Abstract
Despite recent progress in the understanding of systemic lupus erythematosus (SLE), the striking 9:1 female to male ratio of disease incidence remains largely unexplained. In addition, peak SLE incidence rates occur during the early reproductive years in women. Studies which illuminate potential causes underlying this sex difference and characteristic onset during the reproductive years have the potential to fundamentally advance our understanding of disease pathogenesis in SLE. Similarly, progress in this area will likely inform human reproductive immunology. Studies of sex hormone function in the immune system are of obvious importance; however, it seems likely that many other types of sex-related genetic and immunological differences will contribute to SLE. In this review, we will focus on recent work in sex-related differences in cytokine pathways and genetics of these pathways as they relate to SLE pathogenesis. It seems quite possible that many of these sex-related differences could be important to reproductive fitness, which may explain the conservation of these immune system features and the observed female predominance of SLE.
Keywords: Systemic lupus erythematosus, Sex, Cytokines, Genetics, X chromosome, Interferon alpha
Introduction
Systemic lupus erythematosus (SLE) is a chronic inflammatory autoimmune disease affecting approximately one and a half million Americans and five million people worldwide [1]. It may affect almost all organ systems in the form of rash, hair loss, arthralgias, arthritis, glomerulonephritis, pericarditis, pleuritis, psychosis, and seizures. SLE is a prototypic systemic autoimmune disease which involves almost all parts of the immune system; however, humoral autoimmunity with the production of characteristic autoantibodies and serum cytokine dysregulation are hallmarks of the disease. SLE is characterized by a 9:1 female to male ratio of disease incidence, with an even higher female predominance during peak reproductive years [2–4]. The peak of disease incidence for women is during reproductive years (ages 20–30 years), while men tend to have a peak in later middle age (ages 45–60 years) [3, 5]. The large sex and peak incidence differential in SLE hold great promise for increasing our understanding of disease pathogenesis. In this review, we will discuss some current theories for the observed patterns in SLE incidence with an emphasis on human cytokine and genetic studies, including the role of interferon alpha (IFN-α) in the placenta [6], the influence of female sex hormones on IFN-α and Toll-like receptor (TLR) expression [7], fetal and maternal microchimerism [8], aberrant X chromosome inactivation or X chromosome dosage effects [9], and the role of SLE susceptibility genes including the X chromosomal MECP2/IRAK1 locus and the gene–sex influence of the osteopontin locus [10–14].
IFN-α as a causal factor in SLE
Many lines of evidence have implicated IFN-α in SLE pathogenesis. IFN-α is a pleiotropic type I interferon with the potential to break immunologic self-tolerance by activating antigen-presenting cells after uptake of self material [15]. Serum IFN-α is elevated in many SLE patients, and elevations often correlate with disease activity [16, 17]. Recombinant human IFN-α administered as a therapy for chronic viral hepatitis and malignancy is thought to cause de novo SLE in some patients [18]. IFN-α-induced SLE typically resolves after the IFN-α is discontinued [19, 20], supporting the idea that IFN-α was causal. We have previously shown that serum IFN-α is abnormally high in 20% of healthy first-degree relatives of SLE patients as compared to <5% of healthy unrelated individuals [21]. Spouses of SLE patients did not have high serum IFN-α. Taken together, these data suggest that high serum IFN-α is a heritable risk factor for SLE [21]. Additionally, serum IFN-α activity is highest during the ages of peak SLE incidence in both patients and their healthy first-degree relatives [22]. The high IFN-α trait in SLE families is inherited in a complex fashion, suggesting polygenic inheritance which has not been fully characterized. We have begun to genetically map the high IFN-α trait in SLE and found that a number of genetic variants which are associated with susceptibility to SLE are also associated with increased serum IFN-α [23], including variants of IRF5 [24], IRF7 [25], PTPN22 [26], and OPN [27]. We have also shown that the autoimmune disease-associated variant of STAT4 modulates cellular sensitivity to IFN-α signaling [28]. These studies provide further support for the hypothesis that IFN-α pathway dysregulation is a primary causal factor in human SLE.
IFN-α and the placenta
The IFN-α gene cluster has been steadily diversifying and expanding in placental mammals [29]. In fish, it is only encoded by a single gene, while in placental mammals, there has been extensive duplication and diversification of the type I interferon genes, resulting in many subtypes of type I interferon [29]. There are at least 14 human genes that comprise the IFN-α gene cluster [29].
Interferon has antiviral, antiproliferative, and immuno-suppressive effects, and many subtypes of type I interferon are expressed by human placentae, decidua, and fetal membranes [6, 29]. Trophectoderm produces type I interferon in primate peri-implantation [6]. This appears to affect uterine receptivity and placental growth and development via expression of interferon-stimulated genes [6]. Mice lacking the ability to signal through type I interferon usually die in the early embryonic stages [29]. Interferons produced by the human placenta suppress proliferation of T and B cells, suggesting that immune tolerance of the mother to the fetus may be mediated in part by type I interferon [6]. Type I interferons also likely play a role in protecting the fetus from viral infections and suppressing the expression of proto-oncogenes [6]. These studies in aggregate suggest that type I interferon expression by the human placenta has a protective effect on the products of conception and presumably, a direct impact on reproductive fitness [6, 30, 31]. Given these important roles for IFN-α in female reproduction, it seems likely that sex-related differences in the regulation of IFN-α could exist.
Influence of sex hormones upon IFN-α
In some mouse models of SLE, estrogen exacerbates autoimmunity [32]. In vitro studies of human cells have demonstrated increased inflammatory cytokine production in dendritic cells exposed to estrogen [33], although significant differences in IFN-α production in the setting of estrogen stimulation have not been shown to our knowledge [34]. In contrast, progesterone appears to block TLR7-mediated IFN-α production by dendritic cells in murine systems [35]. Women with SLE have abnormally high levels of active estrogen metabolites, while also exhibiting abnormally low levels of progesterone [36, 37]. Thus, in women who have a predisposition for SLE through other factors, low progesterone or high levels of estrogen may facilitate disease development or disease activity by modulating the IFN-α pathway. Sex hormones have been reported to have a number of functions upon other aspects of the immune system which we will not review in depth in this article, but this topic has recently been reviewed extensively [38, 39].
Sex-related differences in the Toll-like receptor pathway of IFN-α production
The endosomal TLRs, including TLR7, TLR8, and TLR9, are pattern recognition receptors, which function in normal immunity by sensing viral nucleic acids and subsequently stimulating IFN-α production [40]. Many lines of evidence suggest that these TLRs can be activated by nucleic acid immune complexes in SLE [41], contributing to the increased levels of serum IFN-α which are observed in the disease. Interestingly, the TLR7 and TLR8 genes are located on the X chromosome in humans and mice. In the BXSB mouse model of SLE, a Y chromosomal element named yaa was discovered to significantly increase the severity of disease [42]. This is curious, given the female predominance observed in human SLE. Characterization of the yaa element revealed that it was in fact a translocation of a pseudoautosomal region of the X chromosome onto the Y chromosome which contained the TLR7 gene [43]. Yaa-bearing B cells overexpress the duplicated X chromosome genes, including TLR7 [43]. This uncompensated duplication of the TLR7 gene was found to accelerate autoimmune disease by increasing IFN-α production, macrophage activation, and autoantibody production [44].
In humans, in vitro studies have shown that peripheral blood mononuclear cells (PBMC) from healthy female subjects produce much more IFN-α after stimulation through TLR7 than PBMC from healthy male subjects [45]. Further investigation showed no evidence of aberrant X inactivation in the region of the TLR7 gene which could explain this phenomenon [45], and the cause of this finding remains mysterious. The importance of TLR signaling in human SLE is supported by a number of observations, including a case report of a woman with SLE who later developed common variable immunodeficiency (CVID). This report describes remission of her SLE after the development of her immunodeficiency [46]. When this patient developed CVID, her double-stranded DNA antibodies became undetectable, and her B cells demonstrated poor proliferation and differentiation in response to TLR7 and TLR9 agonists [46]. This case report provides an interesting human example which demonstrates the importance of the TLR pathway in human disease, as likely the acquired impairment of the TLR pathway due to CVID was related to the clinical improvement in her SLE. Genetic variation in the X chromosomal TLR region has not been implicated in human SLE to date [47], although this possibility has not been ruled out.
Serum IFN-α levels are highest during the reproductive years in SLE patients and healthy SLE family members
Given the data supporting IFN-α as a causal agent in human lupus, we examined IFN-α levels in a large family cohort in the context of age and sex in both healthy and affected family members [22]. Interestingly, in both male and female patient groups as well as male and female healthy relatives, there was a trend toward an inverse relationship between age and serum IFN-α activity [22]. While the general pattern of higher serum IFN-α in younger people was shared, the relationship between age and serum IFN-α appeared to be complex and not well-captured by a simple correlation analysis.
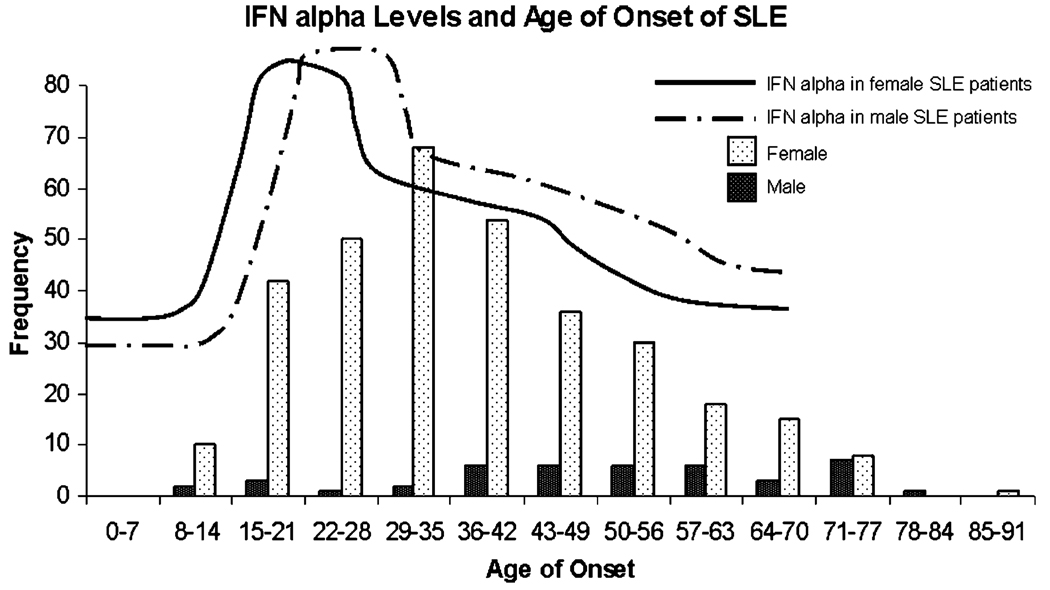
To address this issue, we used a sliding window analysis technique to determine which age ranges were characterized by significant increases in serum IFN-α [22]. This pattern-finding algorithm revealed significantly increased IFN-α activity between the ages of 12 and 22 years for female SLE patients and between the ages of 16 and 29 years for male SLE patients [22]. Both male and female healthy first-degree relatives had significantly decreased IFN-α activity after the age of 50 years [22]. Interestingly, there were no major sex-related differences in the cohort, and the major differences existed between age groups. The age ranges of highest serum IFN-α in the SLE patients corresponded well with the age ranges of peak SLE incidence for each sex (Fig. 1). These data further implicate high serum IFN-α in the disease initiation phase of human SLE and raise the interesting possibility that some influence of IFN-α upon reproduction contributes to the observed incidence patterns of SLE.
Fig. 1.
IFN-α levels and age of onset of SLE. Average serum IFN-α levels by age in a large SLE cohort stratified by sex [22] are shown overlaid upon data regarding the sex-specific age of SLE incidence from a different large population-based study [5]. Serum IFN-α levels seem to correlate with age of SLE onset, as age ranges with higher levels of IFN-α correspond to age ranges of greater SLE incidence. Additionally, there is a time lag for both disease onset and peak serum IFN-α level in men as compared with women. The lines representing average serum IFN-α levels in SLE patients are drawn to approximate the patterns observed in [22], and the bars on the bar graph represent the number of incident SLE cases by age group on the Y axis in the study by Lopez et al. as detailed in [5]
Microchimerism in pregnancy
Microchimerism in pregnancy can involve either transfer of cells from the maternal circulation to the fetus or from the fetal circulation to the mother. Fetomaternal cell trafficking has been implicated in scleroderma [48], another female-predominant autoimmune disease. Scleroderma affects women during and after their childbearing years, and the long persistence of fetal cells in the circulation of these subjects is thought to contribute to the disease pathogenesis [49], although a direct role in scleroderma disease pathogenesis has not been established. In SLE, a case study of a female patient who had two male births and died of SLE-related complications revealed male cells in organs and tissues that were affected by the disease process [50]. Male cells were not found in healthy organs. This raises the possibility that fetal cells play a pathogenetic role in SLE. However, autoimmune disease can also exist in children, men, and nulliparous women, and in larger cohort studies, it is not clear that fetomaternal chimeric cells are playing a pathogenic role in SLE [51].
Maternal chimerism exists in juvenile idiopathic inflammatory myopathies, juvenile dermatomyositis, and neonatal lupus [52–54]. These diseases share many similarities with SLE, including the presence of particular autoantibodies as well as frequent elevations in serum IFN-α [55–57]. A study of men with SLE found persistent maternal microchimerism, documented by visualization of cells with two X chromosomes [49]. In a study of men with SLE and their mothers, bidirectional HLA class II compatibility was significantly increased compared with healthy males and their mothers [8], suggesting that maternal microchimerism could be facilitated in this setting, and the increased prevalence in SLE families would imply that this HLA compatibility results in risk of SLE. As in the case of fetomaternal chimerism, an exact pathogenic role for maternal chimeric cells in SLE has not been defined [58].
X chromosome dosage effects
Complete X monosomy or Turner's syndrome (45, XO) is associated with an increased risk of particular autoimmune conditions, including autoimmune thyroid disease, inflammatory bowel disease, and juvenile idiopathic arthritis [59]. Increased rates of either partial X chromosomal deletions or complete X monosomy have been reported in other autoimmune diseases, such as systemic sclerosis, autoimmune thyroid disease, and primary biliary cirrhosis, which could partially explain some of the female predominance in these diseases [60]. However, an increased rate of X monosomy has not demonstrated in female SLE [61], and reports of the co-existence of Turner's syndrome and SLE is exceedingly rare [62].
Conversely, SLE has been reported in patients with Klinefelter's syndrome (47,XXY) [63], and these male patients have an uncompensated second X chromosome. Klinefelter's syndrome is increased 14-fold in men with SLE when compared with healthy male controls [63]. Thus, while 46,XY men have an approximately 10-fold lower risk of SLE than 46,XX women, men with Klinefelter's syndrome have a risk for SLE that is similar to 46,XX women [63]. Taken together, these data suggest a dose effect of the X chromosome on SLE susceptibility: two X chromosomes confer a higher risk of SLE, while one X chromosome confers a lower risk of SLE. Chagnon et al. report the case of an XX male patient with severe prepubertal SLE who carried a partial Y chromosomal translocation on one X chromosome [9]. Interestingly, in addition to the Y chromosomal translocation, this patient had a duplication of an X chromosome pseudoautosomal region, on the same chromosome, causing a trisomy of 12 genes in that region. Two of these 12 genes, IL3RA and CD99, have function within the immune system, and circulating CD99 levels were higher in this patient than in healthy controls [9], suggesting a potential role for CD99 and this pseudoautosomal region of the X chromosome in SLE pathogenesis.
Skewing of X inactivation
In females, dosage compensation of the X chromosome is achieved via X inactivation, which involves extensive DNA methylation and condensation causing most genes on the inactivated chromosome to not be expressed. The chromosome which is inactivated is chosen randomly during healthy development, and thus, in a given cell, either the paternal or maternal X chromosome may be inactivated. Generally, the proportion of maternal versus paternal X inactivation is similar in a given cell or tissue type. Skewing of X inactivation describes the situation in which one X chromosome is more preferentially inactivated in a given tissue than the other, thus biasing gene expression to either the maternal or paternal X chromosome alleles. Thus, if one X chromosome harbors SLE susceptibility genes and this chromosome is preferentially expressed, then skewed X chromosome inactivation could then potentially predispose to autoimmune disease. While skewed X chromosome inactivation has been observed in systemic sclerosis and autoimmune thyroid disease [64, 65], studies involving both murine models and human PBMC found no skewing of X chromosome inactivation in SLE [66, 67].
MECP2/IRAK1 genetic association
The methyl-CpG-binding protein 2 (MECP2) gene is located on the X chromosome (Xq28), and the gene product suppresses the transcription of particular genes when they are methylated. Interestingly, methylation-sensitive genes are generally overexpressed in SLE [68]. Genetic variation in the MECP2 locus has recently been associated with SLE susceptibility in multiple ancestral backgrounds [10], and although, the causal variant has not yet been determined, it is possible that a loss-of-function variant of MECP2 is driving this association. The MECP2 variants linked to SLE susceptibility are in high linkage disequilibrium with an adjacent gene with immune system relevance, interleukin-1 receptor associated kinase-1 (IRAK1). Association between IRAK1 variants and SLE has also been recently demonstrated in both early and late onset SLE and across different ancestries [11]. In a murine model of SLE, IRAK1 deficiency resulted in lack of autoantibodies, decreased lymphocyte activation, and absence of renal disease [11]. It is unclear if one or both of these X chromosomal loci will harbor the causal variants resulting in risk of human SLE, but these association studies provide a precedent for X chromosomal genetic variation being directly involved in SLE susceptibility.
Osteopontin gene–sex interaction
Osteopontin (SPP1) is known to play an important role in bone biology and has also been shown to regulate inflammation and immunity. Osteopontin1 enhances pro-inflammatory Th1 cell responses and inhibits Th2 responses. Osteopontin also plays an important role in IFN-α production by peripheral dendritic cells downstream of TLR9 ligation in murine models [69]. Osteopontin is overexpressed in biopsies of inflamed tissues in SLE and other autoimmune diseases [70, 71], suggesting pathogenic involvement. A number of candidate gene studies have demonstrated an association between variants of the SPP1 gene and SLE [12–14]. Interestingly, the largest genetic study to date found that the association of osteopontin variants with SLE was particular to male patients, who were only approximately 10% of patients in the study [14]. This result is surprising, as previous genetic studies which found evidence of association did not separate males and females for analysis and had similar proportions of male and female patients [13].
Few studies have examined osteopontin levels in SLE patient serum or the potential influence of osteopontin genetic variants upon serum osteopontin levels. One previous study found that the SLE-associated rs9138 C allele in the 3′ untranslated region was associated with increased serum osteopontin levels in healthy controls; however, this association was not seen in the SLE patients [13]. We studied SLE patients from multiple ancestral backgrounds to determine whether osteopontin genetic variants were linked to altered cytokine profile [27]. Given the previous results suggesting a sex-specific association [14], we separated male and female patients for these analyses. The most consistently replicated SLE-associated SNP (rs9138 C) in the 3′ untranslated region of the gene was associated with increased serum osteopontin in male patients in a dose-response fashion [27]. Surprisingly, female patients as a group did not share this relationship [27]. Osteopontin functions in long bone remodeling [72] and osteopontin expression can be induced by estrogen through a non-classical estrogen receptor-related-α pathway [73]. We hypothesized that age-related regulation of osteopontin in female patients may be present. When the female patients were stratified by age, younger patients (age <23 years) showed a dose-response increase in serum osteopontin in relation to the rs9138 C allele [27]. This relationship was clearly not present in older patients (age ≥23), and risk allele carriers showed a dramatic difference in serum osteopontin levels by age [27]. The same age- and sex-related patterns were present in all ancestral backgrounds [27].
Serum IFN-α levels were correlated with serum osteopontin levels in simultaneous samples, and the relationship between IFN-α and rs9138 genotype mirrored the relationships described above for serum osteopontin [27]. Taken in the context of murine data which suggests an essential role for osteopontin in IFN-α production, we hypothesized that osteopontin may regulate IFN-α in SLE patients. We favor the hypothesis that both intracellular and extracellular osteopontin levels are differentially regulated by the SLE-associated SNP in the 3′ untranslated region of the gene. This SNP could function by altering mRNA poly-adenylation or stability, leading to increased protein translation. Other influences such as promotion of gene expression due to increased estrogen receptor-related-α signaling could then cooperate with increased mRNA stability to result in the higher osteopontin protein levels we detected. Increased osteopontin levels in the setting of the risk variant could subsequently augment downstream signaling from endosomal TLRs, resulting in greater IFN-α production and the correlation between serum IFN-α and serum osteopontin which was observed. The osteopontin gene in SLE provides an example of a genetic risk locus which influences serum levels of two distinct but related inflammatory cytokines. Additionally, genetic variation at this locus should contribute to the age-related pattern we observed in serum IFN-α in the female SLE cohort described above (Fig. 1).
Conclusion
In summary, many theories exist as to why SLE has a female predominance, and it is likely that many of the differences between men and women will relate to the sex disparity observed in the disease. One of the major differences between men and women is the ability to carry out placental reproduction. Elevated IFN-α is thought to play a pathogenic role in SLE [21], and this cytokine is expressed by the placenta, and the gene cluster encoding this cytokine has undergone a dramatic evolution in placental mammals [29]. We postulate that IFN-α contributes to the success of placental reproduction and that potential upregulation of this cytokine system in females could both increase reproductive fitness and simultaneously increase susceptibility to SLE. SLE is rare enough in the general population that the population benefit of an increase in reproductive fitness would likely outweigh the cost of an increase in SLE susceptibility. Another major difference between men and women is the number of X chromosomes, and both the number of X chromosomes and genetic variants on the X chromosome are related to the risk of development of SLE. Two functional X chromosomes, either by sex or by translocation or duplication, appear to confer a greater risk of SLE than one X chromosome [63]. While the exact molecular mechanisms and relative contributions of the above hypotheses are unknown, progress is being made in this exciting area, and via this type of work, much will be learned about both SLE and the sex-related differences in immunity which underlie the disorder.
Acknowledgments
C. E. Weckerle has no grant support or disclosures to declare. Grant support for and disclosures by T. B. Niewold include NIH K08 AI083790, NIAID Clinical Research Loan Repayment AI071651, Arthritis National Research Foundation Eng Tan Scholar Award, Lupus Research Institute Novel Research Grant, University of Chicago CTSA Core Subsidy Grant, and Collaborative University of Chicago/Northshore University Health System Translational Research Pilot Grant from UL1 RR024999.
Contributor Information
Corinna E. Weckerle, Section of Rheumatology, University of Chicago, 5841 S. Maryland Ave. MC 0930, Chicago, IL 60637, USA
Timothy B. Niewold, Email: tniewold@medicine.bsd.uchicago.edu, Section of Rheumatology, University of Chicago, 5841 S. Maryland Ave. MC 0930, Chicago, IL 60637, USA; Gwen Knapp Center for Lupus and Immunology Research, University of Chicago, Chicago, IL, USA.
References
- 1.Lupus Foundation of America. 2009 www.lupus.org. [Google Scholar]
- 2.Petri M. Epidemiology of systemic lupus erythematosus. Best Pract Res Clin Rheumatol. 2002;16(5):847–858. doi: 10.1053/berh.2002.0259. [DOI] [PubMed] [Google Scholar]
- 3.Rus V, Maury EE, Hochberg MC, editors. The epidemiology of systemic lupus erythematosus. Philadelphia: Williams and Wilkins; 2002. [Google Scholar]
- 4.Chakravarty EF, Bush TM, Manzi S, Clarke AE, Ward MM. Prevalence of adult systemic lupus erythematosus in California and Pennsylvania in 2000: estimates obtained using hospitalization data. Arthritis Rheum. 2007;56(6):2092–2094. doi: 10.1002/art.22641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopez P, Mozo L, Gutierrez C, Suarez A. Epidemiology of systemic lupus erythematosus in a northern Spanish population: gender and age influence on immunological features. Lupus. 2003;12(11):860–865. doi: 10.1191/0961203303lu469xx. [DOI] [PubMed] [Google Scholar]
- 6.Bazer FWSTE, Johnson GA, Burghardt RC, Wu G. Comparative aspects of implantation. Reproduction. 2009;138(2):195–209. doi: 10.1530/REP-09-0158. [DOI] [PubMed] [Google Scholar]
- 7.Szyper-Kravitz M, Zandman-Goddard G, Lahita RG, Shoenfeld Y. The neuroendocrine-immune interactions in systemic lupus erythematosus: a basis for understanding disease pathogenesis and complexity. Rheum Dis Clin North Am. 2005;31(1):161–175. doi: 10.1016/j.rdc.2004.10.004. x. [DOI] [PubMed] [Google Scholar]
- 8.Stevens AM, Tsao BP, Hahn BH, Guthrie K, Lambert NC, Porter AJ, et al. Maternal HLA class II compatibility in men with systemic lupus erythematosus. Arthritis Rheum. 2005;52(9):2768–2773. doi: 10.1002/art.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chagnon P, Schneider R, Hebert J, Fortin PR, Provost S, Belisle C, et al. Identification and characterization of an Xp22.33; Yp11.2 translocation causing a triplication of several genes of the pseudoautosomal region 1 in an XX male patient with severe systemic lupus erythematosus. Arthritis Rheum. 2006;54(4):1270–1278. doi: 10.1002/art.21733. [DOI] [PubMed] [Google Scholar]
- 10.Sawalha AH, Webb R, Han S, Kelly JA, Kaufman KM, Kimberly RP, et al. Common variants within MECP2 confer risk of systemic lupus erythematosus. PLoS ONE. 2008;3(3):e1727. doi: 10.1371/journal.pone.0001727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacob CO, Zhu J, Armstrong DL, Yan M, Han J, et al. Identification of IRAK1 as a risk gene with critical role in the pathogenesis of systemic lupus erythematosus. PNAS. 2009;106(15):6256–6261. doi: 10.1073/pnas.0901181106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forton AC, Petri MA, Goldman D, Sullivan KE. An osteopontin (SPP1) polymorphism is associated with systemic lupus erythematosus. Hum Mutat. 2002;19(4):459. doi: 10.1002/humu.9025. [DOI] [PubMed] [Google Scholar]
- 13.D'Alfonso S, Barizzone N, Giordano M, Chiocchetti A, Magnani C, Castelli L, et al. Two single-nucleotide polymorphisms in the 5′ and 3′ ends of the osteopontin gene contribute to susceptibility to systemic lupus erythematosus. Arthritis Rheum. 2005;52(2):539–547. doi: 10.1002/art.20808. [DOI] [PubMed] [Google Scholar]
- 14.Han S, Guthridge JM, Harley IT, Sestak AL, Kim-Howard X, Kaufman KM, et al. Osteopontin and systemic lupus erythematosus association: a probable gene-gender interaction. PLoS ONE. 2008;3(3):e0001757. doi: 10.1371/journal.pone.0001757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294(5546):1540–1543. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 16.Hooks JJ, Moutsopoulos HM, Geis SA, Stahl NI, Decker JL, Notkins AL. Immune interferon in the circulation of patients with autoimmune disease. N Engl J Med. 1979;301(1):5–8. doi: 10.1056/NEJM197907053010102. [DOI] [PubMed] [Google Scholar]
- 17.Kirou KA, Lee C, George S, Louca K, Peterson MG, Crow MK. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 2005;52(5):1491–1503. doi: 10.1002/art.21031. [DOI] [PubMed] [Google Scholar]
- 18.Ronnblom LE, Alm GV, Oberg KE. Possible induction of systemic lupus erythematosus by interferon-alpha treatment in a patient with a malignant carcinoid tumour. J Intern Med. 1990;227(3):207–210. doi: 10.1111/j.1365-2796.1990.tb00144.x. [DOI] [PubMed] [Google Scholar]
- 19.Niewold TB, Swedler WI. Systemic lupus erythematosus arising during interferon-alpha therapy for cryoglobulinemic vasculitis associated with hepatitis C. Clin Rheumatol. 2005;24(2):178–181. doi: 10.1007/s10067-004-1024-2. [DOI] [PubMed] [Google Scholar]
- 20.Niewold TB. Interferon alpha-induced lupus: proof of principle. J Clin Rheumatol. 2008;14(3):131–132. doi: 10.1097/RHU.0b013e318177627d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niewold TB, Hua J, Lehman TJ, Harley JB, Crow MK. High serum IFN-alpha activity is a heritable risk factor for systemic lupus erythematosus. Genes Immun. 2007;8:492–502. doi: 10.1038/sj.gene.6364408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niewold TB, Adler JE, Glenn SB, Lehman TJ, Harley JB, Crow MK. Age- and sex-related patterns of serum interferon-alpha activity in lupus families. Arthritis Rheum. 2008;58(7):2113–2119. doi: 10.1002/art.23619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kariuki SN, Niewold TB. Genetic regulation of serum cytokines in systemic lupus erythematosus. Transl Res. 2009 doi: 10.1016/j.trsl.2009.08.012. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niewold TB, Kelly JA, Flesch MH, Espinoza LR, Harley JB, Crow MK. Association of the IRF5 risk haplotype with high serum interferon-alpha activity in systemic lupus erythematosus patients. Arthritis Rheum. 2008;58(8):2481–2487. doi: 10.1002/art.23613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salloum R, Franek BS, Kariuki SN, Rhee L, Mikolaitis RA, Jolly M, et al. Genetic variation at the IRF7/PHRF1 locus is associated with autoantibody profile and serum interferon alpha activity in lupus patients. Arthritis Rheum. 2009 doi: 10.1002/art.27182. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kariuki SN, Crow MK, Niewold TB. The PTPN22 C1858T polymorphism is associated with skewing of cytokine profiles toward high interferon-alpha activity and low tumor necrosis factor alpha levels in patients with lupus. Arthritis Rheum. 2008;58(9):2818–2823. doi: 10.1002/art.23728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kariuki SN, Moore JG, Kirou KA, Crow MK, Utset TO, Niewold TB. Age- and gender-specific modulation of serum osteopontin and interferon-alpha by osteopontin genotype in systemic lupus erythematosus. Genes Immun. 2009;10(5):487–494. doi: 10.1038/gene.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kariuki SN, Kirou KA, MacDermott EJ, Barillas-Arias L, Crow MK, Niewold TB. Cutting edge: autoimmune disease risk variant of STAT4 confers increased sensitivity to IFN-alpha in lupus patients in vivo. J Immunol. 2009;182(1):34–38. doi: 10.4049/jimmunol.182.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pestka S, Krause CD, Walter MR. Interferons, interferon-like cytokines, and their receptors. Immunol Rev. 2004;202:8–32. doi: 10.1111/j.0105-2896.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- 30.Bazer FWWG, Spencer TE, Johnson GA, Burghardt RC, Bayless K. Novel Pathways for Implantation and Establishment and Maintenance of Pregnancy in Mammals. Mol Hum Reprod. 2009 doi: 10.1093/molehr/gap095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson GA, Bazer FW, Burghardt RC, Spencer TE, Wu G, Bayless KJ. Conceptus-uterus interactions in pigs: endo-metrial gene expression in response to estrogens and interferons from conceptuses. Soc Reprod Fertil. 2009;66 Suppl:321–332. [PubMed] [Google Scholar]
- 32.Peeva E, Grimaldi C, Spatz L, Diamond B. Bromocriptine restores tolerance in estrogen-treated mice. J Clin Invest. 2000;106(11):1373–1379. doi: 10.1172/JCI10420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hughes GC, Clark EA. Regulation of dendritic cells by female sex steroids: relevance to immunity and autoimmunity. Autoimmunity. 2007;40(6):470–481. doi: 10.1080/08916930701464764. [DOI] [PubMed] [Google Scholar]
- 34.Siracusa MC, Overstreet MG, Housseau F, Scott AL, Klein SL. 17beta-estradiol alters the activity of conventional and IFN-producing killer dendritic cells. J Immunol. 2008;180(3):1423–1431. doi: 10.4049/jimmunol.180.3.1423. [DOI] [PubMed] [Google Scholar]
- 35.Hughes GC, Thomas S, Li C, Kaja MK, Clark EA. Cutting edge: progesterone regulates IFN-alpha production by plasmacytoid dendritic cells. J Immunol. 2008;180(4):2029–2033. doi: 10.4049/jimmunol.180.4.2029. [DOI] [PubMed] [Google Scholar]
- 36.Lahita RG. The role of sex hormones in systemic lupus erythematosus. Curr Opin Rheumatol. 1999;11(5):352–356. doi: 10.1097/00002281-199909000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Doria A, Cutolo M, Ghirardello A, Zampieri S, Vescovi F, Sulli A, et al. Steroid hormones and disease activity during pregnancy in systemic lupus erythematosus. Arthritis Rheum. 2002;47(2):202–209. doi: 10.1002/art.10248. [DOI] [PubMed] [Google Scholar]
- 38.Nalbandian G, Kovats S. Understanding sex biases in immunity: effects of estrogen on the differentiation and function of antigen-presenting cells. Immunol Res. 2005;31(2):91–106. doi: 10.1385/IR:31:2:091. [DOI] [PubMed] [Google Scholar]
- 39.Zandman-Goddard G, Peeva E, Shoenfeld Y. Gender and autoimmunity. Autoimmun Rev. 2007;6(6):366–372. doi: 10.1016/j.autrev.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 40.Drexler SK, Foxwell BM. The role of Toll-like receptors in chronic inflammation. Int J Biochem Cell Biol. 2009 doi: 10.1016/j.biocel.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 41.Ronnblom L, Alm GV, Eloranta ML. Type I interferon and lupus. Curr Opin Rheumatol. 2009;21(5):471–477. doi: 10.1097/BOR.0b013e32832e089e. [DOI] [PubMed] [Google Scholar]
- 42.Izui S. Autoimmune accelerating genes, lpr and Yaa, in murine systemic lupus erythematosus. Autoimmunity. 1990;6(1–2):113–129. doi: 10.3109/08916939008993376. [DOI] [PubMed] [Google Scholar]
- 43.Subramanian S, Tus K, Li QZ, Wang A, Tian XH, Zhou J, et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc Natl Acad Sci USA. 2006;103(26):9970–9975. doi: 10.1073/pnas.0603912103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pisitkun P, Deane J, Difilippantonio M, Tarasenko T, Satterthwaite A, Bolland S. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312:1669–1672. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- 45.Berghofer B, Frommer T, Haley G, Fink L, Bein G, Hackstein H. TLR7 ligands induce higher IFN-alpha production in females. J Immunol. 2006;177(4):2088–2096. doi: 10.4049/jimmunol.177.4.2088. [DOI] [PubMed] [Google Scholar]
- 46.Visentini M, Conti V, Cagliuso M, Tinti F, Siciliano G, Trombetta AC, et al. Regression of systemic lupus erythematosus after development of an acquired Toll-like receptor signaling defect and antibody deficiency. Arthritis Rheum. 2009;60(9):2767–2771. doi: 10.1002/art.24760. [DOI] [PubMed] [Google Scholar]
- 47.Kelley J, Johnson MR, Alarcon GS, Kimberly RP, Edberg JC. Variation in the relative copy number of the TLR7 gene in patients with systemic lupus erythematosus and healthy control subjects. Arthritis Rheum. 2007;56(10):3375–3378. doi: 10.1002/art.22916. [DOI] [PubMed] [Google Scholar]
- 48.Jimenez SA, Artlett CM. Microchimerism and systemic sclerosis. Curr Opin Rheumatol. 2005;17(1):86–90. doi: 10.1097/01.bor.0000145516.45854.7b. [DOI] [PubMed] [Google Scholar]
- 49.Lambert NC, Stevens AM, Tylee TS, Erickson TD, Furst DE, Nelson JL. From the simple detection of microchimerism in patients with autoimmune diseases to its implication in pathogenesis. Ann NY Acad Sci. 2001;945:164–171. doi: 10.1111/j.1749-6632.2001.tb03881.x. [DOI] [PubMed] [Google Scholar]
- 50.Johnson KL, McAlindon TE, Mulcahy E, Bianchi DW. Microchimerism in a female patient with systemic lupus eryth-ematosus. Arthritis Rheum. 2001;44(9):2107–2111. doi: 10.1002/1529-0131(200109)44:9<2107::AID-ART361>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 51.Kremer Hovinga IC, Koopmans M, Grootscholten C, van der Wal AM, Bijl M, Derksen RH, et al. Pregnancy, chimerism and lupus nephritis: a multi-centre study. Lupus. 2008;17(6):541–547. doi: 10.1177/0961203308089324. [DOI] [PubMed] [Google Scholar]
- 52.Reed A, Picnorell YJ, Harwood A, Kredich D. Chimerism in children with juvenile dermatomyositis. Lancet. 2000;356:2156–2157. doi: 10.1016/S0140-6736(00)03500-5. [DOI] [PubMed] [Google Scholar]
- 53.Artlett CM, Ramos R, Jiminez SA, Patterson K, Miller FW, Rider LG the Childhood Myositis Heterogeneity Collaborative Group. Chimeric cells of maternal origin in juvenile idiopathic inflammatory myopathies. Lancet. 2000;356:2155–2156. doi: 10.1016/s0140-6736(00)03499-1. [DOI] [PubMed] [Google Scholar]
- 54.Stevens AM, Hermes HM, Rutledge JC, Buyon JP, Nelson JL. Myocardial-tissue-specific phenotype of maternal microchimerism in neonatal lupus congenital heart block. Lancet. 2000;362:1617–1623. doi: 10.1016/S0140-6736(03)14795-2. [DOI] [PubMed] [Google Scholar]
- 55.Walsh RJ, Kong SW, Yao Y, Jallal B, Kiener PA, Pinkus JL, et al. Type I interferon-inducible gene expression in blood is present and reflects disease activity in dermatomyositis and polymyositis. Arthritis Rheum. 2007;56(11):3784–3792. doi: 10.1002/art.22928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Niewold TB, Kariuki SN, Morgan GA, Shrestha S, Pachman LM. Elevated serum interferon-alpha activity in juvenile dermatomyositis: associations with disease activity at diagnosis and after thirty-six months of therapy. Arthritis Rheum. 2009;60(6):1815–1824. doi: 10.1002/art.24555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Niewold TB, Rivera TL, Buyon JP, Crow MK. Serum type I interferon activity is dependent on maternal diagnosis in anti-SSA/Ro-positive mothers of children with neonatal lupus. Arthritis Rheum. 2008;58(2):541–546. doi: 10.1002/art.23191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stevens AM. Microchimeric cells in systemic lupus erythematosus: targets or innocent bystanders? Lupus. 2006;15(11):820–826. doi: 10.1177/0961203306070068. [DOI] [PubMed] [Google Scholar]
- 59.Larizza D, Calcaterra V, Martinetti M. Autoimmune stigmata in Turner syndrome: when lacks an X chromosome. J Autoimmun. 2009;33(1):25–30. doi: 10.1016/j.jaut.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 60.Invernizzi P, Miozzo M, Selmi C, Persani L, Battezzati P, Zuin M, et al. X chromosome monosomy: a common mechanism for autoimmune disease. J Immunol. 2005;175:575–578. doi: 10.4049/jimmunol.175.1.575. [DOI] [PubMed] [Google Scholar]
- 61.Invernizzi P, Miozzo M, Oertelt-Prigione S, Meroni PL, Persani L, Selmi C, et al. X monosomy in female systemic lupus erythematosus. Ann NY Acad Sci. 2007;1110:84–91. doi: 10.1196/annals.1423.010. [DOI] [PubMed] [Google Scholar]
- 62.Cooney CM, Bruner GR, Aberle T, Namjou-Khales B, Myers LK, Feo L, et al. 46, X, del(X)(q13) Turner's syndrome women with systemic lupus erythematosus in a pedigree multiplex for SLE. Genes Immun. 2009;10(5):478–481. doi: 10.1038/gene.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scofield RH, Bruner GR, Namjou B, Kimberly RP, Ramsey-Goldman R, Petri M, et al. Klinefelter's syndrome (47,XXY) in male systemic lupus erythematosus patients: support for the notion of a gene-dose effect from the X chromosome. Arthritis Rheum. 2008;58(8):2511–2517. doi: 10.1002/art.23701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ozbalkan Z, Bagislar S, Kiraz S, Akyerli CB, Ozer HT, Yavuz S, et al. Skewed X chromosome inactivation in blood cells of women with scleroderma. Arthritis Rheum. 2005;52(5):1564–1570. doi: 10.1002/art.21026. [DOI] [PubMed] [Google Scholar]
- 65.Ozcelik T, Uz E, Akyerli CB, Bagislar S, Mustafa CA, Gursoy A, et al. Evidence from autoimmune thyroiditis of skewed X-chromosome inactivation in female predisposition to autoimmunity. Eur J Hum Genet. 2006;14(6):791–797. doi: 10.1038/sj.ejhg.5201614. [DOI] [PubMed] [Google Scholar]
- 66.Invernizzi P, Pasini S, Selmi C, Miozzo M, Podda M. Skewing of X chromosome inactivation in autoimmunity. Autoimmunity. 2008;41(4):272–277. doi: 10.1080/08916930802024574. [DOI] [PubMed] [Google Scholar]
- 67.Chitnis S, Monteiro J, Glass D, Apatoff B, Salmon J, Concannon P, et al. The role of X-chromosome inactivation in female predisposition to autoimmunity. Arthritis Res. 2000;2:399–406. doi: 10.1186/ar118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pan Y, Sawalha AH. Epigenetic regulation and the pathogenesis of systemic lupus erythematosus. Transl Res. 2009;153(1):4–10. doi: 10.1016/j.trsl.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 69.Shinohara ML, Lu L, Bu J, Werneck MB, Kobayashi KS, Glimcher LH, et al. Osteopontin expression is essential for interferon-alpha production by plasmacytoid dendritic cells. Nat Immunol. 2006;7(5):498–506. doi: 10.1038/ni1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Masutani K, Akahoshi M, Tsuruya K, Tokumoto M, Ninomiya T, Kohsaka T, et al. Predominance of Th1 immune response in diffuse proliferative lupus nephritis. Arthritis Rheum. 2001;44(9):2097–2106. doi: 10.1002/1529-0131(200109)44:9<2097::AID-ART360>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 71.Chabas D, Baranzini SE, Mitchell D, Bernard CC, Rittling SR, Denhardt DT, et al. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science. 2001;294(5547):1731–1735. doi: 10.1126/science.1062960. [DOI] [PubMed] [Google Scholar]
- 72.Denhardt DT, Noda M. Osteopontin expression and function: role in bone remodeling. J Cell Biochem. 1998 Suppl 30–31:92–102. [PubMed] [Google Scholar]
- 73.Vanacker JM, Delmarre C, Guo X, Laudet V. Activation of the osteopontin promoter by the orphan nuclear receptor estrogen receptor related alpha. Cell Growth Differ. 1998;9(12):1007–1014. [PubMed] [Google Scholar]