three methods of estimating GFR, namely the Cockcroft–Gault [11], MDRD-4 [12] and CKD-EPI equations, to determine which method would be of most use in our population, and similar populations elsewhere in Africa, in identifying individuals with CKD. Unfortunately, none of the usual gold standards for GFR measurement were available to us as a comparator.
Materials and methods
Population
The study was a cluster-randomised trial in 12 communities in the Ejisu-Juabeng and Ashanti districts near Kumasi [14,15]. From each village, participants aged 40 to 75 were selected using age- and gender-stratified random sampling to ensure that participants from each village matched overall population structure [14]. Individuals with serious mental or physical illness, women pregnant or lactating and individuals declining to participate were excluded from the study. Individuals with hypertension, diabetes or known renal disease were not excluded.
Questionnaire
Participants attended a registration centre in their village. After details of the study had been explained in the local language (Twi), individual written consent was obtained. All participants, with the help of a project assistant, completed the questionnaire, which included sections on demographic background, socioeconomic status, health, diet, lifestyle and past and current drug treatment. Ethical approval was granted by the Committee on Human Research Publication and Ethics of the School of Medical Sciences, Kwame Nkrumah University of Science and Technology, Kumasi, and by Wandsworth Research Ethics Committee, London.
Measurements
Height was measured to the nearest 0.5 cm using a wooden platform with height rule; footwear was removed. Weight was measured to the nearest 0.5 kg with manual Seca 761 scales (Vogel & Halke, Hamburg, Germany) after participants had removed outer garments and footwear. Body mass index (BMI) was calculated from the equation: BMI = weight (kg) / height (m)2. Blood pressure and pulse rate were measured with an automated sphygmomanometer (OMRON HEM705CP, Omron Matsusaka Co, Matsusaka City, Mie-Ken, Japan) using appropriate cuff size after participants had sat undisturbed for at least 5 minutes. Three readings were taken 1 minute apart. The first was discarded, and the mean of the last two used in the analysis.
Blood samples and 24-hour urine collections
Venous blood samples were taken fasting from each participant. The samples were put into Vacutainer tubes and transported without delay to Komfo Anokye Teaching Hospital (KATH). The tubes were centrifuged at 1500 rpm for 10 minutes at room temperature, and the serum transferred into 5-ml aliquot tubes, which were kept frozen at −20°C until analysed. Serum and urine creatinine were measured using a Jaffe alkaline picrate method (Beckman–Coulter). Our method has been validated against isotope dilution mass spectrometry (IDMS) values for creatinine and gave the following correlation: IDMS in μmole/l = (Beckman–Coulter value − 5.94) / 0.994 [16]. The re-calculated creatinine values obtained were used in the modified MDRD-4 and CKD-EPI equations traceable to IDMS values to calculate the eGFR [17].
Two consecutive timed 24-hour urine collections were obtained from each participant. Each collection was started by the participants emptying their bladder, the time of voiding being recorded. A 4.5-litre plastic bottle (containing thymol as preservative) was provided, and the participants instructed to collect into the bottle all urine voided. The 24-hour collections were brought to the study centre the following morning. Each participant was then asked to void for a last time into the bottle; the time of voiding being recorded. The urine collections were retained by the study centre, and each participant was given a new bottle for the second 24-hour urine collection, which was brought to the study centre the next day. The urine samples were transported to KATH where, before storage, electronic scales were used to measure (weigh) the 24-hour urine volume. Five 5-ml aliquots of the urine samples were pipetted into tubes and frozen at −20°C until analysed for creatinine.
Calculations and staging of kidney function
After adjustment for the duration of the collection, Ccr was calculated in millilitre per minute. The means of the two 24-hour calculations were used in the analysis. For Ccr and for eGFR using the Cockcroft–Gault equation, the results have been adjusted for body surface area (BSA). The following equations were used to arrive at the eGFR:
MDRD-4 [12]
GFR (ml/min/1.73 m2) = 175 × {[plasma creatinine (μmol/l) / 88.4] −1.154} × age (years)−0.203 × 0.742 (if female) × 1.212 (if black)
CKD-EPI [13]
The CKD-EPI equation, expressed as a single equation, is: ‘GFR = 141 × min(Scr/k, 1)α × max(Scr / k, 1) −1.209 × 0.993Age × 1.018 (if female) × 1.159 (if black), where Scr is serum creatinine, k is 0.7 for females and 0.9 for males, α is −0.329 for females and −0.411 for males, min indicates the minimum of Scr/k or 1, and max indicates the maximum of Scr/k or 1.’
Modified Cockcroft–Gault [11]
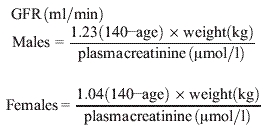 |
The MDRD-4- and CKD-EPI-derived eGFRs are expressed as ml/min/1.73 m2 because the equations were derived by comparison with iothalamate-measured GFR, which itself is expressed as ml/min 1.73 m2. Ccr and e-GFR derived by using the Cockcroft–Gault equation were converted from ml/min to ml/min/1.73 m2 by multiplying calculated values by 1.73, and dividing by BSA. Since the height and weight of each individual was known, it was possible to calculate the individual GFR as ml/min/1.73 m2 for each participant.
[18]
Stages of chronic kidney disease [16]
| Stage | Clinical | GFR (ml/min) |
|---|---|---|
| 1a | Normal | >90 |
| 2a | Mild impairment | 60–89 |
| 3 | Moderate impairment | 30–59 |
| 4 | Severe impairment | 15–29 |
| 5 | End-stage renal failure | <15 |
The correlations between radio-labelled iothalamate measured GFR, and both MDRD-4 and CKD-EPI are close for individuals with GFR <60 ml/min/1.73 m2 but less so for those with GFR >60 ml/min/1.73 m2.
aStages 1 and 2 are usually combined; they apply to patients with evidence of kidney disease (other than reduced GFR), such as proteinuria, haematuria and known kidney disease. In this paper, participants with GFR >60 ml/min/1.73 m2 have been considered together.
Statistical analyses
Results are presented as mean and standard deviation (SD). Males and females were compared using the t-test for continuous variables and by chi-square for categorical variables. Differences in eGFR arrived at by the MDRD-4, CKD-EPI and Cockcroft–Gault equations were compared with Ccr using the paired t-test. Correlations between Ccr and eGFR were estimated using Pearson’s correlation coefficients and fall in GFR with age using linear regression. Limits of agreement between MDRD-4 (both with and without the factor for black race) and Ccr and between Cockcroft–Gault and Ccr were calculated using the Bland–Altman method [20].
Results
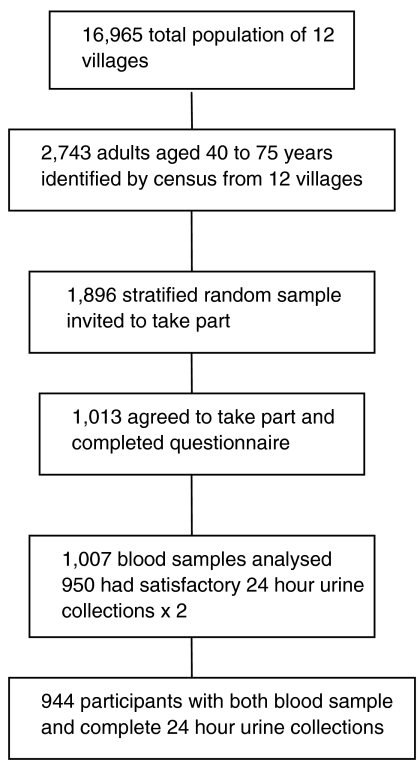
Serum creatinine values were available for 1007 of the 1013 participants, 944 (93.7%) of whom provided two satisfactory 24-hour urine collections (coefficient of variation; 17.7%) (Figure 1). Some samples had to be excluded because of discrepancies in the creatinine excretion of the two samples. Table 1 shows some characteristics of the participants who were lean, the mean body weight being 54.4 kg with mean BMI 21.1 kg/m2. Diastolic blood pressure was higher in the men; there was no statistically significant difference between men and women as regards systolic pressure. Eight hundred eighty-eight participants (94%) were from the same tribe (Ashanti), and most were farmers (n = 629, 67%) or traders (n = 138, 15%).
Fig. 1.
Recruitment of participants.
Table 1.
Characteristics of the population
| Men n = 355 | Women n = 589 | All n = 944 | Men vs. women P values | |
|---|---|---|---|---|
| Age (years) | 54.5 (10.7) | 54.8 (11.5) | 54.7 (11.2) | 0.70 |
| Height (cm) | 166.8 (6.8) | 156.7 (7.0) | 160.5 (8.5) | <0.001 |
| Weight (kg) | 56.4 (9.7) | 53.2 (12.0) | 54.4 (11.3) | <0.001 |
| BMI (kg/m2) | 20.3 (3.1) | 21.7 (4.7) | 21.1 (4.2) | <0.001 |
| Systolic BP (mm Hg) | 125.9 (24.1) | 125.2 (27.1) | 125.5 (26.0) | 0.70 |
| Diastolic BP (mm Hg) | 75.7 (13.6) | 73.6 (13.5) | 74.4 (13.6) | 0.022 |
| Plasma glucose (mmol/l) | 4.1 (1.6) | 4.1 (1.0) | 4.1 (1.3) | 0.91 |
| Current smoker n (%) | 64 (18) | 1 (0.2) | 65 (7) | <0.001 |
| Regular drinkeran (%) | 123 (35) | 41 (7) | 164 (17) | <0.001 |
| Diabetes n (%) | 22 (6.2) | 19 (3.2) | 41 (4) | 0.030 |
Means and (SD) are given.
Once a week or more.
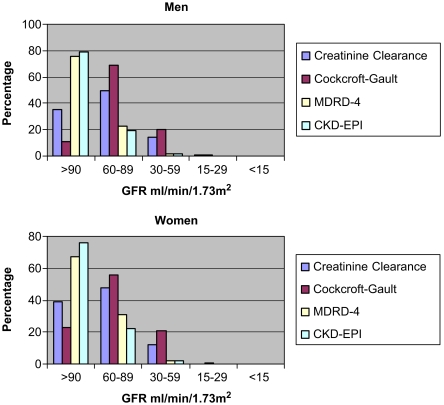
Table 2 shows creatinine measurements, Ccr (ml/min 1.73 m2) and three estimates of GFR (MDRD-4, CKD-EPI and Cockcroft–Gault). The mean Cockcroft–Gault estimate of GFR was 9.4 (95% CI 8.3 to 10.6) ml/min/1.73 m2 lower than the Ccr GFR. The mean MDRD-4 and CKD-EPI eGFRs were higher than Ccr GFR at 18.2 (16.8 to 19.5) and 19.0 (17.8 to 20.2) ml/min/1.73 m2, respectively, and the discrepancy for both equations was greater in the men than the women. Figure 2 gives the distribution by CKD stage.
Table 2.
Serum creatinine, urinary creatinine and GFR
| Men n = 355 | Women n = 589 | All n = 944 | Men vs. women P values | |
|---|---|---|---|---|
| Serum creatinine (μmol/l) | 89.7 (18.3) | 72.5 (13.3) | 79.0 (17.4) | <0.001 |
| 24-hour urinary creatinine (mmol) | 9.8 (2.7) | 7.6 (2.0) | 8.4 (2.6) | <0.001 |
| Creatinine clearance | 82.3 (22.4) | 85.3 (22.7) | 84.1 (22.6) | 0.051 |
| Estimated GFR (ml/min/1.73 m2) | ||||
| Cockcroft–Gault | 72.1 (15.6) | 76.2 (20.0) | 74.7 (18.6) | 0.001 |
| MDRD-4 | 104.7 (23.0) | 100.9 (22.6) | 102.3 (22.8) | 0.014 |
| MDRD-4 without correction for race | 86.5 (19.0) | 83.4 (18.6) | 84.6 (18.8) | 0.014 |
| CKD-EPI | 103.6 (18.2) | 102.8 (19.0) | 103.1 (18.7) | 0.52 |
| CKD-EPI without correction for race | 89.6 (15.8) | 89.2 (16.5) | 89.4 (16.2) | 0.73 |
| Limits of agreement | ||||
| Estimated GFR minus creatinine clearance (ml/min/1.73 m2) | ||||
| Cockcroft–Gault | −10.1 (−44.8 to 24.5) | −9.0 (−44.6 to 26.6) | −9.4 (−44.7 to 25.8) | 0.36 |
| MDRD-4 | 22.4 (−22.1 to 66.8) | 15.6 (−23.6 to 54.8) | 18.2 (−23.6 to 59.9) | <0.001 |
| MDRD-4 without correction for race | 4.2 (−36.7 to 45.1) | −1.9 (−38.4 to 34.6) | 0.4 (−38.2 to 39.0) | <0.001 |
| CKD-EPI | 21.3 (−17.4 to 60.0) | 17.6 (−17.2 to 52.4) | 19.0 (−17.9 to 53.8) | 0.003 |
| CKD-EPI without correction for race | 7.3 (−30.2 to 44.8) | 4.0 (−30.0 to 37.9) | 5.2 (−30.2 to 40.7) | 0.006 |
Means ± SD except for estimated GFR minus creatinine clearance where mean differences and limits of agreement are given.
Fig. 2.
Distribution of renal function by GFR stage.
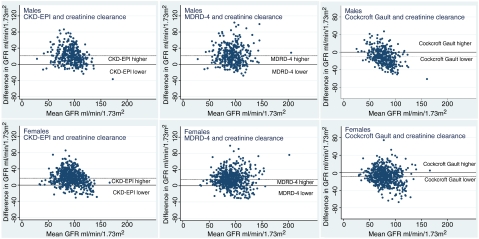
Figure 3 shows Bland–Altman plots for MDRD-4, CKD-EPI and Cockcroft–Gault vs. Ccr. Limits of agreement, given in Table 2, are narrower for the Cockcroft–Gault equation than for either MDRD-4 or CKD-EPI and for the women than the men.
Fig. 3.
Bland–Altman plots for GFR estimated by CKD-EPI, MDRD-4 and Cockcroft–Gault compared with creatinine clearance. Solid line represents equality of the two measurements: dotted line, the mean observed difference, as in Table 2.
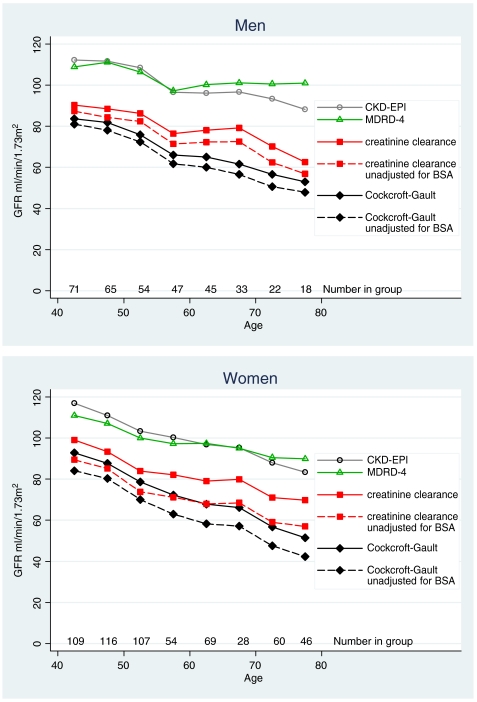
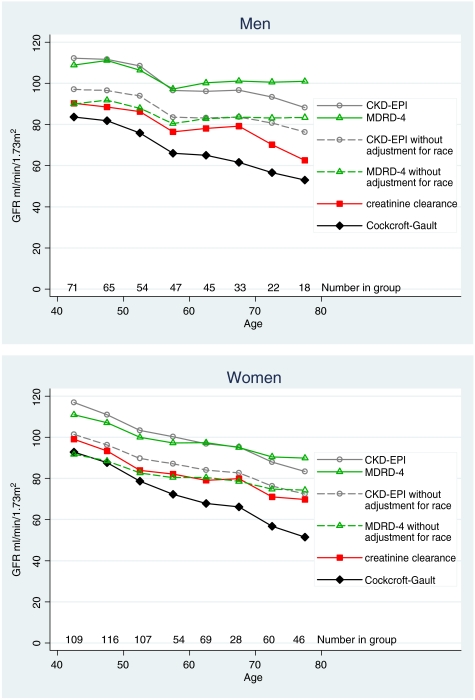
Figure 4 gives mean values by 5-year age group for Ccr, and Cockcroft–Gault, MDRD-4 and CKD-EPI eGFR. With increasing age, all methods show a fall, which was least with MDRD-4 eGFR (5.5 ml/min/1.73 m2/10 years), and greatest with Cockcroft–Gault eGFR (11.0 ml/min/1.73 m2/10 years); the fall for CKD-EPI was 8.7 ml/min/1.73 m2/10 years and for Ccr 7.7 ml/min/1.73 m2/10 years (Table 3). The values for Ccr and the Cockcroft–Gault equation are given both unadjusted and adjusted for BSA (Figure 4), and illustrate the magnitude of the effect of adjusting for BSA.
Fig. 4.
Mean GFR in 5-year age bands by four methods: creatinine clearance and the Cockcroft–Gault equation with and without adjustment for body surface area: also the MDRD-4 and CKD-EPI equations.
Table 3.
Fall in GFR by decade
| Men n = 355 | Women n = 589 | All n = 944 | |
|---|---|---|---|
| Creatinine clearance ml/min/1.73 m2 per 10 years | −6.7 (−8.8 to −6.7) | −8.3 (−9.7 to −6.8) | −7.7 (−8.9 to −6.5) |
| Cockcroft-Gault ml/min/1.73 m2 per 10 years | −9.4 (−10.6 to −8.2) | −11.8 (−12.9 to −10.8) | −11.0 (−11.8 to −10.2) |
| MDRD-4 ml/min/1.73 m2 per 10 years | −3.8 (−6.0 to −1.6) | −6.4 (−7.9 to −4.9) | −5.5 (−6.8 to −4.3) |
| CKD-EPI ml/min/1.73 m2 per 10 years | −7.5 (−9.1 to −5.9) | −9.3 (−10.4 to −8.2) | −8.7 (−9.6 to −7.7) |
95% confidence intervals given in brackets.
The MDRD-4 and CKD-EPI equations comprise four variables—age, gender, creatinine and White/African-American racial group. Age, gender and creatinine are unalterable objective markers. The adjustment for racial group used in the MDRD-4 and CKD-EPI equations may not be appropriate for rural African populations. We therefore tried removing the factor for racial group (Table 2, Figure 5). Without this variable, the values for MDRD-4 and CKD-EPI become much closer to Ccr. Nevertheless, both equations seem to overestimate GFR in the older men.
Fig. 5.
Mean GFR in 5-year age bands using creatinine clearance and the Cockcroft–Gault equation: also using the MDRD-4 and CKD-EPI equations, with and without adjustment for race.
Table 4 shows the effect of removing the factor for race on the absolute numbers of participants allocated to CKD stages 3–5. The unmodified MDRD-4 identified only 15 individuals out of the 944 compared with 125 identified using BSA-adjusted Ccr; when the factor for race was omitted from the MDRD-4 equation, there were 70 individuals in stages 3–5. Comparable figures for CKD-EPI are 16 and (without the factor for race) 44.
Table 4.
Stage of renal function according to method of assessing GFR
| GFR stage | MDRD-4 | CKD-EPI | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ml/min/1.73 m2 | ml/min/1.73 m2 | Creatinine clearance | Cockcroft-Gault | ||||||||||||||
| Omittingcorrectionfor race | Omitting correction for race | ml/min/1.73 m2 | ml/min | ml/min/1.73 m2 | ml/min | ||||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | ||
| Males | 1 | 269 | 75.8 | 139 | 39.2 | 280 | 78.9 | 189 | 53.2 | 123 | 34.7 | 99 | 27.9 | 38 | 10.7 | 34 | 9.6 |
| 2 | 80 | 22.5 | 195 | 54.9 | 68 | 19.2 | 151 | 42.5 | 179 | 50.4 | 183 | 51.5 | 244 | 68.7 | 201 | 56.6 | |
| n = 355 | 3 | 6 | 1.7 | 19 | 5.4 | 7 | 2 | 14 | 3.9 | 49 | 13.8 | 65 | 18.3 | 70 | 19.7 | 115 | 32.4 |
| 4 | 0 | 0 | 2 | 0.6 | 0 | 0 | 1 | 0.3 | 4 | 1.1 | 8 | 2.3 | 3 | 0.9 | 5 | 1.4 | |
| 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – | – | – | – | |
| Females | 1 | 396 | 67.2 | 190 | 32.3 | 448 | 76.1 | 295 | 50.1 | 232 | 39.4 | 148 | 25.1 | 135 | 22.9 | 91 | 15.5 |
| 2 | 184 | 31.2 | 350 | 59.4 | 132 | 22.4 | 265 | 45 | 285 | 48.4 | 278 | 47.2 | 329 | 55.9 | 259 | 44 | |
| n = 589 | 3 | 9 | 1.5 | 47 | 8.0 | 9 | 1.5 | 29 | 4.9 | 71 | 12 | 157 | 26.7 | 122 | 20.7 | 230 | 39 |
| 4 | 0 | 0 | 2 | 0.3 | 0 | 0 | 0 | 0 | 1 | 0.2 | 6 | 1 | 3 | 0.5 | 9 | 1.5 | |
| 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – | – | – | – | – | – | – | – | |
| All | 1 | 665 | 70.4 | 329 | 34.8 | 728 | 77.1 | 484 | 51.3 | 355 | 37.6 | 247 | 26.2 | 173 | 18.3 | 125 | 13. 2 |
| 2 | 264 | 28 | 545 | 57.7 | 200 | 21.2 | 416 | 44.1 | 464 | 49.2 | 461 | 48.8 | 573 | 60.7 | 460 | 48.7 | |
| n = 944 | 3 | 15 | 1.6 | 66 | 7.0 | 16 | 1.7 | 43 | 4.6 | 120 | 12.7 | 222 | 23.5 | 192 | 20.3 | 345 | 36.6 |
| 4 | 0 | 0 | 4 | 0.4 | 0 | 0 | 1 | 0.1 | 5 | 0.5 | 14 | 1.5 | 6 | 0.6 | 14 | 1.5 | |
| 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – | – | – | – | – | – | – | – | |
| All | 3–5 | 15 | 1.6 | 70 | 7.4 | 16 | 1.7 | 44 | 4.7 | 125 | 13.2 | 236 | 25.3 | 198 | 21.0 | 359 | 38.0 |
Discussion
This is the first report on prevalence of reduced GFR in a community study in Sub-Saharan Africa; it shows that, in a Ghanaian village population it is possible to collect reliable data on kidney function. GFR estimated using the MDRD-4 and CKD-EPI equations were 18.2 and 19.0 ml/min/1.73 m2 higher respectively than the value obtained by Ccr, and the difference was more marked in the men, whereas mean GFR estimated using the Cockcroft–Gault equation was 9.4 ml/min/1.73 m2 lower than Ccr.
When the results were compared in 5-year age groups, the eGFRs arrived at by MDRD-4 and CKD-EPI were consistently higher than Ccr, particularly in older age groups, while that produced by the Cockcroft–Gault equation was consistently lower. Without the correction for race, MDRD and CKD-EPI were much closer to Ccr (0.4 and 5.2 ml/min/1.73 m2 higher) with the women showing the greater difference.
In a study of another ethnically homogeneous healthy population, Viktorsdottir et al. [21] reported data on 19,256 adults aged between 33 and 85. They compared three measures of GFR—the MDRD-4 equation, the Cockcroft–Gault equation and the reciprocal of plasma creatinine. Using MDRD-4 and Cockcroft–Gault, the results were similar in the 34–39 and 40–44 age groups. With increasing age, the 5-year group correlations diverged, showing a fall in GFR with age with both methods but greater with Cockcroft–Gault (ca. 10 ml/min/1.73 m2/10 years) than with MDRD-4 (7.2 ml/min/1.73 m2/10 years). In our study, the calculated values for MDRD-4 eGFR were higher than for Ccr (102.3 vs. 84.6 ml/min/1.73 m2/10 years) and the fall in GFR per decade much smaller than that of Ccr (5.5 vs. 7.7 ml/min/1.73 m2/10 years). Our figure for fall in Ccr with age is very close to the findings of a study of 884 healthy men [22].
An example of GFR measurement in an ethnically homogeneous community in a developing country was reported by Jafar et al. [23]. They studied 262 adults ≥40 years from the Pashtun ethnic subgroup in Karachi, and found that 77 (29.9%) had reduced GFR as judged by a BSA-adjusted Ccr of <60 ml/min/1.73 m2—compared with 13.2% (125 out of 944) in our population. Their urban population had a higher prevalence of risk factors for CKD than ours and was heavier (weight 64.7 vs. 54.4 kg; BMI 24.9 vs. 21.1 kg/m2). Diabetes was more common (10.7% vs. 4.3%) as was tobacco use (40.4% vs. 20.1%). Another difference was that both Cockcroft–Gault and MDRD-4 equations gave higher eGFRs than measured Ccr. The authors mentioned the possibility of under-collection of 24-hour urines; also the relatively low urinary creatinines compared with whites.
In the MDRD study [12], the MDRD-4 equation was found to be accurate in predicting GFR for values <60 ml/min/1.73 m2; our intention was to evaluate it in rural African communities, and to determine the prevalence of stage 3–5 CKD.
The MDRD-4 study [12] encompassed 1501 individuals aged 40–90, and the Icelandic study [21] 19 256 individuals aged 33–85 (mean age: male 52.1 years, female 54.1). The CKD-EPI study [13] involved 16 032 individuals but few were elderly. While the age of the participants was not very different from our study (944 individuals, 40–75 years), the studies differ fundamentally in two other areas. The mean body weight of the individuals in the MDRD-4 study [12] was 79.6 kg, in the CKD-EPI study 82 kg and in the Icelandic study [21] 80.3 kg (men) and 66.5 kg (women); in our study, mean body weights were 56.4 kg for men and 53.2 kg for women. The other difference was the ethnic breakdown (MDRD-4 [12]: 13% black; CKD-EPI [13]: ‘few non-white persons’, Icelandic [21] 0% black, our study 100% black). In addition, the CKD-EPI sample was not community based and had a larger number of subjects with CKD stages 3–5.
Strengths of the study
Our study is the first to provide GFR data in a sizeable adult African rural community. The age of the participants means that, as a group, they are likely to be at risk of CKD, and information in such groups is lacking. Our participants live in a geographical area within a 10-mile radius, and population homogeneity is high (94% Ashanti). In the same communities, we have published information on population characteristics [14], prevalence and detection of high blood pressure [15], salt intake [24], body mass index [25] and on our efforts to reduce salt intake and blood pressure [26]. This paper adds important information on the renal aspects.
The data were collected with scrupulous attention to detail. The 24-hour urine collections especially needed supervision at the beginning and end of each collection so that the time could be recorded reliably.
Weaknesses
Most importantly, it is unfortunate that, in this community study in Africa, we were unable, because the methods involve inulin infusion, iodine-labelled iothalamate or other radioactive isotopes, to use a ‘Gold standard’ measurement of GFR.
There were some difficulties with data collection. Though 1007 of the 1013 participants provided a blood sample, rather fewer [944 (93.2%)] provided two satisfactory 24-hour urine collections. Errors in urine collection are always a possibility—especially under-collection; in the study, however, the results obtained do not suggest that there was this particular problem. Another more general difficulty was calculation of age. Many of the older men and women did not have birth certificates so age was determined by reference to well-known national events [14]. In some cases, the age stated at randomisation (provided by the participant) differed from that given on entry to the study itself (sometimes obtained from other family members); hence, there were a small number of individuals outside the intended limits of 40–75 years of age. Consistent underestimation of age could lead to overestimation of eGFR using any of the methods described here. Since this would have a greater effect on Cockcroft–Gault than on MDRD-4, it is unlikely that inaccuracy in estimating age would account for the high values of eGFR we have observed when using MDRD-4.
How is GFR best assessed in African populations?
Our data show that the Cockcroft–Gault equation underestimates GFR, and if used in populations similar to ours, would significantly misclassify individuals as to CKD stage. The unmodified MDRD-4 eGFR appears to be erroneously high in that the values for eGFR are much higher than those obtained Ccr. These observations are not new. It is known that MDRD-4 underestimates GFR in some populations [27], and also that gender, age and BMI can affect the accuracy of predictive equations [28]. It has been pointed out too in a study of the relationship between cardiac risk factors and GFR that the conclusions reached depend on which equation is chosen for estimating GFR [29]. In a study of 2095 non-black individuals, Froissart et al. [30] in France found that the MDRD-4 and Cockcroft–Gault equations, respectively, misclassified 29.2% and 32.4% of their participants.
Our finding of MDRD-4-derived eGFR being higher than GFR obtained by both Ccr and the Cockcroft–Gault equation is in contrast to data of other authors who have found it to be lower. An extrapolation of data from the paper of Verhave et al. [29] suggests that the explanation could be the leanness of our participants. These authors studied 8592 individuals and found that mean GFRs (by Ccr, Cockcroft–Gault and simplified MDRD) were in the range 77.5–94.6 ml/min/1.73 m2, i.e. very similar to the data presented by ourselves. Figure 1 of their paper reveals that as body weight falls from 110 to 65 kg, the simplified MDRD eGFR rises. The lowest mean weight given is 65 kg at which point the GFR by simplified MDRD has almost risen to that of Ccr, which hardly changes across the weight range. From extrapolating the curves in the figure, it seems likely that if Verhave et al. [29] had had participants weighing between 45 and 65 kg (as we did), the curves would have crossed. In other words, their results would be very similar to the data presented by us, i.e. that the highest GFRs would be found with MDRD-4, followed by Ccr and lastly Cockcroft–Gault. These ideas are amplified in a further paper by Verhave et al. [31]. Some of the shortcomings of the MDRD-4 equation probably also apply to the CKD-EPI equation—notwithstanding its better performance—and Levey et al. [13] mention the question of its validity in populations at the extremes of muscle mass. Indeed, they state that ‘All creatinine-based equations should be used with caution in people with abnormally high or low muscle mass’. We suspect our participants would be considered to be such people.
Clearly, it is impractical, in trying to identify individuals with reduced GFR in a community setting, to expect them to perform 24-hour urine collections. We suggest that, until a more credible equation is devised, the CKD-EPI equation is used but without using the factor for racial group, i.e. removing the multiplier that is included because the patients are black. This is an adjustment that has been suggested previously for a population of CKD patients in Sub-Saharan Africa [32]. Figure 5 shows the result of this modification on the MDRD-4 and CKD-EPI slopes in our population. The angle of the slopes has not changed with omission of the racial factor but both are now much closer to the Ccr slope.
Implications
The importance of our study is that to date there are few data on the prevalence of CKD in Sub-Saharan Africa. Such studies are difficult to undertake, and there are few funding sources. Nevertheless, two major risk factors for CKD—high blood pressure and diabetes—are common. The prevalence of both is believed to be increasing so the prevalence of CKD is likely to increase. The nephrological community clearly has an opportunity to develop strategies for detecting and preventing CKD in Africa. We suspect that a coherent, deliverable mechanism for doing this would be welcomed by health policymakers in Africa.
Acknowledgments
We thank the many workers in Ghana who have contributed to the study, whose names are listed elsewhere [14] is Plange-Rhule et al. We would also like to thank the chiefs and elders of the 12 villages for their help. We are grateful to Dr. Gerald Levin for advice during the preparation of the manuscript. The study was supported by the Wellcome Trust (060415/Z/00/Z). F.B.M. was supported by a Wellcome Trust Master’s Research Training Fellowship (069500/Z/02/Z).
Conflict of interest statement. None declared.
References
- 1.Atkins RC. The epidemiology of chronic kidney disease. Kidney Int. 2005;94:S14–S18. doi: 10.1111/j.1523-1755.2005.09403.x. [DOI] [PubMed] [Google Scholar]
- 2.El Nahas AM, Bello AK. Chronic kidney disease: the global challenge. Lancet. 2005;365:331–340. doi: 10.1016/S0140-6736(05)17789-7. [DOI] [PubMed] [Google Scholar]
- 3.Naicker S. End-stage renal disease in sub-Saharan and South Africa. Kidney Int. 2003;83:S119–S122. doi: 10.1046/j.1523-1755.63.s83.25.x. [DOI] [PubMed] [Google Scholar]
- 4.Mate Kole MO, Affram RK. Presentation and clinical course of End-stage renal failure in Ghana. A preliminary prospective study. Ghana Med J. 1990;24:164–168. [Google Scholar]
- 5.The World Health Report . WHO. 2003. Shaping the future. Chapter 6. [Google Scholar]
- 6.Haroun MK, Jaar BG, Hoffman SC, et al. Risk factors for chronic kidney disease: a prospective study of 23,534 men and women in Washington County, Maryland. J Am Soc Nephrol. 2003;14:2934–2941. doi: 10.1097/01.asn.0000095249.99803.85. [DOI] [PubMed] [Google Scholar]
- 7.Plange-Rhule J, Phillips R, Acheampong JW, et al. Hypertension and renal failure in Kumasi, Ghana. J Hum Hypertens. 1999;13:37–40. doi: 10.1038/sj.jhh.1000726. [DOI] [PubMed] [Google Scholar]
- 8.Fogazzi GB, Attolou V, Kadiri S, et al. A nephrological program in Benin and Togo, West Africa. Kidney Int. 2003;83:S56–S60. doi: 10.1046/j.1523-1755.63.s83.12.x. [DOI] [PubMed] [Google Scholar]
- 9.Agaba EI, Lopez A, Ma I, et al. Chronic hemodialysis in a Nigerian teaching hospital: practice and costs. Int J Artif Organs. 2003;26:991–995. doi: 10.1177/039139880302601104. [DOI] [PubMed] [Google Scholar]
- 10.Bello AK, Nwakwo E, El Nahas AM. Prevention of chronic kidney disease: a global challenge. Kidney Int. 2005;98:S11–S17. doi: 10.1111/j.1523-1755.2005.09802.x. [DOI] [PubMed] [Google Scholar]
- 11.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plange-Rhule J, Cappuccio FP, Emmett L, et al. A community study of health promotion in rural West Africa: details of a household survey and population census. Q J Med. 2002;95:445–450. doi: 10.1093/qjmed/95.7.445. [DOI] [PubMed] [Google Scholar]
- 15.Cappuccio FP, Micah FB, Emmett L, et al. Prevalence, detection, management, and control of hypertension in Ashanti, West Africa. Hypertension. 2004;43:1017–1022. doi: 10.1161/01.HYP.0000126176.03319.d8. [DOI] [PubMed] [Google Scholar]
- 16.UK guidelines for GFR estimations: guidance for creatinine slopes and intercepts. www.birminghamquality.org.uk Accessed 4 September 2009. [Google Scholar]
- 17.Levey AS, Coresh J, Greene T, et al. For Chronic Kidney Disease Epidemiology Collaboration Expressing the Modification of Diet in Renal Disease study equation for estimating glomerular filtration rate with standardised serum creatinine values. Clin Chem. 2007;53:766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 18.DuBois D, DuBois EF. A formula to estimate the approximate surface area if height and weight are known. Arch Intern Med. 1916;17:863–871. [Google Scholar]
- 19.Anonymous Kidney Disease Outcome Quality Initiative. Clinical Practice Guidelines for Chronic Kidney Disease: evaluation, classification and stratification. Am J Kidney Dis. 2002;39:S1–S246. [PubMed] [Google Scholar]
- 20.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 21.Viktorsdottir O, Palsson R, Andresdottir MB, et al. Prevalence of chronic kidney disease based on estimated glomerular filtration rate and proteinuria in Icelandic adults. Nephrol Dial Transplant. 2005;20:1799–1807. doi: 10.1093/ndt/gfh914. [DOI] [PubMed] [Google Scholar]
- 22.Rowe JW, Andres R, Tobin JD, et al. The effect of age on creatinine clearance in men: a cross-sectional and longitudinal study. J Gerontol. 1976;31:155–163. doi: 10.1093/geronj/31.2.155. [DOI] [PubMed] [Google Scholar]
- 23.Jafar TH, Schmid CH, Levey AS. Serum creatinine as marker of kidney function in South Asians: a study of reduced GFR in adults in Pakistan. J Am Soc Nephrol. 2005;16:1413–1419. doi: 10.1681/ASN.2004121100. [DOI] [PubMed] [Google Scholar]
- 24.Kerry SM, Emmett L, Micah FB, et al. Rural and semi-urban differences in salt intake, and its dietary sources, in Ashanti, West Africa. Ethn Dis. 2005;15:33–39. [PubMed] [Google Scholar]
- 25.Kerry SM, Micah FB, Plange-Rhule J, et al. Blood pressure and body mass index in lean rural and semi-urban subjects in West Africa. J Hypertens. 2005;23:1645–1651. doi: 10.1097/01.hjh.0000177536.53409.1a. [DOI] [PubMed] [Google Scholar]
- 26.Cappuccio FP, Kerry SM, Micah FB, et al. A community programme to reduce salt intake and blood pressure in Ghana (IRSCTN 88789643) BMC Public Health. 2006;6:13. doi: 10.1186/1471-2458-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rule AD, Larson TS, Bergstralh EJ, et al. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004;141:929–937. doi: 10.7326/0003-4819-141-12-200412210-00009. [DOI] [PubMed] [Google Scholar]
- 28.Cirillo M, Anastasio P, De Santo NG. Relationship of gender, age, and body mass index to errors in predicted kidney function. Nephrol Dial Transplant. 2005;20:1791–1798. doi: 10.1093/ndt/gfh962. [DOI] [PubMed] [Google Scholar]
- 29.Verhave JC, Gansevoort RT, Hillege HL, et al. Drawbacks of the use of indirect estimates of renal function to evaluate the effect of risk factors on renal function. J Am Soc Nephrol. 2004;15:1316–1322. [PubMed] [Google Scholar]
- 30.Froissart M, Rossert J, Jacquot C, et al. Predictive performance of the Modification of Diet in Renal Disease and Cockcroft–Gault equations for estimating renal function. J Am Soc Nephrol. 2005;16:763–773. doi: 10.1681/ASN.2004070549. [DOI] [PubMed] [Google Scholar]
- 31.Verhave JC, Fesler P, Ribstein J, et al. Estimation of Renal function in subjects with normal serum creatinine levels: influence of age and body mass index. Am J Kidney Dis. 2005;46:233–241. doi: 10.1053/j.ajkd.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 32.van Deventer HE, George JA, Paiker JE, et al. Estimating glomerular filtration rate in black South Africans by use of the modification of diet in renal disease and Cockcroft–Gault equations. Clin Chem. 2008;54:1197–1202. doi: 10.1373/clinchem.2007.099085. [DOI] [PubMed] [Google Scholar]