Abstract
Background
In Ethiopia, the utilization of long-lasting insecticide-treated bed nets (LLITN) is hampered by behavioural factors such as low awareness and negative attitude of the community. The aim of this study was to present the design and baseline results of a cluster randomized trial on the effect of training of household heads on the use of LLITN.
Methods
This baseline survey was undertaken from February to March, 2009 as part of a randomized cluster trial. A total of 11 intervention and 11 control Gots (villages) were included in the Gilgel Gibe Field Research Centre, south-west Ethiopia. House to house visit was done in 4135 households to collect information about the use of LLITN and socio-demographic variables. For the diagnosis of malaria and anaemia, blood samples were collected from 2410 under-five children and 242 pregnant women.
Results
One fourth of the households in the intervention and control Gots had functional LLITN. Only 30% of the observed LLITN in the intervention and 28% in the control Gots were hanged properly. Adults were more likely to utilize LLITN than under-five children in the control and intervention Gots. The prevalence of malaria in under-five children in the intervention and control Gots was 10.5% and 8.3% respectively. The intervention and control Gots had no significant difference concerning the prevalence of malaria in under-five children, [OR = 1.28, (95%CI: 0.97, 1.69)]. Eight (6.1%) pregnant women in the intervention and eight (7.2%) in the control Gots were positive for malaria (P = 0.9). Children in the intervention Gots were less likely to have anaemia than children in the control Gots, [OR = 0.75, (95%CI: 0.62, 0.85)].
Conclusion
The availability and utilization of LLITN was low in the study area. The prevalence of malaria and anaemia was high. Intervention strategies of malaria should focus on high risk population and vulnerable groups.
Background
The African region south of the Sahara is heavily affected by malaria. Ethiopia is among the 30 high burden countries in malaria infection and contributed to 6% of the malaria cases in Africa. Due to climatic changes and geographic factors in Ethiopia, malaria occurs everywhere except the central high lands[1].
The use of LLITN is one of the major components of the selective vector control strategy in Ethiopia. LLITN distribution in Ethiopia primarily focuses on households with children less than five years of age and pregnant women in targeted areas[2]. The national malaria control programme had distributed 20 million LLITN between 2005 and 2007, free-of-charge, to households with vulnerable groups [1,2].
Low awareness about malaria and the utilization of the preventive methods are the serious challenges of the malaria control programmes in Africa and Ethiopia[3-7]. The level of knowledge and the use of LLITN in Ethiopia is very low[4,8]. According to the reports of the 2005 Ethiopian Demographic and Health Survey (EDHS), only 2% of rural under-five children and 1% of pregnant women used LLITN[8]. In Gilgel Gibe Field Research Centre, south-west Ethiopia, only 25% of under-five children slept under LLITN the night before the survey[9]. It was observed that many mothers in the study area had used LLITN for scarves and bed sheets to prevent louses and fleas[9]. Utilization of LLITN is also hampered by the low health service coverage, especially in the vast majority of the rural communities[8]. Cognizant of the above facts, effective utilization of LLITN in the community is unlikely in the near future. Therefore, there should be alternative strategy that empowers the community for effective utilization of LLITN to control the burden of malaria in the population particularly in the vulnerable groups (under-five children and pregnant women).
The objective of the trial is to assess the effect of tailored training of the heads of the households on the use of LLITN and community network system on the burden of malaria in vulnerable groups.
Hypothesis of the trial
Training of the heads of the households by village residents on the proper use of LLITN will reduce the burden of malaria in under-five children and pregnant women.
The idea of educating heads of households (decision makers of the household matters) about the proper use of LLITN and assessing its impact on the burden of malaria is a new idea. Previous trials did not address the human behavior concerning the use of LLITN and its impact on the burden of malaria and anaemia among vulnerable groups [10-16]. In this article, the design and baseline results of a cluster randomized trial are presented.
Methods
Study area
The study was conducted in Gilgel Gibe Field Research Centre (GGFRC). This research site is selected since malaria is the major health problem in the area due to ecological disruption[17]. GGFRC is located 260 K.m south-west of Addis Ababa, the capital of Ethiopia. It was established in 2005 to serve as Demographic Surveillance System and field attachment site of Jimma University. The research centre comprised of eight rural and two urban Kebeles (lowest administration unit in Ethiopia) which are located around the reservoir of Gilgel Gibe hydroelectric dam. In the ten Kebeles, there are 52 Gots, 50,000 population and 10500 households. A Got is a village of 140 to 180 households within a Kebele and one Kebele may have five to seven Gots.
Study design and sampling
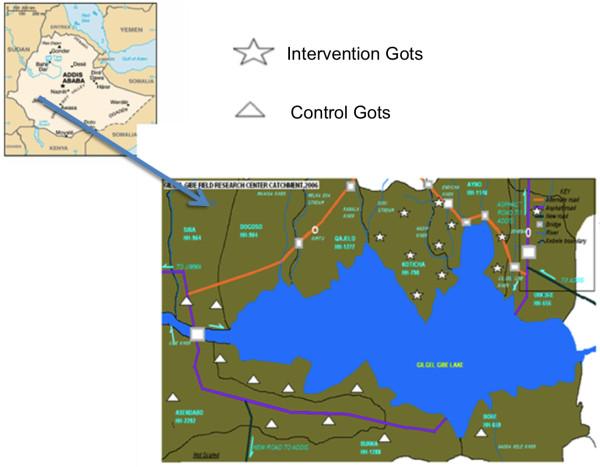
As part of a cluster randomized trial, a baseline survey was conducted in GGFRC from February to March, 2009. The study population consisted of heads of households, under-five children and pregnant women in twenty-two Gots. Heads of the households were selected to be trained on the proper use of LLITN. The major rationale of selecting heads of the households is that they are the decision makers in all matters (including health issues) within a household. In Ethiopian, 80% of the heads of the households in rural areas are males. However, females and children are the direct beneficiaries of this intervention. To avoid contamination, villages in the field research centre were stratified into North and South of the reservoir of the dam (Figure 1).
Figure 1.
Map of the study Gots and Gilgel Gibe Field Research Centre.
Gots in the north direction of the reservoir were selected randomly as intervention whereas those villages in the south direction of the dam were selected to be control groups and both groups are within the same distance (10-15 Km) from the reservoir. For this trail, 22 Gots (11 intervention and 11 control villages) were included. Since the unit of intervention and randomization are Gots, the number of Gots that need to be included in each group is determined using the following equation[18]:
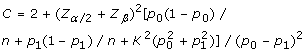 |
Where C is the number of Gots; Zα/2 the standard normal variable at α = 5%; Zβ is (1-β) = 80%; P0 is the prevalence of LLITN utilization (27%)[9], a variable which gives maximum sample size; P1 is the prevalence of LLITN utilization in the intervention Gots (assumed to be increased by 40%); n is number of households which have vulnerable under-five children in the sampled Gots (n = 87); k is coefficient of variation of proportions of the outcome variable between the clusters within each group. Since there is no study to estimate K, it was taken as 0.25[18]. Based on the above information, the total number of cluster/Gots would be 22. All households in each cluster/Gots were included in the study. Detail description of the study profile is given below (Figure 2).
Figure 2.
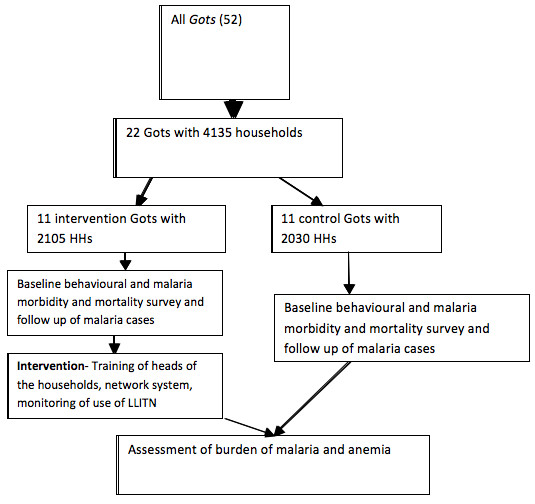
Trial Profile, Gilgel Gibe Field Research Centre.
The intervention and data collection procedures
The Intervention
The study has three phases: preparatory, intervention and evaluation phases. Baseline survey was undertaken from February to March, 2009. After the baseline survey, the intervention phase was started in May 2009 and will continue for two years. The intervention consists of tailored training of heads of the households on the proper use of LLITN and establishing community network system. Nine village residents were identified and given training of trainers (TOT) about the proper utilization of LLITN for five days. The village residents gave training to all heads of the households in each Gots using posters and manuals prepared in local language. After a day of the training, demonstration on use of LLITN was undertaken in the rural houses in the study villages. If the heads of the household were absent, they were trained in another days. The house level training was supervised by malaria experts from the district health offices and the investigators. Community network system was also established in the intervention villages to make the intervention sustainable. The network system links the households and the malaria control programme in the district through the trained village residents. At district level, one focal person from the malaria control team was assigned to monitor and supervise the activities of the trained village residents. The trained village residents had also parallel communication with health extension workers (HEW) who are assigned in each Kebele to give minimum health packages to each household. The village residents report their activities to the malaria control programme and HEW regularly. The investigators, the malaria control team, the trained village residents and the HEW have regular monthly monitoring meeting to discuss progress of activities and challenges.
After the training, 12500 LLITN were distributed to the control and intervention Gots. As of May, 2009, each household in the intervention villages has been visited by the trained village residents monthly to check for the proper use of LLITN and the occurrences of malaria. The proper use of LLITN by under-five children and pregnant women has been evaluated using observation and checklist. The proper hanging of the net in the four angles of the bed/mattress using locally made wooden materials, status of the net (new or old), and the proper placement of the net under the mattress or bed have been checked in each visit. The trained village residents also collect information on the occurrence of malaria using questionnaire.
Mass blood examination of under-five children and pregnant women has been undertaken three times a year in both the intervention and control villages to assess the burden of malaria and anaemia. In the control villages, the occurrence of malaria in each household has been monitored monthly by trained village residents. These trained residents do not give training about LLITN unlike the intervention villages.
Data collection procedure of the baseline survey
Socio-demographic characteristics of the study population, knowledge on malaria, availability and utilization of LLITN was assessed using structured questionnaire prepared in local language. Heads of the households who mentioned at least one symptom, one preventive method and a mosquito as a transmission agent of malaria were categorized as having good knowledge. Utilization of LLITN the night before the survey was assessed by asking the individuals whether they had slept under the net or not. Since there was no observation of the use of bed net during night, the assessment was referred as perceived utilization. However, observations were made in 220 houses (10 in each Gots) to check for the functionality of the net and the place where it was hanged during the survey. LLITN which had no holes and less than 3 years old were labelled as functional. The data was collected by trained high school complete individuals. Four focus group discussions were conducted by public health experts among heads of the household and spouses to explore the barriers of LLITN use.
The baseline blood investigation for all under-five children and pregnant women was done in the study Gots. For malaria parasite identification, two thick and thin films were prepared from a finger prick in the field and stained with Giemsa in Jimma Specialized Hospital. Each slide was read by experienced laboratory technicians. Absence of malaria parasite in 200 high power ocular fields of the thick film was considered as negative[11]. For positive cases, type of species, degree of parasitaemia and presence of gametocytes were reported. A crosscheck reading of all positives and 10% of negatives was conducted to estimate the quality of the first reading. From the positive and negative slides, a discordant rate of 2% and 4% were observed. Slides with discordant results were reread by a third microscopist. Clinical malaria was defined as the presence of malaria parasite in the blood film examination and fever[11].
Haemoglobin concentration was determined using Hemo Cue analyzer in the field (HemoCue Hb 301, Sweden). Moderate and severe anaemia was defined as an haemoglobin level 7-10.9 g/dl and <7 g/dl respectively. Malarial anaemia was defined as presence of anaemia and malaria parasite at the same time[11].
Data management and statistical analysis
Data were entered into computer, edited, cleaned, and analyzed using SPSS-16 and Epi Info 3.5.1 (Centre for Disease Control and Prevention, Atlanta) statistical software. First, univariate analysis was done to describe the socio-demographic characteristics of the study participants, utilization of LLITN and prevalence of malaria and anaemia. Cochran-Mantel-Haenszel chi-square test was used to compare utilization of LLITN and proportion of children and pregnant women with malaria and anaemia in the intervention and control Gots. To control for the effect of confounding variables and clustering of Gots, a stepwise multivariate logistic regression analysis was done. The Omnibus (p < 0.05) and Hosmer-Lomeshow (p > 0.05) tests were used to assess the performance of the logistic regression model. Adjusted odds ratio (OR) and 95%CI was used to interpret the findings. All FGD interviews were transcribed by the experts immediately after the interview. The transcribed data was commented by the investigators. After several readings, key categories & themes were identified. The data was interpreted and presented verbatim.
Ethical considerations
The proposal was approved by Jimma University and the WHO ethical committee. Written consent was obtained from caretakers of under-five children and pregnant women. Patients with anaemia and malaria were given standard [19]treatment by the health extension workers or nearby health centres.
Results
A sample of 4135 households and 21,673 people were included in the baseline survey. Of the sampled population, 49.7% of them were females. Under-five children and pregnant women constituted 18.6% and 2% of the sampled population.
Perceived utilization of LLITN
Seventy-seven percent of households had at least one LLITN. In the intervention villages, similar proportion of males and females perceived that they had utilized LLITN the night before the survey. More females (70%) than males (66.4%) utilized LLITN in the control Gots (P = 0.001). In the intervention and control Gots, adults were more likely to utilize LLITN than the children (P = 0.001). Interestingly, perceived utilization of LLITN in all the age groups in control villages was high as compared to intervention villages. Fifty-six percent of pregnant women and 48.5% of the non-pregnant women of reproductive age group utilized LLITN in the intervention villages (P = 0.015). However, more women in control Gots were using LLITN compared to the intervention Gots (Table 1).
Table 1.
Perceived utilization of long-lasting insecticide-treated bed nets in Gilgel Gibe Field Research Centre
| Socio-demographic characteristics | Perceived utilization of LLITN | |||||
|---|---|---|---|---|---|---|
| Intervention Gots | Control Gots | |||||
| Yes | No | P-Value | Yes | No | P-value | |
| Sex, no (%) | ||||||
| Male | 2948(47.7) | 3234(52.3) | 0.6 | 3125(66.4) | 1578(33.6) | 0.001 |
| Female | 2977(48.1) | 3214(51.5) | 3211(70.4) | 1351(29.6) | ||
| Age in years, no (%) | 0.001 | 0.001 | ||||
| <5 | 1232(52.1) | 1132(47.9) | 1269(76.0) | 401(24.0) | ||
| 5-14 | 1430(38.8) | 2251(61.2) | 1699(61.4) | 1068(38.6) | ||
| 15-24 | 898(41.4) | 1272(58.6) | 951(55.6) | 759(44.4) | ||
| 25-34 | 909(54.6) | 756(45.4) | 854(74.2) | 296(25.8) | ||
| 35-49 | 994(57.8) | 727(42.2) | 1090(80.3) | 267(19.7) | ||
| > = 50 | 455(59.6) | 308(40.4) | 474(78.0) | 134(22.0) | ||
| Women of reproductive age, no (%) | ||||||
| Pregnant | 157(56.1) | 123(43.9) | 0.015 | 121(74.7) | 41(25.3) | 0.2 |
| Not pregnant | 1569(48.5) | 1664(51.5) | 1514(70.3) | 632(29.5) | ||
After controlling for possible confounder, adults of the age group 35-49 [OR = 1.27, (95%CI: 1.11, 1.45)] and > = 50 [OR = 1.29, (95%CI: 1.10, 1.49)] years were more likely to utilize LLITN than under-five children in the intervention villages. Adults in the age group of 35 to 49 were 1.3 times more likely to utilize LLITN than the under-five children in the control villages, [OR = 1.3, (95%CI: 1.08, 1.56)]. Females [OR = 1.2, (95%CI: 1.10, 1.32)] were more likely to utilize LLITN than males in the control villages (Table 2).
Table 2.
Factors independently associated with perceived use of LLITN, Gilgel Gibe Field Research Centre
| Intervention Gots | Control Gots | |||
|---|---|---|---|---|
| Crude OR (95%CI) | Adjusted OR(95%CI) | Crude OR(95%CI) | Adjusted OR(95%CI) | |
| Age in years | ||||
| <5 | 1 | 1 | 1 | 1 |
| 5-14 | 0.58(0.52,0.64) | 0.58(0.52,0.64)* | 0.50(0.44,0.58*) | 0.50(0.44,0.58)* |
| 15-24 | 0.65(0.57,0.73) | 0.64(0.57,0.72)* | 0.39(0.34,0.46)* | 0.39(0.34,0.47) |
| 25-34 | 1.12(0.97,1.25) | 1.10(0.96,1.26) | 0.91(0.77,1.09) | 0.90(0.76,1.07) |
| 35-49 | 1.27(1.10,1.45) | 1.27(1.11,1.45)* | 1.30(1.10,1.56)* | 1.30(1.08,1.56)* |
| > = 50 | 1.29(1.10, 1.49) | 1.29(1.10, 1.49) | 1.12(0.95, 1.42) | 1.17(0.96, 1.44) |
| Sex | ||||
| Males | 1 | 1 | 1 | 1 |
| Females | 1.10(0.9,1.10) | 1.1(0.96,1.12) | 1.20(1.1,1.30)* | 1.21(1.10,1.32)* |
| Women of reproductive age | ||||
| Non- pregnant | 1 | 1 | 1 | |
| Pregnant | 1.35(1.1,1.73) | 1.23(0.95,1.57) | 1.23(0.85,1.77) | 1.10(0.73.1.57) |
* Statistically significant (P < 0.05)
Level of education of the heads of the households and household income did not have statistically significant association with utilization of at least one LLITN in a household. In the intervention villages, utilization of at least one LLITN in a household was more common when the heads of the household is female [OR = 1.92,(95%CI:1.34,2.74)] and has good knowledge on malaria [OR1.96, (95%CI: 1.36,2.8)](Table 3).
Table 3.
Characteristics of household heads and utilization of LLITN, Gilgel Gibe Field Research Centre
| Characteristics of the heads of households | Utilization of at least one LLITN in a household | |||||
|---|---|---|---|---|---|---|
| Intervention Gots | Control Gots | |||||
| Yes | No | Adjusted OR (95%CI) | Yes | No | Adjusted OR (95%CI) | |
| Literacy status | ||||||
| Illiterate | 1093(87.5) | 156(12.5) | 1091(91.5) | 102(8.5) | 1 | |
| Literate | 298(90.0) | 33(10.0) | 1.28(0.86, 1.36) | 255(91.1) | 25(8.9) | 1.10(0.60, 1.66) |
| Sex | ||||||
| Male | 632(84.8) | 113(15.2) | 1 | 517(90.5) | 54(9.5) | 1 |
| Female | 762(91.0) | 75(9.0) | 1.92(1.34,2.74)* | 826(91.9) | 73(8.3) | 1.18(0.81,1.70) |
| Monthly income of the household(in Birr, median income = 500) | ||||||
| <500 | 491(87.5) | 70(12.5) | 1 | 345(90.8) | 35(19.2) | |
| > = 500 | 494(87.1) | 73(12.5) | 1.04(0.67,1.34) | 560(92.9) | 43(7.1) | 1.32(0.82,2.10) |
| Knowledge about malaria | ||||||
| Poor | 968(89.5) | 114(10.5) | 1 | 387(91.7) | 35(8.3) | 1 |
| Good | 428(85.1) | 75(14.9) | 1.96(1.36,2.80)* | 959(91.2) | 92(8.8) | 0.95(0.60, 1.51) |
* Statistically significant (P < 0.05)
More than 95% of the houses were conical shapes, thatched roofs and high ceiling. Observation showed that 26(24%) of the houses in the intervention and 28(26%) in the control Gots had functional LLITN. As a result of the nature of the house, the community were not aware how to hang and put the nets under the bed/mattress. Only 30% of the observed LLITN in the intervention and 28% in the control Gots were hanged properly over the bed/mattress. It was observed that only 39% and 34% of the LLITN in the intervention and control Gots could reach under the mattress/bed while hanged.
The results of the focus group discussion were similar to the observations. Low awareness was one of the major reasons for the low utilization of LLITN. A 30-year old housewife stated, "We have one net in our house, but we do not know how to hang and use it. The ceiling is too high for the net to reach." Most of the FGD participants stated that education and training should be given to them how to utilize LLITN.
A 50-year old male head of household in the control village expressed his idea, "Awareness creation supported by demonstration how to properly use and wash bed nets should be given to us by the government and other stakeholders."
Lack of LLITN was the other major reason of low utilization of LLITN. It was indicated that most of the households had got only one LLITN three years back. A 45-year old female in the intervention Gots says, "The government gave us only one net for a house which has 10 members. How could we share one net for ten people?"
Prevalence of malaria and anaemia in under-five children and pregnant women
A sample of 2750 children gave blood for the diagnosis of malaria and anaemia. However, 2410(87.6%) of them were included in this report. The rest were excluded due to missing information on main variables. The mean age of children was 33.5(SD ± 1.8) and 32.1(SD ± 1.75) months in the intervention and control Gots respectively. The proportion of male and female children was similar in the control and intervention Gots (Table 4).
Table 4.
Socio-demographic characteristics of under-five children (n = 2410) who were investigated for malaria in Gilgel Gibe Field Research Centre
| Variable | Intervention(n = 1207) No (%) | Control(n = 1203) No (%) |
|---|---|---|
| Sex | ||
| Male | 616(51.0) | 607(50.5) |
| Female | 591(49.0) | 596(49.5) |
| Age in months | ||
| <12 | 246(20.4) | 263(21.9) |
| 12-23 | 62(5.1) | 94(7.8) |
| 24-35 | 244(20.2) | 222(18.5) |
| 36-47 | 236(19.6) | 226(18.8) |
| > = 47 | 419(34.7) | 398(33.0) |
| Mean age in months(SD) | 33.5(SD ± 1.8) | 32.1(SD ± 1.75) |
| Birth order | ||
| 1-3 | 665(55.2) | 586(48.8) |
| 4-5 | 364(30.2) | 396(33.0) |
| >5 | 175(14.2) | 219(18.2) |
| Mean Weight(SD) | 11.1(SD ± 3.1) | 11.2(SD ± 3.1) |
The prevalence of malaria in under-five children in the intervention and control Gots was 10.5% and 8.3% respectively. The intervention and control Gots had no significant difference concerning the prevalence of malaria in under-five children, [OR = 1.28, (95%CI: 0.97, 1.69)]. Fifty-nine percent of malaria cases in the intervention Gots and sixty percent of the malaria cases in the control Gots were due to Plasmodium vivax. Four percent and three percent of children had clinical malaria in the intervention and control Gots respectively. After controlling for potential confounding variables, children in the intervention Gots were less likely to have anaemia than children in the control Gots, [OR = 0.75, (95%CI: 0.62, 0.85)]. Under-five children in the intervention Gots were 1.36 times more likely to have fever than children in the control Gots, [OR = 1.36, (95%CI: 1.14, 1.63)] (Table 5). There was no significant difference of the prevalence of malaria among age groups, sex and birth order of the under-five children (Table 6).
Table 5.
Prevalence of malaria and anaemia in under-five children in the intervention and control villages (n = 2410) in Gilgel Gibe Field Research Centre.
| Variable | Intervention Gots No (%) | Control Gots No (%) | Adjusted OR(95%CI)* |
|---|---|---|---|
| Malaria parasitaemia | 1.28(0.97,1.69) | ||
| Yes | 127(10.5) | 100(8.3) | |
| No | 1080(89.5) | 1103(91.7) | |
| Species of malaria parasite | 1.10(0.62,1.90) | ||
| Plasmodium falciparum | 52(40.9) | 38(38.0) | |
| Plasmodium vivax | 75(59.1) | 60(60.0) | |
| Fever | 1.36(1.14,1.63)† | ||
| Yes | 380(31.5) | 304(25.3) | |
| No | 827(68.5) | 899(74.7) | |
| Clinical Malaria | 1.47(0.95,2.27) | ||
| Yes | 52(4.3) | 36(3.0) | |
| No | 1155(95.7) | 1167(97.0) | |
| Anaemia | 0.75(0.62,0.85)† | ||
| Yes | 352(29.2) | 428(35.6) | |
| No | 855(70.8) | 775(64.4) | |
| Severe Anaemia | |||
| Yes | 12(1.0) | 20(1.7) | 0.58(0.28,1.20 |
| No | 1190(99.0) | 1181(98.3) | |
| Malarial Anaemia | 0.81(0.53,1.24) | ||
| Yes | 40(3.3) | 50(4.2) | |
| No | 1159(96) | 1138(94.6) |
*Adjusted for age, sex and birth order of children
†P-value < 0.05
Table 6.
Malaria infection by demographic characteristics of the children in Gilgel Gibe Field Research Centre
| Variable | Malaria parasite | P-value | ||||
|---|---|---|---|---|---|---|
| Intervention Gots | Control Gots | |||||
| Yes | No | yes | No | |||
| Sex | 0.74 | |||||
| Male | 73(11.9) | 543(88.1) | 0.12 | 52(8.6) | 555(91.4) | |
| Female | 54(9.1) | 537(90.9) | 48(8.1) | 548(91.9) | ||
| Age in months | 0.067 | |||||
| <12 | 20(8.1) | 226(91.9) | 0.6 | 23(8.7) | 240(91.3) | |
| 12-24 | 7(11.3) | 55(88.7) | 5(5.3) | 89(94.7) | ||
| 25-34 | 24(9.8) | 220(90.2) | 23(10.4) | 199(89.6) | ||
| 35-47 | 26(11.0) | 210(89.0) | 26(11.5 | 200(88.5) | ||
| > = 47 | 50(11.9) | 369(88.1) | 23(5.8) | 375(94.2) | ||
| Birth order | 0.55 | |||||
| 1-3 | 63(9.5) | 602(90.5) | 0.35 | 48(8.2) | 538(91.8) | |
| 4-5 | 45(12.4) | 319(87.6) | 37(9.3) | 359(90.7) | ||
| >5 | 19(10.9) | 156(89.1) | 15(6.8) | 204(93.2) | ||
A sample of 242 pregnant women was included for the diagnosis of malaria and anaemia. The mean age of these women was 26.1(SD ± 5.7) and 26.4(SD ± 5.3) years in the intervention and control Gots respectively. Eight (6.1%) pregnant women in the intervention Gots and eight (7.2%) in the control Gots had malaria parasite (P = 0.9). Plasmodium falciparum accounted 57% and 50% of the malaria cases in pregnant women in the intervention and control Gots respectively. Twenty-nine percent of the pregnant women in the intervention villages and 36.0% of the women in the control villages had moderate (haemoglobin level of 7 to 11 g/dl) anaemia (P = 0.3). Three percent and five percent of the pregnant women had malarial anaemia in the intervention and control Gots respectively (Table 7).
Table 7.
Prevalence of malaria and anaemia in pregnant women (n = 242), Gilgel Gibe Field Research Centre
| Variable | Intervention Gots | Control Gots | P-value |
|---|---|---|---|
| Mean age in years(SD) | 26.1(SD ± 5.7) | 26.4(SD ± 5.3) | 0.76 |
| Fever in the last two weeks, no (%) | 0.02 | ||
| No | 88(67.2) | 90(81.1) | |
| Yes | 43(32.8)) | 21(18.9) | |
| Parasitaemia, no (%) | 0.9 | ||
| No | 123(93.9) | 103(92.8) | |
| Yes | 8(6.1) | 8(7.2) | |
| Anaemia, no (%) | 0. 36 | ||
| No | 91(70.0) | 71(64.0) | |
| Moderate | 38(29.2) | 40(36.0) | |
| Severe | 1(0.8) | 0(0.0) | |
| Malaria anaemia | 0.8 | ||
| Yes | 4(3.1) | 5(4.5) | |
| No | 127(96.9) | 106(95.5) |
Discussion
Perceived utilization of LLITN is higher in the present study as compared to the findings of other literatures[20-22]. The control villages were better in the perceived utilization of LLITN than the intervention Gots. However, during observation, all villages were poor in utilization of LLITN. Most rural people who lived in traditional round houses (Tukul) did not utilize LLITN properly. As a result of the nature of the houses (mud wall and conical roof), most people do not know how to hang the four angles of the rectangular LLITN. Most of the observed hanged nets could not reach the edge of the bed or mattress which might contribute for the high burden of malaria in the area. Lack of awareness and skill on the use of LLITN and shortage were also mentioned by most of the focus group discussants. Lack of awareness on the utilization of LLITN was also reported by Belay et al in north Ethiopia[21]. Educating the community to use simple locally made methods of hanging LLITN could improve the utilization of LLITN significantly. Unlike other studies, use of LLITN is not associated with the literacy status of heads of the households [21]. However, children less than the age of five years and adolescents were less advantageous than adults in the utilization of LLITN in this study.
In this study, the malaria prevalence survey was undertaken in dry (non-epidemic) season. However, the prevalence of malaria in children (10.5%) in the study area was very high compared to the findings of the national malaria indicator survey (4%)[22], Shargie (2.4%) [23]and Endeshaw et al (4.1%)[24]. The prevalence of malaria in Gilgel Gibe was 10.5% in the epidemic season three years back[17]. This indicates that the burden of malaria is still high in the area unlike the national prevalence, which has dramatically decreased over the last decade[25]. The effect of the lake created by the dam might have contributed for the high burden of malaria in the area[17]. Unlike other studies[22,23], the prevalence of Plasmodium vivax is higher than Plasmodium falciparum. Widespread use of artemether/lumefantrine by the community through the health extension workers may explain the low prevalence of P. falciparum[26]. Prevalence of malaria among pregnant women in this study (6.5%) was also higher compared to the previous findings (1.8%) by Newman et al[27]. Plasmodium falciparum was the common parasite in pregnant women, which can result in serious consequences on the outcome of pregnancy.
Anaemia (haemoglobin level < 11 g/dl) is multifactorial in origin and remain the major public health problem of children and pregnant women in Africa and Ethiopia[22,28,29]. The prevalence and causes of anaemia is not well studied in Ethiopia[30]. The prevalence of anaemia in children in this study was higher than the finding of Wolde et al in north Ethiopia [30] but comparable with the report of EDHS[8]. The prevalence of malarial anaemia in the present study is lower than the findings of other investigators elsewhere[8]. The reversal of malaria parasite prevalence from P. falciparum to P. vivax could explain the lower malarial anaemia in the present study. In major epidemic seasons, the proportion of children with malaria anaemia may be higher than the current finding in the study area.
A significant proportion of pregnant women in the control (36%) and intervention (29%) Gots had moderate anaemia. This finding is much higher than the reports of the EDHS (17%)[8]. In high malaria burden areas like Gilgel Gibe, pregnant mothers may have repeated attacks of malaria, which predispose them to anaemia.
Even though, we tried to document the burden of anaemia in vulnerable groups, the causes of anaemia remain unclear. The definition of malarial anaemia might not explain the real association of anaemia and malaria. Serum ferritin level was not assessed due to resource constraints.
Conclusion
The utilization of LLITN was hampered by lack of awareness in the rural community who lives in traditional Tukul houses. The prevalence of malaria and anaemia in under-five children and pregnant women in the study area was high compared to the national trend of malaria. Intervention strategies of malaria and anaemia should focus on the high risk population like Gilgel Gibe Field Research centre and vulnerable groups.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
AD conceived the study and was involved in the design, coordination, field supervision, analysis and drafted the manuscript. FA participated in the design, field supervision and report writing. ZB was involved in field supervision and writing of the qualitative report. MS and AZ were involved in field supervision and reviewed of the article. NA mobilized the community during the mass blood examination and reviewed the article. AT and LS were involved in field supervision and report writing. FT and TA were involved in data analysis and field supervision and reviewed the article. All authors read and approved the manuscript.
Contributor Information
Amare Deribew, Email: amare_deribew@yahoo.com.
Fessehaye Alemseged, Email: fessahayeat@yahoo.com.
Zewdie Birhanu, Email: zbkoricha@yahoo.com.
Lelisa Sena, Email: lelisase@yahoo.com.
Ayalew Tegegn, Email: ayalewtm@yahoo.com.
Ahmed Zeynudin, Email: azeynudin@yahoo.com.
Tariku Dejene, Email: tarikud@yahoo.com.
Morankar Sudhakar, Email: morankarsn@yahoo.com.
Nasir Abdo, Email: nasirabdo2006@yahoo.co.uk.
Fasil Tessema, Email: fasil.tessema@ju.edu.et.
Acknowledgements
This investigation received financial support from the UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR). All authors acknowledge the financial support of TDR during the field work. TDR gave ethical clearance for the study but has no role in the design, data collection, analysis, report writing and decision on manuscript submission. The authors appreciate the study participants for their cooperation in providing the necessary information. We acknowledge the local administrators and the community for their strong support during the survey.
References
- WHO. World Malaria Report 2008."WHO/HTM/GMP/2008.1". 2008.
- Ministry of Health, Ethiopia. National Strategic Plan for Going to Scale with Coverage & Utilization of ITNs in Ethiopia. Ministry of Health, Addis Ababa, Ethiopia; 2004. [Google Scholar]
- Alemseged FTA, Hileamlak A, Kassahun W. Caretakers' knowledge childhood malaria in Gilgel Gibe Field Research Center. Ethiop J Health Dev. 2008;22(1):49–54. [Google Scholar]
- Ibidapo CA. Perception of causes of malaria and treatment-seeking behaviour of nursing mothers in a rural community. Aust J Rural Health. 2005;13(4):214–218. doi: 10.1111/j.1440-1584.2005.00704.x. [DOI] [PubMed] [Google Scholar]
- Agyepong IA. Malaria: ethnomedical perceptions and practice in an Adangbe farming community and implications for control. Soc Sci Med. 1992;35(2):131–137. doi: 10.1016/0277-9536(92)90160-R. [DOI] [PubMed] [Google Scholar]
- Vundule C, Mharakurwa S. Knowledge, practices, and perceptions about malaria in rural communities of Zimbabwe: relevance to malaria control. Bull World Health Organ. 1996;74(1):55–60. [PMC free article] [PubMed] [Google Scholar]
- Deresssa WAA, Enquoselassie F. Knowledge, attitude and practice about malaria, the mosquito and antimalarial drugs in rural community. Ethiop J Health Dev. 2003;17(2):99–104. [Google Scholar]
- CSA. Ethiopia Demographic and Health Survey 2005. Addis Ababa, Ethiopia and Calverton, Maryland, USA; 2006. [Google Scholar]
- Assefa D. Factors associated with utilization of Insecticide Treated Nets in Gilgel Gibe Field Research Center. Master thesis. Jimma University; 2006. [Google Scholar]
- ter Kuile FO, Terlouw DJ, Phillips-Howard PA, Hawley WA, Friedman JF, Kolczak MS, Kariuki SK, Shi YP, Kwena AM, Vulule JM, Nahlen BL. Impact of permethrin-treated bed nets on malaria and all-cause morbidity in young children in an area of intense perennial malaria transmission in western Kenya: cross-sectional survey. Am J Trop Med Hyg. 2003;68(4 Suppl):100–107. [PubMed] [Google Scholar]
- ter Kuile FO, Terlouw DJ, Kariuki SK, Phillips-Howard PA, Mirel LB, Hawley WA, Friedman JF, Shi YP, Kolczak MS, Lal AA, Vulule JM, Nahlen BL. Impact of permethrin-treated bed nets on malaria, anemia, and growth in infants in an area of intense perennial malaria transmission in western Kenya. Am J Trop Med Hyg. 2003;68(4 Suppl):68–77. [PubMed] [Google Scholar]
- Leenstra T, Phillips-Howard PA, Kariuki SK, Hawley WA, Alaii JA, Rosen DH, Oloo AJ, Nahlen BL, Kager PA, ter Kuile FO. Permethrin-treated bed nets in the prevention of malaria and anemia in adolescent schoolgirls in western Kenya. Am J Trop Med Hyg. 2003;68(4 Suppl):86–93. [PubMed] [Google Scholar]
- Friedman JF, Phillips-Howard PA, Hawley WA, Terlouw DJ, Kolczak MS, Barber M, Okello N, Vulule JM, Duggan C, Nahlen BL, ter Kuile FO. Impact of permethrin-treated bed nets on growth, nutritional status, and body composition of primary school children in western Kenya. Am J Trop Med Hyg. 2003;68(4 Suppl):78–85. [PubMed] [Google Scholar]
- Kariuki SK, Lal AA, Terlouw DJ, ter Kuile FO, Ong'echa JM, Phillips-Howard PA, Orago AS, Kolczak MS, Hawley WA, Nahlen BL, Shi YP. Effects of permethrin-treated bed nets on immunity to malaria in western Kenya II. Antibody responses in young children in an area of intense malaria transmission. Am J Trop Med Hyg. 2003;68(4 Suppl):108–114. [PubMed] [Google Scholar]
- Hawley WA, Phillips-Howard PA, ter Kuile FO, Terlouw DJ, Vulule JM, Ombok M, Nahlen BL, Gimnig JE, Kariuki SK, Kolczak MS, Hightower AW. Community-wide effects of permethrin-treated bed nets on child mortality and malaria morbidity in western Kenya. Am J Trop Med Hyg. 2003;68(4 Suppl):121–127. [PubMed] [Google Scholar]
- Gamble C, Ekwaru JP, ter Kuile FO. Insecticide-treated nets for preventing malaria in pregnancy. Cochrane Database Syst Rev. 2006. p. CD003755. [DOI] [PMC free article] [PubMed]
- Yewhalaw D, Legesse W, Van Bortel W, Gebre-Selassie S, Kloos H, Duchateau L, Speybroeck N. Malaria and water resource development: the case of Gilgel-Gibe hydroelectric dam in Ethiopia. Malar J. 2009;8:21. doi: 10.1186/1475-2875-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes RJ, Bennett S. Simple sample size calculation for cluster-randomized trials. Int J Epidemiol. 1999;28(2):319–326. doi: 10.1093/ije/28.2.319. [DOI] [PubMed] [Google Scholar]
- Ministry of Health, Ethiopia. Malaria Diagnosis and Treatment Guideline for Health Workers in Ethiopia. Addis Ababa, Ethiopia; 2004. [Google Scholar]
- Pettifor A, Taylor E, Nku D, Duvall S, Tabala M, Meshnick S, Behets F. Bed net ownership, use and perceptions among women seeking antenatal care in Kinshasa, Democratic Republic of the Congo (DRC): opportunities for improved maternal and child health. BMC Public Health. 2008;8:331. doi: 10.1186/1471-2458-8-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belay M, Deressa W. Use of insecticide treated nets by pregnant women and associated factors in a pre-dominantly rural population in northern Ethiopia. Trop Med Int Health. 2008;13(10):1303–1313. doi: 10.1111/j.1365-3156.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- Ministry of Health, Ethiopia. Ethiopian Malaria indicator survey. Addis Ababa, Ethiopia; 2007. [Google Scholar]
- Shargie EB, Gebre T, Ngondi J, Graves PM, Mosher AW, Emerson PM, Ejigsemahu Y, Endeshaw T, Olana D, WeldeMeskel A, Teffera A, Taddese Z, Tilahun A, Yohannes G, Richards FO. Malaria prevalence and mosquito net coverage in Oromia and SNNPR regions of Ethiopia. BMC Public Health. 2008;8:321. doi: 10.1186/1471-2458-8-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endeshaw T, Gebre T, Ngondi J, Graves PM, Shargie EB, Ejigsemahu Y, Ayele B, Yohannes G, Teferi T, Messele A, Zerihun M, Genet A, Mosher AW, Emerson PM, Richards FO. Evaluation of light microscopy and rapid diagnostic test for the detection of malaria under operational field conditions: a household survey in Ethiopia. Malar J. 2008;7:118. doi: 10.1186/1475-2875-7-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraha MW, Nigatu TH. Modeling trends of health and health related indicators in Ethiopia (1995-2008): a time-series study. Health Res Policy Syst. 2009;7:29. doi: 10.1186/1478-4505-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes KI, Chanda P, Ab Barnabas G. Impact of the large-scale deployment of artemether/lumefantrine on the malaria disease burden in Africa: case studies of South Africa, Zambia and Ethiopia. Malar J. 2009;8(Suppl 1):S8. doi: 10.1186/1475-2875-8-S1-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman RD, Hailemariam A, Jimma D, Degifie A, Kebede D, Rietveld AE, Nahlen BL, Barnwell JW, Steketee RW, Parise ME. Burden of malaria during pregnancy in areas of stable and unstable transmission in Ethiopia during a nonepidemic year. J Infect Dis. 2003;187(11):1765–1772. doi: 10.1086/374878. [DOI] [PubMed] [Google Scholar]
- Crawley J. Reducing the burden of anemia in infants and young children in malaria-endemic countries of Africa: from evidence to action. Am J Trop Med Hyg. 2004;71(2 Suppl):25–34. [PubMed] [Google Scholar]
- Getahun ZUK, Ganebo T, Nigatu A. Review of the status of malnutrition and trends in Ethiopia. Ethiop J Health Dev. 2001;15(2):55–74. [Google Scholar]
- Wolde-Gebriel Z, West CE, Gebru H, Tadesse AS, Fisseha T, Gabre P, Aboye C, Ayana G, Hautvast JG. Interrelationship between vitamin A, iodine and iron status in schoolchildren in Shoa Region, central Ethiopia. Br J Nutr. 1993;70(2):593–607. doi: 10.1079/BJN19930151. [DOI] [PubMed] [Google Scholar]