Abstract
Background
Exposure to anger in the family is a risk factor for disruptive behavior disorders characterized by ineffective vagal regulation. Effects of anger on developing vagal regulation may be due to direct exposure or to effects on parents’ regulation of emotion as parents support infants’ regulation. Little is known about the impact of anger during infancy when important regulatory systems emerge.
Methods
Six-month-old infants (N = 48) and their mothers were exposed to anger, excitement, or neutral affect then observed in the Still-Face Paradigm (SFP). Vagal tone, indexed by respiratory sinus arrhythmia (RSA), was measured.
Results
Infants exposed to anger subsequently showed greater RSA withdrawal to mothers’ still-face than infants exposed to other emotions. Mothers exposed to anger showed greater RSA withdrawal than other mothers during emotion exposure and across all episodes of the SFP.
Conclusions
Exposure to anger may sensitize infants to stress and lead to increased need for physiological regulation. Exposure to anger makes increased demands on mothers’ self-regulation, which could detract from their abilities to support infants’ regulation.
Keywords: Emotion Regulation, Infancy, Psychophysiology, Parent-child interaction
Anger between adults is associated with greater physiological arousal and less effective vagal regulation in children (e.g., Ballard, Cummings, & Larkin, 1993; El-Sheikh, Ballard, & Cummings, 1994; El-Sheikh, Cummings, & Goetsch, 1989). On the other hand, effective vagal regulation buffers children from negative behavioral outcomes associated with inter-adult anger (El-Sheikh, Harger, & Whitson, 2001; Katz & Gottman, 1995). Because anger in the family is a risk factor for behavior disorders characterized by dysregulation of anger in childhood and adolescence and because ineffective vagal regulation is a consistent biological marker for these disorders (Beauchaine, Gatzke-Kopp, & Mead, 2007), it is critical to understand the impact of inter-adult anger on emerging systems of vagal regulation. Examining these processes during infancy is particularly important, as emotionally challenging environments can have a significant effect on biological regulatory systems as they undergo rapid development early in life (Pollack, 2005).
How anger between adults influences development of vagal regulation is unknown. One theory is that exposure to anger sensitizes developing regulatory systems, making children hyper-reactive initially and eventually leading to less reactive and less effective regulation through regulatory “burn-out.” Another is that spillover of parent conflict diminishes effective parenting support for infants’ regulation (e.g., Davies & Cummings, 2006) by affecting parents’ own regulation of emotion.
The first step in studying these theories is to understand the direct effects of exposure to anger on infants’ and mothers’ vagal regulation. The current study was designed to do this by observing their RSA reactivity, i.e., change in RSA from baseline, during an experimental emotion exposure task (EE) and a subsequent parent-infant interaction task, the Still-Face Paradigm (SFP; Tronick, Als, Adamson, Wise, & Brazelton, 1978). For infants, observing RSA reactivity in a challenge task (mother’s still-face) after anger exposure afforded the opportunity to assess whether anger exposure could sensitize infants to stress in the short-term. Observing mothers’ RSA reactivity in the two tasks afforded the opportunity to assess whether RSA reactivity to anger exposure would carry over into mother-infant interaction.
RSA reactivity was assessed because it is a purported index of physiological regulation of emotion, behavior, and attention, particularly in social interaction (Porges, 2007). RSA reactivity is measured as RSA withdrawal (i.e., decreased RSA) or activation (i.e., increased RSA) and what is normative is a function of expected direction and magnitude of change under specific conditions. In general, atypical RSA reactivity has been found to be related to problem behaviors and family risk factors, with most research focusing on RSA withdrawal in challenge contexts.
RSA withdrawal in challenge contexts is normative and lack of withdrawal or significantly greater than average withdrawal may be considered atypical. For example, infants who failed to show expected RSA withdrawal to mothers’ still-face were less positive and more physiologically aroused during normal play interactions prior to the still-face (Moore & Calkins, 2004). Greater than average RSA withdrawal during a stressor, suggesting a physiological hyper-reactivity, was found to be associated with more anger in three- to five-year-olds (Donzella, Gunnar, Krueger, & Alwin, 2000). In mothers, those rated lower in sensitivity showed a lack of RSA withdrawal during the reunion episode of the SFP, a context in which RSA withdrawal was normative due to the challenge of supporting the resumption of social interaction with infants (Moore, Hill, Propper, Calkins, Mills-Koonce, & Cox, 2009).
To date, little is known about infants’ or mothers’ RSA reactivity to anger, although there is evidence that infants react behaviorally to anger and evidence that qualities of parenting that are likely to be undermined by anger between adults are associated with infants’ physiological regulation.
Infants’ Responses to Anger
Five-month-old infants showed more intense startle responses to bursts of acoustic noise when viewing angry than happy expressions (Balaban, 1995). In an emotion exposure task, 6-month-old infants exposed to anger directed towards their mothers showed more preoccupation and fewer play behaviors than infants exposed to neutral or excited affect (Shred, 1997). This avoidant response has been found to extend to anger toward objects. Infants who observed an adult addressing an object in an angry voice were more hesitant to interact with that object (Repacholi & Meltzoff, 2007) than with objects addressed in other tones of voice.
Indirect evidence of infants’ responses to anger is consistent with this experimental research. Mothers’ reports of infants’ exposure to marital arguments moderated the relation between marital aggression and 6-month-old infants’ avoidance of a novel toy, a response that could be maladaptive (Crockenberg, Leerkes, & Lekka, 2007), although may also have an adaptive function. In a separate paper based on the sample studied in the current report (Moore, in press), infants in higher conflict families showed lower levels of baseline RSA and lower RSA during the SFP, including interactive episodes, which is an atypical response. Together this research suggests that anger may initially sensitize infants to become hyper-reactive and rely more often on self-regulation, as RSA withdrawal facilitates that response.
Consistent with the sensitization perspective, Haley and Stansbury (2003) conducted a modified SFP with two still-face episodes. Although they measured heart rate, not RSA, they found that infants showed greater cardiac arousal during the second still-face than in the first and did not recover physiologically after the second still-face.
In situations that chronically activate physiological regulation, infants and parents could show cumulative negative effects. Infants may develop a physiological hyper-reactivity that could, over time, tax their abilities to effectively regulate emotions (Donovan, Leavitt, & Walsh, 1998; Gottman & Katz, 1989). Regulatory fatigue has been demonstrated in adults over short periods of time in conditions that require self-control (Muraven & Baumeister, 2000), suggesting that chronic activation of self-regulatory mechanisms could undermine parents’ abilities to provide optimal support for their infants’ regulation. Thus, for both infants and parents, anger could detract from physiological resources that promote optimal interaction and development.
The Current Study
The goal was to assess infants’ and mothers’ physiological regulation, measured as RSA reactivity, in response to anger, as an initial step in developing theories to explain relations among inter-adult anger, problems with vagal regulation, and child behavior disorders. Specific aims were to assess: 1) immediate effects of exposure to anger on infants’ and mothers’ RSA reactivity, 2) whether anger increased infants’ RSA reactivity to a subsequent stressor, and 3) whether anger affected mothers’ RSA reactivity during subsequent mother-infant interaction. In addition, because there is evidence that valence and intensity of emotion are processed differently at the physiological and neural level (Lang, Greenwald, Bradley, & Hamm, 1993; Lewis, Critchley, Rothstein, & Dolan, 2006), a fourth aim was to determine whether affective intensity was responsible for any observed effects of anger.
Mother-infant dyads were exposed to different types of emotion: anger (negative valence + high intensity), excitement (positive valence + high intensity), and neutral (neutral valence + low intensity). To determine whether affective intensity was responsible for possible anger effects, two grouping variables were created, Valence (Anger v. Non-Anger, where the Non-Anger group included infants exposed to neutral or excited affect) and Intensity (Low v. High, where the High group included infants exposed to anger and excitement), so that hypotheses could be tested separately for Valence and Intensity models. Overall, we expected that negative, high intensity emotion (i.e., anger) and not intensity alone would elicit regulation, indexed by RSA withdrawal.
Hypotheses
1) Infants and mothers in the Anger group compared to those in the Non-Anger group would show greater RSA withdrawal during the EE task. 2) Based on theory and research suggesting that anger sensitizes infants to subsequent stress (Haley & Stansbury, 2003), infants in the Anger group compared to those in the Non-Anger group would show greater RSA withdrawal during the SFP still-face episode only. 3) Based on a similar theory for mothers that anger effects might carry over to other challenge contexts and on prior research finding that mothers typically showed RSA withdrawal during interactive episodes of the SFP (Moore et al., 2009), mothers in the Anger group would show greater RSA withdrawal during normal play and reunion only of the SFP than mothers in the Non-Anger group. 4) Because prior research suggested that parent conflict may sensitize infants to anger (Moore, in press), we hypothesized that conflict could moderate relations between anger exposure and infants’ and mothers’ responses, such that higher conflict would amplify RSA withdrawal. This hypothesis was tested only where there were significant findings from the first three hypotheses.
Infants’ and mothers’ behaviors were coded and examined but no specific hypotheses were made regarding effects of anger on behavior. First, infants’ behaviors are not consistently correlated with physiological responses (Gunnar, Mangelsdorf, Larson, & Hertsgaard, 1989; Weinberg & Tronick, 1996; Zelenko, Kraemer, Huffman, Gschwendt, Pageler, & Steiner, 2005). Second, it is difficult to link specific behaviors to RSA, in large part due to differences in temporal measurement.
Methods
Participants
Seventy-five mothers who delivered full-term, healthy infants and were married or cohabiting were recruited from birth records and birth/parenting classes. Mothers signed informed consent for their and their infants’ participation and study procedures were approved by the Institutional Review Board.
Complete RSA data across baseline, EE, and all SFP episodes were available for 48 dyads. Data were missing because infants became too distressed to complete the SFP (N = 6), technical problems (N = 4), or invalid data indicated by editing of more than 2% or RSA standard deviation across epochs greater than 1.00, most likely due to movement artifact (N = 17). This amount of missing data is typical of studies of infants’ RSA (e.g., Calkins, 1997; Stifter & Jain, 1996).
Of the 48 dyads with complete data, infants ranged from 6 to 8.5 months (M = 6.81, SD = .68), 29 were male, 25 were European American, and 23 were African American. Mothers ranged from 21 to 37 years (M = 27.87, SD = 5.69). Fifteen mothers (N = 6 European American) reported family incomes below poverty level. There were no differences in demographic or behavior variables or reports of parent conflict between participants with complete and incomplete RSA data. Dyads were randomly assigned to one of three conditions: Anger (N = 21), Excitement (N = 13), Neutral (N = 14), with procedures modified to assign a greater number to the Anger condition. There were no differences in demographic variables among conditions.
Procedures
EE stimuli
Three female European American experimenters were trained by the author to enact EE scripts. After training was complete, to determine whether experimenters’ portrayals were recognized as Anger, Excitement, and Neutral, 35 undergraduate students (68% female) participated in an emotion recognition study for research credit. Two samples of each type of emotion from each experimenter (N = 18 samples) were randomly selected from recordings made at completion of training and copied in random order to CD. After listening to each sample, students selected what emotion they thought the sample represented from: anger, sadness, fear, happiness, excitement/surprise, interest, or neutral, then rated the sample on a scale of 1-5 in terms of valence (very negative to very positive) and intensity (very low to very high).
Overall, participants identified samples accurately as the emotions experimenters intended to portray, χ2(1, 10) = 978.97, p < .001. There were no differences in ratings of valence or intensity among the three experimenters. Anger samples were rated as more negative than Excitement, t(34) = −24.57, p < .001, or Neutral, t(34) = −6.54, p < .001. Anger, t(34) = 11.72, p < .001, and Excitement, t(34) = 12.86, p < .001, were rated as more intense than Neutral. These results supported the theoretical decision to group the three EE conditions according to Valence (Anger v. Neutral/Excited) and Intensity (Neutral v. Anger/Excited).
Parent conflict
Mothers completed a 23 item questionnaire describing partner relationships (Braiker & Kelly, 1979). For the current study, the Conflict subscale, composed of five items, was used (alpha = .76). Total scores ranged from 5 to 34 (M = 15.75, SD = 6.89) of a possible 45, suggesting mild to moderate levels of conflict.
Laboratory procedures
After informed consent was obtained, cardiac monitoring equipment was attached (see below). Mothers placed infants in an infant seat and were seated in front of infants. They were given verbal instructions for the EE and SFP, and then asked to sit quietly and review the same written instructions for 3 minutes. During this period, baseline cardiac data were collected for infants and mothers. The EE and SFP were videorecorded using two cameras. Output from the two cameras was combined using a split-screen generator and a time code was added to the videotape.
The EE procedures were modeled after those developed by Cummings and colleagues (e.g., Cummings, 1987; Shred, 1997). Mothers were instructed to turn in their seats towards the experimenter while the experimenter enacted a script directed towards them in an angry, excited, or neutral tone of voice. The same script was used for each emotion. EE lasted 1 minute and mothers were instructed not to respond in any way.
Immediately following the EE, experimenters left the room and, via intercom, instructed mothers to begin the SFP. Standard SFP procedures (e.g., Weinberg & Tronick, 1996) were followed with 2-minute normal play, still-face, and reunion episodes.
Behavior coding
Positive and negative affect and direction of gaze were coded at 1-s intervals by trained, reliable (κ = .89) coders using microanalytic methods (e.g., Moore et al., 2009) during the EE and SFP for infants and during the interactive episodes of the SFP for mothers, as mothers’ behaviors were constrained to be neutral otherwise.
RSA reactivity
Experimenters placed pediatric electrodes on infants’ chests and showed mothers how to place electrodes on themselves. Separate monitors configured to collect heart interbeat interval data were used for infants and for mothers, and data were transferred to computers for editing and analysis using MXEdit software (Delta Biometrics, Bethesda, MD). RSA was calculated every 30-s epoch for the 3-min baseline period and every 15-sec epoch during EE and each episode of the SFP (Porges, 1985). Mean RSA of the epochs within each episode was used in computation of RSA reactivity.
Following prior research (e.g., Donzella et al., 2000; Moore & Calkins, 2004; Quigley & Stifter, 2006), a set of change scores was computed (ΔRSA) by subtracting baseline RSA from RSA during EE and during each SFP episode. Positive values of ΔRSA represented an increase in RSA (RSA activation); negative values represented a decrease in RSA (RSA withdrawal).
Results
Preliminary Analyses
All analyses were conducted on the subsample of dyads with complete RSA data. Of the 48 infants with complete RSA data, four had missing behavior data due to mothers blocking the camera (N = 2) or video problems (N = 2).
There were no differences in infants’ or mothers’ ΔRSA or behaviors as a function of ethnicity, income, or maternal age. Infant age was related to ΔRSA in EE, r(48) = .43, p < .01, and SFP normal play, r(48) = .34, p < .05. Male infants had higher baseline RSA than females, F(1, 47) = 4.99, p < .05. Mothers’ baseline RSA was higher with male than female infants, F(1, 47) = 5.56, p < .05. Therefore, infant age and sex were added to all main analyses. There was no difference in infants’ or mothers’ baseline RSA or infant sex across EE conditions and no differences in infants’ or mothers’ ΔRSA or behavior as a function of experimenter.
Correlations among ΔRSA and behavior
Infants’ and mothers’ ΔRSA were uncorrelated within or across episodes (all p > .10). Infants’ ΔRSA was uncorrelated with mothers’ behavior and mothers’ ΔRSA was uncorrelated with infants’ behavior within and between adjacent episodes (all p > .10), except mothers’ ΔRSA in EE and infants’ affect in normal play (r(44) = .31, p < .05), such that higher values of ΔRSA, indicating RSA activation, predicted greater negative affect. Infants’ ΔRSA was correlated with infants’ negative affect within each SFP episode (r’s ranged from −.31 to −.34, all p < .05), such that lower values of ΔRSA, indicating RSA withdrawal, were related to greater negative affect, supporting the interpretation that RSA withdrawal is associated with stress and a need to regulate. Mothers’ ΔRSA was uncorrelated with their behaviors. Infants’ and mothers’ positive affect were correlated in reunion (r = .41, p < .01).
Tests of Hypotheses
To determine whether infants’ and mothers’ responses were specific to anger and/or related to general affective intensity, two grouping variables were created, Valence (Anger, N = 21, v. Non-Anger, N = 27, dyads exposed to neutral or excited affect) and Intensity (Low, N = 14, v. High, N = 34, dyads exposed to anger and excitement), so that hypotheses could be tested separately for Valence and Intensity models.
Hypothesis One: Infants and mothers in the Anger compared with Non-Anger group would show greater RSA withdrawal during EE
Univariate general linear models (GLMs) were tested separately for infants and mothers with Valence (Anger/Non-Anger) and infant sex as between-groups factors and infant age as a covariate. To assess whether affective intensity might account for ΔRSA, GLMs were repeated with Intensity (Low/High) groups.
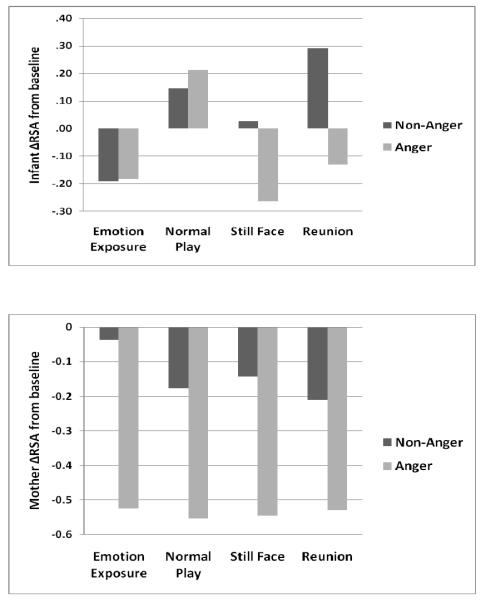
Infants’ ΔRSA during EE did not vary for Valence, η2 = .00, or Intensity, η2 = .02. For mothers, a Valence effect, F(1, 46) = 8.37, p < .01, η2 = .16, indicated that the Anger group showed greater RSA withdrawal than the Non-Anger group (Figure 1). An Intensity effect, F(1, 46) = 6.19, p < .05, η2 = .12, indicated that the High-Intensity group showed greater RSA withdrawal than the Low-Intensity group.
Figure 1.
Infants’ and mothers’ RSA reactivity by Emotion Exposure group
Note. Positive values indicate RSA activation. Negative values indicate RSA withdrawal.
To test Hypothesis Four that parent conflict moderated relations between ΔRSA and Valence and Intensity, two-way interactions were examined between conflict and Valence, η2 = .00, and Intensity, η2 = .11, and found to be non-significant.
Hypothesis Two: Infants in the Anger compared with Non-Anger group would show greater RSA withdrawal during the SFP still-face only (i.e., Valence by episode interaction)
Repeated measures GLMs were conducted separately for infants and mothers, with infant sex and Valence as between-groups factors, SFP episode (normal play, still-face, reunion) as within-subjects factor, and infant age as a covariate. Models were repeated with Intensity as the between-groups factor. There was a significant interaction between episode and Valence, F(2, 84) = 3.72, p < .05, η2 = .15, indicating that the Anger group showed greater RSA withdrawal in the still-face, F(1, 43) = 3.85, p < .05, η2 = .08, and reunion, F(1, 43) = 2.87, p = .098, η2 = .06, episodes than the Non-Anger group (Figure 1), although the effect for reunion did not reach traditional significance. Intensity main, η2 = .001, and interaction, η2 = .02, effects were not significant.
To test the moderation hypothesis (Hypothesis Four) two- and three-way interactions were examined. There were no significant interactions between parent conflict and Valence, η2 = .08, or among parent conflict, Valence, and episode, η2 = .12.
Hypothesis Three. Mothers in the Anger group would show greater RSA withdrawal than mothers in the Non-Anger group during SFP normal play and reunion only (i.e., Valence by episode interaction)
A main effect for Valence, F(1, 43) = 6.15, p < .05, η2 = .12, indicated that the Anger group showed greater RSA withdrawal than the Non-Anger group (Figure 1). The interaction between episode and Valence was not significant, indicating greater RSA withdrawal across the SFP. The Intensity effect was not significant, η2 = .03.
Two- and three-way interactions were not significant between parent conflict and Valence, η2 = .00, or among parent conflict, Valence, and episode, η2 = .05.
Behavior Analyses
Analyses of effects of anger on behaviors were examined using GLMs. Positive and negative affect and gaze were examined for infants. Only positive affect in normal play and reunion were examined for mothers as their behavior was constrained to be neutral otherwise and they were rarely negative or looked away from infants.
EE
A Valence effect, F(1, 38) = 4.31, p < .05, η2 = .10, indicated that infants in the Anger group looked at the experimenter more often (55% of the time) than those in the Non-Anger group (37%). Intensity was not significant, η2 = .08. For negative affect, Valence was not significant, η2 = .07. In the Intensity model, a main effect for sex, F(1, 38) = 10.00, p < .01, η2 = .21, indicated that males showed more negative affect (25%) than females (6%). A sex by Intensity interaction, F(1, 37) = 5.97, p < .05, η2 = .14, indicated that males in the Low Intensity group showed more negative affect (32%) than females in the same group (7%), F(1, 24) = 7.72, p < .05.
SFP
For positive affect, a three-way interaction among episode, sex, and Valence, F(2, 39) = 4.84, p < .05, η2 = .20, indicated that male infants in the Anger group showed less positive affect (17%) during normal play than males in the Non-Anger group (34%), F(1, 26) = 8.24, p < .05. There were no effects for mothers’ positive affect.
Discussion
Theories accounting for associations among parent conflict, children’s vagal regulation, and children’s disruptive behavior disorders propose that exposure to parent conflict may sensitize emerging physiological regulatory systems to be over-reactive to stress (Gottman & Katz, 1989) and that spillover of parent conflict may diminish parents’ abilities to provide support for children’s developing physiology (Davies & Cummings, 2006), leading to ineffective regulation of behavior and emotion (Beauchaine et al., 2007). To date, almost no empirical research has examined vagal regulation in relation to parent conflict during infancy when regulatory systems are undergoing rapid development. As a first step in understanding whether exposure to anger may sensitize infants’ physiologically to stress and may affect parents’ physiological regulation while interacting with their infants, the current study observed infants’ and mothers’ RSA reactivity to anger, compared with exposure to excited or neutral affect, with the emotion exposure procedure designed so that reactivity specifically to anger could be distinguished from reactivity to general affective intensity.
Infants’ and Mothers’ RSA Reactivity
Infants showed no differences in RSA reactivity during emotion exposure as a function of anger or affective intensity. It is possible that brief, mild emotion expressions were insufficient to elicit regulatory responses. Of note, infants exposed to anger looked at experimenters to a greater degree than infants exposed to excitement or neutral affect. This is consistent with research finding longer looking times towards adults who addressed objects in an angry compared with a neutral voice (Repacholi & Meltzoff, 2007). Longer looking times may be due to novelty of anger or because infants may be monitoring adults for further anger signals (Repacholi & Meltzoff, 2007). Although anger exposure did not elicit observable RSA reactivity in infants, effects were apparent in the subsequent SFP.
Consistent with physiological sensitization theory (e.g., Gottman & Katz, 1989) and with prior research (Haley & Stansbury, 2003), infants exposed to anger showed significantly greater RSA withdrawal during the still-face episode than other infants. This effect was specific to anger, not general affective intensity. These findings provide evidence that, in the short-term, anger does appear to prime infants to be physiologically reactive to stressors. With repeated exposure, infants could become hyper-reactive to stress (e.g., Donovan et al., 1998) and eventually develop less reactive and less effective regulation through a process of regulatory burnout (Muraven & Baumeister, 2000), although the long-term impact has yet to be investigated. Because excessive RSA reactivity is a marker for greater anger and negativity in early childhood (Beauchaine, et al., 2007; Donzella, et al., 2000), exposure to anger could have a cumulative effect on the development of physiological regulatory systems that underlie vulnerability to psychopathology.
Mothers showed RSA withdrawal during emotion exposure in response to anger and to affective intensity. During the subsequent SFP, however, they showed greater RSA withdrawal specifically to anger. Although predictions were that this effect would be confined to the interactive episodes (normal play and reunion), greater RSA withdrawal occurred for mothers exposed to anger across the SFP, suggesting a persistent effect on mothers’ physiological regulation.
It is noteworthy that brief and mild anger delivered by female experimenters that was posed and scripted had a persistent physiological impact, although during debriefing, none of the mothers indicated that they felt any subjective reaction to the emotion exposure. In naturalistic settings, mothers may need to regulate their emotions in more intense and personally relevant situations. If infants are present during conflict situations, parents may be faced with the dual task of regulating their own arousal and helping to support their infants’ increased need for regulation.
Together, findings suggest that as early as 6 months of age, anger may have an impact on developing RSA regulation and may do so through reactivity to direct exposure and through effects on parents’ ability to respond sensitively to their infants’ needs. These findings corroborate theories that stressful early experiences may have a substantial effect on developing physiological regulatory systems (e.g., Pollack, 2005). Furthermore they are consistent with prior empirical research finding that parenting sensitivity may mitigate the effects of a genetic vulnerability towards atypical vagal regulation in early infancy (Propper et al., 2008).
Behavioral Responses
There were relatively few findings regarding behavior. In addition to greater attention to anger during emotion exposure, infants showed more negative expressions when exposed to low intensity (neutral) affect, presumably because anger and excitement elicited sufficient arousal to keep them interested. The effect for negative expressions was more pronounced for male than female infants in the low-intensity condition, although there were no sex differences in RSA reactivity in that condition, suggesting male infants were more behaviorally, but not more physiologically, reactive to lack of attention.
During the SFP, the previous emotion exposure had no effect on mothers’ behaviors and little effect on infants’ behaviors, except for male infants exposed to anger, who showed significantly less positive affect with their mothers during the normal play episode immediately following the anger exposure compared with male infants who were exposed to excited or neutral affect. Again, behavioral findings were unrelated to RSA reactivity. Because the number of participants was small and analyses were exploratory, these findings should be interpreted cautiously.
The lack of consistent correlations among infants’ and mothers’ behavior and RSA reactivity suggested that effects of anger on infants’ and mothers’ responses were direct effects of emotion exposure, rather than mediated through each other’s behaviors. However, this may have been a function of sample size and the way in which behaviors were measured in the current study.
Limitations
Specific measures of parenting behaviors and qualities should be incorporated into future work. Research will benefit from larger samples to accommodate the amount of missing data that occurs in physiological research with infants and so that more complex models of relations among anger, RSA reactivity, and child and parent behavior can be tested. Experimental design and human subjects concerns required that the emotion exposure be brief, posed, and relatively low intensity. Naturalistic observations of anger in families are needed as are studies of infants with their mothers and fathers together. High conflict families should be studied to determine if qualitatively different processes occur.
Because previous analyses of this sample indicated that mothers’ reports of parent conflict were related to infants’ RSA (Moore, in press), the interaction between conflict and anger exposure in the laboratory was tested whenever significant main effects were found. In no case was the interaction significant and effect sizes were generally low, perhaps because levels of conflict were low-moderate and reports did not take into account whether infants were actually exposed to conflict. It will be important in future research to assess frequency, intensity, and chronicity of conflict to which infants are exposed in the home. Longitudinal studies are needed to follow the impact of anger and family conflict on developing emotion regulation systems and to identify factors that may moderate or mediate that development, such as timing of exposure, child temperament, and child sex.
There are several caveats when interpreting findings on RSA reactivity. First, it is difficult to link behavior and RSA in a meaningful way, i.e., conclude that a change in RSA preceded a change in behavior or vice versa. Second, there are individual differences in reactivity; some individuals respond with parasympathetic reactivity, some with sympathetic reactivity, and others with both (Berntson & Cacioppo, 2007; Quigley & Stifter, 2006), although, there are methodological limitations on measuring sympathetic responses in infancy (Fox, Schmidt, Henderson, & Marshall, 2007). Given these caveats, conclusions regarding whether infants and mothers were regulating and, if so, how effectively they regulated, are tentative.
Conclusion
Findings suggest that exposure to anger may sensitize infants to greater RSA reactivity when confronted with subsequent stressors and that even brief exposure to anger has persistent effects on mothers’ RSA reactivity. As early as 6 months of age, anger may have an impact on developing RSA regulation and may do so through direct exposure and through effects on parents’ abilities to respond sensitively to their infants’ needs particularly in more personally relevant and intense anger situations. These associations suggest that physiological regulation of behavior, emotion, and attention that develops in early infancy, as indexed by RSA reactivity (Porges, 2007), may mediate the relation between parent conflict and children’s later disruptive behavior disorders.
Key Points.
Exposure to anger may sensitize infants to be hyper-reactive to stress.
Parents are physiologically reactive to anger and these effects persist during subsequent interactions with their children.
Anger in families may contribute to development of disruptive behavior disorders through effects on developing physiological regulation of emotion.
Parent conflict may affect child outcomes through its impact on parenting.
Acknowledgements
This research was supported by a grant from the National Institute of Child Health and Development to the author (RO3HD043181).
Abbreviations
- (RSA)
Respiratory Sinus Arrhythmia
- (ΔRSA)
RSA Reactivity
- (EE)
Emotion Exposure
- (SFP)
Still-Face Paradigm
References
- Balaban MT. Affective influences on startle in five-month-old infants: Reactions to facial expressions of emotion. Child Development. 1995;66:28–36. doi: 10.1111/j.1467-8624.1995.tb00853.x. [DOI] [PubMed] [Google Scholar]
- Ballard ME, Cummings EM, Larkin K. Emotional and cardiovascular-responses to adults’ angry behavior and to challenging tasks in children of hypertensive and normotensive parents. Child Development. 1993;64:500–515. [PubMed] [Google Scholar]
- Beauchaine TP, Gatzke-Kopp L, Mead HK. Polyvagal theory and developmental psychopathology: Emotion dysregulation and conduct problems from preschool to adolescence. Biological Psychology. 2007;74:174–184. doi: 10.1016/j.biopsycho.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT. Integrative physiology: Homeostasis, allostasis, and the orchestration of systemic physiology. In: Cacioppo J, Tassinary L, Berntson G, editors. Handbook of Psychophysiology. Cambridge University Press; Cambridge, UK: 2007. pp. 433–452. [Google Scholar]
- Calkins SD. Cardiac vagal tone indices of temperamental reactivity and behavioral regulation in young children. Developmental Psychobiology. 1997;31:125–135. doi: 10.1002/(sici)1098-2302(199709)31:2<125::aid-dev5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Crockenberg SC, Leerkes EM, Lekka SK. Pathways from marital aggression to infant emotion regulation: The development of withdrawal in infancy. Infant Behavior and Development. 2007;30:97–113. doi: 10.1016/j.infbeh.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Cummings EM. Coping with background anger in early childhood. Child Development. 1987;58(4):976–984. doi: 10.1111/j.1467-8624.1987.tb01433.x. [DOI] [PubMed] [Google Scholar]
- Davies PT, Cummings EM. Interpersonal discord, family process, and developmental psychopathology. In: Cicchetti D, Cohen DJ, editors. Developmental Psychopathology. Second Edition Vol. 3: Risk, Disorder, and Adaptation. Wiley & Sons; New York: 2006. pp. 86–128. [Google Scholar]
- Donovan WL, Leavitt LA, Walsh RO. Conflict and depression predict maternal sensitivity to infant cries. Infant Behavior and Development. 1998;21:505–517. [Google Scholar]
- Donzella B, Gunnar MR, Krueger WK, Alwin J. Cortisol and vagal tone responses to competitive challenge in preschoolers: Associations with temperament. Developmental Psychobiology. 2000;37:209–220. doi: 10.1002/1098-2302(2000)37:4<209::aid-dev1>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Ballard M, Cummings EM. Individual differences in preschoolers’ physiological and verbal responses to videotaped angry interactions. Journal of Abnormal Child Psychology. 1994;22:303–321. doi: 10.1007/BF02168076. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Cummings EM, Goetsch VL. Coping with adults angry behavior - behavioral, physiological, and verbal responses in preschoolers. Developmental Psychology. 1989;25:490–498. [Google Scholar]
- El-Sheikh M, Harger J, Whitson SM. Exposure to interparental conflict and children’s adjustment and physical health: The moderating role of vagal tone. Child Development. 2001;72:1617–1636. doi: 10.1111/1467-8624.00369. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Whitson SA. Longitudinal relations between marital conflict and child adjustment: Vagal regulation as a protective factor. Journal of Family Psychology. 2006;20:30–39. doi: 10.1037/0893-3200.20.1.30. [DOI] [PubMed] [Google Scholar]
- Feldman R, Greenbaum CW, Yirmiya N. Mother-infant affect synchrony as an antecedent of the emergence of self-control. Developmental Psychology. 1999;35:223–231. doi: 10.1037//0012-1649.35.1.223. [DOI] [PubMed] [Google Scholar]
- Fox NA, Schmidt LA, Henderson HA, Marshall PJ. Developmental psychophysiology: Conceptual and methodological issues. In: Cacioppo J, Tassinary L, Berntson G, editors. Handbook of Psychophysiology. Cambridge University Press; Cambridge, UK: 2007. pp. 453–481. [Google Scholar]
- Gottman JM, Katz LF. The effects of marital discord on young children’s peer interaction and health. Developmental Psychology. 1989;25:273–381. [Google Scholar]
- Gunnar MR, Mangelsdorf S, Larson M, Hertsgaard L. Attachment, temperament, and adrenocortical activity in infancy: A study of psychoendocrine regulation. Developmental Psychology. 1989;25:355–363. [Google Scholar]
- Haley DW, Stansbury K. Infant stress and parent responsiveness: Regulation of physiology and behavior during still-face and reunion. Child Development. 2003;74:1534–1546. doi: 10.1111/1467-8624.00621. [DOI] [PubMed] [Google Scholar]
- Katz LF, Gottman JM. Vagal tone protects children from marital conflict. Development and Psychopathology. 1995;7:83–92. [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30:261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Lewis PA, Critchley HD, Rotshtein P, Dolan RJ. Neural correlates of processing valence and arousal in affective words. Cerebral Cortex. 2006;17:742–748. doi: 10.1093/cercor/bhk024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore GA. Parent conflict predicts infants’ vagal regulation in social interaction. Development and Psychopathology. doi: 10.1017/S095457940999023X. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore GA, Calkins SD. Infants’ vagal regulation in the still-face paradigm is related to dyadic coordination of mother-infant interaction. Developmental Psychology. 2004;40:1068–1080. doi: 10.1037/0012-1649.40.6.1068. [DOI] [PubMed] [Google Scholar]
- Moore GA, Hill AL, Propper CB, Calkins SD, Mills-Koonce WR, Cox MJ. Mother-infant vagal regulation in the face-to-face still-face paradigm is moderated by maternal sensitivity. Child Development. 2009;80:209–223. doi: 10.1111/j.1467-8624.2008.01255.x. [DOI] [PubMed] [Google Scholar]
- Muraven M, Baumeister RF. Self-regulation and depletion of limited resources: Does self-control resemble a muscle? Psychological Bulletin. 2000;126:247–259. doi: 10.1037/0033-2909.126.2.247. [DOI] [PubMed] [Google Scholar]
- Pollack SD. Early adversity and mechanisms of plasticity: Integrating affective neuroscience with developmental approaches to psychopathology. Development and Psychopathology. 2005;17:735–752. doi: 10.1017/S0954579405050352. [DOI] [PubMed] [Google Scholar]
- Porges SW. Method and apparatus for evaluating rhythmic oscillations in a periodic physiological response system. No. 4,510,944 Patent. 1985
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter CL, Wouden-Miller M, Silva S. Shizuko, Porter AE. Marital harmony and conflict: Links to infants’ emotional regulation and cardiac vagal tone. Infancy. 2003;4:297–307. [Google Scholar]
- Propper C, Moore GA. The influence of parenting on infant emotionality: A multi-level psychobiological perspective. Developmental Review. 2006;26:427–460. [Google Scholar]
- Propper CB, Moore GA, Mills-Koonce WR, Halpern C, Hill A, Calkins SD, et al. Gene-environment contributions to the development of vagal functioning: An examination of DRD2 and maternal sensitivity. Child Development. 2008;79:1377–1394. doi: 10.1111/j.1467-8624.2008.01194.x. [DOI] [PubMed] [Google Scholar]
- Quigley KS, Stifter CA. A comparative validation of sympathetic reactivity in children and adults. Psychophysiology. 2006;43:357–365. doi: 10.1111/j.1469-8986.2006.00405.x. [DOI] [PubMed] [Google Scholar]
- Repacholi BM, Meltzoff AN. Emotional eavesdropping: Infants selectively respond to indirect emotional signals. Child Development. 2007;78:503–521. doi: 10.1111/j.1467-8624.2007.01012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shred RK. Infants’ emotional and cognitive responses to adults’ anger behaviour (Doctoral dissertation, University of New Brunswick, 1997) Dissertation Abstracts International. 1997;57(7-B):4752. [Google Scholar]
- Stifter CA, Jain A. Psycho physiological correlates of infant temperament: Stability of behavior and autonomic patterning from 5 to 18 months. Developmental Psychobiology. 1996;29:379–391. doi: 10.1002/(SICI)1098-2302(199605)29:4<379::AID-DEV5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Tronick EZ, Als H, Adamson L, Wise S, Brazelton B. The infants’ response to entrapment between contradictory messages in face-to-face interaction. American Academy of Child Psychiatry. 1978;1:1–13. doi: 10.1016/s0002-7138(09)62273-1. [DOI] [PubMed] [Google Scholar]
- Weinberg KM, Tronick EZ. Infants’ affective reactions to the resumption of maternal interaction after the still-face. Child Development. 1996;67:905–914. [PubMed] [Google Scholar]
- Zelenko M, Kraemer H, Huffman L, Gschwendt M, Pageler N, Steiner H. Heart rate correlates of attachment status in young mothers and their infants. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:470–476. doi: 10.1097/01.chi.0000157325.10232.b1. [DOI] [PubMed] [Google Scholar]