Abstract
Insulin receptor substrate-1 (IRS-1) is a cytoplasmic scaffolding protein that is phosphorylated by insulin-like growth factor-I receptor and recruits downstream effectors. Recent evidence suggests that IRS-1 has a nuclear localization and function. Here we investigated whether nuclear and cytoplasmic IRS-1 levels are associated with clinico-pathological characteristics and clinical outcome in breast cancer patients. Tissue microarrays from 1,097 patients with stage I–II breast cancer were stained by immunohistochemistry for IRS-1. Nuclear and cytoplasmic IRS-1 were scored separately according to the Allred score. Nuclear IRS-1 showed a positive association with estrogen receptor (ER) (r = 0.09, P = 0.003) and progesterone receptor (PR) (r = 0.08, P = 0.008) status and a negative correlation with lymph node involvement (r = −0.10, P = 0.001). Cytoplasmic IRS-1 did not correlate with ER or PR but showed a positive correlation with tumor size (r = 0.10, P = 0.001) and S-phase fraction (r = 0.16, P < 0.001). In univariate analysis, tamoxifen-treated patients with tumors showing positive nuclear IRS-1 had a better recurrence-free survival (RFS) (P = 0.009) and overall survival (OS) (P = 0.0007), while no association was shown between cytoplasmic IRS-1 and RFS or OS in the same group of patients. In multivariate analysis of patients receiving tamoxifen, negative nuclear IRS-1 showed a significantly reduced RFS (P = 0.046) and OS (P = 0.018). Combining both PR and nuclear IRS-1, tamoxifen-treated patients with PR+/IRS-1+ tumors had a better RFS (P = 0.0003) and OS (P < 0.0001) when compared with patients with PR–/IRS-1– tumors. In conclusion, nuclear IRS-1 may be a useful marker to predict tamoxifen response in patients with early breast cancer, particularly when assessed in combination with PR.
Keywords: IRS1, IRS-1, Breast cancer, Prognosis, Estrogen receptor
Introduction
Tamoxifen (Tam) is one of the most effective drugs in the endocrine therapy of all stages of estrogen receptor (ER) positive breast cancer [1] and is still frequently prescribed despite the growing use of aromatase inhibitors as hormonal agents. Moreover, Tam remains the endocrine treatment of choice in premenopausal breast cancer patients.
ER is expressed in 80% of breast tumors and is by far the most useful biomarker for predicting Tam treatment response. Nearly half of ER positive tumors co-express progesterone receptor (PR). Patients with ER +/PR + tumors respond better to Tam therapy compared to patients with tumors that are ER–/PR– or ER+/PR– [2]. However, despite hormone receptor positivity, a subset of patients do not benefit from Tam therapy, ultimately resulting in failure of treatment and death. Because treatment is not devoid of side effects it is important to identify new biomarkers that will improve response prediction.
Insulin receptor substrate 1 (IRS-1) is an adaptor protein primarily responsible for transducing signals from insulin and insulin-like growth factor-I (IGF-I) receptors. In addition, IRS-1 is activated by numerous growth factor receptors such as epidermal growth factor receptor [3] and hormones, including growth hormone and estrogen [4]. The importance of IRS-1 in hormone-dependent breast cancer has been emphasized in different models. IRS-1 is an estrogen-regulated gene frequently expressed in ER positive breast cancer cells [5] where it has been involved in anchorage-independent growth, cell survival [6, 7], and estrogen independent growth [8]. ER stabilizes IRS-1 and reduces its ubiquitination [9]. Moreover, in ER positive cells E2 increases IRS-1 levels [10–12], induces IRS-1 phosphorylation and enhances its downstream signaling [10, 11]. IRS-1 levels and signaling are also modulated by antiestrogens such as Tam and Fulvestrant [12–15]. Intriguingly, in addition to its cytoplasmic function, IRS-1 can regulate nuclear processes as well [16–18]. In breast cancer cells, nuclear IRS-1 can directly interact with ER [17, 18] and modulate its transcriptional activity [17]. Nuclear levels of IRS-1 and IRS-1 recruitment on estrogen response element (ERE) motifs are significantly increased by E2 [17, 18]. Preclinical studies have investigated the role of IRS-1 in breast cancer tumorigenesis and metastasis. In vivo transgenic mouse models of breast cancer showed that loss of IRS-1 enhances breast cancer metastasis, supporting the hypothesis that IRS-1 may have a metastasis suppressor function [19].
Despite numerous preclinical studies, there are only very limited and conflicting reports on IRS-1 expression in breast cancer clinical samples and on the role of IRS-1 in patient outcome [20–23]. In order to address this question, we examined cytoplasmic and nuclear IRS-1 expression in a large series of primary human breast tumors with available clinical data and extensive follow-up following either no adjuvant therapy (testing the association of IRS-1 levels with prognosis) or endocrine therapy (testing the ability of IRS-1 levels to predict response to Tam therapy).
Materials and methods
Tumor specimens and patient population
This study was approved by the Baylor College of Medicine Institutional Review Board. Tumor samples were from a prospectively assembled tumor bank (Tumor Bank and Data Network Core in the Lester and Sue Smith Breast Center at Baylor College of Medicine). Tissue specimens were prepared from a cohort of 1,424 frozen tumor specimens as previously described [24]. Individual samples were fixed for 8 h in 10% neutral-buffered formalin and routinely processed to paraffin blocks. Samples were subsequently arrayed (12 samples/array; each core 5 mm in diameter). These uniformly prepared tissue samples have been used to validate other prognostic and predictive factors in breast cancer including PR [25] and ER [26]. The study population consisted of patients who were diagnosed between 1973 and 1998 with stage I and II primary breast cancer with no distant metastasis, treated with mastectomy or lumpectomy plus axillary dissection, with or without post-operative radiation therapy. Complete data on tumor size, number of nodes, receptors, S-phase fraction, ploidy, and use and type of adjuvant therapy were available. Of this population, 695 patients did not receive adjuvant therapy after primary treatment, 402 received Tam only and 327 received chemotherapy or a combination therapy. Median follow-up was 84 months.
Immunohistochemistry (IHC)
Routine 4 μm sections were cut from the blocks, mounted onto slides, deparaffinized and hydrated, then immunostained using standard protocols. Antigen retrieval was performed by heating in 0.1 M Tris–HCl buffer (pH 9.0) using a pressure cooker for 10 min. Slides were incubated with the IRS-1 primary rabbit polyclonal antibody (#06-248, Upstate, Lake Placid, New York, USA) at a dilution of 1:200 for 1 h at room temperature and then incubated with the secondary, biotinylated antibody for 30 min. Sections were then incubated with streptavidin-peroxidase for 30 min and the enzyme was visualized after 15 min of incubation with diaminobenzidine. Finally, slides were lightly counterstained with 0.05% methyl green. Negative controls included omission of the primary antibody or inclusion of a non-specific IgG. The IRS-1 antibody has previously been used by many groups who have shown its specificity in IHC and immunofluorescence using different models including mice [27], human and mouse cell lines lacking IRS-1 [28], and human breast cancers [23].
Scoring of immunohistochemistry
Immunostained slides were evaluated for both nuclear and cytoplasmic IRS-1 according to the Allred score [29] by a pathologist (IM) who was blinded to the clinical data. Briefly, each entire core was evaluated by light microscopy. First, a proportion score was assigned, which represents the estimated proportion of positive-staining tumor cells (0, none; 1,<1/100; 2, 1/100 to 1/10; 3, 1/10 to 1/3; 4, 1/3 to 2/3; and 5 > 2/3). Next, an intensity score was assigned, which represents the average intensity of positive tumor cells (0, none; 1, weak; 2, intermediate; 3, strong). The proportion and intensity scores were then added to obtain a total score, which ranged from 0 to 8.
Statistical analysis
Descriptive statistics were calculated as frequencies and proportions to summarize clinico-pathological characteristics in Tam-treated and untreated patients. In order to identify optimal cut points of nuclear and cytoplasmic IRS-1 Allred scores, median values were calculated and Martingale residual plots were generated to evaluate their functional forms. IRS-1 status was dichotomized as below the median Allred score (IRS-1 negative (−)) versus above median (IRS-1 positive (+)). Nuclear and cytoplasmic IRS-1 were analyzed separately. Correlations between IRS-1 and clinico-pathological characteristics were analyzed as continuous variables using Spearman rank correlation (r).
Univariate analysis of IRS-1 on recurrence-free survival (RFS) and overall survival (OS) was carried out using the Kaplan–Meier method and compared using the log-rank test or generalized Wilcoxon test, which is more appropriate when the assumption of proportional hazards is not met. RFS was calculated from the time of diagnosis to the date of the first proven recurrence. Patients without recurrence were censored at last follow-up or death not due to cancer. OS was calculated from the time of diagnosis to death from any cause or censored at last follow-up. Follow-up was truncated at 180 months for purposes of plotting.
The prognostic and predictive significance of IRS-1 was analyzed by Cox proportional hazards regression models. Tests for proportionality of nuclear and cytoplasmic IRS-1 were performed by including time dependent covariate in the Cox models. All variables of interest were entered into multivariate Cox regression models and model-building proceeded using stepwise selection. Clinico-pathological variables were categorized according to standard cut-offs shown in Table 1. Analyses for RFS and OS were performed separately for Tam-treated and untreated patients.
Table 1.
Distribution of clinical characteristics of all breast cancer patients included in the study and divided according to the treatment
| All (n = 1,097) | Untreated (n = 695) | Tam Treated (n = 402) | |
|---|---|---|---|
| Age (years), No. (%) | |||
| ≤50 | 212 (19.3) | 172 (24.8) | 40 (10.0) |
| >50 | 885 (80.7) | 523 (75.2) | 362 (90.0) |
| Tumor size (cm), No. (%) | |||
| 0–2 | 410 (37.8) | 261 (38.1) | 149 (37.2) |
| >2–5 | 583 (53.7) | 365 (53.3) | 218 (54.5) |
| >5 | 92 (8.5) | 59 (8.6) | 33 (8.3) |
| Missing | 12 | ||
| Nodes, No. (%) | |||
| Node negative | 791 (72.1) | 574 (82.6) | 217 (54.0) |
| Node positive | |||
| 1–3 | 173 (15.8) | 72 (10.4) | 101 (25.1) |
| >3 | 133 (12.1) | 49 (7.0) | 84 (20.9) |
| S phase, No. (%) | |||
| Low (0 to <6%) | 277 (30.8) | 175 (30.8) | 102 (30.7) |
| Intermediate (≥6 to ≤10%) | 261 (29.0) | 151 (26.6) | 110 (33.1) |
| High (>10%) | 362 (40.2) | 242 (42.6) | 120 (36.1) |
| Missing | 197 | ||
| Ploidy, No. (%) | |||
| Diploid | 361 (38.4) | 230 (39.2) | 131 (37.0) |
| Aneuploid | 580 (61.6) | 357 (60.8) | 223 (63.0) |
| Missing | 156 | ||
| ER (fmol/mg), No. (%) | |||
| Negative (<3) | 161 (14.7) | 143 (20.6) | 18 (4.5) |
| Positive (≥3) | 936 (85.3) | 552 (79.4) | 384 (95.5) |
| PR (fmol/mg), No. (%) | |||
| Negative (<5) | 421 (39.6) | 294 (44.0) | 127 (32.2) |
| Positive (≥5) | 642 (60.4) | 374 (56.0) | 268 (67.8) |
| Missing | 34 | ||
| Median follow-up time (mo) | 84 | 85 | 84 |
Results
Patients and tumors characteristics
A total of 1,097 patients were studied including 695 patients who received no adjuvant therapy after primary treatment and 402 patients who were treated with adjuvant Tam monotherapy. The distributions of patient’s clinico-pathological characteristics are summarized in Table 1. The vast majority of studied patients were older than 50 years of age. Most of the patients had tumors less or equal to 5 cm in dimension and were node negative. Eighty-five percent of the tumors expressed ER, while 60% were PR positive. Approximately, 60% of tumors were low to intermediate S-phase and 62% of tumors were aneuploid. The median follow-up was 84 months.
IRS-1 expression
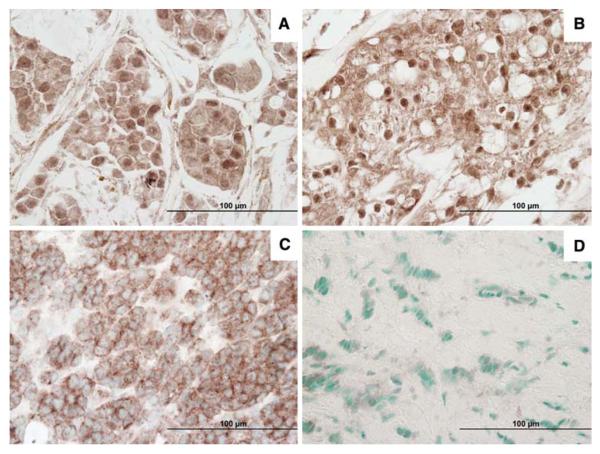
Figure 1 shows representative cases of IRS-1 IHC staining. All cases were evaluated as described in Materials and Methods. Median values of expression were calculated for cytoplasmic and nuclear IRS-1 and IRS-1 expression was classified as positive (above the median value) or negative (below the median value). We found that 33.1% of tumors were nuclear IRS-1 positive and 43.5% were cytoplasmic IRS-1 positive. In addition 44% of tumors were negative for both cytoplasmic and nuclear IRS-1 while 19.9% were double positive.
Fig. 1.
Representative photographs of the immunohistochemical expression of IRS-1 in breast cancer samples: a, b Nuclear and cytoplasmic IRS-1, Magnification 400×; c Cytoplasmic IRS-1 with no nuclear staining, Magnification 400×; d Negative IRS-1, Magnification 400×
Correlation with clinico-pathological characteristics
We assessed the correlation between nuclear and cytoplasmic IRS-1 expression with various clinico-pathological parameters. As shown in Table 2, nuclear and cytoplasmic IRS-1 were moderately correlated with each other (r = 0.36, P < 0.0001). Small, positive correlations were observed between nuclear IRS-1 and ER (r = 0.09, P = 0.003) or PR (r = 0.08, P = 0.008) status, whereas there was a small, negative correlation between nuclear IRS-1 and lymph node involvement (r = −0.10, P = 0.001). However, nuclear IRS-1 did not significantly correlate with age, tumor size, and S-phase fraction. Cytoplasmic IRS-1 demonstrated a significant, albeit small, positive correlation with tumor size (r = 0.10, P = 0.001) and S-phase fraction (r = 0.16, P < 0.001). In addition, we observed a trend for a negative correlation between cytoplasmic IRS-1 and age (r = −0.05, P = 0.092). However, cytoplasmic IRS-1 did not significantly correlate with ER or PR status and lymph node involvement.
Table 2.
Correlation of nuclear and cytoplasmic IRS-1 with all other variables
| Nuclear correlation (P)† | Cytoplasm correlation (P)† | |
|---|---|---|
| Nuclear IRS-1 | 1 | 0.36 (<0.0001) |
| Cytoplasmic IRS-1 | 0.36 (<0.0001) | 1 |
| Age | −0.012 (0.706) | −0.053 (0.092) |
| ER | 0.093 (0.003) | −0.030 (0.332) |
| PR | 0.084 (0.008) | −0.045 (0.148) |
| Tumor size | −0.044 (0.165) | 0.103 (0.001) |
| Nodes | −0.102 (0.001) | −0.012 (0.713) |
| S phase | 0.029 (0.415) | 0.159 (<0.001) |
Spearman rank correlation
Univariate analysis
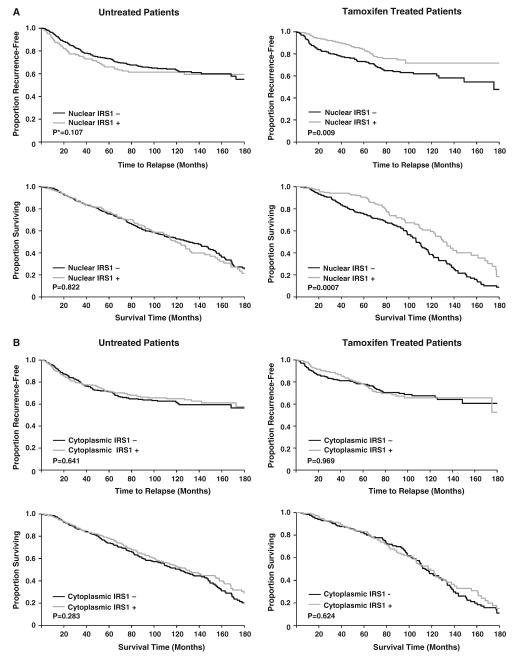
When nuclear and cytoplasmic IRS-1 were analyzed separately, Tam-treated patients with positive nuclear IRS-1 levels showed a significantly better RFS and OS than those with negative nuclear IRS-1 (P = 0.009 and P = 0.007, respectively) (Fig. 2a). However, we did not observe significant differences between positive versus negative nuclear IRS-1 among untreated patients indicating that the effect of nuclear IRS-1 is to predict response to Tam and not simply confer a good prognosis. Interestingly, we found no difference in RFS or OS between positive versus negative cytoplasmic IRS-1 among both Tam-treated and untreated patients (Fig. 2b). We also found no difference in RFS or OS between positive versus negative IRS-1 (both nuclear and cytoplasmic) when untreated patients were analyzed according to ER status (data not shown).
Fig. 2.
Recurrence-free survival and overall survival in untreated and Tam treated patients according to nuclear (a) and cytoplasmic (b) IRS-1 levels. *P-value based upon Wilcoxon test
Multivariate analysis of Tam-treated patients
The predictive effects of clinico-pathological variables (nuclear IRS-1, cytoplasmic IRS-1, age, PR, nodes) on both RFS and OS in Tam-treated patients are shown in Table 3. In multivariate analysis of RFS, negative nuclear IRS-1 was significantly associated with shorter RFS (HR = 1.59; 95% CI, 1.01–2.49, P = 0.046) while cytoplasmic IRS-1 was not associated with RFS. Patients aged 50 or less had a worse RFS than patients aged greater than 50 years old (HR = 3.05; 95% CI, 1.77–5.24, P < 0.0001). PR negativity (defined as <5 fmol/mg) and number of positive nodes were significant indicators of recurrence (P = 0.015 and P < 0.0001, respectively). Similar to RFS, negative nuclear IRS-1 was significantly associated with shorter OS (HR = 1.51; 95% CI, 1.07–2.11, P = 0.018). However, cytoplasmic IRS was not significantly associated with OS. PR negativity and number of positive nodes were indicators of poor OS (P = 0.007 and P < 0.0001, respectively).
Table 3.
Multivariate analysis of Tam treated patients
| Variable | HR | 95% CI | P |
|---|---|---|---|
| RFS | |||
| Nuclear IRS-1 | 0.046 | ||
| Negative (=0) | 1.59 | 1.01–2.49 | |
| Positive (>0) | 1.00 | - | |
| Cytoplasmic IRS-1 | 0.489 | ||
| Negative (≤6) | 0.86 | 0.57–1.31 | |
| Positive (>6) | 1.00 | - | |
| Age | <0.0001 | ||
| ≤50 | 3.05 | 1.77–5.24 | |
| >50 | 1.00 | - | |
| PR | 0.015 | ||
| Negative (<5) | 1.65 | 1.10–2.48 | |
| Positive (≥5) | 1.00 | - | |
| Nodes | <0.0001 | ||
| 0 | 1.00 | - | |
| 1–3 | 1.27 | 0.75–2.14 | |
| >3 | 3.31 | 2.06–5.30 | |
| OS | |||
| Nuclear IRS-1 | 0.018 | ||
| Negative (=0) | 1.51 | 1.07–2.11 | |
| Positive (>0) | 1.00 | - | |
| Cytoplasmic IRS-1 | 0.404 | ||
| Negative (≤6) | 0.88 | 0.64–1.20 | |
| Positive (>6) | 1.00 | - | |
| PR | 0.007 | ||
| Negative (<5) | 1.53 | 1.12–2.08 | |
| Positive (≥5) | 1.00 | - | |
| Nodes | <0.0001 | ||
| 0 | 1.00 | - | |
| 1–3 | 1.15 | 0.80–1.66 | |
| >3 | 2.29 | 1.59–3.29 |
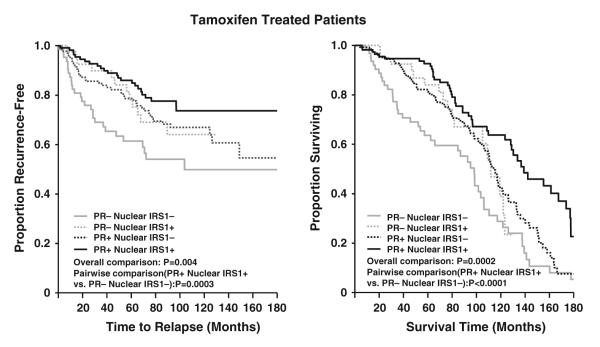
We have previously shown that PR negativity determined by either IHC or ligand binding assay is a predictor of poor response to endocrine therapies [25, 30, 31]. For this reason, secondary analyses were performed to assess the combined effect of PR and nuclear IRS-1 on RFS and OS in Tam-treated patients. As shown in Fig. 3, patients with negative PR and nuclear IRS-1 levels have poorer RFS compared to patients exhibiting a positive status of these combined markers (P = 0.0003). Likewise, the combination of PR and nuclear IRS-1 has an impact on shorter OS (P < 0.0001). The combined effect of PR and nuclear IRS on RFS and OS in Tam-treated patients remained statistically significant after multivariate adjustment in the Cox models. Specifically, patients with negative PR and IRS-1 levels were 2.65 times more likely to experience recurrences than patients showing a positive status on these combined markers (P = 0.001). Similar to RFS, patients with negative PR and IRS-1 levels were 2.47 times more likely to die than patients with a positive combination of the two markers (P < 0.0001).
Fig. 3.
Recurrence-free survival and overall survival in Tam treated patients according to PR and nuclear IRS-1 levels
Discussion
To the best of our knowledge, this is the first study on IRS-1 expression in breast cancer patients designed to reveal its prognostic and predictive role in a large and well-characterized series of breast tumors. This study examined separately the expression of both nuclear and cytoplasmic IRS-1 in relationship with clinico-pathological parameters and patient survival. Although this is not a randomized study, the large number of samples and the comprehensive follow-up give strength to our data.
Cell culture studies have shown that IRS-1 can localize and function in the nucleus and nuclear IRS-1 has already been demonstrated by IHC on clinical breast samples [16–18, 20, 22, 23]. In fact Schnarr et al. [22] noticed the presence of nuclear staining in some breast tumors as well as in normal breast tissue. However, they did not analyze the significance of this finding. Koda et al. [20] also described a strong perinuclear localization of IRS-1 in some breast tumors, but again no association with clinical parameters was addressed by these authors. The most comprehensive analysis of nuclear IRS-1 in human breast cancers was recently performed by Sisci et al. [23] who observed very low levels of nuclear IRS-1 in normal mammary epithelium and an increased expression in breast tumors. These authors also found a positive correlation between nuclear IRS-1 and ER and a negative correlation with grade, size, lymph-node involvement and proliferation rate in the subgroup of ductal cancers [23]. Our results are concordant with Sisci et al. [23] showing that nuclear IRS-1 positively correlates with ER and PR and negatively with lymph-node involvement although we did not found any correlation between nuclear IRS-1 and size of the tumor or proliferation rate. However, our analysis was conducted on the entire population irrespective of tumor subtype perhaps explaining the limited differences between the two studies.
Our results indicate that cytoplasmic IRS-1 is positively associated with tumor size and S-phase fraction and does not correlate with other clinico-pathological characteristics. Previous studies on cytoplasmic IRS-1 have offered contradictory results, with some studies showing correlation with good prognostic factors and some correlating IRS-1 with adverse prognostic factors [20, 22, 23]. Schnarr et al. [22] showed that IRS-1 is expressed at high levels in normal breast tissue and in well and moderately differentiated carcinoma and is low in poorly differentiated breast tumors. On the other hand, Koda et al. [20] found that positive cytoplasmic IRS-1 expression correlates with poorly differentiated tumors and with lymph node involvement. They also showed that in ER positive tumors IRS-1 expression positively correlates with Ki67 [20]. Finally, Sisci et al. [23] found that cytoplasmic IRS-1 positively correlates with ER in ductal carcinomas. Our results indicate that cytoplasmic IRS-1 is associated with adverse prognostic factors therefore representing a marker of more aggressive tumors. While differences in the results from these studies may be simply explained by the study of different tumor types, small numbers of tumors, technical differences in antibodies and staining, it should be noted that in some of these studies the associations are relatively weak and thus may be difficult to replicate. Furthermore, IRS-1 protein levels have been shown to be both increased and decreased by numerous hormones and growth factors, thus making it unlikely that one hormone receptor (e.g., ER) will be a major regulator and thus strongly correlated with IRS-1.
The role of IRS-1 in breast cancer prognosis is still largely unknown. We previously demonstrated, on a series of 195 node-negative primary breast cancers, that patients with tumor expressing higher levels of IRS-1 (analyzed by immunoblot) tended to have a worse disease free survival (DFS) [21] particularly in the subset of ER positive tumors [10]. However, this study was performed on tumors from patients treated with systemic therapy thus making any assessment of IRS-1 and prognosis potentially confounded by response to therapy [21]. The present study on a larger number of patients and IRS-1 assessed by IHC indicates that neither nuclear nor cytoplasm IRS-1 are prognostic markers since they do not affect survival in untreated patients. However, in this study we found that high nuclear IRS-1 levels in tumors from patients treated with Tam are associated with increased RFS and OS. These conflicts with our previous report may reflect the increase in number of samples analyzed (n = 195 vs. n = 1,097) and the different types of breast cancers (the previous study was restricted to node-negative disease). Moreover, our previous study analyzed IRS-1 by immunoblot and did not distinguish between nuclear and cytoplasmic IRS1.
The hypothesis that IRS-1 could be a potential marker of anti-estrogen hormone-therapy response is reasonable given in vitro studies that have established that IRS-1 is regulated by E2, binds ER in the cytoplasm and in the nuclear compartment and regulates ER function [9–12, 17, 18]. IRS-1 may thus reflect the presence of an intact ER signaling pathway in a tumor (similar to PR) and predict response to antiestrogen therapy. Supporting this, IRS-1 levels and signaling are down-regulated by Tam in ER positive cells [14], and Tam inhibits the induction of IRS-1 mRNA by E2 [12]. IRS-1 may also be important in Tam resistance as overexpression of IRS-1 causes MCF-7 cells to become estrogen and Tam-independent, and anti-IRS-1 siRNA treatment increases the cytotoxic effects of Tam [13]. Our results indicate that nuclear IRS-1 is a powerful predictor of better RFS and OS in Tam treated patients. This is confirmed also by our multivariate analysis of Tam treated patients where we found that negative nuclear IRS-1 is significantly associated with shorter RFS and OS. It is interesting to note that combining PR and nuclear IRS-1 levels clearly stratified the Tam-treated patients into four different survival groups and that the survival of patients with tumors PR+/nuclear IRS-1+ was significantly longer than the one of patients whose tumors express neither of these two markers. Therefore, nuclear IRS-1 can potentially give additional information on Tam response when combined with PR.
Our findings show that cytoplasmic levels of IRS-1 do not correlate with ER or PR levels, and are not a predictive marker of response to Tam. At present, there is no clear mechanism to explain why only nuclear IRS-1 can predict a better outcome in Tam treated patients. However, there are in vitro data that suggest that nuclear and cytoplasmic IRS-1 may have different biological roles. In the cytoplasm, IRS-1 acts as a docking protein for a variety of upstream regulators [4]. On the other hand, IRS-1 has been demonstrated to have nuclear functions that are strictly related to ER, at least in breast cancer cells [17, 18]. Indeed, nuclear IRS-1 co-localizes and co-precipitates with ER and can directly interact with the activation function-1/DNA binding domain of ER [17, 18] being able to modulate the ER transcriptional activity at estrogen responsive element (ERE) motifs [17]. E2 significantly increases nuclear levels of IRS-1 and increases IRS-1 recruitment on ERE motifs [17, 18]. Therefore, it can be speculated that nuclear IRS-1 could be a marker of a more effective ER signaling in breast cancer since cytoplasmic IRS-1 levels are regulated by multiple receptors and factors with nuclear IRS-1 levels being more dependent on ER. This observation may be supported also by our survival analysis combining PR and nuclear IRS-1. Positivity for both PR and nuclear IRS-1 might identify those tumors with active ER signaling therefore being more responsive to Tam. On the other hand, the negativity for both PR and IRS-1 may represent a surrogate marker of a less functional ER pathway and, therefore, reduced Tam responsiveness. Clearly, a better understanding of how IRS-1 precisely function in the two different compartments is needed to clarify why only nuclear IRS-1 expression is a predictive marker of Tam response.
In conclusion, our results indicate that in the early-stage of breast cancer, women with tumors with positive nuclear IRS-1 have a better survival after adjuvant Tam-based therapy compared with women with nuclear IRS-1 negative tumors. Here we show the importance of evaluating both nuclear and cytoplasmic IRS-1 immunoreactivity as these might have distinct clinical implications. However, whether IRS-1 expression may help to select endocrine therapy in routine practice will need to be determined in additional studies.
Acknowledgments
This project was supported in part by funding from Susan G Komen for the Cure (A.V.L.), and supported by NIH P50CA58183 (C.K.O) from the National Cancer Institute. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Susan G. Komen for the Cure, the National Cancer Institute, or the National Institutes of Health.
Contributor Information
Ilenia Migliaccio, Lester and Sue Smith Breast Center, Baylor College of Medicine, One Baylor Plaza, BCM:600, Room N1110, Houston, TX 77030, USA; Dipartimento di Scienze Biomorfologiche e Funzionali, Universitá degli Studi di Napoli, “Federico II”, 80131 Napoli, Italy.
Meng-Fen Wu, Dan L. Duncan Cancer Center, Baylor College of Medicine, Houston, TX, USA.
Carolina Gutierrez, Department of Pathology, Baylor College of Medicine, Houston, TX, USA.
Luca Malorni, Lester and Sue Smith Breast Center, Baylor College of Medicine, One Baylor Plaza, BCM:600, Room N1110, Houston, TX 77030, USA; Dipartimento di Endocrinologia e Oncologia Molecolare e Clinica, Universitá degli Studi di Napoli, “Federico II”, 80131 Napoli, Italy.
Syed K. Mohsin, Department of Pathology, Riverside Methodist Hospital, Columbus, OH, USA
D. Craig Allred, Department of Pathology and Immunology, Washington University School of Medicine, St. Louis, MO, USA.
Susan G. Hilsenbeck, Lester and Sue Smith Breast Center, Baylor College of Medicine, One Baylor Plaza, BCM:600, Room N1110, Houston, TX 77030, USA; Dan L. Duncan Cancer Center, Baylor College of Medicine, Houston, TX, USA
C. Kent Osborne, Lester and Sue Smith Breast Center, Baylor College of Medicine, One Baylor Plaza, BCM:600, Room N1110, Houston, TX 77030, USA; Dan L. Duncan Cancer Center, Baylor College of Medicine, Houston, TX, USA.
Heidi Weiss, Department of Preventive Medicine and Community Health, Sealy Center for Cancer Cell Biology, University of Texas Medical Branch, Galveston, TX, USA.
Adrian V. Lee, Lester and Sue Smith Breast Center, Baylor College of Medicine, One Baylor Plaza, BCM:600, Room N1110, Houston, TX 77030, USA; Department of Medicine and Molecular and Cellular Biology, Baylor College of Medicine, Houston, TX, USA
References
- 1.Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998;339(22):1609–1618. doi: 10.1056/NEJM199811263392207. [DOI] [PubMed] [Google Scholar]
- 2.Cui X, Schiff R, Arpino G, Osborne CK, Lee AV. Biology of progesterone receptor loss in breast cancer and its implications for endocrine therapy. J Clin Oncol. 2005;23(30):7721–7735. doi: 10.1200/JCO.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Knowlden JM, Jones HE, Barrow D, Gee JM, Nicholson RI, Hutcheson IR. Insulin receptor substrate-1 involvement in epidermal growth factor receptor and insulin-like growth factor receptor signalling: implication for Gefitinib (‘Iressa’) response and resistance. Breast Cancer Res Treat. 2008;111(1):79–91. doi: 10.1007/s10549-007-9763-9. [DOI] [PubMed] [Google Scholar]
- 4.Dearth RK, Cui X, Kim HJ, Hadsell DL, Lee AV. Oncogenic transformation by the signaling adaptor proteins insulin receptor substrate (IRS)-1 and IRS-2. Cell Cycle. 2007;6(6):705–713. doi: 10.4161/cc.6.6.4035. [DOI] [PubMed] [Google Scholar]
- 5.Jackson JG, White MF, Yee D. Insulin receptor substrate-1 is the predominant signaling molecule activated by insulin-like growth factor-I, insulin, and interleukin-4 in estrogen receptor-positive human breast cancer cells. J Biol Chem. 1998;273(16):9994–10003. doi: 10.1074/jbc.273.16.9994. [DOI] [PubMed] [Google Scholar]
- 6.Nolan MK, Jankowska L, Prisco M, Xu S, Guvakova MA, Surmacz E. Differential roles of IRS-1 and SHC signaling pathways in breast cancer cells. Int J Cancer. 1997;72(5):828–834. doi: 10.1002/(sici)1097-0215(19970904)72:5<828::aid-ijc20>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Surmacz E. Function of the IGF-I receptor in breast cancer. J Mammary Gland Biol Neoplasia. 2000;5(1):95–105. doi: 10.1023/a:1009523501499. [DOI] [PubMed] [Google Scholar]
- 8.Surmacz E, Burgaud JL. Overexpression of insulin receptor substrate 1 (IRS-1) in the human breast cancer cell line MCF-7 induces loss of estrogen requirements for growth and transformation. Clin Cancer Res. 1995;1(11):1429–1436. [PubMed] [Google Scholar]
- 9.Morelli C, Garofalo C, Bartucci M, Surmacz E. Estrogen receptor-alpha regulates the degradation of insulin receptor substrates 1 and 2 in breast cancer cells. Oncogene. 2003;22(26):4007–4016. doi: 10.1038/sj.onc.1206436. [DOI] [PubMed] [Google Scholar]
- 10.Lee AV, Jackson JG, Gooch JL, Hilsenbeck SG, Coronado-Heinsohn E, Osborne CK, Yee D. Enhancement of insulin-like growth factor signaling in human breast cancer: estrogen regulation of insulin receptor substrate-1 expression in vitro and in vivo. Mol Endocrinol. 1999;13(5):787–796. doi: 10.1210/mend.13.5.0274. [DOI] [PubMed] [Google Scholar]
- 11.Mauro L, Salerno M, Panno ML, Bellizzi D, Sisci D, Miglietta A, Surmacz E, Ando S. Estradiol increases IRS-1 gene expression and insulin signaling in breast cancer cells. Biochem Biophys Res Commun. 2001;288(3):685–689. doi: 10.1006/bbrc.2001.5815. [DOI] [PubMed] [Google Scholar]
- 12.Molloy CA, May FE, Westley BR. Insulin receptor substrate-1 expression is regulated by estrogen in the MCF-7 human breast cancer cell line. J Biol Chem. 2000;275(17):12565–12571. doi: 10.1074/jbc.275.17.12565. [DOI] [PubMed] [Google Scholar]
- 13.Cesarone G, Garofalo C, Abrams MT, Igoucheva O, Alexeev V, Yoon K, Surmacz E, Wickstrom E. RNAi-mediated silencing of insulin receptor substrate 1 (IRS-1) enhances tamoxifen-induced cell death in MCF-7 breast cancer cells. J Cell Biochem. 2006;98(2):440–450. doi: 10.1002/jcb.20817. [DOI] [PubMed] [Google Scholar]
- 14.Guvakova MA, Surmacz E. Overexpressed IGF-I receptors reduce estrogen growth requirements, enhance survival, and promote E-cadherin-mediated cell-cell adhesion in human breast cancer cells. Exp Cell Res. 1997;231(1):149–162. doi: 10.1006/excr.1996.3457. [DOI] [PubMed] [Google Scholar]
- 15.Salerno M, Sisci D, Mauro L, Guvakova MA, Ando S, Surmacz E. Insulin receptor substrate 1 is a target for the pure antiestrogen ICI 182, 780 in breast cancer cells. Int J Cancer. 1999;81(2):299–304. doi: 10.1002/(SICI)1097-0215(19990412)81:2<299::AID-IJC21>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 16.Wu A, Chen J, Baserga R. Nuclear insulin receptor substrate-1 activates promoters of cell cycle progression genes. Oncogene. 2008;27(3):397–403. doi: 10.1038/sj.onc.1210636. [DOI] [PubMed] [Google Scholar]
- 17.Morelli C, Garofalo C, Sisci D, del Rincon S, Cascio S, Tu X, Vecchione A, Sauter ER, Miller WH, Jr, Surmacz E. Nuclear insulin receptor substrate 1 interacts with estrogen receptor alpha at ERE promoters. Oncogene. 2004;23(45):7517–7526. doi: 10.1038/sj.onc.1208014. [DOI] [PubMed] [Google Scholar]
- 18.Sisci D, Morelli C, Cascio S, Lanzino M, Garofalo C, Reiss K, Garcia M, Russo A, Ando S, Surmacz E. The estrogen receptor alpha:insulin receptor substrate 1 complex in breast cancer: structure-function relationships. Ann Oncol. 2007;18(Suppl 6):vi81–vi85. doi: 10.1093/annonc/mdm232. [DOI] [PubMed] [Google Scholar]
- 19.Ma Z, Gibson SL, Byrne MA, Zhang J, White MF, Shaw LM. Suppression of insulin receptor substrate 1 (IRS-1) promotes mammary tumor metastasis. Mol Cell Biol. 2006;26(24):9338–9351. doi: 10.1128/MCB.01032-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koda M, Sulkowska M, Kanczuga-Koda L, Sulkowski S. Expression of insulin receptor substrate 1 in primary breast cancer and lymph node metastases. J Clin Pathol. 2005;58(6):645–649. doi: 10.1136/jcp.2004.022590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rocha RL, Hilsenbeck SG, Jackson JG, VanDenBerg CL, Weng C, Lee AV, Yee D. Insulin-like growth factor binding protein-3 and insulin receptor substrate-1 in breast cancer: correlation with clinical parameters and disease-free survival. Clin Cancer Res. 1997;3(1):103–109. [PubMed] [Google Scholar]
- 22.Schnarr B, Strunz K, Ohsam J, Benner A, Wacker J, Mayer D. Down-regulation of insulin-like growth factor-I receptor and insulin receptor substrate-1 expression in advanced human breast cancer. Int J Cancer. 2000;89(6):506–513. doi: 10.1002/1097-0215(20001120)89:6<506::aid-ijc7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 23.Sisci D, Morelli C, Garofalo C, Romeo F, Morabito L, Casaburi F, Middea E, Cascio S, Brunelli E, Ando S, et al. Expression of nuclear insulin receptor substrate 1 in breast cancer. J Clin Pathol. 2007;60(6):633–641. doi: 10.1136/jcp.2006.039107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allred DC, Clark GM, Tandon AK, McGuire WL. Immunohistochemistry on histological sections from small (50 mg) samples of pulverized breast cancer. J Histotech. 1993;16(2):117–120. [Google Scholar]
- 25.Mohsin SK, Weiss H, Havighurst T, Clark GM, Berardo M, le Roanh D, To TV, Qian Z, Love RR, Allred DC. Progesterone receptor by immunohistochemistry and clinical outcome in breast cancer: a validation study. Mod Pathol. 2004;17(12):1545–1554. doi: 10.1038/modpathol.3800229. [DOI] [PubMed] [Google Scholar]
- 26.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17(5):1474–1481. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 27.Lee AV, Zhang P, Ivanova M, Bonnette S, Oesterreich S, Rosen JM, Grimm S, Hovey RC, Vonderhaar BK, Kahn CR, et al. Developmental and hormonal signals dramatically alter the localization and abundance of insulin receptor substrate proteins in the mammary gland. Endocrinology. 2003;144(6):2683–2694. doi: 10.1210/en.2002-221103. [DOI] [PubMed] [Google Scholar]
- 28.Tu X, Batta P, Innocent N, Prisco M, Casaburi I, Belletti B, Baserga R. Nuclear translocation of insulin receptor substrate-1 by oncogenes and Igf-I. Effect on ribosomal RNA synthesis. J Biol Chem. 2002;277(46):44357–44365. doi: 10.1074/jbc.M208001200. [DOI] [PubMed] [Google Scholar]
- 29.Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11(2):155–168. [PubMed] [Google Scholar]
- 30.Arpino G, Weiss H, Lee AV, Schiff R, De Placido S, Osborne CK, Elledge RM. Estrogen receptor-positive, progesterone receptor-negative breast cancer: association with growth factor receptor expression and tamoxifen resistance. J Natl Cancer Inst. 2005;97(17):1254–1261. doi: 10.1093/jnci/dji249. [DOI] [PubMed] [Google Scholar]
- 31.Bardou VJ, Arpino G, Elledge RM, Osborne CK, Clark GM. Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases. J Clin Oncol. 2003;21(10):1973–1979. doi: 10.1200/JCO.2003.09.099. [DOI] [PubMed] [Google Scholar]