Abstract
Introduction
Circulating oxidized LDL, a marker of oxidative stress, is associated with obesity, insulin resistance, metabolic syndrome, and cardiovascular disease in adults. However, little is known about its relation to insulin resistance and cardiovascular risk factors in children. The purpose of this study was to assess the relation of oxidative stress, measured by circulating oxidized LDL, with measures of adiposity and insulin resistance in children.
Methods
Oxidized LDL, measures of body fatness (body mass index: BMI, percent body fat, waist circumference, percent trunk fat, abdominal visceral and subcutaneous fat), insulin resistance with euglycemic insulin clamp (Mlbm), blood pressure, and blood lipids were obtained in 78 children. Oxidized LDL was compared between normal weight children (BMI < 85th percentile) and overweight/obese children (BMI ≥ 85th percentile) and levels were evaluated for associations with body fatness and insulin resistance.
Results
Oxidized LDL levels were significantly higher in overweight/obese vs. normal weight children (p < 0.0001). Oxidized LDL was significantly correlated with BMI, percent body fat, waist circumference, percent trunk fat, abdominal visceral fat, and abdominal subcutaneous fat (all p-values < 0.0001). Moreover, oxidized LDL was negatively correlated with Mlbm, even after adjustment for adiposity (p < 0.01).
Conclusions
Oxidized LDL is significantly associated with adiposity and with insulin resistance, independent of body fatness, in children. Oxidative stress may be independently related to the development of insulin resistance early in life, especially in obese youth.
Keywords: Oxidized LDL, obesity, insulin resistance, children
Introduction
Increased oxidative stress, reflected by elevated levels of oxidized LDL, may precede the development of insulin resistance (1) and therefore be important in the early pathophysiology of type 2 diabetes mellitus. Moreover, oxidative modification of LDL is thought to be a seminal event in the initiation and progression of atherosclerosis. Circulating oxidized LDL facilitates the maturation of macrophages and subsequent conversion to foam cells in the arterial wall (2). In adults, circulating oxidized LDL is associated with obesity (3, 4), insulin resistance (5), metabolic syndrome (6–9), and cardiovascular disease (10–12) but little is known about its relation to insulin resistance and cardiovascular risk factors in children. The purpose of this study was to assess the relation of oxidative stress, as measured by circulating oxidized LDL, with measures of adiposity and insulin resistance in children and adolescents.
Methods
This study included all pediatric participants (N = 78; age 6–18 years) who were consecutively enrolled from June–December 2008 in an ongoing study investigating the early development of obesity, insulin resistance, and other cardiovascular risk factors. The children are offspring of parents who have been participating in a longitudinal study in which initial testing was performed when the parents were children. The protocol was approved by the University of Minnesota Institutional Review Board and consent/assent was obtained from parents/participants. Measures were obtained at the University of Minnesota General Clinical Research Center after participants had been fasting ≥10 hours.
Height and weight were obtained using a standard stadiometer and electronic scale, respectively. Waist and hip circumferences were measured to the nearest 0.5 cm. Seated blood pressure was obtained after five minutes of quiet rest, on the right arm using an automatic sphygmomanometer. Tanner stage was determined by trained pediatricians. Body fat percentage was obtained using dual energy x-ray absorptiometry (DXA) (Prodigy, 3M, Madison, WI, USA). Abdominal visceral and subcutaneous fat were measured with computed tomography (Somatom Sensation, Siemens Medical Solutions, Malvern, PA, USA). Insulin sensitivity was determined by euglycemic insulin clamp as previously described (13). Insulin was infused at a constant rate of 1 mU/kg/min for 3 hours, and glucose was infused at a variable rate to maintain euglycemia. Insulin sensitivity (M) was expressed as the glucose infusion rate (mg/kg/min of glucose), with adjustment for lean body mass (Mlbm). Low Mlbm represents insulin resistance. Fasting lipid profile, glucose, and insulin assays were conducted with standard procedures at the Fairview Diagnostic Laboratories, Fairview-University Medical Center (Minneapolis, MN), a Centers for Disease Control and Prevention–certified laboratory. Plasma oxidized LDL levels (sensitivity ≤ 0.3 U/L) were measured in the University of Minnesota Cytokine Reference Laboratory with competitive ELISA (Mercodia, Inc., Winston-Salem, NC, USA).
Participants were classified as normal weight (BMI < 85th percentile) and/or overweight/obese (BMI ≥ 85th percentile) based on age- and gender-specific pediatric BMI criteria. In the initial analysis, linear regression (GLM procedure, SPSS version 16.0 - SPSS, Inc., Chicago, IL, USA), adjusting for age, gender, and race, was used to compare oxidized LDL levels between the normal weight and overweight/obese groups. Next, the entire cohort of 78 was used for correlation analyses to evaluate the relationship between oxidized LDL and measures of body fatness (adjusted for age, gender, and race) and Mlbm (adjusted for age, gender, race, and percent body fat). Additional adjustments were made and reported separately for LDL cholesterol levels (because LDL is the substrate for oxidized LDL) in an effort to see whether any finding for oxidized LDL was simply a consequence of having higher LDL cholesterol rather than the propensity of LDL to oxidize. Tanner stage data were missing in 17 of the participants. Therefore, we performed a separate analysis with adjustments for Tanner stage with the reduced sample size (N = 61) and results were not significantly different from those unadjusted for Tanner. Other studies from our group have shown that Tanner stage does not affect the relation between BMI and insulin resistance (14). Data are presented as mean ± standard deviation.
Results
The comparison of the clinical variables between the normal weight and overweight/obese groups are shown in the Table. There were no significant differences between the normal weight vs. overweight/obese groups for age or gender. Compared to normal weight participants, overweight/obese children had significantly higher BMI, percent body fat, waist circumference, percent trunk fat, abdominal visceral and subcutaneous fat, diastolic blood pressure, LDL cholesterol, fasting insulin, and significantly lower HDL cholesterol. Oxidized LDL levels were significantly higher in overweight/obese vs. normal weight children (p < 0.0001) (Figure 1) and remained significant, even after adjustment for LDL cholesterol (adjusted p < 0.0001). To evaluate possible interactions of oxidized LDL and weight status by age (children vs. adolescents), we compared means between two different age groups. Although not statistically significant (p = 0.21), mean oxidized LDL was higher in the overweight/obese (N = 12; 67.1 ± 14.4 U/L) vs. normal weight (N = 20; 61.3 ± 11.2 U/L) in the younger (age 6–11) children. Oxidized LDL levels were significantly higher (p < 0.0001) in the overweight/obese (N = 26; 70.1 ± 14.2 U/L) vs. normal weight (N = 20; 51.9 ± 12.1 U/L) in the older (age 12–18) children.
Table.
Clinical Characteristics of Normal Weight vs. Overweight/Obese Children
| Variable | Normal Weight (N = 40) | Overweight/Obese (N = 38) | P-Value |
|---|---|---|---|
| Age (years) | 11.7 ± 3.5 | 12.4 ± 3.3 | 0.318 |
| Gender (male/female) | 25/15 | 26/12 | 0.639 |
| BMI (kg/m2) | 18.3 ± 2.4 | 27.7 ± 6.6 | <0.0001 |
| Body Fat (%) | 19.1 ± 7.2 | 37.5 ± 10.0 | <0.0001 |
| Waist Circumference (cm) | 66.2 ± 7.8 | 87.3 ± 16.0 | <0.0001 |
| Trunk Fat (%) | 17.6 ± 7.8 | 38.9 ± 11.1 | <0.0001 |
| Visceral Fat (cm3) | 12.8 ± 5.7 | 26.5 ± 11.9 | <0.0001 |
| Subcutaneous Fat (cm3) | 37.2 ± 28.1 | 155.3 ± 94.5 | <0.0001 |
| SBP (mmHg) | 104.4 ± 9.6 | 106.6 ± 8.9 | 0.470 |
| DBP (mmHg) | 55.7 ± 8.8 | 60.4 ± 6.0 | 0.008 |
| Total Cholesterol (mg/dL) | 149.1 ± 26.7 | 155.7 ± 28.9 | 0.136 |
| LDL Cholesterol (mg/dL) | 83.0 ± 23.0 | 91.4 ± 24.1 | 0.047 |
| HDL Cholesterol (mg/dL) | 52.8 ± 12.0 | 47.0 ± 11.4 | 0.049 |
| Triglycerides (mg/dL) | 66.6 ± 29.0 | 85.8 ± 57.7 | 0.076 |
| Glucose (mg/dL) | 86.8 ± 6.1 | 88.0 ± 8.0 | 0.498 |
| Insulin (mU/L) | 7.5 ± 4.4 | 11.9 ± 9.9 | 0.024 |
| Mlbm (mg/kg/min) | 13.4 ± 3.6 | 13.7 ± 6.6 | 0.474 |
| Oxidized LDL (U/L) | 56.6 ± 12.4 | 69.1 ± 14.1 | <0.0001 |
Data are presented as mean ± standard deviation. All variables except age and gender are adjusted for age, gender, and race.
Figure 1.
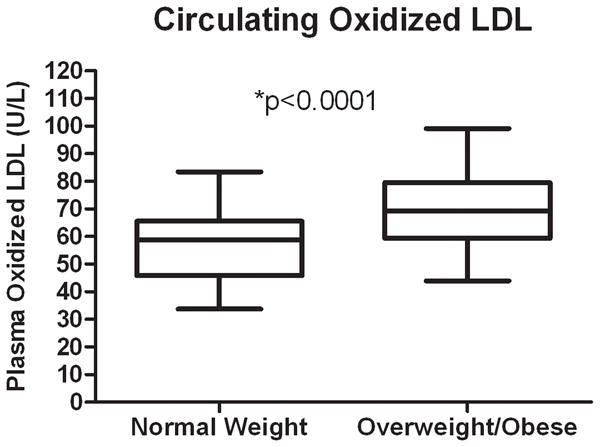
Circulating Oxidized LDL in Normal Weight vs. Overweight/Obese Children
Oxidized LDL was significantly and positively correlated with LDL cholesterol (r = 0.71, p < 0.0001), BMI (r = 0.50, p < 0.0001), percent body fat (r = 0.52, p < 0.0001), waist circumference (r = 0.48, p < 0.0001), percent trunk fat (r = 0.52, p < 0.0001), abdominal visceral fat (r = 0.42, p < 0.0001), and abdominal subcutaneous fat (r = 0.49, p < 0.0001). Oxidized LDL was significantly and negatively associated with Mlbm (r = −0.40, p = 0.006), independent of body fatness (adjusted for total body fat percentage). Oxidized LDL was not significantly related to fasting insulin (r = 0.14, p = 0.32).
Correlations remained significant after additional adjustment for LDL cholesterol levels: BMI (r = 0.43, p < 0.0001), percent body fat (r = 0.44, p < 0.0001), waist circumference (r = 0.43, p < 0.0001), percent trunk fat (r = 0.44, p < 0.0001), abdominal visceral fat (r = 0.45, p < 0.0001), abdominal subcutaneous fat (r = 0.42, p < 0.0001), and Mlbm (r = −0.30, p < 0.05). Results after adjustment for triglycerides were similar to the LDL adjustment. We concluded that the correlation analysis was a good fit to the data since means of body fatness, cardiovascular risk factors, and Mlbm were graded across quartiles of oxidized LDL.
Discussion
The main finding of this study is that oxidative stress, as measured by oxidized LDL, is significantly related to overweight/obesity and insulin resistance (independent of adiposity) in children. Because these findings are independent of LDL cholesterol, they can be considered a property of the propensity of LDL particles to oxidize, rather than the amount of cholesterol these particles contain.
Only a few studies have examined the relation of oxidized LDL with obesity and associated risk factors in children and none have addressed its potential association with insulin resistance. Data from the Cardiovascular Risk in Young Finns Study demonstrated a significant negative association between levels of oxidized LDL and brachial artery nitrate-mediated dilation, a measure of arterial health (15). In a study of First Nation youth, although children with type 2 diabetes mellitus had higher levels of oxidized LDL than controls, the levels were not significantly different in obese children without type 2 diabetes (16). In a study comparing obese and lean adolescents with polycystic ovary syndrome to healthy controls, there were no differences in oxidized LDL levels among the groups (17). Finally, another study reported that levels of oxidized LDL did not differ among groups of normal weight and overweight children with and without risk factors (18). In contrast to those studies, our data, despite using a similar technique for measuring oxidized LDL (ELISA), demonstrate that overweight and obese children have elevated levels of oxidized LDL compared to their normal weight counterparts. While it is not clear why our results differ from prior studies, they may be related to differences in study design and sample size. To our knowledge, our study comparing oxidized LDL levels in a two-group design (normal weight versus overweight/obese children and adolescents), is the largest to date. Alternatively, slightly different procedures for oxidized LDL measurement may detect different types of oxidized LDL (19), possibly explaining the discrepancy.
Oxidized LDL was significantly correlated with all measures of body fatness, with r-values ranging from 0.42 – 0.52. After additional adjustment for LDL cholesterol levels, the range of correlations became even narrower (r = 0.42 – 0.45), suggesting that no single measure of body fatness was superior in its relation to oxidized LDL. Oxidized LDL was significantly associated with insulin sensitivity, independent of body fatness. Although the correlation of oxidized LDL with Mlbm was relatively modest compared to the correlation with measures of body fatness, these findings support the concept that insulin resistance, independent of level of adiposity, is associated with higher levels of oxidative stress early in life. These findings, if confirmed by future investigations, may have implications toward the understanding of the development of insulin resistance in the context of pediatric obesity and risk of future type 2 diabetes mellitus. Recent data in adults suggest that oxidative stress precedes insulin resistance (1); however, data from longitudinal studies will be needed to address this issue in children.
In conclusion, circulating oxidized LDL levels are higher in overweight and obese, versus normal weight, children and adolescents. Body fatness and Mlbm are independently correlated with oxidized LDL suggesting that adiposity and insulin resistance are associated with oxidative stress within the first two decades of life. Future research should seek to better characterize the nature of these associations in children and to determine whether elevated levels of oxidative stress are a cause, or consequence, of obesity and insulin resistance.
Acknowledgments
Funding for this study was provided by the University of Minnesota Vikings Children’s Fund (A.S.K.), National Institutes of Health: 1RO1DK072124-01A3 (J.S.), and GCRC: M01-RR00400, General Clinical Research Center Program, NCRR/NIH. Mercodia, Inc. generously donated the competitive ELISA kits for oxidized LDL analysis.
Footnotes
Disclosures: The authors have no relevant conflicts of interest.
Reference List
- 1.Park K, Gross M, Lee DH, et al. Oxidative stress and insulin resistance: the coronary artery risk development in young adults study. Diabetes Care. 2009;32(7):1302–7. doi: 10.2337/dc09-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yui S, Sasaki T, Miyazaki A, Horiuchi S, Yamazaki M. Induction of murine macrophage growth by modified LDLs. Arterioscler Thromb. 1993;13(3):331–7. doi: 10.1161/01.atv.13.3.331. [DOI] [PubMed] [Google Scholar]
- 3.Couillard C, Ruel G, Archer WR, et al. Circulating levels of oxidative stress markers and endothelial adhesion molecules in men with abdominal obesity. J Clin Endocrinol Metab. 2005;90(12):6454–9. doi: 10.1210/jc.2004-2438. [DOI] [PubMed] [Google Scholar]
- 4.Weinbrenner T, Schroder H, Escurriol V, et al. Circulating oxidized LDL is associated with increased waist circumference independent of body mass index in men and women. Am J Clin Nutr. 2006;83(1):30–5. doi: 10.1093/ajcn/83.1.30. [DOI] [PubMed] [Google Scholar]
- 5.Ho RC, Davy K, Davy B, Melby CL. Whole-body insulin sensitivity, low-density lipoprotein (LDL) particle size, and oxidized LDL in overweight, nondiabetic men. Metabolism. 2002;51(11):1478–83. doi: 10.1053/meta.2002.35577. [DOI] [PubMed] [Google Scholar]
- 6.Holvoet P, Kritchevsky SB, Tracy RP, et al. The metabolic syndrome, circulating oxidized LDL, and risk of myocardial infarction in well-functioning elderly people in the health, aging, and body composition cohort. Diabetes. 2004;53(4):1068–73. doi: 10.2337/diabetes.53.4.1068. [DOI] [PubMed] [Google Scholar]
- 7.Holvoet P, Lee DH, Steffes M, Gross M, Jacobs DR., Jr Association between circulating oxidized low-density lipoprotein and incidence of the metabolic syndrome. JAMA. 2008;299(19):2287–93. doi: 10.1001/jama.299.19.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lapointe A, Couillard C, Piche ME, et al. Circulating oxidized LDL is associated with parameters of the metabolic syndrome in postmenopausal women. Atherosclerosis. 2007;191(2):362–8. doi: 10.1016/j.atherosclerosis.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 9.Van Guilder GP, Hoetzer GL, Greiner JJ, Stauffer BL, Desouza CA. Influence of metabolic syndrome on biomarkers of oxidative stress and inflammation in obese adults. Obesity (Silver Spring) 2006;14(12):2127–31. doi: 10.1038/oby.2006.248. [DOI] [PubMed] [Google Scholar]
- 10.Holvoet P, Vanhaecke J, Janssens S, Van de WF, Collen D. Oxidized LDL and malondialdehyde-modified LDL in patients with acute coronary syndromes and stable coronary artery disease. Circulation. 1998;98(15):1487–94. doi: 10.1161/01.cir.98.15.1487. [DOI] [PubMed] [Google Scholar]
- 11.Holvoet P, Mertens A, Verhamme P, et al. Circulating oxidized LDL is a useful marker for identifying patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2001;21(5):844–8. doi: 10.1161/01.atv.21.5.844. [DOI] [PubMed] [Google Scholar]
- 12.Holvoet P, Jenny NS, Schreiner PJ, Tracy RP, Jacobs DR. The relationship between oxidized LDL and other cardiovascular risk factors and subclinical CVD in different ethnic groups: the Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2007;194(1):245–52. doi: 10.1016/j.atherosclerosis.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Sinaiko AR, Jacobs DR, Jr, Steinberger J, et al. Insulin resistance syndrome in childhood: associations of the euglycemic insulin clamp and fasting insulin with fatness and other risk factors. J Pediatr. 2001;139(5):700–7. doi: 10.1067/mpd.2001.118535. [DOI] [PubMed] [Google Scholar]
- 14.Moran A, Jacobs DR, Jr, Steinberger J, et al. Insulin resistance during puberty: results from clamp studies in 357 children. Diabetes. 1999;48(10):2039–44. doi: 10.2337/diabetes.48.10.2039. [DOI] [PubMed] [Google Scholar]
- 15.Jarvisalo MJ, Lehtimaki T, Raitakari OT. Determinants of arterial nitrate-mediated dilatation in children: role of oxidized low-density lipoprotein, endothelial function, and carotid intima-media thickness. Circulation. 2004;109(23):2885–9. doi: 10.1161/01.CIR.0000129304.98566.D8. [DOI] [PubMed] [Google Scholar]
- 16.Stringer DM, Sellers EA, Burr LL, Taylor CG. Altered plasma adipokines and markers of oxidative stress suggest increased risk of cardiovascular disease in First Nation youth with obesity or type 2 diabetes mellitus. Pediatr Diabetes. 2008 doi: 10.1111/j.1399-5448.2008.00473.x. [DOI] [PubMed] [Google Scholar]
- 17.Demirel F, Bideci A, Cinaz P, et al. Serum leptin, oxidized low density lipoprotein and plasma asymmetric dimethylarginine levels and their relationship with dyslipidaemia in adolescent girls with polycystic ovary syndrome. Clin Endocrinol (Oxf) 2007;67(1):129–34. doi: 10.1111/j.1365-2265.2007.02849.x. [DOI] [PubMed] [Google Scholar]
- 18.Kelishadi R, Cook SR, Amra B, Adibi A. Factors associated with insulin resistance and non-alcoholic fatty liver disease among youths. Atherosclerosis. 2009;204(2):538–43. doi: 10.1016/j.atherosclerosis.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 19.Itabe H, Ueda M. Measurement of plasma oxidized low-density lipoprotein and its clinical implications. J Atheroscler Thromb. 2007;14(1):1–11. doi: 10.5551/jat.14.1. [DOI] [PubMed] [Google Scholar]


