Abstract
Backgroud
Peripartum major depressive disorder (MDD) is a prevalent psychiatric disorder with potential detrimental consequences for both mother and child. Despite its enormous health care relevance, data regarding genetic predictors of peripartum depression are sparse. The aim of this study was to investigate associations of the serotonin-transporter linked polymorphic region (5-HTTLPR) genotype with peripartum MDD in an at-risk population.
Methods
274 women with a prior history of MDD were genotyped for 5-HTTLPR and serially evaluated in late pregnancy (gestational weeks 31-40), early postpartum (week 1-8) and late postpartum (week 9-24) for diagnosis of a current major depressive episode (MDE) and depressive symptom severity.
Results
5-HTTLPR S-allele carrier status predicted the occurrence of a MDE in the early postpartum period only (OR = 5.13, p = 0.017). This association persisted despite continued antidepressant treatment.
Conclusions
The 5-HTTLPR genotype may be a clinically relevant predictor of early postpartum depression in an at-risk population.
Keywords: peripartum depression, pregnancy, serotonin transporter, 5-HTTLPR, polymorphism, at risk population
Introduction
Major Depressive Disorder (MDD) during pregnancy and the postpartum period (peripartum MDD) are common, with estimates of point prevalence ranging between 8 to 13% (CDC 2008; Cox et al 1993; Dietz et al 2007; Evans et al 2001; Gavin et al 2005; O’Hara et al 1991). Moreover, a burgeoning clinical and preclinical literature demonstrates an array of adverse sequelae of maternal MDD and stress during pregnancy and the postpartum period including potential life-long effects on the offspring (Brennan et al 2008; Hammen and Brennan 2003; Murray et al 1996; Newport et al 2002a; Newport et al 2002b). Some have suggested these enduring effects may be epigenetically mediated (Oberlander et al 2008). Similar to non-puerperal MDD, psychological stress and family or personal histories of MDD increase the risk of peripartum MDD (Gotlib et al 1991; Murphy-Eberenz et al 2006; Paykel et al 1980). Despite the high prevalence and negative impact on the child, the biological underpinnings of peripartum MDD are not well-defined. Alterations in stress hormones, gonadal steroids, and serotonergic activity have all been implicated in the etiology of these disorders (Bloch et al 2000; Jolley et al 2007; Maes et al 2002; Newport et al 2004; Steiner et al 2003).
Family studies suggest a genetic contribution to the risk for peripartum MDD (Forty et al 2006; Murphy-Eberenz et al 2006). To date, genetic studies of peripartum MDD have emphasized polymorphisms in genes within the serotonin system, including the serotonin transporter gene (Jones et al 2000; Sanjuan et al 2008; Scheid et al 2007; Sun et al 2004). The serotonin-transporter linked polymorphic region (5-HTTLPR) is one of the best investigated polymorphisms in psychiatric genetics. Briefly, 5-HTTLPR (Lesch et al 1996), a repeat polymorphism in the promoter region of the serotonin transporter gene (SLC6A4) on chromosome 17, has been associated with functional differences in serotonin reuptake. Compared to the long version (16 incomplete 22 base pair repeats), the short version (14 repeats) of the 5-HTTLPR is linked to reduced serotonin transporter gene expression and serotonin uptake (Heils et al 1996). A single nucleotide polymorphism, rs25531, within the long allele has been shown to moderate the functional impact of this genetic variant further (Hu et al 2006).
While case-control associations of the 5-HTTLPR with MDD are largely negative (Lasky-Su et al 2005), an increasing literature supports its interaction with adverse life events to heighten the risk for MDD. While most studies support, at least partially, the initial report of such an interaction by Caspi et al 2003, some studies report negative results (see (Uher and McGuffin 2008) for a recent review). The 5-HTTLPR has also been associated with antidepressant therapeutic response (Serretti et al 2007) but see also (Hu et al 2007). It is thus not surprising that the 5-HTTLPR has also been chosen as a candidate polymorphism in peripartum MDD.
Two existing investigations have reported on the association of the 5-HTTLPR and peripartum MDD, one examining it’s influence on postpartum MDD (Sanjuan et al 2008) and the other gene-environment interactions contributing to mid-pregnancy MDD (Scheid et al 2007). Replicating previously reported gene-environment interactions in non-peripartum populations (Caspi et al 2003), Scheid et al. (2007) reported a higher level of depressive symptoms in mid-pregnancy among women with the 5-HTTLPR short (s-) allele and a history of abuse but no main effects of the polymorphism on depressive symptoms. Sanjuan et al. (2008), investigating not the 5-HTTLPR genotype alone but a combined genotype of the 5-HTTLPR and a second tandem repeat polymorphism in intron 2 of the gene, reported that homozygosity of the long (l-) allele (present in high and medium expressing groups but not a low expressing group) was associated with greater depressive symptoms at 8 but not 32 weeks postpartum. Because postpartum depression has been associated with decreased tryptophan bioavailability (Kohl et al 2005; Maes et al 2002), the authors contend that this finding is congruent with the heightened sensitivity of high function serotonin transporter gene carriers to the depressogenic effects of tryptophan depletion (Moreno et al 2002; Neumeister et al 2006). It is noteworthy, however, that increased sensitivity to tryptophan depletion has also been reported for women carrying the low-expressing S-allele of this polymorphism, especially in combination with a family history of mood disorders (Neumeister et al 2002). Because the two existing investigations of the impact of the 5-HTTLPR on the risk for peripartum MDD have focused on very different phenotypes, no independent replication of either study has yet been reported. In addition, both studies used a non-psychiatric sample.
The goal of the current study was to conduct a prospective longitudinal investigation of the association of the 5-HTTLPR on depressive symptoms in late pregnancy and the postpartum period in an at-risk cohort of women with prior histories of MDD, ascertaining whether this genetic variant might serve to identify women at highest risk for recurrent depressive illness during pregnancy or the postpartum period. We examined both the 5-HTTLPR alone and the functional classification including rs25531 (Hu et al 2006), which has not yet been investigated in peripartum MDD. Our study thus explores mechanisms whereby the previous findings (i.e., interaction of the 5-HTTLPR with a history of abuse to predict MDD during pregnancy and a main effect of this polymorphism on postpartum MDD (Scheid et al 2007; Sanjuan et al 2008)), may be related to an at-risk clinical cohort. Furthermore, the current study adds a longitudinal prospective component utilizing serial data collection from late pregnancy through the postpartum period.
Material and Methods
Patients & Psychometric Assessments
Pregnant women (age >= 18 years) with lifetime histories of MDD, presenting to the Emory Women’s Mental Health Program (WMHP), were enrolled prior to 20 weeks gestation in a prospective observational study of the perinatal course of MDD. Women were excluded from the present study if they were actively suicidal, exhibited current psychotic symptoms, were severely anemic, had a positive urine drug screen, had an abnormal plasma TSH concentration, or were actively abusing alcohol or drugs within the past 12 months. Failure to extract high-quality DNA from blood samples was also an exclusion criterion. Written informed consent for study participation was obtained, and the Institutional Review Board of the Emory University School of Medicine approved the study.
At study entry, current and lifetime psychiatric diagnoses were assessed using the Structured Clinical Interview for DSM-IV (SCID) (First et al 1995).
At the baseline and all subsequent follow-up visits, depressive symptom severity was assessed using the 17-item Hamilton Rating Scale for Depression (HRSD17) (Hamilton 1960), and the presence of a current MDE was determined using the SCID Mood Module. For women agreeing to participate in the genetic analyses, mood was assessed during late pregnancy (≥30 weeks gestation), early postpartum (≤8 weeks), and later postpartum (9 to 24 weeks). Finally, all medications, maternal daily dose, and self-reported adherence were prospectively recorded at each study visit and at delivery.
DNA extraction and 5-HTTLPR genotyping
DNA was extracted from whole blood using the Qiagen M48 biorobot (Qiagen Inc). Genotyping of the 5-HTTLPR used the following primers (forward: 5′-Hex-TGAATGCCAGCACCTAACC -3′; reverse: 5′- ATACTGCGAGGGGTGCAG -3′). PCR was carried out in 384 well plates in a 10 μl volume with 10 ng DNA. Each PCR reaction contained 0.5 μM of each primer, 0.08 μM of dATP, dCTP and dTTP and 0.04 uM of dGTP, 0.2 μM of 7-deaza GTP (Amersham Biosciences), 5% DMSO and 1.25 units of AmpliTaq Gold (Applied Biosystems). The cycling parameters were as follows: 95°C for 5 min, then 94°C for 30sec, 63°C for 30 sec and 72°C for 1 min for 1 cycle, then the annealing temperature was reduced to 62°C for one more cycle and then to 59.5°C for 38 cycles. 5 μl of the resulting PCR products was then digested with 5 U MspI (New England Biolabs) in a total volume of 10 μl for 90 minutes at 37°C to detect the A/G SNP rs25531 shown to influence the functional effects of the long and short alleles (Hu et al 2006). The digested PCR products were then separated using an Applied Biosystems 3100 genetic analyzer and analyzed with Applied Biosystems Genemapper 4.0 software. Fragment lengths for the LA-allele are 291 bp, 148 for the LG and 247 bp for the S-allele. The VL fragment is 335 bp and the XL fragment 375 bp. The L-VL or L-XL genotypes were each observed in one participant; both were excluded from the analysis.
For quality control, the runs included duplicated samples and positive controls established through re-sequencing.
Statistical analysis
Statistical analyses were performed using SPSS version 15.0. We used contingency tables and logistic regression to evaluate differences in polymorphism distribution according to diagnosis of a current MDE. Covariates included maternal age at conception, race, education, marital status, gestational age at delivery, gravidity, and parity. Each of these covariates had been associated with peripartum MDD either in previous reports or in preliminary univariate analyses using our sample. In our sample, a MDE during late pregnancy was associated with education (p < 0.1), race (p < 0.05), and gestational age at delivery (p < 0.05). An early postpartum MDE was associated with race (p < 0.05), gravidity (p < 0.05), and parity (p < 0.05). A late postpartum MDE was associated with race (p < 0.05), marital status (p < 0.05), gravidity (p < 0.05), and maternal age (p < 0.05). Repeated measures general linear models were used to investigate genotype dependent differences in the evolution of depressive symptoms over time. Data are reported as means and standard deviations (Results section and Tables) or means and standard errors (Figures). All tests were two-tailed. Alpha was set a priori to 0.05.
Power analysis
Using the software Quanto 1.1 (http://hydra.usc.edu/gxe), the power to detect a genetic relative risk of 3.0 in a sample of N = 274 with 15% affected individuals with peripartum depression and a minor allele frequency of 40.0 % in a recessive genetic model was 82.6% with an alpha level of 0.05. The power to detect the genetic relative risk of 5.13 observed in the actual sample for early postpartum depression according to the SCID mood module (N = 172) was 95.0% using the above parameters.
Results
A total of 274 women with a lifetime history of MDD were screened for inclusion. Of these women, 206 (75.2%) fulfilled inclusion criteria for the current analysis with high quality DNA and HRSD17 during late pregnancy, while 201 also completed the SCID Mood Module at this time. Attrition following delivery yielded a total of 188 with HRSD17 and 172 with SCID Mood Module ratings during the early postpartum interval, and 183 HRSD17 and 169 SCID Mood Module ratings during the late postpartum interval. No significant differences in any of the descriptive and clinical parameters listed in Table 1 were observed between the screened sample, the included sample, and the post-attrition sample assessed during the late postpartum interval.
Table 1.
Demographic and clinical characteristics of study sample (n=204) at study entry.
| Characteristic | Mean (SD) or Pct. | |
|---|---|---|
| Age (years) | 34.1 (4.3) | |
| Race | White Non-Hispanic Asian Native American African American White Hispanic |
87.8% 2.5% 2.0% 4.4% 3.4% |
| Live Deliveries | 100.0% | |
| Gestational Age at Delivery (weeks) | 38.4 (1.30) | |
| Gravidity | 2.50 (1.60) | |
| Parity | 0.98 (0.99) | |
| Lifetime MDD (per SCID) | 100.0% | |
| MDE at Study Entry | 15.1% | |
| HRSD17 at Study Entry (gestational week 31-40) | 13.0 (5.4) | |
| Treatment at Study Entry |
No Antidepressant Antidepressant Monotherapy Antidepressant Polytherapy |
19.6% 73.0% 7.4% |
Depressive symptoms, diagnostic criteria, and antidepressant treatment
Of the 206 women with DNA and HRSD17 scores, 80.6% were receiving antidepressant therapy at the late pregnancy visit, all but 15 receiving monotherapy. The most common antidepressants were sertraline (22.1%), fluoxetine (13.7%), bupropion (10.3%) and venlafaxine (9.8%). Other antidepressants included citalopram, escitalopram, paroxetine, fluvoxamine, amitriptyline, desipramine, duloxetine and nortriptyline. 83.8% of the participants were taking antidepressant medication at the early postpartum visit and 77.0% at the late postpartum visit. Among the women who were not receiving antidepressant therapy during late pregnancy, 75.8% remained off medication in the early postpartum compared to only 55.3% in the late postpartum period.
At the late pregnancy visit, the mean HRSD17 score was 13.0±5.3, with 14.9% (n=30/201) fulfilling criteria for a current MDE according to the SCID mood module. At the early and late postpartum visits, 13.4% (n=23/172) and 17.8% (n=30/169), respectively, fulfilled criteria for a current MDE. Notably, 65.2% (n=15/23) of the early postpartum MDEs and 75.0% (n=21/28) of the late postpartum MDEs occurred in women who were euthymic during late pregnancy. Compared to late pregnancy (using a paired t-test), the mean HRSD17 scores were lower in the early postpartum period (t = 6.4, df = 179, p < 0.0001), with mean (SD) scores of 13.0 (5.34) in late pregnancy and 10.5 (5.78) in early postpartum as well as the late postpartum period (t = 3.6, df = 172, p < 0.0001) with a mean HRSD17 score of 11.3 (6.09).
Frequency of 5-HTTLPR genotypes
Of the 206 women with high quality DNA and HRSD17 ratings in late pregnancy, two presented with rare genotypes (L-VL (n=1); L-XL (n=1)) and were excluded from all further analyses. The 5-HTTLPR genotype distribution for the remaining 204 women was: 1) 18.4% SS; 2) 49.0% LS; and 3) 31.6% LL, a distribution consistent with the Hardy-Weinberg equilibrium. Of all individuals, 15.1% presented with a G allele at the rs25531 polymorphism (8.8% as LALG and 6.3% as LGS). Using the functional classification according to Hu et al. (2006), 24.3% were classified as low transporter-expressing (SS and LGS), 51.0% as medium expressing (LALG, LAS) and 22.8% as high expressing (LALA).
Association of 5-HTTLPR genotype with diagnosis of peripartum MDE
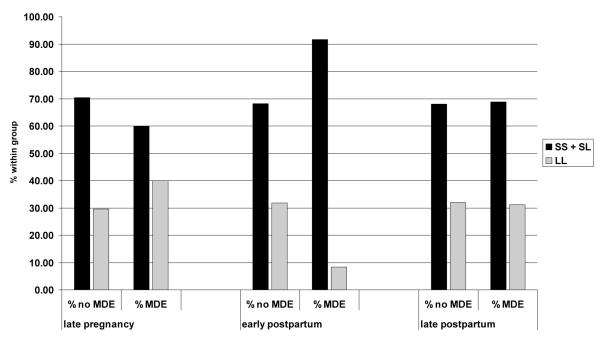
There was no significant association of the 5-HTTLPR LS genotype (additive model), presence of S-allele or functional classification with the occurrence of a MDE in late pregnancy or the late postpartum period; however, significant differences in genotype distribution and presence of S-allele were observed in the early postpartum period (cf. Table 2 and Figure 1). The odds ratio for an early postpartum MDE in the presence of the S-allele (S-carrier model) was 5.13 [1.16 – 22.7] (p = 0.017). To control for potential confounders, a logistic regression was conducted to assess the 5-HTTLPR S-allele and other predictors of a MDE during the early postpartum period. The model identified 5-HTTLPR S-allele (OR=6.71 [1.41-31.95], p = 0.017), maternal age at conception (OR=0.84 [0.95-0.74], p = 0.0081), and gravidity (OR=1.60 [1.22-2.11], p = 0.00070) as significant predictors of an early postpartum MDE. The log likelihood of the main effects model was 116.14 (χ2=22.8, df=3, p<0.0001). The Hosmer and Lemeshow goodness-of-fit statistic was 7.85 (df=8, p = 0.44), indicating that the logistic model fit the data adequately.
Table 2.
Distribution of 5-HTTLPR genotype, presence of S-allele and functional classification according to Hu et al., 2006 between patients with and without current MDE in late pregnancy, early and late postpartum. p = 0.041 for early postpartum and 5-HTTLPR genotype, p = 0.016 (Fishers’ Exact test) for 5-HTTLPR S-allele and p = 0.072 for 5-HTTLPR functional classification. All other tests p > 0.05.
| Assessment Window |
SCID Criteria |
5-HTTLPR Genotype | 5-HTTLPR S-allele |
5-HTTLPR Functional Classification |
|||||
|---|---|---|---|---|---|---|---|---|---|
| SS | SL | LL | SS + SL | LL | Low | Medium | High | ||
|
Late
pregnancy (31 weeks to delivery) |
% No MDE | 20.71 | 49.70 | 29.59 | 70.41 | 29.59 | 26.79 | 51.79 | 21.43 |
| % MDE | 10.00 | 50.00 | 40.00 | 60.00 | 40.00 | 13.79 | 55.17 | 31.03 | |
|
Early
postpartum (1-8 weeks) |
% No MDE | 21.43 | 46.75 | 31.82 | 68.18 | 31.82 | 25.32 | 48.70 | 25.97 |
| % MDE | 20.83 | 70.83 | 8.33 | 91.67 | 8.33 | 27.27 | 68.18 | 4.55 | |
|
Late
postpartum (9-24 weeks) |
% No MDE | 21.53 | 46.53 | 31.94 | 68.06 | 31.94 | 27.78 | 47.22 | 25.00 |
| % MDE | 9.38 | 59.38 | 31.25 | 68.75 | 31.25 | 16.13 | 64.52 | 19.35 | |
Figure 1.
Distribution of 5-HTTLPR S-allele carriers (SS + SL vs LL) between patients with and without current MDE in late pregnancy, early and late postpartum. p = 0.017 (Fishers’ Exact test) for early postpartum and p > 0.05 for the two other time points.
The association between S-allele status and early postpartum MDE was remarkably stable. For example, the distribution of S-allele carriers between those with and without an early postpartum MDE was essentially the same whether the analysis considered all 172 participants with early postpartum SCID assessments (91.7% vs. 68.2%) or was limited to the participants with early postpartum SCID assessments who were euthymic during late pregnancy (92.9% vs. 69.5%). Similarly, when the analysis was limited to those receiving antidepressant therapy during the early postpartum period (n=141), the proportion of S-allele carriers was 90.5% (n=19/21) for those with an early postpartum MDE and 68.3% (n=82/120) for those without an early postpartum MDE.
Because the association with an early postpartum MDE appeared to be driven by S-allele carrier status (i.e., an S-allele-dominant model), all subsequent analyses were based on an S-allele-dominant genetic model. This model is consistent with the reported dominance of the S allele with regard to lower serotonin-transporter expression and activity (Heils et al 1996).
Association of S-allele carrier status with severity of peripartum depressive symptoms
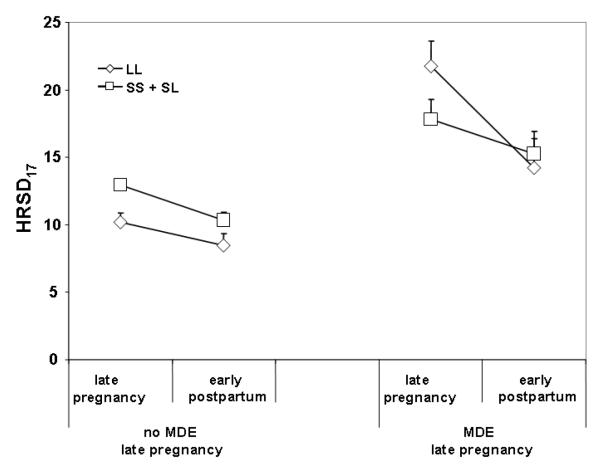
A repeated measures ANOVA of all patients found no significant main effect of carrier status nor time × status effect for change of HRSD17 score from late pregnancy to early postpartum. However, when the analysis was restricted to those who were euthymic during late pregnancy and continuing antidepressant therapy into early postpartum (N = 119), significant main effects of time (F117,1 = 16.33, p < 0.0001) and S-allele carrier status (F117,1 = 8.28, p = 0.005) but no significant interaction were observed (cf. Figure 2). In an exploratory analysis, another repeated measures ANOVA of change in HRSD17 score from late pregnancy to early postpartum was performed to assess the effect of the S-allele in women who were depressed during late pregnancy controlling for antidepressant treatment status. This analysis demonstrated a trend for an interaction between time (late pregnancy vs. early postpartum and 5-HTTLPR S allele (F21,1 = 3.37, p = 0.081) but no main genotype effect (cf. Figure 2).
Figure 2.
HRSD17 scores in late pregnancy and early post-partum according to presence of 5-HTTLPR S-allele in patients without MDE in late pregnancy and on continued antidepressant medication (left panel) and in patients with a MDE in late pregnancy. Sample sizes: No MDE late pregnancy LL = 35 and SS + SL = 84; MDE late pregnancy, LL = 9 and SS + SL = 15.
Association of S-allele carrier status with estimated gestational age at delivery
We observed no genotype effect on estimated gestational age at delivery in the sample as a whole, as well as the subsamples on and off antidepressant medication and with or without current MDE in late pregnancy.
Discussion
The current study provides further evidence for the influence of the 5-HTTLPR genotype on early postpartum depression. In our at-risk sample, S-allele carrier status was a strong predictor (OR = 5.13) for developing a MDE in the early postpartum period, within 8 weeks of delivery. This association persisted for new onset episodes (i.e., those euthymic in late pregnancy but depressed in early postpartum) in women who were receiving antidepressant therapy throughout the entire observation period. Overall, the 5-HTTLPR genotype might therefore be a clinically relevant predictor of postpartum depression in an at-risk psychiatric population, with S-allele carrier status predicting a substantial risk of early postpartum depression in women with prior histories of MDD, despite continued antidepressant medication.
Furthermore, our study, utilizing an at-risk psychiatric sample, supports several of the previously reported findings on the 5-HTTLPR in peripartum depression in non-psychiatric community samples (Scheid et al 2007; Sanjuan et al 2008). Similar to Sanjuan et al. (2008), we observed significantly lower HRSD depressive symptom scores in the postpartum period as compared to mid to late pregnancy. Surprisingly, however, MDE rates as determined by the SCID mood module were highest in the late postpartum. We suspect that these conflicting findings may be a consequence of an inflationary effect of late pregnancy physical symptoms on HRSD scores, producing higher pregnancy euthymic scores than postpartum euthymic scores.
Like Scheid et al. (2007), we report no main effect of the 5-HTTLPR on depressive symptoms in the postpartum period in the sample overall.
On the other hand, our genetic associations in the postpartum period are not consistent with Sanjuan et al,. 2008. While we also found a significant association of 5-HTTLPR genotype with early but not late postpartum depression, the direction of our association was opposite. In our sample, low-expression S-allele carriers rather than high expression genotypes had the highest incidence of MDE and severity of depressive symptoms. This might indicate that our findings in a demographically homogeneous psychiatric sample cannot be extrapolated to women in community settings who have no prior histories of MDD. In fact, the contrasting results might lead to speculation that the change in postpartum depressive symptom severity observed in a community sample has a distinct etiopathogenesis from peripartum recurrence in women with prior histories of MDD. Sanjuan et al. (2008) contend that their data indicate that high function serotonin transporter allele carriers are at heightened risk for postpartum depression because they are more likely to deplete tryptophan stores. In contrast, the current data, in agreement with the majority but not all previously reported pharmacogenetic and gene × environment associations (Caspi et al 2003; Serretti et al 2007), suggest that the low function S-allele is the risk allele.
Contrary to the community setting, the majority of women in this study were receiving continued antidepressant therapy, so that the heightened risk for postpartum MDD in S-allele carriers in our sample may be a product of the reported potential for this group to escape from previously efficacious pharmacological treatment (Serretti et al 2007). However, the time-point specific effects of this genetic association would suggest that non-response to antidepressant treatment cannot be the only explanatory factor but that specific interactions with the biological and/or environmental changes and the 5-HTTLPR in this patients group in early postpartum are likely.
The current study results suggest that the predictive value of the 5-HTTLPR for postpartum depression depends on the timing of the symptom onset. The 5-HTTLPR was a significant predictor of a depressive episode in the early postpartum period, but not in late pregnancy or the later postpartum period. This might be related to the unique set of stressors associated with caring for a newborn, including sleep restriction, which may specifically interact with 5-HTTLPR genotype to predict depressive symptoms. In addition, early postpartum depression (onset before postpartum weeks 6-8) has been identified to be associated with higher familiality and thus possibly genetic load than postpartum depression with later onset (before 6 months postpartum) (Forty et al 2006). This could indicate that the biological underpinnings of early vs. later postpartum depression are different and could explain why the observed genetic association was only seen using the early definition of postpartum onset.
A clear limitation of this study is sample size, and unless replicated in larger independent studies, our findings may be a product of random error. This weakness is partially counterbalanced by the prospective longitudinal nature of the data collection as well as the homogenous sample characteristics. Due to the prospective design, we were able to control for late pregnancy MDD in our analyses of the risk for postpartum MDD. As demonstrated in the results section, over 35% of women fulfilling criteria for a postpartum MDE had fulfilled the same criteria in late pregnancy. An exploratory analysis of women with MDE in late pregnancy suggests that the genotype effects on depressive symptoms might be different in this group (see Figure 2). Separate analyses of postpartum onset depression as compared to depression that is continuous from pregnancy into the postpartum period thus seem warranted.
The current study is the first to report on associations of the 5-HTTLPR genotype in a psychiatric sample followed through the postpartum period. If replicated, these data suggest that 5-HTTLPR polymorphisms have distinct effects on the risk for postpartum MDD in patient vs. general population groups.
Our data may bear significant clinical import. In our homogeneous high risk sample, over 13% of women experienced a MDE during the first eight weeks postpartum, despite continued antidepressant treatment. In this group, S-allele carriers represent over 90% of the cases. If this finding is replicated, more aggressive treatment planning and monitoring could be indicated for at-risk women carrying the 5-HTTLPR S-allele.
Objective.
Peripartum major depressive disorder is a prevalent psychiatric disorder with potential detrimental consequences for both mother and child. Despite its enormous health care relevance, data regarding genetic predictors of peripartum depression are sparse. The aim of this study was to investigate associations of the serotonin-transporter linked polymorphic region (5-HTTLPR) genotype with peripartum MDD in an at-risk population.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bernstein DP, Fink L. Childhood trauma questionnaire manual. Psychological Corporation; San Antoinio, TX: 1998. [Google Scholar]
- Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry. 2000;157:924–930. doi: 10.1176/appi.ajp.157.6.924. [DOI] [PubMed] [Google Scholar]
- Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, et al. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PA, Pargas R, Walker EF, Green P, Newport DJ, Stowe Z. Maternal depression and infant cortisol: influences of timing, comorbidity and treatment. J Child Psychol Psychiatry. 2008;49:1099–1107. doi: 10.1111/j.1469-7610.2008.01914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- CDC Prevalence of self-reported postpartum depressive symptoms--17 states, 2004-2005. MMWR Morb Mortal Wkly Rep. 2008;57:361–366. [PubMed] [Google Scholar]
- Chung EK, Mathew L, Elo IT, Coyne JC, Culhane JF. Depressive symptoms in disadvantaged women receiving prenatal care: the influence of adverse and positive childhood experiences. Ambul Pediatr. 2008;8:109–116. doi: 10.1016/j.ambp.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Cox JL, Murray D, Chapman G. A controlled study of the onset, duration and prevalence of postnatal depression. Br J Psychiatry. 1993;163:27–31. doi: 10.1192/bjp.163.1.27. [DOI] [PubMed] [Google Scholar]
- Dietz PM, Williams SB, Callaghan WM, Bachman DJ, Whitlock EP, Hornbrook MC. Clinically identified maternal depression before, during, and after pregnancies ending in live births. Am J Psychiatry. 2007;164:1515–1520. doi: 10.1176/appi.ajp.2007.06111893. [DOI] [PubMed] [Google Scholar]
- Evans J, Heron J, Francomb H, Oke S, Golding J. Cohort study of depressed mood during pregnancy and after childbirth. BMJ. 2001;323:257–260. doi: 10.1136/bmj.323.7307.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer R, Williams J. Structured clinical interview for DSM-IV (SCID-I) (User’s guide and Interview) Research Version. Biometrics Research Department, New York Psychiatric Institute; New York: 1995. [Google Scholar]
- Forty L, Jones L, Macgregor S, Caesar S, Cooper C, Hough A, et al. Familiality of postpartum depression in unipolar disorder: results of a family study. Am J Psychiatry. 2006;163:1549–1553. doi: 10.1176/ajp.2006.163.9.1549. [DOI] [PubMed] [Google Scholar]
- Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005;106:1071–1083. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Whiffen VE, Wallace PM, Mount JH. Prospective investigation of postpartum depression: factors involved in onset and recovery. J Abnorm Psychol. 1991;100:122–132. doi: 10.1037//0021-843x.100.2.122. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C, Brennan PA. Severity, chronicity, and timing of maternal depression and risk for adolescent offspring diagnoses in a community sample. Arch Gen Psychiatry. 2003;60:253–258. doi: 10.1001/archpsyc.60.3.253. [DOI] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, Lesch KP. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Hranilovic D, Stefulj J, Schwab S, Borrmann-Hassenbach M, Albus M, Jernej B, Wildenauer D. Serotonin transporter promoter and intron 2 polymorphisms: relationship between allelic variants and gene expression. Biol Psychiatry. 2004;55:1090–1094. doi: 10.1016/j.biopsych.2004.01.029. [DOI] [PubMed] [Google Scholar]
- Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet. 2006;78:815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XZ, Rush AJ, Charney D, Wilson AF, Sorant AJ, Papanicolaou GJ, et al. Association between a functional serotonin transporter promoter polymorphism and citalopram treatment in adult outpatients with major depression. Arch Gen Psychiatry. 2007;64:783–792. doi: 10.1001/archpsyc.64.7.783. [DOI] [PubMed] [Google Scholar]
- Jolley SN, Elmore S, Barnard KE, Carr DB. Dysregulation of the hypothalamic-pituitary-adrenal axis in postpartum depression. Biol Res Nurs. 2007;8:210–222. doi: 10.1177/1099800406294598. [DOI] [PubMed] [Google Scholar]
- Jones I, Middle F, McCandless F, Coyle N, Robertson E, Brockington I, et al. Molecular genetic studies of bipolar disorder and puerperal psychosis at two polymorphisms in the estrogen receptor alpha gene (ESR 1) Am J Med Genet. 2000;96:850–853. doi: 10.1002/1096-8628(20001204)96:6<850::aid-ajmg31>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Kohl C, Walch T, Huber R, Kemmler G, Neurauter G, Fuchs D, et al. Measurement of tryptophan, kynurenine and neopterin in women with and without postpartum blues. J Affect Disord. 2005;86:135–142. doi: 10.1016/j.jad.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Lasky-Su JA, Faraone SV, Glatt SJ, Tsuang MT. Meta-analysis of the association between two polymorphisms in the serotonin transporter gene and affective disorders. Am J Med Genet B Neuropsychiatr Genet. 2005;133B:110–115. doi: 10.1002/ajmg.b.30104. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Maes M, Verkerk R, Bonaccorso S, Ombelet W, Bosmans E, Scharpe S. Depressive and anxiety symptoms in the early puerperium are related to increased degradation of tryptophan into kynurenine, a phenomenon which is related to immune activation. Life Sci. 2002;71:1837–1848. doi: 10.1016/s0024-3205(02)01853-2. [DOI] [PubMed] [Google Scholar]
- Moreno FA, Rowe DC, Kaiser B, Chase D, Michaels T, Gelernter J, Delgado PL. Association between a serotonin transporter promoter region polymorphism and mood response during tryptophan depletion. Mol Psychiatry. 2002;7:213–216. doi: 10.1038/sj.mp.4000962. [DOI] [PubMed] [Google Scholar]
- Murphy-Eberenz K, Zandi PP, March D, Crowe RR, Scheftner WA, Alexander M, et al. Is perinatal depression familial? J Affect Disord. 2006;90:49–55. doi: 10.1016/j.jad.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Murray L, Fiori-Cowley A, Hooper R, Cooper P. The impact of postnatal depression and associated adversity on early mother-infant interactions and later infant outcome. Child Dev. 1996;67:2512–2526. [PubMed] [Google Scholar]
- Neumeister A, Hu XZ, Luckenbaugh DA, Schwarz M, Nugent AC, Bonne O, et al. Differential effects of 5-HTTLPR genotypes on the behavioral and neural responses to tryptophan depletion in patients with major depression and controls. Arch Gen Psychiatry. 2006;63:978–986. doi: 10.1001/archpsyc.63.9.978. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Konstantinidis A, Stastny J, Schwarz MJ, Vitouch O, Willeit M, et al. Association between serotonin transporter gene promoter polymorphism (5HTTLPR) and behavioral responses to tryptophan depletion in healthy women with and without family history of depression. Arch Gen Psychiatry. 2002;59:613–620. doi: 10.1001/archpsyc.59.7.613. [DOI] [PubMed] [Google Scholar]
- Newport DJ, Owens MJ, Knight DL, Ragan K, Morgan N, Nemeroff CB, Stowe ZN. Alterations in platelet serotonin transporter binding in women with postpartum onset major depression. J Psychiatr Res. 2004;38:467–473. doi: 10.1016/j.jpsychires.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Newport DJ, Stowe ZN, Nemeroff CB. Parental depression: animal models of an adverse life event. Am J Psychiatry. 2002a;159:1265–1283. doi: 10.1176/appi.ajp.159.8.1265. [DOI] [PubMed] [Google Scholar]
- Newport DJ, Wilcox MM, Stowe ZN. Maternal depression: a child’s first adverse life event. Semin Clin Neuropsychiatry. 2002b;7:113–119. doi: 10.1053/scnp.2002.31789. [DOI] [PubMed] [Google Scholar]
- O’Hara MW, Schlechte JA, Lewis DA, Wright EJ. Prospective study of postpartum blues. Biologic and psychosocial factors. Arch Gen Psychiatry. 1991;48:801–806. doi: 10.1001/archpsyc.1991.01810330025004. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3:97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- Paykel ES, Emms EM, Fletcher J, Rassaby ES. Life events and social support in puerperal depression. Br J Psychiatry. 1980;136:339–346. doi: 10.1192/bjp.136.4.339. [DOI] [PubMed] [Google Scholar]
- Sanjuan J, Martin-Santos R, Garcia-Esteve L, Carot JM, Guillamat R, Gutierrez-Zotes A, et al. Mood changes after delivery: role of the serotonin transporter gene. Br J Psychiatry. 2008;193:383–388. doi: 10.1192/bjp.bp.107.045427. [DOI] [PubMed] [Google Scholar]
- Scheid JM, Holzman CB, Jones N, Friderici KH, Nummy KA, Symonds LL, et al. Depressive symptoms in mid-pregnancy, lifetime stressors and the 5-HTTLPR genotype. Genes Brain Behav. 2007;6:453–464. doi: 10.1111/j.1601-183X.2006.00272.x. [DOI] [PubMed] [Google Scholar]
- Serretti A, Kato M, De Ronchi D, Kinoshita T. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with selective serotonin reuptake inhibitor efficacy in depressed patients. Mol Psychiatry. 2007;12:247–257. doi: 10.1038/sj.mp.4001926. [DOI] [PubMed] [Google Scholar]
- Shea AK, Streiner DL, Fleming A, Kamath MV, Broad K, Steiner M. The effect of depression, anxiety and early life trauma on the cortisol awakening response during pregnancy: preliminary results. Psychoneuroendocrinology. 2007;32:1013–1020. doi: 10.1016/j.psyneuen.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Steiner M, Dunn E, Born L. Hormones and mood: from menarche to menopause and beyond. J Affect Disord. 2003;74:67–83. doi: 10.1016/s0165-0327(02)00432-9. [DOI] [PubMed] [Google Scholar]
- Sun HS, Tsai HW, Ko HC, Chang FM, Yeh TL. Association of tryptophan hydroxylase gene polymorphism with depression, anxiety and comorbid depression and anxiety in a population-based sample of postpartum Taiwanese women. Genes Brain Behav. 2004;3:328–336. doi: 10.1111/j.1601-183X.2004.00085.x. [DOI] [PubMed] [Google Scholar]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: review and methodological analysis. Mol Psychiatry. 2008;13:131–146. doi: 10.1038/sj.mp.4002067. [DOI] [PubMed] [Google Scholar]