Abstract
Presenilin 1 (PS1) mutations are responsible for many early-onset familial Alzheimer’s disease (FAD) cases. While increasing evidence points to impaired synaptic plasticity as an early event in AD, PS1 mutant mice exhibit a paradoxical increase in hippocampal long-term potentiation (LTP). Among PS1 mouse models, PS1 M146V mutant knock-in mice (PS1KI) are particularly interesting in that they exhibit memory impairment in spatial tasks. Here we investigated the effects of aging on two forms of LTP in PS1KI mice, the widely-studied early phase of LTP (E-LTP) and a particular form of LTP called late-LTP (L-LTP) which requires transcription and protein synthesis. L-LTP is thought to be critical for long-term memory. We found a lower L-LTP maintenance phase in PS1KI mice compared to wild type littermates at 3 months of age. As the mice age, they exhibit impairment of both the induction and maintenance phases of LTP. When E-LTP and NMDA receptor-mediated transmission were analyzed, PS1KI mice displayed an increase at 3 months compared to wild type littermates; this difference did not persist at older ages and finally decreased at 12 months. These results reveal an L-LTP decrease in PS1 mutant mice at an early stage, which occurs coincidently with a paradoxical enhancement of E-LTP. The observation of a decrease in both forms of LTP during aging supports the view that PS1KI mice are a valuable model for the study of age-dependent synaptic dysfunction and cognitive decline in AD.
Keywords: aging, Alzheimer’s disease, hippocampus, long-term potentiation, presenilin 1
INTRODUCTION
Mutations in the gene encoding presenilin 1 (PS1) are responsible for many cases of early-onset familial Alzheimer’s disease (FAD), in part by increasing amyloid-β peptide (Aβ) production as a result of aberrant processing of the amyloid-β protein precursor (AβPP). Presenilin-dependent γ-secretase is involved in intramembranous proteolysis of many proteins, which suggests that PS1 may have Aβ-independent roles in regulating synaptic function [1]. FAD mutations have been used to develop various mouse models of AD to replicate Aβ-peptide hypersecretion. A growing body of evidence suggests that the study of excitatory synaptic transmission is essential to understand early events that contribute to the development of AD [2]. Modifications of synaptic plasticity could thus be critical for understanding synaptotoxicity involved in AD.
The physiological substrate of information storage in the hippocampus has been proposed to involve long term potentiation (LTP), an activity-dependent, persistent increase in synaptic transmission. Early LTP (E-LTP) has been extensively studied in various mouse models of AD. Surprisingly, whereas E-LTP is impaired in AβPP mutant mice or by Aβ application [3, 4], it is increased in mice expressing different mutations of PS1 [5, 6]. This increase in E-LTP raised the criticism that PS1 mice are not valid models for the study of memory dysfunctions in AD. However late-LTP (L-LTP), which requires transcription and protein synthesis and is thought to be critical for the storage of long-term memory [7], has never been analyzed in AD mouse models.
Because the mutant PS1 protein is expressed at normal levels in FAD cases, PS1 mutant knock-in mice (PS1KI) that express the M146V mutation have been developed to avoid PS1 overexpression effects [8]. PS1KI mice displayed exaggerated Ca2+ signals and LTP [9, 10]. This toxic gain of function paradoxically coincides with age-related deterioration of hippocampal spatial memory in PS1KI mice [11].
Here, we investigated E-LTP and L-LTP and the possible associated glutamatergic synaptic transmission changes in hippocampal CA1 neurons from 3 to 12 months of age in PS1KI mice. As previously described, we found an increase in E-LTP from 3 to 6 months of age as compared to wild type littermates. By contrast, at 9 months of age, the E-LTP increase did not persist in PS1KI mice and was decreased compared to wild type mice at 12 months of age. We also found an impairment of the maintenance phase of L-LTP in PS1KI mice at 3 months of age. As the mice age, we observed an impairment of both the induction phase and maintenance phase of L-LTP. Because L-LTP is critical for long-term memory, and because PS1KI mice exhibit no Aβ pathology, our findings suggest that mutant PS1 may adversely influence synaptic plasticity by a mechanism in addition to increased amyloidogenesis. Moreover, our data support the view that PS1KI mice are an interesting model for the study of progressive cognitive decline in AD.
MATERIALS AND METHODS
Transgenic mice
The derivation and characterization of the PS1M146V knock-in (PS1KI) mice have been described previously. Mice were genotyped using PCR amplification followed by single-strand conformation polymorphism analysis as described previously [12]. Male (3, 6, 9, and 12 month-old) PS1KI mice and wild type (WT) littermates used in this study were maintained on a common homogeneous genetic background (C57BL/6). The brains from mice expressing mutant PS1, even those older than 16 months, were free from any AD hallmarks (i.e., plaques and tangles) [8]. Mice were bred and housed in the ‘Université Pierre et Marie Curie’ facility, with 12h/12h light/dark cycle and ad libitum access to food and water. All animal procedures were performed according to the regulations of the Comité National d'Ethique pour les Sciences de la Vie et de la Santé, which are in accordance with the European Communities Council Directive (86/609/EEC).
Hippocampal slice preparation
After a brief isofluorane anesthesia, mice were rapidly perfused transcardially with ice-cold dissection buffer containing in mM: 252 sucrose, 3 KCl, 7 MgCl2, 0.5 CaCl2, 1.25 NaH2PO4, 26 NaHCO3, 25 glucose, and 1 kynurenic acid (Sigma-Aldrich, Saint Quentin, France). The brain was rapidly dissected and hippocampal slices (400 µm) were collected using a sliding vibratome (Leica VT1000S, Rueil-Malmaison, France) in the same ice-cold buffer. To optimize the long-term recordings [13], hippocampal slices were placed at 30°C, 3 hours in artificial cerebrospinal fluid (ACSF) containing in mM: 125 NaCl, 2.5 KCl, 26 NaHCO3, 1.25 NaH2PO4, 2.5 CaCl2, 1.5 MgCl2, and 25 glucose. ACSF and dissection buffer were bubbled with 95% O2/5% CO2. For recording, slices were placed in a submersion-recording chamber, maintained at 30°C, and perfused with ACSF.
Input-Output curve recordings
Monopolar stimulation electrodes (2 MΩ, filled with 200 mM NaCl) were placed in the stratum radiatum of the CA1 region. Schaeffer collateral/commissural fibers were stimulated with 100 µs voltage pulses delivered to the pathway at 15 s intervals. Field excitatory post-synaptic potentials (fEPSPs) were monitored using low-resistance glass pipettes (2 MΩ, filled with 200 mM NaCl) placed in the stratum radiatum of CA1. In each experiment, the range of maximal fEPSPs was measured in order to subsequently stimulate at an intensity that yielded 50% of the maximal fEPSP amplitude. Input/output curves were constructed to assess the AMPA/kainate-mediated synaptic responses to electrical stimulation in the presence of the NMDA receptor antagonist D-AP5 (50 µM, Tocris, Bristol, UK). A similar experiment was conducted in the presence of the AMPA receptor antagonist NBQX (10 µM, Sigma, Saint Quentin, France) in low magnesium (0.1 mM). Picrotoxin (10 µM, Sigma, Saint Quentin, France) was added to block inhibitory transmission and the CA3 region was cut to avoid epileptiform activity. Schaeffer collateral/commissural fibers were stimulated with 200 µs voltage pulses delivered to the pathway at 15 s intervals. fEPSPs recorded under these conditions are mediated by NMDA receptors [14]. For I/O curves, the amplitude of four averaged presynaptic fiber volleys and the slope of fEPSPs were plotted as a function of stimulation intensity.
Paired-Pulse Facilitation recordings
For the Paired-Pulse Facilitation (PPF) protocol, intervals between the two pulses were 20, 40, 60, 80, 100, 150, 200, 250, and 300 ms, and stimulus pairs were delivered every 15 s. PPF was calculated as the ratio of the second field potential slope to the first field potential slope.
Early (E) and Late (L) LTP recordings
For E-LTP, slices were subjected to a 15 min period of pre-LTP baseline measures of fEPSPs in which stimuli were elicited at 15 s intervals. E-LTP was induced by one train of 100 Hz for 1 s. Stimulus intensity in the burst protocol was the same as that used during baseline recordings. Field potentials were recorded for at least 60 min after initiation of the burst protocol. For L-LTP, slices were subjected to a 30 min period of pre-LTP baseline measures of fEPSPs in which stimuli were elicited at 15 s intervals. L-LTP was induced by four trains at a frequency of 100 Hz for 1 s every 5 min. Stimulus intensity in the burst protocol was the same as that used during baseline recordings. Field potentials were recorded for at least 4 hours after initiation of the burst protocol. All data consist of fEPSPs slopes.
Statistical analysis
We assessed differences by two-way ANOVA (age and genotype) and post-hoc Scheffé comparisons for NMDA-R and AMPA-R mediated transmission and for PPF. For the LTP study, we used multiple-way ANOVA (age, genotype, and time) analysis and post-hoc Scheffé comparisons. We considered differences to be significant at the 5% level (p<0.05). Data were presented as mean ± standard error of mean (SEM).
RESULTS
Early impairment in the maintenance phase of late-LTP in PS1KI mice
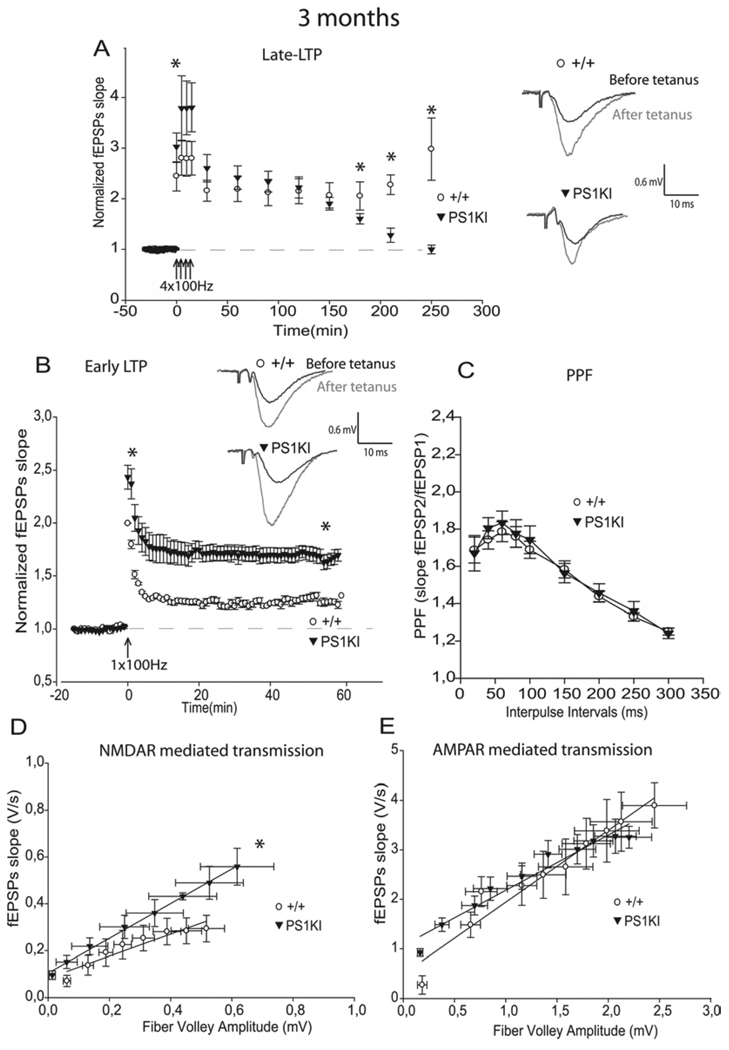
It has been shown that PS1KI mice have age-related impairment of hippocampal spatial memory from 3 to 12 months of age [11]. The late-LTP (L-LTP), is a long lasting form of LTP. L-LTP is induced by four trains at 100 Hz, is protein synthesis-dependent, and is critical for the storage of long term memory [15]. To determine if the PS1 M146V mutation altered this form of potentiation, we investigated L-LTP in the CA1 region of hippocampal slices from WT and PS1KI animals at 3 months of age. A larger L-LTP induction was observed in hippocampal slices from PS1KI mice compared to slices from WT littermates during the first 30 min of recording (p<0.05, Fig. 1A, 5A, Table 1). No significant differences were observed between PS1KI and WT mice for the interval from 30 min to 3 h after HFS (Fig. 1A, 5A). However, a significant difference between the two groups emerged in the maintenance phase of L-LTP at the interval between 3 and 4 h after HFS (p<0.001, Fig. 1A, 5A). PS1KI mice exhibited a decrease in L-LTP and returned progressively to the baseline.
Figure 1.
Induction of L-LTP, E-LTP, and NMDA-R mediated transmission are increased by mutant PS1, while the maintenance phase of L-LTP is decreased at 3 months of age. A) At 3 months, the L-LTP protocol induced a significantly larger induction in PS1KI mice than in +/+ mice during the first 30 min of recording (p<0.05). No significant differences were observed between PS1KI and +/+ mice for the interval from 30 min to 3 h after HFS. However, during the interval from 3 to 4 h the PS1KI mice exhibited a significant decrease in L-LTP which returned progressively to the baseline, compared to +/+ mice (p<0.001). * corresponds to p<0.05. fEPSPs traces of baseline and post-LTP recordings are represented. B) At 3 months, the E-LTP protocol induced a significantly larger response in PS1KI mice than in +/+ littermates (p<0.001). * corresponds to p<0.05. fEPSPs traces of baseline and post-LTP recordings are represented. C) Paired-Pulse Facilitation (PPF) was unchanged at 3 months of age. D) At 3 months, analysis of Input-Output (I-O) slopes demonstrates a significant increase of NMDA receptor mediated responses in PS1KI mice compared +/+ littermates (p<0.05). * corresponds to p<0.05. E) No significant difference was found between the two groups of mice at any stimulus level examined for averaged I-O plots of AMPA receptor mediated responses.
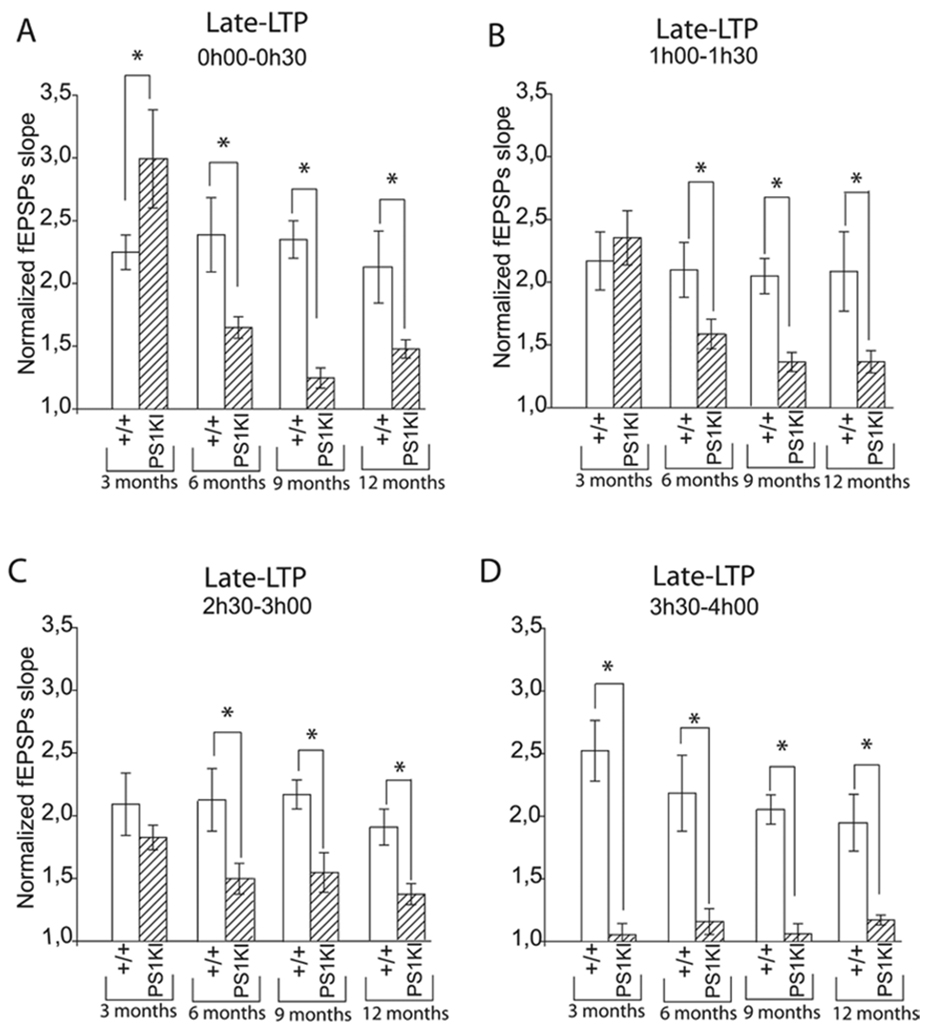
Figure 5.
Means of normalized fEPSP slopes for different periods of L-LTP at 3, 6, 9, and 12 months of age. A) For the recording interval 0h00-0h30, L-LTP is significantly increased in PS1KI mice compared to +/+ mice at 3 months of age (p<0.05). By contrast, at 6, 9 and 12 months of age, L-LTP is significantly decreased in PS1KI mice (p<0.001). * corresponds to p<0.05. B) For the recording interval from 60–90 min after HFS, L-LTP is similar between PS1KI mice and +/+ mice at 3 months of age. By contrast, at 6, 9 and 12 months, L-LTP is significantly decreased in PS1KI mice compared to +/+ littermates (p<0.001). * corresponds to p<0.05. C) Recordings made between 2.5 and 3 h after LTP induction reveal that L-LTP is similar between PS1KI mice and +/+ mice at 3 months of age. By contrast, at 6, 9 and 12 months of age, L-LTP was markedly altered in PS1KI mice compared to +/+ littermates (p<0.001). * corresponds to p<0.05. D) Recordings made between 3.5 and 4 h after HFS showed that, at all ages L-LTP is significantly decreased in PS1KI mice compared to +/+ littermates (p<0.001). * corresponds to p<0.05.
Table 1.
Values of Late-LTP and Early-LTP recordings.
| Age | Genotype | L-LTP | E-LTP | |
|---|---|---|---|---|
| 0h00-0h30 | 3h30-4h00 | 0h30-1h00 | ||
| 3 | +/+ | 2.32±0.18 | 2.52±0.24 (n=6; N=6) | 1.23 ± 0.009 (n=8; N=6) |
| PS1KI | 2.99±0.31* | 1.05±0.08 (n=6; N=6)* | 1.72 ± 0.09 (n=8; N=6)* | |
| 6 | +/+ | 2.38±0.28 | 2.18±0.30 (n=6; N=6) | 1.20 ± 0.01 (n=8; N=5) |
| PS1KI | 1.64±0.08* | 1.15±0.10 (n=5; N=5)* | 1.49 ± 0.17 (n=10; N=6)* | |
| 9 | +/+ | 2.35±0.14 | 2.05±0.11 (n=6; N=6) | 1.24 ± 0.02 (n=9; N=6) |
| PS1KI | 1.24±0.08* | 1.06±0.08 (n=6; N=6)* | 1.26 ± 0.04 (n=8; N=5) | |
| 12 | +/+ | 2.12±0.28 | 1.94±0.22 (n=6; N=6) | 1.23 ± 0.008 (n=7; N=5) |
| PS1KI | 1.47±0.07* | 1.17±0.03 (n=9; N=9)* | 1.09 ± 0.03 (n=9; N=6)* | |
Data are expressed as mean ± SEM, p<0.05*. n, number of slices; N, number of animals.
To determine whether the M146V mutation altered E-LTP, we next analyzed the effect of a simple 100 Hz burst applied to the Schaeffer collateral on the two groups of mice at 3 months of age. A larger E-LTP response was induced in hippocampal slices from PS1KI mice compared to slices from WT littermates, as previously described for other PS1 mutations [5,6,9] (p<0.001, Fig. 1B, Table 1).
We next analyzed paired-pulse facilitation (PPF), a form of short-term plasticity. PPF is a short lasting enhancement in synaptic strength mediated by presynaptic calcium-dependent mechanisms [16] so that the response to a second stimulation is potentiated if it is delivered within 200 ms of the first stimulus [16]. Changes in synaptic release probability may be revealed by changes in PPF. PPF was thus examined in 3 month-old mice. No significant differences were observed between WT and PS1KI mice (Fig. 1C).
To determine if the M146V mutation altered excitatory synaptic transmission, we also analyzed NMDA receptor mediated responses by examining field potentials evoked by stimulation of the Schaeffer collateral/commissural afferent pathway, in the presence of NBQX (10 µM) to block AMPA receptors. Input-output curves were generated by plotting the slope of the field EPSP versus fiber volley amplitude (a measure of the number of presynaptic fibers activated) as stimulus intensity was increased [17]. At 3 months of age, the magnitude of NMDA receptor-dependent responses was significantly increased in PS1KI mice compared to WT littermates (p<0.05, Fig. 1D, Table 2).
Table 2.
Values of NMDA-R mediated transmission recordings.
| Age | Genotype | NMDA-receptor I/O slope |
|---|---|---|
| 3 | +/+ | 0.06 ± 0.006 (n=7; N=4) |
| PS1KI | 0.10 ± 0.008 (n=12; N=5)* | |
| 6 | +/+ | 0.07 ± 0.016 (n=12; N=4) |
| PS1KI | 0.09 ± 0.008 (n=11; N=4) | |
| 9 | +/+ | 0.06 ± 0.005 (n =10; N=4) |
| PS1KI | 0.07 ± 0.009 (n=10; N=4) | |
| 12 | +/+ | 0.07 ± 0.004 (n=8; N=4) |
| PS1KI | 0.05 ± 0.003 (n=6; N=4)* | |
Data are expressed as mean ± SEM, p<0.05*. n, number of slices; N, number of animals.
Because CA1 pyramidal neurons have both AMPA and NMDA receptors, we also specifically analyzed the AMPA/kainate receptor mediated synaptic transmission in the presence of D-AP5 (50 µM) to block NMDA receptors. The averaged input-output slopes of AMPA receptor mediated transmission were not significantly different between the two groups (Fig. 1E).
Thus, at 3 months of age, the PS1 M146V variant induced an increase in NMDA receptor mediated transmission, E-LTP and the induction phase of L-LTP. The maintenance of L-LTP was significantly decreased in PS1KI compared to control littermates.
Progressive age-related impairment of E-LTP, NMDA-R mediated transmission, and L-LTP in PS1KI mice
To determine the progression of the synaptic dysfunctions and the long term effects of the M146V mutation, we next examined WT and PS1KI mice at 6, 9 and 12 months of age.
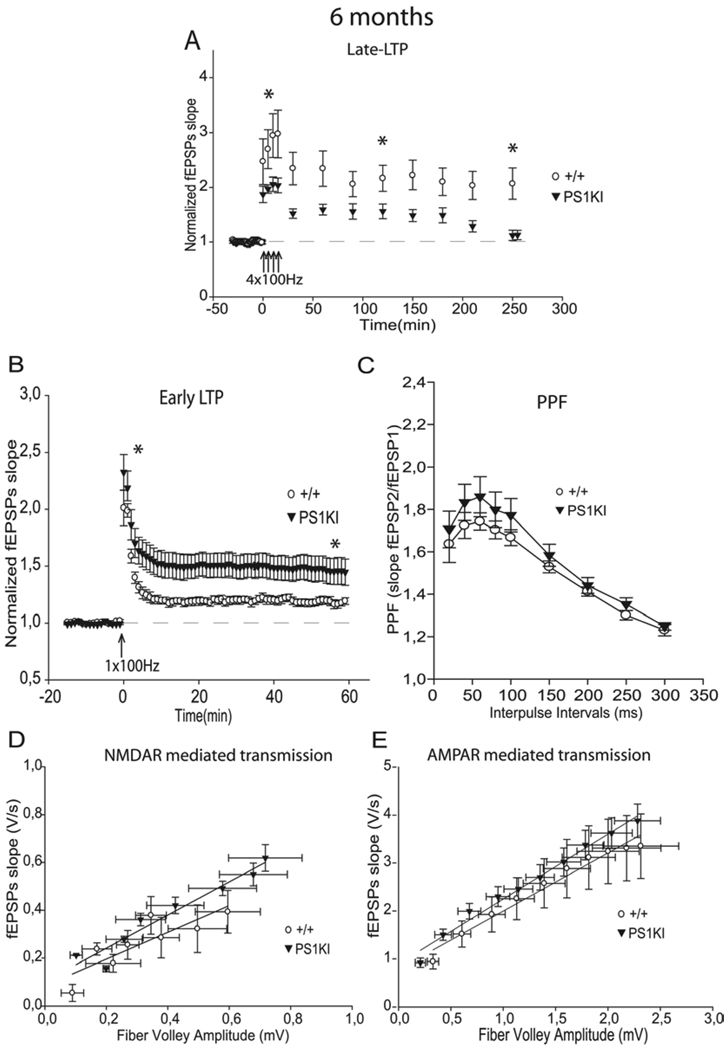
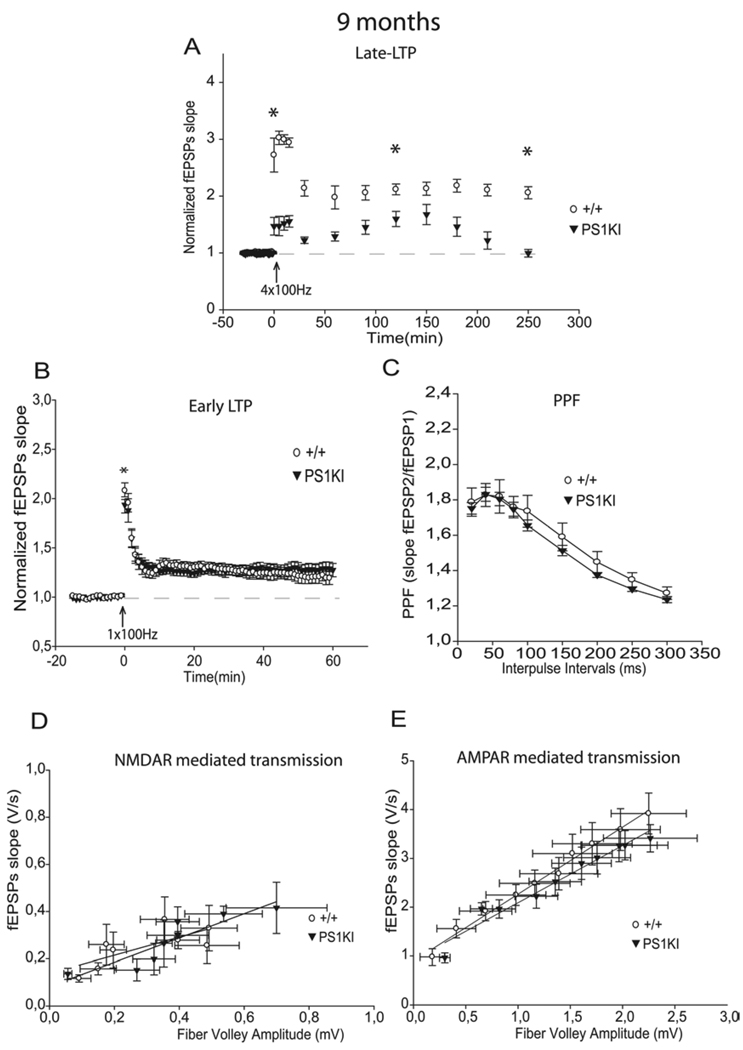
To study the evolution of late-LTP, we examined the effect of the multiple-burst induction protocol at 6 months of age. The larger L-LTP induction observed in hippocampal slices from PS1KI mice compared to slices from WT littermates during the 30 first min of recording did not persist at 6 months (p<0.001, Fig. 2A, 1A, 5A, Table 1). Instead, PS1KI mice exhibited a reduced L-LTP throughout the 4 h of recording compared to WT mice (Fig. 2A). This result suggests that M146V mutation progressively decreases L-LTP with age. This was confirmed by analyzing even older animals. At 9 and 12 months of age, we found a significant decrease of L-LTP in PS1KI mice compared to WT mice (p<0.001, Table 1, Fig. 3A, 4A).
Figure 2.
Induction and maintenance phases of L-LTP are decreased while E-LTP is increased by mutant PS1 at 6 months of age. A) At 6 months, the L-LTP protocol induced a significantly lower induction and maintenance in PS1KI mice than in +/+ mice during the 4 h of recording (p<0.001). * corresponds to p<0.05. B) At 6 months, the E-LTP protocol induced a significantly larger response in PS1KI mice than in +/+ littermates (p<0.05). However, E-LTP levels in PS1KI at 6 months were lower than E-LTP levels in PS1KI at 3 months of age (p<0.05). * corresponds to p<0.05. C) PPF was unchanged at 6 months of age. D) At 6 months, NMDA receptor mediated responses did not differ between the two groups. E) No significant difference was found between the two groups of mice at any stimulus level examined for averaged Input-Output plots of AMPA receptor mediated responses.
Figure 3.
Induction and maintenance phases of L-LTP are decreased while E-LTP is unchanged by mutant PS1 at 9 months of age. A) At 9 months, the L-LTP protocol induced a significantly lower induction and maintenance in PS1KI mice than in +/+ mice during the 4 h of recording (p<0.001). * corresponds to p<0.05. (B) At 9 months, E-LTP analysis revealed no significant differences between the two groups. C) PPF was unchanged at 9 months of age. D) NMDA receptor mediated responses were unchanged at 9 months of age between the two groups. E) No significant difference was found between the two groups of mice at any stimulus level examined for averaged Input-Output plots of AMPA receptor mediated responses.
Figure 4.
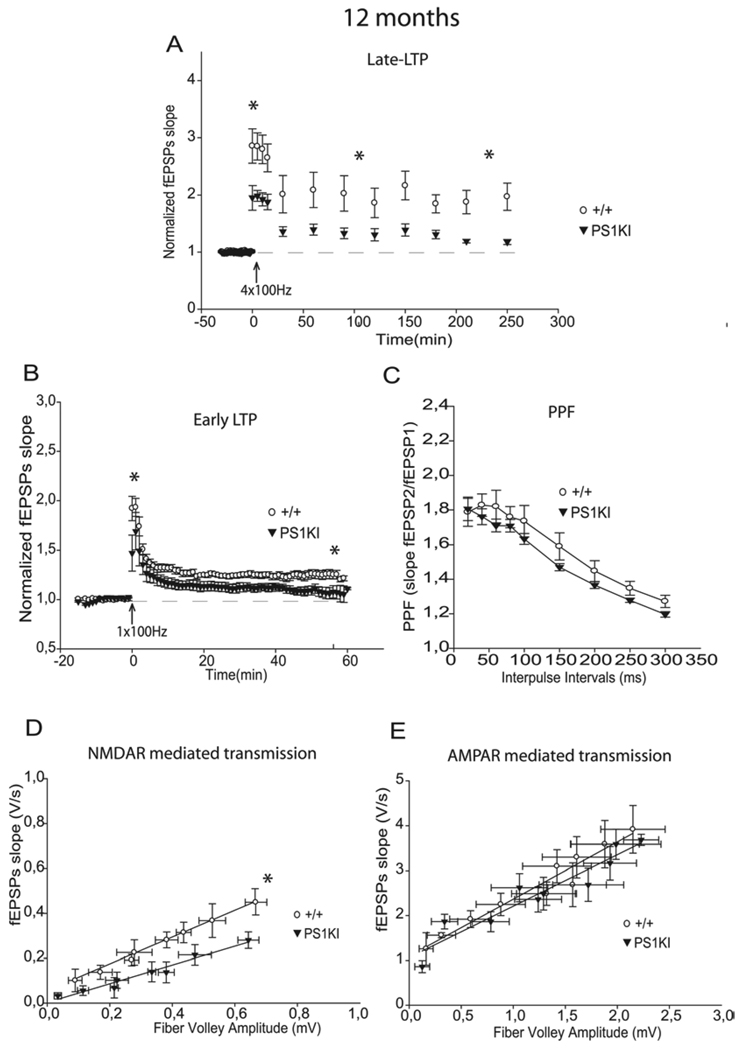
Induction and maintenance phases of L-LTP, E-LTP, and NMDA-R mediated transmission are decreased by mutant PS1 at 12 months of age. A) At 12 months, the L-LTP protocol induced a significantly lower induction and maintenance in PS1KI mice than in +/+ mice during the 4 h of recording (p<0.001). * corresponds to p<0.05. B) At 12 months, E-LTP analysis revealed a significant decrease in PS1KI mice compared +/+ littermates (p<0.05). * corresponds to p<0.05. C) PPF was unchanged at 12 months of age. D) At 12 months, analysis of Input-Output slopes demonstrates a significant decrease of basal NMDA receptor-dependant synaptic transmission in PS1KI mice compared to WT mice (p<0.05). * corresponds to p<0.05. E) No significant difference was found between the two groups of mice at any stimulus level examined for averaged Input-Output plots of AMPA receptor mediated responses.
We next analyzed E-LTP during aging. PS1KI mice at 6 months of age showed a significant increase in E-LTP compared to WT littermates (p<0.05, Fig. 2B, Table 1). However, when ages are compared, E-LTP levels in PS1KI mice at 6 months were lower than in PS1KI mice at 3 months of age, suggesting that E-LTP began to decline with advancing age (p<0.05, Fig. 1B, 2B).
At 9 months of age, no significant difference was found between PS1KI mice and WT littermates (Fig. 3B, Table 1). When we further examined E-LTP at 12 months of age, we finally found a significant decrease of E-LTP in PS1KI mice compared to WT mice (p<0.05, Fig. 4B, Table 1).
To determine if the effects of the PS1 M146V mutation on the different forms of LTP during aging was accompanied by excitatory synaptic transmission changes, we next examined NMDA-R mediated transmission. Among 6 and 9 month-old animals, no significant differences were observed in NMDA-R responses between the two genotypes (Fig. 2D, 3D, Table 2), showing that the increase observed at 3 months of age in PS1KI mice did not persist at 6 months of age (Fig. 1E, 3E). Interestingly, when 12 month-old mice were analyzed, we found a decrease in NMDA-R mediated responses in PS1KI mice compared to WT littermates (Fig. 4D, Table 2).
Moreover, the analysis of PPF (Fig. 2C, 3C, 4C) and AMPA-R mediated transmission (Fig. 2E, 3E, 4E) did not show any differences between the two groups at 6, 9, or 12 months of age.
Overall, these results show that, during aging, PS1 mutation results in progressive decreases in L-LTP, E-LTP, and NMDA receptor mediated transmission.
DISCUSSION
In this study, we elucidated the impact of the M146V PS1 mutation on electrochemical synaptic plasticity during aging by analyzing two distinct forms of hippocampal LTP. Our results identified an early increase in the L-LTP induction phase in PS1KI mice at 3 months of age. At the same age, PS1KI mice displayed an impairment of L-LTP maintenance. At older ages, we found that the mutation severely impaired both induction and maintenance phases of L-LTP (Fig. 5). Moreover, we showed that PS1KI mice exhibited a transient increase of E-LTP and NMDA-R mediated transmission which decreased progressively with age.
Early impairment of L-LTP maintenance in PS1KI mice
LTP is a cellular model of activity-dependent enhancement of synaptic transmission. Hippocampal LTP might at least be described by two different functionally and mechanistically distinct forms [18]. The short term form or E-LTP is usually induced by a single high frequency stimulation (HFS), and lasts up to 1 h in vitro [19]. E-LTP is mediated by phosphorylation of existing proteins without synthesis of new proteins [20]. By contrast, the long-lasting form or L-LTP is induced by multiple HFS, lasts at least 4 h, and requires transcription and protein synthesis [21, 22]. The best studied form of LTP, and the only one studied in mouse models of AD, is the NMDA receptor-dependent E-LTP.
Earlier studies have shown an increase of E-LTP in PS1 mutant mice. It should be noted that, although PS1 mutations lead to hypersecretion of Aβ peptides, these mutant mice express the non-toxic mouse form of Aβ and lack amyloid deposits [9, 23]. By contrast, previous results obtained from hippocampus of mutant human AβPP mouse brains reported decreased LTP at 4 months of age, well before the appearance of Aβ plaques at 18 months [24]. These contrasting results suggest that mutant PS1 influences synaptic plasticity by both Aβ-dependent and Aβ-independent mechanisms. Indeed, expression of AβPP mutations in transgenic mice impairs synaptic transmission by increasing Aβ production [25], and it has recently been shown that soluble Aβ isolated directly from AD brains inhibits E-LTP [26]. This major difference in E-LTP variation was one reason why PS1 mutant mouse models have been criticized as not being relevant models for the study of memory dysfunction in AD. However, L-LTP has never been analyzed in AD mouse models.
In the present study, we showed for the first time that the M146V PS1 mutation induced an early impairment of the L-LTP maintenance (Fig. 5). This result is in agreement with previous results showing that PS1KI mice exhibited subtle deterioration of hippocampal spatial memory as early as 3 months of age [11]. However, in their study, this poorer performance could be overcome by continued training and the spatial impairments only became clearer when the authors analyzed PS1KI animals at 9–11 months of age. Our results showed that synaptic dysfunctions underlying cognitive failure associated with a PS1 mutation can be clearly detected as early as 3 months of age using L-LTP analysis. Hypersecretion of Aβ may contribute to L-LTP impairment in human PS1 FAD patients because it has been shown that a synthetic fibrillar form of Aβ can impair the maintenance phase of L-LTP without affecting the early induction phase [27]. However, our findings in PS1KI mice suggest another Aβ-independent mechanism that may involve alterations in calcium and kinases believed to mediated L-LTP. FAD PS1 mutations result in aberrant elevations of intracellular calcium levels in response to glutamate receptor activation as a result of dysregulation of endoplasmic reticulum calcium stores [8,28,29]. An atypical PKC isoform, protein kinase Mzeta (PKMζ) has been reported to be necessary and sufficient for maintaining L-LTP [30] and a PKMζ inhibitor injected in the hippocampus both reverses LTP maintenance and induces persistent loss of spatial information [7]. Data suggest that PS1 influences the activity of several PKC isoforms implicated in LTP [31] although it remains to be determined whether effects of PS1 mutations on PKC activities are downstream consequences of perturbed calcium homeostasis.
Interestingly, in contrast to its inhibitory effect on L-LTP maintenance, mutant PS1 increased L-LTP induction in young mice. The increased L-LTP induction was associated with an increase in NMDA-R mediated responses and E-LTP in PS1KI mice compared to WT littermate mice. These results suggest that E-LTP and the induction of L-LTP may share similar mechanisms. Indeed, the induction of both E-LTP and L-LTP is known to be NMDA-R dependent [32]. It has previously been shown that 4–6 month-old mice overexpressing mutant PS1 exhibit an increase in NMDA-receptor responses [6, 23].
Age-related progressive dysfunctions of synaptic plasticity in PS1KI mice
When we examined the progression of synaptic dysfunction and the long-term effects of the M146V mutation at older ages, we found that the mutation nearly abolished the induction phase of L-LTP (Fig. 5), suggesting that the aging process itself is involved in the late-LTP degradation. Not surprisingly, L-LTP maintenance was still impaired at older ages.
The increase in the induction phase of L-LTP did not persist at 6 months of age and finally decreased in PS1KI mice. The increase in NMDA-R mediated transmission also did not persist at 6 months. By contrast, when we analyzed the E-LTP at 6 months of age, our results revealed an increase in E-LTP in PS1KI mice compared to non-transgenic littermates. The evolution with age of the two types of LTP implies that there are other mechanisms involved in LTP than the ones underlying the joint increase of E-LTP, L-LTP induction, and NMDA-R mediated transmission at 3 months of age. The possibility that E-LTP and L-LTP induction could be sustained by the same mechanisms is part of the larger unsolved problem of categorizing the different forms of LTP [33].
When we further examined E-LTP at older ages, we found that the increase of E-LTP was transient in mutant PS1 mice and finally decreased at 12 months of age. A previous study from our laboratory also showed that the increase in E-LTP in transgenic mice overexpressing mutant PS1 did not persist at older ages and finally decreased; however, we found that the simple overexpression of human wild-type PS1 progressively decreased E-LTP with aging [6]. In contrast, the present study employed PS1 M146V knock-in mice, thus avoiding possible effects of PS1 overexpression. Overall, a transient increase of E-LTP in mutant PS1 mice which decreases with aging appears to be a consistent finding seemingly related to the mutation itself and not to the overexpression effects.
One possible explanation is the modulatory effect of progressive Aβ peptide accumulation. Recently there has been support for a model of Aβ effects in which low concentrations play a novel positive, modulatory role on E-LTP [34], whereas high concentrations have the well-known detrimental effect [26]. In the same way, E-LTP in mutant PS1 mice was first increased and finally decreased at older ages, although it remains to be determined whether Aβ peptides progressive accumulation in PS1KI modified E-LTP during aging.
Our experiments also showed an increase in NMDA-R mediated responses at 3 months of age in PS1KI mice compared to control littermates. This increase did not persist at 6 and 9 months and finally NMDA-R mediated transmission decreased at 12 months of age. Thus, NMDA-R mediated transmission follows the same pattern as E-LTP and L-LTP induction with age in PS1KI mice. Our results are also consistent with Wang and colleagues [9] who reported that the same PS1 mutant knock-in mice showed decreased NMDA currents at older ages (9–12 months). It is well known that induction of LTP is initiated by calcium influx into the postsynaptic spine via NMDA receptor channels [35]. As PS1 mutations enhance calcium accumulation in the endoplasmic reticulum, disturbances in calcium signaling might explain the transient increase in E-LTP in young animals followed by a decline during aging [36, 38]. Previous studies have shown that there is an optimal level of post-synaptic calcium elevation for LTP expression [39, 40]. Excessive elevation or accumulation of intracellular calcium might be responsible for the progressive age-dependant impairment of NMDA-R responses. In support of this hypothesis, intracellular calcium chelator BAPTA restored NMDA currents in the CA1 neurons of PS1KI mice [9].
CONCLUSION
Overall, this study highlights for the first time an early impairment of the protein synthesis-dependent maintenance phase of L-LTP in a PS1 mouse model of AD, which appears to involve mechanisms other than amyloidogenesis. We documented an impairment of L-LTP coincident with enhanced E-LTP at a young age, followed by progressive impairment of both early and late forms of LTP during aging. The age-dependent nature of these phenotypes is of considerable interest with regard to the crucial role of aging in synaptic dysfunction in AD.
ACKNOWLEDGMENTS
This work was supported by funds from “Centre National de la Recherche Scientifique” (CNRS) and Université Pierre et Marie Curie. A. Auffret was a recipient of a fellowship from “Fondation de la Recherche Médicale” (France). We thank Dr. Ann Lohof for her critical reading of the paper. This research was also supported by the National Institute on Aging Intramural Research Program of the NIH.
Footnotes
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=172).
REFERENCES
- 1.Vetrivel KS, Zhang YW, Xu H, Thinakaran G. Pathological and physiological functions of presenilins. Mol Neurodegener. 2006;1:4. doi: 10.1186/1750-1326-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang EH, Savage MJ, Flood DG, Thomas JM, Levy RB, Mahadomrongkul V, Shirao T, Aoki C, Huerta PT. AMPA receptor downscaling at the onset of Alzheimer's disease pathology in double knockin mice. Proc Natl Acad Sci U S A. 2006;103:3410–3415. doi: 10.1073/pnas.0507313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, Irizarry M, Younkin L, Good MA, Bliss TV, Hyman BT, Younkin SG, Hsiao KK. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci. 1999;2:271–276. doi: 10.1038/6374. [DOI] [PubMed] [Google Scholar]
- 4.Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 5.Parent A, Linden DJ, Sisodia SS, Borchelt DR. Synaptic transmission and hippocampal long-term potentiation in transgenic mice expressing FAD-linked presenilin 1. Neurobiol Dis. 1999;6:56–62. doi: 10.1006/nbdi.1998.0207. [DOI] [PubMed] [Google Scholar]
- 6.Auffret A, Gautheron V, Repici M, Kraftsik R, Mount HT, Mariani J, Rovira C. Age-dependent impairment of spine morphology and synaptic plasticity in hippocampal CA1 neurons of a presenilin 1 transgenic mouse model of Alzheimer's disease. J Neurosci. 2009;29:10144–10152. doi: 10.1523/JNEUROSCI.1856-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313:1141–1144. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- 8.Guo Q, Fu W, Sopher BL, Miller MW, Ware CB, Martin GM, Mattson MP. Increased vulnerability of hippocampal neurons to excitotoxic necrosis in presenilin-1 mutant knock-in mice. Nat Med. 1999;5:101–106. doi: 10.1038/4789. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Greig NH, Yu QS, Mattson MP. Presenilin-1 mutation impairs cholinergic modulation of synaptic plasticity and suppresses NMDA currents in hippocampus slices. Neurobiol Aging. 2007;30:1061–1068. doi: 10.1016/j.neurobiolaging.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stutzmann GE, Caccamo A, LaFerla FM, Parker I. Dysregulated IP3 signaling in cortical neurons of knock-in mice expressing an Alzheimer's-linked mutation in presenilin1 results in exaggerated Ca2+ signals and altered membrane excitability. J Neurosci. 2004;24:508–513. doi: 10.1523/JNEUROSCI.4386-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun X, Beglopoulos V, Mattson MP, Shen J. Hippocampal spatial memory impairments caused by the familial Alzheimer's disease-linked presenilin 1 M146V mutation. Neurodegener Dis. 2005;2:6–15. doi: 10.1159/000086426. [DOI] [PubMed] [Google Scholar]
- 12.Gautheron V, Auffret A, Mattson MP, Mariani J, Garabedian B. A new and simple approach for genotyping Alzheimer's disease presenilin-1 mutant knockin mice. J Neurosci Meth. 2009;30:235–240. doi: 10.1016/j.jneumeth.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sajikumar S, Navakkode S, Frey JU. Protein synthesis-dependent long-term functional plasticity: methods and techniques. Curr Opin Neurobiol. 2005;15:607–613. doi: 10.1016/j.conb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI. Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neurosci. 2004;24:7821–7828. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reymann KG, Frey JU. The late maintenance of hippocampal LTP: requirements, phases, 'synaptic tagging', 'late-associativity' and implications. Neuropharmacology. 2007;52:24–40. doi: 10.1016/j.neuropharm.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 16.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 17.Fitzjohn SM, Morton RA, Kuenzi F, Rosahl TW, Shearman M, Lewis H, Smith D, Reynolds DS, Davies CH, Collingridge GL, Seabrook GR. Age-related impairment of synaptic transmission but normal long-term potentiation in transgenic mice that overexpress the human APP695SWE mutant form of amyloid precursor protein. J Neurosci. 2001;21:4691–4698. doi: 10.1523/JNEUROSCI.21-13-04691.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 19.Huang YY, Kandel ER. Recruitment of long-lasting and protein kinase A-dependent long-term potentiation in the CA1 region of hippocampus requires repeated tetanization. Learn Mem. 1994;1:74–82. [PubMed] [Google Scholar]
- 20.Nicoll RA, Malenka RC. Expression mechanisms underlying NMDA receptor-dependent long-term potentiation. Ann N Y Acad Sci. 1999;868:515–525. doi: 10.1111/j.1749-6632.1999.tb11320.x. [DOI] [PubMed] [Google Scholar]
- 21.Osten P, Valsamis L, Harris A, Sacktor TC. Protein synthesis-dependent formation of protein kinase Mzeta in long-term potentiation. J Neurosci. 1996;16:2444–2451. doi: 10.1523/JNEUROSCI.16-08-02444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Otani S, Abraham WC. Inhibition of protein synthesis in the dentate gyrus, but not the entorhinal cortex, blocks maintenance of long-term potentiation in rats. Neurosci Lett. 1989;106:175–180. doi: 10.1016/0304-3940(89)90222-x. [DOI] [PubMed] [Google Scholar]
- 23.Dewachter I, Ris L, Croes S, Borghgraef P, Devijver H, Voets T, Nilius B, Godaux E, Van Leuven F. Modulation of synaptic plasticity and Tau phosphorylation by wild-type and mutant presenilin1. Neurobiol Aging. 2008;29:639–652. doi: 10.1016/j.neurobiolaging.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 24.Jacobsen JS, Wu CC, Redwine JM, Comery TA, Arias R, Bowlby M, Martone R, Morrison JH, Pangalos MN, Reinhart PH, Bloom FE. Early-onset behavioral and synaptic deficits in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2006;103:5161–5166. doi: 10.1073/pnas.0600948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Venkitaramani DV, Chin J, Netzer WJ, Gouras GK, Lesne S, Malinow R, Lombroso PJ. Beta-amyloid modulation of synaptic transmission and plasticity. J Neurosci. 2007;27:11832–11837. doi: 10.1523/JNEUROSCI.3478-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Puzzo D, Arancio O. Fibrillar beta-amyloid impairs the late phase of long term potentiation. Curr Alzheimer Res. 2006;3:179–183. doi: 10.2174/156720506777632871. [DOI] [PubMed] [Google Scholar]
- 28.Tu H, Nelson O, Bezprozvanny A, Wang Z, Lee SF, Hao YH, Serneels L, De Strooper B, Yu G, Bezprozvanny I. Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer's disease-linked mutations. Cell. 2006;126:981–993. doi: 10.1016/j.cell.2006.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bezprozvanny I, Mattson MP. Neuronal calcium mishandling and the pathogenesis of Alzheimer's disease. Trends Neurosci. 2008;31:454–463. doi: 10.1016/j.tins.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sacktor TC. PKMzeta, LTP maintenance, and the dynamic molecular biology of memory storage. Prog Brain Res. 2008;169:27–40. doi: 10.1016/S0079-6123(07)00002-7. [DOI] [PubMed] [Google Scholar]
- 31.Dehvari N, Cedazo-Minguez A, Isacsson O, Nilsson T, Winblad B, Karlstrom H, Benedikz E, Cowburn RF. Presenilin dependence of phospholipase C and protein kinase C signaling. J Neurochem. 2007;102:848–857. doi: 10.1111/j.1471-4159.2007.04571.x. [DOI] [PubMed] [Google Scholar]
- 32.Reymann KG, Matthies HK, Schulzeck K, Matthies H. N-methyl-D-aspartate receptor activation is required for the induction of both early and late phases of long-term potentiation in rat hippocampal slices. Neurosci Lett. 1989;96:96–101. doi: 10.1016/0304-3940(89)90249-8. [DOI] [PubMed] [Google Scholar]
- 33.Raymond CR. LTP forms 1, 2 and 3: different mechanisms for the "long" in long-term potentiation. Trends Neurosci. 2007;30:167–175. doi: 10.1016/j.tins.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Puzzo D, Privitera L, Leznik E, Fa M, Staniszewski A, Palmeri A, Arancio O. Picomolar amyloid-beta positively modulates synaptic plasticity and memory in hippocampus. J Neurosci. 2008;28:14537–14545. doi: 10.1523/JNEUROSCI.2692-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nicoll RA, Kauer JA, Malenka RC. The current excitement in long-term potentiation. Neuron. 1988;1:97–103. doi: 10.1016/0896-6273(88)90193-6. [DOI] [PubMed] [Google Scholar]
- 36.Begley JG, Duan W, Chan S, Duff K, Mattson MP. Altered calcium homeostasis and mitochondrial dysfunction in cortical synaptic compartments of presenilin-1 mutant mice. J Neurochem. 1999;72:1030–1039. doi: 10.1046/j.1471-4159.1999.0721030.x. [DOI] [PubMed] [Google Scholar]
- 37.LaFerla FM. Calcium dyshomeostasis and intracellular signalling in Alzheimer's disease. Nat Rev Neurosci. 2002;3:862–872. doi: 10.1038/nrn960. [DOI] [PubMed] [Google Scholar]
- 38.Nelson O, Tu H, Lei T, Bentahir M, de Strooper B, Bezprozvanny I. Familial Alzheimer disease-linked mutations specifically disrupt Ca2+ leak function of presenilin 1. J Clin Invest. 2007;117:1230–1239. doi: 10.1172/JCI30447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jouvenceau A, Potier B, Battini R, Ferrari S, Dutar P, Billard JM. Glutamatergic synaptic responses and long-term potentiation are impaired in the CA1 hippocampal area of calbindin D(28k)-deficient mice. Synapse. 1999;33:172–180. doi: 10.1002/(SICI)1098-2396(19990901)33:3<172::AID-SYN2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 40.Tonkikh A, Janus C, El-Beheiry H, Pennefather PS, Samoilova M, McDonald P, Ouanounou A, Carlen PL. Calcium chelation improves spatial learning and synaptic plasticity in aged rats. Exp Neurol. 2006;197:291–300. doi: 10.1016/j.expneurol.2005.06.014. [DOI] [PubMed] [Google Scholar]