SYNOPSIS
There is accumulating epidemiologic and experimental evidence that intrauterine and/or postnatal infection with Ureaplasma species is a significant risk factor for adverse pregnancy outcomes and complications of extreme preterm birth such as BPD and intraventricular hemorrhage. In a cohort of very low birth weight infants, Ureaplasma spp. were detected by culture or PCR in respiratory secretions, blood, and/or CSF of almost half of the subjects, suggesting that this organism is the most common pathogen affecting this population. This review will summarize the evidence supporting the hypothesis that Ureaplasma-mediated inflammation in different compartments (intrauterine, lung, blood, and/or brain) during a common developmental window of vulnerability contributes to preterm labor and lung and brain injury. Appropriate methods for detecting these fastidious organisms and potential strategies to prevent or ameliorate the effects of Ureaplasma infection are discussed.
Keywords: Ureaplasma urealyticum, Ureaplasma parvum, intrauterine infection, prematurity, bronchopulmonary dysplasia, intraventricular hemorrhage
Ureaplasma parvum (serotypes 1, 3, 6, and 14) and U. urealyticum (serotypes 2, 4, 5, 7–13) are closely related species of the Mollicutes class that are among the smallest free-living, self-replicating cells1–3. All serovars lack cell walls, exhibit limited biosynthetic abilities, hydrolyze urea to generate ATP, and adhere to human mucosal surfaces3. U. parvum is the most common species isolated from clinical specimens3, 4. Although Ureaplasma spp. are not typically considered pathogens in early onset neonatal sepsis, they have been implicated in complications of human pregnancy and neonatal outcomes. As observed by Volgmann et al.5 in the title, of a recent review, Ureaplasma is either a “harmless commensal or underestimated enemy of human reproduction”. This review will address this controversy and review evidence that the timing, duration, and intensity of the inflammatory response to Ureaplasma infection are major determinants of pregnancy and neonatal outcomes.
Epidemiology
Ureaplasma spp. are the most common bacteria implicated in human urogenital infections, including non-gonococcal urethritis in men and complications of pregnancy in women5. Ureaplasma spp. can be detected in vaginal flora in 40% of sexually inactive and 67% of sexually active women of reproductive age, and 25% of postmenopausal women6. Despite the detection of viable Ureaplasma on toilets for up to 2 hours7, infection from exposure to contaminated surfaces is unlikely. Known transmission routes involve sexual contact or maternal-infant transfer.
Impact of Ureaplasma Urogenital Tract Colonization on Reproduction
Because Ureaplasma is a commensal in the adult female genital tract, it has been considered of low virulence. However, Ureaplasma urogenital tract colonization has been causally linked to infertility8, 9, early pregnancy loss10, stillbirth11, 12, and preterm birth13–17. The relationship with these outcomes remains controversial because a causal relationship has been difficult to prove for each condition due to 1) accuracy of differentiating the presence of bacteria as specimen contaminants vs. pathogens, 2) the polymicrobial nature of vaginal flora, 3) difficulty in detecting Ureaplasma and other fastidious microbes18, and 4) the contribution of other factors to the pathophysiology of these complex disorders. In vitro and in vivo experimental models have provided additional evidence in support of a role for Ureaplasma in these obstetrical disorders.
Ureaplasma Intrauterine infection/ Chorioamnionitis
Intrauterine infection has been implicated as a major cause of preterm birth, especially in gestations < 30 weeks19. Ureaplasma spp. are the most common organisms isolated from amniotic fluid obtained from women who present with preterm labor (POL) with intact membranes20, 21, preterm premature rupture of membranes (pPROM)22, and short cervix associated with microbial invasion of the amniotic cavity23, and from infected placentas22. The prevalence of infected amniotic fluid with cultivated Ureaplasma as the only microbe ranges from 6–9% for pregnancies complicated by POL with intact membranes21, 24 to 22% for a cohort of women with POL or pPROM25. Detection of cultivated Ureaplasma in placental chorion in pregnancies producing VLBW infants ranges from 6–10% in homogenized frozen tissue26, 27 to 28% in fresh tissue and is inversely related to gestational age13. Recovery of Ureaplasma from the chorion increased with duration of rupture membranes, suggesting an ascending route of infection13. However, Ureaplasma has also been detected in 22% of placentas with duration of rupture of membranes less than one hour, suggesting the possibility of a pre-existing infection. Indeed, Ureaplasma species have been detected in amniotic fluid as early as the time of genetic amniocentesis (16–20 weeks) in up to 13% asymptomatic women16, 28–30. Placentas with the lowest rate of Ureaplasma recovery were from women delivered for preeclampsia or intrauterine growth restriction13. Recent analysis of amniotic fluid from women with preterm labor and intact membranes using advanced molecular techniques has demonstrated a greater prevalence (15%) and diversity of microbes including those uncultivated and uncharacterized compared to culture or species-specific PCR studies18. In this study, only half of Ureaplasma-positive amniotic fluid samples were identified by culture with increased detection by combined culture and PCR.
The presence of Ureaplasma as the only identified microbial isolate in the upper genital tract is significantly associated with adverse pregnancy outcomes, including premature delivery, neonatal morbidity, and perinatal death13, 21, 22, 25. Experimental models of intrauterine Ureaplasma infection in mice31, sheep32, 33, and non-human primates34 have been described. Intra-amniotic inoculation of U. parvum did not stimulate preterm labor in mice or sheep, but did stimulate progressive uterine contractions and preterm delivery in Rhesus macaques inoculated at 136 d gestation (80% term)34, suggesting species differences in the host response or serovar differences in virulence. The Rhesus macaque model is the first experimental model to definitely show a causal link between Ureaplasma intrauterine infection and POL.
Ureaplasma-mediated Inflammation and Preterm Labor
There is accumulating evidence that intrauterine infection-induced preterm labor is the result of an inflammatory cascade initiated by bacterial interaction with host pattern recognition receptors (PRR) such as the toll-like receptors35. Following engagement with PRRs, there is a sequential rise in inflammatory cytokines such as IL-1β, TNF-α, IL-6, and IL-8 followed by leukocyte recruitment, increases in prostaglandins such as PGE2 and PGF2-α̣ and matrix metalloproteinases. These mediators contribute to uterine contractions, cervical dilatation and effacement, and membrane rupture35.
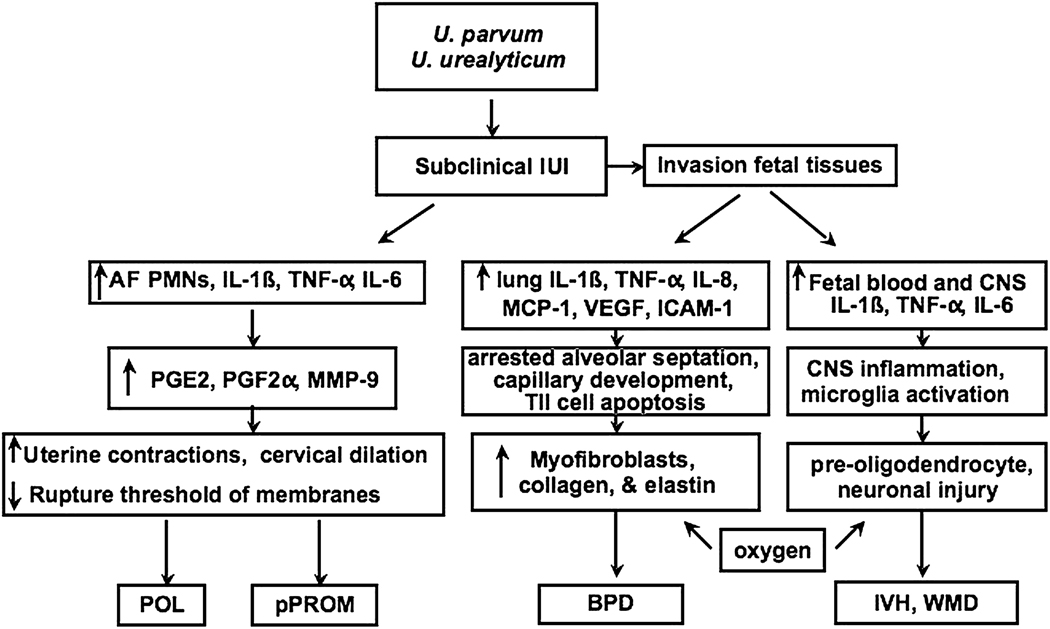
In the presence of pPROM, cultivated Ureaplasma as the sole microbe was associated with increased leukocytes and proinflammatory cytokines (IL-6, IL-1β and TNF-α) in amniotic fluid and increased cord blood IL-6 concentrations, indicating a robust inflammatory response to this infection. While the majority of women in whom subclinical Ureaplasma amniotic cavity infection is detected midtrimester deliver at term16, those with elevated amniotic fluid IL-6 levels have increased risk for adverse pregnancy outcome including fetal loss and preterm delivery36. In the Rhesus U. parvum intrauterine infection model, uterine activity was preceded by a rise in amniotic fluid leukocytes, inflammatory cytokines, prostaglandins PGE2 and PGF2α and matrix metalloproteinase (MMP)-9, demonstrating that Ureaplasma alone stimulates the mediators of preterm labor34. In contrast, heat-killed U. parvum serotype 1 failed to stimulate a significant increase in cytokine and PGE2 response in fetal membrane explants derived from term placentas. High inoculum (106 color changing units (CCU)/ml), but not low inoculum (102 to 104 CCU/ml) heat-killed U. urealyticum serotype 8 stimulated TNF-α, IL-10 and PGE2 production by choriodecidual explants in vitro. The apparent low virulence of Ureaplasma serotypes in these in vitro studies may be due, in part, to the use of laboratory reference strains from the American Tissue Culture Collection (ATCC, Manassas, VA) rather than more virulent clinical isolates, or killed rather than live organisms. Alternatively, a decreased capacity to stimulate an inflammatory response in the intrauterine compartment may allow Ureaplasma infections to persist for long periods of time. Figure 1 summarizes the inflammatory pathways initiated by Ureaplasma infection that may contribute to preterm labor.
Figure 1. Overview of potential inflammatory pathways involved in Ureaplasma-mediated preterm labor, and common neonatal morbidities.
Intrauterine Ureaplasma infections may develop by ascending infection or transplacental route. Engagement with host pathogen recognition receptors initiates a maternal and fetal inflammatory cascade. In the amniotic cavity, there is sequential upregulation of inflammatory cytokines, recruitment of leukocytes, and release of prostaglandins, and metalloproteinases leading to uterine contractions, cervical dilatation and effacement, and membrane rupture34, 35. In the fetal lung, prolonged exposure to inflammatory cytokines inhibits alveolar development110. Systemic invasion via the umbilical cord stimulates a cytokinemia in blood and/or CNS resulting in microglia activation and pre-oligodendrocyte and neuronal injury31.
Association of Ureaplasma spp. and Neonatal Outcomes
The timing and duration of fetal exposure to Ureaplasma and the intensity of the maternal and fetal inflammatory responses may be variable, but these factors likely contribute to the impact of Ureaplasma on neonatal outcomes. The rate of Ureaplasma respiratory tract colonization increases with duration of rupture of membranes37, 38, indicating that for the majority of cases, Ureaplasma is likely vertically transmitted from mothers to their infants just prior to or at the time of preterm birth as the result of an ascending infection from the lower genital tract. However, in a prospective cohort from our institution, we observed that 23% of preterm infants with Ureaplasma respiratory colonization (unpublished observations), and 28% of infants with invasive Ureaplasma detected in blood or CSF by PCR39 experienced rupture of membranes <1 h, suggesting that colonization/infection in these infants was the result of pre-existing (intrauterine) infection40, 41. This is in agreement with Dammann et al.40 who noted that rupture of membranes was <1 h in 31% of pregnancies with Ureaplasma culture-positive placentas. In our cohort, 78% of placentas of infants with Ureaplasma respiratory tract colonization and duration of ROM <1 h showed evidence of histologic chorioamnionitis, in contrast to 36% of culture-negative infants with duration of ROM <1 h. The placentas of Ureaplasma-positive infants in this group were more likely to have advanced maternal and fetal stages, indicating a more long-standing infection42.
These data suggest that fetal exposure to Ureaplasma spp may occur early in pregnancy and may be sustained during critical periods of development. We propose that Ureaplasma-induced injury to the preterm lung and brain occurs during a common developmental-dependent window of vulnerability and is mediated by the inflammatory response in placental, blood, lung, and CNS compartments43. During the period from 23 to 32 weeks gestation, the lung and brain share a vulnerability to injury mediated by infection/inflammation44–46. During this period of saccular lung development45, 46 and predominance of the oxidative stress sensitive pre-oligodendrocyte in the fetal brain47–49, exposure of the fetus to intrauterine Ureaplasma infection-mediated inflammation and post-natal interactions with other inflammatory stimuli may alter developmental signaling. The evidence supporting this hypothesis and potential mechanisms are reviewed in the following sections.
Ureaplasma spp and Neonatal Lung Injury
The rate of respiratory tract colonization with Ureaplasma in infants <1500g birth weight ranges from 20–45%, depending on study entry criteria, frequency of sampling and detection methods50, 51. In a recent cohort of infants <33 wks gestation, Ureaplasma spp were detected during the first week of life in tracheal aspirates or nasopharyngeal specimens in 35% of infants39. Ureaplasma respiratory tract colonization is associated with a peripheral blood leukocytosis52 and early radiographic emphysematous changes of bronchopulmonary dysplasia (BPD)53, 54. These findings may be explained, in part, by an in utero onset of the inflammatory response and lung injury.
The contribution of Ureaplasma respiratory tract colonization to the development of BPD has been debated; however, a meta-analysis of 17 clinical studies published before 1995 supported a significant association between Ureaplasma respiratory tract colonization and development of BPD defined as oxygen dependence at 28 to 30 d postnatal age50. In a meta-analysis of 36 published studies involving ~3000 preterm infants, Schelonka et al.51 observed a significant association between Ureaplasma respiratory colonization and development of BPD whether defined as oxygen-dependence at 28 d or at 36 weeks post menstrual age (PMA). Studies published since the last meta-analysis support the Ureaplasma respiratory colonization-BPD association55, 56, particularly for the subset of Ureaplasma-colonized infants exposed to chorioamnionitis and leukocytosis at birth56.
Evidence from studies of human preterm infants57–59, and intrauterine infection models in mice31, sheep32, 33, and non-human primates34, 60 support that Ureaplasma infection is pro-inflammatory and pro-fibrotic and results in a BPD phenotype. In a review of lung pathology of archived autopsy specimens from Ureaplasma-infected preterm infants, the most striking findings were 1) the presence of moderate to severe fibrosis, 2) increased myofibroblasts, 3) disordered elastin accumulation, and 4) increased numbers of tumor necrosis factor-α̣(TNF-α) and transforming growth factor β1 (TGF̣β1)-immunoreactive cells in all Ureaplasma-infected infants compared to gestational controls and infants who died with pneumonia from other causes58, 59. Experimental murine intrauterine U. parvum exposure stimulated fetal lung cytokine expression and augmented hyperoxia-induced lung injury31. In the 125 d immature baboon model, we observed extensive fibrosis (Fig. 2), an increase in the myofibroblast phenotype and increased expression of pro-inflammatory (TNF-α, interleukin (IL)-1β̣ and pro-fibrotic cytokines (TGFβ1; oncostatin M) in lungs of antenatal Ureaplasma-infected animals compared to gestational controls or non-infected ventilated animals60. In a study of rhesus macaques, histologic changes in the fetus’ lungs depended on the duration of intrauterine exposure to U. parvum34. Infection exposure duration less than 136 h resulted in neutrophil infiltration without epithelial injury. With progressive duration of exposure there was an influx of neutrophils and macrophages, epithelial necrosis, and type II cell proliferation. For exposure duration >10 d, increased collagen and thickened alveolar walls were evident. These data confirm that Ureaplasma infection contributes to chronic inflammation and fibrosis in the preterm lung. Moreover, these data suggest that Ureaplasma acts as a co-inflammatory stimulus by causing an augmented, dysregulated inflammatory response to subsequent inflammatory insults such as hyperoxia and volutrauma.
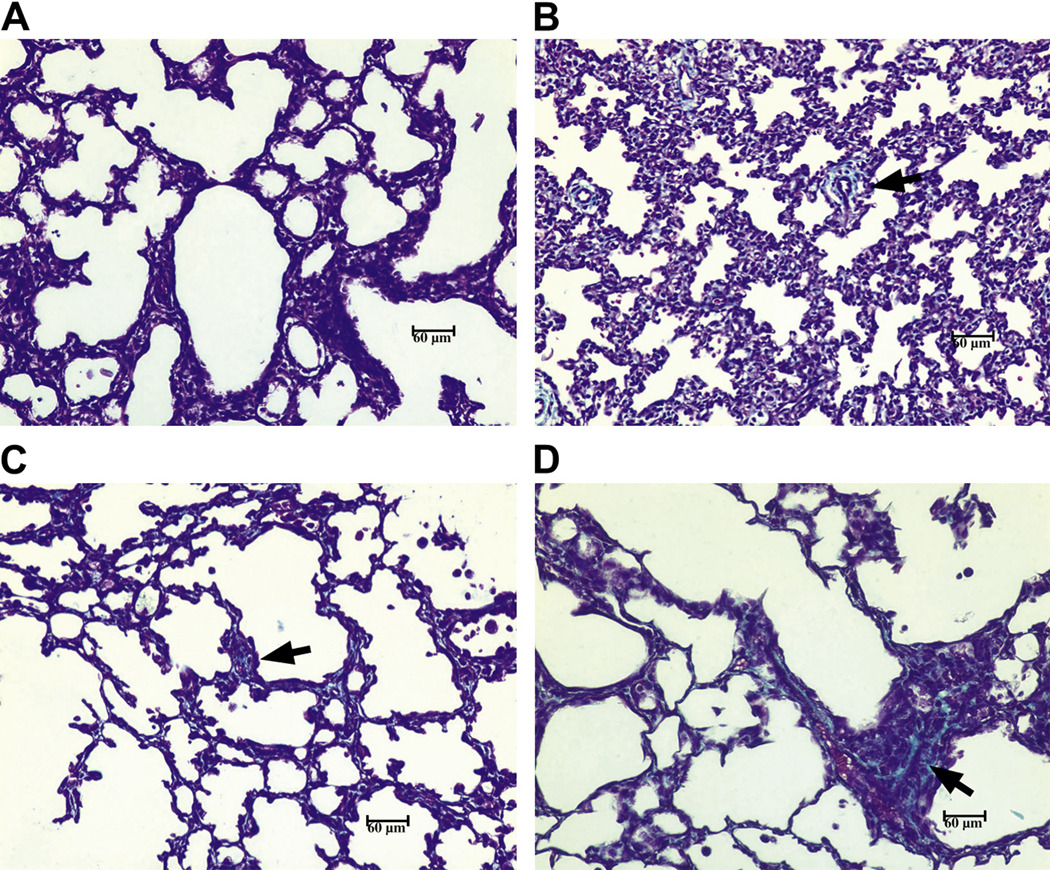
Figure 2. Collagen staining is increased in lungs from antenatal U.parvum-infected baboons.
Lung specimens from 125d gestation baboon newborns ventilated for 14d that were either infected antenatally with U. parvum serovar 1 (D) or noninfected (C) were stained with trichome for collagen and compared to stained lung sections from 125d (A) and 140 d (B) gestational controls. The most marked fibrosis occurred in the U. parvum infected lungs (arrows). Magnification x200.
Insights into how Ureaplasma infection-mediated inflammation produces the BPD phenotype are provided by studies of transgenic mice overexpressing pro-inflammatory cytokines. In transgenic mice, over-expression of TNF-α̣ IL-6, and IL-11 each inhibited alveolarization, indicating that prolonged exposure of the preterm lung to a pro-inflammatory environment may contribute to abnormal alveolar septation, the hallmark of the ‘new BPD’61. In a recently developed bitransgenic CCSP-rtTA-(tetO)-CMV-IL-1β mouse, IL-1β was expressed under conditional control in airway epithelial cells in the fetal and neonatal lung62. IL-1β expression increased on E14.5 and was maximal by E16.5 (pseudoglandular period) and decreased postnatally. In this model, postnatal growth was impaired and mortality was higher in the newborn mice expressing IL-1β. The lungs of these newborn mice demonstrated many features of the BPD phenotype, including disrupted alveolar septation and capillary development, and disordered α-smooth muscle actin and elastin deposition in alveolar septa of distal airspaces62. These observations suggest that an early and prolonged exposure to Ureaplasma-mediated inflammation may be necessary to adversely affect lung development (see Fig. 1).
Ureaplasma spp. and other Neonatal Lung Disorders
There have been case63, 64 and small series reports of culture-confirmed congenital Ureaplasma pneumonia in late preterm infants65. In one series, 3 infants presented with clinical features of pneumonia and persistent pulmonary hypertension65. In each infant, tracheal aspirates and blood samples were Ureaplasma culture-positive and postmortem lung cultures were positive for Ureaplasma in 2 infants who died. In a 5 year series of 159 perinatal autopsies, postmortem lung cultures were positive for Ureaplasma in 9% of cases. Forty-three percent of the autopsied cases were intrauterine deaths and 45% had histologic evidence of congenital pneumonia66. This was in agreement with a larger series of 430 stillborn and neonatal autopsies in which 8% were Ureaplasma-positive in the lungs67. These studies suggest that Ureaplasma species are uncommon causes of pneumonia in term and near-term infants, but cultures for these organisms should be considered in cases of unexplained congenital pneumonia or fetal demise.
Ureaplasmal Invasive Disease
Although Ureaplasma spp. are most commonly isolated from respiratory secretions, the organisms have also been isolated from gastric aspirates68, blood69, 70, cerebrospinal fluid71, 72, lung65 and brain73, 74 tissue. Isolation of Ureaplasma from cord blood was first described in 196975 and isolation from cord or venous blood in association with pneumonia was documented in several other studies65, 69, 76. In 2 recent large prospective cohorts, Ureaplasma was detected in 17% of cord blood cultures70 and 23.6% serum and/or CSF PCR samples39, but invasive disease was not associated with BPD at 36 weeks PMA in either cohort. U. parvum was the predominant species detected in serum and CSF. In infants for whom Ureaplasma respiratory status was known, 19% of serum and 11% CSF samples from infants with Ureaplasma respiratory colonization and 15% serum and 19% CSF samples from infants without Ureaplasma respiratory colonization were PCR-positive. In all cases, the Ureaplasma detected in respiratory samples was the same species as the one detected in serum or CSF from the same subject39.
Ureaplasma-mediated inflammation and neonatal brain injury
Although considerable evidence has been published linking Ureaplasma respiratory colonization with neonatal lung morbidity, less is known about possible links between intrauterine Ureaplasma exposure, invasive disease, and neurologic morbidities such as intraventricular hemorrhage (IVH) and white matter injury. In a prospective series of infants with suspected sepsis/meningitis or hydrocephalus, M. hominis, a related genital mycoplasma, was isolated in 5 and Ureaplasma spp. in 8 CSF samples71. Six infants with Ureaplasma-positive CSF had severe IVH complicated by post-hemorrhagic hydrocephalus in 3. There are additional case reports of IVH complicated by hydrocephalus and/or death in association with Ureaplasma-positive CSF73, 77. In our study of invasive Ureaplasma39, the risk of severe IVH (≥ Grade 3) was 2.5-fold higher in serum PCR-positive than PCR-negative infants after adjustment for gestational age, but there was no association of cranial ultrasound abnormalities with detection of Ureaplasma in CSF. This may have been due to selection bias of lumbar punctures more likely being performed in more stable infants. U. parvum was the species detected in all PCR-positive infants with severe IVH. There was a 5-fold increased risk for severe IVH in the presence of Ureaplasma PCR positive serum combined with elevated serum IL-1β39, suggesting a possible link between invasive Ureaplasma, cytokinemia, and neonatal brain injury (see Figure 1).
A role for Ureaplasma in neonatal brain injury is supported by recent studies in experimental animal models. In the murine intrauterine U. parvum infection model, the brains of fetal and newborn mice showed evidence of microglial activation, delayed myelination, and disturbed neuronal development31. In antenatal- U. parvum-exposed immature rhesus macaques, CSF and brain tissue were culture-positive in 20%34. Although there was no evidence of hemorrhage on gross and microscopic examinations of the brains of these animals, specific immunohistochemical analyses were not performed. Future studies in these models will provide additional insights into the mechanisms of Ureaplasma-mediated brain injury and could evaluate potential therapeutic interventions.
The impact of intrauterine Ureaplasma infection on neurodevelopmental outcome is unknown. A recent study comparing outcomes of infants born < 33 weeks gestation with and without amniotic cavity infection with Ureaplasma or other microbes at time of delivery reported lower psychomotor developmental index scores on the Bayley Scales of Infant Development and a higher rate of cerebral palsy at 2 years adjusted age in infants exposed to intrauterine infection compared to non-exposed infants78. Infants exposed to intrauterine Ureaplasma were more severely affected than those exposed to other bacteria. Additional studies are needed to assess the contribution of Ureaplasma to adverse neurodevelopment.
Ureaplasma spp. and Neonatal Meningitis
The prevalence, clinical risk factors, and CSF parameters in Ureaplasma CNS infections have not been fully elucidated. Most series have focused on the preterm population, but there have been case reports of Ureaplasma isolation in CSF of term infants69, 79. The rate of Ureaplasma culture-positive CSF in prospective series in preterm infants varied from 0.2% to 9%71, 72, 80–82. The prevalence for Ureaplasma is higher than for other causes of bacterial meningitis (1–2%)83, 84. Although CSF pleocytosis has been described, it is absent in most cases69, 82. The utility of CSF parameters in the diagnosis of bacterial meningitis in preterm infants is debated84. In our prospective series39, the CSF white blood cell count, percent neutrophils, and glucose and protein concentrations did not differ between Ureaplasma PCR positive and negative samples (Table 1). Only 25% of Ureaplasma-positive CSF samples had a white blood cell count >20 cells/mm3 compared to 16% of Ureaplasma-negative samples. No other bacteria were detected in these samples. Taken together, these observations suggest that Ureaplasma infections may be the most common cause of CNS infections in neonates, but most infections are asymptomatic. Since most infections appear to resolve without therapy, it is unclear whether culture for these organisms should be included in the routine evaluation of sepsis in the newborn. Additional studies focused on long-term outcomes of Ureaplasma CNS infections are warranted before specific recommendations can be made for clinical management.
Table 1.
CSF Characteristics in infants with invasive Ureaplasma
| CSF PARAMETER | CSF PCR (+) N=36 |
CSF PCR (−) N=153 |
|---|---|---|
| WBC /mm3* | 4 (0–172) | 3 (0–193) |
| % neutrophils | 31 (0–92) | 17 (0–87) |
| % lymphocytes | 14 (0–100) | 16 (0–100) |
| % monocytes | 23 (0–100) | 42 (0–100) |
| Glucose (mg/dL) | 47 (26–105) | 52 (0–171) |
| Protein (mg/dL) | 154 (64–527) | 152 (47–558) |
CSF white blood cell counts were corrected for red blood cells. Data are expressed as median with range in parentheses.
Ureaplasma Infections in Infants and Children
The duration of Ureaplasma respiratory tract colonization is unknown. In a study conducted in 1969, 12 of 21 Ureaplasma-colonized newborns remained culture positive for up to 10 months. In addition, 7 of 52 initially culture-negative infants converted to culture-positive during the same time period, confirming the importance of multiple cultures for definitive diagnosis as well as suggesting the possibility of horizontal transmission. Over a 10 year period, 31% of over 45,000 cultures at a national reference laboratory for mycoplasma cultures were positive for Mycoplasma spp. not including M. pneumoniae4. In children, the majority of Ureaplasma positive respiratory cultures were from children < 12 months (92%), but 4% were from children 1 to 7 years of age. In agreement, Matlow et al85 reported that only 1.8% of non-neonatal respiratory samples submitted to a single institution laboratory were positive for Ureaplasma.
Ureaplasma respiratory tract colonization has also been proposed as an etiologic factor in reactive airway disease in young infants. Wheezing in infants and children less than 3 years of age has been associated with isolation of Ureaplasma from the upper respiratory tract86. Interestingly, in a large study of almost 3000 women and their offspring in Sweden, maternal vaginal colonization with Ureaplasma during pregnancy was associated with a 2-fold increased risk for infant wheezing defined as one or more hospitalizations for asthma during the first 3 years of life87. The investigators proposed that acquisition of microorganisms such as Ureaplasma spp. at birth affects the establishment of infant microflora and subsequent development of allergy and wheezing.
Ureaplasma as a significant cause of sepsis or meningitis beyond the newborn period appears unlikely. In a series of infants less than 3 months of age evaluated for sepsis, Ureaplasma was not detected in any blood or CSF sample, but it was isolated from 6% urine cultures88. Other diseases associated with Ureaplasma include pediatric joint disorders86.
Ureaplasma Diagnostic Methods
Ureaplasma spp. may be isolated from blood, amniotic fluid, CSF, sputum, bronchoalveolar lavage, pleural fluid, and semen. Since these organisms are susceptible to desiccation and are sensitive to temperature changes, attention to sample collection and processing are of utmost importance. Samples should be directly inoculated into 10B broth for transport on ice to the laboratory. The specimen should be inoculated in 10B broth with serial 1:10 dilutions and incubated under atmospheric conditions and on A8 agar and incubated in 5% CO2 at 37°C. Cultures are observed for up to 7 days for broth color change from yellow to pink, indicating pH change due to urease activity in the absence of turbidity. Any broth with color change should be sub-cultured on A8 agar. Colonies of Ureaplasma spp. are identified presumptively by their characteristic brown appearance on A8 agar in the presence of the CaCl2 indicator. Ureaplasma can be differentiated from M. hominis by the larger size, lack of precipitate, and” fried-egg” appearance of M. hominis. Cultures usually grow within 24–48 h. Since most clinical laboratories lack the expertise for culture of these organisms, cultures may need to be shipped for processing to a reference laboratory such as the University of Alabama Mycoplasma Diagnostic Laboratory.
PCR protocols for detection of the common Ureaplasma spp. mba or urease genes are available89–91 as well as species and serovar-specific primers89, 92, 93. These methods have the advantage of more rapid results and potentially increased sensitivity compared to traditional culture.
Therapeutic Considerations
Interventions during pregnancy
Antibiotic therapy is now standard of care for management of pPROM94. However, usual regimens failed to eradicate organisms including Ureaplasma or diminish inflammation in the amniotic cavity in pregnancies complicated by pPROM95. In a small series of women with microbial invasion of the amniotic cavity with short cervix, parenteral azithromycin eradicated Ureaplasma in 3 of 4 cases and delivery occurred at term. Combination of antibiotics with anti-inflammatory drugs may improve efficacy. In an experimental Ureaplasma intraamniotic infection in Rhesus macaques, azithromycin alone or in combination with dexamethasone and indocin prevented fetal lung damage (Novy, MJ et al., Maternal azithromycin (AZI) therapy for Ureaplasma intraamnioitc infection (IAI) prevents advanced fetal lung lesions in rhesus monkeys, 2008 SGI Annual Scientific Meeting, March 26–29, 2008, San Diego, CA, Abstract 438). Whether this approach will be beneficial for CNS outcomes is unknown.
BPD Prevention?
Despite in vitro susceptibility of Ureaplasma to erythromycin96, trials of erythromycin therapy in the first few weeks of life in Ureaplasma colonized preterm infants have failed to demonstrate efficacy to prevent BPD97, 98 or eradicate respiratory tract colonization99. The failure to prevent BPD in these studies may have been due to the small sample size of each study, or to the initiation of erythromycin therapy too late to prevent the lung inflammation and injury that contribute to the pathogenesis of BPD. Demonstration of efficacy of antibiotic therapy to prevent BPD in Ureaplasma-colonized preterm infants will require carefully designed adequately powered studies.
The new 14-membered macrolides that are derivatives of erythromycin and the related 15-member azalides have immunomodulatory effects, including effects on neutrophil function (e.g. chemotaxis, cell adhesion, oxidative burst and phagocytosis) and inhibition of cytokine release (e.g., IL-1β, IL-8, TNFα)100 and nitric oxide production in vitro101. Macrolide antibiotics may exert immunomodulatory anti-inflammatory effects in the setting of infection, and these may occur independently of a direct bactericidal effect102. In addition, azithromycin exhibits higher potency than erythromycin against clinical Ureaplasma isolates in vitro103. Pharmacokinetic studies in mice and humans have shown that azithromycin is preferentially concentrated in pulmonary epithelial lining fluid and alveolar macrophages104–106. Since neutrophil recruitment and activation has been implicated in BPD pathogenesis107, 108, the experimental effects observed with azithromycin in vitro and in vivo indicate that this drug may be beneficial in the treatment of Ureaplasma infection and the prevention of BPD in preterm infants.
Since Ureaplasma-mediated lung injury may be initiated in utero and augmented postnatally by exposure to mechanical ventilation and hyperoxia, therapy to prevent BPD should be initiated as soon as possible after birth in infants at risk. Recently, Walls et al.109 demonstrated that azithromycin, but not erythromycin prophylaxis improved outcomes and reduced inflammation in a murine neonatal Ureaplasma infection model. This suggests that azithromycin may be effective if administered immediately after birth. However, until appropriate pharmacokinetics and efficacy trials are conducted, a dosing regimen for azithromycin in neonates cannot be recommended.
Summary
Ureaplasma species may indeed be an “underestimated enemy of human reproduction”. However, there are many questions yet to be answered before recommendations for changes in clinical practices can be recommended. We need to learn more about the relative contribution of Ureaplasma virulence factors, host immune factors that affect susceptibility to these pathogens and the variability in the inflammatory response, and interactions with environmental factors such as oxygen exposure and mechanical ventilation. Routine antibiotic use should be limited to clinical trials until clear benefit can be established. However, culture or PCR for these organisms should be considered in cases of unexplained fetal demise, congenital pneumonia, or meningitis unresponsive to broad-spectrum antibiotics. Follow-up cultures should be obtained in any treated infant since effective clearance has proven difficult in erythromycin trials.
Acknowledgments
Funding support: This work was funded by NIH grants HL071113 and HL087166
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kong F, James G, Zhenfang M, et al. Phlyogenetic analysis of Ureaplasma urealyticum-support for the establishment of a new species, Ureaplasma parvum. Int J Syst Bacteriol. 1999;49:1879–1889. doi: 10.1099/00207713-49-4-1879. [DOI] [PubMed] [Google Scholar]
- 2.Glass JI, Lefkowitz EJ, Glass JS, et al. The complete sequence of the mucosal pathogen Ureaplasma urealyticum. Nature. 2000;407(6805):757–762. doi: 10.1038/35037619. [DOI] [PubMed] [Google Scholar]
- 3.Waites KB, Katz B, Schelonka RL. Mycoplasmas and ureaplasmas as neonatal pathogens. Clin Microbiol Rev. 2005 Oct;18(4):757–789. doi: 10.1128/CMR.18.4.757-789.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.She RC, Simmon KE, Bender JM, et al. Mollicute infections in neonates. Pediatr Infect Dis J. 2009;28(3):248–250. doi: 10.1097/INF.0b013e31818ddc01. [DOI] [PubMed] [Google Scholar]
- 5.Volgmann T, Ohlinger R, Panzig B. Ureaplasma urealyticum-harmless commensal or underestimated enemy of human reproduction? A review. Arch Gynecol Obstet. 2005;273(3):133–139. doi: 10.1007/s00404-005-0030-1. [DOI] [PubMed] [Google Scholar]
- 6.Iwasaka T, Wada T, Kidera Y, et al. Hormonal status and mycoplasma colonization in the female genital tract. Obstet Gynecol. 1986;68(2):263–266. [PubMed] [Google Scholar]
- 7.Potasman I, Oren A, Srugo I. Isolation of Ureaplasma urealyticum and Mycoplasma hominis from public toilet bowls. Infect Control Hosp Epidemiol. 1999;20(1):66–68. doi: 10.1086/501545. [DOI] [PubMed] [Google Scholar]
- 8.Zeighami H, Peerayeh SN, Yazdi RS, et al. Prevalence of Ureaplasma urealyticum and Ureaplasma parvum in semen of infertile and healthy men. Int J STD AIDS. 2009;20(6):387–390. doi: 10.1258/ijsa.2008.008334. [DOI] [PubMed] [Google Scholar]
- 9.Gupta A, Gupta S, Mittal A, et al. Correlation of mycoplasma with unexplained infertility. Arch Gynecol Obstet. 2009 Mar 26; doi: 10.1007/s00404-009-1042-z. [DOI] [PubMed] [Google Scholar]
- 10.Joste NE, Kundsin RB, Genest DR. Histology and Ureaplasma urealyticum culture in 63 cases of first trimester abortion. Am J Clin Pathol. 1994;102(6):729–732. doi: 10.1093/ajcp/102.6.729. [DOI] [PubMed] [Google Scholar]
- 11.Tafari N, Ross S, Naeye RL, et al. Mycoplasma T strains and perinatal death. Lancet. 1976;1(7951):108–109. doi: 10.1016/s0140-6736(76)93152-4. [DOI] [PubMed] [Google Scholar]
- 12.Gibbs RS. The origins of stillbirth: infectious diseases. Semin Perinatol. 2002;26(1):75–78. doi: 10.1053/sper.2002.29839. [DOI] [PubMed] [Google Scholar]
- 13.Kundsin RB, Leviton A, Allred EN, et al. Ureaplasma urealyticum infection of the placenta in pregnancies that ended prematurely. Obstet Gynecol. 1996;87:122–127. doi: 10.1016/0029-7844(95)00376-2. [DOI] [PubMed] [Google Scholar]
- 14.Yoon BH, Romero R, Chang JW, et al. Microbial invasion of the amniotic cavity with Ureaplasma urealyticum is associated with a robust host response in fetal, amniotic, and maternal compartments. Am J Obstet Gynecol. 1998;179:1254–1260. doi: 10.1016/s0002-9378(98)70142-5. [DOI] [PubMed] [Google Scholar]
- 15.Gerber S, Vial Y, Hohlfeld P, et al. Detection of Ureaplasma urealyticum in second-trimester amniotic fluid by polymerase chain reaction correlates with subsequent preterm labor and delivery. J Infect Dis. 2003;187(3):518–521. doi: 10.1086/368205. [DOI] [PubMed] [Google Scholar]
- 16.Perni SC, Vardhana S, Korneeva I, et al. Mycoplasma hominis and Ureaplasma urealyticum in midtrimester amniotic fluid: association with amniotic fluid cytokine levels and pregnancy outcome. Am J Obstet Gynecol. 2004;191(4):1382–1386. doi: 10.1016/j.ajog.2004.05.070. [DOI] [PubMed] [Google Scholar]
- 17.Witt A, Berger A, Gruber CJ, et al. Increased intrauterine frequency of Ureaplasma urealyticum in women with preterm labor and preterm premature rupture of the membranes and subsequent cesarean delivery. Am J Obstet Gynecol. 2005;193(5):1663–1669. doi: 10.1016/j.ajog.2005.03.067. [DOI] [PubMed] [Google Scholar]
- 18.DiGiulio DB, Romero R, Amogan HP, et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One. 2008;3(8):e3056. doi: 10.1371/journal.pone.0003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342(20):1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 20.Gomez R, Ghezzi F, Romero R, et al. Premature labor and intra-amniotic infection. Clin Perinatol. 1995;22:281–342. [PubMed] [Google Scholar]
- 21.Yoon BH, Chang JW, Romero R. Isolation of Ureaplasma urealyticum from the amniotic cavity and adverse outcome in preterm labor. Obstet Gynecol. 1998;92:77–82. doi: 10.1016/s0029-7844(98)00122-7. [DOI] [PubMed] [Google Scholar]
- 22.Romero R, Yoon BH, Mazor M, et al. A comparative study of the diagnostic performance of amniotic fluid glucose, white blood cell count, interleukin-6, and Gram stain in the detection of microbial invasion in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 1993;169:839–851. doi: 10.1016/0002-9378(93)90014-a. [DOI] [PubMed] [Google Scholar]
- 23.Hassan S, Romero R, Hendler I, et al. A sonographic short cervix as the only clinical manifestation of intra-amniotic infection. J Perinat Med. 2006;34(1):13–19. doi: 10.1515/JPM.2006.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoon BH, Romero R, Lim JH, et al. The clinical significance of detecting Urepasma urealyticum by the polymerase chain reaction in the amniotic fluid of patients with preterm labor. Am J Obstet Gynecol. 2003;189:919–924. doi: 10.1067/s0002-9378(03)00839-1. [DOI] [PubMed] [Google Scholar]
- 25.Kirchner L, Helmer H, Heinze G, et al. Amnionitis with Ureaplasma urealyticum or other microbes leads to increased morbidity and prolonged hospitalization in very low birth weight infants. Eur J Obstet Gynecol Reprod Biol. 2007;134(1):44–50. doi: 10.1016/j.ejogrb.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 26.Onderdonk AB, Delaney ML, DuBois AM, et al. Detection of bacteria in placental tissues obtained from extremely low gestational age neonates. Am J Obstet Gynecol. 2008;198(1):e111–e117. doi: 10.1016/j.ajog.2007.05.044. 110. [DOI] [PubMed] [Google Scholar]
- 27.Olomu IN, Hecht JL, Onderdonk AO, et al. Perinatal correlates of Ureaplasma urealyticum in placenta parenchyma of singleton pregnancies that end before 28 weeks of gestation. Pediatrics. 2009;123(5):1329–1336. doi: 10.1542/peds.2008-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gray DJ, Robinson HB, Malone J, Thomson RB., Jr Adverse outcome in pregnancy following amniotic fluid isolation of Ureaplasma urealyticum. Prenat Diagn. 1992;12(2):111–117. doi: 10.1002/pd.1970120206. [DOI] [PubMed] [Google Scholar]
- 29.Horowitz S, Mazor M, Romero R, et al. Infection of the amniotic cavity with Ureaplasma urealyticum in the midtrimester of pregnancy. J Reprod Med. 1995;40(5):375–379. [PubMed] [Google Scholar]
- 30.Berg TG, Philpot KL, Welsh MS, et al. Ureaplasma/Mycoplasma-infected amniotic fluid: pregnancy outcome in treated and nontreated patients. J Perinatol. 1999;19(4):275–277. doi: 10.1038/sj.jp.7200185. [DOI] [PubMed] [Google Scholar]
- 31.Normann E, Lacaze-Masmonteil T, Eaton F, et al. A novel mouse model of Ureaplasma-induced perinatal inflammation: effects on lung and brain injury. Pediatr Res. 2009;65(4):430–436. doi: 10.1203/PDR.0b013e31819984ce. [DOI] [PubMed] [Google Scholar]
- 32.Moss TJ, Nitsos I, Ikegami M, et al. Experimental intrauterine Ureaplasma infection in sheep. Am J Obstet Gynecol. 2005;192(4):1179–1186. doi: 10.1016/j.ajog.2004.11.063. [DOI] [PubMed] [Google Scholar]
- 33.Moss TJ, Knox CL, Kallapur SG, et al. Experimental amniotic fluid infection in sheep: effects of Ureaplasma parvum serovars 3 and 6 on preterm or term fetal sheep. Am J Obstet Gynecol. 2008;198(1):e121–e128. doi: 10.1016/j.ajog.2007.06.065. 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Novy MJ, Duffy L, Axthelm MK, et al. Ureaplasma parvum or Mycoplasma hominis as sole pathogens cause chorioamnionitis, preterm delivery, and fetal pneumonia in rhesus macaques. Reprod Sci. 2009;16(1):56–70. doi: 10.1177/1933719108325508. [DOI] [PubMed] [Google Scholar]
- 35.Peltier MR. Immunology of term and preterm labor. Reprod Biol Endocrinol. 2003;1:122. doi: 10.1186/1477-7827-1-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bashiri A, Horowitz S, Huleihel M, et al. Elevated concentrations of interleukin-6 in intra-amniotic infection with Ureaplasma urealyticum in asymptomatic women during genetic amniocentesis. Acta Obstet Gynecol Scand. 1999;78(5):379–382. [PubMed] [Google Scholar]
- 37.Grattard F, Soleihac B, De Barbeyrac B, et al. Epidemiologic and molecular investigations of genital mycoplasmas from women and neonates at delivery. Pediatr. Infect. Dis. J. 1995;14:853–858. doi: 10.1097/00006454-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Kafetzis DA, Skevaki CL, Skouteri V, et al. Maternal genital colonization with Ureaplasma urealyticum promotes preterm delivery: association of the respiratory colonization of premature infants with chronic lung disease and increased mortality. Clin Infect Dis. 2004;39(8):1113–1122. doi: 10.1086/424505. [DOI] [PubMed] [Google Scholar]
- 39.Viscardi RM, Hashmi N, Gross GW, et al. Incidence of invasive Ureaplasma in VLBW infants: relationship to severe intraventricular hemorrhage. J Perinatol. 2008;28(11):759–765. doi: 10.1038/jp.2008.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dammann O, Allred EN, Genest DR, et al. Antenatal mycoplasma infection, the fetal inflammatory response and cerebral white matter damage in very-low-birthweight infants. Paediatr Perinat Epidemiol. 2003;17(1):49–57. doi: 10.1046/j.1365-3016.2003.00470.x. [DOI] [PubMed] [Google Scholar]
- 41.McElrath TF, Allred EN, Leviton A. Prolonged latency after preterm premature rupture of membranes: an evaluation of histologic condition and intracranial ultrasonic abnormality in the neonate born at <28 weeks of gestation. Am J Obstet Gynecol. 2003;189(3):794–798. doi: 10.1067/s0002-9378(03)00814-7. [DOI] [PubMed] [Google Scholar]
- 42.Redline RW, Wilson-Costello D, Borawski E, et al. Placental lesions associated with neurologic impairment and cerebral palsy in very low-birth-weight infants. Arch Pathol Lab Med. 1998;122:1091–1098. [PubMed] [Google Scholar]
- 43.Viscardi RM, Muhumuza CK, Rodriguez A, et al. Inflammatory markers in intrauterine and fetal blood and cerebrospinal fluid compartments are associated with adverse pulmonary and neurologic outcomes in preterm infants. Pediatr Res. 2004;55:1009–1017. doi: 10.1203/01.pdr.0000127015.60185.8a. [DOI] [PubMed] [Google Scholar]
- 44.Vigneswaran R. Infection and preterm birth: evidence of a common causal relationship with bronchopulmonary dysplasia and cerebral palsy. J Paediatr Child Health. 2000;36:293–296. doi: 10.1046/j.1440-1754.2000.00536.x. [DOI] [PubMed] [Google Scholar]
- 45.Jobe AH, Ikegami M. Antenatal infection/inflammation and postnatal lung maturation and injury. Respir Res. 2001;2:27–32. doi: 10.1186/rr35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bose CL, Dammann CE, Laughon MM. Bronchopulmonary dysplasia and inflammatory biomarkers in the premature neonate. Arch Dis Child Fetal Neonatal Ed. 2008;93(6):F455–F461. doi: 10.1136/adc.2007.121327. [DOI] [PubMed] [Google Scholar]
- 47.Back SA, Gan X, Li Y, et al. Maturation-dependent vulnerability of oligodentrocytes to oxidative stress-induced death casued by glutathione depletion. J Neurosci. 1998;18:6241–6253. doi: 10.1523/JNEUROSCI.18-16-06241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Back SA, Luo NL, Borenstein NS, et al. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perintal white matter injury. J Neurosci. 2001;21:1302–1312. doi: 10.1523/JNEUROSCI.21-04-01302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Volpe JJ. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res. 2001;50:553–562. doi: 10.1203/00006450-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 50.Wang EL, Ohlsson A, Kellner JD. Association of Ureaplasma urealyticum colonization with chronic lung disease of prematurity: Results of a metaanalysis. J Pediatr. 1995;127:640–644. doi: 10.1016/s0022-3476(95)70130-3. [DOI] [PubMed] [Google Scholar]
- 51.Schelonka RL, Katz B, Waites KB, et al. Critical appraisal of the role of Ureaplasma in the development of bronchopulmonary dysplasia with metaanalytic techniques. Pediatr Infect Dis J. 2005;24(12):1033–1039. doi: 10.1097/01.inf.0000190632.31565.83. [DOI] [PubMed] [Google Scholar]
- 52.Panero A, Pacifico L, Roggini M, et al. Ureaplasma urealyticum as a cause of pneumonia in preterm infants: analysis of the white cell response. Arch Dis Child. 1995;73:F37–F40. doi: 10.1136/fn.73.1.f37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crouse DT, Odrezin GT, Cutter GR, et al. Radiographic changes associated with tracheal isolation of Ureaplasma urealyticum from neonates. Clin Infect Dis. 1993;17 Suppl 1:S122–S130. doi: 10.1093/clinids/17.supplement_1.s122. [DOI] [PubMed] [Google Scholar]
- 54.Pacifico L, Panero A, Roggini M, et al. Ureaplasma urealyticum and pulmonary outcome in a neonatal intensive care population. Pediatr. Infect. Dis. 1997;16:579–586. doi: 10.1097/00006454-199706000-00008. [DOI] [PubMed] [Google Scholar]
- 55.Colaizy TT, Morris CD, Lapidus J, et al. Detection of ureaplasma DNA in endotracheal samples is associated with bronchopulmonary dysplasia after adjustment for multiple risk factors. Pediatr Res. 2007;61(5 Pt 1):578–583. doi: 10.1203/pdr.0b013e318045be03. [DOI] [PubMed] [Google Scholar]
- 56.Honma Y, Yada Y, Takahashi N, Momoi MY, Nakamura Y. Certain type of chronic lung disease of newborns is associated with Ureaplasma urealyticum infection in utero. Pediatr Int. 2007;49(4):479–484. doi: 10.1111/j.1442-200X.2007.02391.x. [DOI] [PubMed] [Google Scholar]
- 57.Patterson AM, Taciak V, Lovchik J, et al. Ureaplasma urealyticum respiratory tract colonization is associated with an increase in IL-1β and TNF-α relative to IL-6 in tracheal aspirates of preterm infants. Pediatr Infect Dis J. 1998;17:321–328. doi: 10.1097/00006454-199804000-00011. [DOI] [PubMed] [Google Scholar]
- 58.Viscardi RM, Manimtim WM, Sun CCJ, et al. Lung pathology in premature infants with Ureaplasma urealyticum infection. Pediatr Devel Pathol. 2002;5:141–150. doi: 10.1007/s10024001-0134-y. [DOI] [PubMed] [Google Scholar]
- 59.Viscardi R, Manimtim W, He JR, et al. Disordered pulmonary myofibroblast distribution and elastin expression in preterm infants with Ureaplasma urealyticum pneumonitis. Pediatr Dev Pathol. 2006;9(2):143–151. doi: 10.2350/10-05-0112.1. [DOI] [PubMed] [Google Scholar]
- 60.Viscardi RM, Atamas SP, Luzina IG, et al. Antenatal Ureaplasma urealyticum respiratory tract infection stimulates proinflammatory, profibrotic responses in the preterm baboon lung. Pediatr Res. 2006;60(2):141–146. doi: 10.1203/01.pdr.0000228322.73777.05. [DOI] [PubMed] [Google Scholar]
- 61.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 62.Bry K, Whitsett JA, Lappalainen U. IL-1beta disrupts postnatal lung morphogenesis in the mouse. Am J Respir Cell Mol Biol. 2007;36(1):32–42. doi: 10.1165/rcmb.2006-0116OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Quinn PA, Gillan JE, Markestad T, et al. Intrauterine infection with Ureaplasma urealyticum as a cause of fatal neonatal pneumonia. Pediatr Infect Dis. 1985;4:538–543. doi: 10.1097/00006454-198509000-00020. [DOI] [PubMed] [Google Scholar]
- 64.Brus F, van Waarde WM, Schoots C, et al. Fatal ureaplasmal pneumonia and sepsis in a newborn infant. Eur J Pediatr. 1991;150:782–783. doi: 10.1007/BF02026711. [DOI] [PubMed] [Google Scholar]
- 65.Waites KB, Crouse DT, Philips JB, et al. Ureaplasmal pneumonia and sepsis associated with persistent pulmonary hypertension of the newborn. Pediatrics. 1989;83:79–85. [PubMed] [Google Scholar]
- 66.Madan E, Meyer MP, Amortequi A. Chorioamnionitis: a study of organisms isolated in perinatal autopsies. Ann Clin Lab Sci. 1988;18(1):39–45. [PubMed] [Google Scholar]
- 67.Madan E, Meyer MP, Amortegui AJ. Isolation of genital mycoplasmas and Chlamydia trachomatis in stillborn and neonatal autopsy material. Arch Pathol Lab Med. 1988;112(7):749–751. [PubMed] [Google Scholar]
- 68.Wang EE, Frayha H, Watts J, et al. Role of Ureaplasma urealyticum and other pathogens in the development of chronic lung disease of prematurity. Pediatr Infect Dis J. 1988;7(8):547–551. [PubMed] [Google Scholar]
- 69.Waites KB, Crouse DT, Cassell GH. Systemic neonatal infection due to Ureaplasma urealyticum. Clin. Infect. Dis. 1993;17 Suppl 1:S131–S135. doi: 10.1093/clinids/17.supplement_1.s131. [DOI] [PubMed] [Google Scholar]
- 70.Goldenberg RL, Andrews WW, Goepfert AR, et al. The Alabama Preterm Birth Study: umbilical cord blood Ureaplasma urealyticum and Mycoplasma hominis cultures in very preterm newborn infants. Am J Obstet Gynecol. 2008;198(1):e41–e45. doi: 10.1016/j.ajog.2007.07.033. 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Waites KB, Crouse DT, Nelson KG, et al. Chronic Ureaplasma urealyticum and Mycoplasma hominis infections of central nervous system in preterm infants. Lancet. 1988;2:17–21. doi: 10.1016/s0140-6736(88)91002-1. [DOI] [PubMed] [Google Scholar]
- 72.Waites KB, Duffy LB, Crouse DT, et al. Mycoplasmal infections of cerebrospinal fluid in newborn infants from a community hospital population. Pediatr Infect Dis J. 1990;9(4):241–245. doi: 10.1097/00006454-199004000-00004. [DOI] [PubMed] [Google Scholar]
- 73.Ollikainen J, Hiekkaniemi H, Korppi M, et al. Ureaplasma urealyticum cultured from brain tissue of preterm twins who died of intraventricular hemorrhage. Scand J Infect Dis. 1993;25:529–531. doi: 10.3109/00365549309008538. [DOI] [PubMed] [Google Scholar]
- 74.Rao RP, Ghanayem NS, Kaufman BA, et al. Mycoplasma hominis and Ureaplasma species brain abscess in a neonate. Pediatr Infect Dis J. 2002;21(11):1083–1085. doi: 10.1097/00006454-200211000-00026. [DOI] [PubMed] [Google Scholar]
- 75.Klein JO, Buckland D, Finland M. Colonization of newborn infants by mycoplasmas. N Engl J Med. 1969;280(19):1025–1030. doi: 10.1056/NEJM196905082801901. [DOI] [PubMed] [Google Scholar]
- 76.Cassell GH, Waites KB, Crouse DT, et al. Association of Ureaplasma urealyticum infection of the lower respiratory tract with chronic lung disease and death in very-low-birth-weight infants. Lancet. 1988;2:240–244. doi: 10.1016/s0140-6736(88)92536-6. [DOI] [PubMed] [Google Scholar]
- 77.Hentschel J, Abele-Horn M, Peters J. Ureaplasma urealyticum in the cerebrospinal fluid of a premature infant. Acta Paediatr. 1993;82(8):690–693. doi: 10.1111/j.1651-2227.1993.tb18042.x. [DOI] [PubMed] [Google Scholar]
- 78.Berger A, Witt A, Haiden N, et al. Intrauterine infection with Ureaplasma species is associated with adverse neuromotor outcome at 1 and 2 years adjusted age in preterm infants. J Perinat Med. 2009;37(1):72–78. doi: 10.1515/JPM.2009.016. [DOI] [PubMed] [Google Scholar]
- 79.Stahelin-Massik J, Levy F, Friderich P, et al. Meningitis caused by Ureaplasma urealyticum in a full term neonate. Pediatr Infect Dis J. 1994 May;13(5):419–421. [PubMed] [Google Scholar]
- 80.Zheng X, Watson HL, Waites KB, et al. Serotype diversity and antigen variation among invasive isolates of Ureaplasma urealyticum from neonates. Infect Immun. 1992;60:3472–3474. doi: 10.1128/iai.60.8.3472-3474.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heggie AD, Jacobs MR, Butler VT, et al. Frequency and significance of isolation of Ureaplasma urealyticum and Mycoplasma hominis from cerebrospinal fluid and tracheal aspirate specimens from low birth weight infants. J Pediatr. 1994;124:956–961. doi: 10.1016/s0022-3476(05)83192-0. [DOI] [PubMed] [Google Scholar]
- 82.Sethi S, Sharma M, Narang A, et al. Isolation pattern and clinical outcome of genital mycoplasma in neonates from a tertiary care neonatal unit. J Trop Pediatr. 1999;45(3):143–145. doi: 10.1093/tropej/45.3.143. [DOI] [PubMed] [Google Scholar]
- 83.Schwersenski J, McIntyre L, Bauer CR. Lumbar puncture frequency and cerebrospinal fluid analysis in the neonate. Am J Dis Child. 1991;145:54–58. doi: 10.1001/archpedi.1991.02160010058016. [DOI] [PubMed] [Google Scholar]
- 84.Smith PB, Garges HP, Cotton CM, et al. Meningitis in preterm neonates: importance of cerebrospinal fluid parameters. Am J Perinatol. 2008;25(7):421–426. doi: 10.1055/s-0028-1083839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Matlow AG, Richardson SE, Quinn PA, et al. Isolation of Ureaplasma urealyticum from nonneonatal respiratory tract specimens in a pediatric institution. Pediatr Infect Dis J. 1996;15(3):272–274. doi: 10.1097/00006454-199603000-00023. [DOI] [PubMed] [Google Scholar]
- 86.Pinna GS, Skevaki CL, Kafetzis DA. The significance of Ureaplasma urealyticum as a pathogenic agent in the paediatric population. Curr Opin Infect Dis. 2006;19(3):283–289. doi: 10.1097/01.qco.0000224824.73223.e7. [DOI] [PubMed] [Google Scholar]
- 87.Benn CS, Thorsen P, Jensen JS, et al. Maternal vaginal microflora during pregnancy and the risk of asthma hospitalization and use of anti-asthma medication in early childhood. J Allergy Clin Immunol. 2002;110(1):72–77. doi: 10.1067/mai.2002.125833. [DOI] [PubMed] [Google Scholar]
- 88.Likitnukul S, Kusmiesz H, Nelson JD, et al. Role of genital mycoplasmas in young infants with suspected sepsis. J Pediatr. 1986;109(6):971–974. doi: 10.1016/s0022-3476(86)80278-5. [DOI] [PubMed] [Google Scholar]
- 89.Kong F, Ma Z, James G, et al. Species identification and subtyping of Ureaplasma parvum and Ureaplasma urealyticum using PCR-based assays. J Clin Microbiol. 2000;38(3):1175–1179. doi: 10.1128/jcm.38.3.1175-1179.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Scheurlen W, Fraunendienst G, Schrod L, et al. Polymerase chain reaction-amplification of urease genes: rapid screening for ureaplasma infection in endotracheal aspirates of ventilated newborns. Eur. J. Pediatr. 1992;151:740–742. doi: 10.1007/BF01959080. [DOI] [PubMed] [Google Scholar]
- 91.Blanchard A, Hentschel J, Duffy L, et al. Detection of Ureaplasma urealyticum by polymerase chain reaction in the urogenital tract of adults, in amnioitic fluid, and in the respiratory tract of newborns. Clin Infect Dis. 1993;17 Suppl 1:S148–S153. doi: 10.1093/clinids/17.supplement_1.s148. [DOI] [PubMed] [Google Scholar]
- 92.Stellrecht KA, Woron AM, Mishrik NG, et al. Comparison of multiplex PCR assay with culture for detection of genital mycoplasmas. J Clin Microbiol. 2004;42(4):1528–1533. doi: 10.1128/JCM.42.4.1528-1533.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mallard K, Schopfer K, Bodmer T. Development of real-time PCR for the differential detection and quantification of Ureaplasma urealyticum and Ureaplasma parvum. J Microbiol Methods. 2005;60(1):13–19. doi: 10.1016/j.mimet.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 94.Kirschbaum T. Antibiotics in the treatment of pretem labor. Am J Obstet Gynecol. 1993;168(4):1239–1246. doi: 10.1016/0002-9378(93)90375-s. [DOI] [PubMed] [Google Scholar]
- 95.Gomez R, Romero R, Nien JK, et al. Antibiotic administration to patients with preterm premature rupture of membranes does not eradicate intra-amniotic infection. J Matern Fetal Neonatal Med. 2007;20(2):167–173. doi: 10.1080/14767050601135485. [DOI] [PubMed] [Google Scholar]
- 96.Renaudin H, Bebear C. Comparative in vitro activity of azithromycin, clarithromycin, erythromycin and lomefloxacin against Mycoplasma pneumoniae, Mycoplasma hominis and Ureaplasma urealyticum. Eur J Clin Microbiol Infect Dis. 1990;9(11):838–841. doi: 10.1007/BF01967388. [DOI] [PubMed] [Google Scholar]
- 97.Bowman ED, Dharmalingam A, Fan WQ, et al. Impact of erythromycin on respiratory colonization of Ureaplasma urealyticum and the development of chronic lung disease in extremely low birth weight infants. Pediatr Infect Dis J. 1998;17:615–620. doi: 10.1097/00006454-199807000-00008. [DOI] [PubMed] [Google Scholar]
- 98.Jonsson B, Rylander M, Faxelius G. Ureaplasma urealyticum, erythromycin and respiratory morbidity in high-risk preterm neonates. Acta Paediatr. 1998;87:1079–1084. doi: 10.1080/080352598750031428. [DOI] [PubMed] [Google Scholar]
- 99.Baier RJ, Loggins J, Kruger TE. Failure of erythromycin to eliminate airway colonization with Ureaplasma urealyticum in very low birth weight infants. BMC Pediatr. 2003;3:10. doi: 10.1186/1471-2431-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rubin BK. Macrolides as biologic response modifiers. J Respir Dis. 2002;23:S31–S38. [Google Scholar]
- 101.Ianaro A, Ialenti A, Maffia P, et al. Anti-inflammatory activity of macrolide antibiotics. J Pharmacol Exp Ther. 2000;292(1):156–163. [PubMed] [Google Scholar]
- 102.Tsai WC, Standiford TJ. Immunomodulatory effects of macrolides in the lung: lessons from in-vitro and in-vivo models. Curr Pharm. 2004;10(25):3081–3093. doi: 10.2174/1381612043383430. [DOI] [PubMed] [Google Scholar]
- 103.Duffy LB, Crabb D, Searcey K, et al. Comparative potency of gemifloxacin, new quinolones, macrolides, tetracycline and clindamycin against Mycoplasma spp. J Antimicrob Chemother. 2000 Apr;45 Suppl 1:29–33. doi: 10.1093/jac/45.suppl_3.29. [DOI] [PubMed] [Google Scholar]
- 104.Girard AE, Cimochowski CR, Faiella JA. Correlation of increased azithromycin concentrations with phagocyte infiltration into sites of localized infection. J Antimicrob Chemother. 1996;37 Suppl C:9–19. doi: 10.1093/jac/37.suppl_c.9. [DOI] [PubMed] [Google Scholar]
- 105.Patel KB, Xuan D, Tessier PR, et al. Comparison of bronchopulmonary pharmacokinetics of clarithromycin and azithromycin. Antimicrob Agents Chemother. 40(10):2375–2379. doi: 10.1128/aac.40.10.2375. 996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Capitano B, Mattoes HM, Shore E, et al. Steady-state intrapulmonary concentrations of moxifloxacin, levofloxacin, and azithromycin in older adults. Chest. 2004;125(3):965–973. doi: 10.1378/chest.125.3.965. [DOI] [PubMed] [Google Scholar]
- 107.Auten RL, Ekekezie II. Blocking leukocyte influx and function to prevent chronic lung disease of prematurity. Pediatr Pulmonol. 2003;35(5):335–341. doi: 10.1002/ppul.10275. [DOI] [PubMed] [Google Scholar]
- 108.Liao L, Ning Q, Li Y, et al. CXCR2 blockade reduces radical formation in hyperoxia-exposed newborn rat lung. Pediatr Res. 2006;60(3):299–303. doi: 10.1203/01.pdr.0000233058.08200.d6. [DOI] [PubMed] [Google Scholar]
- 109.Walls SA, Kong L, Leeming HA, et al. Antibiotic prophylaxis improves Ureaplasma-associated lung disease in suckling mice. Pediatr Res. 2009;66(2):197–202. doi: 10.1203/PDR.0b013e3181aabd34. [DOI] [PubMed] [Google Scholar]
- 110.Viscardi RM, Hasday JD. Role of Ureaplasma species in Neonatal Chronic Lung Disease: Epidemiologic and Experimental Evidence. Pediatr Res. 2009;65:84R–90R. doi: 10.1203/PDR.0b013e31819dc2f9. [DOI] [PMC free article] [PubMed] [Google Scholar]