Abstract
The hippocampus is a part of the limbic system and is important for the formation of associative memories, such as acquiring information about the context (e.g. the place where an experience occurred) during emotional learning (e.g. fear conditioning). Here, we assess whether the hippocampus is responsible for pups’ newly emerging context learning. In all experiments, postnatal day (PN) 21 and PN24 rat pups received 10 pairings of odor-0.5mA shock or control unpaired odor-shock, odor only and shock only. Some pups were used for context, cue or odor avoidance tests, while the remaining pups were used for c-Fos immunohistochemistry to assess hippocampal activity during acquisition. Our results show that cue and odor avoidance learning were similar at both ages, while contextual fear learning and learning-associated hippocampal (CA1, CA3 and dentate gyrus) activity (c-Fos) only occurred in PN24 paired pups. To assess a causal relationship between the hippocampus and context conditioning, we infused muscimol into the hippocampus, which blocked acquisition of context fear learning in the PN24 pups. Muscimol or vehicle infusions did not affect cue learning or aversion to the odor at PN21 or PN24. The results suggest that the newly emerging contextual learning exhibited by PN24 pups is supported by the hippocampus.
Keywords: fear conditioning, odor, cue, shock, odor aversion, development
Introduction
Fear is a highly adaptive emotion, helping animals learn about aversive events and/or the context of where the aversive events took place. Fear conditioning is a procedure where the emotion of fear becomes associated with a neutral stimulus (e.g. odor or tone; called cue learning) but also becomes associated with a neutral context (e.g. a room or conditioning chamber; called contextual fear learning) simply by pairing those stimuli with an aversive stimulus (e.g. electric shock). After repeatedly pairing these neutral and aversive stimuli together, the neutral stimulus and context begin eliciting conditional responses such as freezing (Fanselow, 2000; LeDoux, 2000; Maren, 2001; Rudy et al., 2004; Ji and Maren, 2007). The fear conditioning paradigm has proven to be one of the more prolific and valuable animal models for assessment of human psychiatric disorders associated with abnormally heightened fear and anxiety (LeDoux, 1998; Lissek at al., 2005; Debiec and LeDoux, 2006; Jackson et al., 2006; Mineka and Oehlberg, 2008).
Many studies have demonstrated that the hippocampus is a fundamental structure supporting adult fear conditioning, especially contextual fear learning (O’Keefe and Nadel, 1978; Eichenbaum, 1996; Fanselow, 2000; LeDoux, 2000; Otto et al., 2000; Anagnostaras et a., 2001; Otto and Giardino, 2001; Debiec et al., 2002; Rudy et al., 2002; Sanders et al., 2003; Matus-Amat et al., 2004; Wiltgen et al., 2006). Several studies in infant rats have analyzed contextual fear learning (Rudy, 1993, 1994; Rudy and Morledge, 1994; Brasser and Spear, 1998, 2004; Esmoris-Arranz et al., 2008) and other behaviors that are dependent on the hippocampus in adulthood, such as spontaneous alternation and spatial navigation (Castro et al., 1987; Green and Stanton, 1989; Lilliquist et al., 1993; Kraemer and Randall, 1995; Nair and Gonzalez-Lima, 1999; Brown and Whishaw, 2000). However, no studies to date have directly assessed the role of the hippocampus in mediating the development of these behaviors. While a causal link between the ontogeny of context fear learning and hippocampus may seem to be a foregone conclusion, recent advances in brain development research have highlighted the importance of not assuming that adult functional brain-behavior interactions are consistent throughout development (Johnson, 2000, 2001; Raineki et al., 2009). Indeed, work in our lab on the development of odor malaise learning suggests this learning is initially supported by the olfactory bulb and switches to the amygdala as the animal approaches weaning (Shionoya et al., 2006; Raineki et al., 2009). Thus, the purpose of the present series of experiments was first to correlate the ontogeny of context fear learning with hippocampal plasticity and secondly, to assess a causal link between the hippocampus and emerging context fear learning.
While anatomical development is not necessarily associated with functional development, this assessment can at least provide an age range when a brain area can become functional (Johnson, 2000, 2001). The hippocampus is divided into distinct sections, most basically a trisynaptic circuit relaying information through the dentate gyrus (DG) to CA3, and after to CA1, with each hippocampal subdivision implicated in context fear learning, albeit with different roles (Gilbert and Kesner, 2003; Lee and Kesner, 2004; Daumas et al., 2005; Li et al., 2005; Hunsaker et al., 2008). These anatomical subdivisions of the hippocampus are clearly visible during the first week of life (Bayer, 1980b). Hippocampus development is protracted and continues through adolescence, although each subdivision has a distinct developmental time frame (Nurse and Lacaille, 1999; Swann et al., 1999; Tarazi and Baldessarini, 2000; Toscano et al., 2003; Crews et al., 2007), with long-term potentiation (LTP), which is a presumed measure of plasticity, emerging during the second postnatal week in CA1, and during the third postnatal week in DG (Harris and Teyler, 1984; Wilson, 1984; Swann et al., 1990; Bekenstein and Lothaman, 1991b). Neurotransmitter systems associated with plasticity, such as glutamatergic synapses, achieve maturity after PN14 (Michelson and Lothman, 1989; Kudryashov and Kudryashova, 2001), and GABAergic synaptic transmission has been observed at PN5-6 in CA3, though just at PN9 in CA1 (Swann et al., 1989, 1990).
Since the hippocampus appears sufficiently developed to participate in contextual fear learning at PN21, we assessed the role of the hippocampus in the ontogeny of contextual fear learning. We hypothesize that functional emergence of context fear learning is dependent on hippocampal functional emergence into the fear conditioning circuit.
Materials and Methods
Subjects
We used male and female Long-Evans rat pups (PN21 and PN24) born and bred in our colony at the University of Oklahoma (originally from Harlan Lab Animals, Houston, USA). Animals were housed in polypropylene cages (34 × 29 × 17 cm) with an abundant amount of aspen wood shavings for nest building, and kept in a 20°C environment with a 12:12 light cycle. Food and water were available ad libitum. The day of birth was considered PN0 and the litters were culled to 12 pups (6 males and 6 females) on PN1. For all experiments the animals were not weaned and no more than one male and one female from a litter were used in each experimental condition. The University of Oklahoma Institutional Animal Care and Use Committee, which follows guidelines from the National Institutes of Health, approved all animal care and experimental procedures.
Odor-shock conditioning
Pups were randomly assigned to one of the following classical conditioning groups: (1) Paired odor-0.5mA shock (2) Unpaired odor−0.5mA shock, (3) Odor only and (4) Shock only. All conditioning took place in Lafayette conditioning chambers (Lafayette Instruments, Lafayette, IN; 14 cm long × 20 cm wide × 21 cm high) consisting of one aluminum side and a Plexiglas side, front and back, under a 25W red light bulb. The floor of each chamber had 9 stainless steel rods (3 mm diameter, 1 cm apart) connected to a shock generator (Lafayette Instruments, Lafayette, IN). Animals were placed in the conditioning chamber and given a 10 min adaptation period before initiating the 40 min conditioning session. Pups received 10 presentations of a 30 sec peppermint odor (CS) and a 1 sec 0.5mA footshock, with an intertrial interval (ITI) of 4 min. Peppermint odor was delivered by a flow dilution olfactometer (2 liters/min flow rate) at a concentration of 1:10 peppermint vapor to air. A fume hood provided constant background noise and removed the odor from the conditioning chamber. A chrontrol (ChronTrol Corporation, San Diego, CA), controlled shock and odor delivery. Paired odor-shock pups received 10 pairings of the 30 sec odor with shock overlapping during the last 1 sec of the odor presentation. Unpaired odor-shock pups received the shock 2 min after each onset of odor presentation. Odor only pups received only peppermint odor presentations. Shock only pups received only shock presentations. After conditioning, pups were returned to the home cage until testing, which occurred 24 hours later.
Cue test
Fear to the odor (cue) was assessed by measuring freezing behavior, which was defined as the absence of any visible movement, except that required for breathing (Blanchard and Blanchard, 1969). Pups were removed from the nest, placed in individual 2000 mL beakers and given a 10 min adaptation period. Afterwards, pups received 5 odor presentations with the same ITI, concentration and duration as during conditioning and total time of freezing behavior was observed for 30 sec during odor presentation. Testing occurred under a 25W red light bulb and observations were made blind to the training condition.
Context test
Fear to the context was assessed by measuring freezing behavior. Pups were removed from the nest, placed in Lafayette conditioning chambers (as described in odor-shock conditioning section) and total time of freezing was recorded. Testing lasted for 8 min and was conducted under a 25W red light bulb by an experimenter blind to the training condition. For some unpaired pups, the cue (odor - same ITI, concentration and duration as during training) was presented 2 times during the context test starting at 1 min and 30 sec after beginning the 8 min test to control for learning the cue odor as part of the context.
Y-maze test
A Y-maze test was used to assess pups’ expression of an odor aversion. This test requires pups to choose between two arms of a Plexiglas Y-maze (start box: 9 cm width, 12 cm length, 9 cm height; choice arms: 9 × 30 × 9 cm), one containing the peppermint odor (20 µl of peppermint odor on a Kim Wipe placed at the end of the alley) and the other a familiar odor (20 ml of clean aspen shavings in a Petri dish, same used as bedding). Pups were placed in the start box (direction was counterbalanced) for 5 sec, and the door to each alley was opened, at which time the pups were given 60 sec to choose an arm. A response was considered a choice when a pup’s entire body moved past the entrance to the alley. Each pup received 5 sequential trials and between trials, the pup was placed in a holding cage for 10 sec and the floor was cleaned with water and dried. Observations of each pup were made blind to the training condition.
c-Fos immunohistochemistry
Ninety minutes following conditioning, when c-Fos expression peaks (Moriceau et al., 2004; Roth and Sullivan 2005; Roth et al., 2006; Koehnle and Rinaman, 2007; Lanuza et al., 2008), brains were removed and frozen in 2-methylbutane (−45°C) and placed in a −70°C freezer until cutting and post-fixation. Every sixth section (coronal section, 20 µm) was collected on pretreated slides (Fisherbrand Plus, Fisher) for c-Fos processing, and every seventh section was colleted for cresyl violet staining. c-Fos sections received a 1 h post-fix in 4% paraformaldehyde/0.1 M phosphate buffer (PB, pH 7.2). To eliminate peroxidase activity, sections were incubated in 0.1 M PB saline (PBS, pH 7.2) containing 3% H2O2 and 10% methanol for 5 min. Following PBS rinses and 15 min incubation in 0.2% Triton X-100, slides were incubated in 3% bovine serum albumin for 1 h. Slides were treated overnight at 4°C with the primary antibody (c-Fos, sc-52, Santa Cruz Biotechnology) diluted 1:500 in PBS. Afterward, they were incubated in the secondary biotinylated antibody (goat anti-rabbit, Vector Laboratories) for 2 h at room temperature and then incubated for 90 min in avidin-biotin-peroxidase (ABC) complex solution. Slides were then treated with PB containing 0.1% 3.3’-diaminobenzidine and H2O2 and subsequently dehydrated in alcohol and Histoclear, and coverslipped for microscope examination (Moriceau et al., 2004; Roth and Sullivan 2005; Roth et al., 2006).
c-Fos-positive cells were counted bilaterally using a microscope (Olympus with 10× objective) equipped with a drawing tube. With aid of a stereotaxic atlas (Paxinos and Watson 1986), brain areas were outlined using the correspondent cresyl violet sections. All c-Fos-positive cells were counted without knowledge of the training condition. c-Fos-positive cells were distinguished from the background by density of staining, shape, and size of cells. The mean number of c-Fos cells per brain area for an animal was determined by averaging the counts from all sections (three sections counted for each brain area). Brain areas examined were subdivisions of dorsal hippocampus (CA1, CA3 and DG).
Surgery and hippocampus infusion
On PN19 or PN22, pups were anesthetized by inhalation with isoflurane and place in an adult stereotaxic apparatus modified for use with weanlings. Stainless steel cannulas (30 gauge tubing) were implanted bilaterally in the dorsal hippocampus through holes drilled in the overlying skull. Stereotaxic coordinates, derived from Paxinos and Watson (1986) atlas, were used for implanting cannulas into the hippocampus (caudal: 2.0 mm, lateral: 2.5 mm from bregma). The cannulas were lowered 1.5 mm from the surface of the skull, placing the tip near the hippocampus. The cannulas were fixed to the skull with dental cement. To ensure patency of the cannulas, guide wires were placed in the lumen of the tubing until training. After recovery from surgery (generally within 30 min), pups were returned to the dam and littermates for a 2-day recovery period until conditioning. On PN21 or PN24, in preparation for muscimol infusions, pups’ bilateral cannulas were attached via PE10 tubing to a Harvard syringe pump driving two Hamilton microliter syringes. The cannulas were filled (5 sec at 0.5 µL/min) with muscimol (2mM; GABAA receptor agonist, Sigma, St. Louis, MO) or physiological saline (0.9%). Pups received muscimol or saline infused at 0.1 µL/min for 5 min, for a total infusion volume of 0.5 µL, 45 min before training.
Verifying cannula placement
After testing, brains were removed, frozen, sectioned (20 µm) in a −20°C cryostat, and cresyl violet stained for identification of the cannula placement in relation to dorsal hippocampus using an atlas (Paxinos and Watson, 1986).
Statistical analysis
For all experiments, comparisons were made between groups using analysis of variance (ANOVA) followed by post hoc Fisher tests or Student t-test (Figure 1D).
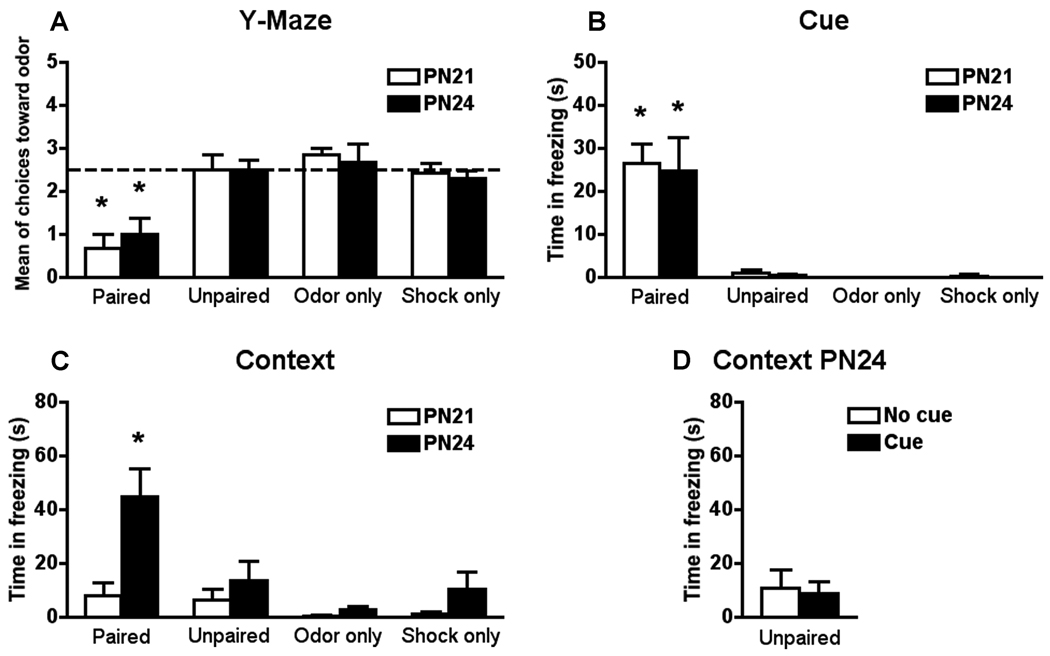
Figure 1.
Odor avoidance (A) and cue fear learning (B) were similar at PN21 and PN24, while contextual fear learning (C) only occurred in PN24 paired pups. Cue presentation during context test did not change the lack of contextual fear learning in PN24 unpaired pup rats (D). *P < 0.05 between groups (n=6-7 for all groups).
Results
Context fear learning emerges at PN24
Our results (Figure 1) show that cue fear learning and odor avoidance learning (Y-maze test) were similar at both ages (PN21 and PN24), while contextual fear learning only occurred in PN24 paired.
Y-maze test
Paired PN21 and PN24 pups showed an odor aversion compared with unpaired, odor only and shock only rat pups (Figure 1A). No significant interaction between condition and age was detected (F(3,42) = 0.316). However, a significant main effect for condition was detected (F(3,42) = 17.562, P < 0.0001). Post hoc Fisher test revealed that all paired groups (PN21 and PN24) differed from the control groups.
Cue test
Paired PN21 and PN24 pups showed an increase in freezing behavior when in the presence of the cue (odor) compared with unpaired, odor only and shock only pups (Figure 1B). No significant interaction between condition and age was detected (F(3,42) = 0.034). However, a significant main effect for condition was detected (F(3,42) = 33.904, P < 0.0001). Post hoc Fisher test revealed that all paired groups (PN21 and PN24) differed from the control groups.
Context test
Only PN24 paired pups showed increased freezing behavior when placed in the same context as conditioning compared with PN21 paired pups and PN21 and PN24 unpaired, odor only and shock only pups (Figure 1C). A significant interaction between condition and age was detected (F(3,42) = 3.979, P < 0.01). Post hoc Fisher test revealed that PN24 paired group differed from all other groups.
The cue (odor) presentation during context testing did not elicit context fear responses in PN24 unpaired rat pups (Figure 1D). No significant difference was detected (t(10) = 0.203) in freezing response to the context between PN24 unpaired rat pups that have cue presentations during the context test and PN24 unpaired rat pups that have no cue presentations during context test.
Fear learning induces hippocampal neural activity (c-Fos) at PN24 but not at PN21
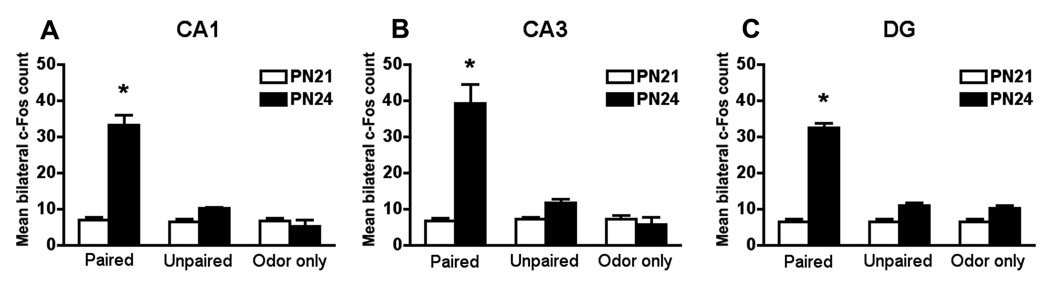
Hippocampal neural activity results correlate well with the context fear behavioral analyses: PN24 paired but not PN21 paired pups show significantly increased neural activity in all subdivisions of the hippocampus (CA1, CA3 and DG; Figure 2). A significant interaction between condition and age was detected for CA1 (F(2,30) = 52.532, P < 0.0001), CA3 (F(2,30) = 30.769, P < 0.0001) and DG (F(2,30) = 117.599, P < 0.0001). Post hoc Fisher test revealed that pairing an odor with shock at PN24 significantly increases the presence of c-Fos positive cells in all subdivisions of the hippocampus compared with all other groups.
Figure 2.
Fear conditioning produces learning-induced changes in c-Fos protein expression in CA1 (A), CA3 (B) and DG (C) subdivisions of the hippocampus just in PN24 paired pups. Bars represent the mean (± SEM) number of c-Fos-positive cells counted bilaterally in the CA1, CA3 and DG of PN21 and PN24 rats. *P < 0.05 between groups (n=6 for all groups).
Muscimol temporary inactivation of the hippocampus prevents context fear learning at PN24
Temporary deactivation of the hippocampus during conditioning with muscimol (a GABAA receptor agonist) blocked context learning in PN24 paired pups and suggests a causal relationship between the hippocampus and contextual fear learning. Intra-hippocampal muscimol or vehicle (saline) infusions did not affect cue learning or aversion to the odor at either PN21 or PN24.
Y-maze test
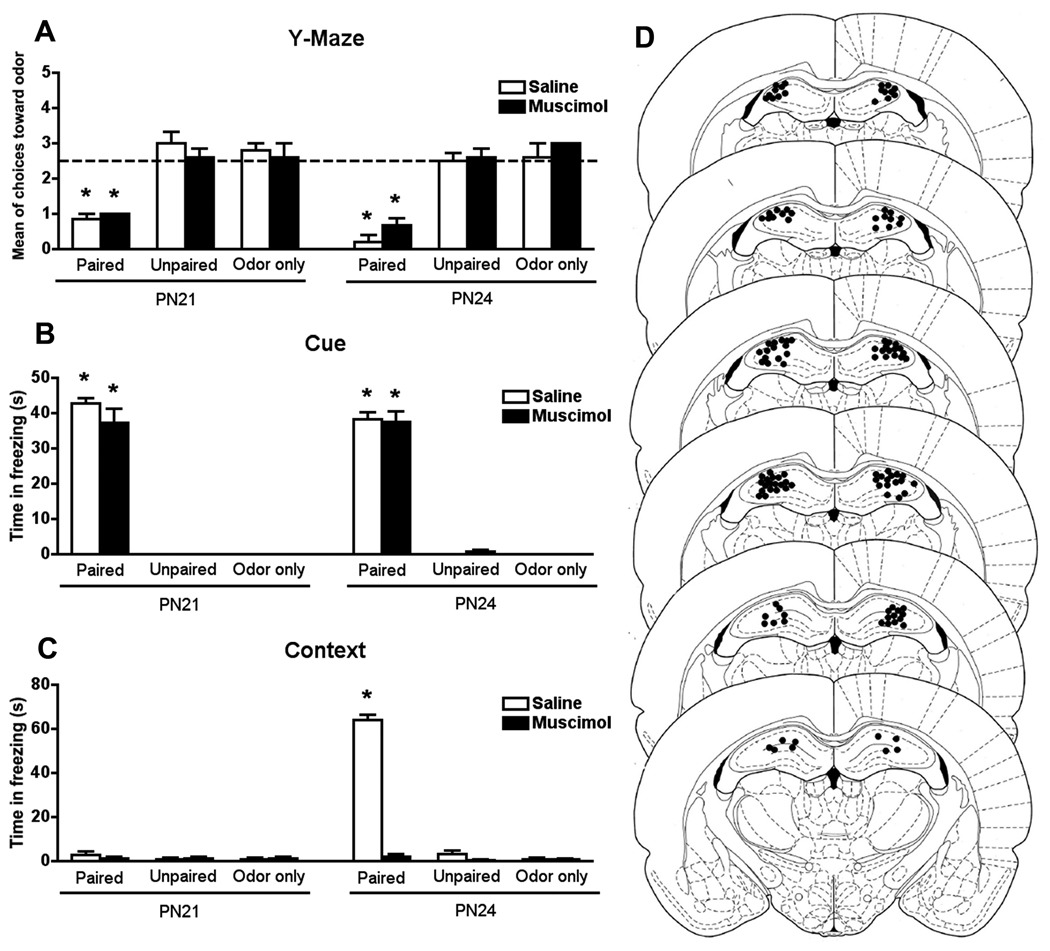
Muscimol infusion into the hippocampus did not prevent the odor aversion in PN21 and PN24 paired pups. PN21 and PN24 paired pups infused with muscimol or saline showed odor aversion compared with unpaired and odor only rat pups (Figure 3A). No significant interaction between condition, age and infusion was detected (F(2,51) = 0.907). However, a significant main effect for condition was detected (F(2,51) = 93.741, P < 0.0001). Post hoc Fisher test revealed that all paired groups (PN21 and PN24, muscimol or saline) differed from the control groups.
Figure 3.
Muscimol infusion into the hippocampus did not change odor avoidance (A) and cue fear learning (B) at PN21 and PN24, though it blocked acquisition of context fear learning (C) in PN24 paired pups. *P < 0.05 between groups (n=5- 6 for all groups). (D) Representative cannula placements in the hippocampus for animals receiving either muscimol or saline infusions at PN21 and PN24. Schematic brain section images are displayed from most rostral to most caudal. Images were adapted from Paxinos and Watson (1986).
Cue test
Muscimol infusion into the hippocampus did not prevent cue freezing in PN21 and PN24 paired pups when in the presence of the cue (odor) compared with unpaired and odor only rat pups (Figure 3B). No significant interaction between condition, age and infusion was detected (F(2,51) = 0.640). However, a significant main effect for condition was detected (F(2,51) = 765.784, P < 0.0001). Post hoc Fisher test revealed that all paired groups (PN21 and PN24, muscimol or saline) differed from the control groups.
Context test
Muscimol infusion into the hippocampus prevented context fear learning in PN24 paired pups. PN24 paired pups infused with saline into the hippocampus showed an increase in freezing behavior when placed in the same context as training. However, hippocampal muscimol infusion prevented the context freezing behavior in PN24 paired pups (Figure 3C). A significant interaction between condition, age and infusion was detected (F(2,51) = 205.186, P < 0.0001). Post hoc Fisher test revealed that the PN24 paired group infused with saline significantly differed from all other groups.
Discussion
The present results show that PN24 paired pups, but not PN21 paired pups, show contextual fear learning following odor-0.5mA shock fear conditioning; however, cue fear learning and odor avoidance learning were similar at both ages. We extend these behavioral results by demonstrating that the contextual fear learning is supported by the hippocampus. Specifically, only pups that showed contextual fear conditioning (PN24 paired pups) presented learning-induced changes (c-Fos expression) in the hippocampus (CA1, CA3 and DG). Importantly, a causal relationship between hippocampus and context learning was demonstrated through temporary deactivation of the hippocampus, with GABAA agonist muscimol infusion, during acquisition and the blocking of contextual fear learning in PN24 paired pups. The specificity of our manipulations is demonstrated by the inability of the intrahippocampal muscimol infusion to alter cue and odor aversion learning.
Behavioral ontogeny of context fear learning
Behavioral dissociation between cue and contextual fear conditioning is well documented in the developmental literature with context conditioning emerging at an older age (Rudy, 1993, 1994; Rudy and Morledge, 1994; Brasser and Spear, 1998, 2004; Sullivan et al., 2000; Moriceau et al, 2006; Esmoris-Arranz et al., 2008; Raineki et al., 2009). Differences in the age of behavioral measures of contextual fear conditioning can be found in the literature, although the age range is consistent with the peri-weanling period (PN17-23). Several variables, including the salience of the contextual environment, appear to modulate the acquisition and expression of context learning during this developmental time period (Rudy, 1993, 1994; Rudy and Morledge, 1994; Brasser and Spear, 1998, 2004; Esmoris-Arranz et al., 2008).
Context fear learning and the hippocampus
Several studies have demonstrated that the hippocampus is a fundamental structure supporting fear conditioning, especially contextual fear learning (O’Keefe and Nadel, 1978; Eichenbaum, 1996; Fanselow, 2000; LeDoux, 2000; Otto et al., 2000; Anagnostaras et a., 2001; Otto and Giardino, 2001; Debiec et al., 2002; Rudy et al., 2002; Sanders et al., 2003; Matus-Amat et al., 2004; Wiltgen et al., 2006). The notion that the hippocampus is involved in contextual fear conditioning is also supported by knockout/transgenic mice studies. Specifically, mutant mice with deficient LTP in the hippocampus also exhibit deficits in contextual fear conditioning (Abeliovich et al., 1993; Bourtchuladze et al., 1994). Additionally, brain-derived neurotropic factor (BDNF) infusion into the hippocampus can rescue contextual fear learning deficits in BDNF knockout mice (Liu et al., 2004).
Hippocampus development supports context fear learning at PN24
In rats, maturation and differentiation of the hippocampus continue into postnatal life (Bayer, 1980a; Baudry et al., 1981; Muller et al., 1989; Altman and Bayer, 1990; DiScenna and Teyler, 1994; Gould and Cameron, 1996). For example, during the first postnatal weeks, neuronal birth, differentiation and migration are ongoing (Altman and Bayer, 1990; Gould and Cameron, 1996). Based on slice physiology, both inhibitory and excitatory responses have been found in the infant hippocampus, although inhibitory responses are just beginning to develop around PN 9-12 and reach adult values between PN14-18 (Baudry et al., 1981; Michelson and Lothman, 1989; Muller et al., 1989; Bekenstein and Lothman, 1991a; DiScenna and Teyler, 1994; Kudryashov and Kudryashova, 2001). Neurogenesis of granule cells peaks just during the second week of life in rodents (Bayer, 1980a) with major synaptic maturation occurring later in the preweaning period (Crain et al., 1973; Muller et al., 1989). Hippocampal LTP similarly emerges during the second postnatal week in CA1 and third postnatal week in DG, both in vivo (Wilson, 1984; Bekenstein and Lothaman, 1991b) and in vitro (Harris and Teyler, 1984; Wilson, 1984; Swann et al., 1990). Neurotransmitter systems, such as GABA, glutamate and BDNF, that are associated with plasticity continue to develop throughout the preweaning period (Swann et al., 1989, 1990; Michelson and Lothman, 1989; Katoh-Semba et al., 1997; Kudryashov and Kudryashova, 2001; Solum and Handa, 2002). The dynamic nature of hippocampal development is also illustrated by excitatory/inhibitory switches in function, as well transformations in the molecular underpinnings of LTP (Harris and Teyler, 1984; Wilson, 1984; Ben-Ari et al., 1989; Michelson and Lothman, 1989; Swann et al., 1989, 1990; Bekenstein and Lothaman, 1991b; Cherubini et al., 1991; Berninger et al., 1995; Kudryashov and Kudryashova, 2001). Thus, the hippocampus seems sufficiently mature to support the emergence of contextual fear learning, despite ongoing, significant development.
It is difficult to determine the age of functional onset for a brain area from these studies, although they can indicate that the hippocampus seems to be sufficiently developed to participate in learning-induced plasticity in the range of age and fear conditioning paradigm that we used in this work. In fact, only PN24 odor-0.5mA shock paired pups show an increase in c-Fos positive cells in CA1, CA3 and DG. This increased neural activity correlates well with the emergence of context fear behavior seen in PN24 paired pups.
Muscimol temporary suppression of hippocampus neural activity prevents context fear learning at PN24
Our results from muscimol infusion into the hippocampus in PN24 pups are consistent with adult literature showing that suppression of hippocampus activity impairs contextual fear conditioning without affecting fear to the cue (Bellgowan and Helmstetter, 1995; Daumas et al., 2005). Electrolytic lesion of the dorsal hippocampus and excitotoxic lesion of the ventral hippocampus also impair acquisition of context fear conditioning in adult rats (Selden et al., 1991; Phillips and LeDoux, 1992, 1994; Kim et al., 1993; Young et al., 1994; Maren and Fanselow, 1997). Lidocaine reversible lesions of the hippocampus (CA1 or CA3) before training decrease contextual fear learning (Daumas et al., 2005), a result that is analogous to our muscimol hippocampal infusions in rat pups. Together, these results indicate that temporary suppression of the hippocampus with muscimol infusions during conditioning can prevent the rat pups from building a unified representation of the environment and thus exhibit decreased contextual learning. Moreover, NMDA-receptor antagonist infusion into the hippocampus of adult rats impairs acquisition of context fear conditioning, but not cue fear learning (Young et al., 1994; Bast et al., 2003; Schenberg and Oliveira, 2008). Similar results were found with a cholinergic antagonist infusion into the hippocampus (Gale et al., 2001; Wallenstein and Vago, 2001). It is important to note, though, that the adult literature on contextual fear learning is not straightforward; there are also reports that prior lesion to the hippocampus has no effect on contextual fear learning (Maren et al., 1997; Richmond et al., 1999; Wiltgen et al., 2006).
Context fear learning in unpaired rats
While our PN24 paired pups learned context fear, both PN24 and PN21 unpaired pups did not. The adult literature consistently shows that unpaired animals display more freezing in a contextual fear test than paired (Phillips and LeDoux, 1994; Brasser and Spear, 1998, 2004; Esmoris-Arranz et al., 2008). Our results, while differing from the adult literature, are consistent with the infant pattern of contextual fear learning, in which paired animals display more freezing than unpaired, indicating a potentiation of context learning by cue learning in infancy (Brasser and Spear, 1998, 2004; Esmoris-Arranz et al., 2008). The absence of context fear learning in unpaired pups also correlates well with hippocampal neural activity, since the PN21 and PN24 unpaired pups have no changes in c-Fos expression in any subdivision of the hippocampus (CA1, CA3 and DG). It is possible that, for the PN24 unpaired rat pups, the unified representation of the context could include the cue (odor) that was presented during conditioning. However, our results show that cue presentation during the context test did not produce contextual fear learning in PN24 unpaired pup rats.
Cue learning and odor aversion are not supported by the hippocampus
At both ages used in this work (PN21 and PN24), paired pups learned an odor aversion and showed an increase in the freezing behavior in the presence of the cue (odor). Additionally, temporary neural activity suppression of the hippocampus with muscimol did not affect the cue learning and odor aversion in all groups, indicating that the hippocampus does not support cue learning. While many parts of the brain are involved in emotional fear learning, the literature suggests that cue learning is dependent upon the amygdala (LeDoux, 1998, 2000; Fanselow and LeDoux, 1999; McGaugh et al., 1999; Fanselow and Gale 2003; Maren, 2003). In fact, previous results from our laboratory (Raineki et al., 2009) show that at PN23, odor aversion induced by odor fear conditioning is associated with increased learning activity in the amygdala. Amygdala-dependent cue fear conditioning emerges at PN10 (Sullivan et al., 2000; Moriceau et al, 2006). These results are supported by developmental experiments showing that amygdala neurogenesis continues until PN14, although major nuclei subdivisions occur around PN7 (Bayer, 1980c; Berdel et al., 1997; Morys et al., 1999; Berdel and Morys, 2000).
Implications for understanding the ontogeny of contextual fear learning
Early life emotional memories are particularly enduring and appear to play an important role in adult mental health. Indeed, adverse experiences during childhood, such as abuse, neglect, or trauma, increase the incidence of fear disorders in children and adults (Kaufman et al., 2000; Heim and Nemeroff, 2001; Bremner, 2003; McEwen, 2003). As part of the limbic system, the hippocampus has been highlighted as an important area mediating these effects of enduring early life trauma (Wilson et al., 1986; Meaney et al., 1996; Tanapat et al., 1998; Bremner, 2003; McEwen, 2003; Ladd et al., 2004). The specific mechanisms underlying early life trauma’s effects, however, are still not completely understood; perhaps in part because of the limited understanding of how the infant processes trauma and aversive stimuli. Work describing the neural basis of fear conditioning learning during development is important as it may help characterize unique ways in which early adverse experience influences the immature organism.
Acknowledgments
This work was funded by grants NICHD-HD33402, NSF IOB-0544406 and Oklahoma Center for Science and Technology - OCAST to RMS; and CAPES (Brazil) to CR.
References
- Abeliovich A, Paylor R, Chen C, Kim JJ, Wehner JM, Tonegawa S. PKA gamma mutant mice exhibit mild deficits in spatial and contextual learning. Cell. 1993;75:1263–1271. doi: 10.1016/0092-8674(93)90614-v. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Prolonged sojourn of developing pyramidal cells in the intermediate zone of the hippocampus and their settling in the stratum pyramidal. J Comp Neurol. 1990;301:343–364. doi: 10.1002/cne.903010303. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and context fear conditioning: Recent controversies and advances. Hippocampus. 2001;11:8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Bast T, Zhang W-N, Feldon J. Dorsal hippocampus and classical fear conditioning to tone and context in rats: Effects of local NMDA-receptor blockade and stimulation. Hippocampus. 2003;13:657–675. doi: 10.1002/hipo.10115. [DOI] [PubMed] [Google Scholar]
- Baudry M, Arst D, Oliver M, Lynch Development of glutamate binding sites and their regulation by calcium in rat hippocampus. Brain Res. 1981;227:37–48. doi: 10.1016/0165-3806(81)90092-4. [DOI] [PubMed] [Google Scholar]
- Bayer SA. Development of the hippocampal region in the rat. I. Neurogenesis examined with 3H-thymidine autoradiography. J Comp Neurol. 1980a;190:87–114. doi: 10.1002/cne.901900107. [DOI] [PubMed] [Google Scholar]
- Bayer SA. Development of the hippocampal region in the rat. II. Morphogenesis during embryonic and early postnatal life. J Comp Neurol. 1980b;190:115–134. doi: 10.1002/cne.901900108. [DOI] [PubMed] [Google Scholar]
- Bayer SA. Quantitative 3H-thymidine radiographic analysis of neurogenesis in the rat amygdala. J Comp Neurol. 1980c;194:845–875. doi: 10.1002/cne.901940409. [DOI] [PubMed] [Google Scholar]
- Bekenstein JW, Lothman EW. A comparison of the ontogeny of excitatory and inhibitory neurotransmission in the CA1 region and dentate gyrus of the rat hippocampal formation. Dev Brain Res. 1991a;63:237–243. doi: 10.1016/0165-3806(91)90083-u. [DOI] [PubMed] [Google Scholar]
- Bekenstein JW, Lothman EW. An in vivo study if the ontogeny of long-term potentiation (LTP) in the CA1 region and in the dentate gyrus of the rat hippocampal formation. Dev Brain Res. 1991b;63:245–251. doi: 10.1016/0165-3806(91)90084-v. [DOI] [PubMed] [Google Scholar]
- Bellgowan PSF, Helmstetter FJ. Effects of muscimol applied to the dorsal hippocampus on the acquisition and expression of cued versus contextual fear conditioning. Soc Neurosci Abstr. 1995;21:1219. [Google Scholar]
- Ben-Ari Y, Cherubini E, Corredetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol. 1989;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdel B, Morys J. Expression of calbindin-D28k and parvalbumin during development of rat’s basolateral amygdaloid complex. Int J Dev Neurosci. 2000;18:501–513. doi: 10.1016/s0736-5748(00)00024-1. [DOI] [PubMed] [Google Scholar]
- Berdel B, Morys J, Maciejewska B. Neuronal changes in the basolateral complex during development of the amygdala of the ratNeuronal changes in the basolateral complex during development of the amygdala of the rat. Int J Dev Neurosci. 1997;15:755–765. doi: 10.1016/s0736-5748(97)00022-1. [DOI] [PubMed] [Google Scholar]
- Berninger B, Marty S, Zafra F, da Penha Berzaghi M, Thoenen H, Lindholm D. GABAergic stimulation switches from enhancing to repressing BDNF expression in rat hippocampal neurons during maturation in vitro. Development. 1995;121:2327–2335. doi: 10.1242/dev.121.8.2327. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Crouching as an index of fear. J Comp Physiol Psychol. 1969;67:370–375. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation in the cAMP-reponsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Brasser SM, Spear NE. A sensory-enhanced context facilitates learning and multiple measures of unconditioned stimulus processing in the preweanling rat. Behav Neurosci. 1998;112:126–140. doi: 10.1037//0735-7044.112.1.126. [DOI] [PubMed] [Google Scholar]
- Brasser SM, Spear NE. Contextual conditioning in infants, but not older animals, is facilitated by the CS conditioning. Neurobiol Learn Mem. 2004;81:46–59. doi: 10.1016/s1074-7427(03)00068-6. [DOI] [PubMed] [Google Scholar]
- Bremner JD. Long-term effects of childhood abuse on brain and neurobiology. Child Adolesc Psychiatr Clin N Am. 2003;12:271–292. doi: 10.1016/s1056-4993(02)00098-6. [DOI] [PubMed] [Google Scholar]
- Brown RW, Whishaw IQ. Similarities in the development of place and cue navigator by rats in a swimming pool. Dev Psychobiol. 2000;37:238–245. doi: 10.1002/1098-2302(2000)37:4<238::aid-dev4>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Castro CA, Paylor R, Rudy JW. A developmental analysis of the learning and short-termmemory processes mediating performance in conditional-spatial discriminative problems. Psychobiology. 1987;15:308–316. [Google Scholar]
- Cherubini E, Gaiarsa JL, Ben-Ari Y. GABA: An excitatory transmitter in early postnatal life. Trends Neurosci. 1991;14:515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- Crain B, Cotman C, Taylor D, Lynch G. A quantitative electron microscopic study of synaptogenesis in the dentate gyrus o the rat. Brain Res. 1973;63:195–204. doi: 10.1016/0006-8993(73)90088-7. [DOI] [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: A critical period of vulnerability for addiction. Pharmacol Biochem Behav. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Daumas S, Halley H, Frances B, Lassalle J-M. Encoding, consolidation, and retrieval of context memory: Differential involvement of dorsal CA3 and CA1 hippocampal subregions. Learn Mem. 2007;12:375–382. doi: 10.1101/lm.81905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec J, LeDoux JE. Noradrenergic signaling in the amygdala contributes to the reconsolidation of fear memory: Treatment implications for PTSD. Ann N Y Acad Sci. 2006;1071:521–524. doi: 10.1196/annals.1364.056. [DOI] [PubMed] [Google Scholar]
- Debiec J, LeDoux JE, Nader K. Cellular and systems reconsolidation in the hippocampus. Neuron. 2002;36:527–538. doi: 10.1016/s0896-6273(02)01001-2. [DOI] [PubMed] [Google Scholar]
- DiScenna PG, Teyler TJ. Development of inhibitory and excitatory synaptic transmission in the rat dentate gyrus. Hippocampus. 1994;17:577–585. doi: 10.1002/hipo.450040506. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Is the rodent hippocampus just for “place”? Curr Opin Neurobiol. 1996;6:187–195. doi: 10.1016/s0959-4388(96)80072-9. [DOI] [PubMed] [Google Scholar]
- Esmoris-Arranz FJ, Mendez C, Spear NE. Contextual fear conditioning differs for infant, adolescent, and adult rats. Behav Processes. 2008;78:340–350. doi: 10.1016/j.beproc.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS. Contextual fear, gestalt memories, and the hippocampus. Behav Brain Res. 2000;10:73–81. doi: 10.1016/s0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Gale GD. The amygdala, fear and memory. Ann N Y Acad Sci. 2003;985:125–134. doi: 10.1111/j.1749-6632.2003.tb07077.x. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23:229–232. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- Gale GD, Anagnostaras SG, Fenselow MS. Cholinergic modulation of Pavlovian fear conditioning: Effects of intrahippocampal scopolamine infusion. Hippocampus. 2001;11:371–376. doi: 10.1002/hipo.1051. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP. Localization of function within the dorsal hippocampus: The role of the CA3 subregion in paired-associate learning. Behav Neurosci. 2003;117:1385–1394. doi: 10.1037/0735-7044.117.6.1385. [DOI] [PubMed] [Google Scholar]
- Gould E, Cameron HA. Regulation of neuronal birth, migration and death in the rat dentate gyrus. Dev Neurosci. 1996;18:22–35. doi: 10.1159/000111392. [DOI] [PubMed] [Google Scholar]
- Green RJ, Stanton ME. Differential ontogeny of working memory and reference memory in the rat. Behav Neurosci. 1989;103:98–105. doi: 10.1037//0735-7044.103.1.98. [DOI] [PubMed] [Google Scholar]
- Harris KM, Teyler TJ. Development onset of long-term potentiation in area CA1 o frat hippocampus. J Physiol (Lond) 1984;346:27–48. doi: 10.1113/jphysiol.1984.sp015005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology if mood and anxiety disorders: Preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Lee B, Kesner RP. Evaluating the temporal context of episodic memory: The role of CA3 and CA1. Behav Brain Res. 2008;188:310–315. doi: 10.1016/j.bbr.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson ED, Payne JD, Nadel L, Jacobs WJ. Stress differentially modulates fear conditioning in healthy men and woman. Biol Psychiatry. 2006;59:516–522. doi: 10.1016/j.biopsych.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Ji J, Maren S. Hippocampal involvement in context modulation of fear extinction. Hippocampus. 2007;17:749–758. doi: 10.1002/hipo.20331. [DOI] [PubMed] [Google Scholar]
- Johnson MH. Functional brain development in humans. Nat Rev Neurosci. 2001;2:475–483. doi: 10.1038/35081509. [DOI] [PubMed] [Google Scholar]
- Johnson MH. Functional brain development in infants: Elements of an interactive specialization framework. Child Develop. 2000;71:75–81. doi: 10.1111/1467-8624.00120. [DOI] [PubMed] [Google Scholar]
- Katoh-Semba R, Takeuchi IK, Semba R, Kato K. Distribution of brain-derived neurtrophic factor in rats and its changes with development in the brain. J Neurochem. 1997;69:34–42. doi: 10.1046/j.1471-4159.1997.69010034.x. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Plotsky PM, Nemeroff CB, Charney DS. Effects of early adverse experiences on the brain structure and function: Clinical implications. Biol Psychiatry. 2000;48:778–790. doi: 10.1016/s0006-3223(00)00998-7. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Rison RA, Fanselow MS. Effects of amygdala, hippocampus, and periaqueductal gray lesions on short- and long-term contextual fear. Behav Neurosci. 1993;107:1093–1098. doi: 10.1037//0735-7044.107.6.1093. [DOI] [PubMed] [Google Scholar]
- Koehnle TJ, Rinaman L. Progressive postnatal increases on Fos immunoreactivity in the forebrain and brain stem of rata after viscerosensory stimulation with lithium chloride. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1212–R1223. doi: 10.1152/ajpregu.00666.2006. [DOI] [PubMed] [Google Scholar]
- Kreamer PJ, Randall CK. Spatial learning in preweanling rats trained on a Morris water maze. Psychobiology. 1995;23:144–152. [Google Scholar]
- Kudryashov IE, Kudryashova IV. Ontogeny of synaptic transmission in the rat hippocampus. Brain Res. 2001;892:263–286. doi: 10.1016/s0006-8993(00)03157-7. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Plotsky PM. Long-term adaptations in glucocorticoid receptor and mineralocorticoid receptor mRNA and negative feedback in the hypothalamo-pituitary-adrenal axis following neonatal maternal separation. Biol Psychiatry. 2004;55:367–375. doi: 10.1016/j.biopsych.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Lanuza E, Moncho-Bogani J, LeDoux JE. Unconditioned stimulus pathways to the amygdala: Effects of lesions of the posterior intralaminar thalamus on footshock-induced c-Fos expression in the subdivisions of the lateral amygdala. Neuroscience. 2008;155:959–968. doi: 10.1016/j.neuroscience.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Ann Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Fear and the brain: Where have we been, and where are we going? Biol Psychiatry. 1998;44:1229–1238. doi: 10.1016/s0006-3223(98)00282-0. [DOI] [PubMed] [Google Scholar]
- Lee I, Kesner RP. Differential contributions of dorsal hippocampus subregions to memory acquisition and retrieval in context fear-conditioning. Hippocampus. 2004;14:301–310. doi: 10.1002/hipo.10177. [DOI] [PubMed] [Google Scholar]
- Li Z, Zhou Q, Li L, Mao R, Wang M, Peng W, Dong Z, Xu L, Cao J. Effects of unconditioned and conditioned aversive stimuli in an intense fear conditioning paradigm on synaptic plasticity in the hippocampal CA1 area in vivo. Hippocampus. 2005;15:815–824. doi: 10.1002/hipo.20104. [DOI] [PubMed] [Google Scholar]
- Lilliquist MW, Burkhalter EC, Lobaugh NJ, Amsel A. Age-dependent effects of hippocampal muscarinic receptor blockade on memory-based learning in the developing rat. Behav Brain Res. 1993;53:119–125. doi: 10.1016/s0166-4328(05)80271-2. [DOI] [PubMed] [Google Scholar]
- Lissek S, Powers AS, McClure EB, Phelps EA, Woldehawariat G, Grillon C, Pine DS. Classical fear conditioning in the anxiety disorders: A meta-analysis. Bahav Res Ther. 2005;43:1391–1424. doi: 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Liu IYC, Lyons WE, Mamounas LA, Thompson RF. Brain-derived neurotropic factor plays a critical role in contextual fear conditioning. J Neurosci. 2004;24:7958–7963. doi: 10.1523/JNEUROSCI.1948-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Maren S. The amygdala, synaptic plasticity, and fear memory. Ann N Y Acad Sci. 2003;985:106–113. doi: 10.1111/j.1749-6632.2003.tb07075.x. [DOI] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning. Behav Brain Res. 1997;88:261–274. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. Electrolytic lesions of the fimbria/fornix, dorsal hippocampus, or entorhinal cortex produce anterograde deficits in contextual fear conditioning in rats. Neurobiol Learn Mem. 1997;67:142–149. doi: 10.1006/nlme.1996.3752. [DOI] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Barrientos RM, Rudy JW. The role of the dorsal hippocampus in the acquisition and retrieval of the context memory representation. J Neurosci. 2004;24:2431–2439. doi: 10.1523/JNEUROSCI.1598-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Early life influences on life-long patterns of behavior and health. Ment Retard Dev Disabil Res Rev. 2003;9:149–154. doi: 10.1002/mrdd.10074. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Roozendaal B, Cahill L. Modulation of memory storage by stress hormones and amygdaloid complex. In: Gazzaniga M, editor. Cognitive neuroscience. Cambrige: MIT Press; 1999. [Google Scholar]
- Meaney MJ, Diorio J, Francis D, Widdowson J, LaPlante P, Caldji C, Sharma S, Seckl JR, Plotsky PM. Early environmental regulation of forebrain glucocorticoid receptor gene expression: Implications for adrenocortical responses to stress. Dev Neurosci. 1996;18:49–72. doi: 10.1159/000111395. [DOI] [PubMed] [Google Scholar]
- Michelson HB, Lothman EW. An in vivo electrophysiological study of the ontogeny of excitatory and inhibitory processes in the rat hippocampus. Dev Brain Res. 1989;47:112–122. doi: 10.1016/0165-3806(89)90113-2. [DOI] [PubMed] [Google Scholar]
- Mineka S, Oehlberg K. The relevance of recent developments in classical conditioning to understanding the etiology and maintenance of anxiety disorders. Acta Psychol (Amst) 2008;127:567–580. doi: 10.1016/j.actpsy.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Moriceau S, Roth TL, Okotoghaide T, Sullivan RM. Corticosterone controls the developmental emergence of fear and amygdala function to predator odors in infant rat pups. Int J Dev Neurosci. 2004;22:415–422. doi: 10.1016/j.ijdevneu.2004.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Wilson DA, Levine S, Sullivan RM. Dual circuitry for odor-shock conditioning during infancy: Corticosterone switches between fear and attraction via amygdala. J Neurosci. 2006;26:6737–6748. doi: 10.1523/JNEUROSCI.0499-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morys J, Berdel B, Jagalska-Majewska H, Luczynska A. The basolateral amygdaloid complex - Its development, morphology and functions. Folia Morphol. 1999;58:29–46. [PubMed] [Google Scholar]
- Muller DM, Oliver M, Lynch G. Development changes in synaptic properties in hippocampus of neonatal rat. Dev Brain Res. 1989;49:105–114. doi: 10.1016/0165-3806(89)90063-1. [DOI] [PubMed] [Google Scholar]
- Nair HP, Gonzalez-Lima F. Extinction of behavior in infant rats: Development of fuctional coupling between septal, hippocampal, and ventral tegmental regions. J Neurosci. 1999;19:8648–8655. doi: 10.1523/JNEUROSCI.19-19-08646.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse S, Lacaille JC. Late maturation of GABAB synaptic transmission in area CA1 of the rat hippocampus. Neuropharmacology. 1999;38:1733–1742. doi: 10.1016/s0028-3908(99)00122-7. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The hippocampus as a cognitive map. New York: Oxford University Press; 1978. [Google Scholar]
- Otto T, Couserns G, Herzog C. Behavioral and neuropsychological foundations of olfactory fear conditioning. Behav Brain Res. 2000;110:119–128. doi: 10.1016/s0166-4328(99)00190-4. [DOI] [PubMed] [Google Scholar]
- Otto T, Giardino ND. Pavlovian conditioning of emotional responses to olfactory and contextual stimuli: A potential model for the development and expression of chemical intolerance. Ann N Y Acad Sci. 2001;933:291–309. doi: 10.1111/j.1749-6632.2001.tb05832.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press; 1986. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Diferential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Lesions o the dorsal hippocampus formation interfere with background but not foreground contextual fear conditioning. Learn Mem. 1994;1:34–44. [PubMed] [Google Scholar]
- Raineki C, Shionoya K, Sander K, Sullivan RM. Ontogeny of odor-LiCl vs. odor-shock learning: Similar behaviors but divergent ages of functional amygdala emergence. Learn Mem. 2009;16:114–121. doi: 10.1101/lm.977909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond MA, Yee B, Pouzet B, Veenman L, Rawlins JNP, Felden J, Bannerman DM. Dissociating context and space within the hippocampus: Effects of complete, dorsal, and ventral excitotoxic hippocampal lesions on conditioned freezing and spatial learning. Behav Neurosci. 1999;113:1189–1203. doi: 10.1037/0735-7044.113.6.1189. [DOI] [PubMed] [Google Scholar]
- Roth TL, Moriceau S, Sullivan RM. Opioid modulation of Fos protein expression and olfactory circuitry plays a pivotal role in what neonates remember. Learn Mem. 2006;13:590–598. doi: 10.1101/lm.301206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Sullivan RM. Memory of early maltreatment: Neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biol Psychiatry. 2005;57:823–831. doi: 10.1016/j.biopsych.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Rudy JW. Contextual conditioning and auditory cue conditioning dissociate during development. Behav Neurosci. 1993;107:887–891. doi: 10.1037//0735-7044.107.5.887. [DOI] [PubMed] [Google Scholar]
- Rudy JW. Ontogeny of context-specific latent inhibition of conditioned fear: Implications for configural associations theory and hippocampal formation development. Dev Psychobiol. 1994;27:367–379. doi: 10.1002/dev.420270605. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Barrientos RM, O’Reilly RC. Hippocampal formation supports conditioning to memory of a context. Behav Neurosci. 2002;116:530–538. doi: 10.1037//0735-7044.116.4.530. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Huff NC, Matus-Amat P. Understanding contextual fear conditioning: insights form a two-process model. Neurosci Biobehav Rev. 2004;28:675–685. doi: 10.1016/j.neubiorev.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Morledge P. Ontogeny of contextual fear conditioning in rats: Implications for consolidation, infantile amnesia, and hippocampal system function. Behav Neurosci. 1994;108:227–234. doi: 10.1037//0735-7044.108.2.227. [DOI] [PubMed] [Google Scholar]
- Sanders MJ, Wiltgen BJ, Fanselow MS. The place of the hippocampus in fear conditioning. Eur J Pharmacol. 2003;463:217–223. doi: 10.1016/s0014-2999(03)01283-4. [DOI] [PubMed] [Google Scholar]
- Schenberg EK, Oliveira GM. Effects of pre or posttraining dorsal hippocampus D-AP5 injection on fear conditioning to tone, background, and foreground context. Hippocampus. 2008;18:1089–1093. doi: 10.1002/hipo.20475. [DOI] [PubMed] [Google Scholar]
- Selden NR, Everitt BJ, Jarrard LE, Robbins TW. Complementary roles for the amygdale and hippocampus in aversive conditioning to explicit and contextual cues. Neuroscience. 1991;42:335–350. doi: 10.1016/0306-4522(91)90379-3. [DOI] [PubMed] [Google Scholar]
- Shionoya K, Moriceau S, Lunday L, Miner C, Roth TL, Sullivan RM. Development switch in neural circuitry underlying odor-malaise learning. Learn Mem. 2006;13:801–808. doi: 10.1101/lm.316006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solum DT, Handa RJ. Estrogen regulates the development of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus. J Neurosci. 2002;22:2650–2659. doi: 10.1523/JNEUROSCI.22-07-02650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Landers M, Yeaman B, Wilson DA. Good memories of bad events in infancy. Nature. 2000;407:38–39. doi: 10.1038/35024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann JW, Brady RJ, Martin DL. Postnatal development of GABAergic synaptic inhibition in rat hippocampus. Neuroscience. 1989;28:551–561. doi: 10.1016/0306-4522(89)90004-3. [DOI] [PubMed] [Google Scholar]
- Swann JW, Pierson MG, Smith KL, Lee CL. Developmental neuroplasticity: Roles in early life seizures and chronic epilepsy. Adv Neurol. 1999;79:203–216. [PubMed] [Google Scholar]
- Swann JW, Smith KL, Brady RJ. Neural networks and synaptic transmission in immature hippocampus. Adv Exp Med Biol. 1990;268:161–171. doi: 10.1007/978-1-4684-5769-8_19. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Galea LAM, Gould E. Stress inhibits the proliferation of granulate cell precursors in the developing dentate gyrus. Int J Dev Neurosci. 1998;16:235–239. doi: 10.1016/s0736-5748(98)00029-x. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Baldessarini RJ. Comparative postnatal development of dopamine D1, D2 and D4 receptors in rat forebrain. Int J Dev Neurosci. 2000;18:29–37. doi: 10.1016/s0736-5748(99)00108-2. [DOI] [PubMed] [Google Scholar]
- Toscano CD, McGlothan JL, Guilarte TR. Lead exposure alters cyclic-AMP response element binding protein phosphorylation and binding activity in the developing rat brain. Dev Brain Res. 2003;145:219–228. doi: 10.1016/j.devbrainres.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Wallenstein GV, Vago DR. Intrahippocampal scopolamine impairs both acquisition and consolidation of contextual fear conditioning. Neurobiol Learn Mem. 2001;75:245–252. doi: 10.1006/nlme.2001.4005. [DOI] [PubMed] [Google Scholar]
- Wilson DA. A comparison of postnatal development of post-activation potentiation in the neocortex and dentate gyrus of the rat. Brain Res. 1984;318:61–68. doi: 10.1016/0165-3806(84)90063-4. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Willner J, Kurz EM, Nadel L. Early handling increases hippocampal long-term potentiation in young rats. Behav Brain Res. 1986;21:223–227. doi: 10.1016/0166-4328(86)90240-8. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Sanders MJ, Anagnostaras SG, Sage JR, Fanselow MS. Context fear learning in the absence of the hippocampus. J Neurosci. 2006;26:5484–5491. doi: 10.1523/JNEUROSCI.2685-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SL, Bohenek DL, Fanselow MS. NMDA processes mediate anterograde amnesia of contextual fear conditioning induced by hippocampal damage: Immunization against amnesia by context preexposure. Behav Neurosci. 1994;108:19–29. doi: 10.1037//0735-7044.108.1.19. [DOI] [PubMed] [Google Scholar]