As research in developmental and clinical sciences has progressed in the last decades, there have been many important technological and methodological advances in the increasingly complimentary fields of molecular genetics and neuroimaging. These advances have facilitated fruitful collaboration across once disparate disciplines, with early results shedding new light on the mechanisms giving rise to individual differences in complex behaviors and related psychiatric disorders. At the leading edge of such efforts is imaging genetics, an experimental strategy for the effective integration of molecular genetics and neuroimaging technologies for the study of biological mechanisms mediating individual differences in behavior and related risk for psychiatric disorders. Imaging genetic studies have the potential to provide a more complex and nuanced understanding of the pathways and mechanisms through which the dynamic interplay of genes, brain, and environment shapes variability in behavior. The broader potential of imaging genetics is to inform risk and resiliency; however, it is likely to be realized only through its orchestrated application within longitudinal developmental studies. To date, no imaging genetic studies of development or of childhood psychiatric disorders have yielded published results, although such studies are underway. The results of these studies may have important implications for the diagnosis and treatment of such psychiatric disorders.
WHY STUDY GENES?
Genes have an unparalleled potential impact on all levels of biology. In the context of disease states, particularly behavioral disorders, genes are fundamental to our understanding of the mechanisms involved in the development of disease. Whereas most human behaviors cannot be explained by genes alone, and certainly much of the variance in aspects of brain information processing will not be genetically determined directly, variations in a genetic sequence that have an impact on gene function will contribute a substantial amount of variance to these more complex phenomena. This conclusion is implicit in results garnered from twin studies that have demonstrated heritabilities of 40% to 70% for various aspects of cognition, temperament, and personality.5 Psychiatric illnesses cluster within families, suggesting a highly heritable component to disease susceptibility.6-8 Genes, therefore, have the potential to identify underlying mechanisms of variability in behavior and disease risk, particularly in cases of child and adolescent psychiatric disorders, which have been shown to be at least similar to, and in some cases, more heritable than adult disorders.9-12 Within this context, imaging genetics is a promising technique representing the specific ability to understand the neurobiological mechanisms through which genes may have an impact on variability in these emergent phenomena.
The classic approach used in genetic association analyses involves the use of candidate genes. A candidate gene is a gene whose variation is suspected of being directly associated with an observable behavioral or clinical (i.e., disease-related) property of the individual (called a phenotype). With this approach, a genetic variant (known as a polymorphism) that potentially has an impact on the function of a behaviorally or clinically relevant biological process is identified, and then, deviations in the frequency of one gene variant (called an allele) in populations expressing the phenotype are determined. Ideally, the genetic variation should have an impact on molecular or cellular function of the gene or protein (i.e., be a functional variation), and the target phenotype should be stable, robust, and quantifiable. Within the imaging genetics framework, the target phenotype is usually a physiological response of the brain during specific behavioral processes (e.g., amygdala reactivity when viewing threatening facial expressions).
WHY FUNCTIONAL IMAGING?
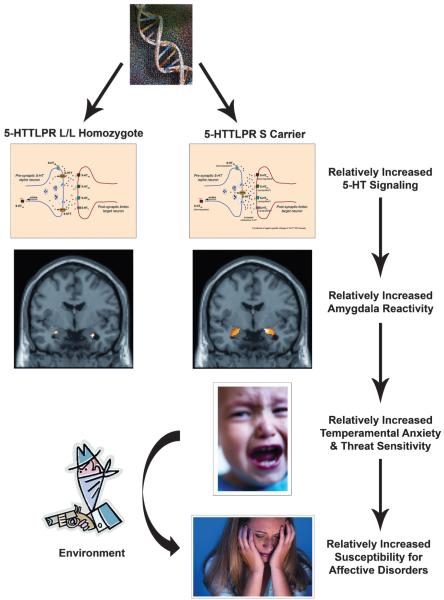
Previous investigations of candidate genes have attempted to associate functional polymorphisms directly with a behavior; however, such findings have been weak and inconsistent (e.g., the 5-HTTLPR short allele and negative emotionality).13 There are considerable interindividual differences in the dimensions of observed behavior, as well as subjectivity in behavioral measures, often requiring daunting sample sizes to detect gene effects.14 More importantly, gene effects are not expressed directly at the level of behavior but rather are mediated by effects on molecular and cellular cascades biasing information processing in brain circuitries mediating behavioral responses to environmental challenge. Functional neuroimaging, using functional magnetic resonance imaging, EEG, or positron emission tomography, provides an efficient and effective tool with which to explore the impact of brain-relevant genetic polymorphisms by quantifying the activity of specific brain regions in association with particular cognitive and emotional tasks that the research participant is asked to perform during the procedure. These techniques promise to identify neural pathways through which these variants contribute to the emergence of variability in behavior and disease risk (Fig. 1).
FIG. 1.
Imaging genetics allows for the identification of how common genetic polymorphisms (e.g., 5-HTTLPR) influencing molecular processes (e.g., serotonin signaling) bias neural pathways (e.g., amygdala reactivity) mediating individual differences in complex behavioral processes (e.g., trait anxiety) related to disease risk in response to environmental adversity. (Reprinted from Trends Cogn Sci. [10:182–191] Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Copyright 2006, with permission from Elsevier.)
BASIC PRINCIPLES
Three basic principles have been articulated for imaging genetics.4 These are selection of candidate genes, control for nongenotype factors, and selection of appropriate tasks for the subject to perform during functional imaging. Well-defined functional polymorphisms (single-nucleotide polymorphisms or other structural variants) in coding or promoter regions previously linked with specific physiological effects at the cellular level and whose impact has been described in distinct brain regions are an ideal starting point (Table 1). Selecting variants that have known neurobiological consequences (e.g., increases in serotonin [5-HT] signaling) is important because of an emphasis in imaging genetics on specifying mechanisms through which genes have an impact on brain and related behavior. Because potential genetic effects are still relatively small compared with typically large effects of age, sex, and IQ, as well as environmental influences (e.g., illness, injury, substance abuse), controlling for these potential confounds is necessary. Furthermore, because imaging genetic studies focus on a single or relatively few polymorphisms against a background of millions, these studies must carefully control for population stratification, which refers to differences in the genetic background of subjects, reflecting their unique ancestry, against which the relation between a specific genotype and phenotype is tested. Because of small genetic effects, choosing a well-characterized behavioral task for subjects to perform during functional neuroimaging that is both sensitive and specific to the brain process under investigation is of crucial importance to the success of identifying functional correlates of genetic variation. The ideal tasks for these investigations are thus ones that have been established to engage specific brain systems robustly in all subjects, as well as display variance both across control subjects and between patients and comparison subjects. Moreover, in child and adolescent populations, tasks that are both developmentally appropriate and acceptable for use with the specific psychiatric sample being studied should be chosen. For example, in previous imaging studies, amygdala reactivity to threat-related emotional facial expressions has been assayed using a well-characterized challenge paradigm that robustly engages the amygdala and interconnected corticolimbic structures. Importantly, this task has been shown to effectively engage the amygdala in control subjects15-19 and to demonstrate altered amygdala function in diverse psychiatric disorders.20-23 Child and adolescent psychiatric populations should also be characterized using behavioral or questionnaire measures that are able to identify relatively homogenous groups for analysis (i.e., separating out a small homogenous group of children with proactive aggression from within the broader and more heterogeneous group of children with conduct disorder). Whereas such steps increase the likelihood of identifying significant genetic regulation of interindividual variability in brain function and related behaviors, multiple genetic polymorphisms (many of which will be of small effect) acting in concert or opposition in the context of unique environmental challenges will ultimately account for the majority of variance in any given neural or behavioral phenotype.
TABLE 1.
Summary of Polymorphisms Impacting Behaviorally Relevant Brain Function
| Gene | Protein | Polymorphisms | Functional Effects |
|---|---|---|---|
| Corticolimbic circuitry for emotional arousal, threat reactivity and stress sensitivity | |||
| SLC6A4 (17q11.1) | 5-HT transporter/facilitates active 5-HT reuptake |
5-HTTLPR short and long alleles |
S allele—increased 5-HT signaling, reduced promoter activity and gene expression, increased amygdala reactivity, decreased functional coupling between amygdala and PFC |
| MAOA (Xp11.3) | Preferentially catalyzes the oxidative deamination of 5-HT |
High (3.5- and 4-repeat) and low (2-, 3-, 5-repeat) activity alleles |
2, 3, and 5–repeat alleles—reduced enzyme activity, increased amygdala reactivity, decreased functional coupling between amygdala and medial PFC |
| TPH2 (12q21.1) | Rate limiting enzyme in neuronal 5-HT synthesis |
G(-844)T | −844T allele—increased amygdala reactivity |
| COMT (22q11.2) | Metabolic degradation of synaptic dopamine |
Val158Met | Met158 allele—decreased enzyme activity, increased functional coupling between amygdala and PFC |
| Mesolimbic circuitry for reward sensitivity and impulsivity | |||
| SLC6A3 (5p15.3) | DA transporter/facilitates active DA reuptake |
DAT1 9- and 10-repeat alleles |
9-repeat allele—reduced DAT1 expression, increased ventral striatum reactivity |
| DRD2 (11q.23) | Inhibitory presynaptic and postsynaptic receptor |
DRD2 −141C Ins/Del | −141C Del—reduced DRD2 function, increased ventral striatum reactivity |
| DRD4 (11p15.5) | Inhibitory postsynaptic receptor |
DRD4 7- and non-7 repeat alleles |
7-repeat allele—reduced DRD4 function, increased ventral striatum reactivity |
Note: 5-HT = serotonin; 5-HTTLPR = serotonin-transporter-linked polymorphic region; COMT = catechol-O-methyl transferase; DA = dopamine; DAT1 = dopamine transporter gene 1; Del = deletion; DRD2 = dopamine receptor D2; DRD4 = dopamine receptor D4; MAOA = monoamine oxidase A; Ins = insertion; PFC = prefrontal cortex; SLC6A3 = solute carrier family 6 (neurotransmitter transporter, dopamine), member 3; SLC6A4 = solute carrier family 6 (neurotransmitter transporter, serotonin), member 4; TPH2 = tryptophan hydroxylase 2. (Reprinted with permission of John Wiley &Sons, Inc., from Fisher PM, Muñoz KE, Hariri AR. Identification of neurogenetics pathways of risk for psychopathology, 1–7. Am J Med Genet C Semin Med Genet. Vol.148, No. 2, 2008, 147–153. Copyright 2008, John Wiley & Sons, Inc.)
SEROTONIN AND EMOTIONAL BEHAVIOR
One of the most replicated findings in the field of imaging genetics is the impact of a common polymorphism in the promoter region (5-HTTLPR) of the 5-HT transporter (5-HTT) gene on amygdala reactivity in adults.24 Abnormal 5-HT neurotransmission has been implicated in the pathophysiology of mood and anxiety disorders and has been a target of pharmacological intervention (e.g., selective serotonin reuptake inhibitors). In comparison to the 5-HTTLPR long (L) allele, the short (S) allele has been associated with alterations conferring relatively increased 5-HT signaling.25 At the behavioral level, possession of either one or two copies of the S allele has been associated with increased levels of temperamental anxiety,26-28 conditioned fear responses,29 and development of depression, particularly in the context of environmental stress.30,31 Against this background, imaging genetics has been used to reveal that threat-related reactivity of the amygdala, a brain region critical in mediating behavioral and physiological arousal, is significantly increased in S allele carriers in comparison to L allele homozygotes.18 In addition, the 5-HTTLPR S allele has been further linked with reduced gray matter volume in and functional coupling between the amygdala and medial prefrontal cortex.32 Because the magnitude of threat-related amygdala reactivity (the response of the amygdala to threat-related signals), as well as its functional coupling with medial prefrontal cortex, is associated with temperamental anxiety, these imaging genetic findings suggest that the 5-HTTLPR S allele may be associated with increased risk for depression upon exposure to environmental stressors because of the polymorphism’s influence on the reactivity of this corticolimbic circuitry. However, no studies have yet been published investigating the association between the 5-HTTLPR and functional brain activity in children or adolescents. The imaging genetic research with the 5-HTTLPR highlights the effectiveness of this strategy in illuminating specific mechanisms that may mediate individual variability in behavior and risk for disease. Additional imaging genetic findings are summarized in Table 1.
DEVELOPMENTAL CONSIDERATIONS
As the field of behavioral and psychiatric genetics transitions to examining interactions between genes and environmental influences in shaping behavior and disease risk,33 consideration of developmental trajectories can no longer be ignored. The functional synergy between genes and brain likely changes throughout development because both experience and biology influence the expression of genes. This process is studied in the rapidly evolving field known as “epigenetics.” For example, these emerging studies on epigenetics34 suggest that certain exogenous factors including environmental stress can literally turn on or off the expression of genes. Gene expression also varies with endogenous shifts such as hormone fluctuations during puberty. Therefore, existing imaging genetic findings in adult populations may not apply to children and adolescents, and further study in these target populations is required because no studies using imaging genetics in children or adolescent populations have yet been published. Additionally, structural imaging studies have shown that cortical development continues into adulthood.35 Therefore, examining the links between genetic polymorphisms and alterations in brain function must also be appreciated across development. Because few psychopathologies arise de novo in adulthood without previous warning signs in childhood, longitudinal applications of imaging genetics have the potential to uncover key neurobiological pathways involved in both disease risk and resiliency. For example, as previously described, functional coupling between the amygdala and regulatory circuits in the medial prefrontal cortex are affected by genetically driven variability in 5-HT function and are also important in the pathophysiology of depression. These prefrontal areas, however, exhibit relatively protracted development,35 and studying genetic effects on the maturation of this coupling during adolescence may help our understanding of the development of depression risk. In turn, such understanding may advance formulation of individually tailored intervention and prevention strategies particularly in high-risk children.
Acknowledgments
This study was supported in part by the National Institute of Mental Health (MH072837 [A.H.]), the National Alliance for Research on Schizophrenia and Depression (A.H.), the Predoctoral Training Program in Behavior Brain Research (GM081760 [L.H.]), and the Multimodal Neuroimaging Training Program Fellowship (1T90DA022761 [K.M.]).
Footnotes
Similar descriptions of this experimental strategy have appeared in American Journal of Medical Genetics (Fisher et al., 2008),1 Development and Psychopathology (Viding et al., 2006),2 Biological Psychiatry (Hariri et al., 2006),3 and British Medical Bulletin (Hariri and Weinberger, 2003).4
Disclosure: The authors report no conflicts of interest.
REFERENCES
- 1.Fisher PM, Muñoz KE, Hariri AR. Identification of neurogenetic pathways of risk for psychopathology. Am J Med Genet C Semin Med Genet. 2008;148:147–153. doi: 10.1002/ajmg.c.30173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Viding E, Williamson DE, Hariri AR. Developmental imaging genetics: challenges and promises for translational research. Dev Psychopathol. 2006;18:877–892. doi: 10.1017/s0954579406060433. [DOI] [PubMed] [Google Scholar]
- 3.Hariri AR, Drabant EM, Weinberger DR. Imaging genetics: perspectives from studies of genetically driven variation in serotonin function and corticolimbic affective processing. Biol Psychiatry. 2006;59:888–897. doi: 10.1016/j.biopsych.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Hariri AR, Weinberger DR. Imaging genomics. Br Med Bull. 2003;65:259–270. doi: 10.1093/bmb/65.1.259. [DOI] [PubMed] [Google Scholar]
- 5.Plomin R, Owen MJ, McGuffin P. The genetic basis of complex human behaviors. Science. 1994;264:1733–1739. doi: 10.1126/science.8209254. [DOI] [PubMed] [Google Scholar]
- 6.Moldin SO, Gottesman II. At issue: genes, experience, and chance in schizophrenia—positioning for the 21st century. Schizophr Bull. 1997;23:547–561. doi: 10.1093/schbul/23.4.547. [DOI] [PubMed] [Google Scholar]
- 7.Weissman MM, Leckman JF, Merikangas KR, Gammon GD, Prusoff BA. Depression and anxiety disorders in parents and children. Results from the Yale family study. Arch Gen Psychiatry. 1984;41:845–852. doi: 10.1001/archpsyc.1984.01790200027004. [DOI] [PubMed] [Google Scholar]
- 8.Williamson DE, Ryan ND, Birmaher B, et al. A case-control family history study of depression in adolescents. J Am Acad Child Adolesc Psychiatry. 1995;34:1596–1607. doi: 10.1097/00004583-199512000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Beaudet AL. Autism: highly heritable but not inherited. Nat Med. 2007;13:534–536. doi: 10.1038/nm0507-534. [DOI] [PubMed] [Google Scholar]
- 10.Coolidge FL, Thede LL, Jang KL. Heritability of personality disorders in childhood: a preliminary investigation. J Personal Disord. 2001;15:33–40. doi: 10.1521/pedi.15.1.33.18645. [DOI] [PubMed] [Google Scholar]
- 11.Lichtenstein P, Annas P. Heritability and prevalence of specific fears and phobias in childhood. J Child Psychol Psychiatry. 2000;41:927–937. [PubMed] [Google Scholar]
- 12.Rhee SH, Waldman ID. Genetic and environmental influences on antisocial behavior: a meta-analysis of twin and adoption studies. Psychol Bull. 2002;128:490–529. [PubMed] [Google Scholar]
- 13.Malhotra AK, Goldman D. Benefits and pitfalls encountered in psychiatric genetic association studies. Biol Psychiatry. 1999;45:544–550. doi: 10.1016/s0006-3223(98)00365-5. [DOI] [PubMed] [Google Scholar]
- 14.Glatt CE, Freimer NB. Association analysis of candidate genes for neuropsychiatric disease: the perpetual campaign. Trends Genet. 2002;18:307–312. doi: 10.1016/S0168-9525(02)02670-7. [DOI] [PubMed] [Google Scholar]
- 15.Bertolino A, Arciero G, Rubino V, et al. Variation of human amygdala response during threatening stimuli as a function of 5’HTTLPR genotype and personality style. Biol Psychiatry. 2005;57:1517–1525. doi: 10.1016/j.biopsych.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 16.Drabant EM, Hariri AR, Meyer-Lindenberg A, et al. Catechol O-methyltransferase val158met genotype and neural mechanisms related to affective arousal and regulation. Arch Gen Psychiatry. 2006;63:1396–1406. doi: 10.1001/archpsyc.63.12.1396. [DOI] [PubMed] [Google Scholar]
- 17.Hariri AR, Drabant EM, Munoz KE, et al. A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry. 2005;62:146–152. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- 18.Hariri AR, Mattay VS, Tessitore A, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 19.Tessitore A, Hariri AR, Fera F, et al. Functional changes in the activity of brain regions underlying emotion processing in the elderly. Psychiatry Res. 2005;139:9–18. doi: 10.1016/j.pscychresns.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Altshuler L, Bookheimer S, Proenza MA, et al. Increased amygdala activation during mania: a functional magnetic resonance imaging study. Am J Psychiatry. 2005;162(6):1211–1213. doi: 10.1176/appi.ajp.162.6.1211. [DOI] [PubMed] [Google Scholar]
- 21.Fakra E, Salgado-Pineda P, Delaveau P, Hariri AR, Blin O. Neural bases of different cognitive strategies for facial affect processing in schizophrenia. Schizophr Res. 2008;100:191–205. doi: 10.1016/j.schres.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 22.Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 2007;164:318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- 23.Wang AT, Dapretto M, Hariri AR, Sigman M, Bookheimer SY. Neural correlates of facial affect processing in children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2004;43:481–490. doi: 10.1097/00004583-200404000-00015. [DOI] [PubMed] [Google Scholar]
- 24.Munafo MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biol Psychiatry. 2008;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends Cogn Sci. 2006;10:182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 26.Munafo MR, Clark T, Flint J. Does measurement instrument moderate the association between the serotonin transporter gene and anxiety-related personality traits? A meta-analysis. Mol Psychiatry. 2005;10:415–419. doi: 10.1038/sj.mp.4001627. [DOI] [PubMed] [Google Scholar]
- 27.Schinka JA, Busch RM, Robichaux-Keene N. A meta-analysis of the association between the serotonin transporter gene polymorphism (5-HTTLPR) and trait anxiety. Mol Psychiatry. 2004;9:197–202. doi: 10.1038/sj.mp.4001405. [DOI] [PubMed] [Google Scholar]
- 28.Sen S, Burmeister M, Ghosh D. Meta-analysis of the association between a serotonin transporter promoter polymorphism (5-HTTLPR) and anxiety-related personality traits. Am J Med Genet B Neuropsychiatr Genet. 2004;127:85–89. doi: 10.1002/ajmg.b.20158. [DOI] [PubMed] [Google Scholar]
- 29.Garpenstrand H, Annas P, Ekblom J, Oreland L, Fredrikson M. Human fear conditioning is related to dopaminergic and serotonergic biological markers. Behav Neurosci. 2001;115:358–364. [PubMed] [Google Scholar]
- 30.Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 31.Kendler KS, Kuhn JW, Vittum J, Prescott CA, Riley B. The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression: a replication. Arch Gen Psychiatry. 2005;62:529–535. doi: 10.1001/archpsyc.62.5.529. [DOI] [PubMed] [Google Scholar]
- 32.Pezawas L, Meyer-Lindenberg A, Drabant EM, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- 33.Caspi A, Moffitt TE. Gene-environment interactions in psychiatry: joining forces with neuroscience. Nat Rev Neurosci. 2006;7:583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- 34.van Vliet J, Oates NA, Whitelaw E. Epigenetic mechanisms in the context of complex diseases. Cell Mol Life Sci. 2007;64:1531–1538. doi: 10.1007/s00018-007-6526-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]