Abstract
Polychlorinated biphenyls (PCBs) are present in the environment as complex mixtures, which make it challenging to identify PCB congeners that may be subject to active transport processes. Here we employ a transgenic mouse model in combination with multivariate analyses to investigate if chiral PCBs 91, 95, 132, 136, 149, 174, 176 and 183 are subject to active (enantioselective) transport by multidrug resistance (MDR) transporters. A synthetic PCB mixture containing these congeners was administered orally to female FVB or mdr1a/1b knockout mice. Due to the short half-life of chiral PCB congeners, mice were euthanized after 24 hours and PCB concentrations and enantiomeric fractions were determined in selected tissues and excreta. Principal component analysis did not reveal differences between wild-type and mdr1a/1b knockout mice. However, Hotelling T2-test revealed significantly lower PCB concentrations and a more pronounced enantiomeric enrichment in the adipose tissue of mdr1a/1b knockout mice. These differences are due to higher body weights and higher fecal fat contents of mdr1a/1b knockout mice. Analysis of the enantiomeric fractions of PCBs 91, 95, 136, 149 and 174 showed a significant enantiomeric enrichment for all five congeners in wild-type and mdr1a/1b knockout mice. Overall, by studying a PCB mixture in a transgenic mouse model in combination with a multivariate data reduction approach, PCBs 91, 95, 136, 149 and 174 could be excluded as substrates of multidrug resistance transporters 1a/b.
Keywords: polychlorinated biphenyls; enantiomeric fraction; atropisomers; fecal fat content; multivariate analysis; chemometrics; principal component analysis; Hotelling T2-test; transgenic mice; active transport, multidrug resistance transporters
1. Introduction
Polychlorinated biphenyls (PCBs) were produced as complex mixtures for a large number of technical applications and are still used in transformers and capacitors in the United States (Robertson and Hansen, 2001). Their widespread use, persistence and semi-volatile character have resulted in worldwide contamination. Physicochemical characteristics, such as lipophilicity and stability towards biological and thermal degradation, contribute to their accumulation in the food chain, thus raising concerns about human health effects. PCBs have been implicated in a number of human disease processes such as carcinogenesis and arteriosclerosis (Robertson and Hansen, 2001). Many questions regarding PCB disposition and toxicity are still unanswered, partly because technical PCB products contain approximately 130 of the 209 possible PCB congeners. Furthermore, PCBs are not a homogenous group of chemicals and differ in their physicochemical and biological properties.
A number of observations suggest that, in addition to passive diffusion (Kelly et al., 2004), some PCB congeners may be subject to active transport processes (i.e., transport processes that transport a xenobiotic against a concentration gradient and require the expenditure of energy). For example, several studies have shown that total PCB concentrations in the brain of wildlife and humans are lower than expected based on the lipid content in the brain (Hayes, 1975; Aguilar, 1985; Ness et al., 1994; Bachour et al., 1998). In addition, selective enrichment of individual PCB congeners has been reported in certain tissues in rodents. For example, PCB 169 does not accumulate in the brain of rats (Saghir et al., 2000) and several higher chlorinated PCB congeners are enriched in the spleen of rats (Kania-Korwel et al., 2005).
Multidrug resistance (MDR) transporters are plasma membrane-bound, adenosine-5'-triphosphate (ATP)-dependent, phosphorylated glycoproteins that prevent the absorption of xenobiotics from the gastrointestinal tract or their transfer from blood to the brain (Klaassen and Lu, 2008). The binding of xenobiotics to MDR transporters largely depends on their lipophilicity and molecular mass (Bain et al., 1997), and substrates of MDR transporters are substrates for cytochrome P-450 3A enzymes and vice versa (Chawla et al., 2001; Abu-Qare et al., 2003). Because of these structure-activity relationships MDR transporters are likely candidates for active transporters of PCBs. To date, only two PCB congeners have been investigated as substrates for MDR transporters in vivo (Tampal et al., 2003). In that study, PCBs 77 and 153 were not subject to active transport in mdr1a knockout mice.
We herein investigate the disposition of a synthetic PCB mixture in female mdr1a/b knockout (MDR) versus FVB wild-type (WT) mice and explore the potential role of MDR transporters in the (enantioselective) tissue distribution of individual PCB congeners. The synthetic PCB mixture contained eight environmentally relevant chiral PCB congeners (i.e., PCBs 91, 95, 132, 136, 149, 174, 176 and 183) in the ratio of a synthetic mixture designed to approximate the PCB profile of fish from the Fox River in Wisconsin, USA (Kostyniak et al., 2005). PCB congener profiles and enantiomeric fractions were determined in abdominal adipose tissue, brain, liver, intestines and kidneys 24 hours after PCB administration. These tissues were selected because mdr1-type P-glycoproteins are expressed in these tissues in mice (Abu-Qare et al., 2003) or because they are storage tissues for PCBs (Matthews and Dedrick, 1984). Subsequently, the data were analyzed using multivariate data reduction tools to identify PCB congeners with different tissue concentrations and/or enantiomeric fractions among treatment groups or tissues.
2. Experimental
2.1. Reagents and materials
Florisil (60-100 mesh), silica gel (70-230 mesh), absolute ethanol (200 proof, 99.5%), dimethylsulfoxide (anhydrous, 99.9%), hydrochloric acid, potassium hydroxide, potassium chloride, phosphoric acid, sodium chloride, sodium sulfate, sulfuric acid, tetrabutylammonium sulfite and pesticide grade solvents were purchased from Fisher Scientific (Pittsburg, PA, USA). Corn oil was purchased from Sigma-Aldrich (St. Louis, MO, USA). 3,5-Dichlorobiphenyl (PCB 14), 2,4,6-trichlorobiphenyl (PCB 30), 2,3,5,6-tetrachlorobiphenyl (PCB 65), 2,2’,3,4’,6-pentachlorobiphenyl (PCB 91), 2,2’,3,5’,6-pentachlorobiphenyl (PCB 95), 2,2’,3,3’,4,6’-hexachlorobiphenyl (PCB 132), 2,2’,3,4’,5’,6’-hexachlorobiphenyl (PCB 149), 2,3,4,4’,5,6-hexachlorobiphenyl (PCB 166), 2,2’,3,3’,4,5,6’-heptachlorobiphenyl (PCB 174), 2,2’,3,3’,4,6,6’-heptachlorobiphenyl (PCB 176), 2,2’,3,4,4’,5’,6,-heptachlorobiphenyl (PCB 183), 2,2’,3,4,4’,5,6,6’-octachlorobiphenyl (PCB 204), 4-hydroxy-2’,3,3’,4’,5,5’-hexachlorobiphenyl (4-OH-PCB 159) and 3-methylsulfonyl-4-methyl-2’,3’,4’,5,5’-pentachlorbiphenyl (3-MeSO2-4-Me-PCB 87) were obtained from AccuStandard (New Haven, CT, USA) in > 98 % purity. 2,2’,3,3’,6,6’-Hexachlorobiphenyl (PCB 136) was synthesized as described previously (Kania-Korwel et al., 2006; Shaikh et al., 2006) and its purity determined using GC-FID (> 99% based on relative peak area).
2.2. Preparation of the synthetic PCB mixture for oral administration
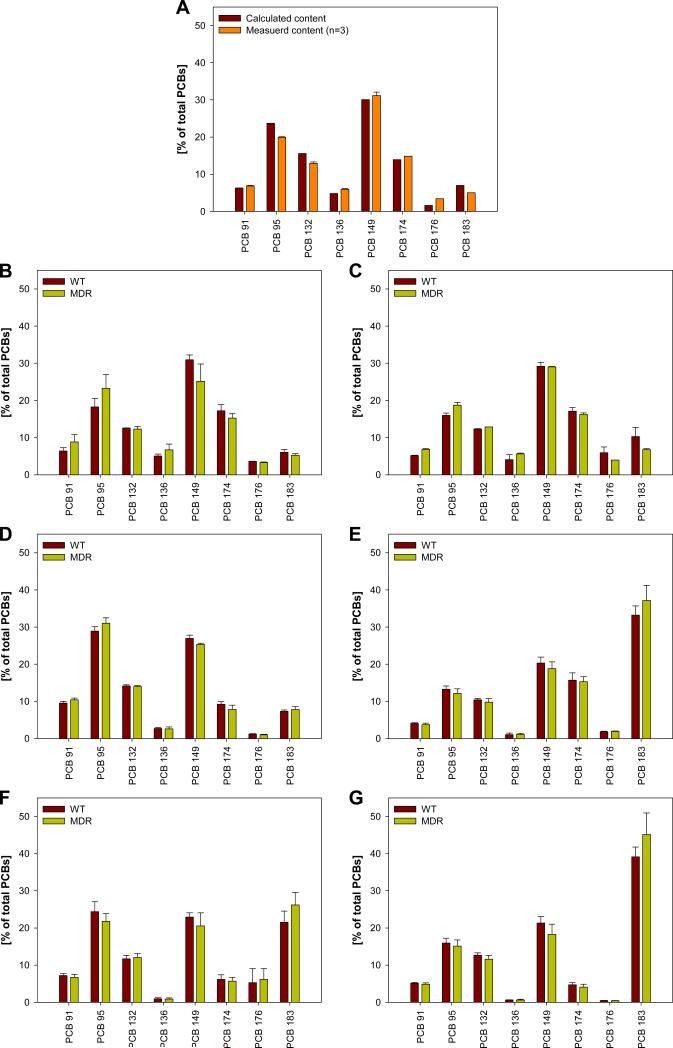
The ratio of the chiral PCB congeners in the synthetic mixture was calculated based on the composition of the synthetic Fox River mixture (Aroclor 1242 : Aroclor 1248 : Aroclor 1254 : Aroclor 1260 = 35 : 35 : 15 : 15, w/w) using published PCB profiles (Frame et al., 1996). The mixture of the chiral PCBs, containing 6.3 % of PCB 91, 23.7 % of PCB 95, 12.6 % of PCB 132, 4.8 % of PCB 136, 30.1 % of PCB 149, 13.9 % of PCB 174, 1.6 % of PCB 176 and 7.0 % of PCB 183 (w/w), was dissolved in corn oil to give a solution with a total PCB concentration of 5 mg/ml (5.45 mg/g). The profile of the target mixture and the actual PCB profile (based on gas chromatographic analysis of the mixture, see Section 2.7) are shown in Figure 1A and are in good agreement with each other (r2 = 0.98; similarity coefficient cos θ = 0.99; for the definition of cos θ see Magar et al., 2005).
Figure 1.
PCB congener profile of A) the calculated and measured profile of the synthetic PCB mixture administered orally in corn oil to the animals in comparison to the profiles of the B) feces samples collected between 0-12 h, C) feces samples collected between 12-24 h, D) adipose tissue, E) brain, F) blood, and G) liver of the WT and MDR mice 24 hours after oral administration of the exposure mixture.
2.3. Animal treatment
All animal experiments and procedures were approved by the Institutional Animal Care and Use Committee of the University of Iowa. Thirteen female mdr1a/b knockout (MDR) and sixteen FVB wild-type (WT) mice, 6 weeks of age, were obtained from Taconic (Germantown, NY, USA). The animals were allowed to acclimatize for 2 weeks and given ad libitum access to diet and water throughout the entire study. The weight of the MDR mice (23.1±1.9 g) was significantly higher than the weight of WT mice (21.6±1.4 g). The animals were randomly divided into treatment and control groups, and the WT (n=8) and MDR (n=7) treatment groups received a single dose of the PCB mixture in corn oil (50 mg/kg body weight) administered by oral gavage. The WT (n=8) and MDR (n=6) control groups received the vehicle (corn oil, 10 ml/kg body weight) alone. Animals were housed in metabolic cages, and feces and urine were collected after 12 and 24 hours. Due to the short half-life of most chiral PCBs, mice were euthanatized 24 hours after PCB administration by asphyxiation with carbon dioxide followed by cervical dislocation. Blood was collected by cardiac puncture and tissues (abdominal adipose tissue, brain, liver, intestines and kidneys) were excised en bloc and their wet weight was determined. A significant decrease in body weight was observed for all treatment groups after 24 hours, most likely because the animals were housed in metabolism cages (Supporting Material, Table S1). However, no significant changes in organ weights were noted due to PCB treatment. The blood, urine and feces samples were stored in glass vials and tissue samples were stored in aluminum foil. All samples were maintained frozen at −80°C until PCB extraction and analysis.
2.4. Extraction of PCBs and lipids from tissue, feces, urine and whole blood samples
A modified extraction method described by Jensen and co-workers was used (Jensen et al., 2003; Kania-Korwel et al., 2008c; Kania-Korwel et al., 2008e). In short, the samples were placed in a 10 ml glass tube, weighed and spiked with PCB 166 (100 ng) as surrogate standard. In addition, 4-OH-PCB 159 (137 ng) and 3-MeSO2-4-Me-PCB 87 (96 ng) were added as surrogate standards to allow the future analysis of the PCB metabolite fractions isolated as part of this study. The tissue and feces samples were homogenized with an IKA homogenizer (Wilmington, NC, USA) in a mixture of 2-propanol:diethyl ether (5:2 v/v, 3.5 ml) for 1 min at 24000 r/min, followed by extraction of the homogenate with hexane:diethyl ether (9:1 v/v, 2.5 ml). The organic extracts were combined and washed with phosphoric acid (0.1 M solution in 0.9% aqueous sodium chloride, 5 ml). The organic phase was separated, and the aqueous phase was re-extracted with hexane:diethyl ether mixture (9:1 v/v, 1 ml). The urine and whole blood samples were denaturated with hydrochloric acid (6 M, 1 ml) and 2-propanol (2 ml) and extracted with hexane:methyl tert-butyl ether (1:1 v/v, 5 ml) and hexane (3 ml). The organic extracts were combined and washed with a saline solution (1% aqueous potassium chloride, 5 ml). Afterwards, extracts were concentrated to dryness and the extractable lipid content was determined gravimetrically in each sample (Supporting Material, Table S2). The nonvolatile residues present in the solvent blanks ranged from 0.5 to 3.7 mg, with an average of 2.2±1.2 mg (n=14).
2.5. Separation of PCBs from PCB metabolites
Tissue extracts were reconstituted in hexane (4 ml) and PCBs were separated from their metabolites using their different physicochemical properties based on a procedure described previously (Hovander et al., 2006; Kania-Korwel et al., 2008c). In short, the fraction containing the OH-PCBs was separated from the fraction containing PCBs and MeSO2-PCBs by partitioning into potassium hydroxide (0.5 M, 50% ethanol in water, 2 ml). Subsequently, PCBs were separated from the MeSO2-PCB fraction by partitioning the MeSO2-PCBs into dimethyl sulfoxide (anhydrous, 0.5 ml). The metabolite fractions were stored at −20°C for future analyses. The PCB fraction was cleaned-up and analyzed as described below.
2.6. Clean-up of extracts and lipid removal
The PCB fractions were subjected to a sulfuric acid clean-up before GC-ECD analysis as described previously (Bunaciu et al., 2007; Kania-Korwel et al., 2007b). Further clean-up of tissues and feces extracts was achieved using SPE columns containing silica gel impregnated with concentrated sulfuric acid (2:1 w/w, 1.0 g), with 0.2 g activated silica gel at the bottom and hexane (10 ml) as an eluent. Prior to GC-ECD analysis, the solvent was exchanged to isooctane and PCB 30 (25 ng) and PCB 204 (25 ng) was added as internal standard.
2.7. Gas chromatography
The PCB concentration in all samples was determined with an Agilent 6890N gas chromatograph equipped with a 63Ni micro-electron-capture detection (μECD) system and a SLB-5MS capillary column (60m × 0.25 mm I.D., 0.25 μm film thickness; Supelco, St. Louis, MO, USA) as described earlier (Bunaciu et al., 2007). The injector and detector temperatures were 280 and 340 °C, respectively. The following temperature program was used for quantification of PCBs: 120 °C for 1 min, 10 °/min to 300 °C, hold for 5 min. The concentrations of PCBs in each tissue were calculated based on tissue wet weight, percentage of the total PCBs administered dose and tissue lipid content (Supporting Material, Tables S4 to S6).
2.8. Enantioselective gas chromatography
Enantiomeric fractions of selected PCBs were determined using enantioselective gas chromatography as described previously (Kania-Korwel et al., 2006). The injector and detector temperatures were set to 250 °C. The oven temperature program was as follows: 150 °C for 2 min, 0.2 °C /min to 185 °C, l5 °C/min to 200 °C, hold for 10 min (Wong and Garrison, 2000). The enantiomeric fraction (EF) (Harner et al., 2000) was calculated as EF = Area(1)/(Area(1) + Area(2)) for PCBs with an unknown elution order of the atropisomers (PCB 91 and PCB 95) or as EF = Area(+)/(Area(+) + Area(−)) for PCBs with a known elution order of the atropisomers (Haglund and Wiberg, 1996). Area(1) and Area(2) are the peak area of the first and second eluting atropisomer; Area(+) and Area(−) are the peak area of the respective (+)- and (−)-enantiomer. PCBs 91, 95, 136, 149 and 174 atropisomers were partially separated (Rs=0.56-0.83) on the Chirasil-Dex column employed in this study. The EF values of the racemic PCBs were 0.49±0.01, 0.50±0.01, 0.51±0.01, 0.50±0.01, and 0.49±0.01 for PCBs 91, 95, 136, 149 and 174 (n=14), respectively. Unfortunately, the resolution and EF values of PCBs 132 and 176 could not be determined because their atropisomers co-eluted on this column. PCB 183 atropisomers were not separated on the Chirasil-Dex column and the enantiomeric enrichment of PCB 183 was not further investigated because no differences between treatment groups were observed for any PCB congener under investigation.
2.9. Quality control
The limits of detection (LOD) for PCBs were calculated based on the method blanks (Kania-Korwel et al., 2007b), with a range of 0.9 to 8.8 ng. The limits of quantification (LOQ) were calculated as 10-times the LOD and ranged from 9.2 to 88.3 ng. The total PCB concentrations in tissues and excreta from control animals were 19.8±16.1 ng/g wet weight for tissues and feces samples and 26.3±10.3 ng/g wet weight for blood and urine samples (Supporting Material, Table S3). The mean recovery rate of the surrogate standard (PCB 166) was 103%±12% for tissues and feces samples (n=103) and 92%±8% for blood and urine samples (n=28). Six feces samples containing an impurity co-eluting with the recovery standard were not included in the calculation of the mean recovery rate. PCB concentrations were corrected for recovery rates below 100%.
2.10. Statistical analysis
Multivariate statistical analyses were performed using R open source statistical software (version 2.7.0, http://www.r-project.org). Principal component analysis was used to observe groupings of tissue samples based on the concentrations or EF values of the PCB congeners analyzed in this study. Two principal components were retained for both datasets. The loadings of the PCA are illustrated in the Supporting Material (Figures S1-S3). Subsequently, Hotelling T2 test (with F distribution for test statistics) was employed to analyze for differences between treatment groups for each congener, considering all tissue concentrations or EFs at once. In all cases, the statistical tests were performed at α=0.05. Finally, univariate statistical analyses were preformed using the STATISTICA data analysis software system, version 8.0 (StatSoft, Inc., Tulsa, OK, USA). Unless stated otherwise, data are presented as mean ± standard deviation. Differences in body and organ weight between treatment and control groups and differences in PCB concentrations and EF values among tissues between treatment groups were analyzed with ANOVA with Tukey multiple comparison test. Differences in the EF values and PCB concentrations among treatment groups were tested using a two-sample t-test (α=0.05). Differences in the EF values in tissues and the racemic standards were tested using a one-sample, one-sided t-test (α=0.05). For all comparisons, p=0.05 was considered to be statistical significant. The normal distribution of data was verified using the Shapiro-Wilk test and outliers were removed using the Grubbs test for outliers. For unknown reasons one WT mouse had consistently higher PCB concentrations compared to other animals from the WT-treatment group and was not included in further analyses.
3. Results and discussion
The present study simultaneously screened a series of PCB congeners as substrates of mdr1a/b transporters by orally administering a PCB mixture to MDR or WT mice. Subsequently, differences in the disposition of chiral PCB congeners between MDR or WT mice were investigated in selected tissues (i.e., adipose, blood, brain, intestines, kidney and liver) and excreta using multivariate data reduction tools (Kania-Korwel et al., 2005; Kania-Korwel et al., 2007b). One drawback of this in vivo approach is that MDR mice differ from WT mice not only in the expression of mdr1a/b genes. For example, MDR mice may express certain cytochrome P-450 enzymes differently (Mankowski et al., 2000; Schuetz et al., 2000). The present study also shows that female MDR and WT mice have i) significantly different body weights and ii) excrete different amounts of fat with their feces (Supporting Material, Table S2). Therefore, it is important to realize the limitations of our approach and that, as discussed below, differences in the (enantioselective) tissue distribution of PCBs are not necessarily only a result of active transport by MDR transporters. Despite these limitations, the use a PCB mixture in a transgenic animal model combined with multivariate analysis tools holds the promise of facilitating the screening of a comparatively large number of potential substrates (and inhibitors) of MDR transporters.
3.1. Comparison of PCB profiles: Differences between treatment groups
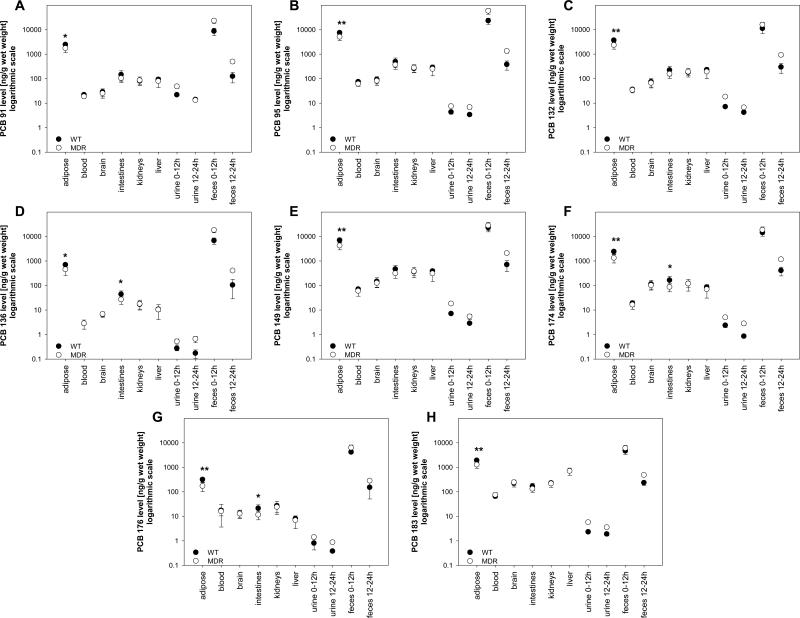
PCA of the PCB profiles depicted in Figure 1 showed no clear grouping according to treatment groups (Figure 2). Similar groupings were observed when the PCA was performed with lipid adjusted PCB concentrations (Supporting Material, Figure S2). However, multivariate analysis of the PCB concentrations in all tissues using the Hotelling T2-test revealed some differences between both treatment groups. Specifically, we observed a significant difference in PCB 95 and PCB 149 concentrations between MDR and WT treatment groups (p < 0.05), independent of how the PCB concentrations were expressed (Supporting Material, Tables S4-S6). Further univariate analysis of the PCB concentrations suggested that these differences between treatment groups are due to significantly higher PCB 95 and 149 concentrations in the adipose tissue in WT mice (Figure 3). Univariate analysis showed that, in addition to PCBs 95 and 149, the other six PCB congeners also had higher concentrations in the adipose tissue of WT mice (Supporting Material, Tables S4-S6). These differences in PCB concentrations in the adipose tissue between MDR and WT mice is due to two factors. First, MDR mice had a significantly higher body weight, thus resulting in a dilution of the dose (Hansen et al., 1977; Drouillard, 2003). Second, a significantly higher percentage of the total dose was excreted by MDR mice compared to WT mice (see below).
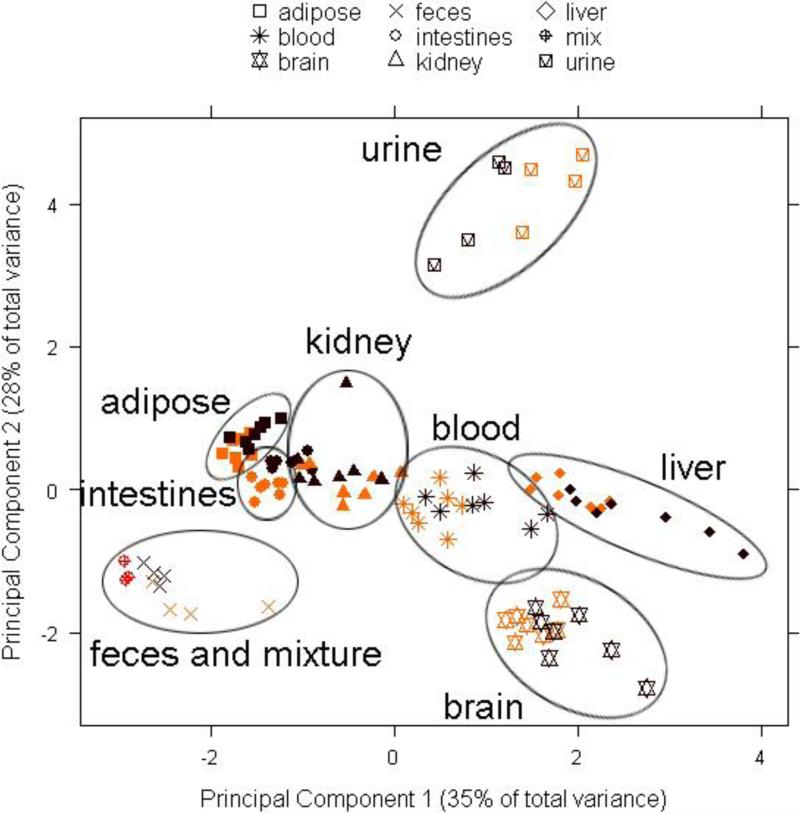
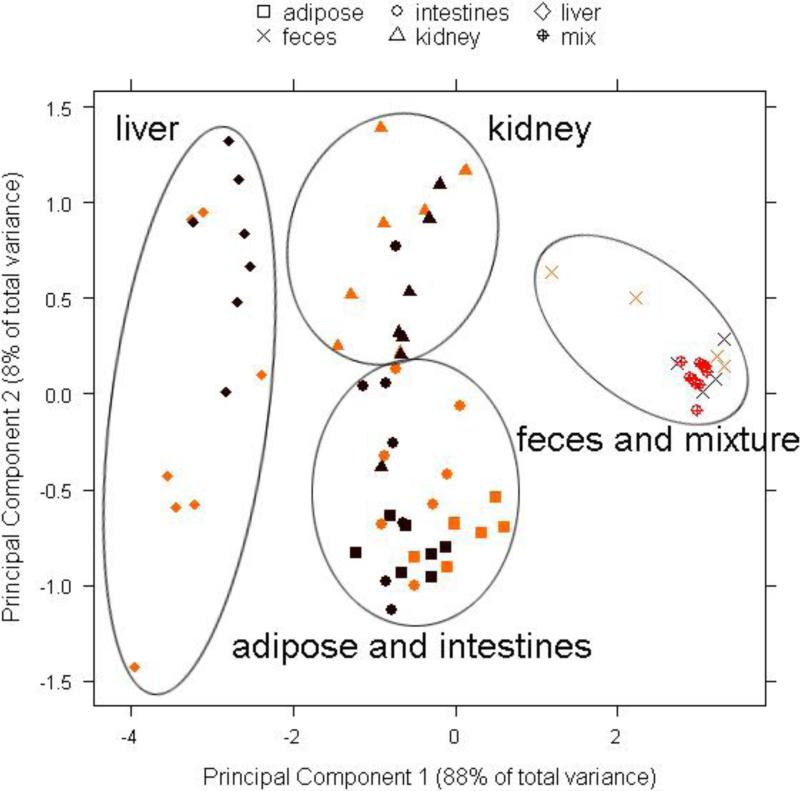
Figure 2.
Projections of principal component 1 versus principal component 2 from the principal component analysis of the concentrations of eight chiral PCB congeners (PCB 91, PCB 95, PCB 132, PCB 136, PCB 149, PCB 174, PCB 176 and PCB 183) in tissues and excreta from WT (yellow) and MDR (brown) mice, normalized for the sum of their concentrations (ng/g wet weight). The synthetic PCB mixture (mix) is shown in orange.
Figure 3.
Comparison of concentrations of the PCB 91 (A), PCB 95 (B), PCB 132 (C), PCB 136 (D), PCB 149 (E), PCB 174 (F), PCB 176 (G) and PCB 183 (H) in tissues, urine and feces of the WT and MDR mice after administration of a single oral dose of chiral PCB mixture (* different from MDR mice; t-test at α=0.05, p<0.05; ** different from MDR mice; t-test at α=0.05, p<0.01).
3.2. Comparison of PCB profiles: Differences between tissues
Although the PCA in Figure 2 did not show a separation by treatment group, it revealed a clear grouping of tissues and excreta by two principal components accounting for 63% of the variability in the dataset. Principal component 1 (PC1) separated brain and liver from adipose tissue, intestines, kidney and feces, whereas principal component 2 (PC2) separated urine from all other tissues. The original PCB mixture was grouped with feces samples.
Analysis of the loading from the PCA indicated that tissues separated by PC1 differ in the relative concentrations of PCBs 95, 136, 149 and 183, whereas PC2 separated urine from all tissues because of different concentrations of PCBs 91, 132 and 174. The PCB profiles (Figures 1) show that the percentage of PCB 183 was higher, whereas the percentages of PCBs 95, 136 and 149 were lower in blood, brain and liver (Figures 1E to 1G) compared to the original, synthetic PCB mixture (Figure 1A). These differences in the congener profiles were also reflected in significantly higher PCB 183 concentrations in the liver of both MDR and WT mice compared to all other tissues, with exception of the adipose (Figure 3H). This is not surprising because, in contrast to the other PCB congeners in the synthetic mixture, PCB 183 does not have vicinal H-atoms and is substituted in both para positions. As a consequence, PCB 183 is not readily metabolized and, based on these structure-activity relationships, is more persistent compared to the other congeners (Kaminski et al., 1981; Brown, 1994; Kannan et al., 1995). Overall, the differences in the PCB profiles most likely reflect the longer half-life of PCB 183 compared to the half-lives of the other PCB congeners in the mixture.
All PCB congeners had the significantly higher concentrations in adipose tissue compared to all other examined matrices (Figure 3), independent of how the PCB concentrations were expressed (Supporting Material, Tables S8-S13). This observation is expected because adipose is the storage tissue of PCBs (Matthews and Dedrick, 1984). Finally, PCB concentrations in urine were low compared to tissue PCB concentrations (Figure 3). Similarly, several animal studies have shown that urinary excretion is a minor route of excretion of parent PCB 136 in rats (Matthews and Tuey, 1980; Birnbaum, 1983), dogs and monkeys (Sipes et al., 1982).
3.3. Comparison of PCB profiles: Fecal PCB concentrations
As mentioned above, feces samples were grouped separately from all other tissues (Figure 2). Comparison of PCB profiles showed that the original mixture and the fecal PCB profiles from both time points were essentially identical (r2 = 0.95 to 0.99; cos = 0.98 to 1.00), both for the MDR and WT treatment group (Figure 1A to 1C). This is not surprising because a significant percentage of the total dose (%TD) of each PCB congener was excreted within the first 12 hours after PCB administration without being absorbed (8.61 to 31.4 %TD; see Supporting Material, Table S4). A smaller but still considerable %TD was excreted between 12-24 hours (0.09 to 0.76 %TD). This is in contrast to earlier studies from our laboratory showing that only <2 %TD of PCB 136 was excreted in C57Bl/6 mice within a 3 day period (Kania-Korwel et al., 2007a; Kania-Korwel et al., 2008a). These differences in the fecal PCB excretion may be due to the different mouse strains employed (i.e., FVB versus C57Bl/6 mice) or a result of the different mode of PCB administration (i.e., oral gavage of a corn oil solution versus administration using a cookie). Because the extractable lipid content in feces decreased 2.6-fold from the 0-12 hour to the 12-24 hour time point (Supporting Material, Table S2), it is most likely that non-absorbed corn oil acted as a PCB reservoir in the gastrointestinal tract, thus resulting in higher fecal PCB concentrations.
One intriguing finding of this study is that PCB concentrations in feces differed between treatment groups (Supporting Material, Tables S4 to S6). MDR mice typically excreted more PCBs with the feces than WT mice (Figure 3). Based on %TD, MDR mice excreted 1.3- to 2.1-times the amount of the individual PCB congeners within the first 12 hours and 1.9 to 4.0-times more in the following 12 hours than WT mice. Similar trends were observed when fecal PCB concentrations were expressed based on tissue wet weight, with MDR mice excreting approximately 1.5 to 4.0-times more of the different PCB congeners than WT mice, respectively. However, only 1.0 to 2.7-times higher fecal PCB concentrations were observed for lipid adjusted PCB concentrations. Thus, the higher fecal PCB concentrations in MDR mice are in part due to 1.5-times higher fecal lipid level, an important factor determining fecal PCB content (Gobas et al., 1993). However, other, currently unknown processes may also play a role because the lipid adjusted PCB concentrations were also slightly higher in the MDR treatment group.
3.4. Enantiomeric enrichment in tissues and excreta
Chiral signatures, i.e., differences in the extent of the enantiomeric enrichment of PCBs, have been used to study sources and environmental transport of this important group of environmental contaminants (Asher et al., 2007; Jamshidi et al., 2007). Similarly, we have proposed that chiral signatures can be used to distinguish physicochemical from biological disposition processes in vivo (Kania-Korwel et al., 2008a). Only biological processes, such as active transport and/or biotransformation, can change chiral signatures due to enantioselective interaction of PCB atropisomers with chiral macromolecules (i.e., proteins), whereas physical processes such as passive diffusion can not.
As shown in Figures 4, all tissues and blood showed a significant enantiomeric enrichment compared to the EF value of the racemic PCB congeners administered to the animals. An enantiomeric enrichment was also observed for PCBs 91 (0.21≤EF≤0.39) and 149 in urine (0.57≤EF≤0.71). PCB 174 was enantiomerically enriched in urine in samples collected between 0-12 hours after PCB administration (0.37≤EF≤0.42), but was below the detection limit in the 12-24 hour urine sample. Some enantiomeric enrichment was observed with PCBs 91, 95 and 149 in the 12-24 hour feces samples; however, all PCB congeners were essentially racemic in the 0-12 hour sample, probably because they are not absorbed in the gastrointestinal tract due to the high fecal fat content after PCB administration in corn oil. Similar observations have been reported by earlier studies in mice (Kania-Korwel et al., 2007a; Kania-Korwel et al., 2008a) and rats (Norström et al., 2006).
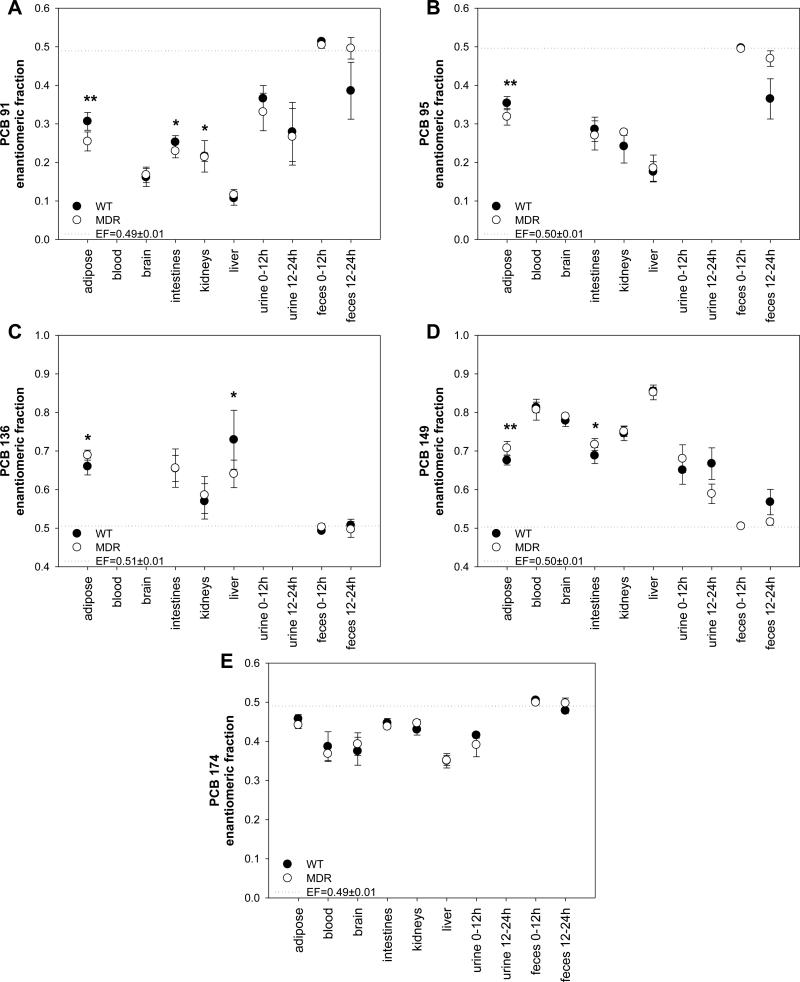
Figure 4.
Comparison of the enantiomeric fraction of the PCB 91 (A), PCB 95 (B), PCB 136 (C), PCB 149 (D), PCB 174 (E) in tissues, urine and feces of the WT and MDR mice after administration of a single oral dose of chiral PCB mixture (* different from the respective WT mice treatment group at a α=0.05; p<0.05; ** different from the respective WT mice treatment group at a α=0.05; p< 0.01). The EF of the racemic PCB congeners (represented as a dotted line) was significantly different from the EF values of all tissues and blood (p<0.001). EFs for PCBs 91, 95 and 136 were not determined in blood or brain due to the low detection limit of enantioselective analyses with the Chirasil-Dex column.
The direction of the enantiomeric enrichment was identical for all five chiral PCBs in all samples and in both treatment groups. An enantiomeric enrichment of the (+)-atropisomer of PCBs 136 and 149, the (−)-atropisomer of PCB 174, and the 1st eluting atropisomer of PCBs 91 and 95 (the elution order of both PCBs is unknown) was observed in both WT and MDR mice. Analogously, we have reported an enrichment of (+)-PCB 136 in tissues, blood, feces and urine from C57Bl/6 mice after oral or intraperitoneal administration of racemic PCB 136 (Kania-Korwel et al., 2007a; Kania-Korwel et al., 2007b). Together, these findings suggest that the enantiomeric enrichment of chiral PCBs is independent of the mouse strain. Interestingly, the extent of the enantiomeric enrichment is congener depended. PCBs 91 and 149, followed by PCBs 95 and 136, displayed the most pronounced enantiomeric enrichment, whereas the PCB 174 had the lowest enantiomeric enrichment in all tissues investigated.
3.5. Enantiomeric enrichment of PCBs: Differences between treatment groups
PCA of the EF values of PCBs 91, 95, 136, 149 and 174 in adipose tissue, feces, intestine, kidneys and liver did not show a grouping according to treatment groups (Figure 5), which is in agreement with our PCA of the PCB profiles (Figure 2). However, multivariate analysis of the EF values in tissues using the Hotelling T2-test revealed differences between both treatment groups. Specifically, we observed significant differences in the EF values for PCBs 91, 136 and 149 (p<0.05). In addition, the EF values of PCB 95 tended to be different between both treatment groups (p=0.06), whereas no such trend was noted for PCB 174 (p=0.14). Univariate analysis of the EF values showed that the differences between treatment groups are in part due to significantly different EF values of PCB 91, 95, 136, 149 and 174 in the adipose tissue (Figure 4; Supporting Material, Table S7). Interestingly, the MDR treatment group displayed a more pronounced enantiomeric enrichment in the adipose tissue for all five PCB congeners analyzed.
Figure 5.
Projections of principal component 1 versus principal component 2 from the principal component analysis of the enantiomeric fractions of five chiral PCB congeners (PCB 91, PCB 95, PCB 136, PCB 149 and PCB 174) in selected tissues and excreta from WT (yellow) and MDR (brown) mice. The synthetic PCB mixture (mix) is shown in orange.
For two reasons it is unlikely that the more pronounced enantiomeric enrichment in the adipose tissue of MDR mice is due to enantioselective transport of PCBs by MDR transporters. First, the adipose tissue is not a major site of expression of mdr1a/b genes (Abu-Qare et al., 2003) and, therefore, is not expected to display MDR transporter-dependent changes in the enantiomeric enrichment. Second, if MDR transporters indeed cause the enantiomeric enrichment of PCBs, their absence should result in a less pronounced enantiomeric enrichment in vivo. Therefore, other factors must explain the small differences in the enantiomeric enrichment between MDR and WT treatment groups.
As mentioned above, MRD mice had a significantly higher body weight compared to WT mice and excreted more PCB with the feces, which resulted in lower PCB (adipose) tissue concentrations compared to WT mice. Because lower PCB tissue concentrations appear to result in an increased enantiomeric enrichment of (+)-PCB 136 in C57Bl/6 mice (Kania-Korwel et al., 2007a; Kania-Korwel et al., 2007b), differences in body weight and, ultimately, PCB tissue concentrations may similarly cause a more pronounced enantiomeric enrichment in MDR mice. Alternatively, MDR mice have been reported to display higher cytochrome P-450 concentrations compared to WT mice (Mankowski et al., 2000; Schuetz et al., 2000). Although levels of P-450 enzymes were not determined in the present study, differences in hepatic P-450 levels may also contribute to a more pronounced enantiomeric enrichment in MDR mice. However, a recent study from our laboratory did not show an increased enantiomeric enrichment after induction of cytochrome P-450 enzymes and, thus, does not support the later hypothesis (Kania-Korwel et al., 2008d).
3.6. Enantiomeric enrichment of PCBs: Differences between tissues
We observed a clear separation of selected tissues and feces by two principal components accounting for 95% of the variability in the dataset with the EF values. PC1 separated the liver from adipose, intestine and kidney (Figure 5). In addition, all tissues were separated from feces samples by PC1. This separation was due to the fact that the enantiomeric enrichment of all five PCB congeners was most pronounced in the liver (Figure 4). At the same time, the enantiomeric enrichment was least pronounced in the feces, with all other tissues (i.e., adipose, intestine and kidney) having intermediate EF values. PC2 separated the kidney from adipose and intestine. This difference is primarily due to a comparatively lower enantiomeric enrichment of (+)-PCB 136 in the kidney. In contrast, all other PCB congeners have similar EF values in all three tissues.
The observation that the most pronounced enantiomeric enrichment of all five PCB congeners is observed in the liver is consistent with previous studies in male and female C57Bl/6 mice showing a pronounced enantiomeric enrichment of (+)-PCB 84 (Lehmler et al., 2003) and (+)-PCB 136 (Kania-Korwel et al., 2007a; Kania-Korwel et al., 2007b) in the liver. A likely explanation for the comparatively high enantiomeric enrichment in the liver is enantioselective metabolism of PCBs by cytochrome P-450 enzymes, as proposed by several authors (Harrad et al., 2006; Kania-Korwel et al., 2008b; Warner et al., 2009).
Another factor contributing to the groupings in the PCA are the near racemic EF values of fecal PCBs at both time points. As mentioned above, these near racemic signatures indicate that the majority of PCBs excreted during the first 24 hours after PCB administration are due to unabsorbed PCBs. This observation argues against an active transport process in the gastrointestinal track that causes a preferential absorption of one PCB atropisomer.
3.7. Enantiomeric enrichment of PCBs: Comparison with other mammalian species
The present study determined the EF values of five PCBs in several tissues and excreta in female mice and, thus, allows a comparison of the direction of the enantiomeric enrichment of these PCB congeners in mice with other species. Although an enantiomeric enrichment of PCBs has been reported in rats, the enrichment in these studies is relatively small (Püttmann et al., 1989; Kania-Korwel et al., 2006; Kania-Korwel et al., 2008c). Animals treated intraperitoneally with Aroclor 1254 displayed an enantiomeric enrichment of the first eluting atropisomer of PCB 95 and an enrichment of (+)-PCB 149 in blood and, possibly, lung tissue, which is in agreement with the direction of the enantiomeric enrichment in the present study. However, (−)-PCB 149 was enriched in adipose, skin and liver tissue from these Aroclor 1254-treated rats. Furthermore, a slight enrichment of (−)-PCB 136 was observed in male and female rats treated intraperitoneally with two doses of racemic PCB 136 (Kania-Korwel et al., 2008c), which is also opposite to the enantiomeric enrichment observed in mice. In agreement with the in vivo studies, a metabolism study with recombinant rat cytochrome P-450 2B1 also showed a preferential elimination of (+)-PCB 136 (Warner et al., 2009).
Chiral signatures in human breast milk and tissue samples also reveal some distinct differences in the direction of the enantiomeric enrichment in comparison to mice. An enrichment of the 2nd eluting atropisomer of PCB 91 and (−)-PCB 174 has been reported for breast milk from Switzerland and Spain (Bordajandi et al., 2008), which is opposite of the enrichment observed in this study (Figure 4). The EF values for PCBs 95 and 149 differ among human samples and point to a significant variation of the extent and the direction of the enantiomeric enrichment among individuals. For example, EF values for PCB 95 range from 0.42-0.50 in feces (Harrad et al., 2006) to 0.51-0.75 in liver (Chu et al., 2003). Similarly, EF values for PCB 149 indicate that (−)-PCB 149 is enriched in the liver (EF=0.42-0.50) (Chu et al., 2003), whereas (+)-PCB 149 is enriched at least in some breast milk samples (EF=0.50-0.66) (Bordajandi et al., 2008). In contrast, the 1st eluting atropisomer of PCB 95 (EF<0.5) and (+)-PCB 149 are enriched in female MDR and WT mice.
Several factors may contribute to the differences in the directions of the enantiomeric enrichment in mice compared to other species, such as rats and humans. For example, the enantiomeric enrichment (in humans) may reflect exposure to enantiomerically enriched foodstuffs (e.g., trout). Alternatively, the process responsible for the enantiomeric enrichment may have different selectivity for PCB atropisomers. The large variability in the enantiomeric enrichment in human samples may also be a result of genetic polymorphisms of the enzymes responsible for the enantiomeric enrichment of PCBs.
4. Conclusions
The present study used a combination of multivariate analysis tools to facilitate the statistical comparison of PCB concentrations in tissue samples from MDR and WT mice after oral administration of a PCB mixture. These analyses revealed several differences in the disposition of PCBs between MDR and WT mice. Specifically, we could show that
fecal excretion within 24 hours of PCB administration is primarily due to non-absorbed PCBs,
PCB 183 is selectively retained in the liver,
PCB concentrations and EF values differ significantly between MDR and WT mice due to higher fecal excretion of PCBs and a dilution of the dose in the larger MDR mice and
differences in tissue PCB concentrations and/or EF values are not indicative of active transport of PCB congeners by MDR transporters.
Overall, this approach can be extended to investigate disposition processes of xenobiotics in many other transgenic animal models and potentially facilitate the screening of a large number of compounds in a single animal experiment. One pitfall of this approach is that changes in the expression of a single gene affect the expression of many other genes and ultimately the physiology of the transgenic animals. This, in turn, may alter the disposition of xenobiotics in a manner that is not related to the knock out of a single gene product (e.g., MDR in this study).
Supplementary Material
ACKNOWLEDGEMENTS
The authors gratefully acknowledge Dr. Mary Vore (Graduate Center for Toxicology, University of Kentucky) for advice and helpful discussions. We also thank Drs. Sanjay Telu and Wei Xie (University of Iowa) for their assistance with the animal experiment. The work was supported by grants ES05605, ES013661 and ES012475 from the National Institute of Environmental Health Sciences and PUMS DS Grant No. 501-01-03314429-50445.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abu-Qare A, Elmasry E, Abou-Donia M. A role for P-glycoprotein in environmental toxicology. J Toxicol Environ Health B Crit Rev. 2003;6:279–288. doi: 10.1080/10937400306466. [DOI] [PubMed] [Google Scholar]
- Aguilar A. Compartmentation and reliability of sampling procedures in organochlorine pollution surveys of cetaceans. Residue Rev. 1985;95:91–114. doi: 10.1007/978-1-4612-5132-3_3. [DOI] [PubMed] [Google Scholar]
- Asher BJ, Wong CS, Rodenburg LA. Chiral source apportionment of polychlorinated biphenyls to the Hudson River Estuary atmosphere and food web. Environ Sci Technol. 2007;41:6163–6169. doi: 10.1021/es070763n. [DOI] [PubMed] [Google Scholar]
- Bachour G, Failing K, Georgii S, Elmadfa I, Brunn H. Species and organ dependence of PCB contamination in fish, foxes, roe deer, and humans. Arch Environ Contam Toxicol. 1998;35:666–673. doi: 10.1007/s002449900429. [DOI] [PubMed] [Google Scholar]
- Bain LJ, McLachlan JB, LeBlanc GA. Structure-activity relationships for xenobiotic transport substrates and inhibitory ligands of P-glycoprotein. Environ Health Perspect. 1997;105:812–818. doi: 10.1289/ehp.97105812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum LS. Distribution and excretion of 2,3,6,2',3',6'- and 2,4,5,2',4',5'-hexachlorobiphenyl in senescent rats. Toxicol Appl Pharmacol. 1983;3:262–272. doi: 10.1016/0041-008x(83)90102-3. [DOI] [PubMed] [Google Scholar]
- Bordajandi LR, Abad E, González MJ. Occurrence of PCBs, PCDD/Fs, PBDEs and DDTs in Spanish breast milk: enantiomeric fraction of chiral PCBs. Chemosphere. 2008;70:567–575. doi: 10.1016/j.chemosphere.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Brown JF. Determination of PCB metabolic, excretion, and accumulation rates for use as indicators of biological response and relative risk. Environ Sci Technol. 1994;28:2295–2305. doi: 10.1021/es00062a013. [DOI] [PubMed] [Google Scholar]
- Bunaciu PR, Tharappel JC, Lehmler HJ, Kania-Korwel I, Robertson LW, Srinivasan C, et al. The effect of dietary glycine on the hepatic tumor promoting activity of polychlorinated biphenyls (PCBs) in rats. Toxicology. 2007;239:147–155. doi: 10.1016/j.tox.2007.06.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294:1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- Chu S, Covaci A, Schepens P. Levels and chiral signatures of persistent organochlorine pollutants in human tissues from Belgium. Environ Res. 2003;93:167–176. doi: 10.1016/s0013-9351(03)00016-1. [DOI] [PubMed] [Google Scholar]
- Drouillard KG, Norstrom RJ. The influence of diet properties and feeding rates on PCB toxicokinetics in the ring dove. Arch Environ Contam Toxicol. 2003;44:97–106. doi: 10.1007/s00244-002-1199-y. [DOI] [PubMed] [Google Scholar]
- Frame GM, Wagner RE, Carnahan JC, Brown JF, May RJ, Smullen LA, et al. Comprehensive, quantitative, congener-specific analyses of eight aroclors and complete PCB congener assignments on DB-1 capillary GC columns. Chemosphere. 1996;33:603–623. [Google Scholar]
- Gobas FAPC, McCorquodale JR, Haffner GD. Intestinal absorption and biomagnification of organochlorines. Environ Toxicol Chem. 1993;12:567–576. [Google Scholar]
- Haglund P, Wiberg K. Determination of the gas chromatographic elution sequences of the (+) and (-) enantiomers of stable enantiomeric PCBs on Chirasil-Dex. J High Resol Chromatogr. 1996;19:373–376. [Google Scholar]
- Hansen LG, Welborn ME, Borchard RE, Teske RH, Metcalf RL. Tissue distribution of PCB components in swine and sheep fed three different rations containing Aroclors 1242 and 1254. Arch Environ Contam Toxicol. 1977;5:257–278. doi: 10.1007/BF02220909. [DOI] [PubMed] [Google Scholar]
- Harner T, Wiberg K, Norstrom R. Enantiomer fractions are preferred to enantiomer ratios for describing chiral signatures in environmental analysis. Environ Sci Technol. 2000;S34:218–220. [Google Scholar]
- Harrad S, Ren J, Hazrati S, Robson M. Chiral signatures of PCB#s 95 and 149 in indoor air, grass, duplicate diets and human faeces. Chemosphere. 2006;63:1368–1376. doi: 10.1016/j.chemosphere.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Hayes WJ. Toxicology of pesticides. Williams and Wilkins; Baltimore: 1975. [Google Scholar]
- Hovander L, Linderholm L, Athanasiadu M, Bignert A, Fangstrom B, Kocan A, et al. Levels of PCBs and their metabolites in the serum of residnets of a highly contaminated area in eastern Slovakia. Environ Sci Technol. 2006;40:3696–3703. doi: 10.1021/es0525657. [DOI] [PubMed] [Google Scholar]
- Jamshidi A, Hunter S, Hazrati S, Harrad S. Concentrations and chiral signatures of polychlorinated biphenyls in outdoor and indoor air and soil in a major U.K. conurbation. Environ Sci Technol. 2007;41:2153–2158. doi: 10.1021/es062218c. [DOI] [PubMed] [Google Scholar]
- Jensen S, Haggberg L, Jorundsdottir H, Odham G. A quantitative lipid extraction method for residue analysis of fish involving nonhalogenated solvents. J Agric Food Chem. 2003;51:5607–5611. doi: 10.1021/jf0301201. [DOI] [PubMed] [Google Scholar]
- Kaminski LS, Kennedy MW, Adams SM, Guengerich FP. Metabolism of dichlorobiphenyls by highly purified isozymes of rat liver cytochrome P-450. Biochemistry. 1981;20:7379–7384. doi: 10.1021/bi00529a009. [DOI] [PubMed] [Google Scholar]
- Kania-Korwel I, Hornbuckle KC, Peck A, Ludewig G, Robertson LW, Sulkowski WW, et al. Congener specific tissue distribution of Aroclor 1254 and a highly chlorinated environmental PCB mixture in rats. Environ Sci Technol. 2005;39:3513–3520. doi: 10.1021/es047987f. [DOI] [PubMed] [Google Scholar]
- Kania-Korwel I, Garrison AW, Avants JK, Hornbuckle KC, Robertson LW, Sulkowski WW, et al. Distribution of chiral PCBs in selected tissues in the laboratory rat. Environ Sci Technol. 2006;40:3704–3710. doi: 10.1021/es0602086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania-Korwel I, Hornbuckle KC, Robertson LW, Lehmler HJ. Dose-dependent enantiomeric enrichment of 2,2',3,3',6,6'-hexachlorobiphenyl in female mice. Environ Toxicol Chem. 2007a;27:299–305. doi: 10.1897/07-359R.1. [DOI] [PubMed] [Google Scholar]
- Kania-Korwel I, Shaikh N, Hornbuckle KC, Robertson LW, Lehmler H-J. Enantioselective disposition of PCB 136 (2,2',3,3',6,6'-hexachlorobiphenyl) in C57BL/6 mice after oral and intraperitoneal administration. Chirality. 2007b;19:56–66. doi: 10.1002/chir.20342. [DOI] [PubMed] [Google Scholar]
- Kania-Korwel I, Hornbuckle KC, Robertson LW, Lehmler HJ. Influence of dietary fat on the enantioselective disposition of 2,2',3,3',6,6'-hexachlorobiphenyl (PCB 136) in female mice. Food Chem Toxicol. 2008a;46:637–644. doi: 10.1016/j.fct.2007.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania-Korwel I, Hrycay EG, Bandiera S, Lehmler H-J. 2,2',3,3',6,6'-hexachlorobiphenyl (PCB 136) atropisomers interact enantioselectively with hepatic microsomal cytochrome P450 enzymes. Chem Res Toxicol. 2008b;21:1295–1303. doi: 10.1021/tx800059j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania-Korwel I, Vyas SM, Song Y, Lehmler H-J. Gas chromatographic separation of methoxylated polychlorinated biphenyl atropisomers. J Chromatogr A. 2008c;1207:146–154. doi: 10.1016/j.chroma.2008.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania-Korwel I, Xie W, Hornbuckle K, Robertson L, Lehmler H-J. Enantiomeric enrichment of 2,2′,3,3′,6,6′-hexachlorobiphenyl (PCB 136) in mice after induction of CYP enzymes. Arch of Environ Contam Toxicol. 2008d;55:510–517. doi: 10.1007/s00244-007-9111-4. [DOI] [PubMed] [Google Scholar]
- Kania-Korwel I, Zhao H, Norstrom K, Li X, Hornbuckle KC, Lehmler H-J. Simultaneous extraction and clean-up of polychlorinated biphenyls and their metabolites from small tissue samples using pressurized liquid extraction. Journal of Chromatography A. 2008e;1214:37–46. doi: 10.1016/j.chroma.2008.10.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan N, Reusch TBH, Schulz-Bull DE, Patrick G, Duinker JC. Chlorobiphenyls: model compounds for metabolism in food chain organisms and their potential use as ecotoxicological stress indicators by application of the metabolic slope concept. Environ Sci Technol. 1995;29:1851–1859. doi: 10.1021/es00007a024. [DOI] [PubMed] [Google Scholar]
- Klaassen CD, Lu H. Xenobiotic transporters: ascribing function from gene knockout and mutation studies. Toxicol Sci. 2008;101:186–196. doi: 10.1093/toxsci/kfm214. [DOI] [PubMed] [Google Scholar]
- Kostyniak PJ, Hansen LG, Widholm JJ, Fitzpatrick RD, Olson JR, Helferich JL, et al. Formulation and characterization of an experimental PCB mixture designed to mimic human exposure from contaminated fish. Toxicol Sci. 2005;88:400–411. doi: 10.1093/toxsci/kfi338. [DOI] [PubMed] [Google Scholar]
- Lehmler H-J, Price DJ, Garrison AW, Birge WJ, Robertson LW. Distribution of PCB 84 enantiomers in C57Bl/6 mice. Fresenius’ Environ Bull. 2003;12:254–260. [Google Scholar]
- Magar VS, Johnson GW, Brenner RC, Quensen JF, Foote EA, Durell G, et al. Long-term recovery of PCB-contaminated sediments at the Lake Hartwell superfund site: PCB dechlorination, 1 end-member characterization. Environ Sci Technol. 2005;39:3538–3547. doi: 10.1021/es048622y. [DOI] [PubMed] [Google Scholar]
- Mankowski DC, Lawton MP, Ekins S. Characterization of transgenic mouse strains using six human hepatic cytochrome P450 probe substrates. Xenobiotica. 2000;30:745–754. doi: 10.1080/00498250050119817. [DOI] [PubMed] [Google Scholar]
- Matthews HB, Tuey DB. The effect of chlorine position on the distribution and excretion of four hexachlorobiphenyl isomers. Toxicol Appl Pharmacol. 1980;53:377–388. doi: 10.1016/0041-008x(80)90351-8. [DOI] [PubMed] [Google Scholar]
- Matthews HB, Dedrick RL. Pharmacokinetics of PCBs. Ann Rev Pharmacol Toxicol. 1984;24:85–103. doi: 10.1146/annurev.pa.24.040184.000505. [DOI] [PubMed] [Google Scholar]
- Ness DK, Schantz SL, Hansen LG. PCB congeners in the rat brain: selective accumulation and lack of regionalization. J Toxicol Environ Health. 1994;43:453–468. doi: 10.1080/15287399409531934. [DOI] [PubMed] [Google Scholar]
- Norström K, Eriksson J, Haglund J, Silvari V, Bergman A. Enantioselective formation of methyl sulfone metabolites of 2,2',3,3',4,6'-hexachlorobiphenyl in rat. Environ Sci Technol. 2006;40:7649–7655. doi: 10.1021/es061584t. [DOI] [PubMed] [Google Scholar]
- Püttmann M, Mannschreck A, Oesch F, Robertson L. Chiral effects in the induction of drug-metabolizing enzymes using synthetic atropisomers of polychlorinated biphenyls (PCBs). Biochem Pharmacol. 1989;38:1345–1352. doi: 10.1016/0006-2952(89)90342-0. [DOI] [PubMed] [Google Scholar]
- Robertson LW, Hansen LG. PCBs: recent advances in environmental toxicology and health effects. The University Press of Kentucky; Lexington: 2001. [Google Scholar]
- Saghir SA, Hansen LG, Holmes KR, Kodavanti PR. Differential and non-uniform tissue and brain distribution of two distinct 14C-hexachlorobiphenyls in weanling rats. Toxicol Sci. 2000;54:60–70. doi: 10.1093/toxsci/54.1.60. [DOI] [PubMed] [Google Scholar]
- Schuetz EG, Umbenhauer DR, Yasuda K, Brimer C, Nguen L, Relling MV, et al. Altered expression of hepatic cytochrome P-450 in mice deficient in one or more mdr1 genes. Mol Pharmacol. 2000;57:188–197. [PubMed] [Google Scholar]
- Shaikh N, Parkin S, Lehmler H-J. The Ullmann coupling reaction: a new approach to tetraarylstannanes. Organometallics. 2006;25:4207–4214. [Google Scholar]
- Sipes I, Slocumb M, Perry D, Carter D. 2,4,5,2',4',5'-Hexachlorobiphenyl: distribution, metabolism, and excretion in the dog and the monkey. Toxicol Appl Pharmacol. 1982;65:264–272. doi: 10.1016/0041-008x(82)90009-6. [DOI] [PubMed] [Google Scholar]
- Tampal NM, Robertson LW, Srinivasan C, Ludewig G. Polychlorinated biphenyls are not substrates for the multidrug resistance transporter-1. Toxicol Appl Pharmacol. 2003;187:168–177. doi: 10.1016/s0041-008x(02)00069-8. [DOI] [PubMed] [Google Scholar]
- Warner NA, Martin JW, Wong CS. Chiral polychlorinated biphenyls are biotransformed enantioselectively by mammalian cytochrome P-450 isozymes to form hydroxylated metabolites. Environ Sci Technol. 2009;43:114–121. doi: 10.1021/es802237u. [DOI] [PubMed] [Google Scholar]
- Wong CS, Garrison AW. Enantiomer separation of polychlorinated biphenyl atropisomers and polychlorinated biphenyl retention behavior on modified cyclodextrin capillary gas chromatography columns. J Chromatogr A. 2000;866:213–220. doi: 10.1016/s0021-9673(99)01104-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.