Abstract
Background. In renal tubules, paracellular permeability is tightly controlled to facilitate solute absorption and urinary concentration and is regulated by tight junctions, which incorporate claudin proteins. There is very limited information confirming the localization of these proteins in the human renal cortex. Most data is inferred from mouse, bovine and rabbit studies and differences exist between mouse and other species.
Methods. A survey of claudin staining was performed on human kidney cortex embedded in glycolmethacrylate resin to enhance tissue morphology and facilitate the cutting of 2 µm serial sections.
Results. Claudin-2, -10 and -11 antibodies labelled renal tubular epithelial cells, correlating with Lotus tetragonolobus and N-cadherin positive proximal tubules. Claudin-3, -10, -11 and -16 antibodies strongly stained a population of tubules that were positive for Tamm Horsfall protein on adjacent sections, confirming expression in the thick ascending limb of the Loop of Henle. Claudin-3, -4 and -8 antibodies reacted with tubules that correlated with the distal nephron markers, E-cadherin, epithelial membrane antigen and Dolichos biflorus and claudin-3, -4, -7 and -8 with the distal tubule marker, calbindin, and the collecting duct marker, aquaporin-2. Claudin-14 was localized in distal convoluted tubules, correlating positively with calbindin but negatively with aquaporin-2, whereas claudin-1 staining was identified in the parietal epithelium of Bowman's capsule, distal convoluted tubule and collecting duct. Cellular and tight junction localization of claudin staining in renal tubules was heterogeneous and is discussed.
Conclusions. Complex variation in the expression of human claudins likely determines paracellular permeability in the kidney. Altered claudin expression may influence pathologies involving abnormalities of absorption.
Keywords: claudins, glycolmethacrylate, paracellular permeability, renal tubule, tight junctions
Introduction
A principal role of the nephron is to regulate the volume and composition of the glomerular filtrate in order to maintain normal physiological homeostasis. The polarized epithelial lining cells of renal tubules have the capacity to actively transport solutes via the transcellular route, whilst also developing a selectively permeable tissue barrier which regulates the movement of solutes and ions paracellularly between cells. The cellular barrier can facilitate large passive solute and ion shifts across the tubule wall during filtrate reabsorption and is also able to inhibit ion and solute movement, when required, to modify the filtrate composition tightly during excretion. In the proximal tubule, around two-thirds of the ultrafiltrate chloride is reabsorbed [1,2] with a significant amount of sodium chloride moving passively by the paracellular route in response to the chloride concentration gradient generated in the early proximal tubule [3,4]. The capacity of the renal tubule lining to maintain tissue integrity, whilst regulating the permeability across the tubule, is dependent on the coordinated assembly of cell–cell tight and adherens junction complexes, which link to the actin cytoskeleton and encircle the tubular epithelial cells. The importance of junctional mechanisms in preventing backleak was demonstrated in proximal tubules of allografts in sustained acute renal failure. These studies showed that downregulation and redistribution of tight and adherens junction complexes away from the apicolateral membrane boundary correlated with significant transtubular backleak of inulin [5]. Characteristic associated transmembrane proteins include the cadherins in adherens junctions [6] and claudins and occludin in tight junctions [7]. In addition to regulating the pathway between cells, tight junctions block the free diffusion of proteins and lipids in the plasma membrane, between the apical and basolateral membrane domains, thus maintaining polarity and correct alignment of epithelial transport mechanisms [8,9] and also contain interacting proteins that regulate differentiation, proliferation and gene expression [10,11].
Tight junctions appear as fusions of opposing plasma membranes in transmission electron microscopy or intramembranous networks of strands and complementary grooves in freeze-fracture electron microscopy [12]. Tight junction transmembrane proteins, occludin, claudins and tricellulin, are tetraspan proteins [13–15]. Claudins have no sequence similarity to the other two, whilst occludin and tricellulin share significant sequence conservation in the COOH terminal 130 amino acids [15]. Claudin proteins comprise a family of at least 24 proteins whose differential expression and properties determine the permeability properties of epithelia [7,16]. When claudins are over expressed in L cells, they polymerize in the membrane to reconstitute paired tight junction strands and they form copolymers of heterogeneous claudins, suggesting that variations in the tightness of individual paired strands are determined by the combinations and ratios of the claudin types [17,18].
We hypothesized that differential expression of claudins is important for the regulation of paracellular permeability in human kidney. Mutations in the human claudin-16 and -19 genes, which are expressed in the Loop of Henle, lead to defective magnesium ion resorption resulting in hypomagnesaemia with nephrocalcinosis and failing kidneys [19,20]. With the exception of claudin-16 and -19, little is known about the expression patterns of claudins in the human nephron. In the kidney, claudin proteins have been identified and mapped in mouse, bovine and rabbit and show various nephron segment-specific expression patterns, which are believed to facilitate the different permeability requirements of various parts of the tubule [16,21–23]. However, some discrepancies exist in the data from these species; therefore, it is important to define the patterns in human kidney. Staining the kidney for these antigens was problematic using frozen and wax sections; however, we found that the glycolmethacrylate (GMA) embedding method [24] gave excellent morphological preservation of 2-µm serial sections for tight junction staining in human kidney. In order to enhance our understanding of the role of claudins in the human cortical nephron, we performed a survey of claudins, based on those known to be expressed in mouse kidney, and compared them with known nephron-specific regional markers [25]. The study demonstrated clear segment-specific distributions of claudins that likely reflects the complexity of ion and solute reabsorption occurring during normal kidney function.
Materials and methods
Tissue acquisition and processing
With ethical approval and obtained consent, cortical renal tissue was excised from nephrectomy specimens, cut into 2 mm3 blocks and immersed in acetone, containing 2 mM phenylmethylsulphonylfluoride and 20 mM iodoacetamide, for up to 16–20 h prior to embedding in GMA resin [24]. After the period of fixation, these specimens were transferred into the GMA monomer and then GMA embedding resin for at least 4 h at 20°C prior to cutting for the preparation of slides and staining. Once polymerized, the blocks were cut into 2-µm sections and mounted on glass slides for immunohistochemistry.
Immunohistochemical staining
The primary antibodies used were mouse monoclonal antibodies against human N- and E-cadherin, claudin-1, -2 and -4, occludin and ZO-1 (Invitrogen, UK), calbindin 28K (Santa Cruz Biotechnology) and human epithelial membrane antigen (Dako, Cambridge, UK). Rabbit polyclonal antibodies against human claudin -3, -7, -8, -11,-14 and -16 (Invitrogen, UK), claudin-10 (Abcam, UK), aquaporin-2 (Lifespan Biosciences) and human Tamm Horsfall protein (THP; Millipore, UK) were used. Biotinylated lectins, Lotus tetragonolobus and Dolichos biflorus, were also used (Vector Labs, UK). Using standard immunohistochemical methods for GMA [24], sections were treated with avidin/biotin blocking kit (Vector Labs, UK) to block endogenous biotin, according to the manufacturer's instructions, and then incubated with Dulbecco's modified Eagle's medium with 20% foetal calf serum (Invitrogen, UK) containing 1% bovine serum albumin (Sigma, UK) for 30 min. Primary antibodies diluted in Tris-buffered saline pH 7.6 (TBS; Sigma, UK) were applied, prior to incubation overnight, at 22°C for monoclonal antibodies or 4°C for polyclonal antibodies. Unspecific negative control antibodies (Vector Labs, UK) of the same monoclonal subclass or polyclonal IgG species were used at the same concentrations as primaries. TBS was used instead of lectin for staining control. The slides were then washed in TBS, 3 × 5 min and incubated with mouse, goat or rabbit biotinylated secondary antibody (Dako, UK) in TBS containing same species normal serum for 2 h, prior to washing 3 × 5 min in TBS. Biotinylated lectins were used to label the proximal tubule (L. tetragonolobus) and distal tubule (D. biflorus) directly by dilution in TBS and incubation overnight at 22°C. StreptABComplex/HRP (Dako, UK) was applied according to the manufacturer’s instructions to both antibody and lectin assays, followed by 3,3′-diaminobenzidine solution (BioGenex, UK) for 10 min and the tissue was counterstained with Meyer's haematoxylin (Sigma, UK). Images were captured and stored using a Zeiss Axioskop light microscope and Axiovision software.
Results
N-cadherin was expressed in the proximal tubule whilst E-cadherin staining predominated in the TAL and distal nephron
Staining of serial 2-µm GMA sections with N-cadherin antibody localized a population of tubules that corresponded to those identified by the proximal tubular marker L. tetragonolobus (Figure 1A and C, asterisks). N-cadherin staining was located at the lateral and basolateral borders of the cells with intense punctate staining at the sub-apical junctional complex region of cells, whereas L. tetragonolobus strongly stained the apical regions and luminal material in the tubules. E-cadherin staining was strongly positive in tubules that correlated with reactivity to the antigen recognized by D. biflorus lectin, which is found in the thick ascending limb (TAL) of the Loop of Henle [26] and a subset of cells in the distal tubule and collecting duct (Figure 1B and D, arrows). E-cadherin staining appeared weak or absent in tubules that corresponded to those clearly positive for L. tetragonolobus and N-cadherin (Figure 1A–C, asterisks). Comparison of the images in Figure 2A and B also showed strong cell border staining for E-cadherin in the N-cadherin negative tubules and weak staining for E-cadherin observed in the N-cadherin positive tubules (Figure 2A and B, asterisks).
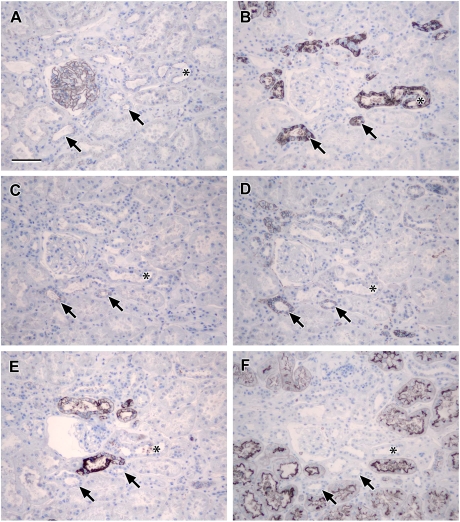
Fig. 1.
Localization of N- and E-cadherin: photomicrographs showing serial sections of human renal cortical tissue immunohistochemically stained for N-cadherin (A), E-cadherin (B), L. tetragonolobus (C) and D. biflorus (D) [note that anatomically similar proximal structures were recognized by N-cadherin antibody and L. tetragonolobus where E-cadherin staining was weak or absent (asterisks, A–C); strong E-cadherin was seen in coincident structures that were negative for N-cadherin and L. tetragonolobus, but positive for D. biflorus (arrows, B and D), indicating strong expression in the distal nephron (TAL or DCT) or collecting duct; lectins L. tetragonolobus and D. biflorus stained in a different cellular pattern to the N- and E-cadherin antibodies, as they label carbohydrates present on the surface of the RTECs whereas the cadherins stain the lateral cell borders; scale bar 100 µm].
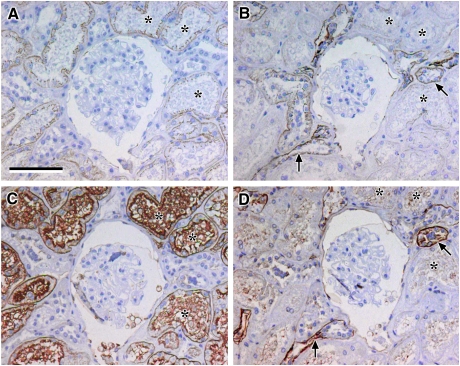
Fig. 2.
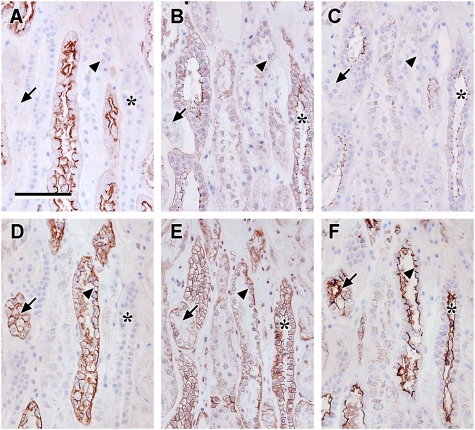
Localization of claudin-2, -10 and -11: photomicrographs showing serial sections of human renal cortical tissue stained for N-cadherin (A), E-cadherin (B), claudin-2 (C), L. tetragonolobus (D), claudin-10 (E), D. biflorus (F) and claudin-11 (G); representative negative control (H) [N-cadherin and claudin-2 stained strongly positive in L. tetragonolobus positive proximal tubules which showed faint discrete junctional staining for claudin-10 and E-cadherin, faint cytoplasmic staining for claudin-11 and negative for D. biflorus (asterisks, A–G); in addition, strongly positive claudin-10 and -11 staining coincided with strong E-cadherin staining in tubules with a subpopulation of cells intensely stained with D. biflorus suggestive of TAL (arrows); scale bar 100 µm].
Immunolocalization of claudin-2 identified a proximal tubular population that was coincident with N-cadherin positive tubules
Claudin-2 staining was localized in a subpopulation of tubules that correlated positively with those identified by N-cadherin antibody and L. tetragonolobus (Figure 2A, C and D, asterisks). Claudin-2 staining was seen at the lateral and basolateral cell borders and often concentrated in a punctate sub-apical pattern characteristic of tight junctions.
Claudin-10 and -11 were detected in similar regions of the nephron and overlap with both claudin-2 and E-cadherin
There was discrete, punctate immunostaining for claudin-10 and weak cytoplasmic claudin-11 staining that corresponded to tubular cells that were strongly stained with N-cadherin, claudin-2 and L. tetragonolobus and weakly stained with E-cadherin (Figure 2A–E and G, asterisks), indicating low proximal tubular expression of claudin-10 and -11. Immunostaining for claudin-10 and -11 also coincided with a subset of strongly E-cadherin positive tubules (Figure 2B, E and G, arrows) where claudin-10 staining was seen at the basolateral and sub-apical borders of the cells and claudin-11 appeared apically located. This stronger staining also corresponded to strong but heterogeneous staining with D. biflorus lectin (Figure 2F, arrows), suggesting that claudin-10 and -11 could be prominently expressed in the TAL of the Loop of Henle.
Immunostaining of claudin-10 and -11 also corresponded to tubules identified as the TAL of the Loop of Henle
To further analyse the location of claudin-10 and -11 staining, serial sections were stained with claudin-10, -11 and -16, THP or uromodulin, calbindin and L. tetragonolobus. The brighter claudin-10 and -11 stained regions of the kidney cortex corresponded to strongly claudin-16 positive regions of the serial sections that were also THP positive, but calbindin and L. tetragonolobus negative, confirming that claudin-10 and -11 were most strongly positive in the TAL of the Loop of Henle and not expressed in human distal convoluted tubule or collecting duct (Figure 3A–F, asterisks). It was noted that claudin-10 and -11 staining was also seen in cells morphologically consistent with the macula densa of the TAL (Figure 3A and C). Claudin-16 staining was often observed basolaterally in corresponding tubules showing discrete occludin-stained punctae characteristic of tight junctions (data not shown). In addition, a weak cytoplasmic stain was seen in the proximal tubules similar to that observed with claudin-11 antibodies, which is most likely non-specific staining.
Fig. 3.
Localization of claudin-10 and -11 compared to claudin-16, THP and calbindin: photomicrographs showing serial sections of human renal cortical tissue immunohistochemically stained for claudin-10 (A), L. tetragonolobus (B), claudin-11 (C), THP (D), claudin-16 (E) and calbindin (F) [very faint discrete claudin-10 staining was seen in junctional areas of tubules coincident with L. tetragonolobus (arrows in A and B); robust claudin-10, -11 and -16 staining (asterisks in A, C and E) was seen in tubules coincident with those stained by THP, but not L. tetragonolobus or calbindin (asterisks in B, D and F); taken together, these data indicate that claudins-10, -11 and -16 are expressed in the TAL; scale bar 100 µm].
Claudin-3, -4 and -8 staining correlated with distal-type E-cadherin positive tubules
Claudin-3, -4 and -8 staining was clearly present in the lateral cell borders of tubules that corresponded to the strongly E-cadherin positive tubules in serial sections (Figure 4A, C, E and G, asterisks). Staining was absent or at background levels, similar to negative controls (Figure 4H), in tubules that corresponded to N-cadherin and L. tetragonolobus positive proximal tubules (Figure 4B and F), suggesting that these claudins are most strongly represented in the distal tubule or collecting ducts. In addition, discrete, weak claudin-3 staining was observed in structures that corresponded to tubules with a subset of cells picked out strongly by D. biflorus lectin, suggesting that claudin-3 may be expressed in the TAL.
Fig. 4.
Localization of claudin-3, -4 and -8: photomicrographs showing serial sections of human renal cortical tissue stained for E-cadherin (A), N-cadherin (B), claudin-8 (C), D. biflorus (D), claudin-3 (E), L. tetragonolobus (F) and claudin-4 (G); representative negative control (H) [discrete tight junction-type claudin-3, -4 and -8 staining coincided with strong positive E-cadherin staining (asterisks, A–G), suggesting a distal nephron or collecting duct distribution; note that the staining was in anatomically different tubules to N-cadherin (B) and L. tetragonolobus (F) positive tubules; claudin-3 also appeared to stain tubules that were coincident with strong E-cadherin and D. biflorus, revealing possible TAL location (arrows in A, C, E and G); scale bar 100 µm].
Claudin-3 staining was positive in cells that corresponded to the TAL of the Loop of Henle identified by THP staining
In view of the indication that claudin-3 was positive in strongly D. biflorus-stained tubules, suggesting a TAL location, claudin-3, -4 and -8 staining was compared to serial sections stained with THP, EMA and L. tetragonolobus. Staining for all three claudins corresponded to tubules negative for L. tetragonolobus and positive for EMA, confirming their distal tubule or collecting duct location. THP recognized a subset of EMA positive tubules (Figure 5D and F, arrows), thus defining them as TAL, which coincided positively with claudin-3 staining and negatively with claudin-4 and -8 (Figure 5B, C and E, arrows). This indicated that claudin -3 was expressed in the TAL in contrast to claudin-4 and -8.
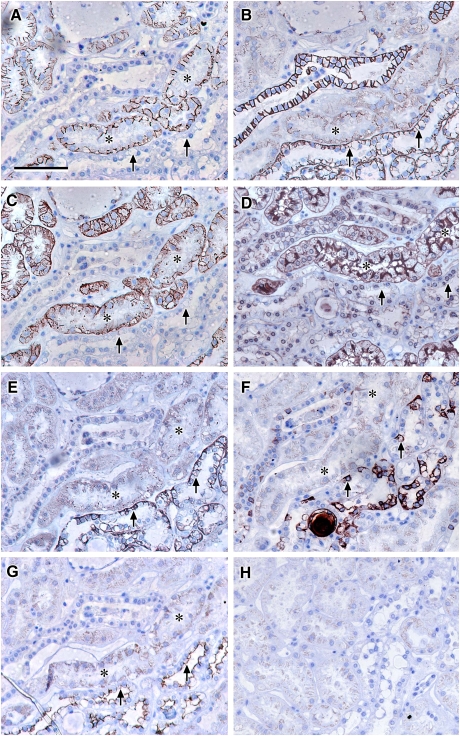
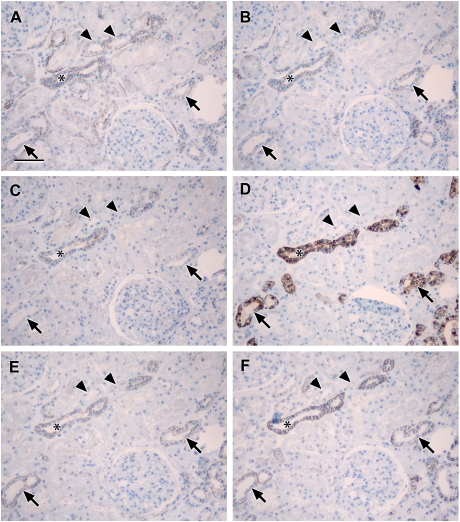
Fig. 5.
Localization of claudin-3, -4 and -8 compared to THP: photomicrographs showing serial sections of human renal cortical tissue stained for L. tetragonolobus (A), claudin-8 (B), claudin-4 (C), THP (D), claudin-3 (E) and EMA (F) [claudin-8, -4 and -3 stained tubules that were coincident with similar ones that were positive for EMA and negative for L. tetragonolobus (asterisks in A–C, E and F), confirming the expression of these claudins in the distal nephron (TAL or DCT) or collecting duct; THP staining was coincident with a subpopulation of EMA positive tubules that was positive for claudin-3 and not claudin-4 or -8 or L. tetragonolobus, indicating that claudin-3 is expressed in the TAL (arrows and arrowheads in A–F); scale bar 100 µm].
Claudin-3, -4, -7 and -8 are expressed in the distal tubule and collecting duct
In order to differentiate between the distal tubule and collecting duct, staining for claudin-3, -4, -7 and -8 was compared with aquaporin-2 to identify the collecting duct and calbindin, a marker of the distal tubule and collecting duct. Claudin-3, -4, -8 and -7 were stained in tubules that were coincident with tubules that were positive for aquaporin-2 and calbindin, confirming expression in the collecting duct (Figure 6A–F, asterisks), but also in calbindin positive, aquaporin-2 negative tubules, confirming the expression of these claudins in distal tubules (Figure 6A–F, arrows). Claudins-3, -4 and -8 showed discrete punctuate junctional staining. In addition, claudin-3 and -7 showed strong basolateral cell membrane staining. Claudin-3 also strongly stained additional tubules, which were negative for claudin-4, -7, -8, aquaporin-2 and calbindin that appeared morphologically similar to TAL (Figure 6A–F, arrowheads). There was also weak basolateral claudin-3 staining in proximal-type tubules in some human specimens (Figures 5E and 6A). In order to obtain evidence of the presence of intact tight junctions in tubules corresponding to those with basolateral claudin staining, serial adjacent sections were stained with claudin-3,-4 and -7 and occludin. This showed that occludin was localized in discrete punctuate patterns indicative of fully assembled tight junctions in similar tubules with basolateral claudin-3 and -7 (Figure 8C–F, asterisks).
Fig. 6.
Localization of claudin-3, -4 -7 and -8 compared to aquaporin-2 and calbindin: photomicrographs showing serial sections of human renal cortical tissue stained for claudin-3 (A), claudin-4 (B), aquaporin-2 (C), calbindin (D), claudin-8 (E) and claudin-7 (F) [claudin-3, -4, -8 and -7 stained tubules that were coincident with similar ones that were positive for aquaporin-2 and calbindin (asterisks in A–F) but were also localized in calbindin positive, aquaporin-2 negative tubules (arrowheads in A–F); claudin-4, -7 and -8 showed similar staining patterns, whereas claudin-3 strongly stained additional tubules which were negative for claudin-4, -7, -8, aquaporin-2 and calbindin (arrows in A–F); scale bar 100 µm].
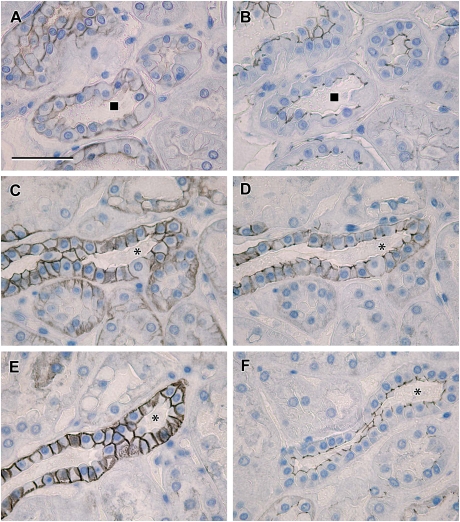
Fig. 8.
High-magnification images comparing claudin localization with ZO-1 and occludin: photomicrographs showing serial sections of human renal cortical tissue stained for claudin-14 (A) and ZO-1 (B) with a second sequence of serial sections showing staining of claudin-3 (C), -4 (D) and -7 (E) with occludin (F) [claudin-14 was seen in basolateral locations in tubules corresponding to those with discrete tight junction staining for ZO-1 (A and B, square symbol marks corresponding tubule); in serial sections from the same tissue sample, claudin-4 was observed to be mostly in discrete tight junction staining patterns (D), whereas claudin-3 and -7 showed strong basolateral cell membrane staining in addition to tight junction staining (C and E, asterisks mark corresponding tubule); in corresponding tubules, occludin was stained in a discrete pattern consistent with tight junction localization (F); scale bar 50 µm].
Claudin-14 is expressed in the distal convoluted tubule in human kidney
Claudin-14 stained tubules were coincident with similar ones that were positive for calbindin and negative for aquaporin-2 (Figure 7A–C, asterisks), confirming the expression of claudin-14 in the distal tubule. Some claudin-14 positive tubules corresponded in serial sections with THP negative tubules, however there were occasional hints of THP staining apically or lumenally (Figure 7D), consistent with the distal convoluted tubule. Claudin-14 staining often appeared in a basolateral membrane location, corresponding with tubules showing discrete junctional ZO-1 staining (Figures 7C and F, asterisks and 8A and B, squares), showing that the tight junctions were intact in these specimens after fixation and staining.
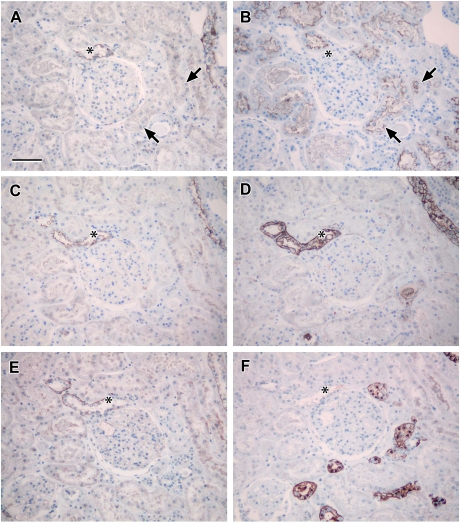
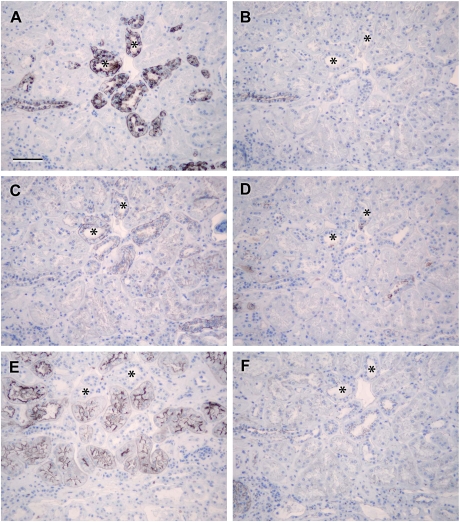
Fig. 7.
Localization of claudin-14: photomicrographs showing serial sections of human renal cortical tissue stained for calbindin (A), aquaporin-2 (B), claudin-14 (C), THP (D), L. tetragonolobus (E) and ZO-1 (F) [claudin-14 stained tubules that were coincident with similar ones that were positive for calbindin (asterisks in A and C) and negative for aquaporin-2, THP and L. tetragonolobus (asterisks in B, D and E), confirming the expression of claudin-14 in the distal tubule; basolateral claudin-14 staining corresponded with tubules showing discrete junctional ZO-1 staining (asterisks in C and F); scale bar 100 µm].
Claudin-1 was expressed in the parietal epithelium of Bowman's capsule, distal tubule and collecting duct
In order to ascertain the expression of claudin-1 in human kidney, staining was done on serial sections with L. tetragonolobus, THP, calbindin, aquaporin-2 and ZO-1. Claudin-1 stained tubules were coincident with similar ones positive for calbindin and aquaporin-2 and negative for THP and L. tetragonolobus (Figure 9B–F, arrows), confirming the expression of claudin-1 in the distal tubule and collecting duct. There were some additional calbindin positive tubules that corresponded to areas negative or intermittent for claudin-1, negative or luminal only for THP and negative for aquaporin-2, suggesting that claudin-1 is not expressed in the entire distal tubule (Figure 9B–E, asterisks). Claudin-1 was also seen in the parietal epithelium of Bowman's capsule. Claudin-1 staining often appeared in a basolateral membrane location, corresponding with tubules showing discrete junctional ZO-1 staining (Figure 9A and D, asterisks). ZO-1 staining was most prominent in glomerular epithelial cells where it appeared continuous with the cell membrane, with faint junctional staining in proximal tubules and discrete stronger junctional staining in distal tubules and collecting ducts (Figure 9A) consistent with previous studies [27].
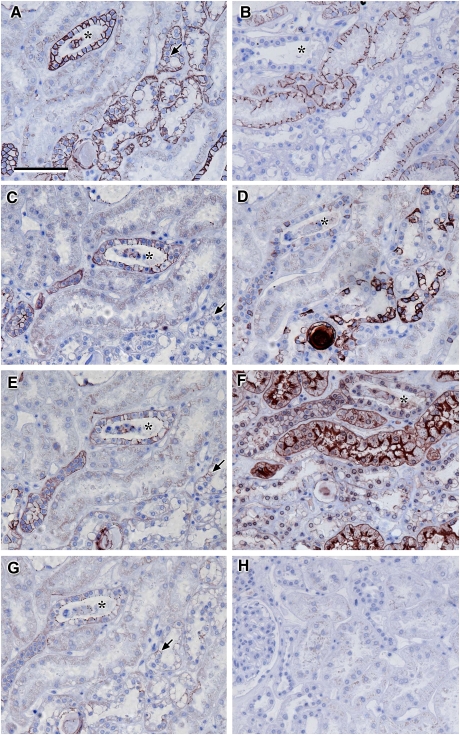
Fig. 9.
Localization of claudin-1: photomicrographs showing serial sections of human renal cortical tissue stained for ZO-1 (A), calbindin (B), aquaporin-2 (C), claudin-1 (D), THP (E) and L. tetragonolobus (F) [claudin-1-, calbindin- and aquaporin-2-stained coincident tubules that were negative for THP and L. tetragonolobus (arrowheads in B–F); there were some calbindin positive tubules that were negative for claudin-1, THP and aquaporin-2 (asterisks in B–E); claudin-1 basolateral membrane location, corresponding with tubules showing discrete junctional ZO-1 staining (arrowheads in A and D); robust ZO-1 staining was seen in glomerular epithelial cells (A); scale bar 100 µm].
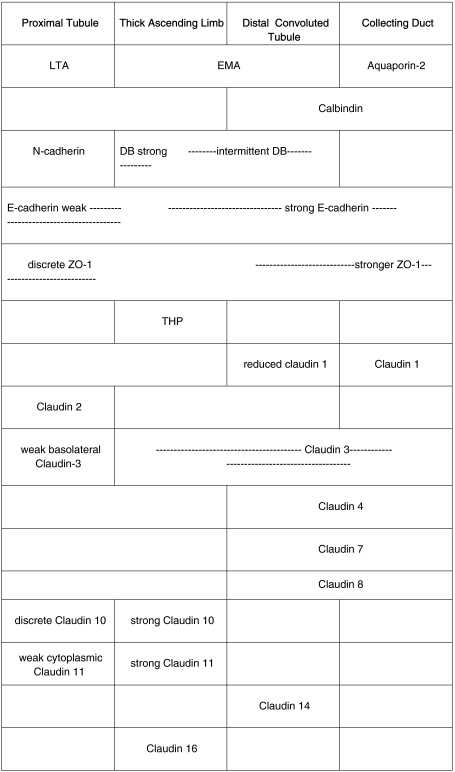
A summary of staining is represented in Table 1.
Table 1.
Expression of claudins in human kidney
Summary of the expression of human claudin-2, -3, -4, -7, -8, -10, -11, -14 and -16 compared with nephron segment-specific marker proteins.
Discussion
To date, there are only sporadic reports directly reporting on claudin expression in human kidney [19,20,28,29]. Therefore, building on previous studies, mainly in mouse [16,22,30–33] but also in rabbit [23] and bovine [34], we hypothesized that claudins would be differentially expressed in human cortical tubular epithelium. Importantly, we have used a method of processing and embedding the tissue in GMA resin, which facilitates the cutting of 2-µm plastic sections which retain antigenicity for many antibodies as fixation is in acetone [24]. These thinner sections exhibited much better preservation of tissue architecture than frozen or wax-embedded kidney tissue (data not shown) and allowed close comparison of antibody labelling patterns with same species antibodies in serial sections, underlining the value of GMA embedding of renal specimens.
This study showed that claudin-1 antibodies stained the parietal epithelium of Bowman's capsule and that claudin-2, -10 and -11 are expressed in the human proximal tubule, as evidenced by staining on serial GMA sections of similar tubules coincident with N-cadherin and L. tetragonolobus, markers of the proximal tubule [25,35]. Claudin-3, -10 and -11 were prominently expressed in the TAL of the Loop of Henle as shown by staining that coincided with THP, D. biflorus, EMA and strong E-cadherin in similar tubules on serial sections [26,35]. Claudin-16 localization in the human TAL was confirmed by positive correlation with THP. Staining of claudin-3, -4 and -8 was identified in the distal tubule and collecting duct, since coincident tubules on serial sections were positive for EMA, E-cadherin and D. biflorus [25,35,36]. Discrimination with calbindin and aquaporin-2 staining [37] indicated that claudin-1, -3, -4, -7 and -8 were present in distal convoluted tubule and collecting duct, whereas claudin-14 could only be confidently confirmed in the distal convoluted tubule. Together, THP, calbindin and aquaporin-2 provided superior TAL, distal tubule and collecting duct markers than the D. biflorus lectin, which was found to give very heterogeneous staining in the TAL, reducing in intensity in more distal structures [26].
Since tight junctions play a central role in the regulation of paracellular permeability [38], these complex patterns of expression are presumably structural adaptations required for the nephron to fulfil its physiological requirements related to control of fluid and ion homeostasis. Claudin-2 is predominantly cation-selective and leaky, claudin-4 is cation-selective and tight [18,39–42], claudin-10a is anion-selective and leaky [32] and combinations of heterologous claudins in cells may be unpredictable in their combined effect on barrier permeability. Therefore, it is envisaged that claudin-2 predominates in the proximal tubule, facilitating the large solute shifts required for sodium reabsorption, whereas claudin-4 and -8 predominate in the distal region of the tubule where the filtrate is more concentrated and paracellular ion flow is attenuated. Claudin-10 was expressed in the human proximal tubule in addition to claudin-2. The antibody to claudin-10 used here would not discriminate between the existence of -10a and -10b splice variants, but hypothetically, the discrete claudin-10 staining in the proximal tubule could be -10a, given its cortical location [32]. The weak cytoplasmic staining of claudin-11 and -16 in proximal tubules was not convincing, although another study showed some cytoplasmic staining with claudin-11 antibodies in mouse kidney [22]. One problem with the use of human tissue is the ischaemia time prior to tissue fixation. Anecdotally, it was observed that, the longer the time before dissection and fixation of human kidney tissue, the more disrupted the tight junction staining became, particularly in the proximal tubule; therefore, some of the cytoplasmic staining in our samples may have been influenced by this factor.
In the human TAL, claudin-3 was expressed in agreement with data from the mouse [22] and differed from bovine kidney [34]. Claudin-10 and -11 were robustly expressed in the TAL and not in the distal convoluted tubule, consistent with previous studies [22,45]. The strong membrane staining for claudin-11 seen in the TAL often appeared to be apical; however, this might be an artefact of sectioning where strong sub-apical staining is present, as has been described for claudin-3 in the mouse distal tubule [22], since there was also evidence of a tight junction location in many cells. An increasing repertoire of claudins has been documented in the human TAL, including claudin-16 and -19 which have been shown to cooperate physically to form cation-selective channels that are important in driving the reabsorption of magnesium ions [19,20,46]. Claudins-3, -10 and -11 may be required to facilitate the generation of ion currents that maintain homeostatic electrolyte balance in the TAL, but are presumably not able to compensate for the loss of functional claudin-16 or -19 [47–50].
Claudin-3, -4, -7 and -8 were strongly expressed in tight junctions in the human distal convoluted tubule and collecting ducts, in agreement with studies in mouse [22], whereas in contrast to mouse [22], claudin-1 was detected heterogeneously staining the human distal convoluted tubule in addition to the parietal epithelium of the capsule. Claudin-14 was confirmed in the distal convoluted tubule in contrast to mouse studies that suggested expression in the collecting duct [31] and proximal tubule and Loop of Henle [51]. This may relate to species differences, and we would not exclude the possibility of detecting expression in proximal tubules with improved reagents. Mutations in human claudin-14 show association with kidney stones and reduced bone mineral density, correlating with increased urinary calcium, suggesting the involvement of claudin-14 in calcium reabsorption [52]. The localization of claudin-14 in the distal convoluted tubule may indicate a direct role for claudin-14, given that regulated calcium reabsorption occurs in the human distal convoluted tubule [53].
In human kidney, claudin subcellular localization was heterogeneous. In the distal tubule and collecting duct, claudin-4 and -8 staining was mostly located at discrete junctional sites; however, a number of other renal claudins were detected junctionally and basolaterally. In the proximal tubule, as defined by L. tetragonolobus, claudin-2 was observed in punctuate spots characteristic of tight junctions, but also strongly expressed at basolateral cell surfaces. This basolateral localization is in contrast to claudin-2 staining seen in other tissue locations, including the colonic epithelium [43], although, consistently with our study, claudin-7 was reported in a basolateral location with claudin-8 in a tight junctional location in mouse [33] and rabbit distal tubules [23]. The staining patterns we observed are unlikely to be an artefact of fixation or staining since we detected discrete junctional ZO-1 and occludin in serial sections of the same tissue blocks with no basolateral staining. Potentially, claudins may be performing other roles or dynamically regulated at tight junctions in response to physiological demands. When large paracellular sodium shifts are required during high rates of glomerular filtration, significant amounts of sodium chloride are transported passively by the paracellular route [3, 4]. In this context, it is of interest that salinity has been reported to regulate claudin expression and localization in the teleost gill [44].
Conclusion
In summary, understanding the functional relationships among claudins and the regulation of their complex renal expression patterns may ultimately explain the changes seen in pathologies associated with tubular dysfunction and aid in the design of novel therapeutic options.
Acknowledgments
This work was funded by the Wessex Renal Research and Transplant Unit, Queen Alexandra Hospital, Cosham, Portsmouth UK. We would like to thank John Ward and Susan Wilson from the University of Southampton Medical School Histochemistry Research Unit for the technical support and Anton Page from the Southampton Medical School Bioimaging Unit for help with the figures.
Conflict of interest statement. None declared.
References
- 1.Rector FC., Jr Sodium, bicarbonate, and chloride absorption by the proximal tubule. Am J Physiol. 1983;244:F461–F471. doi: 10.1152/ajprenal.1983.244.5.F461. [DOI] [PubMed] [Google Scholar]
- 2.Berry CA, Rector FC., Jr Mechanism of proximal NaCl reabsorption in the proximal tubule of the mammalian kidney. Semin Nephrol. 1991;11:86–97. [PubMed] [Google Scholar]
- 3.Aronson PS, Giebisch G. Mechanisms of chloride transport in the proximal tubule. Am J Physiol. 1997;273:F179–F192. doi: 10.1152/ajprenal.1997.273.2.F179. [DOI] [PubMed] [Google Scholar]
- 4.Shah M, Quigley R, Baum M. Maturation of rabbit proximal straight tubule chloride/base exchange. Am J Physiol. 1998;274:F883–F888. doi: 10.1152/ajprenal.1998.274.5.F883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwon O, Nelson WJ, Sibley R, et al. Backleak, tight junctions, and cell–cell adhesion in postischemic injury to the renal allograft. J Clin Invest. 1998;101:2054–2064. doi: 10.1172/JCI772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halbleib JM, Nelson WJ. Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006;20:3199–3214. doi: 10.1101/gad.1486806. [DOI] [PubMed] [Google Scholar]
- 7.Krause G, Winkler L, Mueller SL, et al. Structure and function of claudins. Biochim Biophys Acta. 2008;1778:631–645. doi: 10.1016/j.bbamem.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 8.Dragsten PR, Blumenthal R, Handler JS. Membrane asymmetry in epithelia: is the tight junction a barrier to diffusion in the plasma membrane? Nature. 1981;294:718–722. doi: 10.1038/294718a0. [DOI] [PubMed] [Google Scholar]
- 9.van MG, Simons K. The function of tight junctions in maintaining differences in lipid composition between the apical and the basolateral cell surface domains of MDCK cells. EMBO J. 1986;5:1455–1464. doi: 10.1002/j.1460-2075.1986.tb04382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guillemot L, Paschoud S, Pulimeno P, et al. The cytoplasmic plaque of tight junctions: a scaffolding and signalling center. Biochim Biophys Acta. 2008;1778:601–613. doi: 10.1016/j.bbamem.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 11.Balda MS, Matter K. Tight junctions and the regulation of gene expression. Biochim Biophys Acta. 2009;1788:761–767. doi: 10.1016/j.bbamem.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 12.Staehelin LA. Structure and function of intercellular junctions. Int Rev Cytol. 1974;39:191–283. doi: 10.1016/s0074-7696(08)60940-7. 191–283. [DOI] [PubMed] [Google Scholar]
- 13.Furuse M, Hirase T, Itoh M, et al. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furuse M, Sasaki H, Fujimoto K, et al. A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J Cell Biol. 1998;143:391–401. doi: 10.1083/jcb.143.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikenouchi J, Furuse M, Furuse K, et al. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol. 2005;171:939–945. doi: 10.1083/jcb.200510043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angelow S, Yu AS. Claudins and paracellular transport: an update. Curr Opin Nephrol Hypertens. 2007;16:459–464. doi: 10.1097/MNH.0b013e32820ac97d. [DOI] [PubMed] [Google Scholar]
- 17.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 18.Furuse M, Furuse K, Sasaki H, et al. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J Cell Biol. 2001;153:263–272. doi: 10.1083/jcb.153.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simon DB, Lu Y, Choate KA, et al. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science. 1999;285:103–106. doi: 10.1126/science.285.5424.103. [DOI] [PubMed] [Google Scholar]
- 20.Konrad M, Schaller A, Seelow D, et al. Mutations in the tight-junction gene claudin 19 (CLDN19) are associated with renal magnesium wasting, renal failure, and severe ocular involvement. Am J Hum Genet. 2006;79:949–957. doi: 10.1086/508617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balkovetz DF. Claudins at the gate: determinants of renal epithelial tight junction paracellular permeability. Am J Physiol Renal Physiol. 2006;290:F572–F579. doi: 10.1152/ajprenal.00135.2005. [DOI] [PubMed] [Google Scholar]
- 22.Kiuchi-Saishin Y, Gotoh S, Furuse M, et al. Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J Am Soc Nephrol. 2002;13:875–886. doi: 10.1681/ASN.V134875. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez-Mariscal L, Namorado MC, Martin D, et al. The tight junction proteins claudin-7 and -8 display a different subcellular localization at Henle's loops and collecting ducts of rabbit kidney. Nephrol Dial Transplant. 2006;21:2391–2398. doi: 10.1093/ndt/gfl255. [DOI] [PubMed] [Google Scholar]
- 24.Britten KM, Howarth PH, Roche WR. Immunohistochemistry on resin sections: a comparison of resin embedding techniques for small mucosal biopsies. Biotech Histochem. 1993;68:271–280. doi: 10.3109/10520299309105629. [DOI] [PubMed] [Google Scholar]
- 25.Nouwen EJ, Dauwe S, van dB I, et al. Stage- and segment-specific expression of cell-adhesion molecules N-CAM, A-CAM, and L-CAM in the kidney. Kidney Int. 1993;44:147–158. doi: 10.1038/ki.1993.225. [DOI] [PubMed] [Google Scholar]
- 26.Engel U, Breborowicz D, Bog-Hansen T, et al. Lectin staining of renal tubules in normal kidney. APMIS. 1997;105:31–34. doi: 10.1111/j.1699-0463.1997.tb00536.x. [DOI] [PubMed] [Google Scholar]
- 27.Schnabel E, Anderson JM, MG FARQUHAR. The tight junction protein ZO-1 is concentrated along slit diaphragms of the glomerular epithelium. J Cell Biol. 1990;111:1255–1263. doi: 10.1083/jcb.111.3.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li L, Yao JL, di Sant'agnese PA, et al. Expression of claudin-7 in benign kidney and kidney tumors. Int J Clin Exp Pathol. 2008;1:57–64. [PMC free article] [PubMed] [Google Scholar]
- 29.Yu AS, Kanzawa SA, Usorov A, et al. Tight junction composition is altered in the epithelium of polycystic kidneys. J Pathol. 2008;216:120–128. doi: 10.1002/path.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Enck AH, Berger UV, Yu AS. Claudin-2 is selectively expressed in proximal nephron in mouse kidney. Am J Physiol Renal Physiol. 2001;281:F966–F974. doi: 10.1152/ajprenal.2001.281.5.F966. [DOI] [PubMed] [Google Scholar]
- 31.Ben-Yosef T, Belyantseva IA, Saunders TL, et al. Claudin 14 knockout mice, a model for autosomal recessive deafness DFNB29, are deaf due to cochlear hair cell degeneration. Hum Mol Genet. 2003;12:2049–2061. doi: 10.1093/hmg/ddg210. [DOI] [PubMed] [Google Scholar]
- 32.Van Itallie CM, Rogan S, Yu A, et al. Two splice variants of claudin-10 in the kidney create paracellular pores with different ion selectivities. Am J Physiol Renal Physiol. 2006;291:F1288–F1299. doi: 10.1152/ajprenal.00138.2006. [DOI] [PubMed] [Google Scholar]
- 33.Li WY, Huey CL, Yu AS. Expression of claudin-7 and -8 along the mouse nephron. Am J Physiol Renal Physiol. 2004;286:F1063–F1071. doi: 10.1152/ajprenal.00384.2003. [DOI] [PubMed] [Google Scholar]
- 34.Ohta H, Adachi H, Takiguchi M, et al. Restricted localization of claudin-16 at the tight junction in the thick ascending limb of Henle's loop together with claudins 3, 4, and 10 in bovine nephrons. J Vet Med Sci. 2006;68:453–463. doi: 10.1292/jvms.68.453. [DOI] [PubMed] [Google Scholar]
- 35.Silva FG, Nadasdy T, Laszik Z. Immunohistochemical and lectin dissection of the human nephron in health and disease. Arch Pathol Lab Med. 1993;117:1233–1239. [PubMed] [Google Scholar]
- 36.Abuazza G, Becker A, Williams SS, et al. Claudins 6, 9, and 13 are developmentally expressed renal tight junction proteins. Am J Physiol Renal Physiol. 2006;291:F1132–F1141. doi: 10.1152/ajprenal.00063.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biner HL, Arpin-Bott MP, Loffing J, et al. Human cortical distal nephron: distribution of electrolyte and water transport pathways. J Am Soc Nephrol. 2002;13:836–847. doi: 10.1681/ASN.V134836. [DOI] [PubMed] [Google Scholar]
- 38.Madara JL. Regulation of the movement of solutes across tight junctions. Annu Rev Physiol. 1998;60:143–159. doi: 10.1146/annurev.physiol.60.1.143. 143–159. [DOI] [PubMed] [Google Scholar]
- 39.Van IC, Rahner C, Anderson JM. Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J Clin Invest. 2001;107:1319–1327. doi: 10.1172/JCI12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Itallie CM, Fanning AS, Anderson JM. Reversal of charge selectivity in cation or anion-selective epithelial lines by expression of different claudins. Am J Physiol Renal Physiol. 2003;285:F1078–F1084. doi: 10.1152/ajprenal.00116.2003. [DOI] [PubMed] [Google Scholar]
- 41.Colegio OR, Van Itallie CM, McCrea HJ, et al. Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am J Physiol Cell Physiol. 2002;283:C142–C147. doi: 10.1152/ajpcell.00038.2002. [DOI] [PubMed] [Google Scholar]
- 42.Colegio OR, Van IC, Rahner C, et al. Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture. Am J Physiol Cell Physiol. 2003;284:C1346–C1354. doi: 10.1152/ajpcell.00547.2002. [DOI] [PubMed] [Google Scholar]
- 43.Prasad S, Mingrino R, Kaukinen K, et al. Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Lab Invest. 2005;85:1139–1162. doi: 10.1038/labinvest.3700316. [DOI] [PubMed] [Google Scholar]
- 44.Tipsmark CK, Baltzegar DA, Ozden O, et al. Salinity regulates claudin mRNA and protein expression in the teleost gill. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1004–R1014. doi: 10.1152/ajpregu.00112.2007. [DOI] [PubMed] [Google Scholar]
- 45.Inai T, Sengoku A, Guan X, et al. Heterogeneity in expression and subcellular localization of tight junction proteins, claudin-10 and -15, examined by RT-PCR and immunofluorescence microscopy. Arch Histol Cytol. 2005;68:349–360. doi: 10.1679/aohc.68.349. [DOI] [PubMed] [Google Scholar]
- 46.Weber S, Hoffmann K, Jeck N, et al. Familial hypomagnesaemia with hypercalciuria and nephrocalcinosis maps to chromosome 3q27 and is associated with mutations in the PCLN-1 gene. Eur J Hum Genet. 2000;8:414–422. doi: 10.1038/sj.ejhg.5200475. [DOI] [PubMed] [Google Scholar]
- 47.Angelow S, El-Husseini R, Kanzawa SA, et al. Renal localization and function of the tight junction protein, claudin-19. Am J Physiol Renal Physiol. 2007;293:F166–F177. doi: 10.1152/ajprenal.00087.2007. [DOI] [PubMed] [Google Scholar]
- 48.Kausalya PJ, Amasheh S, Gunzel D, et al. Disease-associated mutations affect intracellular traffic and paracellular Mg2+ transport function of claudin-16. J Clin Invest. 2006;116:878–891. doi: 10.1172/JCI26323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hou J, Renigunta A, Konrad M, et al. Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex. J Clin Invest. 2008;118:619–628. doi: 10.1172/JCI33970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gunzel D, Yu AS. Function and regulation of claudins in the thick ascending limb of Henle. Pflugers Arch. 2009;458:77–88. doi: 10.1007/s00424-008-0589-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Elkouby-Naor L, Abassi Z, Lagziel A, et al. Double gene deletion reveals lack of cooperation between claudin 11 and claudin 14 tight junction proteins. Cell Tissue Res. 2008;333:427–438. doi: 10.1007/s00441-008-0621-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thorleifsson G, Holm H, Edvardsson V, et al. Sequence variants in the CLDN14 associate with kidney stones and bone mineral density. Nat Genet. 2009;41:926–930. doi: 10.1038/ng.404. [DOI] [PubMed] [Google Scholar]
- 53.Friedman PA, Gesek FA. Hormone-responsive Ca2+ entry in distal convoluted tubules. J Am Soc Nephrol. 1994;4:1396–1404. doi: 10.1681/ASN.V471396. [DOI] [PubMed] [Google Scholar]