Congenital diaphragmatic hernia (CDH), a common birth defect affecting as many as 1/3000–1/4000 live born infants has long been recognized as a major congenital anomaly, with drawings and written descriptions dating back to 1700 [Irish et al., 1996]. During the past 50 years, surgical and medical management techniques have greatly improved but so has our understanding of the complexities of CDH including the facts that CDH:(a) does not designate a single or specific type of diaphragmatic defect; (b) almost always co-occurs with serious and often life-threatening abnormalities of pulmonary airway and vascular development; (c) is accompanied by additional major malformations in as many as half of cases; and (d) appears to be etiologically heterogeneous. These factors contribute to the wide discrepancy in outcomes. While some survivors are healthy and have normal development, others either die or have long-term morbidity. The mortality of CDH is persistently high and approaches 50% when all cases of CDH are considered [Stege et al., 2003; Colvin et al., 2005]. The particularly deleterious impact of cardiovascular malformations (CVMs) which commonly co-occur with CDH is well-described in the article by Lin et al.; the authors also provide management guidelines pertaining to clinical and genetic aspects of this potentially life-threatening combination of major malformations. Improved outcomes for individuals with either isolated CDH (e.g., CDH is the only major malformation), or those with complex CDH (e.g., CDH plus one or more additional malformations) will most likely come after greater understanding of basic biology and of pathophysiology have been achieved.
There is increasing evidence that mutations in genes belonging to one or more important developmental pathways contribute to CDH and its accompanying defects. Current knowledge of the genes and pathways associated with CDH and lung development in both humans and model organisms are presented in this issue. For example, Fog2, Gata4, and COUP-TFII likely function in the same developmental pathway, and all three of these genes have been implicated in diaphragm development in mouse models. In humans, a FOG2 mutation has been associated with a posterior diaphragmatic defect while GATA4 and COUP-TFII are strong CDH candidate genes based on their locations at cytogenetic hot spots [Ackerman et al., 2005; Klaassens et al., 2005; Slavotinek et al., 2005; You et al., 2005; Ackerman et al., 2006; Jay et al., 2006]. Numerous perturbations in the retinoic acid pathways are associated with CDH; these include mutations in genes that are part of, or interact with members of, this pathway (such as RXR, RAR, STRA6, COUP-TFII, GATA4, and FOG2) [Mendelsohn et al., 1994; Mascrez et al., 1998; Malpel et al., 2000; Clabby et al., 2003; Scribner et al., 2006; Pasutto et al., 2007] as well as teratogenic exposures such as vitamin A deficiency and the herbicide nitrofen [Andersen, 1941; Kluth et al., 1990]. Although not proven, these findings suggest that genetic abnormalities in the vitamin A pathway may contribute to the most common form of CDH, namely isolated “Bochdalek” hernia and even more intriguingly raise the possibility of a role for preventative supplementation.
However, these articles also point out the likely genetic heterogeneity underlying CDH and indicate that a variety of newer techniques (including molecular cytogenetics, gene sequencing, SNP arrays, and increasingly sophisticated analyses of genetic/teratogenic model organisms) will be required to dissect this complex birth defect. Standard G-banding chromosome studies identify aneuploidy in ~10% of all CDH cases. Array-based comparative genomic hybridization (aCGH) at the 1 Mb resolution has already identified small CDH-associated deletions below the resolution of standard karyotyping [Slavotinek et al., 2005; Kantarci et al., 2006], and it is likely that new higher resolutions platforms will detect additional even smaller areas of anueploidy serving to narrow critical regions that, in turn, will pinpoint genes key for normal diaphragm development.
Since pulmonary hypoplasia and pulmonary hypertension are the major determinants of survival in the neonatal period and predictors of long-term morbidity, several articles (Dr. Kinane, Dr. Khan and co-authors, and Dr. Keller) in this issue pay particular attention to normal and abnormal lung development, to pulmonary vascular development, and to potential pulmonary rescue therapies. The data presented therein make it abundantly clear that CDH-associated pulmonary hypoplasia is not SOLELY due to mechanical compression of the developing lung from herniation of abdominal contents into the chest cavity. Rather, intrinsic pulmonary developmental arrest accounts for at least some degree of the pulmonary hypoplasia seen at birth. Evidence for this hypothesis, coined the “two hit hypothesis,” is based on the discovery that both pulmonary development and diaphragm development are primarily affected by the chemical nitrofen [Keijzer et al., 2000]. There is now genetic evidence supporting this hypothesis (as discussed in the article in this issue by Ackerman and Greer) in that several genes have been identified which play an important role in the development of the lung and of the diaphragm, such as Fog2 and Gata4. At this time, the percentage of CDH patients with primary defects of both lung and diaphragm development versus the percentage of patients with only secondary pulmonary hypoplasia, due to lung compression and abnormal diaphragmatic function, remains unknown. It is also likely, that some of the co-morbidity that occurs with CDH in the gastrointestinal system such as gastroesophageal reflux and dysmotility is not simply secondary to anatomic aberrations caused by herniation. Development of the esophagus and stomach occurs in close proximity to the lung, heart, and diaphragm, and some transcriptional regulatory pathways required for the development of these organs likely also play a role in upper gastrointestinal development [Muratore et al., 2001; Jacobsen et al., 2005; Jay et al., 2006].
The exciting new therapeutic possibility of tracheal occlusion (TO) is discussed in great detail by Khan and co-authors. Although TO appears to promote pulmonary development in model organisms by mechano-transduction (e.g., stretch-induced) acceleration of Type I pneumocyte cell division, the authors cautiously point out not only the lack of long-term outcome studies but also the immediate effect of diminished Type II pneumocytes with consequent diminished surfactant production. Identification of TO-induced genetic changes may ultimately lead to gene therapy targets that can stimulate lung development in utero or shortly after birth. The rationale, implementation, and outcomes for TO in the human population are discussed in the article by Dr. Keller.
GOOD GENETIC STUDIES WILL REQUIRE CAREFUL PHENOTYPING
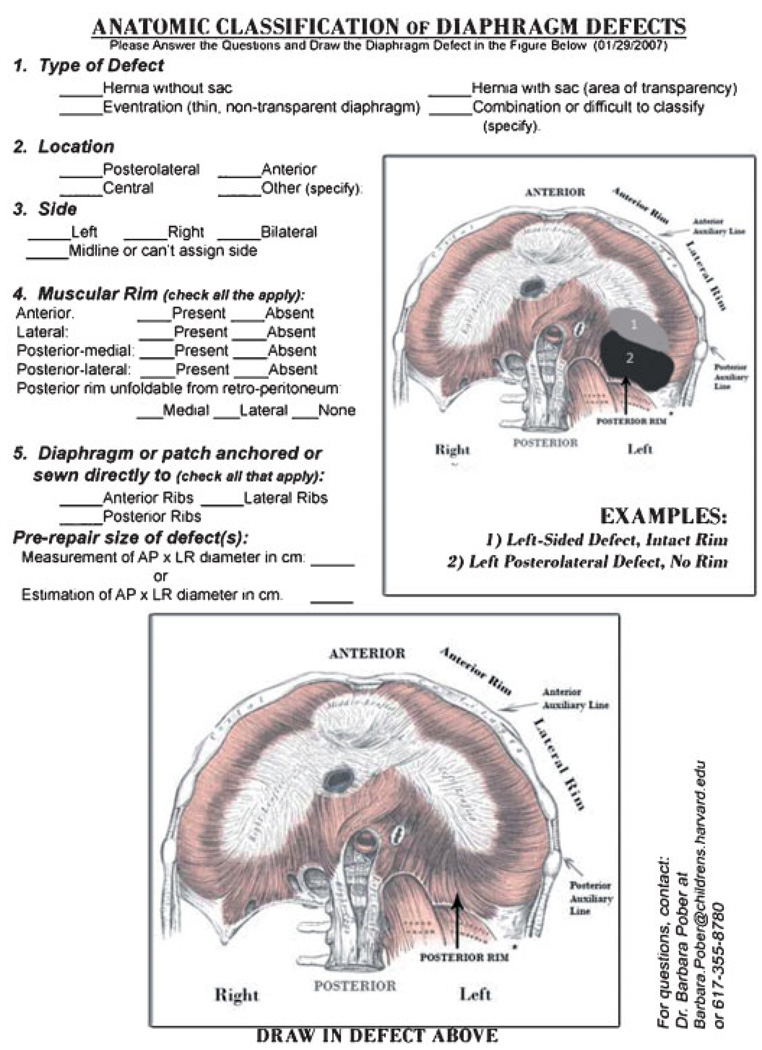
At this time, it is uncertain how many different types of diaphragmatic defects occur in humans or whether certain phenotypes are developmentally related. As part of our ongoing research studies, we review surgical reports and autopsy records from many different medical centers and see that a wide range of hernia phenotypes are categorized, or “lumped” together, as “Bochdalek” hernias. There is also no consensus on describing diaphragmatic defects that are covered with a membrane (and often the description isomitted). Likewise, the description of eventration defects overlaps with that of sac hernia, as both the size of the amuscularized diaphragmatic defect and the extent of herniation are extremely variable.
Since many published reports as well as operative or autopsy reports pertaining to diaphragmatic defects lack sufficient phenotypic information, we have developed a descriptive and pictorial schematic that attempts to capture the full diversity of human diaphragm defects (see Fig. 1, adapted from Anatomy of the Human Body with permission from Bartleby.com, Inc.) [Gray, 1918]. This is currently being tested by pediatric surgeons and pathologists at several institutions, and we encourage others to use it and to provide feedback to us about how it might be improved. We anticipate that widespread use of a standard approach to CDH classification will improve the quality of information in the medical literature, and ultimately will be used to develop genotype–phenotype correlations.
Figure 1.
Pictorial classification of Diaphragmatic Defects. We ask that surgeons and pathologists test this schematic by “drawing in” the exact location of the diaphragm defects and completing the requested information. Completed forms as well as comments to improve this schematic should be sent to us at the contact information provided.
CDH remains a serious, even devastating birth defect, not only for the affected individual and their family, but also for society at large due to the enormous costs associated with providing care [Robbins et al., 2007]. We are fortunate to be working in a time where technological and scientific advances justify increased research attention toward this birth defect, since the goal of deciphering the pathogenesis and genetic mechanisms responsible for diaphragmatic defects and associated pulmonary hypoplasia seems obtainable. The articles in this issue of the Seminar series provide a comprehensive review of the current knowledge in this field. More importantly, we hope that their contents will stimulate further progress, not only in achieving a greater understanding of the spectrum of human CDH but in developing better therapies, especially for the accompanying pulmonary hypoplasia, that will lead to improved outcomes for patients with CDH.
Biographies
Kate G. Ackerman, M.D. is an Assistant Professor of Pediatrics at Harvard Medical School and a member of the Division of Genetics at Brigham and Women’s Hospital and the Division of Emergency Medicine, Department of Medicine at Children’s Hospital Boston. Dr. Ackerman is a pediatric intensivist with post-doctoral research training in mouse genetics. Dr. Ackerman’s research focuses on understanding the pathophysiology of diaphragmatic defects and pulmonary hypoplasia in humans by investigating mechanisms of development in mouse models.
Barbara R. Pober, M.D. is an Associate Professor of Pediatrics at Harvard Medical School, a member of the Department of Surgery and Division of Genetics, Children’s Hospital of Boston, and a member of the Department of Pediatrics at the MassGeneral Hospital for Children in Boston, Massachusetts. Dr. Pober’s interests include the genetic delineation of congenital diaphragmatic hernia, as well as a longstanding interest in Williams syndrome.
REFERENCES
- Ackerman KG, Herron BJ, Vargas SO, Huang H, Tevosian SG, Kochilas L, Rao C, Pober BR, Babiuk RP, Epstein JA, Greer JJ, Beier DR. Fog2 is required for normal diaphragm and lung development in mice and humans. PLoS Genet. 2005;1:58–65. doi: 10.1371/journal.pgen.0010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerman KG, Wang J, Luo L, Fujiwara Y, Orkin SH, Beier DR. Gata4 is necessary for normal pulmonary lobar development. Am J Respir Cell Mol Biol. 2006 doi: 10.1165/rcmb.2006-0211RC. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen DH. Incidence of congenital diaphragmatic hernia in the young of rats bred on a diet deficient in vitamin A. Am J Dis Child. 1941;62:888–889. [Google Scholar]
- Clabby ML, Robison TA, Quigley HF, Wilson DB, Kelly DP. Retinoid X receptor alpha represses GATA-4-mediated transcription via a retinoid-dependent interaction with the cardiac-enriched repressor FOG-2. J Biol Chem. 2003;278:5760–5767. doi: 10.1074/jbc.M208173200. [DOI] [PubMed] [Google Scholar]
- Colvin J, Bower C, Dickinson JE, Sokol J. Outcomes of congenital diaphragmatic hernia: A population-based study in Western Australia. Pediatrics. 2005;116:e356–e363. doi: 10.1542/peds.2004-2845. [DOI] [PubMed] [Google Scholar]
- Gray H. Anatomy of the human body. Philadelphia: Lea & Febiger; 1918. Published in May 2000 by Bartleby.com. [Google Scholar]
- Irish MS, Holm BA, Glick PL. Congenital diaphragmatic hernia. A historical review. Clin Perinatol. 1996;23:625–653. [PubMed] [Google Scholar]
- Jacobsen CM, Mannisto S, Porter-Tinge S, Genova E, Parviainen H, Heikinheimo M, Adameyko II, Tevosian SG, Wilson DB. GATA-4: FOG interactions regulate gastric epithelial development in the mouse. Dev Dyn. 2005;234:355–362. doi: 10.1002/dvdy.20552. [DOI] [PubMed] [Google Scholar]
- Jay PY, Bielinska M, Erlich JM, Mannisto S, Pu WT, Heikinheimo M, Wilson DB. Impaired mesenchymal cell function in Gata4 mutant mice leads to diaphragmatic hernias and primary lung defects. Dev Biol. 2006;301:601–614. doi: 10.1016/j.ydbio.2006.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci S, Casavant D, Prada C, Russell M, Byrne J, Haug LW, Jennings R, Manning S, Blaise F, Boyd TK, Fryns JP, Holmes LB, Donahoe PK, Lee C, Kimonis V, Pober BR. Findings from aCGH in patients with congenital diaphragmatic hernia (CDH): A possible locus for Fryns syndrome. Am J Med Genet Part A. 2006;140A:17–23. doi: 10.1002/ajmg.a.31025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keijzer R, Liu J, Deimling J, Tibboel D, Post M. Dual-hit hypothesis explains pulmonary hypoplasia in the nitrofen model of congenital diaphragmatic hernia. Am J Pathol. 2000;156:1299–1306. doi: 10.1016/S0002-9440(10)65000-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassens M, van Dooren M, Eussen HJ, Douben H, den Dekker AT, Lee C, Donahoe PK, Galjaard RJ, Goemaere N, de Krijger RR, Wouters C, Wauters J, Oostra BA, Tibboel D, de Klein A. Congenital diaphragmatic hernia and chromosome 15q26: Determination of a candidate region by use of fluorescent in situ hybridization and array-based comparative genomic hybridization. Am J Hum Genet. 2005;76:877–882. doi: 10.1086/429842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluth D, Kangah R, Reich P, Tenbrinck R, Tibboel D, Lambrecht W. Nitrofen-induced diaphragmatic hernias in rats: An animal model. J Pediatr Surg. 1990;25:850–854. doi: 10.1016/0022-3468(90)90190-k. [DOI] [PubMed] [Google Scholar]
- Malpel S, Mendelsohn C, Cardoso WV. Regulation of retinoic acid signaling during lung morphogenesis. Development. 2000;127:3057–3067. doi: 10.1242/dev.127.14.3057. [DOI] [PubMed] [Google Scholar]
- Mascrez B, Mark M, Dierich A, Ghyselinck NB, Kastner P, Chambon P. The RXR(alpha) ligand-dependent activation function 2 (AF-2) is important for mouse development. Development. 1998;125:4691–4707. doi: 10.1242/dev.125.23.4691. [DOI] [PubMed] [Google Scholar]
- Mendelsohn C, Lohnes D, Decimo D, Lufkin T, LeMeur M, Chambon P, Mark M. Function of the retinoic acid receptors (RARs) during development (II). Multiple abnormalities at various stages of organogenesis in RAR double mutants. Development. 1994;120:2749–2771. doi: 10.1242/dev.120.10.2749. [DOI] [PubMed] [Google Scholar]
- Muratore CS, Utter S, Jaksic T, Lund DP, Wilson JM. Nutritional morbidity in survivors of congenital diaphragmatic hernia. J Pediatr Surg. 2001;36:1171–1176. doi: 10.1053/jpsu.2001.25746. [DOI] [PubMed] [Google Scholar]
- Pasutto F, Sticht H, Hammersen G, Gillessen-Kaesbach G, Fitzpatrick DR, Nurnberg G, Brasch F, Schirmer-Zimmermann H, Tolmie JL, Chitayat D, Houge G, Fernandez-Martinez L, Keating S, Mortier G, Hennekam RC, von der Wense A, Slavotinek A, Meinecke P, Bitoun P, Becker C, Nurnberg P, Reis A, Rauch A. Mutations in STRA6 cause a broad spectrum of malformations including anophthalmia, congenital heart defects, diaphragmatic hernia, alveolar capillary dysplasia, lung hypoplasia, and mental retardation. Am J Hum Genet. 2007;80:550–560. doi: 10.1086/512203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins JM, Bird TM, Tilford JM, Cleves MA, Hobbs CA, Grosse SD, Correa A. Hospital Stays, hospital charges, and in-hospital deaths among infants with selected birth defects—United States, 2003. MMWR-Weekly. 2007;56:25–29. [PubMed] [Google Scholar]
- Scribner KB, Odom DP, McGrane MM. Nuclear receptor binding to the retinoic acid response elements of the phosphoenolpyruvate carboxykinase gene in vivo: Effects of vitamin A deficiency. J Nutr Biochem. 2006;18:206–214. doi: 10.1016/j.jnutbio.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Slavotinek A, Lee SS, Davis R, Shrit A, Leppig KA, Rhim J, Jasnosz K, Albertson D, Pinkel D. Fryns syndrome phenotype caused by chromosome microdeletions at 15q26.2 and 8p23.1. J Med Genet. 2005;42:730–736. doi: 10.1136/jmg.2004.028787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stege G, Fenton A, Jaffray B. Nihilism in the 1990s: The true mortality of congenital diaphragmatic hernia. Pediatrics. 2003;112:532–535. doi: 10.1542/peds.112.3.532. [DOI] [PubMed] [Google Scholar]
- You LR, Takamoto N, Yu CT, Tanaka T, Kodama T, Demayo FJ, Tsai SY, Tsai MJ. Mouse lacking COUP-TFII as an animal model of Bochdalek-type congenital diaphragmatic hernia. Proc Natl Acad Sci USA. 2005;102:16351–16356. doi: 10.1073/pnas.0507832102. [DOI] [PMC free article] [PubMed] [Google Scholar]