Abstract
Adiponectin (APN) is an adipose tissue-derived cytokine that regulates insulin sensitivity and inflammation. It is also involved in modulation of cell proliferation by binding to various growth factors. Based on its known effects in modulating cell proliferation and oxidative stress, APN may potentially be involved in regulating tissue damage and repair following irradiation. Adiponectin KO mice and their WT littermates were exposed to a single whole-body dose of 3 or 6 Gy gamma radiation. Radiation-induced alterations were studied in jejunum, blood, bone marrow and thymus at day 1 and 5 post-irradiation and compared with sham-irradiated groups. In WT mice, irradiation did not significantly alter serum APN levels while inducing a significant decrease in serum leptin. Irradiation caused a significant reduction in thymocyte cellularity, with concomitant decrease in CD4+, CD8+ and CD4+CD8+ T cell populations, with no significant differences between WT and APN KO mice. Irradiation resulted in a significantly higher increase in the frequency of micronucleated reticulocytes in the blood of APN KO compared with WT mice, whereas frequency of micronucleated normochromatic erythrocytes in the bone marrow at day 5 was significantly higher in WT compared with APN KO mice. Finally, irradiation induced similar alterations in villus height and crypt cell proliferation in the jejunum of WT and APN KO mice. Jejunum explants from sham-irradiated APN KO mice produced higher levels of IL-6 compared with tissue from WT animals, but the difference was no longer apparent following irradiation. Our data indicate that APN deficiency does not play a significant role in modulating radiation-induced gastrointestinal injury in mice, while it may participate in regulation of damage to the hematopoietic system.
Keywords: Adiponectin, gamma radiation, cell proliferation, intestinal inflammation, micronuclei
1. INTRODUCTION
Radiotherapy is an important modality for cancer treatment and more so when surgical removal of the cancer mass is not possible or when surgery might debilitate the patient [1]. Every year it is estimated that nearly 60% of all cancer patients receive radiation therapy, either alone or in conjunction with surgery or chemotherapy [2,3]. At clinically relevant doses of irradiation, bone marrow failure is the most commonly observed life threatening problem. The observed effects are due both to a decrease in the number of hematopoietic stem cell progenitors and a reduction in self-renewal capacity of stem cells [3,4]. Radiation also causes cell attrition of the gastrointestinal epithelium, ulceration, reduced motility and gastrointestinal dysfunction [5]. These cellular changes result in nausea, anorexia, loss of electrolyte balance, diarrhea and bacterial infection [6]. Cumulatively, these effects make radiation-induced enteritis a potentially life-threatening complication to patients [5].
Cytokines and growth factors enhance recovery from radiation syndrome. The administration of some cytokines before irradiation protects mice from radiation-induced death [7–9]. Cytokines and growth factors play an essential role in orchestrating the host’s response to radiation and stimulate the innate defense against ionizing radiation, which confers protection [3,10]. Growth factors like granulocyte colony-stimulating factor (G-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF) are protective when administered therapeutically and have been approved for use in treating acute myelosuppression [11,12]. Overall, preclinical and clinical studies demonstrated that a large number of cytokines and growth factors could serve to accelerate bone marrow restoration after exposure to radiation [3,13].
Adiponectin (APN) is a protein predominantly synthesized and secreted by adipocytes [14,15]. The average levels of plasma APN in humans range from 3 to 30 µg/ml [16,17]. Low APN levels are closely associated with obesity-linked complications, including type 2 diabetes, coronary heart disease and hypertension [18]. Adiponectin is a key regulator of insulin sensitivity through activation of AMP-activated protein kinase (AMPK) [19]. Adiponectin also exerts anti-therogenic effects by suppressing tumor necrosis factor (TNF)-α-induced NF-κB activation through a cAMP-dependent pathway, thereby inhibiting inflammation-induced gene transcription [20,21]. In recent studies APN has been reported as a novel hemopoietic stem cell growth factor [22].
Adiponectin exerts protective effects against oxidative damage in vivo and in vitro [23–25]. Oxidative stress is one of the main mechanisms through which irradiation causes damage to DNA and other cellular components [26]. A relationship between increased oxidative stress and decreased circulating levels of APN in normal weight and metabolically obese humans has been observed [27,28]. Based on the effect of APN as a protective factor against oxidative damage, APN KO mice could develop more severe radiation-induced intestinal and hemopoietic damage when compared with their WT littermates. However, by binding to growth factors that promote tissue regeneration after damage [29,30] APN inhibits cellular proliferation, thus potentially contributing to delaying recovery from gastrointestinal damage following irradiation. In this second hypothesis, APN deficiency could favor epithelial cell proliferation and promote recovery, similarly to what we previously observed using the dextran sulfate sodium (DSS) model of colonic damage [31]. As many cancer patients are obese and subjected to radiotherapy, a relationship between APN and radiation’s effects would be of clinical significance. The primary goal of this study was to evaluate the effect of APN deficiency on radiation-induced damage and cell proliferation in the jejunum by comparing the response of of WT and APN KO mice to whole-body irradiation. In addition, the effect of APN deficiency on radiation-induced thymus atrophy and micronuclei formation was evaluated.
2. MATERIALS AND METHODS
2.1. Animal care and husbandry
Adiponectin KO mice were generated as previously described and kindly provided by Dr. Lawrence Chan at Baylor College of Medicine (Waco, TX) [32]. Female 8–10 week-old APN KO mice and their WT littermates in a C57BL/6J background were used. Care of mice followed institutional guidelines under a protocol approved by the Institutional Animal Care and Use Committee at the University of Illinois at Chicago.
2.2. Irradiation
Mice were whole-body exposed to a single dose of either 3 or 6 Gy gamma radiation at a dose rate of 2 Gy/min using a 137Cs irradiator source in well-ventilated vinyl containers boxes without anesthesia. Control mice were sham-irradiated. Blood was collected from the retroorbital plexus under isoflurane anesthesia and mice were then immediately euthanized by cervical dislocation. Radiation-induced alterations were studied in the jejunum, blood, bone marrow and thymus on days 1 and 5 post-irradiation. Five mice were included in each group for a total number of 50 animals. Each experiment was performed twice to ensure reproducibility of results.
2.3. Leptin and APN measurements
Adiponectin and leptin were measured in serum using specific ELISA kits (R&D Systems, Minneapolis, MN).
2.4. Thymocyte isolation and flow cytometry
Thymocytes were isolated by pressing the thymus through a 100 µm cell strainer (Fisher Scientific, Pittsburgh, PA) and cells were counted using a hemocytometer. Cells were suspended in complete cell culture medium (RPMI 1640 supplemented with 10% FCS, 2 mM l-glutamine, 100 IU/ml penicillin, and 100 µg/ml streptomycin) (Invitrogen Life Technologies, Carlsbad, CA), washed once and counted. For CD4 and CD8 T cell staining, thymocytes (1×106) were incubated with 1 µg of FITC-conjugated anti-CD4 and 1 µg of PE-conjugated anti-CD8 (BD Pharmingen, San Diego, CA) for 20 min at 4 °C and washed with cold PBS three times. Rat IgG conjugated with FITC and PE served as negative control. Cells were then analyzed using a FACSCalibur (Becton Dickinson, San Diego, CA).
2.5. Micronuclei assay in blood reticulocytes
2.5.1. Preparation of acridine orange-coated glass slides
Acridine orange (AO)-coated slides were made as described previously [33]. Briefly, AO was dissolved in distilled water at a concentration of 1 mg/ml and 10 µl placed on a glass slide pre-heated to 70°C. The solution was spread by moving a glass rod back and forth and air-dried.
2.5.2. Peripheral blood cells micronuclei assay
Peripheral blood was collected from the orbital sinus of isoflurane-anesthetized mice and 5 µl aliquots of blood were placed on AO-coated slides and covered with cover slips. Reticulocytes (RETs) and micronucleated reticulocytes (MN-RETs) were monitored using a Carl Zeiss Fluorescent microscope with Axioskop with blue filter. Suitable regions of the slides were selected at 10X magnification and approximately 1000 RETs per animal were scored for micronuclei.
2.5.3. Collection of bone marrow for micronuclei assay
Animals were euthanized at days 1 and 5 post-irradiation with 3 Gy and the femurs dissected, cleaned and the bone marrow flushed. The cell suspension was centrifuged. A few drops of FCS were added and the pellet was mixed thoroughly. Smears were drawn on to slides using a drop of the resultant suspension in FCS. Slides were air-dried and fixed in absolute methanol. Micronuclei were prepared according to the method of Schmid [34], with modifications [35]. Slides were stained with 0.125% AO in Sorensen's buffer (pH 6.8), washed twice and mounted in Sorensen's buffer and observed under a fluorescent microscope using a 40X Neofluar objective. A minimum of 1000 each polychromatic erythrocytes (PCE) and normochromatic erythrocytes (NCE) were counted for the presence of micronuclei for each animal.
2.6. Histological examination of jejunum
A segment of jejunum was resected, washed with 0.9% NaCl by injecting saline through the lumen, fixed in 10% buffered formalin, and embedded in paraffin. Sections were cut at 5 µm and stained with hematoxylin and eosin. Villus height (from the base to the tip of the villus) was measured on individual intestinal segments in five separate microscopic fields for each animal and recorded as the mean value using ocular micrometer-adapted light microscopy.
2.7. Bromodeoxyuridine Incorporation Assay
Mice were injected ip with 1 mg bromodeoxyuridine (BrdU) (Sigma Chemical Co, St. Louis, MO) 2 h prior to euthanasia. To measure cell proliferation, a 0.5–1-cm segment of jejunum was fixed in 10% buffered formalin overnight, embedded in paraffin, and tissue sections were stained for BrdU using a kit from BD Biosciences (San Jose, CA). An index of proliferation was determined as the ratio of crypt cells staining positively for BrdU per 10 crypts.
2.8. Jejunum Organ Culture
A segment of the jejunum was removed, cut open longitudinally, washed in PBS containing penicillin and streptomycin, and placed in tissue culture plates containing RPMI-1640 medium supplemented with penicillin and streptomycin. Cultures were incubated at 37°C in 5% CO2. After 24 h, supernatants were harvested and stored at −70°C for analysis of cytokine levels. IL-6 and chemokine (C-X-C) ligand (CXCL)2 levels in cell supernatants were measured using ELISA kits from BD Bioscience and R&D Systems. Protein concentration was determined using a Bio-Rad (Hercules, CA) protein assay. Concentrations of different mediators were adjusted by total protein content
2.9. Statistical analysis
Statistical significance among treatments was determined using one-way analysis of variance (ANOVA). Bonferroni’s post-hoc test was applied for multiple comparisons, wherever necessary. The data were fitted to linear (Y = α + βD) or linear quadratic (Y = C + αD + βD2) equations to describe the dose–response, if any, where C is control MN frequency, D is DOX dose and α and β are constants.
3. RESULTS AND DISCUSSION
3.1. Effect of irradiation on APN and leptin levels
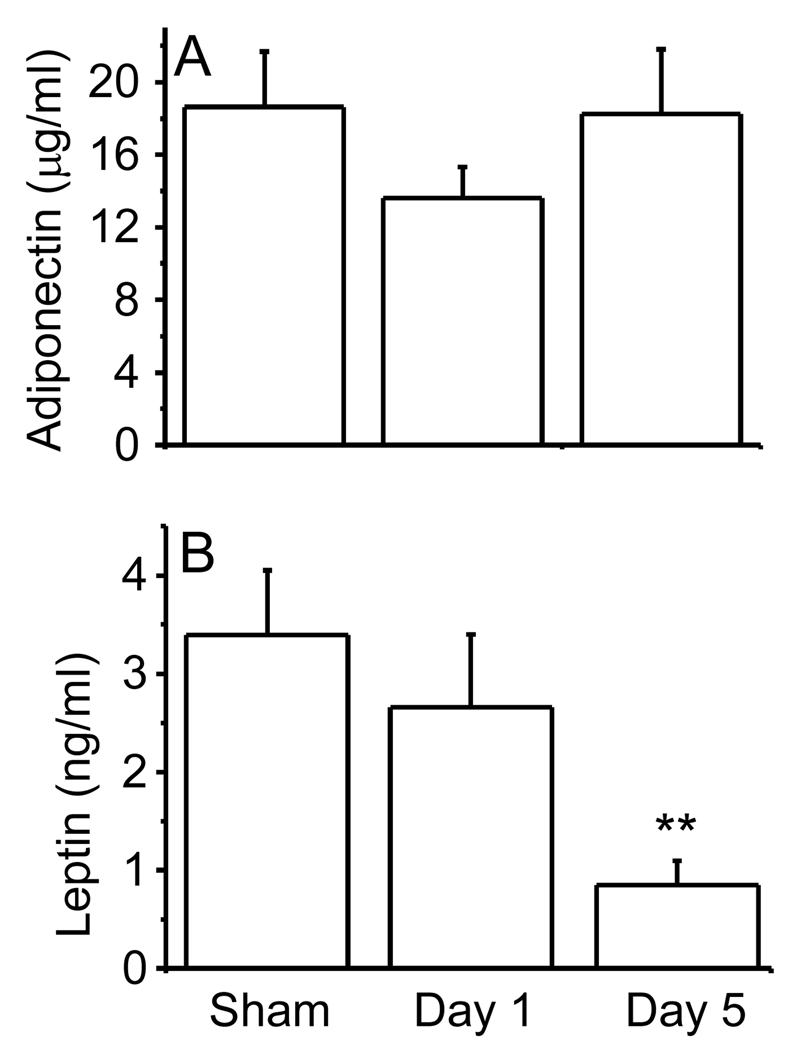
Irradiation of WT mice with 6 Gy did not significantly alter circulating APN levels (Fig. 1A). In contrast, serum levels of another adipokine, leptin, were reduced in a time-dependent manner at day 1 and 5 post-irradiation (Fig 1B). These results indicate that, although whole-body irradiation markedly down regulates leptin production, it does not exert a significant effect on APN levels, thus underlying the profound difference in the mechanisms of regulation of these two adipokines.
Figure 1. Effect of irradiation on serum APN (A) and leptin (B) levels in WT mice exposed to 6 Gy gamma irradiation.
Mice were whole-body irradiated with 6 Gy or sham-irradiated. Blood was collected on day 1 or day 5 post-irradiation and serum obtained for measurement of APN and leptin levels. Data are mean ± SEM; n=5. **p<0.01 vs sham-irradiated.
3.2. Effect of APN deficiency on radiation-induced thymus atrophy
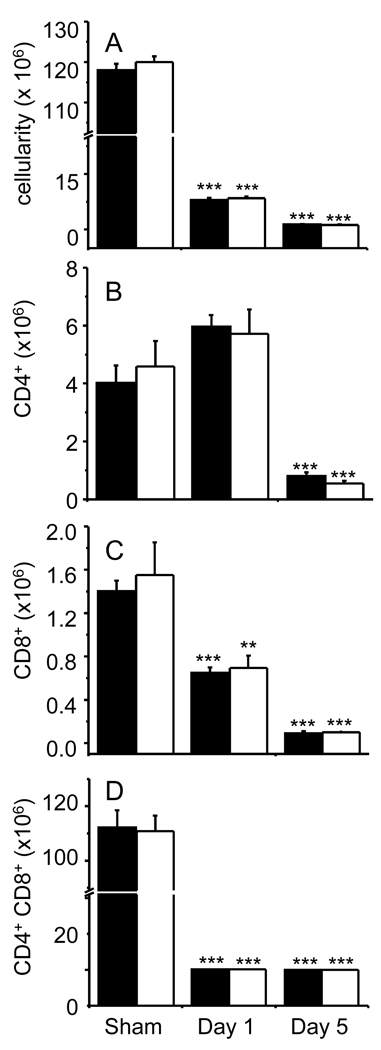
The thymus is one of the most radiosensitive organs and thymocytes rapidly undergo apoptosis after exposure to ionizing radiation. Because important protective effects against thymocyte apoptosis have been described for the adipokine leptin [36], it was of interest to evaluate whether APN is also involved in protection against thymus atrophy Whole-body irradiation with 6 Gy caused a significant reduction in thymus cellularity in both WT and APN KO mice on days 1 and 5 compared to sham-irradiated mice (Fig 2A). A significant reduction in the absolute number of CD4+ T cells was observed on day 5 in irradiated mice (Fig. 2B). Irradiation caused a time-dependent reduction in the CD8+ T cells (Fig. 2C). CD4+CD8+ T cells followed a similar trend as the CD8+ subpopulation (Fig 2D). No significant differences in thymus cellularity or T cell subpopulations was observed between WT and APN KO mice that were sham-irradiated or whole-body irradiated, suggesting that, at variance with leptin, APN is not involved in modulation of baseline thymus cellularity or radiation-induced thymus atrophy.
Figure 2. Effect of irradiation on thymus cellularity and T cell subpopulations in WT and APN KO mice.
WT (black columns) and APN KO (white columns) mice were whole-body irradiated with 6 Gy or sham-irradiated. The thymus was collected on day 1 or day 5 post-irradiation and analyzed by flow cytometry. Total cellularity (A); Number of CD4+ (B), CD8+ (C), and CD4+CD8+ (D) cells. Data are mean ± SEM; n=5. ***p<0.001, **p<0.01 vs respective sham-irradiated.
3.3. Effect of APN deficiency on radiation-induced micronuclei formation
The detection of micronuclei (MN) in circulating erythrocytes has been used as a cytogenetic test for chromosomal aberrations induced by genotoxic or clastogenic effects of chemical or radiation exposure [34,37–39]. Induction of MN in peripheral blood reticulocytes (MN-RET) has also been used as a biological dosimeter in mice exposed to radiation [40,41].
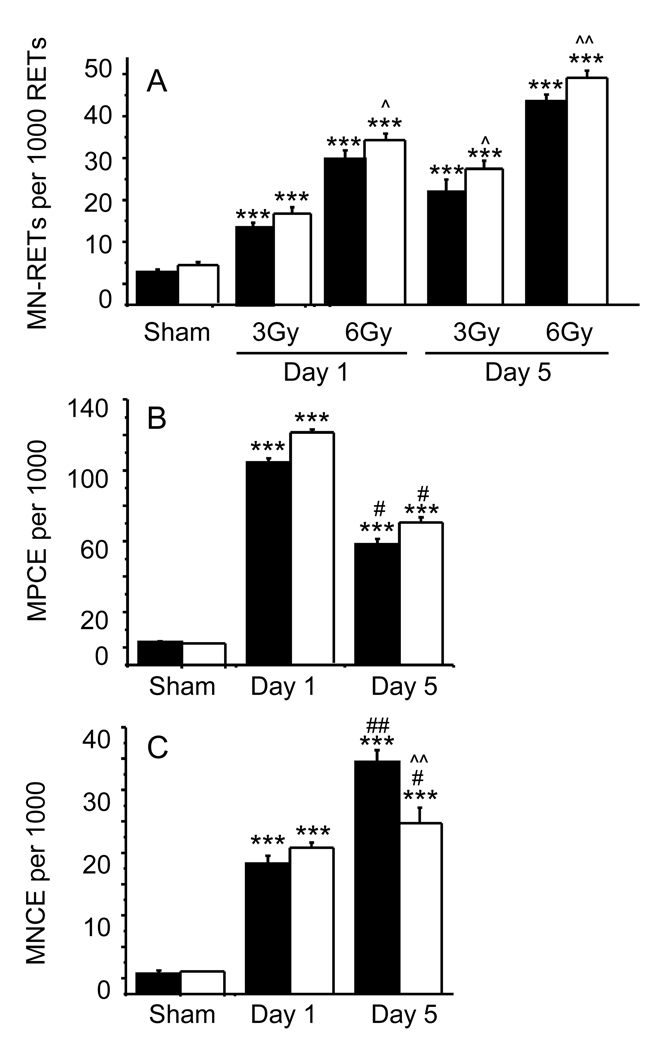
Exposure of WT and APN KO mice to 3 or 6 Gy resulted in a significant, time-dependent induction in the frequency of MN-RET in peripheral blood at days 1 and 5 in both strains (Fig. 3A). There was no significant difference in MN-RET between APN KO and WT mice in the sham-irradiated group. However, APN KO mice had a significantly higher MN-RET frequency compared to WT mice at day 1 post-irradiation with 3 Gy and at both days 1 and 5 when the dose of 6 Gy was used (Fig 3A). Although other mechanisms might contribute to the observed effect, the increased frequency of MN-RET in irradiated APN KO mice is likely secondary to the protective effect of APN against oxidative damage [23–25].
Figure 3. Influence of irradiation on micronuclei formation in WT and APN KO mice.
WT (black columns) and APN KO (white columns) mice were sham-irradiated or whole-body irradiated with either 3 Gy or 6 Gy for evaluation of MN-RET (Panel A) and with 3Gy for MPCE (Panel B) and MNCE (Panel C). Data are mean ± SEM (n=5). *** p<0.001 vs respective sham-irradiated; ^p<0.05, ^^p<0.01 vs respective WT; # p< 0.05, ##p<0.01 vs respective day 1.
Studies from radiotherapy patients have shown that exposure to low doses of radiation causes myelosuppression and induces treatment-related cancers, especially leukemia [4,42,43]. Irradiation of both WT and APN KO mice with 3 Gy resulted in a significant elevation in the frequency of micronucleated polychromatic (MPCE) and normochromatic (MNCE) erythrocytes in the bone marrow at days 1 and 5 post-irradiation (Fig. 3B and C). The frequency of MPCE reached a peak at day 1 and declined thereafter by day 5 post-irradiation in both the WT and APN KO groups. There were no significant differences in MPCE frequency between WT and APN KO mice. The frequency of MNCE was significantly elevated in both WT and APN KO mice at days 1 and 5 when compared to sham-irradiated mice. A significant increase in MNCE frequency was observed in WT compared to APN KO mice at day 5 post-irradiation (Fig. 3C), indicating a less marked delay in erythropoiesis after irradiation in APN KO compared with WT mice. Although no alterations in circulating erythrocytes has been observed in APN KO mice under baseline conditions [44], a possible role for APN in regulating erythropoiesis following irradiation remains to be investigated.
3.4. Effect of APN deficiency on radiation-induced gastrointestinal damage and cell proliferation
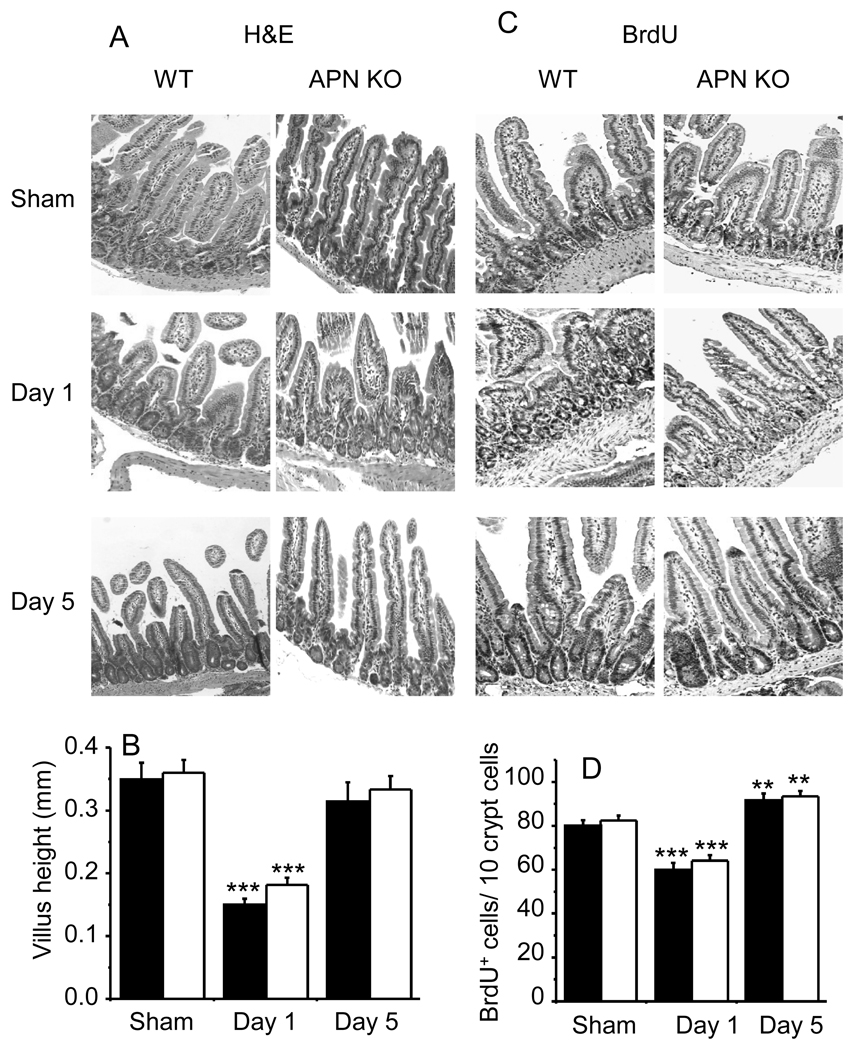
The gastrointestinal tract is an important target for radiation damage as it is a continuum of constantly renewing tissue with rapid rates of cell division, differentiation, migration and attrition throughout the lifetime of an animal [45,46]. No significant difference in jejunum structure was observed between APN KO and WT sham-irradiated mice (Fig. 4A). Irradiation with 6 Gy induced the expected changes in jejunum histological appearance, including a marked edema in the sub-mucosa with mild surface erosion, distortion of the villous and crypt architecture, with depopulation and degeneration of crypts, and reduced villous height (Fig. 4C). Intestinal damage was more pronounced at day 1 compared to day 5 post-irradiation, when signs of regenerating crypts were present. No significant differences in the histology of the jejunum were observed between irradiated WT and APN KO mice at either time point, indicating that APN deficiency exerts neither a protective nor a deleterious function in radiation-induced damage of intestinal structures. When mice where irradiated with 3 Gy, no significant intestinal alterations were observed in either WT or APN KO mice (data not shown).
Figure 4. Influence of irradiation on jejunum histology and cell proliferation in WT and APN KO mice.
WT (black columns) and APN KO (white columns) mice were whole-body irradiated with 6 Gy or sham-irradiated. Jejunum was obtained and stained with H&E for histological examination. A representative staining from each mouse is shown in Panel A and quantification for the whole group in Panel B. The BrdU assay was used for evaluation of cell proliferation (Panels C and D). A representative staining for each group is shown in Panels A and C. Villous height (Panel B) and quantification of cell proliferation (Panel D) were performed as described in the Methods section. Data are mean ± SEM (n=5). **p<0.01, *** p<0.001 vs respective sham-irradiated.
Cell proliferation in the crypts was detected by monitoring DNA replication using the BrdU-incorporation assay (Fig. 4B). The mean number of proliferating cells on day 1 post-irradiation was significantly reduced in both WT and APN KO mice compared with their respective sham-irradiated controls. No significant difference in the response were observed between WT and APN KO mice (Fig. 4D). At day 5 post-irradiation, cell proliferation significantly increased over sham-irradiated mice in both WT and APN KO mice, but no significant effect of APN deficiency was observed (Fig. 4B). Thus, at variance with results we previously obtained in the model of DSS-induced colitis [31], APN deficiency is not associated with enhanced epithelial cell proliferation in response to radiation-induced damage, underlying the presence of either a tissue- or stimulus-dependent effect.
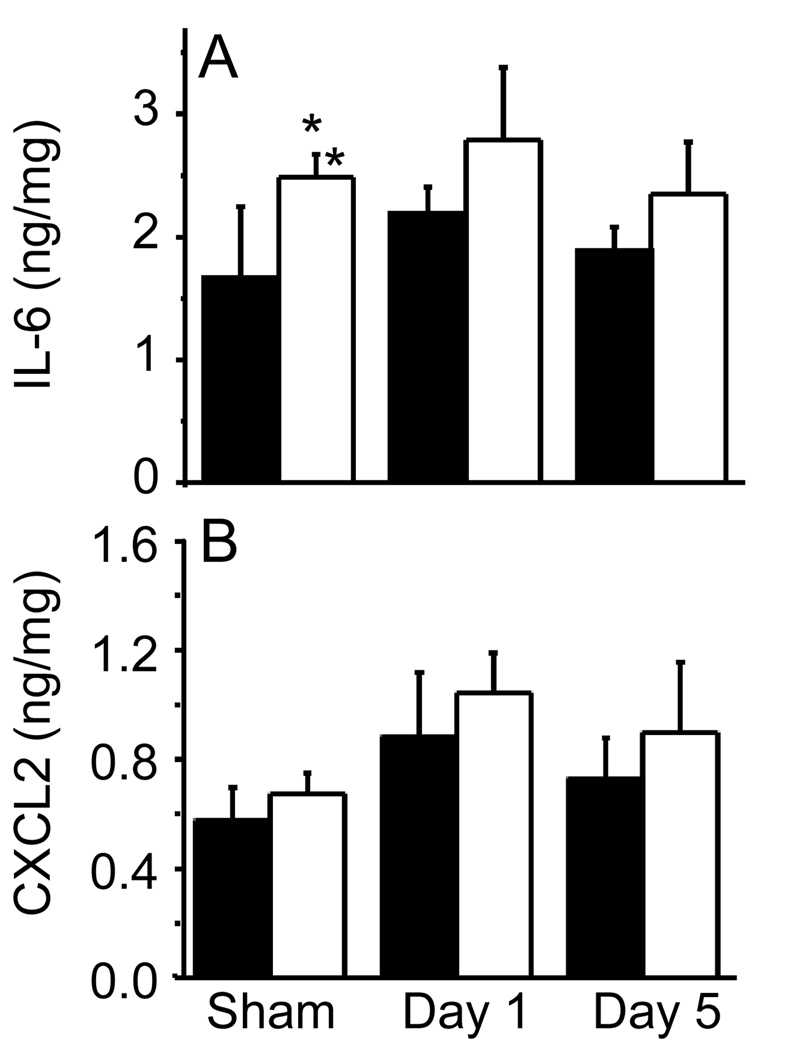
Finally, cultures of jejunum explants obtained from sham-irradiated APN KO mice spontaneously released significantly higher amounts of IL-6 compared with explants from sham-irradiated WT mice (Figure 5A). Irradiation with 6 Gy did not significantly alter IL-6 production from jejunum in either WT or APN KO mice at either 1 or 5 days (Figure 5). Production of the chemokine CXCL2 by jejunum explants did not differ between WT and APN KO mice and no significant effect of irradiation was observed at day 1 or 5 (Figure 5B).
Figure 5. Production of IL-6 and CXCL2 from jejunum explants of WT and APN KO mice.
WT (black columns) and APN KO (white columns) mice were whole-body irradiated with 6 Gy or sham-irradiated. Levels of IL-6 (Panel A) and CXCL2 (Panel B) were measured in the supernatant of jejunum explants cultured overnight. Data are expressed as ng of cytokine/mg of protein and are mean ± SEM (n=5). *p<0.05 vs sham-irradiated WT.
Collectively these data indicate that APN deficiency does not play a critical role in the response of the gastrointestinal tract to irradiation, although APN deficiency was associated with increased production of IL-6 from the jejunum under baseline conditions, in agreement with previous reports demonstrating to an anti-inflammatory role for APN in the gastrointestinal tract and in other organs [20,21,44].
4. CONCLUSIONS
The present results demonstrate that APN deficiency does not play a major role in the response to whole-body irradiation in mice in terms of thymus atrophy and gastrointestinal damage, although a significant effect of APN deficiency was observed in terms of MN-RET and MNCE frequency in response to irradiation. Our initial hypothesis was that APN would modulate radiation-induced intestinal and hemopoietic alterations since APN can protect against oxidative stress and antioxidants reduce radiation-induced damage [27,28,47,48]. On the other hand, APN binds to several growth factors and inhibits their biological activity, thus potentially inhibiting cell repair, particularly in the intestine [29,31]. However our results indicate that absence of APN did not alter radiation-induced injury in the gastrointestinal tract, while leading to significantly enhanced radiation-induced damage in the erythrocyte compartment, possibly secondary to the increase in oxidative stress that is associated with reduced APN levels [23–25].
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement:
The authors declare that are no conflicts of interest.
REFERENCES
- 1.Hall EJ. Radiation, the two-edged sword: cancer risks at high and low doses. Cancer J. 2000;6:343–350. [PubMed] [Google Scholar]
- 2.Hogle WP. The state of the art in radiation therapy. Semin Oncol Nurs. 2006;22:212–220. doi: 10.1016/j.soncn.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Dainiak N, Waselenko JK, Armitage JO, MacVittie TJ, Farese AM. The hematologist and radiation casualties. Hematology Am Soc Hematol Educ Program. 2003:473–496. doi: 10.1182/asheducation-2003.1.473. [DOI] [PubMed] [Google Scholar]
- 4.Tubiana M, Carde P, Frindel E. Ways of minimising hematopoietic damage induced by radiation and cytostatic drugs--the possible role of inhibitors. Radiother Oncol. 1993;29:1–17. doi: 10.1016/0167-8140(93)90167-7. [DOI] [PubMed] [Google Scholar]
- 5.Bismar MM, Sinicrope FA. Radiation enteritis. Curr Gastroenterol Rep. 2002;4:361–365. doi: 10.1007/s11894-002-0005-3. [DOI] [PubMed] [Google Scholar]
- 6.Potten CS. Radiation, the ideal cytotoxic agent for studying the cell biology of tissues such as the small intestine. Radiat Res. 2004;161:123–136. doi: 10.1667/rr3104. [DOI] [PubMed] [Google Scholar]
- 7.Dinarello CA. Interleukin-1 and its biologically related cytokines. Adv Immunol. 1989;44:153–205. doi: 10.1016/s0065-2776(08)60642-2. [DOI] [PubMed] [Google Scholar]
- 8.Neta R, Oppenheim JJ. Cytokines in therapy of radiation injury. Blood. 1988;72:1093–1095. [PubMed] [Google Scholar]
- 9.Neta R, Okunieff P. Cytokine-Induced Radiation Protection and Sensitization. Semin Radiat Oncol. 1996;6:306–320. doi: 10.1053/SRAO00600306. [DOI] [PubMed] [Google Scholar]
- 10.Booth D, Potten CS. Protection against mucosal injury by growth factors and cytokines. J Natl Cancer Inst Monogr. 2001:16–20. doi: 10.1093/oxfordjournals.jncimonographs.a003433. [DOI] [PubMed] [Google Scholar]
- 11.Wingard JR, Demetri GD. Clinical Applications of Cytokines and Growth Factors. 1999 [Google Scholar]
- 12.Ozer H, Armitage JO, Bennett CL, Crawford J, Demetri GD, Pizzo PA, Schiffer CA, Smith TJ, Somlo G, Wade JC, Wade JL, 3rd, Winn RJ, Wozniak AJ, Somerfield MR. 2000 update of recommendations for the use of hematopoietic colony-stimulating factors: evidence-based, clinical practice guidelines. American Society of Clinical Oncology Growth Factors Expert Panel. J Clin Oncol. 2000;18:3558–3585. doi: 10.1200/JCO.2000.18.20.3558. [DOI] [PubMed] [Google Scholar]
- 13.MacVittie TJ, Farese AM. Cytokine-based treatment of radiation injury: potential benefits after low-level radiation exposure. Mil Med. 2002;167:68–70. [PubMed] [Google Scholar]
- 14.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 15.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 16.Ouchi N, Kihara S, Funahashi T, Nakamura T, Nishida M, Kumada M, Okamoto Y, Ohashi K, Nagaretani H, Kishida K, Nishizawa H, Maeda N, Kobayashi H, Hiraoka H, Matsuzawa Y. Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue. Circulation. 2003;107:671–674. doi: 10.1161/01.cir.0000055188.83694.b3. [DOI] [PubMed] [Google Scholar]
- 17.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 18.Ouchi N, Walsh K. Adiponectin as an anti-inflammatory factor. Clin Chim Acta. 2007;380:24–30. doi: 10.1016/j.cca.2007.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 20.Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, Hotta K, Nishida M, Takahashi M, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–1301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 21.Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, Ouchi N, Kihara S, Funahashi T, Tenner AJ, Tomiyama Y, Matsuzawa Y. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000;96:1723–1732. [PubMed] [Google Scholar]
- 22.DiMascio L, Voermans C, Uqoezwa M, Duncan A, Lu D, Wu J, Sankar U, Reya T. Identification of adiponectin as a novel hemopoietic stem cell growth factor. J Immunol. 2007;178:3511–3520. doi: 10.4049/jimmunol.178.6.3511. [DOI] [PubMed] [Google Scholar]
- 23.Jung TW, Lee JY, Shim WS, Kang ES, Kim JS, Ahn CW, Lee HC, Cha BS. Adiponectin protects human neuroblastoma SH-SY5Y cells against acetaldehyde-induced cytotoxicity. Biochem Pharmacol. 2006;72:616–623. doi: 10.1016/j.bcp.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 24.Lam KS, Xu A. Adiponectin: protection of the endothelium. Curr Diab Rep. 2005;5:254–259. doi: 10.1007/s11892-005-0019-y. [DOI] [PubMed] [Google Scholar]
- 25.Li R, Wang WQ, Zhang H, Yang X, Fan Q, Christopher TA, Lopez BL, Tao L, Goldstein BJ, Gao F, Ma XL. Adiponectin improves endothelial function in hyperlipidemic rats by reducing oxidative/nitrative stress and differential regulation of eNOS/iNOS activity. Am J Physiol Endocrinol Metab. 2007;293:E1703–E1708. doi: 10.1152/ajpendo.00462.2007. [DOI] [PubMed] [Google Scholar]
- 26.Gutteridge JM, Halliwell B. Free radicals and antioxidants in the year 2000. A historical look to the future. Ann N Y Acad Sci. 2000;899:136–147. doi: 10.1111/j.1749-6632.2000.tb06182.x. [DOI] [PubMed] [Google Scholar]
- 27.Nakanishi S, Yamane K, Kamei N, Nojima H, Okubo M, Kohno N. A protective effect of adiponectin against oxidative stress in Japanese Americans: the association between adiponectin or leptin and urinary isoprostane. Metabolism. 2005;54:194–199. doi: 10.1016/j.metabol.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 28.Katsuki A, Suematsu M, Gabazza EC, Murashima S, Nakatani K, Togashi K, Yano Y, Adachi Y, Sumida Y. Increased oxidative stress is associated with decreased circulating levels of adiponectin in Japanese metabolically obese, normal-weight men with normal glucose tolerance. Diabetes Res Clin Pract. 2006;73:310–314. doi: 10.1016/j.diabres.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Lam KS, Xu JY, Lu G, Xu LY, Cooper GJ, Xu A. Adiponectin inhibits cell proliferation by interacting with several growth factors in an oligomerization-dependent manner. J Biol Chem. 2005;280:18341–18347. doi: 10.1074/jbc.M501149200. [DOI] [PubMed] [Google Scholar]
- 30.Masaie H, Oritani K, Yokota T, Takahashi I, Shirogane T, Ujiie H, Ichii M, Saitoh N, Maeda T, Tanigawa R, Oka K, Hoshida Y, Tomiyama Y, Kanakura Y. Adiponectin binds to chemokines via the globular head and modulates interactions between chemokines and heparan sulfates. Exp Hematol. 2007;35:947–956. doi: 10.1016/j.exphem.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Fayad R, Pini M, Sennello JA, Cabay RJ, Chan L, Xu A, Fantuzzi G. Adiponectin deficiency protects mice from chemically induced colonic inflammation. Gastroenterology. 2007;132:601–614. doi: 10.1053/j.gastro.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 32.Ma K, Cabrero A, Saha PK, Kojima H, Li L, Chang BH, Paul A, Chan L. Increased beta -oxidation but no insulin resistance or glucose intolerance in mice lacking adiponectin. J Biol Chem. 2002;277:34658–34661. doi: 10.1074/jbc.C200362200. [DOI] [PubMed] [Google Scholar]
- 33.Hayashi M, MacGregor JT, Gatehouse DG, Blakey DH, Dertinger SD, Abramsson-Zetterberg L, Krishna G, Morita T, Russo A, Asano N, Suzuki H, Ohyama W, Gibson D. In vivo erythrocyte micronucleus assay III. Validation and regulatory acceptance of automated scoring and the use of rat peripheral blood reticulocytes, with discussion of non-hematopoietic target cells and a single dose-level limit test. Mutat Res. 2007;627:10–30. doi: 10.1016/j.mrgentox.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 34.Schmid W. The micronucleus test. Mutat Res. 1975;31:9–15. doi: 10.1016/0165-1161(75)90058-8. [DOI] [PubMed] [Google Scholar]
- 35.Jagetia GC, Jacob PS. Vinblastine treatment induces dose-dependent increases in the frequency of micronuclei in mouse bone marrow. Mutat Res. 1992;280:87–92. doi: 10.1016/0165-1218(92)90003-i. [DOI] [PubMed] [Google Scholar]
- 36.Fantuzzi G. Three questions about leptin and immunity. Brain Behav Immunity. 2008 doi: 10.1016/j.bbi.2008.10.007. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jenssen D, Ramel C. Dose response at low doses of X-irradiation and MMS on the induction of micronuclei in mouse erythroblasts. Mutat Res. 1976;41:311–320. doi: 10.1016/0027-5107(76)90104-4. [DOI] [PubMed] [Google Scholar]
- 38.Jenssen D, Ramel C. Factors affecting the induction of micronuclei at low doses of X-rays, MMS and dimethylnitrosamine in mouse erythroblasts. Mutat Res. 1978;58:51–65. doi: 10.1016/0165-1218(78)90095-2. [DOI] [PubMed] [Google Scholar]
- 39.Schlegel R, MacGregor JT. The persistence of micronuclei in peripheral blood erythrocytes: detection of chronic chromosome breakage in mice. Mutat Res. 1982;104:367–369. doi: 10.1016/0165-7992(82)90171-3. [DOI] [PubMed] [Google Scholar]
- 40.Lenarczyk M, Slowikowska MG. The micronucleus assay using peripheral blood reticulocytes from X-ray-exposed mice. Mutat Res. 1995;335:229–234. doi: 10.1016/0165-1161(95)00025-9. [DOI] [PubMed] [Google Scholar]
- 41.Lenarczyk M, Goddu SM, Rao DV, Howell RW. Biologic dosimetry of bone marrow: induction of micronuclei in reticulocytes after exposure to 32P and 90Y. J Nucl Med. 2001;42:162–169. [PubMed] [Google Scholar]
- 42.Weiss JF, Landauer MR. Protection against ionizing radiation by antioxidant nutrients and phytochemicals. Toxicology. 2003;189:1–20. doi: 10.1016/s0300-483x(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 43.Soares DP, Gilligan P. Ionizing radiation. The question of responsible use: Pandora's box revisited. West Indian Med J. 2004;53:118–121. [PubMed] [Google Scholar]
- 44.Gove M, Pini M, Fayad R, Cabay RJ, Fantuzzi G. Adiponectin deficiency modulates adhesion molecules expression and cytokine production but does not affect disease severity in the transfer model of colitis in mice. Cytokine. 2009 doi: 10.1016/j.cyto.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Potten CS, Morris RJ. Epithelial stem cells in vivo. J Cell Sci Suppl. 1988;10:45–62. doi: 10.1242/jcs.1988.supplement_10.4. [DOI] [PubMed] [Google Scholar]
- 46.Jonathan EC, Bernhard EJ, McKenna WG. How does radiation kill cells? Curr Opin Chem Biol. 1999;3:77–83. doi: 10.1016/s1367-5931(99)80014-3. [DOI] [PubMed] [Google Scholar]
- 47.Urakawa H, Katsuki A, Sumida Y, Gabazza EC, Murashima S, Morioka K, Maruyama N, Kitagawa N, Tanaka T, Hori Y, Nakatani K, Yano Y, Adachi Y. Oxidative stress is associated with adiposity and insulin resistance in men. J Clin Endocrinol Metab. 2003;88:4673–4676. doi: 10.1210/jc.2003-030202. [DOI] [PubMed] [Google Scholar]
- 48.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]