Abstract
Regional deterioration of brain structure is a typical feature of aging, but emerging evidence suggests that exercise may mitigate the decline. The purpose of the present investigation was to examine the moderating influence of exercise engagement on cross-sectional estimates of age-related brain atrophy at both global and regional levels. Estimates of exercise engagement over the past 10 years and MRI-based measures of global (gray and white) and regional volumes were obtained in a sample of 52 healthy older adults aged 55 – 79. Volume estimates were obtained in prefrontal, parietal, temporal, occipital, neostriatal, and medial temporal regions. Higher levels of exercise engagement were related to larger superior frontal volumes. Most critically, exercise engagement selectively moderated age-related medial temporal lobe atrophy. Specifically, significant age-related atrophy was observed for older adults who engaged in low levels of exercise, but not for those who engaged in high levels of exercise. This novel finding extends support for the efficacy of exercise to the potential maintenance of medial temporal lobe integrity in older adults.
Keywords: Aging, Exercise, Fitness, Brain Atrophy, Hippocampus
1. Introduction
Advancing age is accompanied by a pattern of differential brain changes that are observed in both cross-sectional and longitudinal investigations (see Raz & Rodrigue, 2006 for a review). There are disproportionate declines in the prefrontal cortex, moderate declines in temporal cortex and only mild declines in occipital regions. Greater age-related atrophy also tends to be observed in hippocampal and neostriatal regions compared to other subcortical structures such as the paleostriatum. In addition to this inter-regional variation, there are also patterns of inter-individual variation in atrophy. Even in nondemented older adult samples, differential degrees of age-related atrophy have been observed when comparing older adults within a narrow age range (Raz, 2005). Such patterns raise the question of what factors might moderate the variability in the magnitude of age-related atrophy observed across individuals. Here we focus on engagement in exercise as one potential factor based on emerging evidence that exercise may mitigate age effects on neural integrity (see Hillman, Erickson, & Kramer, 2008 for a review). Consistent with the regional variability in age effects on volume, the evidence thus far suggests that exercise has differential effects on brain regions. We next briefly review the extant evidence on these issues and then describe the goals of the present investigation.
1.1 Moderating effects of exercise
Past research indicates that lifestyle factors such as engagement in exercise are related to enhanced performance on tasks of executive control, controlled attention, spatial ability, and processing speed in older adults (Kramer et al., 1999; see Colcombe & Kramer, 2003 for a review). Similarly, there is growing but limited evidence that suggests exercise may have a positive effect on the structural integrity of the human brain. Not only are positive correlations observed between physical fitness and gray matter volumes (Gordon et al., 2008), most critically, some evidence suggests that exercise may moderate age-related brain atrophy with a regionally selective pattern. Using a cross-sectional design, Colcombe et al. (2003) found that physical fitness moderated the effects of age on tissue density in prefrontal, parietal, and temporal cortices, and anterior white matter tracts; areas that also evidenced the largest negative effects of age. Furthermore, aerobic fitness training involving a 6-month walking intervention in sedentary older adults led to significant increases in brain volumes in frontal and temporal regions, and anterior white matter tracts (Colcombe et al., 2006). In contrast, cardiorespiratory fitness has been found to be unrelated to whole brain, global gray or global white matter volumes in nondemented older adults (Burns et al., 2008). These studies suggest a positive and causal relationship between engagement in aerobic exercise and the sparing of brain volume in older adults in select regions of the cortex.
While the cross-sectional and intervention studies in older adult humans reveal exercise effects in the cortex, an extensive animal literature indicates that exercise produces increases in brain-derived neurotrophic factor (BDNF) (Neeper, Gomez-Pinilla, Chol, & Cotman, 1995; Vanyman, Ying, & Gomez-Pinilla, 2004), insulin-like growth factor (IGF-1) and nerve growth factor (NGF) (for review see Ang & Gomez-Pinilla, 2007 and Cotman & Berchtold, 2002), promotes neurogenesis (van Praag, Christie, Sejnowski, & Gage, 1999; van Praag, Shubert, Zhao, & Gage, 2005), angiogenesis (Gomez-Pinilla, So, & Kesslak, 1998), and synaptic plasticity and expression of enzymes that underlie glucose use and metabolism (for review see Cotman, Berchtold, & Christie, 2007). While these effects have been particularly observed in the hippocampal region of rats, there are also reports of exercise-related effects in the entorhinal cortex (Stranahan et al., 2007) and the amygdala (Greenwood et al., 2009). Limited, but promising, evidence is emerging regarding the effects of exercise on medial temporal lobe integrity in humans. Recently, selective increases in cerebral blood volume (a potential marker of neurogenesis in humans) were observed in the dentate region of the hippocampus in a small sample of young and middle-aged adults following a 3-month aerobic exercise intervention (Pereira et al., 2007). As the first suggestion of exercise-related neurogenesis in humans of any age, the findings of Pereira et al. (2007) raise the intriguing possibility that exercise may also have a moderating influence on age-related atrophy of the hippocampus or other medial temporal regions. Recently observed associations between cardiorespiratory fitness and medial temporal lobe regions in healthy older adults (Gordon et al., 2008) and in individuals with early-stage Alzheimer’s disease (Honea et al., 2008) provide preliminary support for the benefits of exercise in these regions in aged populations. However, these studies (Honea et al., 2008, Gordon et al., 2008) did not assess for an interaction between exercise and age leaving the role of exercise in moderating age-related atrophy of the medial temporal lobe in nondemented older adults unclear.
1.2 Goals and hypotheses of current study
The primary goal of the current study was to evaluate the relationship between engagement in physical exercise and cross-sectional age-related decline in global gray and white matter volume and regional brain volumes in an older adult sample. The preponderance of previous work reviewed above localized exercise-related effects to voxel clusters within frontal, temporal and parietal cortex using voxel-based morphometry (VBM). In the current study, we used an automated labeling technique (Desikan et al., 2006; Fischl, Salat et al., 2002; Fischl, van der Kouwe, et al., 2004) that estimates regional volumes, similar to those derived from manual morphometry. Accordingly, a novel component of the current study is the a priori examination of the moderating influence of exercise on neuroanatomically defined regions rather than voxel-wise analyses. To decrease the likelihood of a Type 1 error, we restricted our analysis to a limited set of cortical and subcortical regions. We selected cortical regions that were consistent with voxel clusters found to be sensitive to exercise in previous VBM studies (Colcombe et al., 2003; 2006). We also included the pericalcarine cortex, which has not been associated with exercise effects, as a comparison region. Accordingly, we hypothesized that cross-sectional age-related decline would be less marked for older adults with higher levels of physical exercise engagement, and that this moderating effect would be observed in dorsal/ventral lateral prefrontal, superior frontal, superior temporal, lateral parietal, and medial temporal regions, but not in the pericalcarine cortex. The predicted pattern of broad yet selective (i.e., all regions except the pericalcarine cortex) effects of exercise engagement on regional cortical volumes thus mirrors the obtained patterns found in previous reports (e.g., Colcombe et al., 2003; 2006). As for the main effect of exercise engagement, based on the mixed prior findings (e.g., Colcombe et al., 2003; Gordon et al., 2008), it was unclear as to whether a significant effect would be observed for any region. Similarly, given the null finding from the single study that examined the effects of exercise on global gray and white matter volumes in nondemented older adults (Burns et al., 2008), it was also uncertain whether exercise engagement alone would be related to global measures of volume in the current study.
2. Methods
2.1 Participants
Fifty-two older adults (15 males, 37 females) aged 55 – 79 (M = 69.0; SD = 6.7) were recruited from the Washington University’s Alzheimer’s Disease Research Center (ADRC) and screened for neurologic illness, head injury, depression and medical conditions that might produce cognitive impairment (e.g., cerebrovascular disease, Parkinson’s disease). Based on the Washington University Clinical Dementia Rating (CDR) scale, a validated and reliable interview-based measurement that is sensitive in detecting the earliest stages of dementia (Morris, 1993, Storandt et al., 2006), all participants were classifed as nondemented (CDR=0). Years of education and socioeconomic status (SES) were obtained. SES category (1 – 5) was determined via the Hollingshead two-factor index of social position where one refers to high-privilege (Hollingshead, 1958). All participants consented to participation in accordance with guidelines of the Washington University Human Studies Committee.
2.2 Measurement of physical exercise engagement
2.2.1 Reliability and validity
A questionnaire on engagement in a running, walking, or jogging exercise program over the past 10 years was administered (Bowles, FitzGerald, Morrow, Jackson, & Blair, 2004). The questionnaire has been validated with a sample of 5063 individuals aged 18 to 80. Most critically, the questionnaire has good construct validity. Self-reported engagement in running, walking, and jogging is significantly correlated (rs = .40 – .61) with cardiorespiratory fitness, as measured via a treadmill test, and other indices of health including body mass index and triglyceride levels. Importantly, participants within the wide age range investigated by Bowles et al. (2004) appeared capable of accurately self-reporting running, walking, and jogging activities for up to 10 years, as indicated by the stable correlations between fitness and questionnaire scores for each of the ten 1-year assessment periods.
2.2.2 Procedure
Participants engaged in a 30 – 40 minute phone interview during which the historical physical activity questionnaire was administered. Participants reported the number of months during which they engaged in running, walking, or jogging for exercise during each of the 10 years preceding the interview. For each year in which they reported engagement for at least one month, they were further asked to report the number of times per week that they engaged in running, walking, or jogging, the average number of miles covered during each session, and the average time per mile. Following the procedure outlined in Bowles et al. (2004), participants’ data from the running, walking, and jogging questionnaire were converted to estimated metabolic equivalent (MET) values using the compendium of physical activities (Ainsworth et al., 2000). A MET approximates the energy cost of engaging in a physical activity. A physical exercise engagement score, stated in average MET hours per week for the past 10 years, was derived for each participant. As an example, an individual who on average engaged in moderate intensity activity 5 days a week for 30 minutes would be assigned a score of approximately 7.5 (Bowles et al., 2004).
Years of participation in strenuous sports other than running, walking, and jogging were also recorded. Examples included racquet sports, cycling, swimming, aerobic dance, or strenuous sports involving running such as basketball and soccer. Participants reported which (if any) of the past 10 years they participated at least twice a week for 6 consecutive months in strenuous sports.
2.3 MR acquisition and image processing
Images were obtained, on average, 2.2 years prior to the physical activity interview. All imaging was performed using a Siemens 1.5 Tesla Vision scanner (Erlangen, Germany). Cushions and a thermoplastic mask were used during scanning to reduce head movement. A scout image (TR = 15 ms, TE = 6 ms, flip angle = 30°, 2.34 × 1.17 × 8 mm resolution) was acquired first in order to center the field of view on the brain. Two to four T1-weighted sagittal MP-RAGE (Mugler & Brookeman, 1991) scans (TR = 9.7 ms, TE = 4 ms, flip angle = 10°, TI = 20 ms, TD = 200 ms, 1 × 1 × 1.25 mm resolution) were acquired in each subject. Image processing steps have been described in detail in previous publications (Buckner et al., 2004; Fotenos et al., 2005; Head et al., 2005) and include inter- and intra-scan motion correction, atlas transformation, and averaging and inhomogeneity correction. Processing resulted in registered structural data resampled to 1 mm3 voxels in the atlas space of Talairach and Tournoux (Talairach & Tournoux, 1998). Atlas normalization is equivalent to normalization based on intracranial volume and is minimally biased by global atrophy (Buckner et al., 2004).
2.4 Regional volumetry
Regional volume estimates were obtained using the Freesurfer image analysis suite, which implements an automated probabilistic labeling procedure (Desikan et al., 2006; Fischl, Salat et al., 2002; Fischl, van der Kouwe, et al., 2004). Each voxel in an MR image is assigned a neuroanatomical label based on probabilistic information from a manually labeled training set. This technique generates volumes with a high correspondence to manually generated volumes (Fischl et al., 2004). Regions-of-interest (ROIs) included cortical gray matter, cortical white matter, dorsal/ventral lateral prefrontal cortex (combined caudal middle frontal and inferior frontal gyri), superior frontal gyrus (medial and lateral portions), lateral parietal cortex (combined inferior parietal and supramarginal gyrus), superior temporal cortex, pericalcarine cortex, neostriatum (combined caudate and putamen), medial temporal lobe (combined hippocampus, amygdala and parahippocampus) and the hippocampus separately.
2.5 Analytical approach
2.5.1 Derivation of exercise groups
The distribution of exercise engagement scores was heavily skewed due to the presence of a large number of participants (n = 18) who scored below 1 (including zero) on the questionnaire, and transformations (e.g. logarithmic) could not resolve these distributional issues. Thus, rather than treating the exercise engagement score as a continuous variable, we assigned participants to low and high exercise engagement groups. Group assignment was based on normative data from a larger sample of 109 older adults (32 males, 77 females; aged 55 – 79 (M = 68.6, SD = 6.7)) who engaged in a phone interview evaluating exercise engagement. We used the full sample’s exercise data so as to better gauge population estimates of exercise engagement. The mean physical exercise engagement score (i.e., MET value) for the sample of 109 older adults was 4.56 (SD = 5.76) and the range was 0 to 29.72. We used the median score (2.44) from the full sample to assign participants in the current study to low and high exercise engagement groups. While a participant who engaged in moderate intensity activity approximately 2 days a week for 10 minutes on average would just exceed the lower limit for inclusion in the high exercise engagement group, the average exercise participation of this group entailed moderate intensity activity for approximately 5 days a week for 30 minutes.
2.5.2 Statistical analyses
Following Baron and Kenny (1986), we used a series of hierarchical regression analyses to examine the independent effects of age, exercise engagement, and the interaction of age and exercise engagement on the volumes of each ROI. Age was standardized and the product of the standardized age and exercise variables was used to create the age × exercise interaction term. The three variables were then entered into the regression model with age in the first step, followed by exercise engagement, and then the interaction term. Importantly, a significant interaction indicates that exercise group exerts a moderating effect on the cross-sectional age slope on volume above and beyond the influence of either age or exercise engagement alone.
3. Results
Table 1 presents the demographic information for the low (n = 24) and high (n = 28) exercise engagement groups. Importantly, the groups were matched in age, years of education, and socioeconomic status, ps > .60 and had a similar age range (Low = 55 to 79; High = 59 to 79). In addition, there was not a significant difference across groups in the proportion of females (χ 2<1). As expected, the physical exercise engagement score was significantly higher for the high exercise engagement group. The high and low exercise engagements groups did not differ in years of participation in strenuous sports other than running, walking, and jogging, p = .10.
Table 1.
Characteristics of the exercise engagement groups
| Variable | Low Exercise Engagement(n = 24) | High Exercise Engagement (n = 28) | p-value for independent t-test comparing groups |
|---|---|---|---|
| Age | 68.9 (7.4) | 69.1 (6.1) | .90 |
| Gender | 6 M, 18 F | 9 M, 19 F | -- |
| Years of Education | 16.0 (2.9) | 15.8 (2.6) | .75 |
| Socioeconomic Status | 2.3 (.9) | 2.1 (1.1) | .60 |
| Physical Exercise Engagement Score | .63 (.74) | 7.76 (3.94) | < .001 |
| Years of Participation in Strenuous Sports | 1.63 (3.06) | 3.46 (4.5) | .10 |
Note: Data are presented as Mean (SD).
3.1 Global gray and white matter volume
As can be seen in Table 2, the regression analyses indicated that age accounted for a significant amount of variance in both gray and white matter volume. A marginally non-significant effect of exercise engagement was found for gray matter volume, B = 17732.73 (SE = 9085.77), p = .057. Gray matter volume was larger for the high (M =520 cm3, SD = 36) relative to the low (M =503 cm3, SD = 43) exercise engagement group. Exercise engagement alone did not account for a significant amount of variance in white matter volume, p = .52.
Table 2.
Unique proportion of variance (R2) accounted for by age, exercise engagement, and the interaction of age and exercise engagement.
| Brain Structure | Age | Exercise Engagement | Age × Exercise Engagement |
|---|---|---|---|
| Global Volumes | |||
| Cerebral Gray Matter | .31*** | .05 | .01 |
| Cerebral White Matter | .41*** | .01 | .00 |
| Regional Cortical Volumes | |||
| Dorsal/Ventral Lateral Prefrontal Cortex | .19*** | .02 | .00 |
| Superior Frontal Cortex | .23*** | .07* | .01 |
| Lateral Parietal Cortex | .34*** | .01 | .01 |
| Superior Temporal Cortex | .34*** | .02 | .01 |
| Pericalcarine Cortex | .04 | .07* | .02 |
| Regional Subcortical Volumes | |||
| Medial Temporal Lobe | .24*** | .00 | .07* |
| Hippocampus | .34*** | .01 | .02 |
| Amygdala | .20*** | .00 | .05 |
| Parahippocampus | .02 | .01 | .06 |
| Neostriatal Nuclei | .16** | .02 | .01 |
Note: p ≤ .05;
p ≤ .01;
p ≤ .001
The bivariate correlations between age and volume for the low and high exercise engagement groups are displayed in Table 3. For gray matter volume, a negative correlation with age was numerically greater for the low exercise engagement group than the high exercise group; however, the correlations were not significantly different (age × exercise engagement interaction, p = .32). Significant negative correlations of similar magnitude were observed between age and white matter volume across the low and high exercise engagement groups, (age × exercise engagement interaction, p = .98).
Table 3.
Bivariate correlations (rs) between age and normalized brain volumes for the low and high exercise engagement groups
| Brain Structure | Low Exercise Engagement Group | High Exercise Engagement Group |
|---|---|---|
| Global Volumes | ||
| Cerebral Gray Matter | −.69*** | −.43* |
| Cerebral White Matter | −.69*** | −.60*** |
| Regional Cortical Volumes | ||
| Dorsal/Ventral Lateral Prefrontal Cortex | −.43* | −.47** |
| Superior Frontal Cortex | −.59** | −.38* |
| Lateral Parietal Cortex | −.56** | −.61*** |
| Superior Temporal Cortex | −.66** | −.50** |
| Pericalcarine Cortex | −.09 | −.32 |
| Regional Subcortical Volumes | ||
| Medial Temporal Lobe | −.65*** | −.24 |
| Hippocampus | −.64*** | −.52** |
| Amygdala | −.62*** | −.21 |
| Parahippocampus | −.35 | .14 |
| Neostriatal Nuclei | −.52** | −.27 |
Note: p ≤ .05;
p ≤ .01;
p ≤ .001
3.2 Regional cortical volumes
Age accounted for a significant amount of variance in all cortical ROIs, with the exception of pericalcarine cortex (see Table 2). Exercise engagement alone accounted for a significant amount of variance in superior frontal cortex, B = 1833.90 (SE = 846.21). The volume of superior frontal cortex was larger for the high (M = 50 cm3, SD = 3) relative to the low (M = 48 cm3, SD = 4) exercise engagement group. A similar trend was observed for pericalcarine cortex, B = 470.00 (SE = 242.47), p = .058. Again, the volume of pericalcarine cortex was larger for the high (M = 5.7 cm3, SD = .90) than the low (M = 5.3 cm3, SD = .86) exercise engagement group. Exercise engagement did not account for a significant amount of variance in dorsal/ventral lateral prefrontal, lateral parietal, or superior temporal regions, ps ≥ .21. Contrary to expectations, the interaction of age and exercise engagement did not account for a significant amount of variance in dorsal/ventral lateral prefrontal, superior frontal, lateral parietal or superior temporal cortices, ps ≥ .39. As expected, there was not a significant age × exercise interaction (p=.32) for the pericalcarine cortex.
Examination of the bivariate correlations in Table 3 indicates that the pattern for superior frontal and superior temporal volumes was consistent with the expectation that the magnitude of age effects would be greater for the low exercise engagement group. Although the formal moderator (i.e. interaction) analysis presented above indicates that these correlations did not differ significantly between groups, these patterns suggest further study is warranted. The age-volume correlations were similar across exercise groups for the dorsal/ventral lateral prefrontal and lateral parietal regions. As expected, for pericalcarine cortex, the correlation between age and volume was non-significant for both groups.
3.3 Regional subcortical volumes
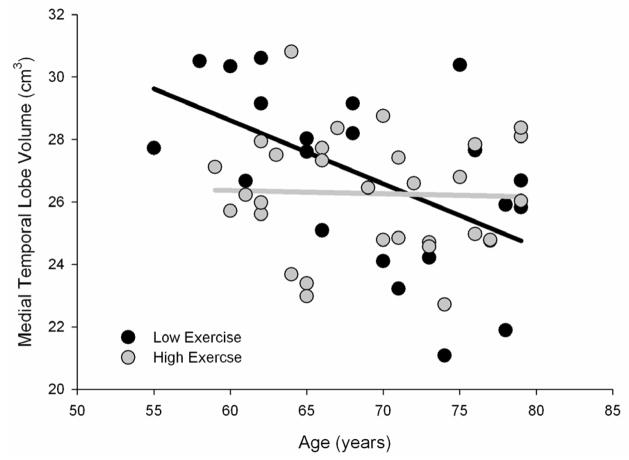
There were significant negative age effects observed for each of the three subcortical ROIs. No significant effects of exercise engagement alone were observed, ps ≥ .34. For the neostriatal nuclei, the age × exercise engagement interaction was not significant (p >. 45). Most critically, however, there was a significant age × exercise engagement interaction for medial temporal lobe volume, B = 977.76 (SE = 456.99), indicating that exercise engagement had a moderating effect on age-related decline in this region (see Figure 2). The interaction accounted for approximately 7% of the variance in medial temporal lobe volume. For older adults with low levels of exercise engagement, the bivariate correlation between age and medial temporal lobe volume was significant (r = −.65, p = .001). This correlation was markedly reduced for older adults with high levels of exercise engagement (r = −.24, ns). For hippocampus proper, the moderating influence of exercise engagement on age-related atrophy was not significant (p > .25). As the age × exercise engagement interaction was significant for the medial temporal lobe volume but not the hippocampus, we ran separate regressions for the two additional regions (amygdala and parahippocampus) that comprised the medial temporal lobe ROI to further gauge the locus of the significant interaction. The age × exercise engagement interaction approached significance for both amygdala (p = .098) and parahippocampus (p = .078). Although the moderating influence of exercise engagement on age-related atrophy of neostriatal nuclei, hippocampus, amygdala and parahippocampus were not significant, the bivariate correlations between age and these volumes were in the appropriate direction for such an effect (see Table 3).
4. Discussion
The purpose of the present investigation was to examine the moderating influence of exercise engagement on cross-sectional estimates of age-related brain atrophy, both at a global and regional level. The primary finding was that engagement in exercise had a selective moderating effect on age-related medial temporal lobe atrophy. A significant age-related decrease in the volume of the medial temporal lobe was observed for older adults who engaged in low levels of exercise but not for those who engaged in higher levels of exercise. While previous investigations have demonstrated an association between exercise and medial temporal lobe volume in healthy older adults (Gordon et al., 2008) and early-stage Alzheimer’s disease (Honea et al., 2008), our novel finding provides an important suggestion of a moderating influence of exercise on the deleterious effects of age in this region.
Considering the preponderance of evidence from the animal literature indicates that exercise effects such as neurogenesis, angiogenesis and increases in BDNF (Gomez-Pinilla et al., 1998; Neeper et al., 1995, 1996; van Praag et al., 1999, 2005; Vaynman et al., 2004) are observed in the hippocampus and the evidence of potential exercised-induced neurogenesis in the hippocampus of young/middle-aged humans (Pareiera et al., 2007), we expected a moderating effect of exercise to be observed for the hippocampus proper. While the bivariate correlations between age and hippocampal volume were in the expected direction for such an effect, the interaction was not significant. In the remaining regions comprising the medial temporal lobe volume (i.e., amygdala and parahippocampus), trends toward a significant interaction between age and exercise engagement were observed. This is consistent with previous reports of significant exercise effects for rats (Stranahan et al., 2007) and nonsignificant trends for humans (Pereira et al., 2007) in regions of the parahippocampus, such as the entorhinal cortex and also with reports of exercise-related increases in BDNF in the amygdala in rats (Greenwood et al., 2009). The finding of a significant moderating influence on the medial temporal lobe volume as a whole, but not the subregions, may relate to differential reliability. Specifically, it is possible that only the larger, summed estimate from the current morphometric technique was a sufficiently reliable, and thus sensitive, measure of the effect of interest.
The second major finding was that higher levels of exercise engagement were related to larger superior frontal cortex volume, with similar patterns observed for global gray matter volume and pericalcarine cortex volume. Prior reports are mixed with some indicating that fitness alone is predictive of brain volume in cortical regions including parietal and frontal areas (Gordon et al., 2008), while others have indicated that fitness alone is not related to regional (Colcombe et al., 2003) or global (Burns et al., 2008) cortical volumes. While the positive associations observed here between select volumes and exercise support exercise-related benefits on brain structure, engagement in exercise did not moderate age-related volumetric reductions in the cortex, neither at a global (gray or white matter) or regional level. The absence of a moderating effect on regional cortical volumes is surprising in light of previous findings (e.g., Colcombe et al., 2003, 2006). One potential factor that may have contributed to the apparent discrepancy is the measurement of exercise. Colcombe et al. (2003, 2006) used a treadmill test to quantify cardiorespiratory fitness while we used a self-report measure of exercise engagement that is correlated with fitness. It is possible that fitness rather than self-reported physical activity is relevant for neural integrity, as has been found in the prediction of cognitive decline (Barnes et al. 2003). However, we specifically evaluated engagement in aerobic activities that have been linked to fitness (Bowles et al., 2004), cognition (Kramer et al., 1999), and brain structure (Colcombe et al., 2003, 2006) instead of physical activity more broadly including activities (e.g., household, gardening) evaluated in the Barnes et al. (2003) study that may not necessarily significantly enhance aerobic fitness. In addition, it is unlikely that the sole determinant for the discrepancy is our measurement of exercise rather than fitness as the estimate of exercise engagement was sufficiently sensitive to detect a main effect on superior frontal volume and a moderating effect on age-related atrophy of the medial temporal lobe.
The discrepant findings may instead relate more to the technique used to localize aging and exercise-related effects in the brain. Previous studies used VBM (e.g. Colcombe et al., 2003, 2006) to assess tissue density or volume in a voxel-wise manner across the whole brain. In contrast, the current study implemented an automated labeling technique that produces estimates of neuroanatomically defined regional volumes comparable to those obtained with manual morphometry (Fischl et al., 2004). While VBM has advantages, it is also associated with several limitations, such as substantial smoothing resulting in a reduction of spatial resolution and potential misregistration errors that may be particularly problematic for smaller structures (e.g., hippocampus, amygdala) and regions adjacent to ventricles (Bookstein, 2001; Davatzikos, 2004; Kennedy et al., 2008; Mechelli et al., 2005). A recent comparison between VBM and manual volumetry methods suggested that the typical VBM approach may overestimate age effects (Kennedy et al., 2008). However, VBM was also noted to be more sensitive in detecting plausible hypertension-related effects (Kennedy et al., 2008). A direct comparison between VBM and neuronatomically defined volumes is necessary to gain a better understanding of the discrepant findings across techniques in the examination of exercise-related effects in older adults.
4.1 Limitations
While the cross-sectional approach implemented here commonly represents an important first step in identifying moderators of age-related effects, such as those produced by exercise, this approach cannot determine causality. Therefore, we are unable to conclude that exercise engagement produced the differential patterns of age-related atrophy of medial temporal lobe. Ultimately, studies that include sensitive and reliable measures of atrophy, such as the volumetric approach introduced here, with a pre-post exercise intervention design are needed to determine whether exercise has a causal influence on medial temporal lobe atrophy in older adults. Longitudinal studies that measure the individual trajectories of age-related medial temporal lobe atrophy in older adults who engage in exercise versus those who are sedentary would also be informative.
Another limitation in the current study is the relatively small sample size. There were several regions for which the expected pattern of older adults with high exercise engagement evidencing less age-related atrophy than those with low exercise engagement was observed. These included superior frontal, superior temporal, and neostriatal regions, in addition to the hippocampus proper. Thus, future investigations with larger sample sizes are necessary to replicate current results and provide sufficient power to detect any potentially moderating effects in these regions.
Equally important for future studies will be the inclusion of direct measures of cardiorespiratory fitness such as VO2 max. The self-report measure of exercise engagement used in the present study is significantly, though not perfectly, related to cardiorespiratory fitness (Bowles et al., 2004). While the self-report measure was sensitive to exercise effects in superior frontal cortex and the medial temporal lobe, limitations of this measure may also in part be responsible for the absence of significant moderating effects in hippocampus proper as well as the other regions included in the current analyses. For example, older adult participants’ ability to accurately report running, walking, and jogging engagement over a 10-year span may limit the sensitivity of the self-report measure.
4.2 Implications and future directions
The current report in combination with previous work (e.g., Colcombe et al., 2003; 2006) demonstrates the benefits of exercise engagement for cortical and medial temporal regions in older adult samples that might be considered to be at the younger end of the older adult range; it remains to be determined whether the benefits extend to older adult samples aged 80 and above. Age-related reductions in plasticity (i.e., the potential for the neurocognitive system to adapt) may be apparent not only for older relative to younger adults, but also for old-old relative to young-old adults (e.g., Baltes & Kliegl, 1992; Nyberg, 2005; Verhaeghen et al., 1992; Verhaeghen & Marcoen, 1996). For example, young-old adults have been shown to exhibit greater improvements in cognition as a function of engagement in cognitive training programs (Bissig & Lustig, 2007; Verhaeghen et al., 1992). It is conceivable that age-related reductions in plasticity could limit the degree to which exercise can moderate age-related atrophy. Participants’ age was a significant moderator of exercise effects on cognition in a recent meta-analysis (Colcombe & Kramer, 2003); however, there were no individuals above the age of 80. While it was of interest to compare the moderating effects of exercise engagement in a young-old sample to those obtained in an old-old sample (aged 80 and above), we were able to recruit only a small sample (N = 12) of old-old adults who met exclusion criteria precluding examination of this issue in the present study. Thus, the extent of exercise-related effects in old-old adults warrants investigation in future studies.
The current study demonstrated that cross-sectional estimates of age-related medial temporal atrophy are affected by level of engagement in running, walking, and jogging exercises in older adults. This finding suggests the possibility that not only may exercise be associated with medial temporal volume in Alzheimer’s disease as observed by Honea et al. (2008), but it might also moderate the medial temporal shrinkage that occurs early in the course of the disease (Braak & Braak, 1991). This possibility is buttressed by the potential for exercise to decrease amyloid burden as observed in a mice model of Alzheimer’s disease (Adlard et al., 2005). Furthermore, our finding raises the prospect that exercise, in conjunction with cognitive training, may be an especially effective way for curtailing age-related medial temporal lobe atrophy in addition to improving memory functions associated with these regions. Animal work suggests that the combination of exercise and cognitive activity may best foster neurogenesis in the hippocampus (Fabel & Kempermann, 2008). Examination of the interactive effects of memory training and exercise is an important direction for future research in humans.
Figure 1.
Exercise engagement moderates age effects on the medial temporal lobe. Scatter plot of the significant age × exercise engagement interaction with separate regression lines for low (black) and high (gray) exercise groups.
Acknowledgments
We thank the Clinical Core of the Washington University Alzheimer Disease Research Center for the clinical assessments and the Imaging Core for the structural MRI data. We thank Lauren Ruth, Marlisa Isom, Matt Riedel, Lindsay Casmaer, and William Janes for assistance with this project. Supported by NIH grants P50 AG05861, P01 AG 03991. Julie Bugg was supported by National Institute on Aging Grant 5T32AG00030.
Footnotes
The authors and their institution have no conflicts of interest related to this work. The data contained in this manuscript have not previously been published, nor has the manuscript been submitted elsewhere, nor will it be submitted elsewhere while under review. Appropriate ethical guidelines were followed with regard to the treatment of human subjects. All authors have reviewed the manuscript and approve of its contents and validate the accuracy of the data.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adlard PA, Perreau VM, Pop V, Cotman CW. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. The Journal of Neuroscience. 2005;25(17):4217–4221. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O’Brien WL, Bassett DR, Schmitz KH, Emplaincourt PO, Jacobs DR, Leon AS. Compendium of physical activities: An update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498–S516. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- Ang ET, Gomez-Pinilla F. Potential therapeutic effects of exercise. Current Medicinal Chemistry. 2007;14:2564–2571. doi: 10.2174/092986707782023280. [DOI] [PubMed] [Google Scholar]
- Baltes PB, Kliegl R. Further testing of limits of cognitive plasticity: Negative age differences in a mnemonic skill are robust. Developmental Psychology. 1992;28:121–125. [Google Scholar]
- Barnes DE, Yaffe K, Satariano WA, Tager IB. A longitudinal study of cardiorespiratory fitness and cognitive function in healthy older adults. Journal of the American Geriatrics Society. 2003;51:459–465. doi: 10.1046/j.1532-5415.2003.51153.x. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bissig A, Lustig C. Who benefits from psychological training? Psychological Science. 2007;18(8):720–726. doi: 10.1111/j.1467-9280.2007.01966.x. [DOI] [PubMed] [Google Scholar]
- Bookstein FL. Voxel-based morphometry should not be used with imperfectly registered images. Neuroimage. 2001;14:1454–1462. doi: 10.1006/nimg.2001.0770. [DOI] [PubMed] [Google Scholar]
- Bowles HR, FitzGerald SJ, Morrow JR, Jackson AW, Blair SN. Construct validity of self-reported historical physical activity. American Journal of Epidemiology. 2004;160(3):279–286. doi: 10.1093/aje/kwh209. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: Reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23(2):724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Burns JM, Cronk BB, Anderson HS, Donnelly JE, Thomas GP, Harsha A, Brooks WM, Swerdlow RH. Cardiorespiratory fitness and brain atrophy in early Alzheimer disease. Neurology. 2008;71:210–216. doi: 10.1212/01.wnl.0000317094.86209.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychological Science. 2003;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, Kramer A. Aerobic fitness reduces brain tissue loss in aging humans. Journal of Gerontology: Medical Sciences. 2003;58A(2):176–180. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. Journal of Gerontology: Medical Sciences. 2006;61A(11):1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: A behavioral intervention to enhance brain health and plasticity. Trends in Neurosciences. 2002;25(6):295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, Christie L. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends in Neurosciences. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Davatzikos C. Why voxel-based morphometric analysis should be used with great caution when characterizing group differences. Neuroimage. 2004;23:17–20. doi: 10.1016/j.neuroimage.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Fabel K, Kempermann G. Physical activity and the regulation of neurogenesis in the adult and aging brain. Neuromol Med. 2008;10:59–66. doi: 10.1007/s12017-008-8031-4. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl BA, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat D, Busa E, Seidman L, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically parcellating the human cerebral cortex. Cerebral Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fotenos AF, Snyder AZ, Girton LE, Morris JC, Buckner RL. Normative estimates of cross-sectional and longitudinal brain volume decline in aging and AD. Neurology. 2005;64(6):1032–1039. doi: 10.1212/01.WNL.0000154530.72969.11. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, So V, Kesslak JP. Spatial learning and physical activity contribute to the induction of fibroblast growth factor: Neural substrates for increased cognition associated with exercise. Neuroscience. 1998;85:53–61. doi: 10.1016/s0306-4522(97)00576-9. [DOI] [PubMed] [Google Scholar]
- Gordon BA, Rykhlevskaia EI, Brumback CR, Lee Y, Elavsky S, Konopack JF, McAuley E, Kramer AF, Colcombe S, Gratton G, Fabiani M. Neuroanatomical correlates of aging, cardiopulmonary fitness level, and education. Psychophysiology. 2008;45:825–838. doi: 10.1111/j.1469-8986.2008.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Strong PV, Foley TE, Fleshner M. A behavioral analysis of the impact of voluntary physical activity on hippocampus-dependent contextual conditioning. Hippocampus. 2009 doi: 10.1002/hipo.20534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head D, Snyder AZ, Girton LE, Morris JC, Buckner RL. Frontal- hippocampal double dissociation between normal aging and Alzheimer’s disease. Cerebral Cortex. 2005;15(6):732–739. doi: 10.1093/cercor/bhh174. [DOI] [PubMed] [Google Scholar]
- Honea RA, Thomas G, Harsha A, Cronk B, Donnelly J, Brooks WM, Burns JM. Physical fitness is associated with preservation of brain volume in Alzheimer’s disease; The International Conference on Alzheimer’s Disease; Chicago, IL. Jul, 2008. Poster presented at . [Google Scholar]
- Hollingshead AB. Social Class and Mental Illness. New York, NY: John Wiley & Sons; 1958. [Google Scholar]
- Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: Exercise effects on brain and cognition. Nature Reviews Neuroscience. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Erickson KI, Rodrigue KM, Voss MW, Colcombe SJ, Kramer AF, Acker JD, Raz N. Age-related differences in regional brain volumes: A comparison of optimized voxel-based morphometry to manual volumetry. Neurobiology of Aging. 2008 doi: 10.1016/j.neurobiolaging.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, Chason J, Vakil E, Bardell L, Boileau RA, Colcombe A. Aging, fitness, and neurocognitive function. Nature. 1999;400:418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Price CJ, Friston KJ, Ashburner J. Voxel-based morphometry of the human brain: Methods and applications. Current Medical Imaging Reviews. 2005:105–113. [Google Scholar]
- Morris JC. The clinical dementia rating scale (CDR): current version and scoring rules. Neurology, 43. 1993;2412:2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Mugler JP, Brookeman JR. Rapid three-dimensional T1-weighted MR imaging with the MP-RAGE sequence. J Magn Reson Imaging. 1991;1:561–567. doi: 10.1002/jmri.1880010509. [DOI] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Chol J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Chol J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Research. 1996;726:49–56. [PubMed] [Google Scholar]
- Nyberg L. Cognitive training in healthy aging: A cognitive neuroscience perspective. In: Cabeza R, Nyberg L, Park D, editors. Cognitive Neuroscience of Aging: Linking Cognitive and Cerebral Aging. New York, NY: Oxford University Press; 2005. pp. 309–324. [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proceedings of the National Academy of Sciences. 2007;104(13):5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N. The aging brain observed in vivo: Differential changes and their modifiers. In: Cabeza R, Nyberg L, Park D, editors. Cognitive Neuroscience of Aging: Linking Cognitive and Cerebral Aging. New York, NY: Oxford University Press; 2005. pp. 19–57. [Google Scholar]
- Raz N, Rodrigue K. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storandt M, Grant EA, Miller JP, Morris JC. Longitudinal course and neuropathological outcomes in original vs revised MCI and in preMCI. Neurology. 2006;67:467–479. doi: 10.1212/01.wnl.0000228231.26111.6e. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus. 2007;17:1017–1022. doi: 10.1002/hipo.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishers; 1998. [Google Scholar]
- van Praag H, Christie B, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proceedings of the National Academy of Sciences. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. The Journal of Neuroscience. 2005;25(38):8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanyman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. European Journal of Neuroscience. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Marcoen A, Goossens L. Improving memory performance in the aged through mnemonic training: A meta-analytic study. Psychology and Aging. 1992;7:242–251. doi: 10.1037//0882-7974.7.2.242. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Marcoen A. On the mechanisms of plasticity in young and older adults after instruction in the method of loci: Evidence for an amplification model. Psychology and Aging. 1996;11:164–178. doi: 10.1037//0882-7974.11.1.164. [DOI] [PubMed] [Google Scholar]