Abstract
During early pregnancy, placentation occurs in a relatively hypoxic environment that is essential for appropriate embryonic development. Intervillous blood flow increases around 10 to 12 weeks of gestation and results in exposure of trophoblast cells to increased oxygen tension. Before this time, low oxygen appears to prevent trophoblast differentiation toward an invasive phenotype. Using human villous explants of 5–8 weeks’ gestation, we found that low oxygen tension triggered trophoblast proliferation, fibronectin synthesis, α5 integrin expression, and gelatinase A activity. These biochemical markers were barely detectable under oxic conditions. We therefore examined the placental expression of hypoxia-inducible factor-1 (HIF-1), a master regulator of oxygen homeostasis, and determined that expression of HIF-1α subunit during the first trimester of gestation parallels that of TGFβ3, an inhibitor of extravillous trophoblast differentiation. Expression of both molecules is high in early pregnancy and falls around 9 weeks of gestation, when placental pO2 levels are believed to increase. Increasing oxygen tension induced a similar decrease in expression in cultured explants. Moreover, antisense inhibition of HIF-1α expression in hypoxic explants inhibited expression of TGFβ3, arrested cell proliferation, decreased α5 expression and gelatinase A activity, and triggered biochemical markers of an invasive trophoblast phenotype such as α1 integrin and gelatinase B expression. These data suggest that the oxygen-regulated early events of trophoblast differentiation are in part mediated by TGFβ3 through HIF-1 transcription factors.
Introduction
During placentation cytotrophoblast cells localized in floating and anchoring villi follow 2 distinct pathways of differentiation (1, 2). Villous cytotrophoblasts fuse to form the highly specialized syncytiotrophoblast layer that contributes to gas, nutrient, and waste exchange. In anchoring villi cytotrohoblast generates a multilayered column of highly invasive extravillous trophoblasts [EVT] that later migrate into the decidua and invade the first third of the myometrium. Within the myometrium the EVT induce remodeling of the spiral arterioles to produce the low-resistance vascular system that is essential for fetal growth (3). This period in development is characterized by an important physiological switch in oxygen tension at the opening of the intervillous space.
During the first weeks of gestation EVT exists in a relatively low-oxygen environment. Maternal blood flow to the placenta is limited and endovascular EVT invasion is minimal (4). This low-oxygen environment is essential for normal embryonic and placental development because the early conceptus has little protection against oxygen-generated free radicals. Genbacev et al. (5) have provided in vitro evidence to support a role for low oxygen tension in maintaining trophoblasts in a proliferative, noninvasive, and immature phenotype. In mammalian systems, the adaptive response to hypoxia is accompanied by an increase in the expression of a variety of genes, including the hematopoietic growth factor erythropoietin gene, vascular endothelial growth factor, glycolytic enzymes, and inducible nitric oxide synthetase (6–9). Most of these genes are regulated by a common oxygen-sensing pathway, the formation of the hypoxia-inducible factor-1 (HIF-1) protein complex (10, 11). HIF-1, a basic helix-loop-helix PAS (bHLH-PAS) transcription factor, binds to a short DNA motif identified in the 5′-flanking regions of many of the hypoxia-induced genes (12). HIF-1 binds DNA as a heteromeric complex composed of 2 subunits, the constitutively expressed HIF-1β (ARNT) and HIF-1α, which is present in hypoxic conditions and is rapidly degraded by the proteasome under normoxic conditions through an interaction with the tumour suppressor protein (von Hippel-Lindau) VHL (13). There are no data on the expression of HIF-1α in the placenta or on its role in regulating trophoblast differentiation.
Around 10 to 12 weeks of gestation there is a critical physiologic increase in oxygen tension as the intervillous space opens and the conceptus is exposed to maternal blood. It is at this time that EVT differentiates towards a more invasive phenotype. We demonstrated recently that TGFβ3 is highly expressed during early placentation (6-8 weeks) when oxygen tension is low and declines at the end of the first trimester (10–12 weeks) when oxygen tension increases (14). TGFβ3 inhibits the early events of trophoblast differentiation along the invasive pathway. In pregnancies complicated by early-onset preeclampsia, TGFβ3 expression remains abnormally elevated and trophoblasts are arrested to an intermediate immature phenotype. The mechanisms that regulate expression of placental TGFβ3 during the first trimester of gestation remain to be determined.
Based on these data we hypothesize that early in the first trimester (< 10 weeks) the low oxygen tension environment maintains trophoblasts in a relatively immature, proliferative state, mediated by TGFβ3 through HIF-1α. Subsequently, trophoblast exposure to increased oxygen tension reduces HIF-1α and TGFβ3 expression, which in turn releases the block to EVT differentiation and invasion into the uterine wall. Data presented herein provide support for this hypothesis.
Methods
Human chorionic villous explant culture.
Villous explant cultures were established from first-trimester human placentas obtained from elective terminations of pregnancies as described previously (15). Placental tissue from 5 to 14 weeks of gestation was dated according to the criteria of the Carnegie classification evaluating the length of the embryo and external characteristics of embryonic/fetal parts. Briefly, placental tissue was placed in ice-cold PBS and processed within 2 hours of collection. The tissue was aseptically dissected to remove decidual tissue and fetal membranes. Small fragments of placental villi (15–25 mg wet weight) were teased apart and placed on Millicell-CM culture dish inserts (Millipore Corp, Bedford, Massachusetts, USA), precoated with 0.2 mL of undiluted Matrigel (Collaborative Biomedical Products, Bedford, Massachusetts, USA), and placed in a 24-well culture dish. Explants were cultured in serum-free DMEM/F12 (GIBCO-BRL, Grand Island, New York, USA), supplemented with 100 μg/mL streptomycin, 100 U/mL penicillin, and 0.25 μg/mL ascorbic acid, pH 7.4. Villous explants were placed at 37°C in either standard tissue culture condition (5% CO2 in 95% air) or maintained in an atmosphere of 3% O2/93% N2/5% CO2. Morphological integrity and viability of villous explants and their EVT differentiation were monitored daily for up to 6 days as reported previously (15).
Antisense oligonucleotides and their effects on trophoblast differentiation along the invasive pathway.
Phosphorothioate oligonucleotides (ON) were synthesized on a DNA synthesizer and purified by capillary electrophoresis. Oligonucleotides of 15 bp targeted against sequences adjacent to the AUG initiation codon of human TGFβ3 and HIF-1α mRNA were synthesized. Previous studies have demonstrated that antisense oligonucleotides targeted to sequences adjacent to initiation codons are most efficient in inhibiting translation (16). Furthermore, 16-mer oligonucleotides are short enough to be taken up efficiently and provide sufficient specificity for hybridization to the corresponding target mRNA (16). The sequences of the antisense and sense HIF-1α and TGFβ3 oligonucleotides were HIF-1α: 5′-GCCGGCGCCCTCCAT-3′ and 5′-ATGGAGGGCGCCGGC-3′; TGFβ3: 5′-CCTTTGCAAGTGCATC-3′ and 5′-GATGCACTTGCAAAGG-3′, respectively. Oligonucleotides were dissolved in water and their concentration was estimated by optical density at OD260. Villous explants, prepared from placentas of 5–14 weeks’ gestation were incubated in DMEM/F12 at 3% O2 overnight and then incubated in medium alone or medium containing antisense or sense oligonucleotides (10 μM). Culture media with and without oligonucleotides were routinely changed every 48 hours.
RT-PCR and Southern blot analysis.
Total RNA was extracted from placental tissue and from villous explants, reverse transcribed, and amplified by 15 cycles of PCR using HIF-1α– and TGFβ3-specific primers. RT-PCR products were analysed by Southern blotting using TGFβ3 and HIF-1α cDNAs labeled with 32P (17). The primer sets chosen for amplification of TGFβ3 and HIF-1α were based on human mRNA sequences. Primers used for amplification were: (a) TGFβ3 cDNA: (forward primer) 5′- CAAAGGGCTCTGGTGGTCCTG-3′, (reversed primer) 5′-CTTGGAGGTAATTCCTTTAGGG-3′ (predicted product size = 374 bp); (b) HIF-1α cDNA: (forward primer) 5′- ATCTCGGCGAAGTAAAGAATCTG-3′, (reversed primer) 5′-GTCACCATCATCT GTGAGAACC-3′ (predicted product size = 243 bp); (c) β-actin cDNA: (forward primer) 5′- CTTCTACAATGAGCTGGGTG-3′, (reversed primer) 5′-TCATGAGGTAGTCAGTCAGG-3′ (predicted product size = 307 bp). The identity of the PCR reaction products was confirmed by sequencing. The RT-PCR assay was linear for up to 25 cycles of amplification, and no signals were detected without the initial addition of reverse transcriptase.
Immunohistochemistry.
Placental tissue from first trimester (5–14 weeks) and from villous explants, kept in culture for 6 days at either 3 or 20% O2, was fixed for 2–4 hours at 4°C in 4% (vol/vol) paraformaldehyde, embedded in paraffin, and cut into 5-micron sections. To verify the quality of the tissue and select the most representative sections, every 10th one was stained with hematoxylin and eosin. Neighbouring sections were selected and stained using the avidin-biotin immunoperoxidase method. Endogenous peroxidase enzyme activity was quenched with 1.5% (vol/vol) hydrogen peroxide in methanol for 30 minutes. Antigen was retrieved by incubation with 0.125% trypsin in PBS for 10 minutes at room temperature. Nonspecific binding sites were blocked using 5% (vol/vol) normal goat serum (NGS) and 1% (wt/vol) BSA in Tris buffer for 30 minutes at room temperature. Optimal antibody concentrations were established in preliminary experiments by titration. A purified mouse mAb directed against human Ki67 (Boehringer Mannheim, Montreal, Quebec, Canada) and rat mAb to cytokeratin (7D3; gift of Jay Cross, Samuel Lunenfeld Research Institute) were used, each at 1:20 dilution, whereas the mouse mAb to human MMP9 (mAb Ab-2; Oncogene Research Products, Calbiochem, Cambridge, Massachusetts, USA) and mAb to human α1 integrin (VLA-1; T Cell Diagnostics, Woburn, Massachusetts, USA) were used at 1:100 and 1:10 dilution, respectively. Mouse mAbs against HIF-1α (mgc3) (18) were used at 1:50 dilution and mouse mAbs against α5 integrin subunit (P1D6; Chemicon International, Temecula, California, USA) were used at 1:200 dilution. For the HIF-1α staining, antigen was retrieved by microwaving the section in 10 mM sodium citrate, pH 6.0, for 5 minutes, and endogenous peroxidase was quenched using 3% (vol/vol) hydrogen peroxide in methanol for 30 minutes. In all the experiments slides were washed 3 times with Tris buffer, pH 7.6, and incubated with a 30-fold dilution of biotinylated anti-mouse IgG or a 50-fold dilution of biotinylated anti-rat IgG for 2 hours at room temperature; after washing 3 times with Tris buffer, the slides were incubated with an avidin-biotin complex for 1 hour. Slides were washed again in Tris buffer and developed in 0.075% (wt/vol) 3,3-diaminobenzidine in Tris buffer containing 0.002% (vol/vol) H2O2, giving rise to a brown product. After light counterstaining with toluidine blue, slides were dehydrated in an ascending ethanol series, cleared in xylene, and mounted. In control experiments, primary antibodies were replaced with blocking solution, 5% (vol/vol) NGS and 1% (wt/vol) BSA.
In situ hybridization.
Antisense and sense digoxigenin-labeled HIF-1α riboprobes (17) were generated as described in the RNA-labeling and detection kits (nonradioactive) from Boehringer Mannheim. In situ hybridization to placental tissue from the first trimester (5–14 weeks) was performed according to Braissant and Wahli (19).
Fibronectin synthesis and release.
Villous explants of 5–14 weeks’ gestation were incubated overnight at 37°C in DMEM/F12. Explants were placed in either standard tissue culture condition (5% CO2 in 95% air) or maintained in an atmosphere of 3% O2/93% N2/5% CO2. The medium was changed on various days of culture and replaced by methionine/cysteine–free DMEM containing 25 μCi/mL of [35S]methionine/cysteine. The cultures were metabolically labeled for 18 hours. Conditioned culture media were collected and diluted with an equal amount of 25 mM Tris-HCl buffer, pH 7.4, 0.15 M NaCl, and 0.5% (vol/vol) Triton X-100 (Tris/Triton X-100 buffer). Fibronectin was isolated using gelatin-Sepharose as described previously (15, 20). Briefly, 50 μL of the gelatin-Sepharose suspension was added to 500 μL of medium, and the samples were incubated overnight at 4°C. The gelatin-Sepharose beads were centrifuged and washed 3 times in Tris/Triton X-100 buffer. Fibronectin was eluted by boiling for 5 minutes in 1% (vol/vol) SDS and electrophoresed on 4–12% (wt/vol) polyacrylamide gradient gels. Radiolabeled fibronectin was revealed by autoradiography and quantitated using a PhosphoImager (410A and Image Quant software; Molecular Dynamics, Sunnyvale, California, USA).
Detection of metalloproteases by zymography.
Analysis of gelatinolytic activity was performed using 10% (wt/vol) polyacrylamide gel impregnated with 0.1% (wt/vol) gelatin (Novex, San Diego, California, USA) as described previously (21). Four microliters of conditioned media harvested from the explant cultures at day 1 and 3 of treatment were mixed with 10% (vol/vol) glycerol, 2% (wt/vol) SDS, 0.0025% (wt/vol) bromophenol blue, 0.5 M Tris, pH 6.8, and subjected to substrate-gel electrophoresis. Gels were then washed twice in 2% (vol/vol) Triton X-100 for 30 minutes at room temperature to remove the SDS. Then the gels were equilibrated with developing buffer (50 mM Tris-HCl, 0.2 M NaCl, 5 M CaCl2, Brij 35, pH 7.2) for 30 minutes and incubated overnight with the same buffer at 37°C. Gels were then stained with 0.1% (wt/vol) Coomassie brilliant blue G-250 after destaining to reveal zones of gelatinase activity.
Western blot analysis.
For Western blot analysis of metalloprotease expression, 5 μL of conditioned media were subjected to gel electrophoresis using 10% (wt/vol) polyacrylamide gels (SDS-PAGE). Proteins were then blotted to Western PVDF membrane. Primary antibodies were used at 1:100 dilution (mouse mAb to human MMP9, mAb Ab-2, and MMP2, mAb 75-7F7), detected using horse radish peroxidase–conjugated anti-mouse IgG (1:10.000-fold dilution), and enhanced by chemiluminescence (ECL; Amersham Pharmacia Biotech, Oakville, Ontario, Canada).
For Western blot analysis of HIF-1α, 100 μg of 7–15-week placental proteins were subjected to 10% (wt/vol) SDS-PAGE. After electrophoresis, proteins were transferred to nitrocellulose membranes. Nonspecific binding was blocked by incubation in 5% (wt/vol) nonfat dry milk in Tris-buffered saline containing 0.1% (vol/vol) Tween-20 (TBST) for 60 minutes. Membranes were then incubated with 1:1000 diluted anti–HIF-1α mAb (mcg3) in 5% (wt/vol) nonfat dry milk in TBST at 4°C. After overnight incubation, membranes were washed with TBST and incubated for 60 minutes at room temperature with 1:1000 diluted horseradish peroxidase–conjugated anti-mouse IgG in 5% (wt/vol) nonfat dry milk in TBST. After washing with TBST, blots were detected with chemiluminescent reagent.
Statistical analysis.
All data are presented as means ± SE of at least 3 separate experiments carried out in triplicate. Statistical significance was determined by Student’s t test for paired groups and by 1-way ANOVA followed by assessment of differences using Student-Newman-Keuls test for nonpaired groups. Significance was defined as P < 0.05.
Results
Low oxygen tension induces the onset of trophoblast proliferation in villous explants.
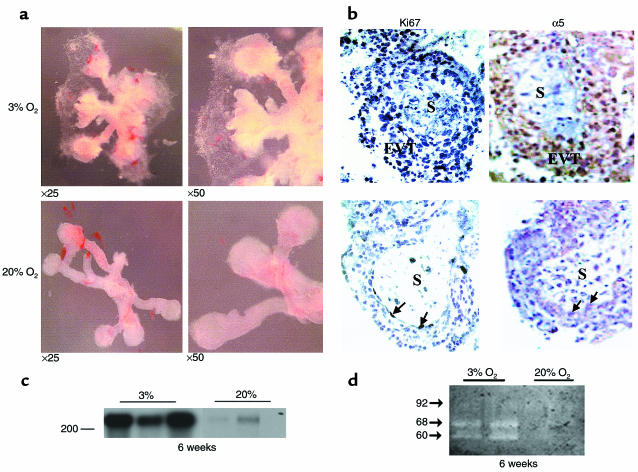
Intervillous blood flow is limited in early placentation but increases at around 10 to 12 weeks of gestation, resulting in exposure of the trophoblast to increased oxygen tension. Because recent studies (5, 22) have suggested that a switch in oxygen tension regulates trophoblast proliferation and differentiation along the invasive pathway, we first investigated the effect of different oxygen tensions on the early events of trophoblast differentiation. Human villous explants (5–8 weeks of gestation) cultured on Matrigel under normoxic conditions (20% O2) in serum-free medium remained viable for approximately 7–10 days and morphologically exhibited minimal EVT budding from the distal end of the villous tips. Exposure of villous explants to low oxygen (3% O2) resulted in an increase in EVT outgrowth from the distal end of the villous tips when compared with explants cultured under normoxic conditions (20% O2) (Figure 1a). Whereas low oxygen tension increased EVT outgrowth, no migrating cells were visible after villous explant exposure to low oxygen (Figure 1a). The increase in EVT outgrowth in low pO2 was associated with an increase in cell proliferation, assessed by Ki67 immunoreactivity (Figure 1b), α5 integrin expression (Figure 1b), fibronectin synthesis (Figure 1c), and gelatinase A/MMP2 gelatinolytic activity (Figure 1d).
Figure 1.
Low oxygen tension induces trophoblast outgrowth and increases proliferation, fibronectin synthesis, and gelatinase activity in villous explant cultures. (a) Villous explants from 5 to 8 weeks of gestation were maintained in culture for 5 days under normal (20% O2) or low (3% O2) oxygen tension. Note that exposure of villous explants to 3% O2 dramatically increases budding and outgrowth of EVT from the distal end of the villous tips when compared with villous explants kept at 20% O2. ×25, ×50. (b) Section of explants, cultured in either 3% or 20% O2, were stained for Ki67, a marker of cellular proliferation and α5 integrin. Strong positive nickel-enhanced immunoreactivity for Ki67 was observed in EVT of the outgrowth (EVT) of explants cultured in 3% O2. Similar immunolocalization was observed for α5 integrin. A few immunopositive cells for both Ki67 and α5 integrin were noted in explants kept at 20% O2 (arrows). S, villous stroma. (c) Explants incubated for 4 days in either low or normal pO2 conditions were metabolically labeled with [35S]methionine for 18 hours. Fibronectin was isolated from conditioned medium using gelatin-Sepharose beads. Samples were subjected to SDS-PAGE, and the position of the marker (with Mr = 200 × 103) is indicated. (d) Samples of medium conditioned by explants, cultured in either 3 or 20% O2, were collected at day 5 and subjected to analysis by gelatin zymography. Arrows indicate positions of gelatinase activity (gelatinase A/MMP-2: 60 and 68 kDa; gelatinase B/MMP-9: 92 kDa).
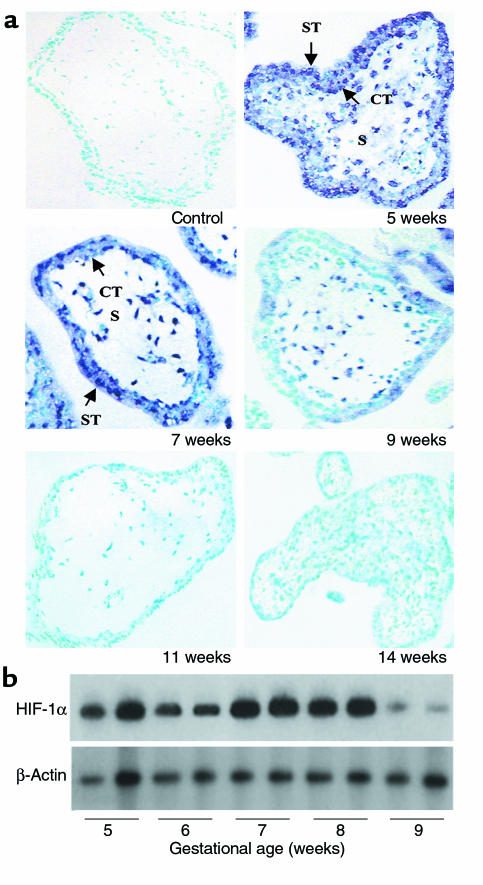
Ontogeny of HIF-1α mRNA expression in placenta during the first trimester.
To define the mechanism(s) by which low oxygen tension induces an increase in proliferation, fibronectin synthesis, α5 integrin expression, and MMP2 activity, we first studied the expression of HIF-1α in first-trimester placentas using nonradioactive in situ hybridization (Figure 2a) and low-cycle RT-PCR, followed by Southern blot analysis (Figure 2b). Expression of HIF-1α mRNA exhibited a striking pattern of developmental regulation with levels high at 5–8 weeks, but declining markedly at 9 weeks of gestation and absent at 11–14 weeks. HIF-1α mRNA was predominantly localized to the trophoblast layers with limited expression in the mesenchyme (Figure 2a). Western blot analysis revealed that the HIF-1α mAb recognized a protein of approximately 116 kDa (Figure 3d), in agreement with previous studies (18). HIF-1α protein showed a similar developmental expression pattern as HIF-1α mRNA, i.e., high at 7 weeks but low/absent at 15 weeks of gestation.
Figure 2.
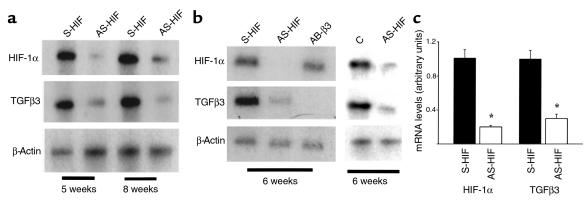
Expression of HIF-1α in human placenta in the first trimester of gestation (a) Expression of HIF-1α mRNA was also assessed by in situ hybridization to placental sections at 5–14 weeks of gestation with digoxigenin-labeled sense and antisense HIF-1α riboprobes. Endogenous alkaline phosphatase was blocked by the addition of levamisole. Sections were counterstained with methyl green. Note that HIF-1α mRNA expression, revealed by blue staining, is high at 5–7 weeks in chorionic villi, decreases around 9 weeks, and is absent at 11–14 weeks. Controls using a sense HIF-1α riboprobe were negative. ×100. (b) Message expression of HIF-1α and TGFβ3 were assessed by low-cycle RT-PCR followed by Southern blot analysis using specific probes for HIF-1α and TGFβ3 and the control housekeeping gene β-actin. Note that mRNA expression of HIF-1α is high between 5 to 8 weeks of gestation and declines thereafter.
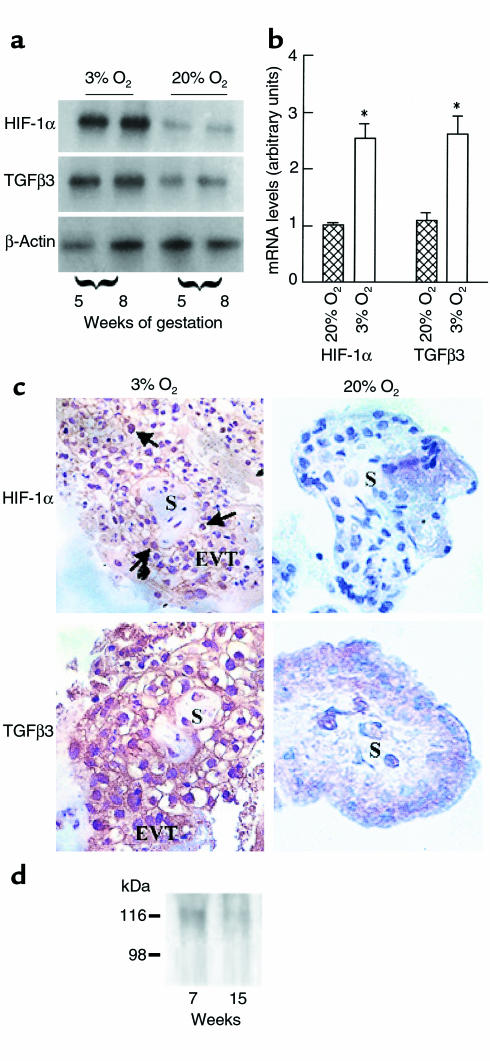
Figure 3.
Expression of HIF-1α villous explants exposed to different oxygen tension (a) Exposure of villous explants to 20% O2 downregulates the mRNA expression for HIF-1α and TGFβ3. (b) Bands were quantified by densitometric analysis. Shown are the changes in HIF-1α and TGFβ3 mRNA after normalization to control cultures kept at 20% O2. All data are expressed as the mean ± SEM of 5 separate experiments carried out in triplicate. (c) Immunoperoxidase staining of HIF-1α and TGFβ3 was performed in sections from explants kept at either 3% or 20% O2.. Sections of explants kept at 3% O2 show positive HIF-1α and TGFβ3 immunoreactivity in EVT cells of the outgrowth (EVT), whereas no immunoreactivity for HIF-1α and low immunoreactivity for TGFβ3 are noted in sections of explants kept at 20% O2. S, villous stroma. (d) Western blot analysis for HIF-1α in human placental tissue. HIF-1α protein (116 kDa) was detected in placentas of 7 weeks’ gestation, but not 15 weeks’ gestation.
Effect of low oxygen tension on HIF-1α and TGFβ3 expression in villous explants.
To define whether HIF-1α plays a role in modulating trophoblast function in low oxygen tension we studied the effect of different oxygen tensions on HIF-1α gene expression in the villous explant system. Villous explants from 5 to 8 weeks’ gestation, cultured at 3% O2, demonstrated significantly greater mRNA expression of HIF-1α when compared with explants cultured at 20% O2 (Figure 3, a and b). The effect of low pO2 on HIF-1α gene expression was noted as early as at 24 hours of incubation (data not shown). Because the ontogeny of placental HIF-1α mRNA expression (Figure 2) was similar to that of TGFβ3 (14), we also determined the effect of low oxygen tension on TGFβ3 mRNA expression in villous explants. Three percent oxygen upregulated the number of TGFβ3 transcripts when compared with 20% O2 (Figure 3, a and b). The abundant expression of HIF-1α and TGFβ3 in villous explants, cultured at 3% O2, was further confirmed by histochemical analysis. Immunoreactive HIF-1α protein localized to the EVT in the low oxygen–induced outgrowth (Figure 3c). In contrast, explants cultured at 20% O2 demonstrated no HIF-1α immunoreactivity (Figure 3c). Positive immunoreactivity for TGFβ3 was observed in explants cultured at either 3 or 20% O2; however, TGFβ3 immunoreactivity was markedly stronger in explants kept at 3% O2 (Figure 3c).
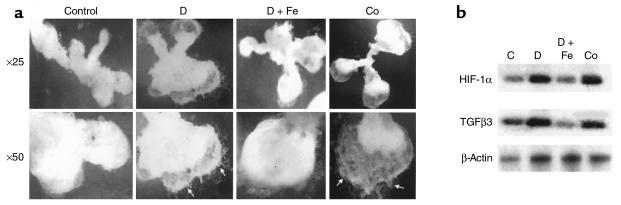
To determine whether low pO2 regulates HIF-1α and TGFβ3 gene expression by way of an iron (heme)-containing protein, villous explants were cultured at 20% O2 in the presence of either cobalt chloride or the iron chelator desferoxamine, both of which interfere with binding of molecular oxygen to heme proteins, thus mimicking hypoxia. Explants cultured in the presence of either 100 μM cobalt chloride or 10 μM desferoxamine showed an increase in the EVT outgrowth (Figure 4a) similar to that observed with explants maintained at 3% O2 (Figure 1a). Treatment of villous explants with either cobalt chloride or desferoxamine resulted in an increase of both HIF-1α and TGFβ3 mRNA expression (Figure 4b). Addition of iron (20 μM) to the desferoxamine-treated explants reversed both the stimulatory effect on EVT outgrowth (Figure 4a) and HIF-1α and TGFβ3 mRNA expression (Figure 4b). These data suggest that hypoxia upregulates HIF-1α and TGFβ3 gene expression in human villous explants and that this is mediated by way of a ferroprotein-dependent mechanism.
Figure 4.
Desferoxamine and cobalt chloride mimic the low-oxygen effect on EVT outgrowth and HIF-1α and TGFβ3 expression. (a) Exposure of villous explants, cultured in 20% O2 to either desferoxamine (10 μM) or CoCl2 (100 μM) increased EVT outgrowth (arrows). Addition of FeCl2 (20 μM) to the desferoxamine-treated cultures prevented the outgrowth. (b) Exposure of villous explants to either desferoxamine (10 μM) or cobalt chloride (100 μM) increased mRNA expression for HIf-1α and TGFβ3. C, control medium; D, desferoxamine; Co, cobalt chloride; D+Fe, desferoxamine + iron chloride. ×25, ×50.
Antisense inhibition of HIF-1α downregulates TGFβ3 mRNA expression in villous explants.
To determine whether HIF-1α mediates the hypoxia-induced TGFβ3 expression, we examined HIF- 1α and TGFβ3 mRNA expression in explants exposed to antisense HIF-1α and TGFβ3 oligonucleotides, respectively. Treatment of villous explants, cultured in 3% O2 with antisense oligonucleotides to HIF-1α (but not sense oligonucleotide–treated or control-untreated explants) abolished both TGFβ3 and HIF-1α mRNA expression (Figure 5, a–c), but not that of β-actin (Figure 5, a and b). In contrast, treatment of villous explants with antisense oligonucleotides to TGFβ3 abolished TGFβ3 mRNA expression but not that of HIF-1α (Figure 5b). These data corroborate the specificity of the antisense HIF-1α effect and suggest that HIF-1α may regulate TGFβ3 expression during early EVT outgrowth.
Figure 5.
Antisense oligonucleotides to HIF-1α inhibit HIF-1α and TGFβ3 mRNA expression. Explants of 5–8 weeks’ gestation were treated for 5 days with 10 μM antisense oligonucleotides to HIF-1α (AS-HIF) and antisense oligonucleotides to TGFβ3 (AS-β3). Control experiments were run in parallel using either sense oligonucleotides to HIF-1α (S-HIF) and TGF-β3 (S-β3) or medium alone (C). Expression of HIF-1α and TGFβ3 mRNA was measured by low-cycle RT-PCR followed by Southern blot analysis using specific probes for HIF-1α, TGF-β3, and the control housekeeping gene β-actin. (a) Antisense HIF-1α treatment of villous explants inhibits both HIF-1α and TGFβ3 mRNA. (b) Antisense TGFβ3 treatment of villous explants inhibits TGFβ3 mRNA expression but not that of HIF-1α, whereas antisense HIF-1α exposure results in inhibition of both HIF-1α and TGFβ3 mRNA expression. (c) Bands were quantified by densitometric analysis. Shown are the changes in HIF-1α and TGFβ3 mRNA after normalization to control cultures using sense oligonucleotides. All data are expressed as the mean ± SEM of 3 separate experiments carried out in triplicate.
Antisense inhibition of HIF-1α expression triggers a switch from a proliferative to an invasive trophoblast phenotype.
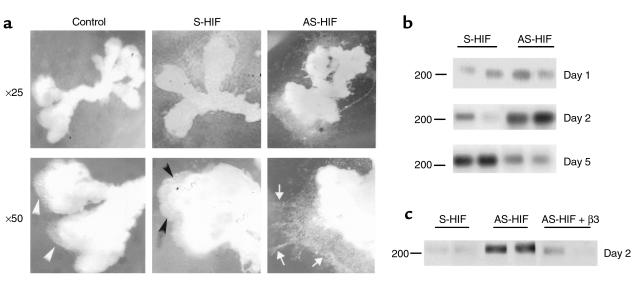
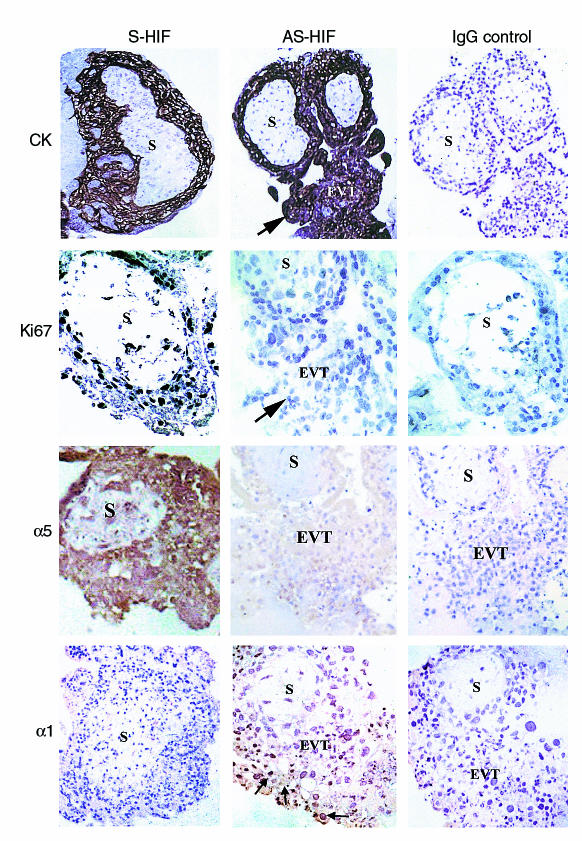
To determine the functional significance of HIF-1α expression during early placentation, we monitored trophoblast response to antisense-induced inhibition of HIF-1α expression in explants at 5–8 weeks of gestation. EVT outgrowth and proliferation without cell invasion was visible in control explants kept in 3% O2 in the presence of medium alone or sense oligonucleotides (Figure 6a). In contrast, explants exposed to antisense HIF-1α oligonucleotides displayed prominent EVT outgrowth from the distal end of the villous tip and an increased number of columns of cells migrating and invading into the surrounding matrix (Figure 6a). We next performed experiments to characterize the invasive phenotype of trophoblast cells in the outgrowth of the antisense-treated explants to confirm that trophoblasts in the outgrowth underwent a differentiation pathway typical of the extravillous trophoblast cells in vivo. Villous explants kept at 3% O2 showed an increase in newly synthesized fibronectin after 5 days of culture (Figures 1a and 6b). In contrast, explants kept at 3% O2 in the presence of antisense oligonucleotides to HIF-1α showed increased fibronectin production as early as 2 days of culture with a decrease thereafter, suggesting that downregulation of HIF-1α results in trophoblast differentiation along the invasive pathway (Figure 6b). Of interest, addition of recombinant TGFβ3 to the antisense HIF-1α–treated explants reversed the antisense stimulatory effect on fibronectin synthesis, which is in agreement with TGFβ3 being downstream of HIF-1α (Figure 6c). Similar to previous reports (5, 22), exposure of first-trimester villous explants to low oxygen tension resulted in increased proliferation (assessed by Ki67 immunoreactivity) at the proximal site of the column before trophoblasts start to migrate away from the anchoring villus (Figures 1b and 7). However, when explants in low oxygen tension were exposed to antisense HIF-1α oligonucleotides, we observed stimulated EVT outgrowth together with an increase in expression of biochemical markers of the invasive trophoblast phenotype such as gelatinase B/MMP9 (23, 24) and α1 integrin (24) (Figures 7 and 8), the latter being found in the EVT at the distal edge of the Matrigel (Figure 6). At the same time downregulation of HIF-1α resulted in markedly reduced Ki67 and α5 integrin immunoreactivity, suggesting a less proliferative phenotype (Figure 7). All trophoblast cells stained positively for cytokeratin, indicating the epithelial-like nature of the proliferating trophoblast cells in the low oxygen tension–induced outgrowth as well as in the invasive column of EVT after antisense HIF-1α treatment (Figure 7).
Figure 6.
Antisense oligonucleotides to HIF-1α induce extravillous trophoblast invasion and fibronectin production in villous explant cultures. (a) Villous explants of 5–8 weeks’ gestation were maintained in culture for 5 days at 3% O2 in the presence of 10 μM antisense oligonucleotides to HIF-1α (AS-HIF). Control experiments were run in parallel using explants from the same placentas cultured with medium alone or medium containing sense oligonucleotides (S-HIF). Note that antisense HIF-1α–treatment dramatically increases EVT invasion into the surrounding Matrigel (arrows) when compared with control villous explants, which show the typical low pO2-induced outgrowth (arrowheads). ×25, ×50. (b) Antisense HIF-1α treatment increases fibronectin synthesis as early as 2 days after oligonucleotide exposure. Note that after 5 days of treatment the antisense-stimulatory effect on fibronectin synthesis is lost. (c) Shown is a representative experiment demonstrating that addition of recombinant TGFβ3 to the antisense HIF-1α–treated explants abolished the early antisense-stimulatory effect on fibronectin production.
Figure 7.
Antisense oligonucleotides to HIF-1α inhibit proliferation and α5 integrin expression and induce α1 integrin expression in villous explant cultures. Immunoperoxidase staining of cytokeratin, Ki67, integrin α5, and α1 integrin was performed in placental sections from villous explants cultured under hypoxic condition with antisense and control sense oligonucleotides to HIF-1α. Sections of explants, cultured with antisense oligonucleotides, have no positive Ki67 immunoreactivity and markedly reduced staining for α5 integrin but are immunopositive for α1 integrin in EVT cells of the invading column (arrow, EVT). Sections of control cultures in the presence of sense oligonucleotides show positive immunoreactivity for Ki67 and α5 integrin but no positive α1 integrin staining in the EVT of the outgrowth induced by 3% O2 exposure. Independent of treatment, trophoblast cells stained positive for cytokeratin (CK), a marker for epithelial cells. Control immunostaining experiments were performed using control IgG. S, villous stroma.
Figure 8.
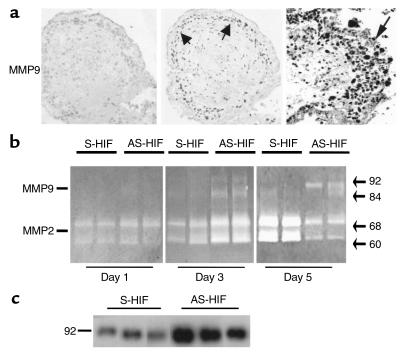
Antisense oligonucleotides to HIF-1α trigger gelatinase expression and activity in villous explants cultured at 3% O2. Explants of 5–8 weeks’ gestation were treated with antisense (AS-HIF) or control sense (S-HIF) oligonucleotides to HIF-1α (a) Section of explants treated for 5 days with antisense HIF-1α show positive immunoreactivity for gelatinase B/MMP9 in the invading EVT (arrow, EVT) when compared with control sense–treated explants. Samples of conditioned medium were collected at days 1, 3, and 5 and subjected to analysis by gelatin zymography (b) or at day 5 for Western blotting with gelatinase B/MMP9 antisera (c). Arrows indicate positions of gelatinase activity (gelatinase A/MMP-2: 60 and 68 kDa; gelatinase B/MMP-9: 84 and 92 kDa).
Antisense treatment markedly increased MMP9 immunoreactivity in the EVT within the outgrowth. Control sense oligonucleotide–treated explants showed weak immunoreactivity for MMP9 in the villous cytotrophoblast and in the stroma (Figure 8a). Gelatin zymography analysis of conditioned media from sense- and antisense-treated explants after 1 day of culture revealed gelatinase A/MMP2 activities at 60 and 68 kDa. After 3 days of culture, exposure of villous explants to antisense HIF-1α oligonucleotides resulted in an increase of gelatinase A/MMP2 activity and appearance of gelatinase B/MMP9 activities at 84 and 92 kDa (Figure 8b). It is interesting that after 5 days of culture antisense-HIF-1α oligonucleotide treatment decreased gelatinase A/MMP2 activity and increased gelatinaseB/MMP9 activity when compared with control cultures kept at 3% O2 in the presence of sense oligonucleotides (Figure 8b). Western blot analysis confirmed the upregulation of the MMP9 expression in explants treated for 5 days with antisense–HIF-1α (Figure 8c).
Discussion
In this report we demonstrate that oxygen tension is a critical regulator of trophoblast differentiation and that HIF-1α is upstream of TGFβ3 in mediating the effects of oxygen upon EVT differentiation. This conclusion is based on 3 observations: first, the parallel expression patterns of TGFβ3 and HIF-1α are seen during EVT development; second, increasing oxygen tension downregulates expression of both HIF-1α and TGFβ3; and third, the trophoblast invasive phenotype can be prematurely triggered in first trimester placentas by downregulation of TGFβ3 by way of antisense inhibition of HIF-1α.
During the invasion process, trophoblast cells undergo striking and rapid changes in cellular functions that are temporally and spatially regulated along the invasive pathway. During the formation of the anchoring villi, proliferating cytotrophoblasts break through the syncytium and form columns of nonpolarized extravillous trophoblast cells. This initial proliferative process is associated with a switch in the repertoire of integrins and extracellular matrix remodeling. EVT subsequently differentiate to acquire an invasive phenotype (25), expressing markers such as gelatinase B/MMP9 and α1 integrin (23, 26, 27) that permit the cells to invade and surround the maternal vessels in the decidua. Using a human villous explant system, we demonstrated that incubation of villous explants from early first-trimester placentas in their assumed physiologic pO2 (3% O2) environment is accompanied by changes in EVT outgrowth, cell proliferation, fibronectin synthesis, and gelatinase A/MMP2 activity (15, 21). Our data agree with previous observations that persistent low oxygen tension arrests trophoblast differentiation at this initial proliferative noninvasive stage, characterized by α5 integrin expression and low α1 integrin expression (5, 28). In contrast, in explants cultured at 20% oxygen, cytotrophoblasts of the proximal column do maintain a proliferative state beyond 24 hours (29). Several other studies document responses of trophoblasts to low oxygen tension, including increased production of the inflammatory cytokines (e.g., TNFα, IL-1α, and IL-1β [30, 31]), VEGF (32), plasminogen activator inhibitor-1 (33), and inhibition of cytotrophoblast differentiation toward syncytium (34).
HIF-1α has been shown to be a major regulator of cell function and differentiation in a number of in vivo animal models and cell systems (10–13). Our data suggest that HIF-1α may also mediate the effects of oxygen on human trophoblast development. Thus, expression of HIF-1α mRNA in placental trophoblast is high early on between 5 to 8 weeks and then falls precipitously around 10 to 12 weeks, precisely at the time when the intervillous space is perfused by maternal blood and pO2 levels are believed to increase (4). Moreover, HIF-1α mRNA and protein are high in trophoblast of villous explants cultured under physiologic low oxygen tension, and this expression is dramatically decreased when explants are exposed to higher oxygen levels. Janatpour et al (35), using isolated second-trimester trophoblasts, recently reported that HIF-1α expression was elevated after 60 hours of culture in both 2 and 20% oxygen. These data are surprising because reports from a wide variety of mammalian systems (including our own study on first-trimester trophoblasts) indicate that HIF-1α is upregulated by hypoxia. It is possible that this unusual response is unique to second-trimester trophoblasts. Alternatively, because it has been reported that stress can induce expression of HIF-1α (36), it may be that the particular conditions under which these isolated trophoblasts were cultured may have imposed a stress resulting in elevated HIF-1α expression irrespective of the levels of oxygen tension.
The decrease in HIF-1α expression in vivo is associated with the period of maximal trophoblast invasion into the maternal decidua. Interestingly, antisense inhibition of HIF-1α expression induced a premature switch to the invasive phenotype in human villous explants at 6–8 weeks of pregnancy, similar to that which normally occurs at 10–12 weeks (when endogenous expression of HIF-1α is reduced). This antisense HIF-1α–induced switch occurred despite the continued exposure of the explants to a low-oxygen environment that otherwise maintains them in a phase of arrested early development. We found that the switch is accompanied by inhibition of EVT proliferation, α5 integrin expression, a transient increase of fibronectin synthesis, MMP2 activity and an induction of MMP9, and α1 integrin expression. Note that treatment of explants with antisense HIF-1α inhibited proliferation and early EVT events (5). This observation is consistent with a recent report showing that hypoxic exposure of isolated trophoblasts induces changes in the expression of proteins that control the cell cycle (37). Moreover, HIF-1α has recently been reported to control proliferation and apoptosis of embryonic stem cells by regulating genes such as p53, p21, and Bcl-2 (36). Together these data suggest a key role for HIF-1α in regulating trophoblast proliferation under conditions of low oxygen tension.
Data from gene-targeting studies also support a role for the HIF family of transcription factors in placental development and uterine vascular remodeling. The disruption of the HIF-1α gene in mice results in embryonic lethality after cardiovascular and neuronal defects (38), whereas ARNT (HIF-1β)-deficient mice die after abnormalities related to angiogenesis (39) or from a failure in placental vascular development and formation of the labyrinthine spongiotrophoblast (40). The pattern of expression of HIF-1α during the first trimester and the initiation of trophoblast differentiation in vitro upon knockdown of HIF-1α levels paralleled data we published recently on TGFβ3 (14), leading us to investigate whether the effects of HIF-1α were in fact mediated by TGFβ3. Our data support such a role. As with HIF-1α, explants exposed to physiologic low oxygen tension expressed high levels of TGFβ3 mRNA and protein, but this expression was dramatically reduced upon exposure to high (20%) oxygen. Moreover, in addition to triggering trophoblast differentiation along the invasive pathway, antisense knockdown of HIF-1α expression led to inhibition of TGFβ3 expression. Critically, the effects of HIF-1α knockdown could be reversed by TGFβ3. The demonstration that antisense TGFβ3 treatment also induced markers of trophoblast invasion (14), but did not affect HIF-1α expression, further supports this cytokine acting downstream of HIF-1α.
The mechanisms by which HIF-1α expression is regulated in the trophoblast remain to be determined. A recent report showed that HIF-1α protein is continuously synthesized but rapidly degraded in normoxia by the proteasome system by way of an iron-dependent pVHL/HIF-1 interaction (13, 41). Thus, it is possible that in addition to downregulation of HIF-1α mRNA expression degradation of HIF-1α protein occurs in 20% O2. Hypoxia, cobalt, iron chelators, and various antioxidants have been shown to stabilize the HIF-1α subunit, thereby inducing the formation of the active HIF-1 complex (13, 41). However, the mechanisms of oxygen sensing are yet unclear. Studies analysing the erythropoietin gene (EPO), the first gene described to be responsive to hypoxia, have suggested that the oxygen sensor resides in a heme-containing molecule (11, 42). Herein, we demonstrate that treatment of villous explants, cultured at 20% O2 with either cobalt chloride or the iron chelator desferoxamine, induced EVT outgrowth from the distal end of the villous tips and increased expression of HIF-1α and TGFβ3 mRNA, thus mimicking the hypoxic response. Furthermore, we found that these effects were specific because addition of iron to the desferoxamine-treated explants abolished these stimulatory effects. Taken together, these data suggest that under reduced oxygen tension trophoblast cells activate HIF-1α, which in turn may upregulate TGFβ3 expression, and this oxygen effect appears to be mediated through a ferroprotein-dependent mechanism.
Mounting evidence suggests that the extravillous trophoblasts from early preeclamptic placentas are arrested at a relatively immature phenotype, possibly because of a failure to undergo complete differentiation along the invasive pathway during the first trimester of gestation. Preeclamptic placentas fail to complete integrin switching; i.e., the trophoblast remains positive for α5 and fails to express α1 (43), does not acquire an endovascular adhesion phenotype (44), demonstrates an excess of proliferative immature intermediate trophoblasts (45), and overexpresses fibronectin (14, 46). Data from our laboratory indicate that abnormalities in TGFβ3 expression are associated with preeclampsia and that downregulation of TGFβ3 with antisense oligonucleotides restores the invasive capability of preeclamptic trophoblasts (14).
In summary, our in vivo and in vitro HIF-1α expression data strongly suggest that the early conceptus and placenta develop in a relative hypoxic environment. The data are further consistent with a model of normal placentation in which the placental pO2 increases around 10 to 12 weeks of gestation, reduces HIF-1α expression, and in turn downregulates trophoblast TGFβ3 expression, thereby releasing the block to complete trophoblast differentiation into invasive EVT that invades deep into the maternal uterus. This invasion contributes to the remodeling of the uterine spiral arteries and, ultimately, enables the establishment of increased vascular perfusion of the placenta. A failure in a developmental switching in oxygen tension or a defect in the ability of the trophoblast to respond appropriately to this switch could result in abnormally elevated HIF-1α,and hence maintenance of TGFβ3 expression, for which we have shown results in an arrest of trophoblasts in a relatively immature state of differentiation. As a direct consequence, trophoblast invasion into the uterus is shallow, and uteroplacental perfusion is reduced. This may result in the clinical manifestations of preeclampsia, including shallow trophoblast invasion into the uterus and abnormally high uteroplacental vascular resistance.
Acknowledgments
We thank Christine Botsford for providing the placental samples. We also thank John Kingdom, James Copeman, and Jay Cross for carefully reading the manuscript and Knox Ritchie for the constant support. This work was supported by the Department of Obstetrics and Gynaecology, Medical Research Council of Canada (MRC) grant MT-14096 (to I. Caniggia), and MRC Group Grant in Lung Development (to M. Post). I. Caniggia is an MRC Scholar.
References
- 1.Cross JC, Werb Z, Fisher SJ. Implantation and the placenta: key pieces of the development puzzle. Science. 1994;266:1508–1518. doi: 10.1126/science.7985020. [DOI] [PubMed] [Google Scholar]
- 2.Strickland S, Richards WG. Invasion of trophoblasts. Cell. 1992;71:355–357. doi: 10.1016/0092-8674(92)90503-5. [DOI] [PubMed] [Google Scholar]
- 3.Aplin JD. Implantation, trophoblast differentiation and haemochorial placentation: mechanistic evidence in vivo and in vitro. J Cell Sci. 1991;99:681–692. doi: 10.1242/jcs.99.4.681. [DOI] [PubMed] [Google Scholar]
- 4.Rodesch F, Simon P, Donner C, Jauniaux E. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet Gynecol. 1992;80:283–285. [PubMed] [Google Scholar]
- 5.Genbacev O, Joslin R, Damsky CH, Polliotti BM, Fisher SJ. Hypoxia alters early gestation human cytotrophoblast differentiation/invasion in vitro and models the placental defects that occur in preeclampsia. J Clin Invest. 1996;97:540–550. doi: 10.1172/JCI118447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forsythe JA, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Semenza GL, Roth PH, Fang H-M, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia inducible factor 1. J Biol Chem. 1994;269:23757–23763. [PubMed] [Google Scholar]
- 9.Melillo G, et al. A hypoxia responsive element mediates a novel pathway of activation of the inducible nitric oxide synthase promoter. J Exp Med. 1995;182:1683–1693. doi: 10.1084/jem.182.6.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wenger RH, Gassmann M. Oxygen(es) and the hypoxia-inducible factor-1. J Biol Chem. 1997;378:609–616. [PubMed] [Google Scholar]
- 11.Wang GL, Semenza GL. General involvement of hypoxia inducible factor1 in transcriptional response to hypoxia. Proc Natl Acad Sci USA. 1993;90:4304–4308. doi: 10.1073/pnas.90.9.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang GL, Jiang BH, Rueand EA, Semenza GL. Hypoxia inducible factor 1 is a basic helix-loop-helix PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maxwell PH, et al. The tumor suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 14.Caniggia I, Grisaru-Gravnoski S, Kuliszewski M, Post M, Lye SJ. Inhibition of TGFβ3 restores the invasive capability of extravillous trophoblast in preeclamptic pregnancies. J Clin Invest. 1999;103:1641–1650. doi: 10.1172/JCI6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caniggia I, Taylor CV, Ritchie JWK, Lye SJ, Letarte M. Endoglin regulates trophoblast differentiation along the invasive pathway in human placental villous explants. Endocrinology. 1997;138:4977–4988. doi: 10.1210/endo.138.11.5475. [DOI] [PubMed] [Google Scholar]
- 16.Malcolm AD. Uses of antisense nucleic acids: an introduction. Biochem Soc Trans. 1992;20:745–746. doi: 10.1042/bst0200745. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, et al. Cloning and characterization of glucocorticoids induced genes in fetal rat lung fibroblasts: transforming growth factor β3. J Biol Chem. 1995;270:2722–2728. doi: 10.1074/jbc.270.6.2722. [DOI] [PubMed] [Google Scholar]
- 18.Camenisch G, et al. General applicability of chicken egg yolk antibodies: the performance of IgY immunoglobulins raised against the hypoxia-inducible factor 1α. FASEB J. 1999;13:81–88. doi: 10.1096/fasebj.13.1.81. [DOI] [PubMed] [Google Scholar]
- 19.Braissant O, Wahli W. A simplified in situ hybridization protocol using non-radioactive labeled probes to detect abundant and rare mRNAs on tissue sections. Biochemica. 1998;1:10–16. [Google Scholar]
- 20.Engvall E, Ruoslhati E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977;20:1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- 21.Caniggia I, Lye SJL, Cross JC. Activin is a local regulator of human cytotrophoblast cell differentiation. Endocrinology. 1997;138:3976–3986. doi: 10.1210/endo.138.9.5403. [DOI] [PubMed] [Google Scholar]
- 22.Zhou Y, Genbacev O, Damsky CH, Fisher SJ. Oxygen regulates human cytotrophoblast differentiation and invasion: implications for endovascular invasion in normal pregnancy and in pre-eclampsia. J Reprod Immunol. 1998;39:197–213. doi: 10.1016/s0165-0378(98)00022-9. [DOI] [PubMed] [Google Scholar]
- 23.Fisher SJ, et al. Adhesive and degradative properties of human placental cytotrophoblast cells in vitro. J Cell Biol. 1989;109:891–902. doi: 10.1083/jcb.109.2.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Librach CL, et al. 92-kD type IV collagenase mediates invasion of human cytotrophoblast. J Cell Biol. 1991;113:437–449. doi: 10.1083/jcb.113.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou Y, et al. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. J Clin Invest. 1997;99:2139–2151. doi: 10.1172/JCI119387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Damsky CH, et al. Integrin switching regulates normal trophoblast invasion. Development. 1994;120:3657–3666. doi: 10.1242/dev.120.12.3657. [DOI] [PubMed] [Google Scholar]
- 27.Bischof P, Redard M, Gindre P, Vassilakos P, Campana A. Localization of alpha2, alpha5 and alpha6 integrin subunits in human endometrium, decidua and trophoblast. Eur J Obstet Gynecol Reprod Biol. 1993;51:217–226. doi: 10.1016/0028-2243(93)90038-e. [DOI] [PubMed] [Google Scholar]
- 28.Fox H, Path MC. Effect of hypoxia on trophoblast in organ culture. Am J Obstet Gynecol. 1970;107:1058–1064. doi: 10.1016/0002-9378(70)90629-0. [DOI] [PubMed] [Google Scholar]
- 29.Aplin JD, Haigh T, Jones CJP, Church HJ, Vicovac L. Development of cytotrophoblast columns from explanted first-trimester human placental villi: role of fibronectin and integrin α5β1. Biol Reprod. 1999;60:828–838. doi: 10.1095/biolreprod60.4.828. [DOI] [PubMed] [Google Scholar]
- 30.Benyo DF, Miles TM, Conrad KP. Hypoxia stimulates cytokine production by villous explants from the human placenta. J Clin Endocrinol Metab. 1997;82:1582–1588. doi: 10.1210/jcem.82.5.3916. [DOI] [PubMed] [Google Scholar]
- 31.Conrad KP, Benyo DB. Placental cytokines and the pathogenesis of preeclampsia. Am J Reprod Immunol. 1997;37:240–249. doi: 10.1111/j.1600-0897.1997.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 32.Taylor CM, Stevens H, Anthony FW, Wheeler T. Influence of hypoxia on vascular endothelial growth factor and chorionic gonadotrophin production in trophoblast-derived cell lines: JEG, Jar and BeWo. Placenta. 1997;18:451–458. doi: 10.1016/s0143-4004(97)80047-1. [DOI] [PubMed] [Google Scholar]
- 33.Fitzpatrick TE, Graham CH. Stimulation of plasminogen activator inhibitor-1 expression in immortalized human trophoblast cells cultured under low levels of oxygen. Exp Cell Res. 1998;245:155–162. doi: 10.1006/excr.1998.4240. [DOI] [PubMed] [Google Scholar]
- 34.Alsat E, et al. Hypoxia impairs cell fusion and differentiation process in human cytotrophoblast, in vitro. J Cell Physiol. 1996;168:346–353. doi: 10.1002/(SICI)1097-4652(199608)168:2<346::AID-JCP13>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 35.Janatpour MJ, et al. A repertoire of differentially expressed transcription factors that offers insight into mechanisms of human cytotrophoblast differentiation. Dev Genet. 1999;25:146–157. doi: 10.1002/(SICI)1520-6408(1999)25:2<146::AID-DVG9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 36.Carmeliet P, et al. Role of HIF-1α in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485–490. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 37.Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science. 1997;277:1669–1672. doi: 10.1126/science.277.5332.1669. [DOI] [PubMed] [Google Scholar]
- 38.Ryan HE, Lo J, Johnson RS. HIF-1α is required for solid tumor formation and embryonic vascularization. EMBO J. 1988;17:3005–3015. doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maltepe E, Schmidt JV, Baunoch D, Bradfieldand CA, Simon MC. Abnormal angiogenesis and response to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature. 1997;386:403–407. doi: 10.1038/386403a0. [DOI] [PubMed] [Google Scholar]
- 40.Kozak KR, Abbott B, Hankinson O. ARNT deficient mice and placental differentiation. Dev Biol. 1997;191:297–305. doi: 10.1006/dbio.1997.8758. [DOI] [PubMed] [Google Scholar]
- 41.Salceda S, Caro J. Hypoxia-inducible factor 1α (HIF-1α) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilizatoin by hypoxia depends on redox-induced changes. J Biol Chem. 1997;272:22642–22647. doi: 10.1074/jbc.272.36.22642. [DOI] [PubMed] [Google Scholar]
- 42.Goldberg MA, Dunning SP, Bunn HF. Regulation of the erythropoietin gene: evidence that the oxygen sensor is a heme protein. Science. 1988;242:1412–1415. doi: 10.1126/science.2849206. [DOI] [PubMed] [Google Scholar]
- 43.Zhou Y, Damsky CH, Chiu K, Roberts JM, Fisher SJ. Preeclampsia is associated with abnormal expression of adhesion molecules by invasive cytotrophoblasts. J Clin Invest. 1993;91:950–960. doi: 10.1172/JCI116316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou Y, Damsky CH, Fisher SJ. Preeclampsia is associated with failure of human cytotrophoblast to mimic a vascular adhesion phenotype. J Clin Invest. 1997;99:2152–2164. doi: 10.1172/JCI119388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Redline RW, Patterson P. Pre-eclampsia is associated with an excess of proliferative immature intermediate trophoblast. Hum Pathol. 1995;26:594–600. doi: 10.1016/0046-8177(95)90162-0. [DOI] [PubMed] [Google Scholar]
- 46.Kupferminc MJ, Peaceman AM, Wigton TR, Rehnberg KA, Socol MA. Fetal fibronectin levels are elevated in maternal plasma and amniotic fluid of patients with severe preeclampsia. Am J Obstet Gynecol. 1995;172:649–653. doi: 10.1016/0002-9378(95)90587-1. [DOI] [PubMed] [Google Scholar]