Abstract
Several recent studies have documented age-related changes in brain activity - less amygdala activity and higher prefrontal activity in response to emotional stimuli. Using functional magnetic resonance imaging (fMRI), we examined whether aging also affects the maintenance of activity to emotional stimuli and whether maintenance differs by the valence (negative, neutral, positive) of the pictures. Younger participants had a larger volume of activity in the amygdala but less in the prefrontal cortex than the old. The old showed more habituation to highly arousing negative but not positive or neutral stimuli in prefrontal cortex as compared to younger participants. Thus prefrontal cortex activity indexes emotion in the elderly, but not the young. Amplified prefrontal activity suggests elderly increase cognitive control for negative, highly arousing emotional stimuli, but it is not maintained. Taken together, age-related increases in prefrontal activity and reduced amygdala activity may underlie observed affective changes in aging.
Keywords: aging, amygdala, emotion, fMRI, habituation, prefrontal cortex
1. Introduction
Aging affects emotion and emotional memory (Charles et al., 2003;Lawton et al., 1992;Leigland et al., 2004;Levenson et al., 1994;Mroczek and Kolarz, 1998). Older adults experience less negative affect, attend less to negative stimuli, rate negative pictures as less arousing and show disproportionately better memory for positive stimuli than for negative or neutral stimuli as compared to younger adults (Charles et al., 2003;Leigland et al., 2004;Mather et al., 2004;Mather and Carstensen, 2003). Both psychological and physiological mechanisms have been proposed for these changes. Psychological studies of well-being suggest that increasing age brings greater emotional control and less negative affect, resulting in a more positive shift in outlook (Lawton et al., 1992;Mroczek and Kolarz, 1998). Older adults have greater regulation of their emotional experiences, as shown by their maintenance of positive states and the greater likelihood that they will shift out of negative states as compared to the young (Carstensen et al., 2000;Lawton et al., 1992). Carstensen's socioemotional selectivity theory suggests that awareness of endings promotes emotional goals, which then accounts for older adults' greater focus on positive affect (Carstensen, 1995;Carstensen et al., 1999;Carstensen, 2006).
Physiological changes in the amygdala and prefrontal cortex may also explain age-related emotional changes. In young adults the amygdala is critical for the processing of emotion, particularly negative emotions including fear (Davis and Whalen, 2001;Murphy et al., 2003;Whalen et al., 1998) and anger (Adams, Jr. et al., 2003;Whalen et al., 2001). This has been shown by both lesion (Adolphs et al., 1997;Adolphs nd Tranel, 2004;Aggleton et al., 1992;Gale et al., 2004;Phelps et al., 1997) and neuroimaging studies (Calder et al., 2001;Gunning-Dixon et al., 2003;Gur et al., 1994;Hariri et al., 2002;Phan et al., 2003;Whalen et al., 2001;Wright et al., 2006b). The prefrontal cortex (PFC) is also implicated in the production of emotional states. (Phillips et al., 2003;Price, 1999;Rolls, 2006). Both of these regions atrophy and undergo functional changes with aging (Allen et al., 2005;Grieve et al., 2005;Mu et al., 1999;Salat et al., 1999a;Salat et al., 2001).
Less amygdala activity and more prefrontal activity characterize age-related responses to emotion. Amygdala activity is greater in healthy young adults than in older adults in response to negative emotional stimuli (Gunning-Dixon et al., 2003;Iidaka et al., 2002;Mather et al., 2004) such as passive viewing of angry faces (Fischer et al., 2005a). Comparison of amygdala activity to positive and negative stimuli in the old reveals greater activity to positive than negative while in younger adults the amygdala activates similarly to either emotional valence (Mather et al., 2004). In contrast, the old have more activity to emotional stimuli in prefrontal cortex as compared to the young.(Gunning-Dixon et al., 2003;Iidaka et al., 2002;Tessitore et al., 2005;Williams et al., 2006). For example, the young activate the amygdala and medial temporal regions whereas the old recruit PFC during emotional face processing (Gunning-Dixon et al., 2003). The old have more PFC activity for positive than negative stimuli whereas the opposite is true in the young (Leclerc & Kensinger, 2008) and connectivity analyses show that PFC and amygdala influence hippocampal activity for positive stimuli in the old but not the young (Addis et al., 2009).
Age-related changes in the anatomical and functional interaction of the amygdala and PFC affects emotional regulation across the lifespan. Reappraisal or regulation of negative emotional scenes results in more PFC activity and less amygdala activity in the young (Ochsner et al., 2002; Hariri et al., 2003) and old (Urry et al., 2006). However, it remains unclear what conditions are necessary for PFC suppression of amygdala activity and the behavioral consequences of suppression (Williams et al., 2006). Better memory for negative stimuli in the elderly is the result of more DLPFC activity and greater functional connectivity between the amygdala and DLPFC, but between the amygdala and medial temporal lobe in the young (St. Jacques et al., 2009). However, it is not known whether age-related loss of amygdala activity induces higher prefrontal activity (e.g. compensatory recruitment), or whether a “top-down” age-related amplification of prefrontal activity reduces amygdala activity (for review see Ochsner & Gross, 2005). In either case, the result of greater emotional regulation is shown by less responsiveness to negative information and/or more responsiveness for positive information. It is also unclear why lower amygdala activity and higher prefrontal activity results in the emotional asymmetry that has been called the “positivity bias.” Certainly the amygdala is responsive to both negative and positive stimuli, and it is not clear why its suppression or prefrontal amplification would result in more activity, arousal or memory for positive information.
One possibility is that aging modifies the quality of brain activity; its maintenance or habituation. Maintenance of brain activity has been shown to be critical in other cognitive domains. For example, a study that used repetition at encoding to improve memory in the elderly found that initial presentation of a face-name pair resulted in activity of the hippocampus and prefrontal cortex and this activity was related to memory performance, as others have shown (Rand-Giovannetti et al., 2006). However, with subsequent presentations, sustained activity in prefrontal and other cortical regions was related to successful memory, which was not the case for the hippocampus. This suggests that the hippocampus can respond in the elderly, but that enhanced learning requires sustained cortical activity (Rand-Giovannetti et al., 2006). Habituation of brain activity may be due to active inhibition, a shift of attention toward stimuli of more salience, or a loss of cognitive control (Paxton et al., 2008). Studies in the young show rapid loss (habituation) of amygdala activity to repeated emotional stimuli (Breiter et al., 1996;Fischer et al., 2000;Fischer et al., 2003;Wedig et al., 2005;Wright et al., 2002). For example, habituation occurs when the same affective picture is presented multiple times (Wright et al., 2002) and when multiple examples of the same valence category (e.g. negative pictures) are shown (Houtveen et al., 2001). The sole study of habituation of amygdala activity in aging found that it is similar in old and young, but only repeated neutral face stimuli were studied (Wedig et al., 2005). Age-related effects have not been examined for multiple emotional valences, non-face stimuli, nor with regard to habituation of prefrontal activity. The goal of this study was to examine aging related responses to both negative and positive emotional stimuli and to examine the quality of brain activity, specifically whether there is differential habituation or maintenance of activity across regions or by valence.
2. Materials and Methods
2.1. Participants
Participants were 22 healthy right-handed elderly adults (13 female; 9 male) and 14 healthy young adults (7 female; 7 male) recruited from an urban population. Health histories were obtained via phone interview. Inclusion criteria required that young individuals be between 21 and 35 years of age and elderly individuals be between 65 and 80 years of age. These age brackets permitted recruitment of relatively healthy elderly, and groups that significantly differed in age but had experienced relatively comparable life styles, including levels of education and employment. All subjects understood English and had adequate hearing and vision (with correction if necessary) to view computer and paper-pencil tasks. Young and old did not differ on the vocabulary subtest of the Wechsler Adult Intelligence Scale-Revised (Table 1). This sub-test provides a standardized approximation of functional intelligence and is highly correlated with both verbal and full-scale IQ scores (Wechsler, 1981). Matching subjects on this measure controls for cohort-related differences in education environments.
Table 1.
Demographic characteristics of all participants.
| Young (n=14) | Old (n=22) | |
|---|---|---|
| Age (years) | 25.2 (2.8) | 72.5 (4.4) |
| Gender (Male/Female) | 7/7 | 9/13 |
| Handedness (Right/Left) | 14/0 | 22/0 |
| Education (years) | 16.3 (.7) | 15.6 (2.1) |
| WAIS-Ra | 12.6 (1.7) | 13.1 (3.0) |
Note: Age, education and WAIS-R expressed as: Mean (SD)
WAIS-R: Weschler Adult Intelligence Scale-Revised (Scaled scores)
Participants were excluded if they had a self-reported history of neurological problems (e.g., stroke, seizure, or head trauma), significant medical problems (e.g. uncontrolled hypertension), current or previous psychiatric conditions (e.g., schizophrenia), or current use of medications likely to affect mood or cognition such as anti-anxiety agents (e.g. SSRIs). Participants were also excluded if they had conditions contrary to MRI such as internal metal, risk of metal in the eyes, non-removable hearing aids or implants, pacemakers or reported claustrophobia. The Mini-Mental Status Examination (MMSE) and Geriatric Depression Scale (GDS) were used as screening measures to exclude older participants with possible dementia (MMSE > 26) or depression (GDS>10). However, no older subjects had abnormal MMSE or GDS scores nor did MMSE or GDS scores correlate with any measure of brain activity. All participants provided written informed consent and were paid for their time and participation in the study.
2.2. Stimuli
Using a design similar to previous studies that is sensitive to habituation (Fischer et al., 2000;Fischer et al., 2005b;Wedig et al., 2005;Wright et al., 2002;Wright et al., 2001) participants passively viewed a 10-minute presentation of emotion-laden pictures during fMRI acquisition. Pictures were selected from the International Affective Picture System (IAPS; See list of pictures in Supplementary Material #1;Lang et al., 1999). Eighty unique pictures were used for each valence category (negative, neutral and positive) and were presented in blocks. Pictures were selected based on IAPS normative valence ratings based on a 1 (negative) to 9 (positive) scale. Valence ratings for the pictures used were negative = 1.45–2.98, neutral 4.53–5.93 and positive = 6.82–8.34. Arousal level differed by valence category (F2, 78=219.54, P <.01; negative M= 5.83, neutral M=3.36, positive M=5.09). Negative pictures were more arousing than both neutral (t39 =20.38, P <.01) and positive (t39 =5.15, P <.01) pictures; positive pictures were also more arousing than neutral pictures (t39 =18.45, P <.01). Negative, neutral and positive pictures were matched for luminosity. Because others had shown little responsiveness of the amygdala in the elderly (Gunning-Dixon et al., 2003;Iidaka et al., 2002;Mather et al., 2004) we used exceedingly negative and arousing stimuli to try to obtain activity and thus also permit an examination of habituation in the young and old.
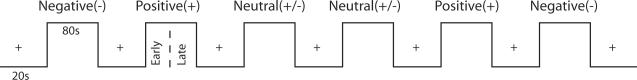
Each trial was 2000ms and each picture was presented for the first 1500ms allowing for 500ms transition to the next picture. The series of pictures began with 10 cross-hair control trials (20 seconds) followed by 40 affective pictures (80 seconds) all of a single valence category (e.g. negative) and ended with 10 cross-hair control trials in a blocked design (Fischer et al., 2005b;Wedig et al., 2005;Williams et al., 2006;Wright et al., 2001). The sequence was repeated until pictures of all three valence categories (negative, positive, and neutral) occurred. The valence order in the second set of blocks was then reversed (neutral, positive, negative) with new pictures for each valence (see Figure 1). The reversal of order permitted acquisition of two datasets per valence while controlling for the possibility of a general drift in scanner signal over the course of the study that would preclude interpretation of habituation among valence categories. Subjects were instructed to focus on the pictures at the level of the fixation cross. In order to assess habituation or other signal changes, pictures within a valence category were divided into Early Pictures (stimuli 1–20) and Late Pictures (stimuli 21–40). Early and Late pictures of each valence category were a priori matched for normative arousal and valence ratings. Thus, any changes in brain activity between early and late pictures are not due to differences in valence or arousal.
Figure 1.
Examples of stimuli used in this block design experiment. A series of pictures began with 10 cross-hair control trials (20 seconds) followed by 40 affective pictures (80 seconds) all of a single valence category (e.g. negative) and ended with 10 cross-hair control trials. This sequence was repeated until pictures of all three valence categories (negative, positive, and neutral) occurred. Each picture was presented for 1500ms.
Pictures were back-projected into the MR scanner using a video projector (PLC-XP50L, Sanyo, Inc.) fitted with a custom long-throw lens and viewed via a mirror mounted on the head-coil. Picture and timing were controlled using Presentation Software (version 10.1, Neurobehavioral Systems, http//:www.neurobs.com).
After MRI scanning was complete, subjects rated all 240 IAPS pictures for valence and arousal. Pictures appeared for 2 s after which they were rated on a 9-point scale for valence (1: negative, 5: neutral, 9: positive) and arousal (1: calming, 5: neutral, 9: exciting). Order of presentation was the same as during MR scanning. Mean valence and arousal ratings were calculated by taking the average subject ratings across each valence. Participants were not told that they would be asked to remember the pictures later.
After a 1- week retention interval (mean for old = 6.92 days, range 6–11 days; mean for young= 7.09 days, range = 6–8 days), participants performed a yes/maybe/no recognition test. The retention interval did not differ between young and old (p>.10) and its length insured that memory performance would not be at ceiling or floor for either group. During the recognition test participants viewed 180 pictures comprised of the 120 pictures they rated previously and 60 novel pictures. The 60 novel pictures were matched for a priori valence and arousal to the target pictures. Order of picture presentation was randomized with respect to target/novel status and valence.
2.3 fMRI Data Acquisition
A Siemens TIMS Trio 3T MR scanner (Erlangen, Germany) with a 12-channel head-coil was used to collect anatomical and functional imaging data. Details of the scanning protocol are provided in Supplementary Material #2.
2.4 fMRI Data Analysis
The fMRI data was processed and analyzed using Brain Voyager QX1.9 (Brain Innovations, http://www.brainvoyager.com). Prior to statistical analysis, each subjects' functional data underwent linear trend removal (sinc interpolation), high-pass temporal filtering (> 0.016 Hz) to remove low frequency oscillations in the signal timecourse in each voxel, slice scan time correction, motion correction and spatial smoothing with a Gaussian kernel of 8mm FWHM (Hamann et al., 2004). The individual fMRI scans were normalized and then co-registered to the individual's high resolution (MPRAGE) anatomical brain scan and resampled into 3 mm3 voxels. Next, each brain dataset was convolved into standard stereotaxic coordinate system (Talairach and Tournoux, 1988) to allow the pooling of data across subjects. Results of the spatial normalization were visually inspected to ensure acceptable anatomical registration with the standard brain.
The activity data were analyzed in two steps. First, a qualitative whole brain analysis used Random Effects Analysis of Variance (ANOVA) within a General Linear Model (GLM) framework to identify significant brain activation (number of active voxels/timecourse). GLM analysis included 12 regressors; one for each half of each valence block (e.g. Regressor 1 = Negative Early (Pictures 1–20 in the Negative block 1), Regressor 2 = Negative Late (Pictures 21–40 in the Negative Block 1)… through to Regressor 12 = Negative Late (Pictures 260–300 in the Negative Block 2)) and were modeled using a canonical hemodynamic response function. All EPI data was visually inspected prior to analysis for data integrity. After coregistration, EPI data that did not extend below the anatomical location (below Z=−22) for either the amygdala or orbitofrontal cortex was removed from the whole brain data analysis. Seven older subjects (3 men; 4 women) and one young male subject were excluded from the whole brain analysis due to susceptibility artifacts close to the amygdala or orbitofrontal cortex. Thus, the whole brain functional analyses included 15 old and 13 young participants.
Whole brain contrasts were performed within and between each age group for each valence category versus baseline (i.e. negative – baseline, neutral – baseline, positive – baseline). In addition, contrasts of emotion versus neutral (i.e. negative – neutral, positive – neutral) and negative versus positive were performed for each group. Contrasts for valence category versus baseline were thresholded at an alpha level of p < 10−10. This value was chosen based on a false discovery rate (FDR) correction of q<.001 for the statistical map (Benjamini and Hochberg, 1995). We chose a stringent p-value to reduce false positives as we have a limited number of subjects. Comparisons between age groups (e.g. Young negative – baseline vs. Old negative – baseline) were assessed by using BrainVoyager RFX ANOVA and contrasts. Group analyses and contrasts for emotion versus neutral were corrected for multiple comparison by using a threshold of p<.01 and a minimum cluster size of 10 voxels. Peak voxel (Talairach coordinate), hemisphere, lobe, region, and a Brodmann Area (BA) as computed using Talairach Daemon software (Lancaster et al., 2000) are reported in table format (Table 2 and Supplemental Tables 2 & 3).
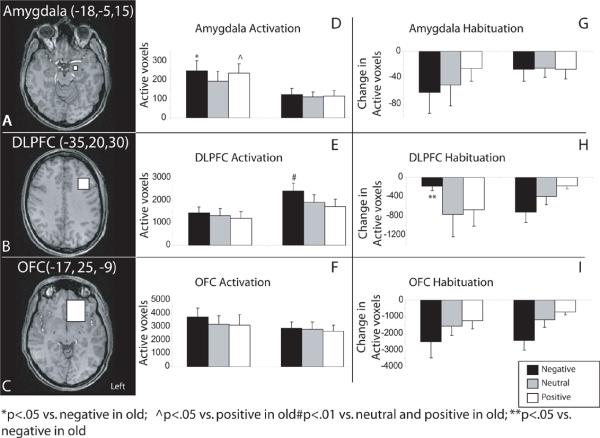
Subsequently, a priori hypotheses regarding prefrontal and amygdala activity were investigated with a region of interest analysis (ROI). Three ROIs, (1) amygdala, (2) dorsolateral prefrontal cortex (DLPFC) and (3) orbitofrontal cortex (OFC; Figure 4A,–C) were identified anatomically on each subjects' high-resolution MPRAGE and then applied to each subjects' functional data by placing the brain in standard space. Landmarks to aid in placement of the ROIs were chosen using neuroanatomical atlases (Talairach and Tournoux, 1988). An 8mm cube was placed in the amygdala, a 20mm cube was placed in the DLPFC (Herrington et al., 2005;Wright et al., 2001) and a 15 × 30 × 45mm ROI was placed in the OFC. The boundaries of the ROIs were selected based on previous literature (Hamann et al., 2004;Herrington et al., 2005;Wedig et al., 2005;Wright et al., 2007;Wright et al., 2001) and were adjusted where necessary according to visual inspection. All ROI placements were within the structure of interest in each individual as verified visually and with the Talaraich coordinates, and they included the active regions found in the whole-brain analysis. We choose to employ the cube ROI drawing tool within Brain Voyager for all ROIs (amygdala, DLPFC and OFC). Previous studies assessing amygdala function have used similarly shaped ROIs in the amygdala in elderly subjects (Cook et al., 2007). Other studies have employed spherical ROIs (Hamann et al., 2004;Iidaka et al., 2006), although these have not included elderly subjects. This ROI method permits us to ask whether equal amounts of tissue within a given area are equally active between two groups and alleviates concerns about age-related atrophy driving differential activity. We used anatomically, not functionally, defined ROIs to extract activity data. A previous emotion study in the elderly (Wright et al., 2006a) analyzed activity from both functionally and anatomically defined regions of the amygdala. The authors showed that both the functional and anatomical regions-of-interest resulted in similar findings when comparing activity to emotional faces in both the young and the old.
Figure 4.
Left: Axial brain slices documenting the midpoint of the three regions of interest: A) Amygdala, B) Dorsolateral Prefrontal Cortex (DLPFC) and C) Orbitofrontal Cortex (OFC). Right: ROI activity (D–F) and habituation (G–I) in the amygdala, DLPFC and OFC for young and old for negative (black), neutral (grey) and positive (grey) emotional pictures. The volume of amygdala activity (D) was greater in younger than older adults to both negative and positive pictures. In contrast, older adults had more active regions and a larger total volume of activity in the DLPFC (E) as compared to the young. There were no group differences in OFC activity (F). Habituation of amygdala activity did not differ between young and old (G). The old had a greater decline in the volume of activity to negative stimuli in the DLPFC and less habituation (or persistence) of activity to repeated positive pictures as compared to the young (H). Both groups showed overall valence effects in habituation (neg>neu>pos). The young and old did not differ in habituation to repeated emotional pictures within the OFC (I).
The extent of activation (number of active voxels) and the percent signal change (time-course signal) for each valence (negative, neutral, positive) as compared to crosshair, emotion (negative or positive as compared to neutral), and negative as compared to positive was examined. Habituation was determined by measuring the change in activity within the series of pictures for each valence. Pictures in the Early (first 20 pictures of a valence block) portion of each series were compared to pictures in the Late (last 20 pictures) portion of each series by calculating a change score (Early-Late). The p-value of <.05 in the ROI analyses are reasonable based on the a priori delineation of a limited number of ROIs, the specificity of the contrasts, and as a follow-up from our whole-brain analysis. Correction for multiple comparisons was accomplished by using an additional cluster filter of 50 cubic millimeters for within-group analysis (Forman et al., 1995). As with the whole brain analysis, the amygdala and OFC ROI data included 15 elderly and 13 young due to susceptibility artifact in the amygdala or OFC for other participants. The ROI analyses for the DLPFC included all subjects, 22 older and 14 young participants.
2.5 Statistical Comparisons
Comparisons were made using repeated measures ANOVAs and follow up t-tests (SPSS v15.0). Valence categories (negative, neutral and positive) and time (Early or Late) were the within group repeated variables, and group (young or old) was the independent measure for behavioral and fMRI data. The groups (young and old) were compared for: 1) behavioral ratings and memory 2) ROI activation (amygdala, DLPFC, OFC) to emotional pictures, and 3) Habituation: the change in activity within each valence. All measures were tested for sphericity and equal variances (Levene's test); appropriate statistical corrections were used when necessary and are noted in the results. As an exploratory analysis, we assessed laterality effects in activity. For this, the data was re-entered into mixed-model ANOVA using group as the between subjects factor and valence and hemisphere as within-subjects factors. This data is presented as supplementary material # 3. Pearson correlations were performed within each valence category for young and old groups separately to assess for associations between brain activity and behavioral measures, and for associations in activity among brain regions. For the correlational analyses Bonferroni corrections were used to adjust for multiple comparisons.
3. Results
3.1. Behavioral Results
3.1.1. Valence and Arousal Ratings
Young and old had similar valence ratings for negative, neutral and positive pictures. The ratings differed across valence categories (F2,68=292.87, p <.001;Figure 2A). Negative pictures had lower ratings than neutral pictures (t35=−15.97, p <.001), which had lower ratings than positive pictures (t35=−13.46, p <.001; Figure 2a). There were no differences between early or late ratings nor any interactions between valence, early-late or age (Supplemental Table 1). Others have shown a “positivity bias” by the elderly in some studies (Charles et al., 2003;Leigland et al., 2004;Mather et al., 2004;Mather and Carstensen, 2003). Therefore, we performed an exploratory comparison of each valence separately that showed that the old made more positive valence ratings to positive pictures than the young (t34= 2.22, p < .03) but no group differences were found for negative or neutral pictures.
Figure 2.
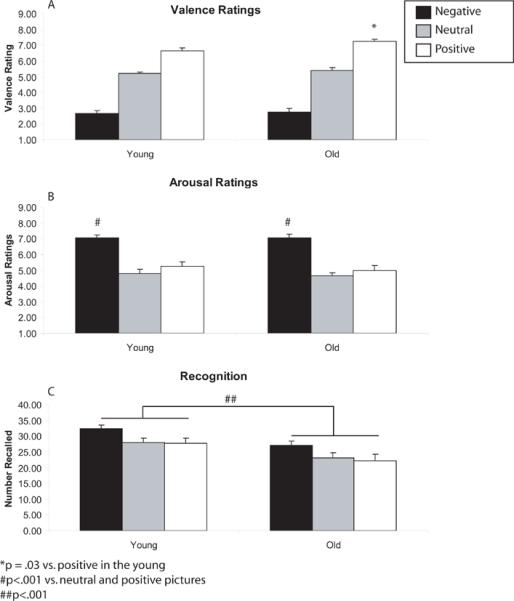
Behavioral performance of the young and old on negative (black), neutral (grey) and positive (white) pictures. (A) Both groups showed similar valence ratings for negative, neutral and positive pictures. The old had more positive valence ratings to positive pictures than the young. (B) Arousal ratings differed across valence categories. Negative pictures elicited higher arousal ratings than neutral or positive pictures in both young and old. (C) The young had better memory for emotional pictures for all valence categories as compared to the old.
Young and old did not differ in arousal ratings of the emotional pictures. Arousal ratings differed across valence categories (F2,68=50.03, p <.001). Negative pictures elicited higher arousal ratings than neutral (t35=10.13, p <.001) or positive pictures (t35=6.91, p <.001;Figure 2B). Neutral pictures were marginally less arousing than positive pictures (t35=1.98, p =.06). There were no differences among valences for arousal ratings of early versus late pictures or any interactions with age (Table 2).
3.1.2. Recognition Memory
The young had better memory than the old for emotional pictures (F2,68=13.70, p <.001;Figure 2C). Post-hoc t-test showed that the young had better recognition memory than the old for negative (t34= 2.68, p < .01), neutral (t34=2.08, p < .05) and positive (t33.16= 2.819 p < .01) pictures. Memory was also affected by valence (F2,70= 14.92, p < .001). Both age groups remembered negative pictures better than neutral (t35=4.12, p <.01) or positive (t35=4.89, p <.01) pictures. There was no difference in recognition memory between neutral and positive pictures. There was no interaction between valence and age. There were no differences among valences for arousal ratings of early versus late pictures or any interactions with age (Table 2).
3.2. Functional Imaging Results
3.2.1. Whole brain analysis
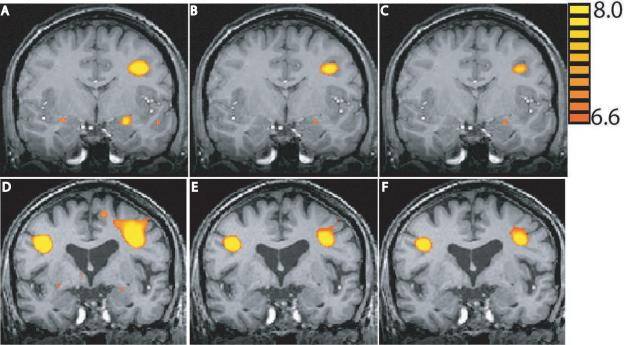
Both the young (Figure 3A–C) and old (Figure 3D–F) had significant activation of the amygdala and prefrontal regions in response to viewing emotional pictures as compared to crosshairs Qualitatively the young activated the amygdala to negative and positive, but not neutral emotional pictures whereas the old only showed amygdala activity for negative pictures (Table 2a). Both groups activated regions of the prefrontal cortex to all emotional stimuli, including inferior frontal, superior frontal, middle and medial frontal gyri. In addition, young and old activated similar regions of the visual cortex. Between-group comparisons for each valence separated by Early and Late are shown in Table 2b. In summary, for negative pictures the old showed a habituation-like pattern: more regions of greater activation as compared to the young during Early negative pictures than for Late negative pictures (Early as compared to Late). For positive pictures the old showed the opposite: more regions of greater activity as compared to the young during Late positive pictures than for Early positive pictures. Both groups showed similar change to early and late neutral pictures. Furthermore, the old showed the typical posterior-to-anterior (PASA; Davis et al., 2008) shift as they show more activation in frontal regions as compared to posterior regions.
Figure 3.
Random effects group statistical maps of brain responses in the young (A–C) and old (D–F) to negative (A,D), neutral (B,E) and positive (C,F) emotional pictures as compared to baseline. Activity for the young is displayed on a representative young brain and activity for the old is displayed on a representative old brain. The statistical maps are superimposed on a representative coronal T1 structural image from their respective group. The young have significant fMRI signal in the amygdala and left frontal cortex for all emotional stimuli. The old have significant fMRI signal in the amygdala only for negative stimuli and bilateral frontal activity for all emotional scenes. All images are presented in standard stereotaxic space (Talaraich). The coordinate of the coronal slice shown is y = −5 for each of the representative young and old brains.
Table 2A.
Regions of activity (emotional pictures > crosshair) in young and old. Coordinates are based on activity at the peak voxel.
| Young | ||||||||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | T-stat | p-value | Voxels | Hemi | Peak Voxel GM | BA |
| Negative>Baseline | ||||||||
| 28 | −58 | 0 | 16.83 | 1.00E-23 | 108737 | Right | Lingual Gyrus | BA 19 |
| 27 | −4 | −7 | 7.75 | 1.20E-13 | 323 | Right | Lentiform Nucleus | Putamen |
| −24 | −4 | −10 | 9.94 | 1.69E-20 | 872 | Left | Parahippocampal Gyrus | Amygdala |
| −39 | 27 | −1 | 10.60 | 9.51E-23 | 10405 | Left | Inferior Frontal Gyrus | BA 47 |
| 39 | 5 | 29 | 10.19 | 2.42E-21 | 1488 | Right | Inferior Frontal Gyrus | BA 9 |
| 45 | 23 | 23 | 9.28 | 2.49E-18 | 1181 | Right | Middle Frontal Gyrus | BA 46 |
| −3 | 53 | 33 | 7.58 | 3.60E-13 | 390 | Left | Superior Frontal Gyrus | BA 9 |
| Neutral>Baseline | ||||||||
| 30 | −61 | −4 | 17.68 | 1.00E-23 | 85689 | Right | Lingual Gyrus | BA 19 |
| −39 | 29 | −1 | 9.92 | 2.00E-20 | 2734 | Left | Inferior Frontal Gyrus | BA 47 |
| 39 | 5 | 29 | 8.62 | 3.11E-16 | 556 | Right | Inferior Frontal Gyrus | BA 9 |
| −39 | −4 | 35 | 9.44 | 7.64E-19 | 1712 | Left | Precentral Gyrus | BA 6 |
| 42 | 23 | 23 | 8.68 | 2.02E-16 | 550 | Right | Middle Frontal Gyrus | BA 46 |
| −42 | 20 | 20 | 10.25 | 1.51E-21 | 1421 | Left | Middle Frontal Gyrus | BA 46 |
| Positive>Baseline | ||||||||
| 28 | −61 | −1 | 16.83 | 1.00E-23 | 88457 | Right | Lingual Gyrus | BA 19 |
| −24 | −4 | −10 | 8.69 | 1.81E-16 | 289 | Left | Parahippocampal Gyrus | Amygdala |
| −39 | 29 | −1 | 9.19 | 4.74E-18 | 2152 | Left | Inferior Frontal Gyrus | BA 47 |
| −39 | −4 | 35 | 8.50 | 6.99E-16 | 880 | Left | Precentral Gyrus | BA 6 |
| −42 | 20 | 20 | 9.84 | 3.58E-20 | 1110 | Left | Middle Frontal Gyrus | BA 46 |
| Old | ||||||||
|---|---|---|---|---|---|---|---|---|
| Negative>Baseline | ||||||||
| 21 | −10 | −1 | 8.83 | 6.70E-17 | 511 | Right | Lentiform Nucleus | Globus Pallidus |
| 23 | −57 | −6 | 17.02 | 1.00E-30 | 101197 | Right | Parahippocampal Gyrus | BA 19 |
| 30 | 29 | 2 | 7.67 | 1.97E-13 | 344 | Right | Inferior Frontal Gyrus | BA 47 |
| −36 | 29 | −4 | 10.03 | 8.27E-21 | 2178 | Left | Inferior Frontal Gyrus | BA 47 |
| 36 | 2 | 29 | 13.26 | 2.30E-32 | 6524 | Right | Precentral Gyrus | BA 6 |
| −36 | 0 | 32 | 12.54 | 1.09E-29 | 13007 | Left | Precentral Gyrus | BA 6 |
| −6 | 2 | 53 | 9.87 | 3.02E-20 | 755 | Left | Medial Frontal Gyrus | BA 6 |
| −12 | 44 | 35 | 10.83 | 1.5E-23 | 6278 | Left | Superior Frontal Gyrus | BA 8 |
| Neutral>Baseline | ||||||||
| 25 | −59 | −6 | 16.86 | 1.00E-28 | 87293 | Right | Fusiform Gyrus | BA 19 |
| 45 | 26 | 20 | 7.98 | 2.58E-14 | 475 | Right | Middle Frontal Gyrus | BA 46 |
| 36 | 2 | 29 | 11.75 | 8.78E-27 | 2850 | Right | Precentral Gyrus | BA 6 |
| −6 | 2 | 54 | 9.43 | 8.44E-19 | 639 | Left | Medial Frontal Gyrus | BA 6 |
| −9 | 41 | 38 | 7.88 | 5.01E-14 | 665 | Left | Medial Frontal Gyrus | BA 8 |
| −36 | −1 | 32 | 11.71 | 1.16E-26 | 9072 | Left | Precentral Gyrus | BA 6 |
| −36 | 29 | −4 | 9.64 | 1.68E-19 | 1388 | Left | Inferior Frontal Gyrus | BA 47 |
| Positive>Baseline | ||||||||
| 36 | −61 | −8 | 16.92 | 1.00E-25 | 77303 | Right | Declive | * |
| 39 | −1 | 26 | 10.91 | 8.07E-24 | 1861 | Right | Precentral Gyrus | BA 6 |
| −30 | −10 | 44 | 10.53 | 1.7E-22 | 6590 | Left | Middle Frontal Gyrus | BA 6 |
a X Y and Z are coordinates in a standard stereotaxic space (Talairach & Tournoux, 1988). Positive values indicate right (X), anterior (Y), and superior (Z) to the anterior commisure.
b Volume is the number of voxels (mm3) that reached statistical significance in each volume.
c Hemi refers to Hemisphere
d BA refers to Brodmann areas.
No associated Brodmann area.
Table 2B.
Between group comparisons for early and late emotional stimuli. Coordinates are based on activity at the peak voxel.
| X | Y | Z | T-stat | p-value | Voxels | Hemi | Peak Voxel GM | BA |
|---|---|---|---|---|---|---|---|---|
| Negative EARLY | ||||||||
| Young>Old | ||||||||
| 9 | −76 | 11 | 3.7314 | 2.25E-04 | 394 | Right | Cuneus | BA 17 |
| 3 | 14 | 2 | 3.0701 | 2.32E-03 | 394 | Right | Caudate | Caudate Head |
| −3 | 29 | 11 | 3.5414 | 4.56E-04 | 477 | Left | Anterior Cingulate | BA 24 |
| −57 | 11 | 17 | 3.9119 | 1.12E-04 | 992 | Left | Inferior Frontal Gyrus | BA 44 |
| 51 | 28 | −13 | 3.9963 | 8.00E-05 | 1043 | Right | Inferior Frontal Gyrus | BA 47 |
| −2 | 56 | −1 | 2.9631 | 3.27E-03 | 314 | Left | Medial Frontal Gyrus | BA 10 |
| Old>Young | ||||||||
| −18 | −67 | −28 | −4.056 | 6.30E-05 | 4671 | Left | Pyramis | * |
| −12 | −64 | −4 | −3.362 | 8.67E-04 | 304 | Left | Culmen | * |
| 18 | −31 | −25 | −4.295 | 2.30E-05 | 2481 | Right | Culmen | * |
| 39 | −64 | −31 | −4.934 | 1.00E-06 | 7701 | Right | Cerebellar Tonsil | * |
| −27 | −52 | −10 | 4.3876 | 1.60E-05 | 1437 | Left | Declive | * |
| 24 | −76 | −7 | 5.4439 | 1.03E-07 | 3641 | Right | Lingual Gyrus | BA 18 |
| −45 | −13 | −16 | −4.411 | 1.40E-05 | 2137 | Left | Sub-Gyral | BA 20 |
| 15 | −34 | 2 | 4.1259 | 4.70E-05 | 585 | Right | Parahippocampal Gyrus | BA 27 |
| 30 | 29 | 2 | −5.088 | 6.14E-07 | 3901 | Right | Inferior Frontal Gyrus | BA 47 |
| −36 | −28 | 35 | −5.172 | 4.06E-07 | 117676 | Left | Postcentral Gyrus | BA 2 |
| Negative LATE | ||||||||
| Young>Old | ||||||||
| 42 | −49 | −19 | 5.9737 | 6.10E-09 | 45179 | Right | Culmen | * |
| 12 | −28 | −10 | 3.5941 | 3.76E-04 | 601 | Right | Culmen | * |
| 1 | −80 | −1 | 4.1003 | 5.20E-05 | 2718 | Right | Lingual Gyrus | BA 18 |
| −24 | −76 | −7 | 3.7383 | 2.19E-04 | 2318 | Left | Lingual Gyrus | BA 18 |
| −48 | −4 | −13 | 4.173 | 3.90E-05 | 1687 | Left | Middle Temporal Gyrus | BA 21 |
| 36 | −1 | −32 | 3.7488 | 2.10E-04 | 950 | Right | Middle Temporal Gyrus | BA 21 |
| 57 | −7 | −17 | 3.4528 | 6.28E-04 | 708 | Right | Inferior Temporal Gyrus | BA 21 |
| −42 | −16 | −19 | 3.2845 | 1.13E-03 | 391 | Left | Sub-Gyral | BA 20 |
| 21 | −28 | −1 | 3.0219 | 2.71E-03 | 278 | Right | Thalamus | * |
| 9 | −4 | −7 | 4.0517 | 6.40E-05 | 682 | Right | * | Hypothalamus |
| 15 | 35 | 2 | 3.2418 | 1.31E-03 | 629 | Right | Anterior Cingulate | BA 24 |
| −27 | −10 | 23 | 3.4409 | 6.56E-04 | 439 | Left | Insula | BA 13 |
| −42 | 27 | −16 | 3.9123 | 1.11E-04 | 1892 | Left | Inferior Frontal Gyrus | BA 47 |
| 24 | −7 | 47 | 3.6002 | 3.68E-04 | 1230 | Right | Middle Frontal Gyrus | BA 6 |
| 30 | 23 | 29 | 4.0358 | 6.80E-05 | 1569 | Right | Middle Frontal Gyrus | BA 9 |
| −30 | 41 | −1 | 4.0774 | 5.70E-05 | 1008 | Left | Middle Frontal Gyrus | BA 6 |
| 0 | 53 | 23 | 3.4647 | 6.02E-04 | 310 | Left | Medial Frontal Gyrus | BA 9 |
| 27 | 54 | 8 | 3.4642 | 6.03E-04 | 988 | Right | Superior Frontal Gyrus | BA 10 |
| Old>Young | ||||||||
| −45 | −37 | 32 | −3.259 | 1.24E-03 | 434 | Left | Supramarginal Gyrus | BA 40 |
| 45 | −35 | 32 | −4.193 | 3.60E-05 | 977 | Right | Inferior Parietal Lobule | BA 40 |
| −39 | 26 | 35 | −4.447 | 1.20E-05 | 3986 | Left | Precentral Gyrus | BA 9 |
| 51 | 23 | −11 | −4.433 | 1.30E-05 | 1363 | Right | Inferior Frontal Gyrus | BA 47 |
| Neutral_EARLY | ||||||||
| Young>Old | ||||||||
| 33 | −58 | 26 | 4.8041 | 2.00E-06 | 564 | Right | Sub-Gyral | BA 39 |
| 21 | −76 | −7 | 4.7224 | 3.00E-06 | 1869 | Right | Lingual Gyrus | BA 18 |
| −39 | −67 | −19 | 3.3583 | 8.78E-04 | 338 | Left | Declive | BA 10 |
| 9 | −46 | 23 | 4.2212 | 3.20E-05 | 817 | Right | Posterior Cingulate | BA 19 |
| −54 | 11 | −10 | 3.9698 | 8.90E-05 | 1381 | Left | Superior Temporal Gyrus | BA 42 |
| −63 | −16 | 8 | 3.6882 | 2.65E-04 | 557 | Left | Transverse Temporal Gyrus | * |
| 27 | −52 | −4 | 4.0745 | 5.80E-05 | 1041 | Right | Parahippocampal Gyrus | BA 38 |
| 39 | −19 | 11 | 3.0264 | 2.67E-03 | 394 | Right | Insula | BA 13 |
| 24 | 41 | 14 | 3.3002 | 1.07E-03 | 632 | Right | Medial Frontal Gyrus | BA 9 |
| 33 | 50 | −7 | 4.3473 | 1.80E-05 | 453 | Right | Middle Frontal Gyrus | BA 30 |
| 36 | 23 | 23 | 4.8408 | 2.00E-06 | 1864 | Right | Middle Frontal Gyrus | BA 30 |
| Old>Young | ||||||||
| 39 | −46 | −31 | −4.095 | 5.30E-05 | 949 | Right | Cerebellar Tonsil | * |
| −42 | −43 | −37 | −4.312 | 2.10E-05 | 611 | Left | Cerebellar Tonsil | * |
| 6 | −61 | −28 | −3.836 | 1.50E-04 | 945 | Right | Uvula | * |
| −12 | 6 | −7 | −3.795 | 1.76E-04 | 862 | Left | * | * |
| −18 | −70 | 20 | −3.588 | 3.84E-04 | 1450 | Left | Precuneus | BA 31 |
| −42 | −10 | −10 | −4.027 | 7.00E-05 | 508 | Left | Sub-Gyral | BA 21 |
| 12 | −34 | 17 | −4.651 | 5.00E-06 | 9869 | Right | Thalamus | Pulvinar |
| −27 | 57 | 14 | −3.165 | 1.70E-03 | 384 | Left | Superior Frontal Gyrus | BA 10 |
| Neutral_LATE | ||||||||
| Young>Old | ||||||||
| 21 | −31 | 23 | 3.6778 | 2.75E-04 | 382 | Right | Caudate | Caudate Tail |
| −12 | −13 | 29 | 3.473 | 5.85E-04 | 802 | Left | Caudate | Caudate Body |
| −27 | −49 | −4 | 4.0417 | 6.60E-05 | 297 | Left | Parahippocampal Gyrus | BA 19 |
| −12 | 50 | 5 | 3.7598 | 2.02E-04 | 1219 | Left | Medial Frontal Gyrus | BA 10 |
| Old>Young | ||||||||
| −42 | −68 | −22 | −3.578 | 3.98E-04 | 2133 | Left | Declive | * |
| 5 | −76 | −31 | −3.692 | 2.61E-04 | 13827 | Right | Uvula | * |
| −33 | −38 | 41 | −3.816 | 1.63E-04 | 271 | Left | Inferior Parietal Lobule | BA 40 |
| 39 | 8 | 8 | −4.639 | 5.00E-06 | 38982 | Right | Insula | BA 13 |
| −42 | 26 | 11 | −3.336 | 9.49E-04 | 341 | Left | Inferior Frontal Gyrus | BA 13 |
| −30 | 38 | 32 | −4.177 | 3.80E-05 | 77294 | Left | Middle Frontal Gyrus | BA 9 |
| Positive_EARLY | ||||||||
| Young>Old | ||||||||
| 27 | −79 | −12 | 5.4887 | 8.18E-08 | 40578 | Right | Lingual Gyrus | BA 18 |
| −48 | −34 | −1 | 4.2561 | 2.70E-05 | 3894 | Left | Middle Temporal Gyrus | * |
| 60 | −40 | −1 | 4.1723 | 3.90E-05 | 693 | Right | Middle Temporal Gyrus | BA 21 |
| 51 | −16 | 8 | 3.9743 | 8.70E-05 | 2666 | Right | Superior Temporal Gyrus | BA 22 |
| −48 | 14 | 8 | 3.7025 | 2.51E-04 | 775 | Left | Precentral Gyrus | BA 44 |
| −21 | −64 | 5 | 3.6578 | 2.97E-04 | 1004 | Left | Posterior Cingulate | BA 30 |
| −3 | −1 | 5 | 3.6728 | 2.81E-04 | 419 | Left | Thalamus | Anterior Nucleus |
| −9 | 8 | 17 | 3.5619 | 4.23E-04 | 1409 | Left | Caudate | Caudate Body |
| 3 | 32 | 26 | 3.4636 | 6.05E-04 | 2969 | Right | Cingulate Gyrus | BA 32 |
| −39 | 35 | −1 | 3.7109 | 2.43E-04 | 1908 | Left | Inferior Frontal Gyrus | BA 47 |
| 30 | 17 | −13 | 4.157 | 4.10E-05 | 16792 | Right | Inferior Frontal Gyrus | BA 47 |
| −33 | 47 | 17 | 3.7496 | 2.10E-04 | 766 | Left | Middle Frontal Gyrus | BA 10 |
| −24 | 38 | −1 | 3.6653 | 2.89E-04 | 403 | Left | Medial Frontal Gyrus | BA 10 |
| −21 | 41 | 29 | 3.5501 | 4.42E-04 | 1080 | Left | Superior Frontal Gyrus | BA 9 |
| Old>Young | ||||||||
| 18 | −31 | 29 | −3.805 | 1.70E-04 | 1037 | Right | Cingulate Gyrus | BA 32 |
| Positive_LATE | ||||||||
| Young>Old | ||||||||
| 30 | 11 | −29 | 4.2489 | 2.80E-05 | 806 | Right | Superior Temporal Gyrus | BA 38 |
| −33 | 5 | −22 | 4.2454 | 2.90E-05 | 617 | Left | Superior Temporal Gyrus | BA 38 |
| 30 | −40 | 5 | 3.6057 | 3.60E-04 | 733 | Right | Sub-Gyral | Hippocampus |
| Old>Young | ||||||||
| 57 | −28 | 14 | −5.274 | 2.45E-07 | 281214 | Right | Superior Temporal Gyrus | BA 42 |
| 6 | −40 | 11 | −3.581 | 3.95E-04 | 1138 | Right | Posterior Cingulate | BA 29 |
| 12 | −13 | −1 | −4.139 | 4.50E-05 | 1336 | Right | Thalamus | * |
| −18 | 29 | −1 | −2.928 | 3.66E-03 | 338 | Left | Anterior Cingulate | BA 24 |
aX, Y and Z are coordinates in a standard stereotaxic space (Talairach & Tournoux, 1988). Positive values indicate right (X) anterior (Y), and superior (Z) to the anterior commisure.
bVolume is the number of voxels (mm3) that reached statistical significance in each volume.
cHemi refers to Hemisphere
dBA refers to Brodmann areas.
No associated Brodmann area.
Analyses of activity specific to an emotion within each group (e.g. negative - neutral or positive - neutral) are shown in Supplementary Table 2. To summarize this data: the young showed more activity to negative and positive pictures as compared to neutral pictures. The old activated more regions to negative, but not positive pictures, as compared to neutral pictures. The young activated more and larger regions in response to negative as compared to positive pictures. The old activated one large region centered in the thalamus to negative as compared to positive picture, but also activated more regions, including frontal regions to positive as compared to negative pictures (Supplementary Table 3). These qualitative whole brain analyses were followed up with ROI analyses.
3.2.2. ROI Analysis
Negative, Positive and Neutral vs. Baseline
Younger subjects had more active voxels in the amygdala than the old (F1,26= 4.05, p<.05; Figure 4D); a result that is similar to that found in the whole-brain analysis. Activation did not differ across valence and there was no interaction (ps> .10). However, individual exploratory comparisons suggest that the young activate more voxels than the old in response to negative (t26= 2.07, p< .05) and positive (t26= 2.12, p<.05), but not neutral pictures.
The number of active voxels in the DLPFC ROI did not differ between the young and the old. The number of active voxels differed across valence categories (F2,68=7.06, p <0.01). Across all participants negative pictures elicited more activity than did neutral (t35=2.58, p <.014) or positive pictures (t35=3.93, p <.001; Figure 4E), which did not differ from each other. Activation across the valence categories was similar for old and young as the interaction was not significant (p> .10). A post-hoc exploratory within-group analysis showed that the young had similar amounts of DLPFC activity to negative, neutral, and positive pictures (p > .10) whereas the activity differed in the old across valence categories (F2,42=7.97, p <.001). The old activated more voxels in DLPFC to negative pictures than to neutral (t21=2.76, p <.01) or positive (t21=3.65, p <.01) pictures, which did not differ from each other (Figure 4E). Indeed a direct comparison of the young and old for each valence category shows that the old activate the DLPFC more to negative pictures (t33.98=2.22, P =.03) than the young but the groups do not differ in activity for neutral or positive pictures. There were no between or within subject differences, nor any interactions for the volume of activity within OFC (Figure 4F).
Emotion: Negative/Positive vs. Neutral
The young and old did not differ in number of active voxels among valences in the amygdala, DLPFC or OFC. Overall, the number of active voxels within the DLPFC (F1,34 =6.05, p<.02) and OFC (F1,34 =4.74, p<.04) differed among the valences, but not in the amygdala (Supplemental Table 2). Within the DLPFC, negative pictures elicited greater activity than positive pictures (p<.02; Supplemental Table 3). The opposite pattern was seen in the OFC; positive pictures elicited greater activity than negative (p<.04). There were no interactions between age-group and valence for any ROI.
3.2.3. ROI Analysis
Habituation (Early vs. Late)
There were no main effects or interactions of habituation in the amygdala, as measured by number of active voxels. These results remained the same when amygdala habituation was corrected for differences in overall amygdala activity between old and young. Habituation of the number of active voxels did not differ between groups or among valence categories in the DLPFC. However, there was an interaction between age group and valence category (F1.636, 55.62=3.24, p<.05). Sphericity could not be assumed (Mauchly's W= .77, p<.01), thus a Greenhouse-Geisser correction was used. Post-hoc analysis showed that the young had similar habituation to all three valence categories (p>.31). The old showed marginal habituation across the three valences (p=.09), with more habituation to negative as compared to positive pictures (p=.08), but no differences between negative and neutral or positive and neutral pictures. Within the DLPFC, post hoc t-tests show that the old had more habituation to repeated negative pictures (t28.03=2.26, p<.05) than the young (Figure 4H). There were no differences in habituation between the young and old to neutral or positive pictures.
The difference among valence categories in DLPFC habituation in the old could be the result of comparable activity of each valence in the early phase but failure to maintain activity only for negative scenes. Alternatively, it may be there is more DLPFC activity in the early phase to negative than positive or neutral scenes but that the greater activity is not maintained. To investigate potential differences in early or late phase activity we used within-group paired t-tests. These results are shown in Figure 5. The young showed no valence differences in early or late phase recruitment within the DLPFC (all ps>.10). The old showed greater activity in the DLPFC in the early phase to negative scenes as compared to neutral (t21=3.01,p=.007) or positive (t21=5.42, p<.001) scenes; positive and neutral early phase activity did not differ. There were no valence differences in late phase activity within the old (all ps>.10).
Figure 5.
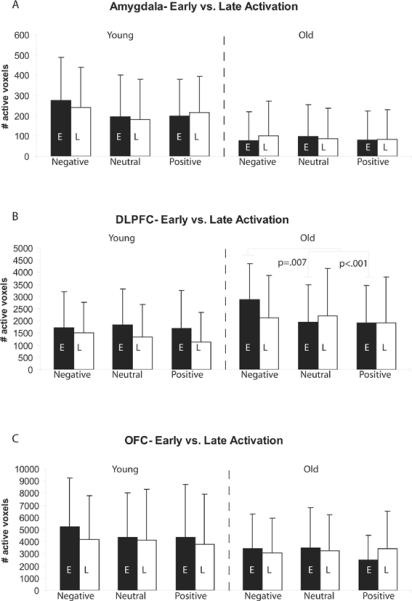
Means and standard deviations for the number of active voxels to early (black bars) and late (white bars) emotional scenes as compared to baseline in young and old. The old, but not the young show more activation of the DLPFC during early emotional scenes as compared to neutral or positive scenes. However, this activity to negative in the old is not maintained during late emotional scenes resulting in habituation.
There were no differences in habituation between the young and old in the OFC using the number of active voxels as the measure. There was a significant difference in habituation across the three valence categories (F1.72,58.67=4.91, p<0.01). Sphericity could not be assumed (Mauchly's W= .84, p<.06), thus a Greenhouse-Geisser correction was used. Post hoc t-tests showed that there was more habituation for negative than neutral (t35=2.32, p<.026) or positive (t35 =2.83, p<.008) pictures but no difference in habituation between neutral and positive pictures (Figure 4). There were no interactions between age group and valence category.
Percent Signal Change
Percent signal change data is displayed in Supplemental Table 3. There were no group, valence or significant interactions for percent change signal in the amygdala or OFC. Percent signal change within the DLPFC differed across the valences (F2,68 =9.04, p<.01), but there were no difference between groups nor any interactions. Within the DLPFC, percent signal change to negative pictures was greater than neutral (t35=2.80, p<.01) or positive pictures (t35=4.52, p<.01), which did not differ from each other. There were no group, valence, or significant interactions for the habituation measure in the amygdala (Figure 5D), DLPFC or OFC. There were no differences in percent signal change between early and late blocks of emotional stimuli.
Due to the high variability observed in percent signal change measures effect sizes between each group for each ROI were calculated. Effect size measures indicate a moderate effect of age in the amygdala (d'=0.45; young>old), DLPFC (d'= −0.34; old>young) and OFC (d'=.31; young>old) to negative scenes. Effect sizes between groups for neutral and positive scenes were smaller (d'≤.27; Supplemental Table 4).
3.2.4 Comparison among stimulus blocks
Since the blocks of emotional pictures were not fully counterbalanced we assessed brain activity during the first and last block of each valence category to ensure this was not affecting our results. There was a difference in number of active voxels between the first and last negative blocks (last block>first block), but not neutral or positive blocks. This difference was present in all three ROI's amygdala (p=.05), DLPFC (p=.01) and OFC (p=.02) and was true for both young and old. There was no interaction with age group.
3.2.5. Relationship among regions of activation
The correlations reported here are based on number of active voxels. Percent signal change correlations are detailed in Supplemental material #4. In the old, activity in the amygdala to positive pictures during the late phase, was correlated with activity to positive pictures in the OFC in the late phase (r=.72, p<.003), but no other correlations between regions for negative or neutral pictures were significant. Activity in the young was correlated between late DLPFC and late OFC for positive and neutral (ps<.001), but not negative pictures. No other correlations were found in the young.
There were no correlations among brain regions for habituation in the old. In young, habituation to negative pictures in the amygdala was correlated with habituation in the OFC (r=.95, p<.001), but not for the DLPFC; habituation to neutral (r=.81, p<.001) and positive (r=.72, p<.009) pictures was correlated between the amygdala and DLPFC, but not OFC.
3.2.6. Relationship among regions of activation and behavior
There were no correlations among regions of activation and behavioral measures for either young or old.
4. Discussion
We found that the old have reduced amygdala activity compared to the young and no differential activity by valence, despite highly arousing negative pictures. This was accompanied by more prefrontal activity in the old for early negative scenes that then habituated. The old, but not the young, had more activity in the DLPFC in the early phase of negative, but not neutral or positive scenes. The young did not show differential habituation among the valences in the DLPFC. In addition, exploratory analyses suggested that the young do not have differential prefrontal activity for negative, neutral and positive scenes whereas the old do, with negative scenes inducing more activity than neutral or positive scenes. To our knowledge, this is the first paper that simultaneously quantified age-related changes in the amygdala and prefrontal cortex in response to emotional pictures and examined whether activity was maintained over time.
The findings confirm previous studies that show less amygdala or more prefrontal activity in aging and add new insights about aging and emotion. It is not solely that the old activate the amygdala less (Gunning-Dixon et al., 2003;Iidaka et al., 2002;Tessitore et al., 2005;Williams et al., 2006), and the PFC more, as they do in many situations (Cabeza et al., 2002;Grady et al., 1994;Grady et al., 1998;Reuter-Lorenz et al., 2000), but that activity is sustained or declines depending on the valence and arousal quality of the stimuli. These results suggest a reorganization of the neural processing of emotion in aging. The loss of emotion-induced amygdala activity and the amplified activity in prefrontal cortex, particularly for negative stimuli, suggests that the prefrontal cortex and not the amygdala registers emotion in aging. We suggest this changes the character of emotional perceptions. The prefrontal cortex has an evaluative role in cognition; assessing multimodal information and planning actions (Wood & Grafman, 2003). This is quite different than the amygdala's registration of emotion. These prefrontal functions would permit it to regulate emotion in aging, as described by others (Gunning-Dixon et al., 2003 Iidaka et al., 2002; Tessitore et al., 2005) and our data suggests that this did not occur in the young.
Still unclear is whether the pattern of activity is due to top down inhibition of the amygdala or as a compensatory response to loss of amygdala function. Our correlations among brain regions and between brain activity and behavior did not aid in resolving this issue. Our findings are consistent with “top-down” cognitive control, or emotional regulation (Mather & Knight, 2005). Indeed, higher activity of the prefrontal cortex is linked to voluntary suppression of sadness in healthy young women (Levesque et al., 2003) suggesting that prefrontal activity can regulate emotional state. Alternatively, there are ample studies that suggest higher prefrontal activity in aging is compensatory and in some cases is associated with cognitive improvements that normalize performance to that of the young (Grady et al., 1995, Cabeza et al., 1997, Rypma & D'Esposito 2000, Persson et al., 2004). One possibility then, is that higher prefrontal activity, whether due to top down or bottom up mechanisms, improves cognition in the elderly through better control of a variety of processes including attention, but that it qualitatively modifies emotion, decreasing negative affect.
This scenario leaves two further questions; why does the signal habituate and why is it selective to negative stimuli? We showed that the higher cognitive control was short-lived, at least as shown by brain activity. Our finding of habituation only in the PFC, not the amygdala, and only to negative pictures is in contrast to previous studies in younger adults that found habituation in the amygdala to repeated negative and positive emotional faces (Breiter et al., 1996;Fischer et al., 2000;Fischer et al., 2003;Phillips et al., 2001;Wright et al., 2001), and in older adults who had similar habituation to neutral faces as the young (Wedig et al., 2005). Higher arousal for the negative pictures may explain the higher prefrontal activity that habituates, but would not explain the lower activity of the amygdala in the elderly. It may also be that pictures of emotional scenes have special features. Indeed, face stimuli are not as arousing as picture stimuli when directly compared (Britton et al., 2006). It also does not explain differences between the old and young as both groups received the same stimuli and reported similar behavioral ratings of these stimuli. One explanation is that the elderly have a reduced ability to maintain effortful controlled processes (Braver et al., 2001;Braver et al., 2005;Braver and Barch, 2002;Craik et al., 1990;Daigneault and Braun, 1993;Paxton et al., 2008;Rush et al., 2006;Salthouse et al., 1989), in this case for highly arousing negative stimuli, and this results in valence specific habituation. Most of the studies that have shown “compensatory” higher prefrontal activity (Grady et al., 1995, Cabeza et al., 1997, Rypma & D'Esposito 2000, Persson et al., 2004) did not examine whether the higher activity was maintained. However, one study found that older adults failed to maintain prefrontal activity during the maintenance phase of a working memory task due to difficulty in maintaining goal-relevant information (Paxton et al., 2008). A second explanation is that the enhanced cognitive control permits a shift of attention to positive stimuli and thus response to the negative stimuli fades and attention is allocated toward stimuli of more emotional salience (i.e. positive). For example, previous studies show that the old use their attention resources in favor of positive information as compared to negative (Mather and Knight, 2005) especially over long periods of time (Isaacowitz et al., 2009). A switch in attention driven by failure to maintain a response to negative emotions, particularly without valence specific input from the limbic system (e.g. amygdala) may drive the old toward positive emotional stimuli. The neural source of habituation remains unknown. It could be due to inhibition from another region, or lack of maintenance of neural activity. Additional studies will be able to elucidate these issues.
We did not measure anatomical volume of the amygdala or PFC regions in this study, but we know from our own work, and studies by others, that both regions atrophy with aging (Allen et al., 2005;Raz et al., 2004;Salat et al., 1999a;Salat et al., 1999b). We do not believe atrophy within these regions explains the differential fMRI signal among regions. We showed activity is less in the amygdala but greater in the PFC, whereas both structures atrophy. We would not expect atrophy to result in changes in activity in opposite directions across regions. In addition, the age-related differences are among valence categories within a region. Thus, only a very complicated explanation (with little empirical evidence) would support atrophic changes alone as an underlying mechanism. For example, the explanation would have to propose that atrophy in the amygdala results in less activity, while prefrontal atrophy results in amplification of activity, but failure to maintain activity for only the negative valence category. Thus, some combination of atrophic changes, the underlying role of these structures in emotion, and the interaction among regions likely underlies the age-related changes described.
We did not find age-related effects on arousal ratings and we only found valence rating differences in exploratory analyses in this study. We suspect this is due to the choice of highly arousing negative pictures, which was done to ensure we would obtain amygdala activity in the elderly. This may override more subtle changes in behavioral ratings of negative versus positive stimuli that we and others have reported (Charles et al., 2003;Leigland et al., 2004;Mather et al., 2004;Mather and Carstensen, 2003). It may also override the ability to maintain cognitive control and thus behaviorally, the old perceived the stimuli similarly to the young.
Our fMRI data where we show age-related changes in the extent of activation but not percent signal change is in contrast to previous studies that showed higher percent signal change for negative stimuli in the amygdala (Breiter et al., 1996;Fischer et al., 2000;Fischer et al., 2003;Phillips et al., 2001;Wedig et al., 2005;Wright et al., 2001). Effect size comparison of the percent signal change data shows that the young have modestly higher percent signal change as compared to the old in the amygdala and the old have a modestly higher signal change in the DLPFC as compared to the young. However, examination of our time course data suggests that there is greater variability of the BOLD signal in the old and this may preclude findings of group differences (see Supplementary Table 4). Our study suggests that the old continue to use similar brain regions for emotion processing as the young (amygdala and PFC), but utilize a larger extent of the PFC to evaluate emotional stimuli as compared to the young. Future studies should further examine this relationship.
A few limitations should be noted when interpreting the results of the current study. The order of presentation of pictures was not completely counterbalanced. However, an order effect would most likely have resulted in finding greater habituation to neutral stimuli as those two blocks were contiguous, which we did not find. The sample size for the ROI analysis of the amygdala was limited due in part to susceptibility artifact during MRI acquisition. Still, our subject numbers are within the range of other studies of amygdala activity (Fischer et al., 2005a;Iidaka et al., 2002;Tessitore et al., 2005;Wright et al., 2001), and our findings of little activity in the elderly is the same as others have found (Gunning-Dixon et al., 2003, Iidaka et al., 2002, Tessitore et al., 2005). A study with additional participants in the future would help verify these findings. Finally, this is a cohort study, and while the young and old were matched for intellect and educational attainment, there may be other factors that covary with age and mediate the brain activity differences we found.
The unique aspect of this study is that we show habituation to a valence category, and that there is differential response and habituation among emotional valence categories between young and old. In this study the elderly modulated their responses to the class of stimuli that were highly arousing and negative. While it is clear that the precise mechanism underlying the age-related changes remains elusive, a shift in the neural basis of emotion to a dominate role of the prefrontal cortex occurs with aging. We suggest this shift, and whether prefrontal activity is maintained depending on the valence or arousal quality of the stimuli, results in age-related changes in the perception of emotional events.
Supplementary Material
Acknowledgements
The authors thank Laura Young for her assistance with data collection and Mathew Snodgrass for his assistance with data processing. We also thank the Advanced Imaging Research Center at OHSU for their support of the neuroimaging in this study, and funding agencies: National Institutes of Health (AG12611 (J.J.), AG18843 (J.J.), Neuroscience of Aging Training Grant (T32 AG023477 to D.R.). Multidisciplinary Training Grant in Neuroscience (5T32NS007466-08 to T.P.), National Defense Science and Engineering Graduate Fellowship funded by the Department of Defense (T.P.), and ARCS Foundation, Inc. Portland Chapter (T.P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement The authors certify that they have no actual or potential conflicts of interest including any financial, personal or other relationships with other people or organizations within three years of beginning the work submitted that could inappropriately influence our work. This experiment was approved for ethical treatment of human participants by the Institutional Review Board at Oregon Health & Science University and all participants provided written consent.
REFERENCES
- Adams RB, Jr., Gordon HL, Baird AA, Ambady N, Kleck RE. Effects of gaze on amygdala sensitivity to anger and fear faces. Science. 2003;300:1536. doi: 10.1126/science.1082244. [DOI] [PubMed] [Google Scholar]
- Addis DR, Leclerc CM, Muscatell K, Kensinger EA. There are age-related changes in neural connectivity during the encoding of positive, but not negative, information. Cortex. 2009 doi: 10.1016/j.cortex.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Cahill L, Schul R, Babinsky R. Impaired declarative memory for emotional material following bilateral damage in humans. Learning and Memory. 1997;4:291–300. doi: 10.1101/lm.4.3.291. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D. Impaired judgments of sadness but not happiness following bilateral amygdala damage. J.Cogn Neurosci. 2004;16:453–462. doi: 10.1162/089892904322926782. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Shaw C, Gaffan EA. The performance of postencephalitic amnesic subjects on two behavioural tests of memory: concurrent discrimination learning and delayed matching-to-sample. Cortex. 1992;28:359–372. doi: 10.1016/s0010-9452(13)80146-3. [DOI] [PubMed] [Google Scholar]
- Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: the major lobes and a parcellation of the temporal region. Neurobiol.Aging. 2005;26:1245–1260. doi: 10.1016/j.neurobiolaging.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 1995;57:289–300. [Google Scholar]
- Braver TS, Barch DM. A theory of cognitive control, aging cognition, and neuromodulation. Neurosci.Biobehav.Rev. 2002;26:809–817. doi: 10.1016/s0149-7634(02)00067-2. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Keys BA, Carter CS, Cohen JD, Kaye JA, Janowsky JS, Taylor SF, Yesavage JA, Mumenthaler MS, Jagust WJ, Reed BR. Context processing in older adults: evidence for a theory relating cognitive control to neurobiology in healthy aging. J.Exp.Psychol.Gen. 2001;130:746–763. [PubMed] [Google Scholar]
- Braver TS, Satpute AB, Rush BK, Racine CA, Barch DM. Context processing and context maintenance in healthy aging and early stage dementia of the Alzheimer's type. Psychol.Aging. 2005;20:33–46. doi: 10.1037/0882-7974.20.1.33. [DOI] [PubMed] [Google Scholar]
- Breiter H, Etcoff N, Whalen P, Kennedy W, Rauch S, Buckner R, Strauss M, Hyman S, Rosen B. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Britton JC, Taylor SF, Sudheimer KD, Liberzon I. Facial expressions and complex IAPS pictures: common and differential networks. Neuroimage. 2006;31:906–919. doi: 10.1016/j.neuroimage.2005.12.050. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Grady CL, Nyberg L, McIntosh AR, Tulving E, Kapur S, Jennings JM, Houle S, Craik FI. Age-related differences in neural activity during memory encoding and retrieval: a positron emission tomography study. J.Neurosci. 1997;17:391–400. doi: 10.1523/JNEUROSCI.17-01-00391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder AJ, Lawrence AD, Young AW. Neuropsychology of fear and loathing. Nat.Rev.Neurosci. 2001;2:352–363. doi: 10.1038/35072584. [DOI] [PubMed] [Google Scholar]
- Carstensen LL. The influence of a sense of time on human development. Science. 2006;312:1913–1915. doi: 10.1126/science.1127488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen LL. Evidence for a life-span theory of socioemotional selectivity. Current Directions in Psychological Science. 1995;4:151–155. doi: 10.1177/09637214211011468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen LL, Isaacowitz DM, Charles ST. Taking time seriously. A theory of socioemotional selectivity. Am.Psychol. 1999;54:165–181. doi: 10.1037//0003-066x.54.3.165. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, Pasupathi M, Mayr U, Nesselroade JR. Emotional experience in everyday life across the adult life span. J.Pers.Soc.Psychol. 2000;79:644–655. [PubMed] [Google Scholar]
- Charles ST, Mather M, Carstensen LL. Aging and emotional memory: the forgettable nature of negative images for older adults. J.Exp.Psychol.Gen. 2003;132:310–324. doi: 10.1037/0096-3445.132.2.310. [DOI] [PubMed] [Google Scholar]
- Cook IA, Bookheimer SY, Mickes L, Leuchter AF, Kumar A. Aging and brain activation with working memory tasks: an fMRI study of connectivity. Int.J.Geriatr.Psychiatry. 2007;22:332–342. doi: 10.1002/gps.1678. [DOI] [PubMed] [Google Scholar]
- Craik FI, Morris RG, Gick M. Adult age differences in working memory. In: Vallar G, Shallice T, editors. Neuropsychological impairments of short-term memory. Cambridge University Press; Cambridge: 1990. pp. 247–267. [Google Scholar]
- Daigneault S, Braun CM. Working memory and the Self-Ordered Pointing Task: further evidence of early prefrontal decline in normal aging. J.Clin.Exp.Neuropsychol. 1993;15:881–895. doi: 10.1080/01688639308402605. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol.Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior-anterior shift in aging. Cereb.Cortex. 2008;18:1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H, Furmark T, Wik G, Fredrikson M. Brain representation of habituation to repeated complex visual stimulation studied with PET. Neuroreport. 2000;11:123–126. doi: 10.1097/00001756-200001170-00024. [DOI] [PubMed] [Google Scholar]
- Fischer H, Sandblom J, Gavazzeni J, Fransson P, Wright CI, Backman L. Age-differential patterns of brain activation during perception of angry faces. Neurosci.Lett. 2005b;386:99–104. doi: 10.1016/j.neulet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Fischer H, Sandblom J, Gavazzeni J, Fransson P, Wright CI, Backman L. Age-differential patterns of brain activation during perception of angry faces. Neurosci.Lett. 2005a;386:99–104. doi: 10.1016/j.neulet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Fischer H, Wright CI, Whalen PJ, McInerney SC, Shin LM, Rauch SL. Brain habituation during repeated exposure to fearful and neutral faces: a functional MRI study. Brain Res.Bull. 2003;59:387–392. doi: 10.1016/s0361-9230(02)00940-1. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a clustersize threshold. Magn Reson.Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Gale GD, Anagnostaras SG, Godsil BP, Mitchell S, Nozawa T, Sage JR, Wiltgen B, Fanselow MS. Role of the basolateral amygdala in the storage of fear memories across the adult lifetime of rats. J.Neurosci. 2004;24:3810–3815. doi: 10.1523/JNEUROSCI.4100-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Maisog JM, Horwitz B, Ungerleider LG, Mentis MJ, Salerno JA, Pietrini P, Wagner E, Haxby JV. Age-related changes in cortical blood flow activation during visual processing of faces and location. J.Neurosci. 1994;14:1450–1462. doi: 10.1523/JNEUROSCI.14-03-01450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Bookstein F, Horwitz B, Rapoport SI, Haxby JV. Age-related changes in regional cerebral blood flow during working memory for faces. Neuroimage. 1998;8:409–425. doi: 10.1006/nimg.1998.0376. [DOI] [PubMed] [Google Scholar]
- Grieve SM, Clark CR, Williams LM, Peduto AJ, Gordon E. Preservation of limbic and paralimbic structures in aging. Hum.Brain Mapp. 2005;25:391–401. doi: 10.1002/hbm.20115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Gur RC, Perkins AC, Schroeder L, Turner T, Turetsky BI, Chan RM, Loughead JW, Alsop DC, Maldjian J, Gur RE. Age-related differences in brain activation during emotional face processing. Neurobiol.Aging. 2003;24:285–295. doi: 10.1016/s0197-4580(02)00099-4. [DOI] [PubMed] [Google Scholar]
- Gur RC, Skolnick BE, Gur RE. Effects of emotional discrimination tasks on cerebral blood flow: regional activation and its relation to performance. Brain Cogn. 1994;25:271–286. doi: 10.1006/brcg.1994.1036. [DOI] [PubMed] [Google Scholar]
- Hamann S, Herman RA, Nolan CL, Wallen K. Men and women differ in amygdala response to visual sexual stimuli. Nat.Neurosci. 2004;7:411–416. doi: 10.1038/nn1208. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage. 2002;17:317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biol.Psychiatry. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Herrington JD, Mohanty A, Koven NS, Fisher JE, Stewart JL, Banich MT, Webb AG, Miller GA, Heller W. Emotion-modulated performance and activity in left dorsolateral prefrontal cortex. Emotion. 2005;5:200–207. doi: 10.1037/1528-3542.5.2.200. [DOI] [PubMed] [Google Scholar]
- Houtveen JH, Rietveld S, Schoutrop M, Spiering M, Brosschot JF. A repressive coping style and affective, facial and physiological responses to looking at emotional pictures. Int.J.Psychophysiol. 2001;42:265–277. doi: 10.1016/s0167-8760(01)00150-7. [DOI] [PubMed] [Google Scholar]
- Iidaka T, Matsumoto A, Ozaki N, Suzuki T, Iwata N, Yamamoto Y, Okada T, Sadato N. Volume of left amygdala subregion predicted temperamental trait of harm avoidance in female young subjects. A voxel-based morphometry study. Brain Res. 2006;1125:85–93. doi: 10.1016/j.brainres.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Iidaka T, Okada T, Murata T, Omori M, Kosaka H, Sadato N, Yonekura Y. Age-related differences in the medial temporal lobe responses to emotional faces as revealed by fMRI. Hippocampus. 2002;12:352–362. doi: 10.1002/hipo.1113. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum.Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P, Bradley M, Cuthbert B. The International Affective Picture System (IAPS): Instruction manual and affective ratings. The Center for Research in Psychophysiology; University of Florida: 1999. [Google Scholar]
- Lawton MP, Kleban MH, Rajagopal D, Dean J. Dimensions of affective experience in three age groups. Psychol.Aging. 1992;7:171–184. doi: 10.1037//0882-7974.7.2.171. [DOI] [PubMed] [Google Scholar]
- Leclerc CM, Kensinger EA. Age-related differences in medial prefrontal activation in response to emotional images. Cogn Affect.Behav.Neurosci. 2008;8:153–164. doi: 10.3758/cabn.8.2.153. [DOI] [PubMed] [Google Scholar]
- Leigland LA, Schulz LE, Janowsky JS. Age related changes in emotional memory. Neurobiol.Aging. 2004;25:1117–1124. doi: 10.1016/j.neurobiolaging.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Carstensen LL, Gottman JM. The influence of age and gender on affect, physiology, and their interrelations: a study of long-term marriages. J.Pers.Soc.Psychol. 1994;67:56–68. doi: 10.1037//0022-3514.67.1.56. [DOI] [PubMed] [Google Scholar]
- Levesque J, Eugene F, Joanette Y, Paquette V, Mensour B, Beaudoin G, Leroux JM, Bourgouin P, Beauregard M. Neural circuitry underlying voluntary suppression of sadness. Biol.Psychiatry. 2003;53:502–510. doi: 10.1016/s0006-3223(02)01817-6. [DOI] [PubMed] [Google Scholar]
- Mather M, Canli T, English T, Whitfield S, Wais P, Ochsner K, Gabrieli JD, Carstensen LL. Amygdala responses to emotionally valenced stimuli in older and younger adults. Psychol.Sci. 2004;15:259–263. doi: 10.1111/j.0956-7976.2004.00662.x. [DOI] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. Aging and attentional biases for emotional faces. Psychol.Sci. 2003;14:409–415. doi: 10.1111/1467-9280.01455. [DOI] [PubMed] [Google Scholar]
- Mather M, Knight M. Goal-directed memory: the role of cognitive control in older adults' emotional memory. Psychol.Aging. 2005;20:554–570. doi: 10.1037/0882-7974.20.4.554. [DOI] [PubMed] [Google Scholar]
- Mroczek DK, Kolarz CM. The effect of age on positive and negative affect: a developmental perspective on happiness. J.Pers.Soc.Psychol. 1998;75:1333–1349. doi: 10.1037//0022-3514.75.5.1333. [DOI] [PubMed] [Google Scholar]
- Mu Q, Xie J, Wen Z, Weng Y, Shuyun Z. A quantitative MR study of the hippocampal formation, the amygdala, and the temporal horn of the lateral ventricle in healthy subjects 40 to 90 years of age. AJNR Am.J.Neuroradiol. 1999;20:207–211. [PMC free article] [PubMed] [Google Scholar]
- Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: a meta-analysis. Cogn Affect.Behav.Neurosci. 2003;3:207–233. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J.Cogn Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Paxton JL, Barch DM, Racine CA, Braver TS. Cognitive control, goal maintenance, and prefrontal function in healthy aging. Cereb.Cortex. 2008;18:1010–1028. doi: 10.1093/cercor/bhm135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Sylvester CY, Nelson JK, Welsh KM, Jonides J, Reuter-Lorenz PA. Selection requirements during verb generation: differential recruitment in older and younger adults. Neuroimage. 2004;23:1382–1390. doi: 10.1016/j.neuroimage.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Phan KL, Taylor SF, Welsh RC, Decker LR, Noll DC, Nichols TE, Britton JC, Liberzon I. Activation of the medial prefrontal cortex and extended amygdala by individual ratings of emotional arousal: a fMRI study. Biol.Psychiatry. 2003;53:211–215. doi: 10.1016/s0006-3223(02)01485-3. [DOI] [PubMed] [Google Scholar]
- Phelps E, LaBar K, Spencer D. Memory for emotional words following unilateral temporal lobectomy. Brain and Cognition. 1997;35:85–109. doi: 10.1006/brcg.1997.0929. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol.Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Medford N, Young AW, Williams L, Williams SC, Bullmore ET, Gray JA, Brammer MJ. Time courses of left and right amygdalar responses to fearful facial expressions. Hum.Brain Mapp. 2001;12:193–202. doi: 10.1002/1097-0193(200104)12:4<193::AID-HBM1015>3.0.CO;2-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL. Prefrontal cortical networks related to visceral function and mood. Ann.N.Y.Acad.Sci. 1999;877:383–96. doi: 10.1111/j.1749-6632.1999.tb09278.x. 383–396. [DOI] [PubMed] [Google Scholar]
- Rand-Giovannetti E, Chua EF, Driscoll AE, Schacter DL, Albert MS, Sperling RA. Hippocampal and neocortical activation during repetitive encoding in older persons. Neurobiol.Aging. 2006;27:173–182. doi: 10.1016/j.neurobiolaging.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Head D, Kennedy KM, Acker JD. Differential aging of the medial temporal lobe: a study of a five-year change. Neurology. 2004;62:433–438. doi: 10.1212/01.wnl.0000106466.09835.46. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, Koeppe RA. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J.Cogn Neurosci. 2000;12:174–187. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- Rolls ET. Brain Mechanisms of emotion and decision-making. International Congress Series. 2006;1291:3–13. [Google Scholar]
- Rush BK, Barch DM, Braver TS. Accounting for cognitive aging: context processing, inhibition or processing speed? Neuropsychol.Dev.Cogn B Aging Neuropsychol.Cogn. 2006;13:588–610. doi: 10.1080/13825580600680703. [DOI] [PubMed] [Google Scholar]
- Rypma B, D'Esposito M. Isolating the neural mechanisms of age-related changes in human working memory. Nat.Neurosci. 2000;3:509–515. doi: 10.1038/74889. [DOI] [PubMed] [Google Scholar]
- Salat D, Kaye J, Janowsky J. Selective preservation and degeneration of the prefrontal cortex in aging and Alzheimer's disease. Archives of Neurology. 2001;58:1403–1408. doi: 10.1001/archneur.58.9.1403. [DOI] [PubMed] [Google Scholar]
- Salat D, Stangl P, Kaye J, Janowsky J. Sex differences in prefrontal volume with aging and Alzheimer's disease. Neurobiol.Aging. 1999a;20:591–596. doi: 10.1016/s0197-4580(99)00067-6. [DOI] [PubMed] [Google Scholar]
- Salat DH, Kaye JA, Janowsky JS. Prefrontal gray and white matter volumes in healthy aging and Alzheimer disease. Arch.Neurol. 1999b;56:338–344. doi: 10.1001/archneur.56.3.338. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Mitchell DR, Skovronek E, Babcock RL. Effects of adult age and working memory on reasoning and spatial abilities. J.Exp.Psychol.Learn.Mem.Cogn. 1989;15:507–516. doi: 10.1037//0278-7393.15.3.507. [DOI] [PubMed] [Google Scholar]
- Jacques PL, Bessette-Symons B, Cabeza R. Functional neuroimaging studies of aging and emotion: Fronto-amygdalar differences during emotional perception and episodic memory. J.Int.Neuropsychol.Soc. 2009a:1–7. doi: 10.1017/S1355617709990439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques PL, Dolcos F, Cabeza R. Effects of aging on functional connectivity of the amygdala for subsequent memory of negative pictures: a network analysis of functional magnetic resonance imaging data. Psychol.Sci. 2009b;20:74–84. doi: 10.1111/j.1467-9280.2008.02258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Thieme Medical; New York: 1988. [Google Scholar]
- Tessitore A, Hariri AR, Fera F, Smith WG, Das S, Weinberger DR, Mattay VS. Functional changes in the activity of brain regions underlying emotion processing in the elderly. Psychiatry Res. 2005;139:9–18. doi: 10.1016/j.pscychresns.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 2006;26:4415–25. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale - Revised. Psychological Corporation; New York: 1981. [Google Scholar]
- Wedig MM, Rauch SL, Albert MS, Wright CI. Differential amygdala habituation to neutral faces in young and elderly adults. Neurosci.Lett. 2005;385:114–119. doi: 10.1016/j.neulet.2005.05.039. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL. A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion. 2001;1:70–83. doi: 10.1037/1528-3542.1.1.70. [DOI] [PubMed] [Google Scholar]
- Whalen P, Rauch S, Etcoff N, McInerney S, Lee M, Jenike M. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, Brown KJ, Palmer D, Liddell BJ, Kemp AH, Olivieri G, Peduto A, Gordon E. The mellow years?: neural basis of improving emotional stability over age. J.Neurosci. 2006;26:6422–6430. doi: 10.1523/JNEUROSCI.0022-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CI, Dickerson BC, Feczko E, Negeira A, Williams D. A Functional Magnetic Resonance Imaging Study of Amygdala Responses to Human Faces in Aging and Mild Alzheimer's Disease. Biol.Psychiatry. 2007 doi: 10.1016/j.biopsych.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Wright CI, Fischer H, Whalen PJ, McInerney SC, Shin LM, Rauch SL. Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. Neuroreport. 2001;12:379–383. doi: 10.1097/00001756-200102120-00039. [DOI] [PubMed] [Google Scholar]
- Wright CI, Martis B, Shin LM, Fischer H, Rauch SL. Enhanced amygdala responses to emotional versus neutral schematic facial expressions. Neuroreport. 2002;13:785–790. doi: 10.1097/00001756-200205070-00010. [DOI] [PubMed] [Google Scholar]
- Wright CI, Wedig MM, Williams D, Rauch SL, Albert MS. Novel fearful faces activate the amygdala in healthy young and elderly adults. Neurobiol.Aging. 2006a;27:361–374. doi: 10.1016/j.neurobiolaging.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Wright CI, Wedig MM, Williams D, Rauch SL, Albert MS. Novel fearful faces activate the amygdala in healthy young and elderly adults. Neurobiol.Aging. 2006b;27:361–374. doi: 10.1016/j.neurobiolaging.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Wood JN, Grafman J. Human prefrontal cortex: processing and representational perspectives. Nat.Rev.Neurosci. 2003;4:139–147. doi: 10.1038/nrn1033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.