Abstract
Trains of action potentials in CA1 pyramidal neurons are followed by a prolonged calcium-dependent post-burst afterhyperpolarization (AHP) that serves to limit further firing to a sustained depolarizing input. A reduction in the AHP accompanies acquisition of several types of learning and increases in the AHP are correlated with age-related cognitive impairment. The AHP develops primarily as the result of activation of outward calcium-activated potassium currents; however the precise source of calcium for activation of the AHP remains unclear. There is substantial experimental evidence suggesting that calcium influx via voltage-gated L-type calcium channels (L-VGCCs) contributes to the generation of the AHP. Two L-VGCC subtypes are predominately expressed in the hippocampus, CaV1.2 and CaV1.3, however it is not known which L-VGCC subtype is involved in generation of the AHP. This ambiguity is due in large part to the fact that at present there are no subunit-specific agonists or antagonists. Therefore, using mice in which the gene encoding CaV1.2 or CaV1.3 was deleted, we sought to determine the impact of alterations in levels of these two L-VCGG subtypes on neuronal excitability. No differences in any AHP measure were seen between neurons from CaV1.2 knockout mice and controls. However, the total area of the AHP was significantly smaller in neurons from CaV1.3 knockout mice as compared to neurons from wildtype controls. A significant reduction in the amplitude of the AHP was also seen at the 1 sec time point in neurons from CaV1.3 knockout mice as compared to those from controls. Reductions in both the area and 1 sec amplitude suggest the involvement of calcium influx via CaV1.3 in the slow AHP (sAHP). Thus, the results of our study demonstrate that deletion of CaV1.3, but not CaV1.2, significantly impacts the generation of the sAHP.
Keywords: hippocampus, neuronal excitability, afterhyperpolarization, mouse, voltage-gated ion channel
INTRODUCTION
In the hippocampus, as well as other brain regions, bursts of action potentials are followed by a prolonged post-burst afterhyperpolarization (AHP) that serves to limit further firing to a sustained depolarizing input (Alger and Nicoll, 1980; Hotson and Prince, 1980; Madison and Nicoll, 1984; Schwartzkroin and Stafstrom, 1980). A reduction in the AHP in CA1 pyramidal neurons accompanies learning in a variety of hippocampal-dependent tasks in multiple species including rabbits, rats and mice (Moyer et al., 1996; Oh et al., 2003; Ohno et al., 2006). Similarly, a reduction in the AHP has also been seen in piriform cortical neurons following odorant-discrimination learning (Saar et al., 1998).
There are also numerous reports documenting an age-related increase in the AHP (for recent review see Disterhoft and Oh, 2007) and this age-related increase in AHP is correlated with a decline in performance during learning and memory tasks that require the hippocampus (Moyer et al., 2000; Tombaugh et al., 2005). Finally, genetic and pharmacological manipulations that functionally oppose the age-related increase in the AHP (Murphy et al., 2004; Oh et al., 1999) act to ameliorate cognitive impairments in spatial learning that are typically observed in aged rodents (Dachir et al., 1997; Murphy et al., 2004). These experimental results have led to the suggestion that plasticity of the AHP is a neurobiological substrate of learning which is sensitive to aging (Wu et al., 2002).
The post-burst AHP in CA1 hippocampal pyramidal neurons is often described as having three components: a fast, medium and a slow AHP. The fast AHP (fAHP) occurs immediately after individual action potentials and lasts only 1–5 ms. The current underlying the fAHP (termed Ic) is calcium and voltage dependent, is blocked by low concentrations of TEA (Lancaster and Nicoll, 1987; Storm, 1987), iberiotoxin ((Zhang and McBain, 1995), and paxilline (Knaus et al., 1994; Shao et al., 1999) indicating that the underlying channels are BK-type channels. In addition, voltage-gated potassium channels that underlie the A-type current have been implicated in rapid action potential repolarization (Hoffman et al., 1997; Johnston et al., 2000).
The medium AHP (mAHP) is typically observed after a burst of action potentials and has a decay constant of approximately 100 ms. Currently there is some debate as to the exact current or currents that underlie the mAHP. There are numerous reports implicating an apamin sensitive calcium-activated potassium current (Gu et al., 2005; Pedarzani et al., 2001; Sailer et al., 2002; Shah and Haylett, 2000; Stackman et al., 2002; Stocker et al., 1999). It seems likely that the apamin sensitive current (IAHP) is gated by the small-conductance calcium-activated potassium channel SK2 because knockout mice in which the gene that codes for SK2 (Kcnn2) is deleted do not exhibit an apamin sensitive current (Bond et al., 2004). In addition, there are a number of reports demonstrating that bath application of apamin reduces the amplitude of the mAHP in CA1 pyramidal neurons (Kaczorowski et al., 2007; Kramar et al., 2004; Norris et al., 1998; Oh et al., 2000). However, other reports have failed to find an affect of apamin on the mAHP (Gu et al., 2005; Storm, 1989) and further suggest that the mAHP is not calcium dependent but is instead dependent upon the activation of KCNQ (IM) and HCN (Ih) channels (Gu et al., 2005).
The slow AHP (sAHP) has a rather long time constant (1–5 seconds) and is voltage-independent (Storm, 1990). The current that underlies the sAHP, the IsAHP, is calcium dependent and is influenced by a wide variety of neuromodulators including serotonin and norepinephrine (e.g. Pedarzani and Storm, 1993). In spite of numerous efforts, the channels that give raise to the sAHP remain elusive.
At present, the precise source of calcium required for the activation of the various calcium-activated potassium currents that underlie the post-burst AHP remains unclear. Several studies using L-type voltage-gated calcium channel (L-VGCC) blockers have shown that blockade of these channels leads to a reduction in the currents underlying the AHP in hippocampal neurons, suggesting that the AHP is generated by calcium influx via L-VGCCs (Lima and Marrion, 2007; Marrion and Tavalin, 1998; Power et al., 2002; Rascol et al., 1991; Shah and Haylett, 2000). In addition, an increase in both the activity and expression of L-VGCC in the hippocampus occurs with aging (Landfield and Pitler, 1984; Thibault and Landfield, 1996; Veng and Browning, 2002) and application of the L-VGCC blocker nimodipine results in a reduction of the AHP in neurons from aging animals (Moyer et al., 1992).
Despite the evidence suggesting a link between calcium influx via L-VGCCs and the AHP in neurons from both young and aging animals, it is not known which L-VGCC subtype is involved in generation of the AHP. In the rodent hippocampus, two L-VGCC subtypes are expressed: CaV1.2 and CaV1.3 (Hell et al., 1993). CaV1.2 is preferentially expressed in pyramidal cell soma and dendritic fields of areas CA1–CA3 and in granule cell soma and fibers in the dentate gyrus (Tippens et al., 2008), while CaV1.3 is expressed in cell bodies and proximal dendrites in CA1–CA3 (Hell et al., 1993). In addition, CaV1.3 is evenly distributed throughout the cell body of the neuron, while CaV1.2 is found in clusters in this region (Hell et al., 1993) and is observed in dendrites, axons and axon terminals (Tippens et al., 2008). Taken together, these data suggest possible different functional roles for CaV1.2 and CaV1.3. Currently, it is not possible to assess the relative contributions of CaV1.2 and CaV1.3 to neuronal excitability with respect to the AHP, due to the lack of isoform-specific agonists or antagonists. In light of the role ascribed to the AHP during learning and the now well documented age-related changes in the AHP, we have completed a series of experiments using mice in which the gene encoding CaV1.3 (Clark et al., 2003; Platzer et al., 2000) or CaV1.2 was deleted (McKinney et al., 2008; White et al., 2008) to determine the impact of deletion of these two L-VCGG subtypes on the AHP and neuronal excitability. The results of our study demonstrate that deletion of CaV1.3, but not CaV1.2, has a significant impact on the generation of the AHP in CA1 pyramidal neurons.
MATERIALS & METHODS
Mice
For the CaV1.2 study, conditional knockout mice with a forebrain-specific (hippocampus and forebrain) deletion of CaV1.2 were used (McKinney et al., 2008; White et al., 2008). For these experiments mice heterozygous for the floxed CaV1.2 exon two allele were maintained on a 129SvEv genetic background. Experimental animals were generated by crossing heterozygous floxed CaV1.2 mice (CaV1.2f/+ mice) with transgenic mice that expressed Cre-recombinase (Chen et al., 2006). Cre-recombinase expression was regulated by the calcium calmodulin kinase IIα (CaMKIIα) promoter which reaches peak expression levels postnatally (Kelly et al., 1987; Sugiura and Yamauchi, 1992) and restricted Cre-recombinase expression to glutamatergic neurons of the forebrain (Chen et al., 2006). These mice (termed here as the CaMK-Cre mice) were maintained on a C57BL/6 background (10+ generations). Offspring from the F1 cross that were heterozygous floxed and Cre positive (i.e. CaV1.2f/+, CaMK-CreCre/+) were then intercrossed (non-sibling) with mice heterozygous floxed and Cre negative (i.e. CaV1.2f/+, CaMK-Cre+/+) to achieve homozygous conditional knockout mice (CaV1.2f/f, CaMK-CreCre/+) and wild-type mice (CaV1.2+/+, CaMK-Cre+/+) on an F2 129Sve:C57Bl/6 hybrid background. For ease of reading, conditional knockout mice are referred to as CaV1.2−/− and wild-type mice are referred to as CaV1.2+/+ throughout the remainder of the text. For the CaV1.3 study, knockout mice with a global deletion of the gene encoding CaV1.3 were used (Clark et al., 2003; McKinney and Murphy, 2006; Platzer et al., 2000). Mice were maintained on a C57BL/6 background by successively crossing heterozygous offspring with C57BL/6 wild-type mice. Experimental animals were generated by crossing heterozygous CaV1.3 mice with wild-type 129SvEv mice. Heterozygous offspring from the F1 cross were then intercrossed (non-sibling) to achieve homozygous knockout mice (CaV1.3−/−) and wild-type mice (CaV1.3+/+) on an F2 129Sve:C57Bl/6 hybrid background. For all experiments approximately equal numbers of adult (approx. 6 months) male and female mice were used. All comparisons were made between knockout mice and wildtype littermates and the experimenter was kept blind as to the genotype throughout the experiment. All experiments were conducted in accordance with the guidelines set forth by the University of Michigan Committee on Use and Care of Animals.
Slice Preparation
Mice were anesthetized with isoflurane and decapitated. Whole-brain (minus the cerebellum) 400 μm sagittal sections were cut on a vibratome and rapid micro-dissection of both hippocampi was completed in ice-cold (<1°C) oxygenated sucrose-based cutting solution containing the following (in mM): 2.8 KCl, 1 MgCl2, 2 MgSO4, 1.25 NaH2PO4, 1 CaCl2, 206 sucrose, 26 NaHCO3, 10 D-glucose, 0.40 ascorbic acid. Slices were transferred to a holding chamber filled with artificial cerebrospinal fluid (aCSF) containing the following (in mM): 124 NaCl, 2.8 KCl, 2 MgSO4, 1.25 NaH2PO4, 2 CaCl2, 26 NaHCO3, 10 D-glucose, 0.40 ascorbic acid at room temperature and remained there for at least 1 hour before being individually transferred to a submersion chamber and continuously perfused (~1.5 ml/min) with oxygenated aCSF heated to 31°C.
Electrophysiology
Whole-cell recordings were made from CA1 pyramidal neurons using a Dagan 3900A amplifier in bridge mode. Neurons were visualized with an Olympus BX51WI upright microscope equipped with infrared differential interference contrast optics. Patch-pipettes made from Clark Borosilicate Standard Wall glass (Warner Instruments) and pulled using a P-97 Flaming-Brown pipette puller (Sutter Instruments) with resistances of 5–8 MΩ were used and filled with the following internal solution (in mM): 120 potassium methylsulfate, 20 KCl, 10 HEPES, 4 Na2-ATP, 2 MgCl2, 0.3 GTP, 0.2 EGTA, 7 phosphocreatine. Seal resistances of >2 GΩ were achieved prior to rupturing into whole-cell mode. Action potentials were measured from rest and were analyzed for spike threshold, amplitude and width. Spike width was measured at ½ of the action potential amplitude. Cells were held at 5 mV below action potential threshold and the AHP was studied using a 100 ms current step sufficient to elicit 5 action potentials. AHP measurements were made using the average of 10 successive traces from each neuron (10 sec inter-sweep interval). Total integral area of the AHP was calculated from the time that the voltage trace crosses the baseline from the depolarized state until it returned to baseline. The duration of the AHP was measured as the time required for the AHP to return to baseline for at least 10 ms (maximum duration 10 s), starting at the offset of the stimulus. The peak AHP amplitude was calculated as the maximum negative voltage deflection (occurring at any time point during the entire AHP), relative to resting potential. Latency was calculated as the time at which the AHP achieved peak amplitude. Amplitude of the AHP was also calculated at 200 ms and 1 sec after the offset of the stimulus used to generate the AHP to assess alterations in the medium and slow AHP, respectively (Oh et al., 2003). Accommodation was studied at rest in these neurons using a series of 500 ms current injection of increasing intensity (−0.05 nA to 0.4nA, .05 nA steps).
Data was acquired and analyzed using pClamp 9.2. The number of neurons recorded from an individual animal ranged from 1–5 (average = 2.4 ± 0.2). For analysis, when recordings from multiple neurons were obtained, the values from individual neurons for each animal were averaged such that each animal contributed a single data point to the group average and thus the sample size reported refers to the number of animals and not the number of neurons. All values are expressed as mean ± standard error of the mean (S.E.M.). Statistical analysis was performed using student t-tests or 2-way repeated ANOVA using Fisher's post hoc tests where appropriate.
RESULTS
Deletion of CaV1.2 does not impact generation of the AHP
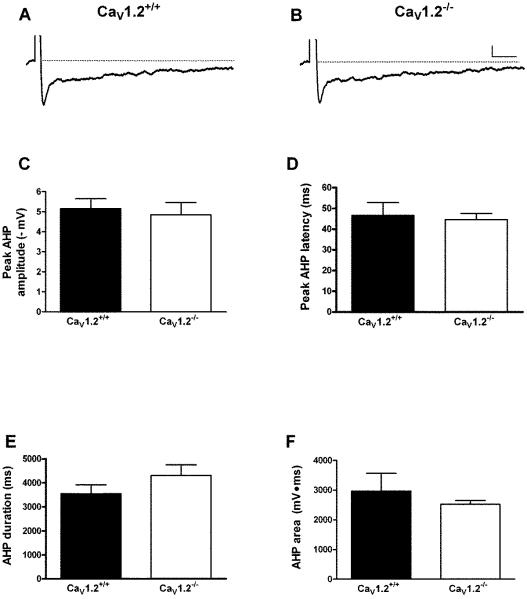
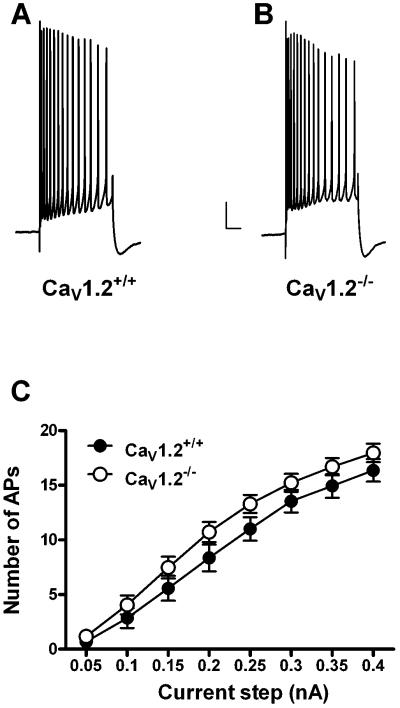
Whole-cell current clamp recordings were made from 11 CaV1.2+/+ mice (24 neurons) and 9 CaV1.2−/− mice (19 neurons). Conditional deletion of CaV1.2 did not significantly impact resting membrane potential or input resistance (Table 1). Action potential threshold, height and width were also not altered in CaV1.2 knockout mice when compared to wildtype littermates (Table 1). Deletion of CaV1.2 did not significantly impact the AHP recorded in CA1 pyramidal neurons. There were no differences in any of the AHP measures examined. We examined peak amplitude (CaV1.2+/+: 4.8 ± .6 mV; Cav1.2−/−: 5.2 ± .5 mV), latency to peak amplitude (CaV1.2+/+: 44.5 ± 3.0 ms; CaV1.2−/−: 46.5 ± 6.2 ms), area (CaV1.2+/+: 2965.4 ± 595.3 mV•ms; CaV1.2−/−: 2527.7 ± 119.1 mV•ms) and duration (CaV1.2−/−: 4303.0 ± 450.0 ms; CaV1.2−/−: 3544.3 ± 371.8 ms) in neurons from CaV1.2−/− and CaV1.2+/+ neurons (Fig. 1A–F). In addition, we examined accommodation in neurons from both groups (Fig. 2). There was no difference in number of action potentials fired in response to a range of current steps in neurons from CaV1.2−/− mice as compared to those from Cav1.2+/+ mice (F [1,17] = 2.0, P = 0.17 main effect of genotype, 2-way repeated measures ANOVA). In addition, we examined the peak and area of the AHP during these longer current steps and found no difference between CaV1.2−/− and Cav1.2+/+mice (data not shown).
Table 1.
Biophysical properties of CA1 pyramidal neurons from CaV1.2+/+ and CaV1.2−/− mice. There was no significant difference in any property measured. Abbreviations: RMP, resting membrane potential, IR, input resistance, AP, action potential. RMP and AP threshold values were not corrected for liquid junction potential. All data is presented as mean ± S.E.M
| RMP (mV) | IR (mΩ) | AP Threshold (mV) | AP Width (ms) | AP Height (mV) | |
|---|---|---|---|---|---|
| CaV1.2 +/+ | 58.9 ± 1.0 | 150.6 ± 8.6 | 52.3 ± 0.7 | 1.4 ± 0.1 | 84.6 ± 1.8 |
| CaV1.2 −/− | 56.8 ± 1.8 | 151.1 ± 11.0 | 52.7 ± 0.7 | 1.3 ± 0.1 | 88.1 ± 3.1 |
Figure 1.
Deletion of CaV1.2 has no effect on the AHP in CA1 pyramidal neurons. Representative AHP traces (average of 10 successive sweeps) recorded from a CA1 neuron from a CaV1.2+/+ (A) and CaV1.2−/− (B) mouse. There was no difference in any AHP measure, including peak amplitude (C), latency to peak (D) duration (E) or area of the AHP (F) between neurons from knockout and wild-type mice. Scale bar: 1 mV/500 ms. All data presented as mean ± S.E.M.
Figure 2.
Deletion of CaV1.2 does not alter accommodation in CA1 pyramidal neurons. Representative accommodation traces from a neuron from a CaV1.2+/+ (A) and CaV1.2−/− (B) mouse in response to a 0.35 nA current step (500 ms). No difference was seen in accommodation in neurons from either group (C); neurons from Ca 1.2+/+ (closed circles) and CaV1.2−/− (open circles) mice fired an equal number of action potentials in response to a given current step. Scale bar: 10 mV/500 ms. All data presented as mean ± S.E.M.
Deletion of CaV1.3 significantly reduces the AHP
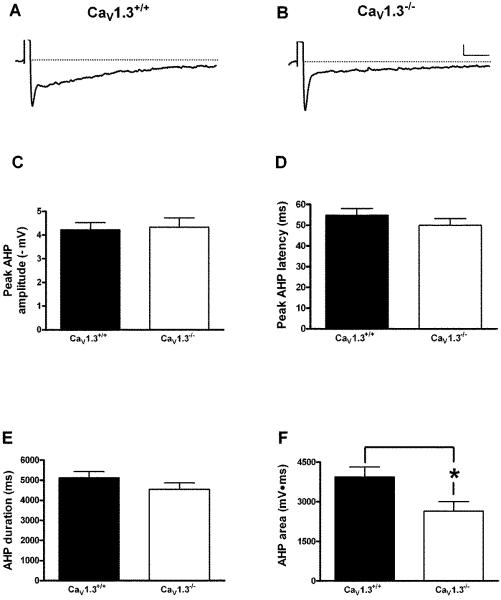
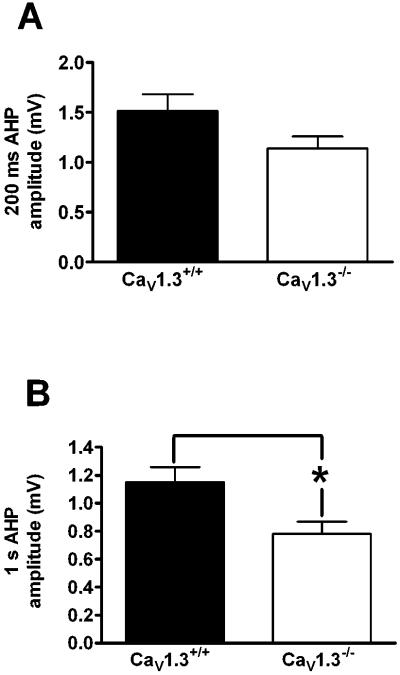
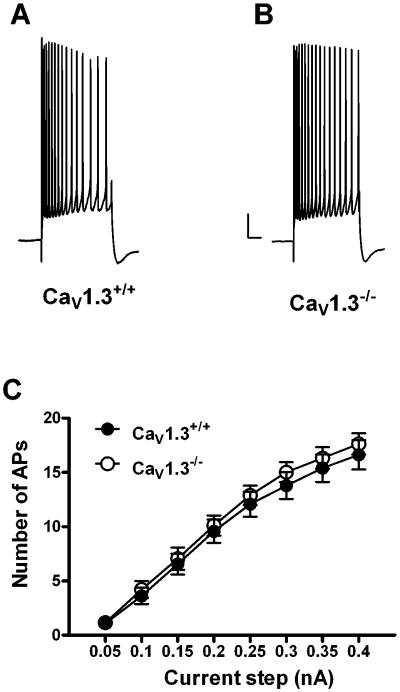
Whole cell recordings were made from 14 Cav1.3+/+ mice (35 neurons) and 14 Cav1.3−/− mice (40 neurons). Similar to that observed in neurons from Cav1.2−/− mice, deletion of CaV1.3 did not have an effect on membrane properties such as resting membrane potential, input resistance, action potential threshold or width when compared to wildtype littermates (Table 2). In addition we found no significant difference in the peak amplitude (Cav1.3+/+: 4.3 ± 0.4 mV; Cav1.3−/− : 4.2 ± 0.3 mV), latency to peak (Cav1.3+/+: 54.8 ± 3.7 ms; Cav1.3−/−: 49.4 ± 3.2 ms) or duration of the AHP (Cav1.3+/+: 5115.8 ± 309.8 ms; Cav1.3−/−: 4541.7 ± 320.2 ms) in CA1 pyramidal neurons (Fig. 3A – E). However, a significant difference was observed in the area of the AHP in neurons from Cav1.3−/− mice as compared to those from Cav1.3+/+ mice (Fig. 3F). Neurons from Cav1.3−/− mice had a significantly smaller AHP area as compared to those from Cav1.3+/+ mice (Cav1.3+/+: 3943.7 ± 370.6 mV•ms; Cav1.3−/−: 2647.2 ± 355.5 mV•ms, P < 0.02, unpaired t-test). As a change in the area of the AHP could result from alterations in either the medium or slow AHP, we measured the amplitude of the AHP at both 200 ms (measure of the medium AHP) and 1 sec (measure of the slow AHP) after current stimulus offset. As shown in Fig. 4A, there was a strong trend for a reduced AHP amplitude at 200 ms in neurons from Cav1.3−/− mice as compared to those from Cav1.3+/+ mice, although this difference was not statistically significant (Cav1.3 +/+: 1.5 ± 0.2 mV; Cav1.3−/−: 1.1 ± 0.1 mV, P =.07, unpaired t-test). Importantly, analysis of the AHP amplitude at 1 second (Fig. 4B) revealed a significantly smaller amplitude in neurons from Cav1.3−/− mice as compared to those from Cav1.3+/+ mice (Cav1.3+/+: 1.2 ± 0.1 mV; Cav1.3−/−: 0.8 ± .09 mV, P = 0.01, unpaired t-test) further suggesting a preferential role for CaV1.3 in the generation of the sAHP. We also examined accommodation in neurons from both groups of mice (Fig. 5) and found no difference in accommodation in neurons from Cav1.3 −/− mice as compared to those from Cav1.3+/+ mice (F[1,22] = 0.32, p = 0.6). Similar results were obtained when the interspike interval was analyzed (data not presented).
Table 2.
Biophysical properties of CA1 pyramidal neurons from CaV1.3+/+ and CaV1.3−/− mice. Deletion of CaV1.3 did not significantly alter any of the properties that were measured. Abbreviations: RMP, resting membrane potential, IR, input resistance, AP, action potential. RMP and AP threshold values were not corrected for liquid junction potential. All data is presented as mean ± S.E.M
| RMP (mV) | IR (mΩ) | AP Threshold (mV) | AP Width (ms) | AP Height (mV) | |
|---|---|---|---|---|---|
| CaV1.3 +/+ | 60.3 ± 0.7 | 150.3 ± 8.6 | 54.3 ± 0.4 | 1.4 ± 0.04 | 87.8 ± 2.1 |
| CaV1.3 −/− | 59.3 ± 0.7 | 155.6 ± 15.5 | 54.8 ± 0.5 | 1.4 ± 0.03 | 86.7 ± 2.6 |
Figure 3.
Deletion of CaV1.3 has a significant impact on generation of the AHP in CA1 pyramidal neurons. Representative AHP traces (average of 10 successive sweeps) in a neuron from a CaV1.3+/+ (A) and CaV1.3−/− (B) mouse. No difference was seen in peak amplitude (C), latency to peak (D) or duration (E) of the AHP between neurons from CaV1.3+/+ and CaV1.3−/− mice. However, a significant difference was seen in the area of the AHP (F) between neurons from both groups. *p < 0.02, unpaired t-test. Scale bar: 1 mV/500 ms. All data presented as mean ± S.E.M.
Figure 4.
Deletion of CaV1.3 preferentially impacts the slow AHP. A strong trend was observed for a smaller AHP amplitude at 200 ms in neurons from CaV1.3−/− mice as compared to those from CaV1.3+/+ mice, suggesting a modest reduction in mAHP in the knockout animals (A). A significant reduction was seen in the AHP amplitude at 1 s between neurons from CaV1.3−/− mice as compared neurons from CaV1.3+/+ mice, demonstrating a reduction in sAHP in knockout animals (B). *p = 0.02, unpaired t-test. All data presented as mean ± S.E.M.
Figure 5.
Deletion of CaV1.3 does not impact accommodation in CA1 pyramidal neurons. Representative accommodation traces from a neuron from a CaV1.3+/+ (A) and CaV1.3−/− (B) mouse in response to a 0.35 nA current step (500 ms). No difference was seen in accommodation in neurons from either group (C); neurons from CaV1.3+/+ (closed circles) and CaV1.3−/− (open circles) mice fired an equal number of action potentials in response to a given current step. Closed circles: CaV1.3+/+, open circles: CaV1.3−/−. Scale bar: 10 mV/500 ms. All data presented as mean ± S.E.M.
DISCUSSION
The principal finding of this study is that deletion of the L-type calcium channel pore-forming subunit CaV1.3 results in a significant reduction in the post-burst AHP in pyramidal neurons within the CA1 region of the mouse hippocampus. In addition we found that conditional deletion of the gene encoding the CaV1.2 L-type calcium channel subtype has no impact on generation of the AHP. This study is the first to our knowledge to demonstrate the requirement for the CaV1.3, but not CaV1.2 subtype for generation of the AHP. Specifically, neurons from CaV1.3−/− mice had a significantly reduced AHP area as compared to neurons from CaV1.3+/+ mice. In addition, there was a significant reduction of the AHP amplitude measured at 1 second after current stimulus offset in neurons from knockout mice as compared to wildtype controls demonstrating that deletion of CaV1.3 primarily impacts the sAHP. In addition, we observed a strong trend toward a reduction when similar measurements were made at 200 ms after the current stimulus offset, suggesting that deletion of CaV1.3 may also have a modest impact on the mAHP. These effects on the AHP in CA1 neurons from CaV1.3−/− mice were seen in the absence of any effects on basic membrane properties of these neurons. Conversely, we did not observe any significant changes in the AHP or spike accommodation in neurons from CaV1.2−/− mice. These results are significant in that they suggest that the calcium that is gated by CaV1.2 does not have functional access to the calcium activated potassium channels that underlie the sAHP. This might reflect the differential cellular distribution of the two different CaV calcium channel subtypes (Hell et al., 1993) and would suggest that CaV1.2 is not located in the same cellular compartment as the calcium activated potassium channels that generate the sAHP. Alternatively, it is possible that the both channels reside in the same cellular compartment but the diffusion distance between the CaV1.2 channels and the potassium channels that underlie the sAHP is sufficiently great that under normal physiological conditions (i.e. brief repetitive spiking) the relative contribution of CaV1.2 is negligible.
Interestingly, deletion of CaV1.3 had no effect on the peak amplitude of the AHP. In rats and rabbits, the peak AHP amplitude in CA1 pyramidal neurons is thought to be representative of the mAHP and is a result of overlapping activation of IAHP and sIAHP. In both rats and rabbits, the peak AHP occurs around ~100–200 ms, consistent with activation of both IAHP and sIAHP (Coulter et al., 1989; Oh et al., 2003). However, in the current study we found that the peak amplitude of the AHP in mouse CA1 pyramidal neurons occurs around ~50 ms. Recent studies have revealed that IM is predominately active during this phase of the AHP (Kuo et al., 2007; Peters et al., 2005). Given that IM is not a calcium-activated potassium current, it is not surprising that deletion of CaV1.3 did not impact the peak amplitude of the AHP.
Despite the impact of deletion of CaV1.3 on the sAHP, no concomitant reduction was seen in accommodation. This may seem surprising given previous reports (Madison and Nicoll, 1984; Storm, 1990) that the sAHP modulates accommodation in CA1 pyramidal neurons (but see also Colling et al., 1996; Jones and Heinemann, 1988; Oh et al., 2003). One possibility is that in our accommodation experiments the duration of the current steps was limited to 500 ms whereas many previous studies have used longer pulses ranging from 600 ms to 1000 ms and thus had we used longer pulses this may have revealed a significant decrease in accommodation in the CaV1.3 knockout mice. This seems unlikely given that in mice this 500 ms window brackets the largest portion of the hyperpolarizing deflection. In addition, we have recently demonstrated that in principle neurons in the amygdala, deletion of CaV1.3 decreases the sAHP as well as spike accommodation (McKinney et al., 2009). The major difference between these two studies is the relative size of the sAHP. In the current study, the sAHP (measured at 1000 ms) is approximately 1.2 mV whereas in the amygdala the sAHP is over double that—approximately 2.9 mV. Although the sAHP in our recordings is somewhat small when compared to that recorded in rats with sharp electrodes, it is in line with recent reports from using mice with comparable recording methods (Kaczorowski et al., 2007; Kaczorowski and Disterhoft, 2009; Weiss et al., 2005). Therefore, it seems likely that in mouse CA1 hippocampal pyramidal neurons where the sAHP is relatively small, deletion of CaV1.3 has minimal impact upon spike accommodation.
Although deletion of CaV1.3 did result in a significant reduction of the sAHP, a residual sAHP was still observed in neurons from CaV1.3−/− mice. This finding is consistent with previous studies demonstrating the lack of complete abolition of the IsAHP using pharmacological blockade of L-VGCCs (Lima and Marrion, 2007; Power et al., 2002; Shah and Haylett, 2000), suggesting that additional calcium sources may also contribute to generation of the sAHP. One possibility is that the residual sAHP observed in our study results from calcium influx through other voltage gated calcium channels. Indeed it has been previously reported that bath application of omega-conotoxin GIVA significantly reduced the IsAHP (Shah and Haylett, 2000) in rat pyramidal neurons in culture, suggesting that N-type calcium channels may also contribute to the sAHP (but see also (Dutar et al., 1989). To date, little work has been done elucidating the calcium sources of the AHP in mouse CA1 pyramidal neurons; it is possible that in mouse hippocampal neurons N, P/Q, R and/or T-type calcium channels play a greater role in generation of the AHP than previously observed in rat neurons.
Although our experiments with the CaV1.2 knockout mice indicate that CaV1.2 does not normally modulate the sAHP, an alternate possibility is that genetic ablation of CaV1.3 results in some sort of compensatory upregulation of CaV1.2. This seems unlikely as it has been previously reported that deletion of CaV1.3 in these mice does not lead to compensatory up-regulation of CaV1.2 as measured by high affinity (+)[3H]isradipine binding assays, in situ hybridization and western blot analysis (Clark et al., 2003). In addition, CaV1.2 and CaV1.3 differ significantly in their gating properties and thresholds of activation (Helton et al., 2005; Xu and Lipscombe, 2001) as well as their cellular distributions (Hell et al., 1993). Thus, a compensatory shift would not only mean a change in expression levels but would also necessitate a significant change in biophysical properties and distribution for CaV1.2.
Finally, as is the case with all experiments that utilize knockout mice, some consideration regarding potential non-specific changes need be made. In light of the fact that the identity of the channel (or channels) that mediate the sAHP remains unknown, it is possible that deletion of CaV1.3 resulted in a concomitant reduction in the expression or trafficking of the calcium activated potassium channels that underlie the sAHP. While our data do not specifically rule this out as a possibility, if this were the case, then the CaV1.3 knockout mice might be a useful tool in the establishment of the identity of these channels.
As previously mentioned, there are multiple reports documenting an age-related increase in the sAHP (Disterhoft and Oh, 2007). Furthermore, there is ample evidence that the age-related increase in the sAHP results from an increase in L-type calcium channel density (recently reviewed by Thibault et al., 2007). The best evidence to date is that an increase in the CaV1.3 pore-forming subunit is responsible for the age-related increase in the sAHP. Landfield and colleagues have demonstrated that magnitude of the age-related increase in L-type currents was correlated with mRNA levels of CaV1.3—as determined by RT-PCR—in young and aged animals (Chen et al., 2000) which supports earlier experiments from the same group utilizing in situ hybridization and ribonuclease protection assays, that demonstrated that there is an up-regulation of hippocampal CaV1.3 subunit mRNA in aged rodents (Herman et al., 1998). In addition using antibodies directed against CaV1.3, Browning and colleagues have reported finding an age-related increase in expression of CaV1.3 protein within the CA1 region of the hippocampus in rats (Veng and Browning, 2002; Veng et al., 2003). Conversely, there does not appear to be an up-regulation of CaV1.2 protein in hippocampal tissue harvested from aged mice that are cognitively impaired (Murphy et al., 2006a; Murphy et al., 2006b). Although we did not examine aged animals in the present study, our results would support the hypothesis that the age-related increases observed in the sAHP are directly attributable to an increase in channel density of CaV1.3.
Acknowledgments
Grant Sponsor: National Institutes on Aging: T32-AG000114 (AEG); R21AG025471 (GGM); R01AG028488 (GGM); General Medical Sciences: T32GM008322 (BCM)
REFERENCES
- Alger BE, Nicoll RA. Epileptiform burst after hyperpolarization: Calcium-dependent potassium potential in hippocampal CA1 pyramidal cells. Science. 1980;210(4474):1122–1124. doi: 10.1126/science.7444438. [DOI] [PubMed] [Google Scholar]
- Bond CT, Herson PS, Strassmaier T, Hammond R, Stackman R, Maylie J, Adelman JP. Small conductance Ca2+-activated K+ channel knock-out mice reveal the identity of calcium-dependent afterhyperpolarization currents. J Neurosci. 2004;24(23):5301–5306. doi: 10.1523/JNEUROSCI.0182-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AP, Ohno M, Giese KP, Kuhn R, Chen RL, Silva AJ. Forebrain-specific knockout of B-raf kinase leads to deficits in hippocampal long-term potentiation, learning, and memory. J Neurosci Res. 2006;83(1):28–38. doi: 10.1002/jnr.20703. [DOI] [PubMed] [Google Scholar]
- Chen KC, Blalock EM, Thibault O, Kaminker P, Landfield PW. Expression of α1D subunit mRNA is correlated with L-type Ca2+ channel activity in single neurons of hippocampal “zipper” slices. Proc Natl Acad Sci U S A. 2000;97(8):4357–4362. doi: 10.1073/pnas.070056097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark NC, Nagano N, Kuenzi FM, Jarolimek W, Huber I, Walter D, Wietzorrek G, Boyce S, Kullmann DM, Striessnig J. Neurological phenotype and synaptic function in mice lacking the CaV1.3 alpha subunit of neuronal L-type voltage-dependent Ca2+ channels. Neuroscience. 2003;120(2):435–442. doi: 10.1016/s0306-4522(03)00329-4. [DOI] [PubMed] [Google Scholar]
- Colling SB, Collinge J, Jefferys JG. Hippocampal slices from prion protein null mice: disrupted Ca2+-activated K+ currents. Neurosci Lett. 1996;209(1):49–52. doi: 10.1016/0304-3940(96)12596-9. [DOI] [PubMed] [Google Scholar]
- Coulter DA, Lo Turco JJ, Kubota M, Disterhoft JF, Moore JW, Alkon DL. Classical conditioning reduces amplitude and duration of calcium-dependent afterhyperpolarization in rabbit hippocampal pyramidal cells. J Neurophysiol. 1989;61(5):971–981. doi: 10.1152/jn.1989.61.5.971. [DOI] [PubMed] [Google Scholar]
- Dachir S, Schmidt B, Levy A. Effects of metrifonate on radial arm maze acquisition in middle-aged rats. Brain Res. 1997;777(1–2):251–254. doi: 10.1016/s0006-8993(97)01234-1. [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Oh MM. Alterations in intrinsic neuronal excitability during normal aging. Aging Cell. 2007;6(3):327–336. doi: 10.1111/j.1474-9726.2007.00297.x. [DOI] [PubMed] [Google Scholar]
- Dutar P, Rascol O, Lamour Y. ω-Conotoxin GVIA blocks synaptic transmission in the CA1 field of the hippocampus. Eur J Pharmacol. 1989;174(2–3):261–266. doi: 10.1016/0014-2999(89)90318-x. [DOI] [PubMed] [Google Scholar]
- Gu N, Vervaeke K, Hu H, Storm JF. Kv7/KCNQ/M and HCN/h, but not KCa2/SK channels, contribute to the somatic medium after-hyperpolarization and excitability control in CA1 hippocampal pyramidal cells. J Physiol. 2005;566(3):689–715. doi: 10.1113/jphysiol.2005.086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell JW, Westenbroek RE, Warner C, Ahlijanian MK, Prystay W, Gilbert MM, Snutch TP, Catterall WA. Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel α1 subunits. J Cell Biol. 1993;123(4):949–962. doi: 10.1083/jcb.123.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helton TD, Xu W, Lipscombe D. Neuronal L-type calcium channels open quickly and are inhibited slowly. J Neurosci. 2005;25(44):10247–51. doi: 10.1523/JNEUROSCI.1089-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Chen KC, Booze R, Landfield PW. Up-regulation of alpha1D Ca2+ channel subunit mRNA expression in the hippocampus of aged F344 rats. Neurobiol Aging. 1998;19(6):581–7. doi: 10.1016/s0197-4580(98)00099-2. [DOI] [PubMed] [Google Scholar]
- Hoffman DA, Magee JC, Colbert CM, Johnston D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature. 1997;387(6636):869–875. doi: 10.1038/43119. [DOI] [PubMed] [Google Scholar]
- Hotson JR, Prince DA. A calcium-activated hyperpolarization follows repetitive firing in hippocampal neurons. J Neurophysiol. 1980;43(2):409–419. doi: 10.1152/jn.1980.43.2.409. [DOI] [PubMed] [Google Scholar]
- Johnston D, Hoffman DA, Magee JC, Poolos NP, Watanabe S, Colbert CM, Migliore M. Dendritic potassium channels in hippocampal pyramidal neurons. J Physiol. 2000;525(1):75–81. doi: 10.1111/j.1469-7793.2000.00075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RS, Heinemann U. Verapamil blocks the afterhyperpolarization but not the spike frequency accommodation of rat CA1 pyramidal cells in vitro. Brain Res. 1988;462(2):367–371. doi: 10.1016/0006-8993(88)90567-7. [DOI] [PubMed] [Google Scholar]
- Kaczorowski CC, Disterhoft J, Spruston N. Stability and plasticity of intrinsic membrane properties in hippocampal CA1 pyramidal neurons: effects of internal anions. J Physiol. 2007;578(3):799–818. doi: 10.1113/jphysiol.2006.124586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczorowski CC, Disterhoft JF. Memory deficits are associated with impaired ability to modulate neuronal excitability in middle-aged mice. Learn Mem. 2009;16(6):362–366. doi: 10.1101/lm.1365609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly PT, Shields S, Conway K, Yip R, Burgin K. Developmental changes in Calmodulin-Kinase II activity at brain synaptic junctions: alterations in holoenzyme composition. J Neurochem. 1987;49(6):1927–1940. doi: 10.1111/j.1471-4159.1987.tb02456.x. [DOI] [PubMed] [Google Scholar]
- Knaus H-G, McManus OB, Lee SH, Schmalhofer WA, Garcia-Calvo M, Helms LMH, Sanchez M, Giangiacomo K, Reuben JP, et al. Tremorgenic indole alkaloids potently inhibit smooth muscle high-conductance calcium-activated potassium channels. Biochemistry. 1994;33(19):5819–5828. doi: 10.1021/bi00185a021. [DOI] [PubMed] [Google Scholar]
- Kramar EA, Lin B, Arai AC, Gall CM, Lynch G. A novel mechanism for the facilitation of theta-induced long-term potentiation by brain-derived neurotrophic factor. J Neurosci. 2004;24(22):5151–5161. doi: 10.1523/JNEUROSCI.0800-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo AG, Lee G, McKay BM, Disterhoft JF. Enhanced neuronal excitability in rat CA1 pyramidal neurons following trace eyeblink conditioning acquisition is not due to alterations in I(M) Neurobiol Learn Mem. 2007;89(2):125–133. doi: 10.1016/j.nlm.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster B, Nicoll RA. Properties of two calcium-activated hyperpolarizations in rat hippocampal neurones. J Neurophysiol. 1987;389(1):187–203. doi: 10.1113/jphysiol.1987.sp016653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landfield PW, Pitler TA. Prolonged Ca2+-dependent afterhyperpolarizations in hippocampal neurons of aged rats. Science. 1984;226(4678):1089–1092. doi: 10.1126/science.6494926. [DOI] [PubMed] [Google Scholar]
- Lima PA, Marrion NV. Mechanisms underlying activation of the slow AHP in rat hippocampal neurons. Brain Res. 2007;1150:74–82. doi: 10.1016/j.brainres.2007.02.067. [DOI] [PubMed] [Google Scholar]
- Madison DV, Nicoll RA. Control of the repetitive discharge of rat CA1 pyramidal neurones in vitro. J Physiol. 1984;354:319–331. doi: 10.1113/jphysiol.1984.sp015378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrion NV, Tavalin SJ. Selective activation of Ca2+-activated K+ channels by co-localized Ca2+ channels in hippocampal neurons. Nature. 1998;395(6705):900–905. doi: 10.1038/27674. [DOI] [PubMed] [Google Scholar]
- McKinney BC, Murphy GG. The L-Type voltage-gated calcium channel CaV1.3 mediates consolidation, but not extinction, of contextually conditioned fear in mice. Learn Mem. 2006;13(5):584–589. doi: 10.1101/lm.279006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney BC, Sze W, Lee B, Murphy GG. Impaired long-term potentiation and enhanced neuronal excitability in the amygdala of CaV1.3 knockout mice. Neurobiol Learn Mem. 2009 doi: 10.1016/j.nlm.2009.06.012. In Press, Uncorrected Proof:doi:10.1016/j.nlm.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney BC, Sze W, White JA, Murphy GG. L-type voltage-gated calcium channels in conditioned fear: A genetic and pharmacological analysis. Learn Mem. 2008;15(5):326–334. doi: 10.1101/lm.893808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer JR, Jr., Power JM, Thompson LT, Disterhoft JF. Increased excitability of aged rabbit CA1 neurons after trace eyeblink conditioning. J Neurosci. 2000;20(14):5476–82. doi: 10.1523/JNEUROSCI.20-14-05476.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer JR, Jr., Thompson LT, Black JP, Disterhoft JF. Nimodipine increases excitability of rabbit CA1 pyramidal neurons in an age- and concentration-dependent manner. J Neurophysiol. 1992;68(6):2100–2109. doi: 10.1152/jn.1992.68.6.2100. [DOI] [PubMed] [Google Scholar]
- Moyer JR, Jr., Thompson LT, Disterhoft JF. Trace eyeblink conditioning increases CA1 excitability in a transient and learning-specific manner. J Neurosci. 1996;16(17):5536–5546. doi: 10.1523/JNEUROSCI.16-17-05536.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy GG, Fedorov NB, Giese KP, Ohno M, Friedman E, Chen R, Silva AJ. Increased neuronal excitability, synaptic plasticity, and learning in aged Kvβ1.1 knockout mice. Curr Biol. 2004;14(21):1907–1915. doi: 10.1016/j.cub.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Murphy GG, Rahnama NP, Silva AJ. Investigation of age-related cognitive decline using mice as a model system: behavioral correlates. American Journal of Geriatric Psychiatry. 2006a;14(12):1004–11. doi: 10.1097/01.JGP.0000209405.27548.7b. [DOI] [PubMed] [Google Scholar]
- Murphy GG, Shah V, Hell JW, Silva AJ. Investigation of age-related cognitive decline using mice as a model system: neurophysiological correlates. American Journal of Geriatric Psychiatry. 2006b;14(12):1012–21. doi: 10.1097/01.JGP.0000209404.54310.b3. [DOI] [PubMed] [Google Scholar]
- Norris CM, Halpain S, Foster TC. Reversal of age-related alterations in synaptic plasticity by blockade of L-type Ca2+ channels. J Neurosci. 1998;18(9):3171–3179. doi: 10.1523/JNEUROSCI.18-09-03171.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MM, Kuo AG, Wu WW, Sametsky EA, Disterhoft JF. Watermaze learning enhances excitability of CA1 pyramidal neurons. J Neurophysiol. 2003;90(4):2171–2179. doi: 10.1152/jn.01177.2002. [DOI] [PubMed] [Google Scholar]
- Oh MM, Power JM, Thompson LT, Disterhoft JF. Apamin increases excitability of CA1 hippocampal pyramidal neurons. Neurosci Res Commun. 2000;27(2):135–142. [Google Scholar]
- Oh MM, Power JM, Thompson LT, Moriearty PL, Disterhoft JF. Metrifonate increases neuronal excitability in CA1 pyramidal neurons from both young and aging rabbit hippocampus. J Neurosci. 1999;19(5):1814–1823. doi: 10.1523/JNEUROSCI.19-05-01814.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno M, Sametsky EA, Silva AJ, Disterhoft JF. Differential effects of αCaMKII mutation on hippocampal learning and changes in intrinsic neuronal excitability. Eur J Neurosci. 2006;23(8):2235–2240. doi: 10.1111/j.1460-9568.2006.04746.x. [DOI] [PubMed] [Google Scholar]
- Pedarzani P, Mosbacher J, Rivard A, Cingolani LA, Oliver D, Stocker M, Adelman JP, Fakler B. Control of electrical activity in central neurons by modulating the gating of small conductance Ca2+-activated K+ channels. J Biol Chem. 2001;276(13):9762–9769. doi: 10.1074/jbc.M010001200. [DOI] [PubMed] [Google Scholar]
- Pedarzani P, Storm JF. PKA mediates the effects of monoamine transmitters on the K+ current underlying the slow spike frequency adaptation in hippocampal neurons. Neuron. 1993;11(6):1023–1035. doi: 10.1016/0896-6273(93)90216-e. [DOI] [PubMed] [Google Scholar]
- Peters HC, Hu H, Pongs O, Storm JF, Isbrandt D. Conditional transgenic suppression of M channels in mouse brain reveals functions in neuronal excitability, resonance and behavior. Nat Neurosci. 2005;8(1):51–60. doi: 10.1038/nn1375. [DOI] [PubMed] [Google Scholar]
- Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, Zheng H, Striessnig J. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 2000;102(1):89–97. doi: 10.1016/s0092-8674(00)00013-1. [DOI] [PubMed] [Google Scholar]
- Power JM, Wu WW, Sametsky E, Oh MM, Disterhoft JF. Age-related enhancement of the slow outward calcium-activated potassium current in hippocampal CA1 pyramidal neurons in vitro. J Neurosci. 2002;22(16):7234–7243. doi: 10.1523/JNEUROSCI.22-16-07234.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascol O, Potier B, Lamour Y, Dutar P. Effects of calcium channel agonist and antagonists on calcium-dependent events in CA1 hippocampal neurons. Fundam Clin Pharmacol. 1991;5(4):299–317. doi: 10.1111/j.1472-8206.1991.tb00725.x. [DOI] [PubMed] [Google Scholar]
- Saar D, Grossman Y, Barkai E. Reduced after-hyperpolarization in rat piriform cortex pyramidal neurons is associated with increased learning capability during operant conditioning. Eur J Neurosci. 1998;10(4):1518–1523. doi: 10.1046/j.1460-9568.1998.00149.x. [DOI] [PubMed] [Google Scholar]
- Sailer CA, Hu H, Kaufmann WA, Trieb M, Schwarzer C, Storm JF, Knaus H-G. Regional differences in distribution and functional expression of small-conductance Ca2+-activated K+ channels in rat brain. J Neurosci. 2002;22(22):9698–9707. doi: 10.1523/JNEUROSCI.22-22-09698.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzkroin PA, Stafstrom CE. Effects of EGTA on the calcium-activated afterhyperpolarization in hippocampal CA3 pyramidal cells. Science. 1980;210(4474):1125–1126. doi: 10.1126/science.6777871. [DOI] [PubMed] [Google Scholar]
- Shah M, Haylett DG. Ca2+ channels involved in the generation of the slow afterhyperpolarization in cultured rat hippocampal pyramidal neurons. J Neurophysiol. 2000;83(5):2554–2561. doi: 10.1152/jn.2000.83.5.2554. [DOI] [PubMed] [Google Scholar]
- Shao L-R, Halvorsrud R, Borg-Graham L, Storm JF. The role of BK-type Ca2+-dependent K+ channels in spike broadening during repetitive firing in rat hippocampal pyramidal cells. J Physiol. 1999;521(1):135–146. doi: 10.1111/j.1469-7793.1999.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackman RW, Hammond RS, Linardatos E, Gerlach A, Maylie J, Adelman JP, Tzounopoulos T. Small conductance Ca2+-activated K+ channels modulate synaptic plasticity and memory encoding. J Neurosci. 2002;22(23):10163–10171. doi: 10.1523/JNEUROSCI.22-23-10163.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker M, Krause M, Pedarzani P. An apamin-sensitive Ca2+-activated K+ current in hippocampal pyramidal neurons. Proc Natl Acad Sci U S A. 1999;96(8):4662–4667. doi: 10.1073/pnas.96.8.4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm JF. Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. J Physiol. 1987;385(1):733–759. doi: 10.1113/jphysiol.1987.sp016517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm JF. An after-hyperpolarization of medium duration in rat hippocampal pyramidal cells. J Physiol. 1989;409(1):171–190. doi: 10.1113/jphysiol.1989.sp017491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm JF. Potassium currents in hippocampal pyramidal cells. Prog Brain Res. 1990;83:161–187. doi: 10.1016/s0079-6123(08)61248-0. [DOI] [PubMed] [Google Scholar]
- Sugiura H, Yamauchi T. Developmental changes in the levels of Ca2+/calmodulin-dependent protein kinase II α and β proteins in soluble and particulate fractions of the rat brain. Brain Res. 1992;593(1):97–104. doi: 10.1016/0006-8993(92)91269-k. [DOI] [PubMed] [Google Scholar]
- Thibault O, Gant JC, Landfield PW. Expansion of the calcium hypothesis of brain aging and Alzheimer's disease: minding the store. Aging Cell. 2007;6(3):307–17. doi: 10.1111/j.1474-9726.2007.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault O, Landfield PW. Increase in single L-type calcium channels in hippocampal neurons during aging. Science. 1996;272(5264):1017–20. doi: 10.1126/science.272.5264.1017. [DOI] [PubMed] [Google Scholar]
- Tippens AL, Langwieser N, Moosmang S, Milner TA, Smith Y, Lee A. Ultrastructural Evidence for Pre- and Postsynaptic Localization of CaV 1.2 L-type Ca2+ Channels in the Rat Hippocampus. J Comp Neurol. 2008;506(4):569–583. doi: 10.1002/cne.21567. [DOI] [PubMed] [Google Scholar]
- Tombaugh GC, Rowe WB, Rose GM. The slow afterhyperpolarization in hippocampal CA1 neurons covaries with spatial learning ability in aged Fisher 344 rats. J Neurosci. 2005;25(10):2609–16. doi: 10.1523/JNEUROSCI.5023-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veng LM, Browning MD. Regionally selective alterations in expression of the α1D subunit (CaV1.3) of L-type calcium channels in the hippocampus of aged rats. Mol Brain Res. 2002;107(2):120–127. doi: 10.1016/s0169-328x(02)00453-9. [DOI] [PubMed] [Google Scholar]
- Veng LM, Mesches MH, Browning MD. Age-related working memory impairment is correlated with increases in the L-type calcium channel protein alpha1D (Cav1.3) in area CA1 of the hippocampus and both are ameliorated by chronic nimodipine treatment. Brain Res Mol Brain Res. 2003;110(2):193–202. doi: 10.1016/s0169-328x(02)00643-5. [DOI] [PubMed] [Google Scholar]
- Weiss C, Sametsky E, Sasse A, Spiess J, Disterhoft JF. Acute stress facilitates trace eyeblink conditioning in C57BL/6 male mice and increases the excitability of their CA1 pyramidal neurons. Learn Mem. 2005;12(2):138–143. doi: 10.1101/lm.89005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JA, McKinney BC, John MC, Powers PA, Kamp TJ, Murphy GG. Conditional forebrain deletion of the L-type calcium channel CaV1.2 disrupts remote spatial memories in mice. Learn Mem. 2008;15(1):1–5. doi: 10.1101/lm.773208. [DOI] [PubMed] [Google Scholar]
- Wu WW, Oh MM, Disterhoft JF. Age-related biophysical alterations of hippocampal pyramidal neurons: implications for learning and memory. Ageing Res Rev. 2002;1(2):181–207. doi: 10.1016/s1568-1637(01)00009-5. [DOI] [PubMed] [Google Scholar]
- Xu W, Lipscombe D. Neuronal CaV1.3 α1 L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. J Neurosci. 2001;21(16):5944–5951. doi: 10.1523/JNEUROSCI.21-16-05944.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, McBain CJ. Potassium conductances underlying repolarization and after-hyperpolarization in rat CA1 hippocampal interneurones. J Physiol. 1995;488(Pt 3):661–672. doi: 10.1113/jphysiol.1995.sp020998. [DOI] [PMC free article] [PubMed] [Google Scholar]