Abstract
Purpose
To estimate the association of age with maximal heart rate (MHR).
Methods
Data were obtained in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Participants were black and white men and women aged 18-30 in 1985-86 (year 0). A symptom-limited maximal graded exercise test was completed at years 0, 7, and 20 by 4969, 2583, and 2870 participants, respectively. After exclusion 9622 eligible tests remained.
Results
In all 9622 tests, estimated MHR (eMHR, beats/minute) had a quadratic relation to age in the age range 18 to 50 years, eMHR=179+0.29*age-0.011*age2. The age-MHR association was approximately linear in the restricted age ranges of consecutive tests. In 2215 people who completed both year 0 and 7 tests (age range 18 to 37), eMHR=189–0.35*age; and in 1574 people who completed both year 7 and 20 tests (age range 25 to 50), eMHR=199–0.63*age. In the lowest baseline BMI quartile, the rate of decline was 0.20 beats/minute/year between years 0-7 and 0.51 beats/minute/year between years 7-20; while in the highest baseline BMI quartile there was a linear rate of decline of approximately 0.7 beats/minute/year over the full age of 18 to 50 years.
Conclusion
Clinicians making exercise prescriptions should be aware that the loss of symptom-limited MHR is much slower at young adulthood and more pronounced in later adulthood. In particular, MHR loss is very slow in those with lowest BMI below age 40.
Keywords: prediction equations, graded exercise test, mixed models, epidemiologic study
Introduction
Fitness testing is used in a variety of circumstances, often with a goal of estimating physiologic maximum heart rate (MHR). MHR is commonly used in the medical field for preventive or diagnostic purposes. For example, the recommendation for maintenance or improvement of cardiorespiratory fitness is based on functions of MHR, resulting in a target heart rate, calculated either as a percentage of MHR or as a percentage of maximum heart rate reserve (9, 23). Additionally, MHR minus heart rate 2 minutes later defines heart rate recovery, an assessment of vagal tone which is associated with risk of death beyond the risk predicted by Framingham and European risk scores(1, 14). Because of the potential risks of undergoing true maximal treadmill testing (e.g. in a heart disease patient being tested for a cardiac rehabilitation prescription) and the discomfort of reaching true maximal exertion, cardiovascular stress tests commonly use symptom-limited maximal testing or endpoints associated with cut-off percentages of age-predicted MHR (e.g. achieving 85% of predicted MHR) (9).
The inverse association of age and maximum heart rate was initially described in the 1970s by the formula generated by Fox and Haskell that maximum heart rate = 220-age (6). Tanaka and colleagues later refined the formula to 208-0.7*age (22). Gellish et al. (8), recommended an association similar to that of Tanaka in a longitudinal study of 132 adults who completed a symptom-limited exercise test, between the ages of 27 and 78, but reported that a quadratic association of age with MHR fit the data better.
In this article we studied the association of MHR with age and other correlates, based on 1-3 symptom-limited maximal exercise tests completed over 20 years in a large population-based sample. Our primary objective was to provide a formula that linked MHR to age based on the repeated testing in this large cohort and to compare our formula to existing formulae (22, 8). Our hypothesis was that our findings would be similar to those of Tanaka et al. (22), which were based on a large number of true maximal tests studied in cross-sectional designs. We examined whether change in MHR with age within person is concordant with cross-sectional associations of MHR regressed on age. Therefore a larger goal was to characterize how the information about MHR in symptom-limited maximal testing compares to that in true maximal testing (22), considering that not every participant in a symptom-limited test performs at full effort.
Methods
Study design
Coronary Artery Risk Development in Young Adults (CARDIA) is a multi-center longitudinal cohort study established to understand the precursors and development of coronary heart disease risk among adults between 18-30 years of age. CARDIA examined 5115 participants with balanced subgroups of race (black and white), gender, education and age (18-24 and 25-30) at baseline (year 0) in 1985-1986. Recruitment was at random from the general population in Birmingham, AL, Chicago, IL, and Minneapolis, MN, and from members of the Kaiser Permanente Medical Care Plan in Oakland, CA (7, 11). Exclusions from the baseline CARDIA exam were done for the following reasons: blindness, deafness, muteness, inability to communicate, and permanent inability to walk. Six follow-up examinations were conducted at years 2, 5, 7, 10, 15 and 20, with 91%, 86%, 81%, 79%, 74%, and 72% of the surviving cohort returning, respectively. This article focuses on the symptom-limited maximal graded treadmill exercise tests performed at years 0, 7, and 20. The Year 20 exercise tests were obtained as part of an ancillary study, the CARDIA Fitness Study. The institutional review boards for the protection of human subjects for the participating study sites provided approval for the study, and written informed consent was obtained from all participants.
Data collection
Age, race, and sex were self-reported. At each examination, height and weight were measured to the nearest 0.5 cm and 0.09 kg, respectively. After 5 minutes of rest, seated blood pressure was measured three times with the average of the last two measurements used in analysis. Smoking status was self-reported; current smoking or nonsmoking were used as covariates in these analyses. Concurrent use of systemic β-blocker medications was obtained from self reports, with examination of medication bottles when available, at each time period. The diagnosis of asthma was made if the subject was receiving asthma medication (medicine containers examined) or self-reported doctor or nurse diagnosis of asthma (3).
Physical activity was measured at each examination by an interviewer administered questionnaire regarding the frequency of 13 different activities during the past 12 months. (19, 12) Since participants were not asked specifically about duration of physical activity, the exact energy expenditure cannot be estimated; however, the activity level was estimated by “Exercise Units” (EU). Duration of TV viewing was assessed by the answers to the related questions on a self-administered questionnaire at years 5, 10 and 20 (20). Lung function was measured at years 0, 10, and 20 using a Collins Survey 8-L watersealed spirometer and an Eagle II Microprocessor (Warren E. Collins, Inc., Braintree, MA) and following the standard procedures of the American Thoracic Society (2). Daily checks for leaks and volume calibration with a 3-L syringe and weekly calibration in the 4 to 7 L range were undertaken to minimize methodological artifacts between exams.
Graded Exercise testing
The graded symptom-limited maximal exercise test used at years 0, 7, and 20 followed a modified Balke protocol, which consisted of up to nine 2-minute stages of gradually increasing difficulty (18). Stage 1 was 2% grade at 3 miles per hour, stages 2-6 were 6, 10, 14, 18, and 22% grade at 3.4 miles per hour, stages 7 and 8 were 22 and 25% grade at 4.2 miles per hour, and stage 9 was 25% grade at 5.6 miles per hour. Heart rate (beats/minute) was measured by ECG at the end of each stage and upon cessation of the test. At each stage, participants were asked their rating of perceived exertion (RPE) using a 15 point Borg scale (range 6-20) (4). Participants were determined to be ineligible for the test primarily for the following reasons: history of heart disease, elevated resting blood pressure, abnormal ECG, acute illness with a fever (and not possible to reschedule for a later date), (See Table, Supplemental Digital Content 1, which shows the exclusion criteria for taking treadmill tests). Overall, test termination was due to fatigue or shortness of breath in about 98% of participants at all years.
The exercise test was performed by 4968, 3560, and 2870 participants (97%, 70%, and 56% of the CARDIA cohort) at years 0, 7, and 20, respectively. A systematic protocol violation of the year 7 tests was discovered in the Minnesota clinic after the examination period ended (21) which prompted the exclusion of 977 year 7 tests, leaving 2583 tests at year 7. Participants who took the tests in the Minnesota clinic tended to have longer maximal treadmill test duration (711.8 seconds versus 545.7 seconds) with lower MHR (174.4 beats/minute versus 177.9 beats/minute) than those who took tests at other clinics at year 7 examination. Even though the difference in MHR between these 977 Minnesota participants and the remaining participants from other clinics was similar at baseline (177.3 beats/minute versus 180.7 beats/minute) as at year 7, other characteristics differed. At baseline the 977 participants who were subject to the Minnesota protocol violation were more likely to be current smokers (36% versus 25%), and had lower maximal treadmill test duration (583.4 seconds versus 600.8 seconds), than the 2583 people from other clinics who were included at year 7. Additionally, we excluded 266 tests of participants with concurrent beta blocker medication use and 572 tests that were terminated for medical reasons including abnormal ECG manifestations (n=111 tests), abnormal blood pressure changes (n=45 tests), and several other medical reasons such as back pain, nausea or dizziness. We also excluded all 109 tests of participants who were younger than 18 or older than 30 years of age at the time of baseline testing. Some participants met more than one exclusion criteria.
Statistical Analysis
We first examined characteristics of participants according to treadmill test completion status. Because the focus of Table 1 was on the willingness and ability to be tested during a longitudinal epidemiologic study, we included the year 7 tests in Minnesota in the categorization of treadmill test attendance. This analysis included 4969 participants, excluding 72 who never took the treadmill test and 74 participants who took the tests only during follow up. The remaining analyses in this paper included 9622 tests in 4844 participants. To characterize the age relationship with MHR, we plotted mean, standard deviation, and 5th percentile of MHR at each year of age (Figure 1). To account for correlations between repeated tests within person, we analyzed the association of maximum heart rate (MHR) with age using a repeated measures regression analysis, with toeplitz covariance structure and random intercept (SAS, PROC MIXED). The predictor variable was current age; the quadratic form was used when testing for curvature. In accord with the hypothesis that the association of MHR with age is nonlinear, we have strong power to predict a difference in the change in MHR by age. The minimal detectable difference (5% type I error (two tailed) with 85% power) was 0.14 beats/minute/year in the difference of the MHR slope per year starting at average age 25 and followed for 7 years minus the difference of the MHR slope per year starting at average age 32 and followed for 13 years.
Table 1.
Baseline characteristics [mean (standard deviation) or percentage] of the participants according to treadmill test attendance status.
| Baseline and follow-up treadmill test performance status* | ||||
|---|---|---|---|---|
| Characteristic at baseline, age 18-30 | Baseline only (n=928) | Baseline+1 follow-up (n=1480†) | Baseline+2 follow-ups (n=2182†) | P-value |
| Exercise test Characteristics | ||||
| Resting heart rate (beats/min) | 70.0(11.0) | 69.5(11.0) | 68.9(10.6) | 0.03 |
| Maximum heart rate (beats/min) | 175.7(16.6) | 178.2(15.4) | 181.0(14.4) | <.0001 |
| Test duration (second) | 554.0(178.2) | 576.0(167.8) | 612.1(167.4) | <.0001 |
| Demographic Characteristics | ||||
| Age (years) | 24.4(3.7) | 24.6(3.6) | 25.0(3.5) | <.0001 |
| Gender (% women) | 54.7 | 54.7 | 55.6 | 0.86 |
| Race (% black) | 66.0 | 55.8 | 42.8 | <.0001 |
| Body Mass Index (BMI) (kg/m2) | 25.0 (6.1) | 24.7(5.0) | 24.0(4.3) | <.0001 |
| Weight Status | ||||
| Normal Weight (<25kg/m2) (%) | 64.3 | 61.8 | 69.1 | <.0001 |
| Overweight (25-30kg/m2) (%) | 19.7 | 24.8 | 22.8 | 0.02 |
| Obese (≥30kg/m2) (%) | 16.0 | 13.5 | 8.1 | <.0001 |
| Systolic Blood Pressure (mmHg) | 111.1(11.2) | 110.6(10.7) | 109.6(10.4) | 0.0004 |
| Current smoker (%) | 39.9 | 32.8 | 23.9 | <.0001 |
| Current asthma (%) | 3.8 | 5.1 | 4.4 | 0.31 |
| Physical activity score (exercise units) | 403.2(305.4) | 410.8(294.6) | 426.7(299.8) | 0.09 |
| TV watching (hours/week)‡ | 10.9(13.6) | 9.3(12.1) | 7.3(10.0) | <.0001 |
| Lung Function | ||||
| FVC (L) | 4.1(0.9) | 4.3(1.0) | 4.4(1.0) | <.0001 |
| FEV1(L) | 3.4(0.7) | 3.5(0.8) | 3.6(0.8) | <.0001 |
| FEV1/FVC | 83.6% (6.2%) | 83.1% (6.6%) | 83.1% (6.4%) | 0.10 |
Among 5115 participants of CARDIA study, 72 participants never took the exercise treadmill tests at baseline or during 20 years of follow-up and 74 participants took the tests only during follow-up. Since the number is relatively small compared to the three groups listed in the table, the data concerning these two groups of participants are not shown in the table.
403 of the participants in the baseline+1 follow-up group and 553 of the participants in the baseline + 2 follow-up group did the exercise treadmill test in Minnesota at year 7 and are included in this table. Because of a protocol violation (see methods), these test results did not contribute to further analysis.
TV watching was measured at year 5 and is available in 447, 1272, and 2111 of the respective treadmill attendance grouping: baseline only, baseline+1 follow-up, and baseline+2 follow-ups.
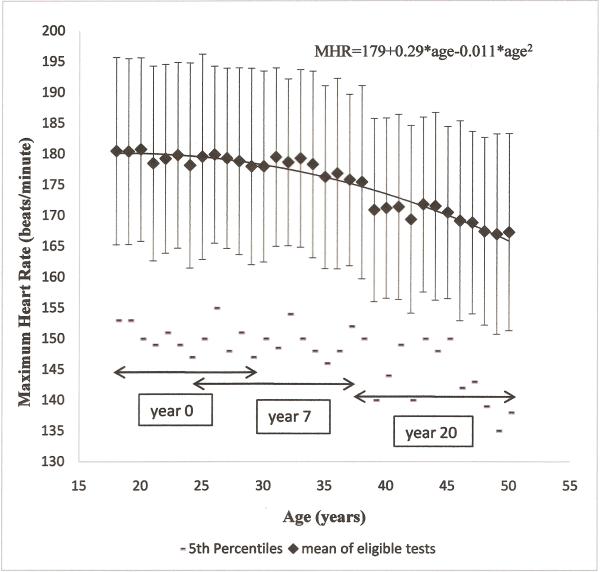
Figure 1.
Distribution of Maximum Heart Rate (1 standard deviation error bars and age-specific 5th percentiles) of eligible tests across 20 year follow-up by age at examination (9622 tests in 4844 participants).
In true maximal testing, achievement of MHR can be objectively verified (e.g., by verifying lack of change of heart rate despite increasing workload). However, most people do not voluntarily perform at maximal level of exertion, indicated by a plateauing of heart rate or oxygen consumption. Symptom-limited maximal tests vary in how close they come to maximal effort, but the percent of maximum achieved cannot be measured. Therefore, restricting to tests that most likely represented full effort could help interpret the association of age and observed MHR in CARDIA. Our investigation of this topic concluded that such restriction was not helpful (see Text, Supplemental Digital Content 2, which shows the findings of this methodological investigation).
The MHR-age relationship was further analyzed according to quartile of baseline BMI, smoking status, forced vital, capacity, physical activity, television watching, and baseline treadmill duration. In addition, general linear models were used to examine the relationship of MHR at each visit and the changes of MHR between consecutive visits, as dependent variables, with several predictors at baseline or changes between visits, as shown in the tables.
Results
Participant characteristics and selection bias
During 20-year follow-up, 928 underwent exercise treadmill testing only at baseline, 1480 at baseline and one follow-up visit (year 7 or year 20), and 2182 at baseline and both follow-up visits (year 7 and year 20) (table 1). The baseline treadmill exercise test duration was (mean±SD) 589±171 seconds and maximum heart rate achieved was 179±15 beats/minute. Several baseline characteristics suggest selection bias in those who were willing or able to undergo multiple treadmill tests during the study. Participants who had higher maximum heart rate or longer treadmill duration at baseline were more likely to undergo follow-up testing. Other baseline characteristics associated with completing more than one treadmill test were older age, white race, lower BMI, lower systolic blood pressure, higher forced vital capacity (FVC) and forced expiratory volume in 1 second, higher self reported activity, less TV watching, and not smoking.
Distribution of maximal heart rate
Mean MHR declined with age, with age-specific standard deviation ranging from 14beats/minute to 16beats/minute (Figure 1). As illustrated by age-specific 5th percentiles, observed MHR for 5% of participants was <155 beats/minute at age 18, declining to below 140 beats/minute at age 50. The age-specific first percentile of MHR ranged from 113 to 142 beats/minute without any age pattern (data not shown). The age-specific mean levels in Figure 1 display a quadratic pattern estimating MHR (eMHR). Observed MHR tracked over examinations, with intraclass correlation (ICC) between 0.4 and 0.55 in all analyses. Maximum heart rate and age
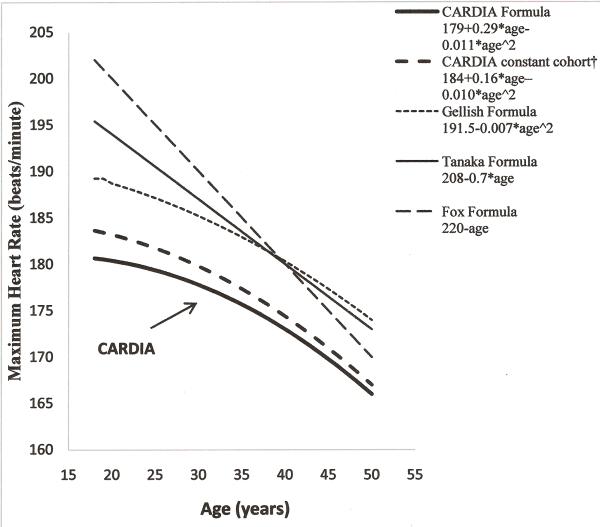
Among the 4844 participants who completed 1 to 3 eligible tests, the relationship between age and MHR was significantly quadratic (eMHR=179+0.29*age-0.011*age2). While restricting to those who completed all 3 tests during follow-up, the association maintained its quadratic shape (eMHR=184+0.16*age–0.010*age2). The overall MHR observed in CARDIA are substantially lower than the Tanaka, Gellish and Fox predicted MHR (figure 2). However, the association was linear over restricted age ranges. For the 2216 participants who completed both year 0 and year 7 treadmill tests (age range 18 to 37), the age-MHR relationship was linear (eMHR=190-0.37*age). Also, for the 1574 participants who participated in both of the treadmill tests at year 7 and year 20 (age range 25 to 30), this association was linear (eMHR=199-0.63*age). The mean MHR in people who completed tests at both year 0 and 7 exams was 181.0±14.8 and 178.3±15.1 beats/minute, and for people who did both year 7 and 20 exams was 179.4±14.6 and 171.0±14.7 beats/minute, at the respective examinations. Additionally, in restriction sets, obtained by excluding less than full effort tests based on RPE, stage achieved, certain percentage of the Tanaka eMHR, or any other rule, the relationship between age and MHR was quadratic (see Table, Supplemental Digital Content 3, which demonstrates prediction of maximum heart rate (MHR) from age in all available data and in some restriction sets obtained by different trimming methods; see Figure, Supplemental Digital Content 4 which shows distribution of MHR by age after excluding those with values below the 85% TANAKA estimate of MHR (eMHR)).
Figure 2.
Distribution of Maximum Heart Rate of treadmill tests predicted by existing formulae using CARDIA data.
†Constant cohort represents 1479 participants who took treadmill tests at year 0, 7 and 20.
Maximum heart rate and age across BMI quartiles
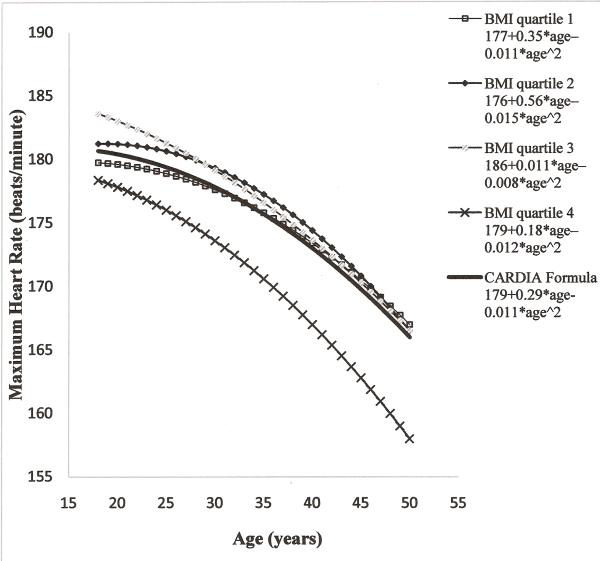
The age-MHR relationship was further analyzed across baseline BMI quartiles (table 2). The rate of decline of MHR was significantly different across baseline BMI quartiles (P for interaction <0.0001). In quartile 1, the pattern of the relationship between age and MHR was quadratic, but with shallower rate of decline (0.24 beats/minute/year in years 0-7 and 0.51 beats/minute/year in years 7-20) than the results for all participants (0.35 beats/minute/year in years 0-7 and 0.63 beats/minute/year in years 7-20). The rate of decline of MHR with age became steeper as BMI increased, particularly in the younger ages, with the result that in the highest baseline BMI quartile there was a linear rate of decline of 0.6–0.7 beats/minute/year over the full age of 18 to 50 years. Similar analysis conducted among those who completed all three tests yielded a similar pattern of decline of MHR with age across BMI quartiles (data not shown).
Table 2.
MHR-age formula and number of participants according to quartile of baseline BMI.
| Baseline BMI quartile | Participants | Tests | Formula |
|---|---|---|---|
| 1st quartile (<21.1 kg/m2) | |||
| All years | 1224 | 2472 | Quadratic: 177+0.35* age–0.011*age2 |
| Yr 0-7 | 576 | 1152 | Linear: 187 – 0.24*age |
| Yr 7-20 | 412 | 824 | Linear: 197 – 0.51*age |
| 2nd quartile (21.1 – 23.3 kg/m2) | |||
| All years | 1209 | 2428 | quadratic: 176+0.56* age–0.015*age2 |
| Yr 0-7 | 537 | 1074 | Linear: 189 – 0.27*age |
| Yr 7-20 | 428 | 856 | Linear: 200 – 0.63*age |
| 3rd quartile (23.4 – 26.3 kg/m2) | |||
| All years | 1216 | 2468 | quadratic: 186+0.011*age–0.008*age2 |
| Yr 0-7 | 581 | 1162 | Linear: 191 – 0.37*age |
| Yr 7-20 | 426 | 852 | Linear: 202 –0.69*age |
| 4th quartile (≥26.4 kg/m2) | |||
| All years | 1183 | 2229 | quadratic: 179+0.18*age–0.012*age2 |
| Yr 0-7 | 516 | 1032 | Linear: 193 – 0.60*age |
| Yr 7-20 | 303 | 606 | Linear: 197 – 0.71*age |
Figure 3 shows the BMI quartile specific eMHR. The overall quadratic solution predicts lower MHR than the estimates of lower BMI quartiles at younger age and overlaps with the estimates of lower BMI quartile at older age. People in the highest BMI quartile have lowest predicted MHR. Those who completed all 3 tests during 20 year follow-up had relatively higher eMHR than the overall cohort.
Figure 3.
Distribution of Maximum Heart Rate of treadmill tests by age across quartiles of baseline BMI in CARDIA.
Differences in rate of decline of MHR according to other baseline characteristics were much less than those shown in Table 2. The rate of decline of MHR with age was somewhat steeper in those with initially lower FVC than in those with initially higher FVC (data not shown, P for interaction <0.0001). Those with shorter versus longer year 0 treadmill duration tended to have less decrease in MHR with age (data not shown, P for interaction <0.0001), suggesting regression towards the mean or a learning effect, i.e., those who reached a lower proportion of true MHR at year 0 tended to reach a somewhat higher proportion of true MHR on repeat testing). There was no age interaction with baseline physical activity, TV watching, or smoking status.
Other correlates of Maximum heart rate
Besides age, several variables were related to MHR either cross-sectionally or longitudinally (Table 3; see Table, Supplemental Digital Content 5, which showed the equation for tests at year 0, year 0 and 7, and then year 0, 7, and 20). Women had lower MHR than men, although mean MHR increased in women relative to men between years 7 and 20. Blacks had lower MHR than whites, but this difference was attenuated between years 7 and 20. BMI was consistently inversely associated with MHR in all analyses. Taller people tended to have lower MHR cross-sectionally, but the change in MHR was not predicted by height. Those with higher pre-exercise heart rate achieved higher mean MHR than those with lower pre-exercise heart rates. However, higher pre-exercise heart rate at baseline predicted a decrease in MHR over time. Similar to pre-exercise heart rate, higher systolic blood pressure , cross-sectionally, and increases in systolic blood pressure over time were associated with higher MHR and change in MHR, respectively; yet those with higher baseline blood pressure had a faster decline in MHR over time. Participants whose FVC was higher had higher MHR. Increasing FVC between years 0 and 7 was related to increasing MHR over time. Neither physical activity nor TV watching showed strong relationships with MHR or its changes. Current smokers (consistent across visits) and those who recently started smoking had lower MHR. The slope of MHR on age was substantially attenuated after adjustment for these factors. Nevertheless, relatively little of the variance of MHR was predictable in any of the models in Table 3, the maximum r2 being 0.22 for the baseline cross-sectional model.
Table 3.
Linear regression analysis of MHR(beats/min) and its changes during 20 years (dependent variables) in relation to baseline (year 0 or year 7) variables and their changes. A. Cross-sectional and change in MHR on baseline levels
| Year 0 cross section | MHR (Year 7-Year 0) regressed on Year 0 variables | MHR (Year 20-Year 7) regressed on Year 7 variables | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable (year 0 or year 7) | Slope | SE | P-value | Slope | SE | P-value | Slope | SE | P-value |
| Intercept | 180 | 6.88 | <.0001 | 31 | 8.93 | 0.0001 | 20 | 9.87 | 0.04 |
| Age (years) | −0.17 | 0.07 | 0.01 | −0.14 | 0.08 | 0.08 | −0.12 | 0.09 | 0.20 |
| Gender (women) | −2.89 | 0.74 | 0.0001 | −2.93 | 0.97 | 0.003 | 2.21 | 1.04 | 0.03 |
| Race (black) | −3.05 | 0.58 | <.0001 | −1.78 | 0.74 | 0.02 | 1.97 | 0.84 | 0.02 |
| BMI (kg/m2) | −0.31 | 0.05 | <.0001 | −0.20 | 0.07 | 0.003 | −0.18 | 0.07 | 0.005 |
| Height (m) | −0.11 | 0.04 | 0.01 | −0.02 | 0.05 | 0.77 | −0.001 | 0.06 | 0.99 |
| Pre-exercise heart rate (beats/min) | 0.23 | 0.02 | <.0001 | −0.14 | 0.02 | <.0001 | −0.16 | 0.02 | <.0001 |
| Systolic Blood Pressure (mmHg) | 0.06 | 0.02 | 0.02 | −0.05 | 0.03 | 0.13 | −0.09 | 0.03 | 0.01 |
| Forced vital capacity (L)* | 2.58 | 0.45 | <.0001 | −0.94 | 0.58 | 0.11 | −0.34 | 0.62 | 0.59 |
| Physical activity score (100 exercise units) | 0.38 | 0.08 | <.0001 | −0.01 | 0.10 | 0.91 | −0.2 | 0.1 | 0.17 |
| TV watching (hours/week)† | −0.11 | 0.02 | <.0001 | −0.04 | 0.03 | 0.23 | 0.09 | 0.04 | 0.01 |
| Smoking status (vs. never) | |||||||||
| Current | −8.07 | 0.53 | <.0001 | 0.79 | 0.71 | 0.27 | 1.93 | 0.86 | 0.03 |
| Former | −1.87 | 0.68 | 0.006 | −1.65 | 0.93 | 0.07 | 1.88 | 1.16 | 0.11 |
| R2 | 0.22 | 0.05 | 0.06 | ||||||
| B. Concurrent changes | ||||||
|---|---|---|---|---|---|---|
| MHR (Year 7-Year 0) regressed on change in variables (Year 7-Year 0)‡ | MHR (Year 20-Year 7) regressed on change in variables (Year 20-Year 7)‡ | |||||
| Variable | Slope | StdErr | P-value | Slope | StdErr | P-value |
| Intercept | 0.46 | 0.61 | 0. 46 | −9.52 | 0.77 | <.0001 |
| ΔBMI ( kg/m2) | −0.36 | 0.11 | 0.001 | −0.18 | 0.07 | 0.008 |
| ΔPre-exercise heart rate (beats/min) | 0.17 | 0.02 | <.0001 | 0.11 | 0.02 | <.0001 |
| ΔSystolic Blood Pressure (mmHg) | 0.13 | 0.03 | <.0001 | 0.02 | 0.03 | 0.40 |
| ΔFVC (L)* | 3.86 | 1.07 | 0.0003 | −0.44 | 1.03 | 0.67 |
| ΔPhysical activity score (exercise units) | 0.001 | 0.001 | 0.19 | 0.002 | 0.001 | 0.20 |
| ΔTV watching (hours/week)† | −0.04 | 0.03 | 0.17 | −0.08 | 0.03 | 0.01 |
| ΔSmoking status (vs. never) | ||||||
| Long term former | −1.11 | 0.91 | 0.22 | 0.30 | 0.94 | 0.75 |
| Recent quitter | 2.09 | 1.29 | 0.10 | 0.11 | 1.40 | 0.94 |
| Consistently current | 0.53 | 0.82 | 0.52 | −2.37 | 1.03 | 0.02 |
| Recent starter | −5.85 | 1.73 | 0.0008 | −3.95 | 2.25 | 0.08 |
| R2 | 0.08 | 0.06 | ||||
Since year 7 lung function data were not collected, year 10 lung function data were used in the analysis when baseline was year 7.
Year 5 data were used in the analysis when baseline was year 0; year 10 data were used when baseline was year 7.
Gender and race were included as covariates in the models of change in MHR on change in other characteristics. Coefficients are not shown, but are similar to those given for models of change in MHR on baseline levels of other characteristics.
Discussion
The primary finding in this study was that, in repeat exercise treadmill testing between ages 18 and 50, the decline of MHR with age was less at younger ages than at older ones. The fitted rates of loss of MHR according to a quadratic age function, 179+0.29*age-0.011*age2, are similar to the observed rates of loss between years 0 and 7 (0.37 beats/minute/year) and between years 7 and 20 (0.63 beats/minute/year), respectively. Another important observation is that the rate of decline of MHR with age was slower in people who were thinner at baseline than in those who had higher BMI.
Our findings are consistent with those of two other longitudinal studies. Plowman et al. (15) reported on 36 healthy women retested after an interval of 6.1 years. MHR in symptom-limited testing remained relatively steady during ages in the 20’s and 30’s, then declined at a faster rate in the 50- and 60-year-old age groups. Gellish et al. (8) studied 908 symptom-limited maximal exercise treadmill tests in 132 men and women attending a university fitness center over several years. Although they concluded that eMHR = 207 – 0.7 x age fit their data well, eMHR = 191.5-.007*Age2 fit the data better. There is a negligible difference between the curvature of our formula and that of Gellish among ages 27-50 that occur in both studies. In their study, the rate of decline in MHR was close to zero through about age 40, and was about 0.5 beats/minute/year between ages 40 and 50. We showed a reduced rate of decline in CARDIA participants who were thinner or fitter at baseline, at ages 18 to 30 years. Consistent with this finding, it is likely that the participants in the Gellish study were thinner and more fit than were members of the general population studied in CARDIA, which may account for the slower rate of loss of MHR in the Gellish study than in CARDIA.
On the other hand, our findings conflict with those of Tanaka et al., who found that eMHR = 208- 0.7 × age in a summary of 492 groups that included true maximal exercise tests in 18,712 persons, confirmed in their own specific study of true maximal tests in 514 individuals. There was no sign of curvature in the MHR and age relationship in this monumental study. Tanaka's (22) finding differs from ours in important ways. Tanaka's results were based on cross-sectional study designs, so could not capture individual changes over time, whereas the longitudinal study designs used by Plowman (15), Gellish (8), and CARDIA were able to assess individual changes. In individual changes, the slope of MHR over age was consistently lower in persons initially younger than at older ages. Thus, in this respect, the CARDIA study that included tests in 97% of the general population sample recruited into the study, suggests curvature in the MHR and age relationship that is missed in cross-sectional study designs.
The true maximal exercise test is a superior measure of cardiorespiratory capacity compared to the symptom-limited test that CARDIA, Plowman (15), and Gellish (8) used, but it may restrict the sample to those who are willing and able to do the test, and in this way skew the estimate of the relation of MHR to age in the general population. A characteristic of the symptom-limited maximal exercise protocol is that it does not in most cases achieve true maximum, as reflected in Figure 2 by the higher age-specific eMHR using the Tanaka or Fox formula than using any of the CARDIA findings. Furthermore, in a symptom limited test, the extent of full effort is a differential proportion of true maximum that varies by person. A common strategy is to eliminate from statistical analysis all tests deemed to be “less than full effort” (eg using 85% of the Tanaka prediction). As shown in the Supplement, all restriction methods considered, whether based on RPE, stage achieved, excluding tests with MHR less than 85% of the Tanaka eMHR, or any other rule for excluding less than full effort tests, yielded a quadratic relationship between MHR and age. However, viewed from a population perspective, we think that the volunteers in Tanaka's paper (22) almost certainly were highly selected, excluding those for whom a true maximal test would be uncomfortable. Since CARDIA was able to test 97% of its participants at baseline, we were able to assess selection bias in future testing. Among important baseline characteristics of those least likely to complete 1 or 2 more tests over 20 years were reduced fitness and lung capacity, adiposity, smoking, and sedentary lifestyle. Consequently, participants who completed 3 tests, who tended to be the healthiest at baseline, had relatively higher eMHR than the overall CARDIA cohort. Therefore, the formula derived from these participants was less generalizable than the formula derived from the whole CARDIA cohort. Moreover, the CARDIA formula may also be more realistic for the general population than the Gellish (8) formula. CARDIA showed a slightly steeper rate of decline in MHR with age at each age than did Gellish et al., but the Gellish et al. sample was considerably selected compared to the population-based CARDIA sample as is visually apparent in Table 2.
The decrease of MHR with advancing age is a reasonable expectation for physiologic change. Rodeheffer et al. (16) performed serial gated blood pool scans at rest and during exercise treadmill tests for 61 participants aged 25 to 79 years. They found an age-related increase in end-diastolic volume and stroke volume, and an age-related decrease in heart rate during treadmill tests, such that cardiac output both at rest and during exercise was maintained across ages. The changes in the cardiovascular system associated with aging (5) and adiposity, (13, 17) tend to decrease heart rate and heart rate response to exercise. These changes include apoptosis of sinoatrial node pacemaker cells, decreased responsiveness to β-adrenergic receptor stimulation and decreased reactivity to baroreceptors and chemoreceptors (5, 16, 17).
Furthermore, the quadratic component to the age-related MHR decrease, implying more rapid MHR decrease in older than in younger ages, might be partly explained by physiologic change. Cheitlin pointed out that apoptosis of sinoatrial node pacemaker cells leads to a loss of 50% - 75% of cells by age 50, resulting in slower intrinsic heart rate. Since the exact rate of the loss of cells is not available and has not been proven to be linear, it is possible that this rate of loss is quadratic, leading to greater loss of intrinsic heart rate at older than at younger ages and thus contributing to greater loss of MHR at older than at younger ages. In addition, Cheitlin et al. (5) pointed to decreased responsiveness to β-adrenergic receptor stimuli with aging, partly compensated by an increase in circulating catecholamines. However, whether this compensation is at a constant rate with aging or not was not assessed. Therefore, further cardiovascular physiological studies would be helpful, related to the change, especially the change rate of atrial pacemaker cells, responsiveness to β-adrenergic receptor stimuli and compensation by circulating catecholamines with aging.
We previously reported a finding that is consistent with slower loss of cardiorespiratory capacity in thinner than in heavier people, namely that the people in the lowest quartile of baseline BMI maintained their lung function through their mid-30s (24). Thus structure and function of cardiovascular and pulmonary tissue was maintained in the smaller leaner participants. The steeper rate of decline in the participants with higher BMI was not due to reduced physical activity, since physical activity itself was not a strong correlate of MHR. Another possible explanation of the more rapid rate of decline in people with higher BMI is that they did not work as hard on the treadmill as thinner people, especially as they aged, as would be evidenced by stopping the test at lower RPE. However, adjustment for RPE did not much alter the pattern of the decline of MHR with age across baseline BMI quartiles. Furthermore, the rate of decline of the MHR at older ages was likely underestimated because the people with higher BMI had tendency not to return for retesting (Table 1).
Apart from age and BMI, several variables were related to MHR. The underlying mechanisms are not well studied. For example, smoking was related to a reduced MHR, but smokers did not have a greater rate of decline of MHR than nonsmokers. It is known that nicotine increases heart rate, myocardial contractility, and blood pressure by stimulation of sympathetic neurotransmission. However, the norepinephrine-releasing effect of nicotine cannot explain the smoking-MHR association in this study (10). We hypothesize that during exercise nicotine acts as a beta blocker, which lowers MHR acutely (21), but is not associated with MHR decreases over time.
The large, population-based sample of adults and the 20 year follow-up of participants with 3 tests in many cases are the strengths of this study. Our results may be more generalizable than previous studies due to the population-based sampling of participants, the substantial proportion of women and black participants, and the inclusion of smokers and obese persons. While the repeat testing is a strength, a limitation for determining repeatability is that testing was completed only 3 times by each participant spread out over 20 years. Due to the symptom-limited maximal test protocol that was used, it is difficult in these data to distinguish differences among participants in true maximal heart from differences in proportion of true maximum achieved. However, use of the symptom-limited test allowed us to perform testing in nearly everyone in the study.
In conclusion, for those using exercise testing for providing a target heart rate during exercise, one implication from this study is that people with lower BMI lose MHR very slowly through their 20s and 30s, then start to lose more quickly in the 40s, yet at a slower rate than was observed in Tanaka's equation. People with higher BMI lose MHR throughout age 18-50 at about the rate of 0.7 beats/minute per year of age as specified by Tanaka et al. (22). While no precise alteration to the formula 208-0.7*age is possible from these CARDIA data, partly because such an alteration would only apply to ages 18-50. Clinicians making exercise prescriptions should be aware that the loss of MHR is quadratic in most people, and is very slow in the smallest younger individuals. For those with higher BMI, the clinician should be aware that the rate of loss of MHR is steep, even at younger ages. Future study involving participants at older ages (>50 years) is needed. Further characterization of the distribution of the percent of true MHR achieved in submaximal testing would be helpful.
Supplementary Material
Supplemental Digital Content 2.
Text. Estimated maximum heart rate after deleting tests that are presumably less than full effort
Methods
Supplemental Paragraph Number 1 Including tests that represent less than full effort may impede assessment of the association of age and observed MHR. We explored several strategies for forming restriction sets that eliminated tests that were less than full effort: 1) If RPE was low, the participant did not perceive working at full effort during the treadmill test; as an example of RPE-based restriction, we studied a restriction set that eliminated any test with RPE <15. 2) Tests of short duration may not represent full effort; we studied a duration-based restriction set that excluded those who did not finish stage 2 of the test, and for another excluding those who did not finish stage 3. 3) We formed restriction sets by excluding tests with low age-specific MHR, using a “trimline”. One such “trimline” strategy excludes tests with MHR <85% of predicted MHR using the Tanaka formula 208- 0.7*age, as depicted in Supplemental Digital Content 4. This restriction eliminates tests in 18 year olds with MHR < 166 beats/minute. The cutpoint for exclusion declines to 147 at age 50 by 0.7 beats/minute per year of age. We designate this “trimline” by the cutpoints at age 18 and at age 50, that is trimline (166,147).
Supplemental Paragraph Number 2 Trimline (166,147) makes exclusion proportional to predicted MHR at each age. We explored 600 additional non-proportional “trimlines” in order to see whether a linear age-MHR association emerged in this CARDIA dataset similar to the equations reported by Tanaka and Gellish (8, 22). This exploration consisted of examining the repeated measures regression of MHR on quadratic age for each trimline (X, Y), with X (the cutpoint at age 18) ranging from 160 to 189 and Y (the cutpoint at age 50) ranging from 120 to 139 (30 × 20 = 600 “trimlines” in total). We recorded size and statistical significance of the coefficient for age2, with focus on a cutoff line that yielded a small and nonsignificant coefficient for age2 (indicating a linear age-MHR relationship), then repeated the regression omitting the age2 term to characterize the linear solution.
Interpretation
Supplemental Paragraph Number 3 We further studied the shape of the eMHR curve under several restriction strategies based on RPE, highest stage of the treadmill test attained, or trimming using a percent of the Tanaka age-predicted MHR (eg, trimline (166,147)) (see Table, Supplemental Digital Content 3, which demonstrates prediction of maximum heart rate (MHR) from age in all available data and in some restriction sets obtained by different trimming methods; see Figure, Supplemental Digital Content 4 which shows distribution of MHR by age after excluding those with values below the 85% TANAKA estimate of MHR (eMHR)). All shapes were quadratic. We explored a wide range of non-proportional “trimlines” and found that many yielded a linear slope similar to what was reported previously (8,22). For example, linearity of the MHR age relationship was maintained for the age 18 cutoff 180-186 combined with any age 50 cutoffs in the range 120-139; the age slope was close to (−0.7) for all these trimlines. Specifically, the trimline (182,130) yielded eMHR=203-0.70*age. However, further adjustment for baseline characteristics that may have been involved in selection bias, including sex, race, BMI, physical activity, smoking status, lung function and treadmill test duration, restored a quadratic association with quadratic coefficient −0.003. We concluded that we never know what proportion of a true maximal test was performed; even a person achieving a relatively high MHR might have achieved a relatively low proportion of true MHR. Therefore we were not able to distinguish less than full effort from full effort tests, and we deemed restriction strategies for all but a very few low level tests not to be helpful in understanding MHR.
List of Supplemental Digital Content
Supplemental Digital Content 1. Table showing number of people excluded from the treadmill tests according to different criteria in CARDIA study. doc.
Supplemental Digital Content 2. Text explaining several strategies for forming restriction sets that eliminated tests that might be less than full effort. doc.
Supplemental Digital Content 3. Table demonstrating prediction of maximum heart rate (MHR) from age in all available data and in some restriction sets obtained by different trimming methods. doc.
Supplemental Digital Content 4. Figure showing distribution of MHR by age after excluding those with values below the 85% TANAKA estimate of MHR (eMHR). tif.
Supplemental Digital Content 5. Table showing the equations for year 0, year 0 and 7, and then for year 0, 7, and 20 with multiple baseline variables. doc.
Supplemental Digital Content 4.
Figure. Distribution of Maximum Heart Rate (1 standard deviation error bars) of treadmill tests by age at examination after excluding those with values below the 85% eMHR as predicted by Tanaka formula, 208-0.7*age (trimline (166,147)).
Supplemental Digital Content 1.
Table. Number of people excluded from the treadmill tests according to different criteria in the CARDIA study.
Supplemental Digital Content 3.
Table. Prediction of MHR from age in all available data and in some restriction sets obtained by different trimming methods.
Supplemental Digital Content 5
Table. The predicting equation of Maximum Heart Rate at year 0, year 0 and 7, and year 0, 7, and 20 from multiple baseline variables.
Acknowledgement
This study is supported by the CARDIA contract (N01-Hc-48047-N01-HC-48050 and N01-HC-95095) and a grant for the CARDIA fitness study R01 HL 078972.
Funding received for this work is from National Institutes of Health (NIH).
Footnotes
The authors do not have a professional relationship with companies or manufacturers that may benefit from the results of this study. The results of the present study do not constitute endorsement by ACSM.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aktas MK, Ozduran V, Pothier CE, Lang R, Lauer MS. Global risk scores and exercise testing for predicting all-cause mortality in a preventive medicine program. JAMA. 2004;292(12):1462–84. doi: 10.1001/jama.292.12.1462. [DOI] [PubMed] [Google Scholar]
- 2.ATS statement–Snowbird workshop on standardization of spirometry. Am Rev Respir Dis. 1979;119(5):831–8. doi: 10.1164/arrd.1979.119.5.831. [DOI] [PubMed] [Google Scholar]
- 3.Beckett WS, Jacobs DR, Yu X, Iribarren C, Williams OD. Asthma is associated with weight gain in females but not males, independent of physical activity. Am J Respir Crit Care Med. 2001;164(11):2045–50. doi: 10.1164/ajrccm.164.11.2004235. [DOI] [PubMed] [Google Scholar]
- 4.Borg GA. Perceived exertion. Exerc Sport Sci Rev. 1974;2:131–53. [PubMed] [Google Scholar]
- 5.Cheitlin MD. Cardiovascular physiology-changes with aging. Am J Geriatr Cardiol. 2003;12(1):9–13. doi: 10.1111/j.1076-7460.2003.01751.x. [DOI] [PubMed] [Google Scholar]
- 6.Fox SM, 3rd, Naughton JP, Haskell WL. Physical activity and the prevention of coronary heart disease. Ann Clin Res. 1971;3(6):404–32. [PubMed] [Google Scholar]
- 7.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: Study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105–16. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 8.Gellish RL, Goslin BR, Olson RE, McDonald A, Russi GD, Moudgil VK. Longitudinal modeling of the relationship between age and maximal heart rate. Med Sci Sports Exerc. 2007;39(5):822–9. doi: 10.1097/mss.0b013e31803349c6. [DOI] [PubMed] [Google Scholar]
- 9.Gibbons RJ, Balady GJ, Bricker JT, et al. ACC/AHA 2002 guideline update for exercise testing: Summary article. A report of the American college of Cardiology/American heart association task force on practice guidelines (committee to update the 1997 exercise testing guidelines). J Am Coll Cardiol. 2002;40(8):1531–40. doi: 10.1016/s0735-1097(02)02164-2. [DOI] [PubMed] [Google Scholar]
- 10.Haass M, Kübler W. Nicotine and sympathetic neurotransmission. Cardiovasc Drugs Ther. 1997;10(6):657–65. doi: 10.1007/BF00053022. [DOI] [PubMed] [Google Scholar]
- 11.Hughes GH, Cutter G, Donahue R, et al. Recruitment in the Coronary Artery Disease Risk Development in Young Adults (Cardia) Study. Control Clin Trials. 1987;8(4 Suppl):68S–73S. doi: 10.1016/0197-2456(87)90008-0. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs DR, Hahn L, Haskell WL, Pirie P, Sidney S. Validity and reliability of short physical activity history: CARDIA study and the Minnesota Heart Health Program. J Cardiopulmonary Rehabil. 1989;9(11):448–59. doi: 10.1097/00008483-198911000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JA, Park YG, Cho KH, Hong MH, Han HC, Choi YS, Yoon D. Heart rate variability and obesity indices: emphasis on the response to noise and standing. J Am Board Fam Pract. 2005;18(2):97–103. doi: 10.3122/jabfm.18.2.97. [DOI] [PubMed] [Google Scholar]
- 14.Lauer M, Froelicher ES, Williams M, Kligfield P, American Heart Association Council on Clinical Cardiology, Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention Exercise testing in asymptomatic adults: A statement for professionals from the American heart association council on clinical cardiology, subcommittee on exercise, cardiac rehabilitation, and prevention. Circulation. 2005;112(5):771–6. doi: 10.1161/CIRCULATIONAHA.105.166543. [DOI] [PubMed] [Google Scholar]
- 15.Plowman SA, Drinkwater BL, Horvath SM. Age and aerobic power in women: a longitudinal study. J Gerontol. 1979;34(4):512–20. doi: 10.1093/geronj/34.4.512. [DOI] [PubMed] [Google Scholar]
- 16.Rodeheffer RJ, Gerstenblith G, Becker LC, Fleg JL, Weisfeldt ML, Lakatta EG. Exercise cardiac output is maintained with advancing age in healthy human subjects: cardiac dilatation and increased stroke volume compensate for a diminished heart rate. Circulation. 1984;69(2):203–13. doi: 10.1161/01.cir.69.2.203. [DOI] [PubMed] [Google Scholar]
- 17.Salvadori A, Fanari P, Palmulli P, et al. Cardiovascular and adrenergic response to exercise in obese subjects. J Clin Basic Cardiol. 1999;2:229–236. [Google Scholar]
- 18.Sidney S, Haskell WL, Crow R, et al. Symptom-limited graded treadmill exercise testing in young adults in the CARDIA study. Med Sci Sports Exerc. 1992;24(2):177–83. [PubMed] [Google Scholar]
- 19.Sidney S, Jacobs DR, Jr, Haskell WL, et al. Comparison of two methods of assessing physical activity in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Epidemiol. 1991;133(12):1231–45. doi: 10.1093/oxfordjournals.aje.a115835. [DOI] [PubMed] [Google Scholar]
- 20.Sidney S, Sternfeld B, Haskell WL, Jacobs DR, Jr, Chesney MA, Hulley SB. Television viewing and cardiovascular risk factors in young adults: the CARDIA study. Ann Epidemiol. 1996;6(2):154–9. doi: 10.1016/1047-2797(95)00135-2. [DOI] [PubMed] [Google Scholar]
- 21.Sidney S, Sternfeld B, Haskell WL, Quesenberry CP, Jr, Crow RS, Thomas RJ. Seven-year change in graded exercise treadmill test performance in young adults in the CARDIA study. Cardiovascular Risk Factors in Young Adults. Med Sci Sports Exerc. 1998;30(3):427–33. doi: 10.1097/00005768-199803000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol. 2001;37(1):153–6. doi: 10.1016/s0735-1097(00)01054-8. [DOI] [PubMed] [Google Scholar]
- 23.Thompson WR, Gordon NF, Pescatello LS, American College of Sports Medicine . ACSM's Guidelines for Exercise Testing. 8th ed Lippincott Williams & Wilkins; Philadelphia(PA): 2010. p. 160. [Google Scholar]
- 24.Thyagarajan B, Jacobs DR, Jr, Apostol GG, et al. Longitudinal association of body mass index with lung function: the CARDIA study. Respir Res. 2008;9:31. doi: 10.1186/1465-9921-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 2.
Text. Estimated maximum heart rate after deleting tests that are presumably less than full effort
Methods
Supplemental Paragraph Number 1 Including tests that represent less than full effort may impede assessment of the association of age and observed MHR. We explored several strategies for forming restriction sets that eliminated tests that were less than full effort: 1) If RPE was low, the participant did not perceive working at full effort during the treadmill test; as an example of RPE-based restriction, we studied a restriction set that eliminated any test with RPE <15. 2) Tests of short duration may not represent full effort; we studied a duration-based restriction set that excluded those who did not finish stage 2 of the test, and for another excluding those who did not finish stage 3. 3) We formed restriction sets by excluding tests with low age-specific MHR, using a “trimline”. One such “trimline” strategy excludes tests with MHR <85% of predicted MHR using the Tanaka formula 208- 0.7*age, as depicted in Supplemental Digital Content 4. This restriction eliminates tests in 18 year olds with MHR < 166 beats/minute. The cutpoint for exclusion declines to 147 at age 50 by 0.7 beats/minute per year of age. We designate this “trimline” by the cutpoints at age 18 and at age 50, that is trimline (166,147).
Supplemental Paragraph Number 2 Trimline (166,147) makes exclusion proportional to predicted MHR at each age. We explored 600 additional non-proportional “trimlines” in order to see whether a linear age-MHR association emerged in this CARDIA dataset similar to the equations reported by Tanaka and Gellish (8, 22). This exploration consisted of examining the repeated measures regression of MHR on quadratic age for each trimline (X, Y), with X (the cutpoint at age 18) ranging from 160 to 189 and Y (the cutpoint at age 50) ranging from 120 to 139 (30 × 20 = 600 “trimlines” in total). We recorded size and statistical significance of the coefficient for age2, with focus on a cutoff line that yielded a small and nonsignificant coefficient for age2 (indicating a linear age-MHR relationship), then repeated the regression omitting the age2 term to characterize the linear solution.
Interpretation
Supplemental Paragraph Number 3 We further studied the shape of the eMHR curve under several restriction strategies based on RPE, highest stage of the treadmill test attained, or trimming using a percent of the Tanaka age-predicted MHR (eg, trimline (166,147)) (see Table, Supplemental Digital Content 3, which demonstrates prediction of maximum heart rate (MHR) from age in all available data and in some restriction sets obtained by different trimming methods; see Figure, Supplemental Digital Content 4 which shows distribution of MHR by age after excluding those with values below the 85% TANAKA estimate of MHR (eMHR)). All shapes were quadratic. We explored a wide range of non-proportional “trimlines” and found that many yielded a linear slope similar to what was reported previously (8,22). For example, linearity of the MHR age relationship was maintained for the age 18 cutoff 180-186 combined with any age 50 cutoffs in the range 120-139; the age slope was close to (−0.7) for all these trimlines. Specifically, the trimline (182,130) yielded eMHR=203-0.70*age. However, further adjustment for baseline characteristics that may have been involved in selection bias, including sex, race, BMI, physical activity, smoking status, lung function and treadmill test duration, restored a quadratic association with quadratic coefficient −0.003. We concluded that we never know what proportion of a true maximal test was performed; even a person achieving a relatively high MHR might have achieved a relatively low proportion of true MHR. Therefore we were not able to distinguish less than full effort from full effort tests, and we deemed restriction strategies for all but a very few low level tests not to be helpful in understanding MHR.
List of Supplemental Digital Content
Supplemental Digital Content 1. Table showing number of people excluded from the treadmill tests according to different criteria in CARDIA study. doc.
Supplemental Digital Content 2. Text explaining several strategies for forming restriction sets that eliminated tests that might be less than full effort. doc.
Supplemental Digital Content 3. Table demonstrating prediction of maximum heart rate (MHR) from age in all available data and in some restriction sets obtained by different trimming methods. doc.
Supplemental Digital Content 4. Figure showing distribution of MHR by age after excluding those with values below the 85% TANAKA estimate of MHR (eMHR). tif.
Supplemental Digital Content 5. Table showing the equations for year 0, year 0 and 7, and then for year 0, 7, and 20 with multiple baseline variables. doc.
Supplemental Digital Content 4.
Figure. Distribution of Maximum Heart Rate (1 standard deviation error bars) of treadmill tests by age at examination after excluding those with values below the 85% eMHR as predicted by Tanaka formula, 208-0.7*age (trimline (166,147)).
Supplemental Digital Content 1.
Table. Number of people excluded from the treadmill tests according to different criteria in the CARDIA study.
Supplemental Digital Content 3.
Table. Prediction of MHR from age in all available data and in some restriction sets obtained by different trimming methods.
Supplemental Digital Content 5
Table. The predicting equation of Maximum Heart Rate at year 0, year 0 and 7, and year 0, 7, and 20 from multiple baseline variables.