Abstract
The nuclear vitamin D receptor (VDR) mediates the actions of 1,25-dihydroxyvitamin D3 (1,25D) to regulate gene transcription. Recently, the secondary bile acid, lithocholate, was recognized as a novel VDR ligand. Using reporter gene and mammalian two-hybrid systems, immunoblotting, competitive ligand displacement, and quantitative real time PCR, we identified curcumin (CM), a turmeric-derived bioactive polyphenol, as a likely additional novel ligand for VDR. CM (10−5 M) activated transcription of a luciferase plasmid containing the distal vitamin D responsive element from the human CYP3A4 gene at levels comparable to 1,25D (10−8 M) in transfected human colon cancer cells (Caco-2). While CM also activated transcription via a retinoid X receptor (RXR) responsive element, activation of the glucocorticoid receptor (GR) by CM was negligible. Competition binding assays with radiolabeled 1,25D confirmed that CM binds directly to VDR. In mammalian two hybrid assays employing transfected Caco-2 cells, CM (10−5 M) increased the ability of VDR to recruit its heterodimeric partner, RXR, and steroid receptor coactivator-1 (SRC-1). Real time PCR studies revealed that CM-bound VDR can activate VDR target genes CYP3A4, CYP24, p21, and TRPV6 in Caco-2 cells. Numerous studies have shown chemoprotection by CM against intestinal cancers via a variety of mechanisms. Small intestine and colon are important VDR-expressing tissues where 1,25D has known anticancer properties that may, in part, be elicited by activation of CYP-mediated xenobiotic detoxification and/or up-regulation of the tumor suppressor p21. Our results suggest the novel hypothesis that nutritionally-derived CM facilitates chemoprevention via direct binding to, and activation of, VDR.
Keywords: curcumin, vitamin D receptor, retinoid X receptor, cancer prevention, anticancer diet, tumeric
1. Introduction
The active hormonal metabolite of vitamin D, 1α,25-dihydroxyvitamin D3 (1,25D), has a broad spectrum of biological actions which have been extensively reviewed [1–3]. 1,25D stimulates intestinal calcium and phosphate absorption, bone calcium and phosphate resorption, and renal calcium and phosphate reabsorption. These actions of 1,25D effect calcium and phosphate homeostasis and ensure proper remodeling of the mineralized skeleton. An obligate mediator of 1,25D action is a transcription factor, the vitamin D receptor (VDR), a member of the nuclear and steroid receptor superfamily. Binding of 1,25D elicits conformation changes in VDR which lead to recruitment of its co-receptor, the retinoid X receptor (RXR). A liganded VDR-RXR heterocomplex then binds to short sequences of DNA, termed vitamin D responsive elements (VDREs), that are typically in the vicinity of 1,25D-regulated genes. Once bound to a VDRE, the VDR-RXR duplex induces transcription by recruiting coactivators with histone acetyl transferase activity, such as steroid receptor coactivator-1 (SRC-1), as well as helping assemble other components of the RNA polymerase promoter complex.
In addition to the classic target genes regulated by 1,25D-VDR that mediate bone and mineral homeostasis, of particular interest is the capacity of liganded VDR to regulate cell growth and division. The ability of 1,25D analogs, such as calcipotriol, to prevent hyperproliferation and induce differentiation has been successfully employed to treat psoriasis [4–6]. Moreover, 1,25D and its analogs exhibit potential in chemoprevention of a variety of cancers, particularly those of the prostate and colon [7, 8]. Mechanistically, it has been shown that 1,25D controls cell division by arresting cells in the G1/G0 phase of the cell cycle and by upregulating powerful tumor suppressor genes such as p21 [9, 10]. An important pathway for prevention of colon cancer by VDR may be detoxification of the carcinogenic secondary bile acid, lithocholate (LCA) [11]. LCA activates self-detoxification by inducing transcription of cytochrome P450-3A4 (CYP3A4) via direct binding to VDR. LCA acts as a low-affinity VDR ligand that is able to perform some of the same traditional functions of 1,25D in vitamin D-deficient rats [12]. The establishment of LCA as a bona fide VDR ligand led us to hypothesize that there may be additional novel ligands for VDR which may play a role in cancer chemoprevention. In this study, we evaluate curcumin as a potential VDR ligand.
Curcumin (CM) is a major biologically active component of turmeric, which is abundant in the traditional Indian diet. A number of studies reveal that curcumin inhibits tumor initiation by suppressing proinflammatory pathways and inducing phase II conjugating enzymes, such as sulfotransferase and glutathione-S-transferase, that facilitate the excretion of carcinogens (reviewed in [13]). The conjugated nature of the CM molecule that leads to its ability to act as an antioxidant is another important factor for cancer chemoprevention [14]. CM exhibits particular promise as a therapeutic and preventative agent for gastrointestinal cancer, where it displays a modest bioavailability in the colon following oral administration [15]. Notably, there is a significant overlap among the molecular targets of 1,25D and CM; thus, both molecules prevent TNF-induced degradation of IκB, leading to attenuated activity of NF-κB, a well-known cancer-promoter [16–18]. CM has also been shown to activate p21 in a p53-independent fashion in breast (MCF-7), prostate (PC-3) and colon (Colo-205) cancer cells [19–21]. The upregulation of p21 by 1,25D and its analogs also has been documented in MCF-7 and PC-3 cells [22, 23].
In the current study, we present the first evidence which supports a novel hypothesis that CM is a nutritionally-derived ligand of VDR. CM is able to bind VDR, induce recruitment of its co-receptor RXR, co-activator SRC-1, and activate transcription of a VDR-target gene, CYP3A4, in colon cancer cells. Moreover, we provide data that upregulation of p21 by CM may be at least in part mediated by VDR, and we investigate the molecular mechanism that is perhaps responsible for the previously reported synergism between 1,25D and CM in promoting differentiation of HL-60 cells [24].
2. Methods and materials
2.1. Transfection of cultured mammalian cells and transcriptional activation assays
Cells were grown at 37 °C under a humidified atmosphere of 5% carbon dioxide. All cell lines in this study originated from the ATCC (Manassas, VA) and were transfected in Costar® polystyrene 24 well plates from Corning Inc. (Corning, NY) using Lipofectamine Transfection Reagent in combination with Plus Reagent, both supplied by Invitrogen (Carlsbad, CA). Human colorectal adenocarcinoma cells (Caco-2) were plated at a density of 40,000 cells/well approximately 24 hours prior to transfection in minimum essential medium (MEM), supplemented with 20% fetal bovine serum (FBS), 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 100 units/mL penicillin and 100 μg/mL streptomycin. The transfection procedure was adapted from the manufacturer’s protocol. Briefly, each well received 1 μL of Lipofectamine Reagent, 2 μL of Plus Reagent, 500 ng of pTZ18U carrier DNA plasmid, and 20 ng of pRL-null (constitutively expressing low levels of Renilla reniformis luciferase) to monitor transfection efficiency. Each well also received 250 ng of pLuc-MCS plasmid(Stratagene, La Jolla, CA) containing an oligonucleotide (cloned between the HindIII and BglII sites) with two copies of a nuclear receptor responsive element upstream of the firefly (Photinus pyralis) luciferase gene. The VDRE, designated XDR3, was the distal element from the human cytochrome P450 (CYP) 3A4 gene [25]. The entire sequence inserted into pLuc-MCS reporter vector was CAGAGGGTCAGCAAGTTCATTCACAGAGGGTCAGCAAGTTCATTCA, with the half elements underlined. A glucocorticoid responsive element (GRE) derived from the rat tyrosine aminotransferase gene was employed, and the sequence cloned into pLuc-MCS was AGCTAGAACATCCTGTACAGCAGAGCTAGAACATCCTGTACAGCAG [26]. The reporter construct containing a retinoid X receptor responsive element (RXRE) was based on a naturally occurring double repeat responsive element from the rat cellular retinol binding protein II gene [27]. The sequence used was AAAATGAACTGTGACCTGTGACCTGTGACCTGTGAC. In addition to the responsive element reporter constructs, cells were also cotransfected with 50 ng of a pSG5-based expression plasmid containing the appropriate nuclear receptor. The cells received both 50 ng of pSG5-VDR, and 20 ng of pSG5-RXRα when the VDRE-containing reporter was employed. The cells were treated with known nuclear receptor ligands or curcumin, as described in the figure legends, 18 hours after completion of transfection; treatment times ranged from 24 to 30 hours. After incubation with ligands, cells were collected and the amount of reporter gene product (luciferase) produced in the cells was measured using the Dual-Luciferase® Reporter Assay System according to the manufacturer’s protocol (Promega, Madison, WI). To control for transfection efficiency, general cell death and ligand-induced cellular toxicity, the amount of luminescence produced by inducible firefly luciferase was divided by luminescence created by constitutively expressed Renilla luciferase; this ratio was then multiplied by 10,000 to simplify data presentation. The mean ratio of firefly luciferase to Renilla luciferase signal was determined for each experimental group, and the standard deviation was calculated using MS Excel (expressed as error bars). An asterisk denotes data which display statistically significant differences compared to the appropriate vehicle control (P<0.05 in a Student’s t-test). All data are reported as either the average of two or more experiments, or are representative of two or more trials. Each experimental treatment group was replicated in at least three, and often as many as six wells.
2.2. Mammalian two hybrid transfections
Caco-2 cells were transfected with components of the Mammalian Two Hybrid® system from Stratagene, employing similar procedures to those outlined above. RXRα was cloned into pCMV-BD (bait) and VDR into pCMV-AD (prey). SRC-1 was cloned into the pM vector from Clontech (Mountain View, CA), which is similar to the pCMV-BD construct, and is compatible with pCMV-AD. Each well received 50 ng of bait, 50 ng of prey vectors and 20 ng of pRL-Null; 500 ng of pFR-luc, a firefly luciferase reporter construct, was also introduced into the cells. The cells were assayed for luciferase activity and results were analyzed as described in the previous section. Negative controls employing “empty” AD and BD vectors were also tested as described previously [28].
2.3. Competitive binding assays
To prepare cell lysates for competitive ligand binding assays, VDR-deficient COS-7 cells were plated at 2.5×106 cells per 150 mm plate and transfected approximately five hours later by the calcium-phosphate DNA coprecipitation method [29]. Each plate received 5 μg of pSG5-hVDR, 5 μg of pSG5-hRXRα and 40 μg of pTZ18U plasmids. The growth medium was replaced 24 hours after the cells were plated. The transfected cells were then allowed to grow for an additional 24 hours, followed by three 10 mL washes with cold PBS, and harvested by scraping (three times, each in 5 mL of PBS). A cell pellet was obtained after a five minute spin at 2000 rpm (performed at 4 °C); PBS was removed and the cells were resuspended in 1.5 mL of KTEZ0.3+5 buffer (10 mM Tris-HCl, pH 7.5, 1 mM EDTA, 0.3 mM zinc acetate, 0.3 M KCl, 5 mM DTT, 0.2 mg/mL Pefabloc SC, 2 μg/mL of aprotinin, 1 μg/mL of leupeptin, 1 μg/mL of pepstatin A and 2.5 μg/mL of E64). Resuspended cells were sonicated to disrupt plasma membranes and centrifuged at 58,000 rpm at 4 °C for 30 minutes. The supernatant containing VDR and RXR was used for competitive ligand binding assays.
The prepared lysate (10 μL) was diluted 1:20 in KTEZ0.3+5 buffer and mixed with 5 μL of a 51 Ci/mmol solution of 1α,25-dihydroxy[26,27-methyl-3H]cholecalciferol (Amersham, Piscataway, NJ) (final concentration approximately 4.0×10−10 M). Ligands of interest were added to the resulting solution and allowed to equilibrate with radiolabeled 1,25D for 15 hours. The unbound 1,25D was removed by addition of 80 μL of Dextran coated charcoal (Sigma-Aldrich; St. Louis, MO) for 15 minutes. The samples were then centrifuged at 10,000 rpm for 2 min, and 200 μL of the supernatant were transferred to a scintillation vial along with 4 mL of scintillation cocktail (ScintiSafe 30%) (Fisher Scientific, Pittsburgh, PA). After a one hour incubation, the samples were assayed using a Beckman (Fullerton, California) LS 5801 scintillation counter and analyzed with Prism4 (GraphPad Software, Inc.; San Diego, CA).
2.4. Western blotting
Rat osteosarcoma cells (ROS 17/2.8) were plated in DMEM/F12 (1/2 Dulbecco’s modified Eagle’s medium; 1/2 Ham’s F12), supplemented with 10% FBS, 100 units/mL penicillin and 100 μg/mL streptomycin at 320,000 cells/well (6-well plate) 18 hours prior to the treatment with 1,25D or CM. Caco-2 cells were grown as described in section 2.1, except using 6-well plates when preparing extracts for immunoblotting. After incubating with a ligand for 4 or 24 hours, the cells were washed with PBS and lysed in a solution of 2% SDS, 0.125 M Tris-HCl, pH 6.8, and 20% glycerol. The protein content of the lysates was determined using a BCA assay (Thermo Fisher Scientific, Rockford IL) and 100 μg of protein from each sample were run on a 5%–15% gradient SDS-polyacrylamide gel, followed by electrotransfer to Immobilon P membrane (Millipore Corp., Bedford, MA). The transfer was performed using a Transblot apparatus in 25 mM Tris-HCl, pH 7.4, 192 mM glycine, 0.01% SDS, and 20% methanol. The membrane was then blocked by incubation for 1 hour with 3% dry milk, 10 mM Tris-HCl, pH 7.5 and 150 mM NaCl, followed by treatment for 3 hours at room temperature with a 1:10,000 dilution of the anti-VDR monoclonal antibody, 9A7γ[30] or 1:500 dilution of anti-CYP24 polyclonal antibody (H-87; Santa Cruz Biotechnology, Santa Cruz, CA). A horseradish peroxidase conjugated anti-rat IgG (against 9A7γ) or anti-rabbit IgG (against H-87) was incubated with the membrane overnight at 4 °C followed by washes and visualization using the Enhanced Chemiluminescence (ECL) detection system (Amersham, Piscataway, NJ).
2.5. Real-time PCR
Real-time PCR was performed on: the human CYP3A4 gene using 5′-CCAGTATGGAGATGTGTTGGTGAG-3′ and 5′-TCTTGTGGATTGTTGAGAGAGTCG-3′ primers (153 bp product); the human CYP24 gene using 5′-CAGCGAACTGAACAAATGGTCG-3′ and 5′-TCTCTTCTCATACAACACGAGGCAG-3′ primers (58 bp product); the human p21 gene using 5′-AGGAAGACCATGTGGACCTGTCAC-3′ and 5′-GGCGTTTGGAGTGGTAGAAATCTG-3′ primers (147 bp product); the human TRPV6 gene using 5′-CCTCAAGCCCAGGACCAATAAC-3′ and 5′-TCTACCAGCAGGATGATGATAGCC-3′ primers (156 bp product), and the rat VDR gene using 5′-GCAAAGGTTTCTTCAGGCGG-3′ and 5′-CTTGGTGATGCGGCAATCTC-3′ primers (80 bp product). Total RNA was isolated from 2×106 Caco-2 or ROS 17/2.8 cells (treated with ligands for 4 or 24 hours in serum free medium) using an Aurum Total RNA Mini Kit (Bio-Rad, Hercules, CA). The RNA obtained was quantified using A260/A280 spectrophotometry. DNase treated RNA (2 μg) was reverse transcribed using the iScript cDNA Synthesis Kit (Bio-Rad). The obtained cDNA was used in 20 μL PCR reactions containing 10 L iQ SYBR Green Supermix (Bio-Rad), 1 μL primers, 2 μL of cDNA template sample, and 7 μL of molecular grade water. Reactions were performed in 96-well PCR plates and read on a Bio-Rad iCycler iQ Real-Time PCR detection system or an ABI 7500 Fast instrument. Data were analyzed using the comparative Ct method as a means of relative quantitation, normalized to an endogenous reference (GAPDH cDNA) and relative to a calibrator (normalized Ct value obtained from vehicle-treated cells) and expressed as 2−ΔΔCt according to Applied Biosystems User Bulletin 2: Rev B, “Relative Quantitation of Gene Expression.”
2.6. Epithelial cell migration assay
Cell migration assays were performed essentially as described previously [31]. Caco-2 cells were grown to 90% confluency in minimum essential medium (MEM), supplemented with 20% fetal bovine serum (FBS), 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 100 units/mL penicillin, and 100 μg/mL streptomycin in 6-well plates. Cells were starved in serum-free media for 24 h, and a linear “lesion” was generated by scratching the monolayers with a sterile cell scraper along a line drawn on each plate. Detached cells were removed by gently washing in PBS. The cells were then incubated in MEM containing 1% FBS in the presence or absence of 10−7M 1,25D or a range of CM concentrations (10−6 to 10−4 M) for 48 h. Cell migration across the lesion line was assessed under a microscope and recorded by color photography using Kodak UltraMAX 400 film.
3. Results
3.1. Curcumin activates VDR and stimulates VDRE-mediated transcription
We hypothesized that VDR may be one of the direct mediators of curcumin bioactions. Several experiments were performed to test this hypothesis, including the use of transcription assays employing a VDRE-firefly luciferase reporter plasmid, mammalian two hybrid assays, competition binding assays to evaluate the direct association of CM and VDR in vitro, and quantitative real time PCR assessment of CM-mediated induction of VDR target genes, as well as a Caco-2 cell migration assay.
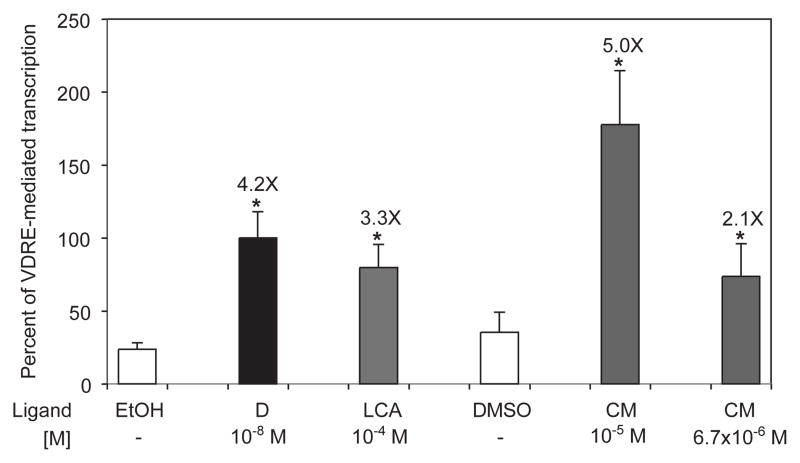
Fig. 1 shows the pooled results of three independent experiments in which Caco-2 human colon cancer cells were transfected with a reporter plasmid and treated with curcumin and other known VDR ligands. Treatment with 10−8 M 1,25D and 10−4M LCA for 30 hours in complete medium, including serum, boosted transcription of the reporter plasmid 4.2- and 3.3-fold, respectively. Cells treated with 6.7×10−6 M and 10−5 M CM also demonstrated a dose dependent increase (2.1- and 5.0-fold, respectively) in the level of transcription of the VDRE-reporter plasmid.
Fig. 1.
Curcumin activates transcription of an XDR3 reporter construct in a concentration dependent fashion. Human colon cancer cells (Caco-2) were transfected with a firefly luciferase plasmid containing two copies of the distal vitamin D responsive element, XDR3, from the human cytochrome P450 (CYP) 3A4 gene. The cells were treated for 30 hours in complete media with 10−8 M 1,25D (black bar; D), 10−4 M lithocholic acid (grey bar; LCA), and two concentrations of curcumin (grey bar; CM). Ethanol was the vehicle for D and LCA, while curcumin was reconstituted in DMSO. The data were normalized for transfection efficiency as described in Methods and Materials and expressed as percent of 1,25D-stimulated transcription. The mean fold effect of each ligand was calculated with respect to the appropriate vehicle and is shown above each bar; error bars represent standard deviation, and asterisks indicate statistically significant differences (p < 0.05) from the vehicle control. The data represent the mean of three independent experiments.
3.2. VDR heterodimerizes with RXR and interacts with a nuclear receptor coactivator in the presence of curcumin
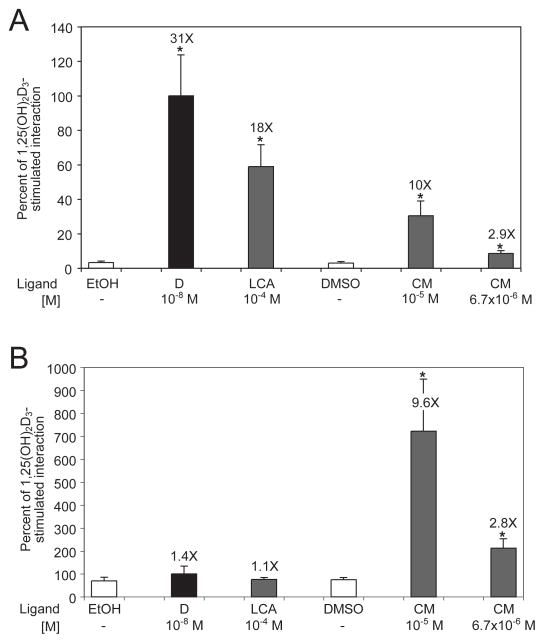
The next approach to evaluating curcumin as a potential ligand for VDR was assessment of its ability to promote recruitment of the VDR co-receptor, RXR, in the mammalian two hybrid system. Formation of the VDR-RXR heterodimer is a step necessary for initiation of VDR-mediated transcription [1, 2]. The data in Fig. 2A indicate that 10−5 M CM is 32% as effective as 10−8 M 1,25D and 56% as effective as 10−4 M LCA in recruiting RXR upon binding to VDR. Decreasing the CM concentration from 10−5 M to 6.7×10−6 M weakened its ability to induce VDR-RXR heterodimerization; however, it was still significantly greater than the heterodimerization observed in the cells treated with DMSO vehicle (P<0.001).
Fig. 2.
Evaluation of curcumin in the mammalian two hybrid system. (A) Curcumin induces heterodimerization of VDR and RXR. Human colon cancer cells, Caco-2, were transfected with expression vectors encoding RXRα bait (BD) and VDR prey (AD) fusion constructs. A firefly luciferase reporter vector (pFR-luc) and Renilla luciferase control plasmid were also introduced into the cells to measure the amount of ligand-stimulated VDR-RXR interaction and to measure transfection efficiency, respectively. The cells were treated for 30 hours in complete media with 10−8 M 1,25D (black bar; D), 10−4 M lithocholic acid (grey bar; LCA) and two concentrations of curcumin (grey bar; CM). Negative controls included the use of BD-empty and AD-empty expression vectors which resulted in low background levels of luciferase activity (not shown). (B) Curcumin induces heterodimerization of VDR and SRC-1. Caco-2 cells were transfected with expression vectors encoding SRC-1 bait (BD) and VDR prey (AD) fusion constructs. The cells were treated for 30 hours as in A. The data were normalized for transfection efficiency and expressed as a percent of 1,25D-stimulated interaction between VDR and RXR (A) or VDR and SRC-1 (B), with error bars indicating standard deviation. Differences statistically significant from control are indicated by asterisks as described in the legend to Fig. 1.
To further evaluate curcumin as a bona fide ligand that stimulates VDR-mediated transcription of target genes, the capacity of CM to facilitate recruitment of the SRC-1 coactivator was measured in the mammalian two hybrid system. In transfected Caco-2 cells, neither 10−8 M 1,25D nor 10−4 M LCA was able to induce significant dimerization of VDR and SRC-1 expressed from the two hybrid constructs when the cells were treated with those ligands in complete medium including serum for 30 hours (Fig. 2B). In stark contrast, treatment with 10−5 CM produced an almost 10-fold increase in SRC-1 recruitment compared to the cells treated with DMSO vehicle. Similar to the trend observed with RXR recruitment, when a lower concentration of curcumin was used (6.7×10−6 M), CM-bound VDR was significantly less efficient in attracting SRC-1, but still more efficacious than 1,25D-bound VDR. Since 1,25D is transcriptionally active in Caco-2 (Fig. 1), and VDR-mediated transcription is not possible without coactivators [1,2], 1,25D may stimulate preferential recruitment of other cell-type specific coactivators, such as SRC-2 and SRC-3, which were not assessed in this mammalian two hybrid system. Unlike 1,25D, curcumin may conform VDR in a way that favors interaction with SRC-1 rather than interactions with other types of coactivators, explaining the high level of VDR-SRC-1 association observed in Fig. 2B.
3.3. Curcumin binding displays nuclear receptor specificity
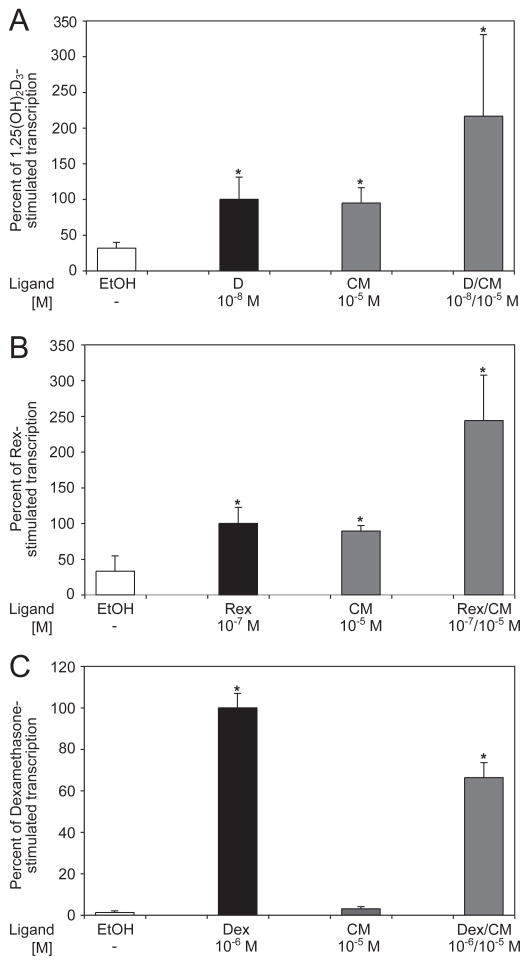
To determine whether curcumin specifically activates gene expression via VDR or has a more generic effect on all nuclear receptors, the ability of curcumin to induce transcription of reporter constructs containing a VDRE, RXRE or GRE was evaluated. Caco-2 cells were transfected with the indicated reporter constructs (described in the Methods and Materials) to compare the amount of transcription induced by curcumin in the same experiment. The results shown in Fig. 3A demonstrate that 10−5 M CM is 95% as efficient as 10−8 M 1,25D in inducing transcription of the reporter plasmid containing two distal vitamin D responsive elements from the human CYP 3A4 gene. When cells transfected with the same reporter vector are treated with a mixture containing 10−5 M CM and 10−8 M 1,25D, the level of transcription observed is 2.16-fold greater than in cells treated with 10−8 M 1,25D alone, and this difference is statistically significant (P<0.05). Curcumin had a similar effect on the transcription of a reporter plasmid containing the RXRE (Fig. 3B). CM at 10−5 M was 89% as potent as 10−7 M LG101305 (Rex), a synthetic ligand for RXR, in stimulating the transcription of the reporter gene. When cells containing the same reporter plasmid were treated with both CM and Rex, 2.44 times more transcription was observed than in cells treated with Rex alone.
Fig. 3.
Specificity test of curcumin binding to the retinoid X receptor and glucocorticoid receptor. Human Caco-2 cells were transfected with appropriate firefly luciferase reporter constructs and nuclear receptor expression plasmids, as well as Renilla luciferase to normalize for transfection efficiency. The cells were treated with the indicated combinations of ligands for 24 hours and a dual luciferase assay was performed. A total of three independent experiments were carried out with each reporter construct. (A) Transcriptional activation by VDR. The cells received VDR expression vector and the luciferase reporter plasmid containing two copies of the XDR3. The results were normalized for transfection efficiency and plotted as a percent of 1,25D-stimulated transcription. (B) Transcriptional activation by RXR. The cells received an RXR expression vector and the luciferase reporter plasmid containing the RXRE from the rat cellular retinol binding protein, type II, gene. The results were normalized for transfection efficiency and plotted as a percent of rexinoid LG101305 (Rex)-stimulated transcription. (C) Transcriptional activation by GR. A GR expression vector and the luciferase reporter plasmid containing two copies of the GRE from the rat tyrosine aminotransferase gene were introduced into the cells. The results were normalized for transfection efficiency and plotted as a percent of dexamethasone (Dex)-stimulated transcription. Error bars represent standard deviation.
In contrast to the effect of curcumin on vitamin D- and retinoid X-responsive element reporter constructs, CM did not appear to significantly activate transcription of a reporter plasmid containing a glucocorticoid responsive element. Despite the observation that 10−5 M CM treatment displayed a modest fold increase in transcriptional activity compared to the vehicle control, this increase was only 3% of the reporter transcription elicited by treatment with 10−6M dexamethasone (Dex), a high affinity ligand of glucocorticoid receptor (Fig. 3C). In addition, treatment with both CM and Dex produced less transcription than treatment with Dex alone. Thus, curcumin displays at least some level of nuclear receptor specificity when tested under the conditions of these assays.
3.4. Mechanism of curcumin-mediated potentiation of 1,25D action
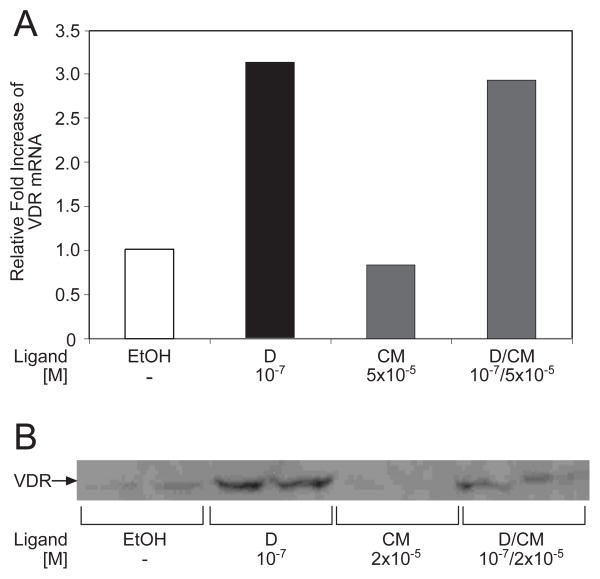
In order to investigate the molecular basis for the large increase in the level of VDR-mediated transcription by the combined treatment of cells with CM and 1,25D, two routes were pursued. Employing real-time PCR, we explored the potential of 24 hour treatment with 5×10−5 M CM to upregulate expression of VDR mRNA in osteosarcoma (ROS 17/2.8) and Caco-2 cells. Increased levels of VDR mRNA could lead to a higher receptor concentration and more robust transcriptional initiation. However, the graph depicted in Fig. 4A indicates that CM alone did not increase ROS 17/2.8 VDR mRNA content, while dual treatment with 1,25D and CM was slightly less effective than 1,25D alone. Similar results were obtained with ligand treatment for 4 hours in ROS 17/2.8 cells or for 4 or 24 hours in Caco-2 cells (data not shown). Thus, curcumin is unlikely to potentiate the action of 1,25D-VDR by increasing the level of receptor mRNA.
Fig. 4.
Evaluation of VDR mRNA and protein levels in 1,25D- and curcumin-treated cells. (A) Real-time PCR. Rat osteoblast-like osteosarcoma cells (ROS 17/2.8) cells were treated with 10−7 M 1,25D, 5×10−5 M CM, or a combination of both compounds for 24 hours in order to evaluate potential regulation of VDR mRNA. Relative levels of VDR mRNA were measured using quantitative real-time PCR as described in Methods and Materials. (B) Western blots. ROS 17/2.8 were treated as in A, followed by preparation of cell lysates in a solution of 2% SDS, 0.125 M Tris-HCl, pH 6.8 and 20% glycerol. The protein content of the lysates was determined using a BCA assay, and 100 μg of total protein from each sample were run on a 5%–15% gradient SDS-polyacrylamide gel, followed by Western blotting with an antibody (9A7γ) directed against VDR (see Methods and Materials). This result is representative of three independent experiments.
An alternative mechanism for CM potentiation of VDR action is at the protein level (for example, by protecting VDR from degradation), rather than at the transcriptional level. We therefore performed Western blots to investigate directly the effect of CM on VDR content in ROS 17/2.8 cells. This cell line was used instead of Caco-2 because of abundant endogenous VDR levels that can be more readily detected by Western blotting [32]. Based on a representative experiment shown in Fig. 4B, 24 hour treatment with CM (2×10−5 M) did not increase levels of VDR compared to the vehicle control. The cells treated with both 1,25D and CM had, in fact, a lower VDR content than cells treated with 1,25D alone. Thus, effects of CM on VDR mRNA or protein cannot explain the marked increase in transcriptional activity induced by the dual ligand treatment with 1,25D and CM observed in Fig. 3A.
3.5. Curcumin can compete with 1,25D for direct binding to VDR
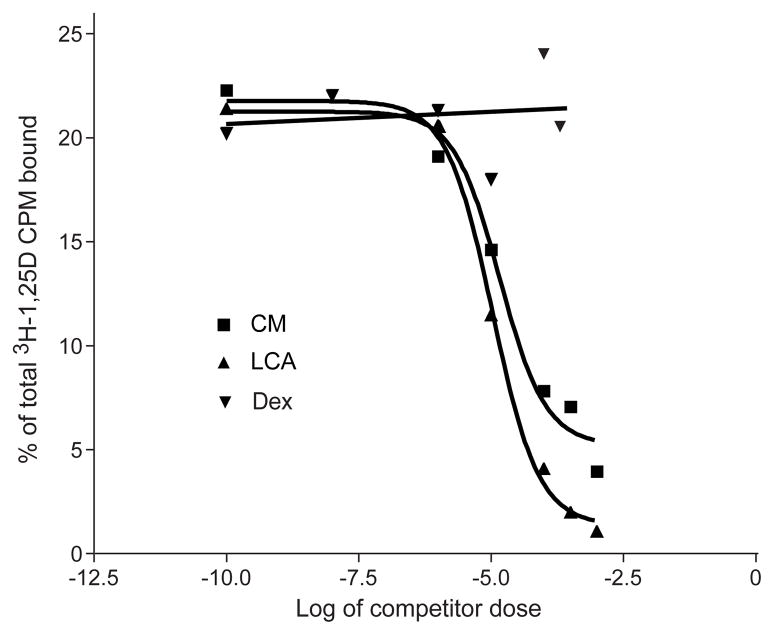
To obviate the possibility that curcumin is not a VDR ligand, but rather affects VDRE-mediated transcription indirectly, perhaps through direct binding to only RXR, in vitro competition binding assays were performed (see Methods and Materials). As illustrated in Fig. 5, which contains data from a single representative experiment from three independent tests, curcumin successfully competed with radiolabeled 1α,25-dihydroxy[26,27-methyl-3H]cholecalciferol for binding to VDR in a transfected cell lysate containing both human VDR and RXR proteins. At 10−4 M, the CM ligand effectively competed more than 50% of the amount of radiolabeled 1,25D bound to VDR, compared to negligible competition by Dex, a lipophilic ligand without appreciable binding affinity for VDR. LCA, a recognized novel ligand of VDR, was included for comparison with curcumin, and was only slightly more effective than CM in competing with 1α,25-dihydroxy[26,27-methyl-3H]cholecalciferol for VDR binding (inhibition constant (Ki) = 2.1 μM and 2.9 μM, respectively, assuming a Kd for 1,25D-VDR = 10−10 M). Overall, these findings not only indicate that curcumin directly binds to VDR, but also strongly suggest that CM activates transcription via a VDRE by recruiting co-receptor RXR and coactivator SRC-1.
Fig. 5.
Ability of curcumin to compete with 1,25D for binding to VDR. Competition curves display the concentration range in which curcumin is able to compete for binding to VDR with ≈ 4.0×10−10 M [3H]1,25D. Dexamethasone (Dex) is a GR ligand and has no appreciable binding to VDR, thus serving as a non-competing negative control. Lithocholic acid (LCA) was included as a positive (competing) control. This plot was generated in Prism4 (GraphPad Software, Inc.) and is representative of three independent experiments.
3.6. Curcumin activates 1,25D target genes as assessed by RT-PCR
If curcumin is truly a biologically relevant VDR ligand, it must activate transcription of VDR target genes in their natural chromatin context. To test this hypothesis, we employed quantitative real-time PCR to measure the effect of CM treatment in Caco-2 cells on the mRNA levels of CYP3A4, CYP24, p21, and TRPV6, genes that are known to be regulated by 1,25D-VDR [24, 25]. To test whether upregulation of these genes is by a direct effect of CM on VDR or elicited by some other mechanism, the measurements were performed in cells containing either endogenous or additional co-transfected VDR. Table 1 summarizes the results obtained from 4 independent experiments expressed as fold induction of the mRNAs by 4 hour treatment (in serum free medium) with 10−7 M 1,25D or 5×10−5 M CM as compared to the vehicle control. CYP3A4 was upregulated 1.6-fold in the presence of 1,25D, p21 was only induced 1.2-fold, and TRPV6 was stimulated 2.4-fold under the same conditions. Providing exogenous VDR via cotransfection yielded a more potent induction of CYP3A4, p21, and TRPV6 mRNAs by 1,25D (3.1-, 2.5- and 5.3-fold, respectively). In untransfected cells, CM had a 1.4- and 2.2-fold effect on CYP3A4 and TRPV6, respectively. However, increased VDR content boosted its ability to activate these gene to new levels of 3.0- and 4.1-fold, respectively. Induction of p21 by CM is strong in the presence of endogenous VDR (7.9-fold), while CM treatment in the presence of additional co-transfected VDR leads to an even greater 11.6-fold increase in p21 mRNA. Taken together, these data indicate that CM treatment not only leads to upregulation of these target genes, but also that this upregulation is VDR-dependent.
Table 1.
Induction of VDR-target genes by 1,25D and CM in Caco-2 cells as measured via real-time PCR.
| Gene | Ligand | Average (± S.D.) Relative Fold Increase in Target Gene mRNA (n=3) | |
|---|---|---|---|
| Endogenous VDR | Co-transfected VDR | ||
| CYP3A4 (Xenobiotic detoxification) | +D (10−7 M) | 1.55 ± 0.29 | 3.10 ± 0.56 |
| +CM (5 × 10−5 M) | 1.41 ± 0.19 | 3.02 ± 0.64 | |
| p21 (Cell differentiation and division) | +D (10−7 M) | 1.22 ± 0.09 | 2.52 ± 0.45 |
| +CM (5 × 10−5 M) | 7.91 ± 1.67 | 11.58 ± 1.92 | |
| TRPV6 (Intestinal calcium transport) | +D (10−7 M) | 2.41 ± 0.53 | 5.32 ± 1.12 |
| +CM (5 × 10−5 M) | 2.17 ± 0.44 | 4.11 ± 0.98 | |
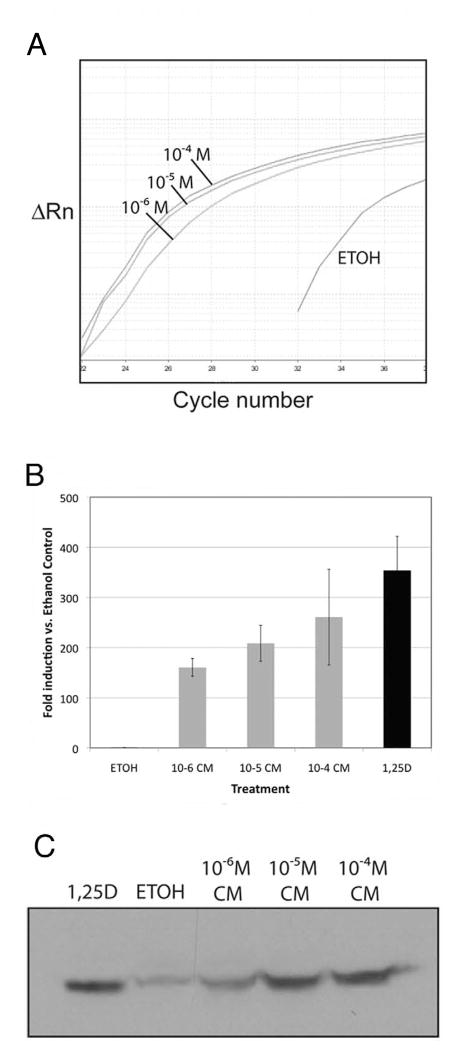
Given the VDR-augmented increase in mRNA from vitamin D target genes by treatment of cells with 1,25D or CM, we next sought to determine if a classic vitamin D-regulated gene such as CYP24 could be induced by CM in a dose-dependent fashion. Figure 6A illustrates an actual amplification plot from a real-time PCR experiment using Caco-2 cells that were treated with increasing amounts of CM. When these data are analyzed using the comparative Ct method as means of relative quantitation, and normalized to an endogenous reference (GAPDH), the results demonstrate a classic CM dose-dependent enhancement of both CYP24 (Fig. 6B) and p21 expression (data not shown), with the highest level of CM statistically comparable to 10−7M 1,25D treatment (Fig. 6B, black bar). The curcumin dose-dependent transactivation effect is nearly perfectly reflected at the protein level as shown in the CYP24 immunoblot of extracts of Caco-2 cells (Fig. 6C) treated under the same conditions as in Fig. 6B. These results provide additional corroborative evidence that CM is a bona fide VDR ligand that can induce traditional vitamin D target genes at both the RNA and protein level.
Fig. 6.
Curcumin regulates CYP24 (25-hydroxyvitamin D 24-hydroxylase) in human Caco-2 cells in a dose-dependent fashion. (A) Results of a real-time PCR experiment using human CYP24 primers and cDNA prepared from Caco-2 cells incubated for 4 h with the indicated concentration of CM or ethanol vehicle. Results are plotted as ΔRn vs. cycle number where ΔRn is the reporter dye signal normalized to the passive reference dye, and Rn is Rn from which the baseline dye signal has been subtracted. (B) Induction of CYP24 mRNA by the indicated ligands as compared to the ethanol control. Data were obtained using Ct values from real time PCR as described in A; error bars represent triplicate determinations ± SD. (C) Western blotting of CYP24 protein detected in cell lysates from Caco-2 cells incubated with the indicated ligand.
3.6. Curcumin stimulates Caco-2 cell migration in vitro
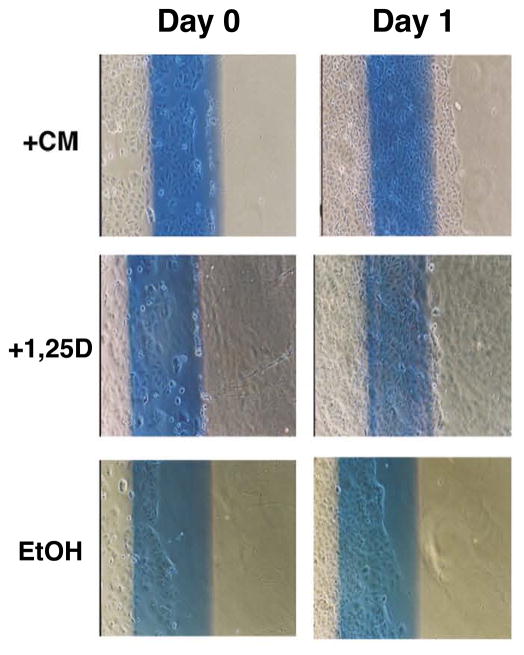
Intestinal epithelial repair is thought to be driven, in part, by 1,25D-activated, VDR-dependent pathways [31]. Thus, another readout of VDR activation in the context of a cellular assay employing Caco-2 cells is the ability of a putative VDR ligand to stimulate cell migration in a cell culture “scratch” assay. Migration is measured after cells are starved for 24 hours in serum-free medium, then wounded by scratching, followed by growth in 1% serum-containing medium for 24–48 hours. Treatment with 10−7 M 1,25D stimulated cell migration after the monolayer was scratched (indicated by line) and allowed to grow, when compared to the vehicle-treated (EtOH) control cells (Fig. 7). Importantly, the CM-treated cells also displayed significant cell migration comparable, and in some cases superior to that observed with the 1,25D treatment group. These results suggest that both 1,25D and CM can serve to enhance VDR-mediated epithelial restitution in mucosal wound healing using Caco-2 as a model in cellulo system.
Fig 7.
1,25D and curcumin modulate Caco-2 cell migration. Caco-2 cells were grown in MEM to 90% confluency. Cells were starved in serum-free media for 24 h, and a linear lesion (“scratch”) was generated by removing the monolayer with a sterile cell scraper along a line drawn on each plate. The cells were then incubated with MEM+1% FBS and either ethanol (EtOH), 10−7 M 1,25D (+1,25D), or 10−4 M curcumin (+CM) for 24 h. Cell migration was assessed under an inverted phase contrast microscope (magnification: 40X).
4. Discussion
The biologically active component of turmeric, curcumin, was investigated as a potential novel nutritionally-derived VDR ligand which may play a role in colon cancer chemoprevention. This study demonstrates that CM competes with radiolabeled 1,25D for binding to VDR as effectively as LCA, a known alternative ligand for VDR [11]. Further, CM is capable of occupying the VDR ligand binding pocket and conforming the receptor into its transcriptionally active form. In human colon cancer cells (Caco-2), CM induced recruitment of VDR transcriptional comodulators, RXR and SRC-1, and activated transcription of a VDRE-containing reporter construct. CM treatment upregulates known VDR target genes such as CYP3A4, CYP24, p21, and TRPV6 in the context of their natural promoters as measured by quantitative RT-PCR in Caco-2 cells. Finally, CM is as effective as 1,25D in stimulating cell migration in a cell monolayer “scratch” assay, suggesting that CM-VDR is a biologically relevant complex in colonic cells that could facilitate mucosal wound healing [31].
Activation of VDR by CM required a much higher concentration (10−6 to 10−5 M) than the endocrine 1,25D ligand (10−8M), suggesting that a relatively low-affinity ligand may not achieve physiologic levels. However, up to 8 g of CM can be safely administered orally to human subjects on a daily basis, potentially leading to plasma concentrations greater than 10−6M [33], and even greater levels of CM in colonic tissue [15]. When CM is administered intraperitoneally to mice, the concentration of CM in the intestine is about 200 times greater than in plasma [34]. Thus, in the intestinal tract, where CM has shown promise in cancer chemoprevention, CM concentrations may be sufficient to activate VDR, especially in individuals consuming a diet high in turmeric.
Current data suggest that CM is poorly absorbed from the GI tract and is also rapidly metabolized by glucuronidation [34]. Some human [35] and animal [36] trials have attempted to boost the bioavailability of CM by administering it along with piperine, which is known to inhibit glucuronidation of xenobiotics. Co-administration of piperine was shown to increase serum concentrations of CM as well as lengthen the half-life of CM in the body [35, 36]. Also, absorption of CM in a topical skin application was enhanced by the use of a gel containing either eugenol or terpeniol [37]. These studies suggest that there may be mechanisms for enhancing the bioavailability of CM by either improving its uptake or by inhibiting its metabolism.
CM is also metabolized in mammals by sulfation [38] and reduction to tetra-, hexa- and octahydro derivatives [39]. The rapid and consistent metabolism of CM to other forms raises the possibility that a metabolite of CM may, in fact, be the biologically most active ligand for VDR. In such a case, using an agent to increase CM bioavailability might not be advisable if the action of that agent is to suppress CM metabolism.
Cancer chemoprevention by CM may be attributed, at least in part, to activation of tumor-suppressor genes like p21. Since curcumin has a wide range of molecular targets (reviewed in [40]), p21 induction could be caused by a secondary effect on the p21 promoter. Recently C/EBPβ (a member of the CCAAT/enhancer binding protein family of transcription factors) has been shown to induce p21 in a p53-independent manner in the presence of antioxidants, such as vitamin E [41]. It has been reported that CM simultaneously induces C/EBPβ and p21 in a dose-dependent fashion, suggesting that C/EBPβ may be one of the mediators of the effect of curcumin on p21 expression [20]. However, our results demonstrate that overexpression of VDR leads to a greater upregulation of p21 by CM. Thus it is possible that CM can stimulate p21 transcription not only via a secondary mechanism, but also directly through a VDRE in the p21 promoter by binding to VDR. Additionally, there is evidence that 1,25D-VDR induces C/EBPβ, hinting at another potential connection between VDR, 1,25D, CM and p21 [42].
Yet another novel mechanism by which CM might exert anti-tumor effects is via upregulation of intestinal TRPV6, as shown in the current study. TRPV6 is a major calcium transporter in the small intestine [43], where its role in vitamin D-stimulated calcium transport has been well demonstrated [44]. Several studies have indicated that high dietary calcium protects against risk for colon cancer [45, 46]. If CM is, in fact, a VDR ligand that upregulates TRPV6 in vivo, then it is conceivable that CM may perhaps play a role similar to that of 1,25D in promoting calcium uptake as part of the protective effect against colon cancer.
Vitamin D and its analogs continue to be evaluated as colon cancer chemopreventative and treatment agents [47]. Of particular interest is identification of compounds that can be employed in combination with vitamin D analogs, allowing the application of lower doses to minimize undesired toxicity and side effects such as hypercalcemia, and providing a greater treatment efficiency. CM has been shown to act synergistically with 1,25D to elicit differentiation of human promyelocytic leukemia HL-60 cells [24]. In the current study, cells treated with 1,25D and CM had a higher level of transcription of a transfected VDRE-reporter construct than cells treated with 1,25D alone (Fig. 3A). Additive stimulation of the receptor is unlikely to explain this, because most of the ligand binding sites of VDR may be occupied by the high affinity 1,25D ligand. There are several other potential molecular mechanisms for this phenomenon. One that we have discounted is the possible ability of CM to upregulate VDR mRNA and VDR protein content since CM increased neither VDR mRNA nor protein levels. Another possibility is that CM could potentiate VDR transactivation via its demonstrated ability to bind to RXR. Finally, the observation that CM evidently configures VDR in such a way as to attract SRC-1 more strongly than does 1,25D-bound VDR (Fig. 2B) may be a feature of CM-bound VDR that could allow it to potentiate transactivation by 1,25D-bound VDR, assuming that CM-bound VDR and 1,25D-bound VDR complexes are in close proximity, as they might be on our reporter construct that contains two VDREs. It is intriguing to note that, in this respect, many genes naturally regulated by 1,25D-VDR (and potentially by CM) contain several VDREs that could be brought into close apposition by looping out of the intervening chromatin to form a transcriptional “hub”, thus allowing CM-VDR to attract SRC-1 to a multi-receptor complex that also contains 1,25D-bound VDR and RXR [48, 49].
In summary, the results of this study clearly indicate that curcumin binds directly to and activates VDR, inducing the VDR target genes CYP3A4, CYP24, p21, and TRPV6. In the colon, some of these as well as other, yet-to-be identified genes may play a role in cancer chemoprevention, thereby providing additional molecular explanations for beneficial effects of 1,25D and CM to lower the risk of colon cancer. Furthermore, activation of VDR by CM may elicit unique, 1,25D-independent signaling pathways that assist in mediating the bioeffects of this compound in colon, an important VDR expressing organ. The present results support a new hypothesis that implicates VDR as a dietary sensor of CM and perhaps other nutritionally-derived beneficial lipids to effect tissue-specific chemoprevention, further challenging the notion that nuclear receptors solely bind to and mediate the activity of high affinity endocrine ligands.
Acknowledgments
LG101305 (Rex) was a generous gift from Ligand Pharmaceuticals (San Diego, CA).
This work was supported by the National Institutes of Health [Grants DK 063930 and DK 33351].
Nonstandard abbreviations
- VDR
nuclear vitamin D receptor
- 1,25D
1,25-dihydroxyvitamin D3
- CM
curcumin
- RXR
retinoid X receptor
- GR
glucocorticoid receptor
- SRC-1
steroid receptor coactivator-1
- VDREs
vitamin D responsive elements
- LCA
lithocholate
- CYP3A4
cytochrome P450-subfamily 3A polypeptide 4
- GRE
glucocorticoid responsive element
- RXRE
retinoid X receptor response element
- ECL
enhanced chemiluminescence
- Rex
RXR specific ligand LG101305
- Dex
dexamethasone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Whitfield GK, Jurutka PW, Haussler CA, Hsieh JC, Barthel TK, Jacobs ET, et al. Nuclear vitamin D receptor: structure-function, molecular control of gene transcription, and novel bioactions. In: Feldman D, Pike JW, Glorieux FH, editors. Vitamin D. Oxford, UK: Elsevier Academic Press; 2005. pp. 219–61. [Google Scholar]
- 2.Jurutka PW, Whitfield GK, Hsieh JC, Thompson PD, Haussler CA, Haussler MR. Molecular nature of the vitamin D receptor and its role in regulation of gene expression. Rev Endocr Metab Disord. 2001;2:203–16. doi: 10.1023/a:1010062929140. [DOI] [PubMed] [Google Scholar]
- 3.Haussler MR, Whitfield GK, Haussler CA, Hsieh JC, Thompson PD, Selznick SH, et al. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res. 1998;13:325–49. doi: 10.1359/jbmr.1998.13.3.325. [DOI] [PubMed] [Google Scholar]
- 4.Holick MF. Will 1,25-dihydroxyvitamin D3, MC 903, and their analogues herald a new pharmacologic era for the treatment of psoriasis? Arch Dermatol. 1989;125:1692–7. [PubMed] [Google Scholar]
- 5.El-Domyati M, Barakat M, Abdel-Razek R, El-Din Anbar T. Apoptosis, P53 and Bcl-2 expression in response to topical calcipotriol therapy for psoriasis. Int J Dermatol. 2007;46:468–74. doi: 10.1111/j.1365-4632.2007.03099.x. [DOI] [PubMed] [Google Scholar]
- 6.Kragballe K, Gjertsen BT, De Hoop D, Karlsmark T, van de Kerkhof PC, Larko O, et al. Double-blind, right/left comparison of calcipotriol and betamethasone valerate in treatment of psoriasis vulgaris. Lancet. 1991;337:193–6. doi: 10.1016/0140-6736(91)92157-w. [DOI] [PubMed] [Google Scholar]
- 7.Vijayakumar S, Mehta RR, Boerner PS, Packianathan S, Mehta RG. Clinical trials involving vitamin D analogs in prostate cancer. Cancer J. 2005;11:362–73. doi: 10.1097/00130404-200509000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Guyton KZ, Kensler TW, Posner GH. Vitamin D and vitamin D analogs as cancer chemopreventive agents. Nutr Rev. 2003;61:227–38. doi: 10.1301/nr.2003.jul.227-238. [DOI] [PubMed] [Google Scholar]
- 9.Eelen G, Verlinden L, van Camp M, van Hummelen P, Marchal K, de Moor B, et al. The effects of 1alpha,25-dihydroxyvitamin D3 on the expression of DNA replication genes. J Bone Miner Res. 2004;19:133–46. doi: 10.1359/JBMR.0301204. [DOI] [PubMed] [Google Scholar]
- 10.Liu M, Lee MH, Cohen M, Bommakanti M, Freedman LP. Transcriptional activation of the Cdk inhibitor p21 by vitamin D3 leads to the induced differentiation of the myelomonocytic cell line U937. Genes Dev. 1996;10:142–53. doi: 10.1101/gad.10.2.142. [DOI] [PubMed] [Google Scholar]
- 11.Makishima M, Lu TT, Xie W, Whitfield GK, Domoto H, Evans RM, et al. Vitamin D receptor as an intestinal bile acid sensor. Science. 2002;296:1313–6. doi: 10.1126/science.1070477. [DOI] [PubMed] [Google Scholar]
- 12.Nehring JA, Zierold C, DeLuca HF. Lithocholic acid can carry out in vivo functions of vitamin D. Proc Natl Acad Sci U S A. 2007;104:10006–9. doi: 10.1073/pnas.0703512104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thangapazham RL, Sharma A, Maheshwari RK. Multiple molecular targets in cancer chemoprevention by curcumin. Aaps J. 2006;8:E443–9. doi: 10.1208/aapsj080352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruby AJ, Kuttan G, Babu KD, Rajasekharan KN, Kuttan R. Anti-tumour and antioxidant activity of natural curcuminoids. Cancer Lett. 1995;94:79–83. doi: 10.1016/0304-3835(95)03827-j. [DOI] [PubMed] [Google Scholar]
- 15.Sharma RA, Gescher AJ, Steward WP. Curcumin: the story so far. Eur J Cancer. 2005;41:1955–68. doi: 10.1016/j.ejca.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected] J Biol Chem. 1995;270:24995–5000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 17.Szeto FL, Sun J, Kong J, Duan Y, Liao A, Madara JL, et al. Involvement of the vitamin D receptor in the regulation of NF-kappaB activity in fibroblasts. J Steroid Biochem Mol Biol. 2007;103:563–6. doi: 10.1016/j.jsbmb.2006.12.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inoue J, Gohda J, Akiyama T, Semba K. NF-kappaB activation in development and progression of cancer. Cancer Sci. 2007;98:268–74. doi: 10.1111/j.1349-7006.2007.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aggarwal BB, Banerjee S, Bharadwaj U, Sung B, Shishodia S, Sethi G. Curcumin induces the degradation of cyclin E expression through ubiquitin-dependent pathway and up-regulates cyclin-dependent kinase inhibitors p21 and p27 in multiple human tumor cell lines. Biochem Pharmacol. 2007;73:1024–32. doi: 10.1016/j.bcp.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Hour TC, Chen J, Huang CY, Guan JY, Lu SH, Pu YS. Curcumin enhances cytotoxicity of chemotherapeutic agents in prostate cancer cells by inducing p21(WAF1/CIP1) and C/EBPbeta expressions and suppressing NF-kappaB activation. Prostate. 2002;51:211–8. doi: 10.1002/pros.10089. [DOI] [PubMed] [Google Scholar]
- 21.Su CC, Lin JG, Li TM, Chung JG, Yang JS, Ip SW, et al. Curcumin-induced apoptosis of human colon cancer colo 205 cells through the production of ROS, Ca2+ and the activation of caspase-3. Anticancer Res. 2006;26:4379–89. [PubMed] [Google Scholar]
- 22.Bratland A, Risberg K, Maelandsmo GM, Gutzkow KB, Olsen OE, Moghaddam A, et al. Expression of a novel factor, com1, is regulated by 1,25-dihydroxyvitamin D3 in breast cancer cells. Cancer Res. 2000;60:5578–83. [PubMed] [Google Scholar]
- 23.Campbell MJ, Elstner E, Holden S, Uskokovic M, Koeffler HP. Inhibition of proliferation of prostate cancer cells by a 19-nor-hexafluoride vitamin D3 analogue involves the induction of p21waf1, p27kip1 and E-cadherin. J Mol Endocrinol. 1997;19:15–27. doi: 10.1677/jme.0.0190015. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Chang RL, Cui XX, Newmark HL, Conney AH. Synergistic effects of curcumin on all-trans retinoic acid- and 1 alpha,25-dihydroxyvitamin D3-induced differentiation in human promyelocytic leukemia HL-60 cells. Oncol Res. 1997;9:19–29. [PubMed] [Google Scholar]
- 25.Thompson PD, Jurutka PW, Whitfield GK, Myskowski SM, Eichhorst KR, Dominguez CE, et al. Liganded VDR induces CYP3A4 in small intestinal and colon cancer cells via DR3 and ER6 vitamin D responsive elements. Biochem Biophys Res Commun. 2002;299:730–8. doi: 10.1016/s0006-291x(02)02742-0. [DOI] [PubMed] [Google Scholar]
- 26.Jantzen HM, Strahle U, Gloss B, Stewart F, Schmid W, Boshart M, et al. Cooperativity of glucocorticoid response elements located far upstream of the tyrosine aminotransferase gene. Cell. 1987;49:29–38. doi: 10.1016/0092-8674(87)90752-5. [DOI] [PubMed] [Google Scholar]
- 27.Mangelsdorf DJ, Umesono K, Kliewer SA, Borgmeyer U, Ong ES, Evans RM. A direct repeat in the cellular retinol-binding protein type II gene confers differential regulation by RXR and RAR. Cell. 1991;66:555–61. doi: 10.1016/0092-8674(81)90018-0. [DOI] [PubMed] [Google Scholar]
- 28.Jurutka PW, Thompson PD, Whitfield GK, Eichhorst KR, Hall N, Dominguez CE, et al. Molecular and functional comparison of 1,25-dihydroxyvitamin D3 and the novel vitamin D receptor ligand, lithocholic acid, in activating transcription of cytochrome P450 3A4. J Cell Biochem. 2005;94:917–43. doi: 10.1002/jcb.20359. [DOI] [PubMed] [Google Scholar]
- 29.Kingston RE. Transfection of DNA into eucaryotic cells: Calcium phosphate transfection. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Siedman JG, Smith JA, et al., editors. Current Protocols in Molecular Biology. New York: Greene Publishing and Wiley-Interscience; 1990. pp. 9.1–9.1.4. [Google Scholar]
- 30.Pike JW. Monoclonal antibodies to chick intestinal receptors for 1,25-dihydroxyvitamin D3. Interaction and effects of binding on receptor function. J Biol Chem. 1984;259:1167–73. [PubMed] [Google Scholar]
- 31.Kong J, Zhang Z, Musch MW, Ning G, Sun J, Hart J, et al. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol. 2008;294:G208–16. doi: 10.1152/ajpgi.00398.2007. [DOI] [PubMed] [Google Scholar]
- 32.Sriussadaporn S, Wong MS, Whitfield JF, Tembe V, Favus MJ. Structure-function relationship of human parathyroid hormone in the regulation of vitamin D receptor expression in osteoblast-like cells (ROS 17/2.8) Endocrinology. 1995;136:3735–42. doi: 10.1210/endo.136.9.7649079. [DOI] [PubMed] [Google Scholar]
- 33.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–900. [PubMed] [Google Scholar]
- 34.Pan MH, Huang TM, Lin JK. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab Dispos. 1999;27:486–94. [PubMed] [Google Scholar]
- 35.Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Medica. 1998;64:353–6. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 36.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–18. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 37.Fang JY, Hung CF, Chiu HC, Wang JJ, Chan TF. Efficacy and irritancy of enhancers on the in-vitro and in-vivo percutaneous absorption of curcumin. J Pharm Pharmacol. 2003;55:593–601. doi: 10.1211/002235703765344496. [DOI] [PubMed] [Google Scholar]
- 38.Ravindranath V, Chandrasekhara N. Absorption and tissue distribution of curcumin in rats. Toxicology. 1980;16:259–65. doi: 10.1016/0300-483x(80)90122-5. [DOI] [PubMed] [Google Scholar]
- 39.Hoehle SI, Pfeiffer E, Solyom AM, Metzler M. Metabolism of curcuminoids in tissue slices and subcellular fractions from rat liver. J Agric Food Chem. 2006;54:756–64. doi: 10.1021/jf058146a. [DOI] [PubMed] [Google Scholar]
- 40.Shishodia S, Chaturvedi MM, Aggarwal BB. Role of curcumin in cancer therapy. Curr Probl Cancer. 2007;31:243–305. doi: 10.1016/j.currproblcancer.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 41.Chinery R, Brockman JA, Peeler MO, Shyr Y, Beauchamp RD, Coffey RJ. Antioxidants enhance the cytotoxicity of chemotherapeutic agents in colorectal cancer: a p53-independent induction of p21WAF1/CIP1 via C/EBPbeta. Nat Med. 1997;3:1233–41. doi: 10.1038/nm1197-1233. [DOI] [PubMed] [Google Scholar]
- 42.Dhawan P, Peng X, Sutton AL, MacDonald PN, Croniger CM, Trautwein C, et al. Functional cooperation between CCAAT/enhancer-binding proteins and the vitamin D receptor in regulation of 25-hydroxyvitamin D3 24-hydroxylase. Mol Cell Biol. 2005;25:472–87. doi: 10.1128/MCB.25.1.472-487.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peng JB, Zhuang L, Berger UV, Adam RM, Williams BJ, Brown EM, et al. CaT1 expression correlates with tumor grade in prostate cancer. Biochemical and Biophysical Research Communications. 2001;282:729–34. doi: 10.1006/bbrc.2001.4638. [DOI] [PubMed] [Google Scholar]
- 44.Meyer MB, Watanuki M, Kim S, Shevde NK, Pike JW. The human transient receptor potential vanilloid type 6 distal promoter contains multiple vitamin D receptor binding sites that mediate activation by 1,25-dihydroxyvitamin D3 in intestinal cells. Mol Endocrinol. 2006;20:1447–61. doi: 10.1210/me.2006-0031. [DOI] [PubMed] [Google Scholar]
- 45.Slattery ML, Sorenson AW, Ford MH. Dietary calcium intake as a mitigating factor in colon cancer. Am J Epidemiol. 1988;128:504–14. doi: 10.1093/oxfordjournals.aje.a114998. [DOI] [PubMed] [Google Scholar]
- 46.Lipkin M, Newmark H. Calcium and the prevention of colon cancer. J Cell Biochem Suppl. 1995;22:65–73. doi: 10.1002/jcb.240590810. [DOI] [PubMed] [Google Scholar]
- 47.Guyton KZ, Kensler TW, Posner GH. Cancer chemoprevention using natural vitamin D and synthetic analogs. Annu Rev Phamacol Toxicol. 2001;41:421–42. doi: 10.1146/annurev.pharmtox.41.1.421. [DOI] [PubMed] [Google Scholar]
- 48.Chambeyron S, Bickmore WA. Does looping and clustering in the nucleus regulate gene expression? Curr Opin Cell Biol. 2004;16:256–62. doi: 10.1016/j.ceb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 49.Kim S, Yamazaki M, Zella LA, Meyer MB, Fretz JA, Shevde NK, et al. Multiple enhancer regions located at significant distances upstream of the transcriptional start site mediate RANKL gene expression in response to 1,25-dihydroxyvitamin D3. J Steroid Biochem Mol Biol. 2007;103:430–4. doi: 10.1016/j.jsbmb.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]