Abstract
Purpose
To determine the natural end-point for refractive development during childhood.
Methods
Cycloplegic (1% cyclopentolate) autorefraction was performed on 38,811 children aged 5 and 15 in population-based samples at eight sites in the Refractive Error Study in Children (RESC). Refractions (right eye) were categorized as myopic (≤ −0.5D), emmetropic (> −0.5D to ≤ +0.5D), mildly hyperopic (> +0.5D to ≤ +2.0D and hyperopic (> +2.0D).
Results
At 5 sites (Jhapa – rural Nepal, New Delhi -urban India, Mahabubnagar - rural India, Durban - semi-urban South Africa, and La Florida – urban Chile), there was <20% myopia by age 15. Mild hyperopia was the most prevalent category at all ages, except for Mahabubnagar where emmetropia became the marginally most prevalent category at ages 14 and 15. At the other sites (Gombak – semi-urban Malaysia, Shunyi – semi-rural China, and Guangzhou - urban China) there was substantial (>35%) myopia by age 15. At these sites, mild hyperopia was the most prevalent category during early childhood, and myopia became the predominant category later. In Gombak district and Guangzhou, emmetropia was a minor category at all ages, with myopia increasing as mild hyperopia decreased. In Shunyi district, emmetropia was the most prevalent category over the ages 11-14.
Conclusion
Emmetropia was not the predominant outcome for refractive development in children. Instead, populations were either predominantly mildly hyperopic, or substantial amounts of myopia appeared. This suggests that mild hyperopia is the natural state of refractive development in children, and that emmetropia during childhood carries the risk of subsequent progression to myopia.
Keywords: myopia, hyperopia, emmetropia, emmetropisation, refractive error, development, children
Introduction
Emmetropia is classically defined as a state between myopia and hyperopia, in which “when parallel rays strike a physiologically normal eye, they are refracted so as to converge upon the retina, where they focus, forming a circle of least confusion …….. with the eye in a state of rest…”(Duke-Elder 1993). This concept of emmetropia should not be confused with the range of refractive states which are compatible with normal visual acuity, because normal visual acuity is compatible with mild myopia, emmetropia and even substantial hyperopia, where accommodation can be used to bring the image into focus on the retina.
The process of achieving this optical state of emmetropia during development has been termed “emmetropisation” (Wildsoet 1997). The concept of emmetropisation has been given strong support from animal experimentation. In particular, during development, animals as diverse as chickens (Schaeffel et al. 1988), tree shrews (Shaikh et al. 1999) and primates (Hung et al. 1995) have been shown to compensate with considerable precision for experimental changes in optical power by increasing or decreasing the rate of eye growth in response to hyperopic or myopic defocus respectively, thus minimising refractive error. These findings have led to quite wide-spread view that the mechanisms underlying refractive development lead to precise emmetropia, or a plano refraction, in which the image of distant objects falls with great precision on the photosensitive layer of the retina in the absence of accommodation (see, for example Rada et al. 2006; Tkatchenko et al. 2006). The existence of signals that slow eye growth in response to myopic defocus could then provide a mechanism for maintaining spherical equivalent refraction (SER) at emmetropia, implying that the appearance of myopic refractions is due to a failure of this mechanism.
However, studies on refractive development in humans, suggest a somewhat different and more complex picture. Firstly, there is evidence of multiple mechanisms at work. Over the first year or two of life, the predominantly hyperopic refractive errors and the much rarer myopic errors of neonates are rapidly reduced towards emmetropia (Gwiazda et al. 1993; Mayer et al. 2001; Mutti et al. 2005). This process appears to be under visual feedback control, and involves coordinated changes in corneal power and axial length, such that the distribution of SER changes from a normal to a highly peaked, leptokurtotic distribution, at mean SER values which are significantly hyperopic (Mayer et al. 2001; Mutti et al. 2005). Reductions in lens power over this period have also been documented (Mutti et al. 2005).
After that, corneal power stabilizes. Subsequent refractive development then appears to involve continued axial elongation, combined with reductions in lens power and increases in anterior chamber depth that could compensate, at least partially, for the myopic shift that would normally be associated with axial elongation (Sorsby et al. 1961; Garner et al. 1995; Zadnik et al. 2003; Ojaimi et al. 2005; Garner et al. 2006; Ip et al. 2008). It should be noted that, because these compensatory developments occur while refractions are still hyperopic, the reductions in lens power and increases in anterior chamber depth are effectively anti-emmetropic, since they slow the rate at which SER approaches plano with continued axial elongation.
Secondly, the data on human refractive development suggest that the normal end-point of refractive development may be mild hyperopia, rather than emmetropia. Interpretation of much of the earlier data in the literature on human refractive development is complicated by the frequent failure to use adequate cycloplegia, as well as the frequent failure to use large population-based samples (for a review of the earlier literature, see Hirsch and Weymouth 1991). However, the early studies, carried out on populations with low prevalences of myopia, suggested that mean SER tended to stabilize at mildly hyperopic rather than emmetropic values.
More recent studies using cycloplegia suggest the same conclusion; that mean SER is hyperopic rather than emmetropic in childhood (Sorsby et al. 1961; Zadnik et al. 2003; Ojaimi et al. 2005; Ip et al. 2008), and even into young adult life (Sorsby et al. 1960; Hashemi et al. 2004). But in other populations, where the prevalence of myopia is high, the mean cycloplegic SER becomes substantially myopic by the end of childhood (Matsumura and Hirai 1999; Lin et al. 2004).
The picture of tight emmetropisation derived from animal studies suggests that under natural conditions, refractions should be predominantly emmetropic. In this paper, we test this prediction using data obtained in the Refractive Error Study in Children (RESC) series (Maul et al. 2000; Negrel et al. 2000; Pokarel et al. 2000; Zhao et al. 2002; Dandona et al. 2002; Murthy et al. 2002; Naidoo et al. 2003; He et al. 2004; Goh et al. 2005), which has achieved cycloplegia with cyclopentolate, to measure refractive status during childhood on large population-based samples of children from a range of ethnic backgrounds.
METHODS
The RESC studies used a standard protocol with randomized cluster sampling to provide a representative, population-based sample of children combined with high examination participation rates and the use of cycloplegia to determine spherical equivalent refraction. The methodology of these studies and the specific details of each survey, including details of approval by human experimentation committees or institutional review boards have been described elsewhere (Maul et al. 2000; Negrel et al. 2000; Pokarel et al. 2000; Zhao et al. 2002; Dandona et al. 2002; Murthy et al. 2002; Naidoo et al. 2003; He et al. 2004; Goh et al. 2005). Informed consent for each examined child was obtained from a parent or other responsible adult. The research followed the tenets of the Declaration of Helsinki.
The studies were carried out in eight sites as shown in Table 1, arranged in increasing order of the prevalence of myopia at age 15. There were two sites in China (one urban, Liwan District in Guangzhou (He et al. 2004); and one semi-rural, Shunyi District near Beijing (Zhao et al. 2000)), two sites in India (one urban, Trilokupi segment in New Delhi (Murthy et al. 2003); 31 and one rural, Mahabubnagar District in Southern India (Dandona et al. 2002), one site in Chile (the urban La Florida area of Santiago (Maul et al. 2001)), one site in Malaysia (the Gombak District near Kuala Lumpur (Goh et al. 2005)), one site in Nepal (the rural Jhapa District in Eastern Nepal (Pokharel et al. 2002)), and one site in South Africa (a semi-urban contiguous area within the South and West Regions of Durban (Naidoo et al. 2004)). Approximately 500 children at each age from 5 to 15 years were examined, except in Southern India and Kuala Lumpur where only children aged 7 to 15 were examined. The prevalences of refractive error at each site for children at 5 (or 7) and 15 years of age are shown.
Table 1.
Prevalence of refractive categories in right eyes with cycloplegic auto-refraction.
| Study Site | Number of children examined |
Percentage with myopia at age 5 |
Percentage with myopia at age 15 |
Maximum emmetropic percentage (age) |
|---|---|---|---|---|
| Jhapa District, Eastern Nepal (rural) |
4991 | 0.45 | 0.79 | 20.1 (12) |
| Mahabubnagar District, Southern India (rural) |
3972 | 2.63* | 7.84 | 47.2 (14) |
| Durban, South Africa (semi- urban) |
4102 | 3.88 | 8.16 | 39.4 (15) |
| Trilokupi Segment, New Delhi, India (urban) |
5692 | 4.29 | 10.8 | 26.9 (13) |
| La Florida Area, Santiago, Chile (urban) |
5293 | 3.23 | 14.9 | 22.9 (14) |
| Gombak District, Kuala Lumpur, Malaysia (urban) |
4573 | 9.11* | 35.9 | 28.5 (12) |
| Shunyi District, Beijing, China (semi-rural) |
5866 | 1.94 | 48.7 | 43.8 (12) |
| Liwan District, Guangzhou, China (urban) |
4322 | 3.03 | 72.9 | 31.6 (10) |
Prevalence of myopia in children at age 7 years.
Cycloplegia was induced with two drops of 1% cyclopentolate administered 5 minutes apart to each eye. After 20 minutes, if a pupillary light reflex was still present, a third drop was administered. Light reflex and pupil dilation were checked after an additional 15 minutes. Cycloplegia was considered complete if the pupil dilated to 6mm or greater and a light reflex was absent. Refractions were measured by both retinoscopy and auto-refraction.
For this paper, auto-refraction data in right eyes with complete cycloplegia were analysed. Spherical equivalent refractive error categories were defined as: myopia: ≤ −0.50 D; emmetropia: > −0.50 D but ≤ +0.50 D; mild hyperopia: > +0.50 D but ≤ +2.00 D; and significant hyperopia: > +2.00 D.
The definition chosen for emmetropia is based on the postulated process of emmetropisation, in which defocus-driven changes in eye growth operate to produce tight emmetropia, with refractions clustering at or around plano, and takes into account the analysis of refractive development carried out by Thorn et al.(2005) and Rose et al. (Rose K, et al. IOVS 2007;48:ARVO E-Abstract 1535) which suggests that normal control over eye growth is lost at low hyperopic refractions. The emmetropic range has been set to cover those who are not yet clearly clinically myopic, and who are within the range of refractions in which higher “myopic” progression rates appear, with allowance for measurement errors.
RESULTS
The results at the 8 sites are summarised in Table 1. In all the sites for which there is data on children at the age of 5, the initial prevalence of myopia is quite low (<5%), but by the age of 15, the prevalences of myopia range from 0.79% in Jhapa District, Nepal to 72.9% in Liwan District, Guangzhou. Across all sites, the maximum percentage of emmetropia reached was 47.2% in Mahabubnagar District in India. However, in a majority of sites the maximum prevalence of emmetropia obtained was less than 35%, even when, as in the case of Liwan District in Guangzhou, the majority of children progressed to become myopic.
The distributions of refractive error by category are shown for each of the sites in Figures 1 to 8. They are ordered on the basis of the prevalence of myopia at age 15 so that changes in the distribution of refractive error categories can be analysed in relation to the development of myopia through childhood.
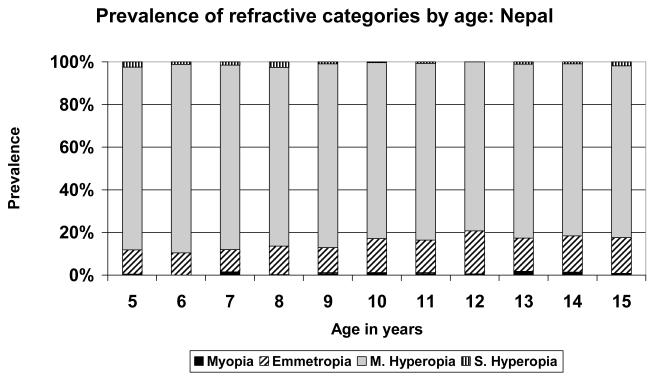
FIGURE 1.
Prevalence of refractive categories by age: Eastern Nepal (rural).
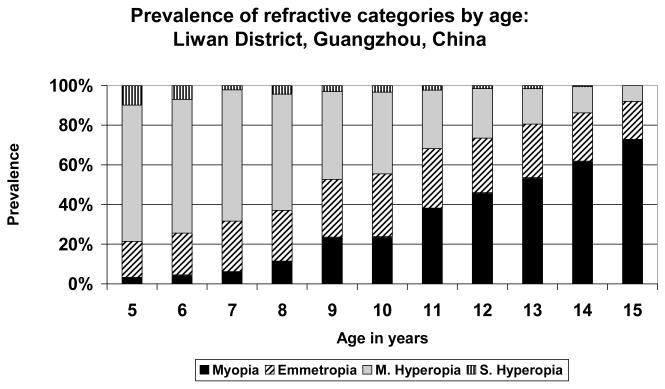
FIGURE 8.
Prevalence of refractive categories by age: Guangzhou, China (urban).
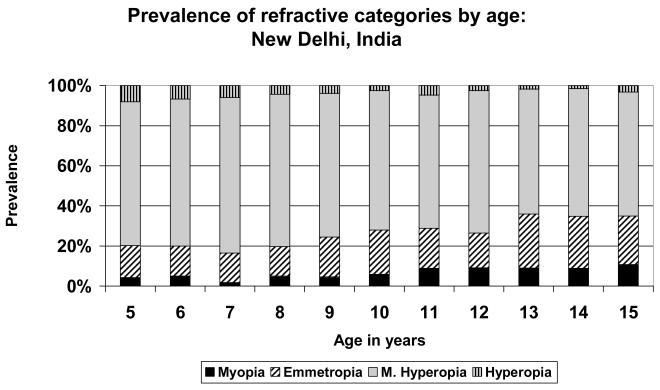
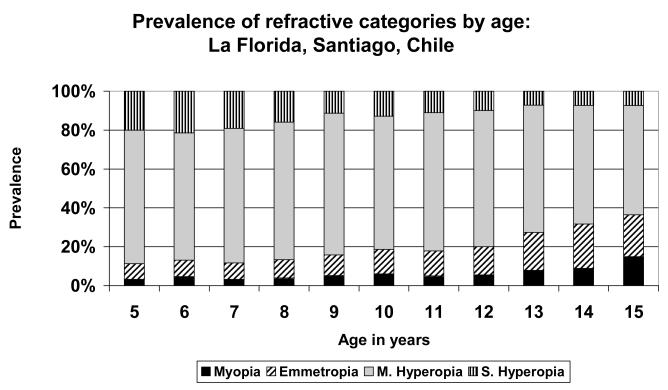
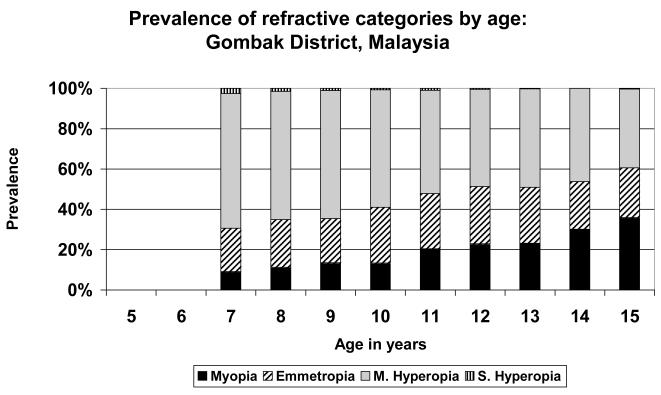
A very simple pattern is seen in the data from Eastern Nepal (Figure 1), where the population was predominantly mildly hyperopic at all stages. There was little myopia at any age, but the maximum prevalence of emmetropia, just over 20%, was reached at age 12. Similar patterns were seen in the data from Durban (Figure 3), where the population was predominantly hyperopic at all ages, with the maximum emmetropic proportion (39.4%) at age 15; in New Delhi (Figure 4), where the population was predominantly hyperopic at all ages, with the maximum proportion emmetropic (26.9%) at age 13; in Santiago (Figure 5), where the population was predominantly hyperopic at all ages, with the maximum proportion emmetropic (22.9%) at age 14; and in Kuala Lumpur (Figure 6), where the population was predominantly hyperopic at all ages, with the maximum proportion emmetropic (28.5%) reached at age 12..
FIGURE 3.
Prevalence of refractive categories by age: Durban, South Africa (semi-urban).
FIGURE 4.
Prevalence of refractive categories by age: New Delhi, India (urban).
FIGURE 5.
Prevalence of refractive categories by age: Santiago, Chile (urban).
FIGURE 6.
Prevalence of refractive categories by age: Kuala Lumpur, Malaysia (urban).
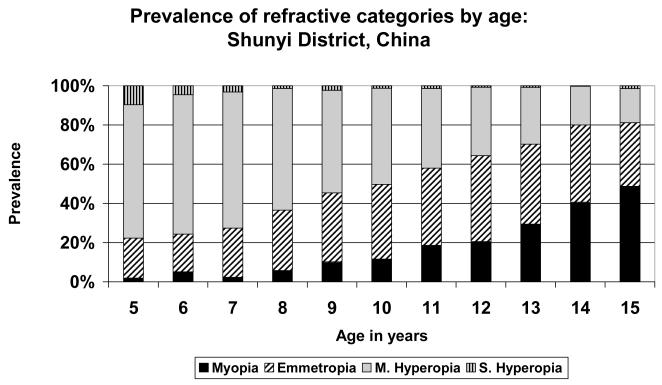
The other extreme is represented by the data from Shunyi District (Figure 7) and Guangzhou (Figure 8), the two study sites in China. In Guangzhou, the prevalence of myopia was over 70% at the age of 15, from a very low level of 3% at the age of 5. The population shifted from predominantly hyperopic at age 5 to predominantly myopic at age 15, with a cross-over taking place around the ages of 11-12. At no stage was the population predominantly emmetropic, with the maximum proportion emmetropic (31.6%) at age 10. It appears as though the proportion of children shifting from the mildly hyperopic state to the emmetropic state is generally balanced by the proportion shifting from the emmetropic category into myopia. A rather similar picture was seen in the data from Shunyi District (Figure 7), where the prevalence of myopia attained almost 50% at age 15. In this case, the proportion of children in the emmetropic category reached somewhat higher levels than in Guangzhou, and emmetropia was the most prevalent category at ages 12 and 13, before declining and being supplanted by the increasing proportion of children myopic at age 14.
FIGURE 7.
Prevalence of refractive categories by age: Shunyi District, China (semi-rural).
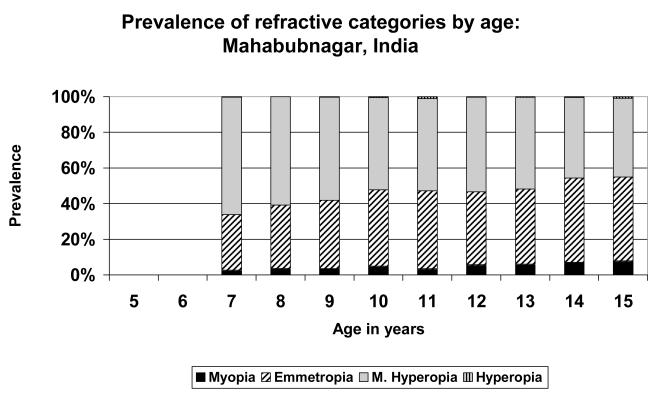
The only substantially different pattern was seen in the data from rural Southern India (Figure 2). In this case, the proportion emmetropic was already high (31.2%) at the age of 7, although the population was still predominantly mildly hyperopic. The proportion of children with emmetropia then increased steadily to slightly over-shadow the proportion mildly hyperopic at ages 14 and 15. However, in contrast to the situation in Shunyi and Guangzhou, there was little increase in the proportion of children who were myopic.
FIGURE 2.
Prevalence of refractive categories by age: Southern India (rural).
It is also worth noting that while there were substantial differences in the prevalence of significant hyperopia in the younger age groups, there was no particular pattern of association with ethnicity or the rural/urban nature of the study site. Early significant hyperopia was rapidly cleared to insignificant levels by the age of 15, except in the study sample from Chile.
DISCUSSION
These data give a comprehensive picture of how spherical equivalent refraction changes with age, in a range of different ethnic groups, at several different sites around the world, with population-based samples and rigorous cycloplegia. These data are cross-sectional, not longitudinal, and hence, if there were major birth-cohort effects, their interpretation would be problematic. However, the narrow age span in the study suggests that major birth-cohort effects, within a population and site, are unlikely; and we have therefore interpreted these data to give insight into the likely patterns of longitudinal change. These patterns need, however, to be confirmed in longitudinal studies.
In general, the pattern of development of refraction by categories was in line with a more complex model of human refractive development, rather than the model of tight emmetropisation that has emerged from studies on animal models, based on the apparently precise compensation which occurs in response to imposed defocus. In only rare cases were the age samples predominantly emmetropic. Instead, by the age of 15, the populations were either predominantly mildly hyperopic (Nepal (Pokharel et al. 2000), Chile (Maul et al. 2000), New Delhi (Murthy et al. 2002), Durban (Naidoo et al. 2003); Malaysia (Goh et al. 2005), or the population quite rapidly shifted to predominant myopia (Shunyi (Zhao et al. 2000) and Guangzhou (He et al. 2004)).
At seven of the eight sites, movement into the emmetropic category from the mildly hyperopic category with increasing age appears to be counter-balanced by movement from the emmetropic category into the myopic, keeping the proportion of the population in the optically emmetropic range relatively low. The only substantial deviation from this pattern is seen in the data from Mahabubnagar in rural Southern India (Dandona et al. 2002). In this case, the proportion of children with emmetropia was already high at the age of 7, the earliest age studied, and it continued to increase up to the age of 15, without a large amount of transfer into the myopic category. A similar pattern could possibly be emerging in the data from Durban (Naidoo et al. 2003), although this population was still predominantly mildly hyperopic at the age of 15. To the extent that high rates of axial elongation are associated with movement from mild hyperopia to emmetropia, it is difficult to understand how these do not subsequently lead to substantial increases in the prevalence of myopia, and the pattern of refractive development in these cases needs further documentation and analysis.
Overall, these data strongly support to the idea that emmetropia, as defined optically, is not the preferred end-point for refractive development in humans. In sites with a low prevalence of myopia, the population tends to remain predominantly mildly hyperopic, rather than emmetropic, even at the age of 15. This however, has little impact on visual acuity, at least in the short-term, because of the high accommodative capacities of children (Anderson et al. 2008). In sites where the prevalence of myopia becomes high, myopia tends to become the predominant category, and children appear to progress through emmetropia to become myopic, rather than being trapped in the emmetropic category, as tight definitions of emmetropisation would imply.
A restricted range for emmetropia was used for analysis because it corresponds to the classical definition of emmetropia, and in order to test whether emmetropisation in humans is precisely directed to produce a clustering of SER values around plano. The clearest demonstration that this is not the case comes from the data from Nepal, where around 80% of the subjects have refractions in the range from > +0.50 to ≤ +2.00D over the age range studied. The level of urbanization and education in Nepal is the lowest of the various sites examined, and the absence of any significant increase in the prevalence of myopia up to the age of 15 suggests that there is not a natural tendency for myopia to increase during childhood, unless the natural environment that children are exposed to is altered sufficiently to challenge the mechanisms that regulate eye growth.
Changing the definition of emmetropia would have an obvious impact on this analysis. In particular, increasing the upper limit for emmetropia would naturally include an increasing proportion of the mild hyperopes in the emmetropic category, increasing the proportion of emmetropes at each site. However, this sort of analysis would simply reinforce the point that SER values are not clustered around plano, but are spread over a range of values in which accommodation can provide normal visual acuity for 4-5 decades of life, with no indication of great precision in the end-point.
The results suggest that by the time that children become myopic, there may be little or no capacity to maintain emmetropia. This does not appear to be due to specific genetic characteristics of ethnic groups, since the prevalence of myopia is highly variable within and across ethic groups, depending on environmental exposures to educational pressures (Morgan and Rose 2005) and to outdoor environments (Jones et al. 2007; Rose et al. 2008a,b; Dirani et al. 2009). Instead, we suggest when axial elongation has reached a certain point, the capacity to limit further progression by reducing lens power has been exhausted. The sites at which mildly hyperopic refractions are maintained to the age of 15 share the characteristic of having relatively poorly developed educational systems, whereas those where myopia becomes predominant are in countries where prolonged and intensive education is the norm. An overview of international educational standards can be obtained at the UNESCO website (http://www.ibe.unesco.org/en/access-by-country.html), but more work needs to be done to characterize the amount of time children spend outdoors across the various sites.
Data from longitudinal studies have supported the common clinical intuition that early achievement of emmetropia is a risk factor for subsequent progression to myopia (Zadnik et al. 1999). Because of the potential for future progression to myopia once near-emmetropia is achieved (Thorn et al. 2005), the strategy favoured by evolution appears to be one of maintaining eyes in a state of mild hyperopia, where clear vision can be obtained through accommodation. From this evolutionary perspective, a refractive state which does not significantly impair distance visual acuity, where hyperopic errors can be cleared by accommodation, would not be selected against, and indeed all the RESC populations over most of the period from age 5 to age 15 are in a state in which the prevailing refractive error would have little functional significance, except where substantial levels of myopia have appeared in the population. The problems associated with this strategy when presbyopia emerges later in life are not important from an evolutionary perspective.
This picture of refractive development in humans is not consistent models of refractive development derived from animal studies, which suggest that optical emmetropia should be the end point, due to the interplay of growth-promoting and growth-inhibiting signals (Wallman and Winawer 2004). This view of emmetropisation may be relevant to the rapid adjustments of refractive error which take place in the first year of life (Gwiazda et al. 1993; Mayer et al. 2001; Mutti et al. 2005), but may have little relevance to human refractive development after this time. Recent studies on non-human primates suggest that, after a rapid phase of elimination of neo-natal refractive errors, mild hyperopia is the preferred end-point for refractive development (Qiao-Grider et al. 2007).
It could be argued that one explanation of these findings is that once spherical equivalent refractions are in the mildly hyperopic range, the use of accommodation would minimise hyperopic defocus and hence minimise the drive towards axial elongation. However, this view is not compatible with the precise compensation that occurs in animal models to imposed hyperopic defocus (Wallman and Winawer 2004), where accommodation can also be used to clear vision. Moreover, at many of the RESC sites, there is continued incident myopia up the age of 15 and increases in mean SER, which suggests that axial elongation has not ceased.
One limitation of this study is that the oldest children examined were 15. Over the years, the age at which refractive development has been regarded as terminating, at least in terms of the development of myopia, has tended to increase, with early studies proposing 12-13 as a typical age for the end of refractive development (Curtin 1985), and more recent studies proposing the later teen-age years (Goss et al. 1990). There is now sound evidence from a population-based study which used cycloplegic refraction (Hashemi et al. 2004) that the prevalence of myopia can increase for another decade, and there is also evidence for continued myopic progression, at least in selected groups (Lin et al. 1996). The study by Sorsby et al. (1960) of cycloplegic refractions in national servicemen provides a useful point of reference, even though the refractive categories cannot be precisely aligned with ours. This study reported that 73.4% of these young adults had refractions in the range from 0.00 to <2.00D, in a sample where the overall prevalence of myopia (<0.00D) was only 11.7% These data suggest that the basic characteristics of the distribution of refractive errors documented in this paper are preserved into young adult life, at least when the overall prevalence of myopia is low. No studies have examined young adults in a population-based sample with cycloplegic refraction where the prevalence of myopia is high. We believe that continued transition of low hyperopes into emmetropes and emmetropes into myopes is likely to be observed. Overall, more detailed studies of this important young adult age range, in which cyloplegia is essential for accurate refraction (Hashemi et al. 2004), are required. This is, of course, not the end of refractive development, since there is a later phase of development, after the age of around 30, in which there are hyperopic shifts in refraction over several decades (Lee et al. 2002; Hashemi et al. 2009), and, later still, myopic shifts in refraction associated with nuclear opacities (Wong et al. 2001).
These results have several implications. They emphasise that mild hyperopia is not an abnormal state during childhood, which has implication for the debate over if, and when, to treat hyperopia in children (Mutti 2007). In contrast, early onset emmetropia is a problem which may lead to the development of myopia, and thus may be an appropriate trigger for myopia prevention when acceptable methods become available. They also suggest that future research needs to concentrate on the biological basis and control of the two main factors which appear to be at play in human refractive development after the first year or two of life. The first is axial elongation, which, judged from continuing refractive changes, can continue into the third decade of life (Lin et al. 1996; Hashemi et al. 2004), and which may be sensitive to environmental exposures (Morgan and Rose 2005). The second is the process of reduction in lens power, which has so far not been studied experimentally.
Acknowledgements
This study was supported by the World Health Organization under National Institutes of Health contract N01-EY-2103; International Centre for Eyecare Education; Christoffel-Blindenmission; Sight Savers International; Helen Keller International; Ministry of Health, Government of India; Ministry of Health, Government of Malaysia; and the Australian Research Council (ARC Centre of Excellence in Vision Science, COE561903). The sponsors or funding organizations had no role in the design or conduct of this research.
APPENDIX
RESC Survey Group principal investigators and their organizational affiliation during the conduct of the surveys:
Gopal P. Pokharel, MD, MPH. Foundation Eye Care Himalaya, Kathmandu, Nepal.
Jialiang Zhao, MD. Peking Union Medical College Hospital, Beijing, China.
Eugenio Maul, MD. Pontificia Universidad Catolica de Chile, Santiago, Chile.
Lalit Dandona, MD, MPH. L. V. Prasad Eye Institute, Hyderbad, India.
G. V. S. Murthy, MD. Dr. R. P. Centre for Ophthalmic Sciences, New Delhi, India.
Kovin S. Naidoo, OD, MPH. University of Durban-Westville, Durban, South Africa.
Mingguang He, MD, PhD. Zhongshan Ophthalmic Center, Guangzhou, China.
Pik-Pin Goh, MD, MPH. Hospital Selayang, Selangor, Malaysia
Footnotes
No conflicting relationship exists for any author.
References
- Anderson HA, Hentz G, Glasser A, Stuebing KK, Manny RE. Minus-lens-stimulated accommodative amplitude decreases sigmoidally with age: a study pf objectively measured accommodative amplitude from age 3. Invest Ophthalmol Vis Sci. 2008;49:2919–26. doi: 10.1167/iovs.07-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin BJ. Basic Science and Clinical Management. Harper and Row; Philadelphia: 1985. The Myopias. [Google Scholar]
- Dandona R, Dandona L, Srinivas M, Sahare P, Narsaiah S, Munoz SR, Pokharel GP, Ellwein LB. Refractive error in children in a rural population in India. Invest Ophthalmol Vis Sci. 2002;43:615–22. [PubMed] [Google Scholar]
- Dirani M, Tong L, Gazzard G, Zhang X, Chia A, Young TL, Rose KA, Mitchell P, Saw SM. Outdoor activity and myopia in Singapore teenage children. Br J Ophthalmol. 2009 doi: 10.1136/bjo.2008.150979. Epub Feb 11. [DOI] [PubMed] [Google Scholar]
- Duke-Elder S. In: Practice of refraction. 10th revised edition Abrams D, editor. Elsevier Health Sciences; 1993. [Google Scholar]
- Garner LF, Yap MK, Kinnear RF, Frith MJ. Ocular dimensions and refraction in Tibetan children. Optom Vis Sci. 1995;72:266–71. doi: 10.1097/00006324-199504000-00007. [DOI] [PubMed] [Google Scholar]
- Garner LF, Stewart AW, Owens H, Kinnear RF, Frith MJ. The Nepal Longitudinal Study: biometric characteristics of developing eyes. Optom Vis Sci. 2006;83:274–80. doi: 10.1097/01.opx.0000215251.27409.16. [DOI] [PubMed] [Google Scholar]
- Goh PP, Abqariyah Y, Pokharel GP, Ellwein LB. Refractive error and visual impairment in school-age children in Gombak District, Malaysia. Ophthalmol. 2005;112:678–85. doi: 10.1016/j.ophtha.2004.10.048. [DOI] [PubMed] [Google Scholar]
- Goss DA, Cox VD, Herrin-Lawson GA, Nielsen ED, Dolton WA. Refractive error, axial length, and height as a function of age in young myopes. Optom Vis Sci. 1990;67:332–8. doi: 10.1097/00006324-199005000-00006. [DOI] [PubMed] [Google Scholar]
- Gwiazda J, Thorn F, Bauer J, Held R. Emmetropization and the progression of manifest refraction in children followed from infancy to puberty. Clin Vis Sci. 1993;8:337–44. [Google Scholar]
- He M, Zeng J, Liu Y, Xu J, Pokharel GP, Ellwein LB. Refractive error and visual impairment in urban children in southern China. Invest Ophthalmol Vis Sci. 2004;45:793–9. doi: 10.1167/iovs.03-1051. [DOI] [PubMed] [Google Scholar]
- Hashemi H, Fotouhi A, Mohammad K. The age- and gender-specific prevalences of refractive errors in Tehran: the Tehran Eye Study. Ophthalmic Epidemiol. 2004;11:213–25. doi: 10.1080/09286580490514513. [DOI] [PubMed] [Google Scholar]
- Hashemi H, KhabazKhoob R, Iribarren R, Morgan IG, Mohammad K, Fotouhi A. Hyperopic shift in the elderly: the Tehran Eye Study. Brit J Ophthalmol. 2009 doi: 10.1136/bjo.2009.160465. Epub, August 18. [DOI] [PubMed] [Google Scholar]
- Hirsch M, Weymouth F. Prevalence of refractive anomalies. In: Grosvenor T, Flom M, editors. Refractive anomalies. Research and clinical applications. Butterworth-Heinemann; Boston: 1991. pp. 15–38. [Google Scholar]
- Hung LF, Crawford ML, Smith EL. Spectacle lenses alter eye growth and the refractive status of young monkeys. Nat Med. 1995;1:761–5. doi: 10.1038/nm0895-761. [DOI] [PubMed] [Google Scholar]
- Ip JM, Huynh SC, Robaei D, Kifley A, Rose KA, Morgan IG, Wang JJ, Mitchell P. Ethnic differences in refraction and ocular biometry in a population-based sample of 11-15-year-old Australian children. Eye. 2008;22:649–56. doi: 10.1038/sj.eye.6702701. [DOI] [PubMed] [Google Scholar]
- Jones LA, Sinnott LT, Mutti DO, Mitchell GL, Moeschberger ML, Zadnik K. Parental history of myopia, sports and outdoor activities, and future myopia. Invest Ophthalmol Vis Sci. 2007;48:3524–32. doi: 10.1167/iovs.06-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KE, Klein BE, Klein R, Wong TY. Changes in refraction over 10 years in an adult population: the Beaver Dam Eye Study. Invest Ophthalmol Vis Sci. 2002;43:2566–71. [PubMed] [Google Scholar]
- Lin LL, Shih YF, Lee YC, Hung PT, Hou PK. Changes in ocular refraction and its components among medical students--a 5-year longitudinal study. Optom Vis Sci. 1996;73:495–8. doi: 10.1097/00006324-199607000-00007. [DOI] [PubMed] [Google Scholar]
- Lin LL, Shih YF, Hsiao CK, Chen CJ. Prevalence of myopia in Taiwanese schoolchildren: 1983 to 2000. Ann Acad Med Singapore. 2004;33:27–33. [PubMed] [Google Scholar]
- Matsumura H, Hirai H. Prevalence of myopia and refractive changes in students from 3 to 17 years of age. Surv Ophthalmol. 1999;44(Suppl 1):S109–15. doi: 10.1016/s0039-6257(99)00094-6. [DOI] [PubMed] [Google Scholar]
- Maul E, Barroso S, Munoz SR, Sperduto RD, Ellwein LB. Refractive Error Study in Children: results from La Florida, Chile. Am J Ophthalmol. 2000;129:445–54. doi: 10.1016/s0002-9394(99)00454-7. [DOI] [PubMed] [Google Scholar]
- Mayer DL, Hansen RM, Moore BD, Kim S, Fulton AB. Cycloplegic refractions in healthy children aged 1 through 48 months. Arch Ophthalmol. 2001;119:1625–8. doi: 10.1001/archopht.119.11.1625. [DOI] [PubMed] [Google Scholar]
- Morgan I, Rose K. How genetic is school myopia? Prog Retin Eye Res. 2005;24:1–38. doi: 10.1016/j.preteyeres.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Murthy GV, Gupta SK, Ellwein LB, Munoz SR, Pokharel GP, Sanga L, Bachani D. Refractive error in children in an urban population in New Delhi. Invest Ophthalmol Vis Sci. 2002;43:623–31. [PubMed] [Google Scholar]
- Mutti DO. To emmetropise or not to emmetropise? The question for hyperopic development. Optom Vis Sci. 2007;84:97–102. doi: 10.1097/OPX.0b013e318031b079. [DOI] [PubMed] [Google Scholar]
- Mutti DO, Mitchell GL, Jones LA, Friedman NE, Frane SL, Lin WK, Moeschberger ML, Zadnik K. Axial growth and changes in lenticular and corneal power during emmetropization in infants. Invest Ophthalmol Vis Sci. 2005;46:3074–80. doi: 10.1167/iovs.04-1040. [DOI] [PubMed] [Google Scholar]
- Naidoo KS, Raghunandan A, Mashige KP, Govender P, Holden BA, Pokharel GP, Ellwein LB. Refractive error and visual impairment in African children in South Africa. Invest Ophthalmol Vis Sci. 2003;44:3764–70. doi: 10.1167/iovs.03-0283. [DOI] [PubMed] [Google Scholar]
- Negrel AD, Maul E, Pokharel GP, Zhao J, Ellwein LB. Refractive Error Study in Children: sampling and measurement methods for a multi-country survey. Am J Ophthalmol. 2000;129:421–6. doi: 10.1016/s0002-9394(99)00455-9. [DOI] [PubMed] [Google Scholar]
- Ojaimi E, Rose KA, Morgan IG, Smith W, Martin FJ, Kifley A, Robaei D, Mitchell P. Distribution of ocular biometric parameters and refraction in a population-based study of Australian children. Invest Ophthalmol Vis Sci. 2005;46:2748–54. doi: 10.1167/iovs.04-1324. [DOI] [PubMed] [Google Scholar]
- Pokharel GP, Negrel AD, Munoz SR, Ellwein LB. Refractive Error Study in Children: results from Mechi Zone, Nepal. Am J Ophthalmol. 2000;129:436–44. doi: 10.1016/s0002-9394(99)00453-5. [DOI] [PubMed] [Google Scholar]
- Qiao-Grider Y, Hung LF, Kee CS, Ramamirthan R, Smith EL. Normal ocular development in young rhesus monkeys (Macaca mulatta) Vision Res. 2007;47:1424–44. doi: 10.1016/j.visres.2007.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada J, Shelton S, Norton TT. The sclera and myopia. Exp Eye Res. 2006;82:185–200. doi: 10.1016/j.exer.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Rose KA, Morgan IG, Smith W, Burlutsky G, Mitchell P, Saw SM. Myopia, lifestyle, and schooling in students of Chinese ethnicity in Singapore and Sydney. Arch Ophthalmol. 2008a;126:527–30. doi: 10.1001/archopht.126.4.527. [DOI] [PubMed] [Google Scholar]
- Rose KA, Morgan IG, Ip J, Kifley A, Huynh S, Smith W, Mitchell P. Outdoor activity reduces the prevalence of myopia in children. Ophthalmol. 2008b;115:1279–85. doi: 10.1016/j.ophtha.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Glasser A, Howland HC. Accommodation, refractive error and eye growth in chickens. Vision Res. 1988;28:639–57. doi: 10.1016/0042-6989(88)90113-7. [DOI] [PubMed] [Google Scholar]
- Shaikh AW, Siegwart JT, Jr., Norton TT. Effect of interrupted lens wear on compensation for a minus lens in tree shrews. Optom Vis Sci. 1999;76:308–15. doi: 10.1097/00006324-199905000-00019. [DOI] [PubMed] [Google Scholar]
- Sorsby A, Sheridan M, Leary GA, Benjamin B. Vision, visual acuity, and ocular refraction of young men. Br Med J. 1960;1:1394–8. doi: 10.1136/bmj.1.5183.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorsby A, Benjamin B, Sheridan M, Stone J, Leary GA. Refraction and its components during the growth of the eye from the age of three. Memo Med Res Council. 1961;301:1–67. (Special) [PubMed] [Google Scholar]
- Thorn F, Gwiazda J, Held R. Myopia progression is specified by a double exponential growth function. Optom Vis Sci. 2005;82:286–97. doi: 10.1097/01.opx.0000159370.66540.34. [DOI] [PubMed] [Google Scholar]
- Tkatchenko AV, Walsh PA, Tkatchenko TV, Gustincich S, Raviola E. Form deprivation modulates retinal neurogenesis in primate experimental myopia. Proc Natl Acad Sci U S A. 2006;103:4681–6. doi: 10.1073/pnas.0600589103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43:447–68. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Wildsoet CF. Active emmetropization--evidence for its existence and ramifications for clinical practice. Ophthalmic Physiol Opt. 1997;17:279–90. [PubMed] [Google Scholar]
- Wong TY, Klein BE, Klein R, Tomany SC, Lee KE. Refractive errors and incident cataracts; the Beaver Dam Eye Study. Invest Ophthalmol Vis Sci. 2001;42:1449–54. [PubMed] [Google Scholar]
- Zadnik K, Mutti DO, Friedman NE, Qualley PA, Jones LA, Qui P, Kim HS, Hsu JC, Moeschberger ML. Ocular predictors of the onset of juvenile myopia. Invest Ophthalmol Vis Sci. 1999;40:1936–43. [PubMed] [Google Scholar]
- Zadnik K, Manny RE, Yu JA, Mitchell GL, Cotter SA, Quiralte JC, Shipp H, Friedman NE, Kleinstein RN, Walker TW, Jones LA, Moeschberger ML, Mutti DO. Ocular component data in schoolchildren as a function of age and gender. Optom Vis Sci. 2003;80:226–36. doi: 10.1097/00006324-200303000-00012. [DOI] [PubMed] [Google Scholar]
- Zhao J, Pan X, Sui R, Li F, Munoz SR, Ellwein LB. Refractive Error Study in Children: results from Shunyi District, China. Am J Ophthalmol. 2000;129:427–35. doi: 10.1016/s0002-9394(99)00452-3. [DOI] [PubMed] [Google Scholar]