Abstract
Purpose
Systemic metastatic retinal lymphoma (SMRL) is exceptionally rare, as systemic lymphomas most often metastasize to the uvea. We have evaluated a series of SMRL cases to elucidate the clinical and pathological features of SMRL.
Methods
The pathologic specimens of intraocular lymphomas (IOLs) at the National Eye Institute from 1991–2009 were retrospectively reviewed. These cases were diagnosed by cytology, cytokine measurement (ELISA for interleukin (IL)-10 and IL-6 levels), and Immunoglobulin-Heavy (IgH) and T-cell-receptor (TCR) gene analyses.
Results
There were 9 B-SMRLs among 96 B-cell retina lymphomas (9.4%) and 3 T-SMRLs among 5 T-cell retinal lymphomas (60%) from a total of 116 IOLs. The original sites were nasopharynx (3), testis (2), skin (2), breast (1), blood (1), retroperitoneum (1), ileo-cecum (1) and stomach (1). Cytology of vitreous samples illustrated atypical lymphoma cells with either B- or T- monoclonality. More B-SMRLs had a high ratio of vitreal IL-10 to IL-6 than T-SMRLs. Molecular pathology demonstrated lymphoma cells with gene rearrangements of IgH in all B-SMRLs and TCR in all T-SMRLs.
Conclusions
SMRL and primary retinal lymphoma present with similar clinical manifestations. Systemic T-cell lymphoma invades the retina and vitreous more aggressively than systemic B-cell lymphoma. A diagnosis of SMRL is made when there is a clinical history of systemic lymphoma (particularly from nasopharynx, testis, and skin) and lymphoma cells are identified in the vitreous or retina. Molecular analysis is more useful than vitreal cytokine measurement for SMRL diagnosis.
Keywords: Systemic Metastatic Retinal Lymphoma, Intraocular Lymphoma, Cytokine, IgH gene rearrangement, TCR gene rearrangement
Introduction
Lymphomas are derived from a monoclonal proliferation of B- or T-lymphocytes. Lymphomas inside the eye are relatively uncommon and account for 1% of non-Hodgkin’s lymphomas and less than 1% of all intraocular tumors (Bardenstein 1998). The term intraocular lymphoma (IOL) describes a lymphoma located inside the eye, which arises from the central nervous system (CNS), including the retina, the uvea (extremely rare), or a metastatic systemic lymphoma. The former, primary intraocular lymphoma (PIOL) is a subset of primary CNS lymphoma (PCNSL) and is also called primary retinal lymphoma (PRL). PIOL and has a significantly higher prevalence than the other forms of IOLs (Chan 2007; Coupland et al. 2009). Primary uveal lymphoma is extremely rare and has a much lower prevalence than the above two IOLs. Primary choroidal lymphoma is an extranodal marginal zone B-cell lymphoma (EMZL). Only few cases of primary choroidal MALT (mucosa-associated lymphoid tissue) lymphoma have been studied (Coupland & Damato 2008). Primary iridal or ciliary lymphomas are extremely rare, and few cases have been documented (Chan et al. 2008). Systemic lymphomas usually metastatize though blood into the uveal tissues.
The incidence rate of PIOL is estimated to be approximately 21 per 100,000 patients with ocular disorders who present in a referral eye center per year (Mochizuki & Singh 2009). Among the IOLs, PCNS/PIOL is most common (61%), followed by PIOL alone (17%), IOL secondary to metastatic systemic lymphoma (17%), and IOL secondary to both metastatic systemic and CNS lymphoma (5%) (Singh et al. 2007).
PIOL may initially present in the eye with or without simultaneous CNS involvement. Most PIOLs/PCNSLs are extranodal, non-Hodgkin, diffuse large B-cell lymphomas (DLBCLs). PIOL cells are located in the retina, vitreous and optic nerve (Chan 2007). In contrast, metastatic systemic lymphoma is a lymphoma that metastasizes into the eye via hematogenous spread. Typically, small B-cell IOLs present with advanced systemic disease. In general, intraocular T-cell lymphomas are rare and can be secondary to metastatic systemic T-cell lymphomas, including primary cutaneous peripheral T-cell lymphoma and occasionally adult T-cell leukemia/lymphoma (Kumar et al. 1994; Shibata et al. 1997; Levy-Clarke et al. 2002; Buggage 2003). The uvea is the most common site for metastatic systemic lymphomas, and metastatic systemic lymphoma to the retina and vitreous without clinical uveal or conjunctival involvement is exceedingly rare, making the diagnosis of systemic metastatic retinal lymphoma (SMRL) very difficult and challenging (Levy-Clarke et al. 2005; Calfa et al. 2007). No study has fully investigated the clinical features and pathological diagnosis of SMRL as compared to PIOL. Herein we report a small series of SMRL cases to elucidate the clinical and pathological features of SMRL.
Material and Methods
The study was approved by the National Eye Institute Institutional Review Board for human subjects, and informed consent was obtained from all patients.
The medical records of IOL cases that were diagnosed at the National Eye Institute (NEI), National Institutes of Health, from January 1991 to April 2009 were reviewed retrospectively. These cases were diagnosed by cytology, cytokine measurement (ELISA for interleukin (IL)-10 and IL-6 levels), and Immunoglobulin-Heavy (IgH) and T-cell-receptor (TCR) gene analyses as reported previously (Shen et al. 1998; Chan & Wallace 2004; Levy-Clarke et al. 2005; Gonzales & Chan 2007). The detailed protocol of processing the vitreous sample has been documented (Gonzales & Chan 2007). Briefly, the vitreous specimens were centrifuged at 1,000 rpm. The supernatant was collected and IL-10 and IL-6 levels were measured by enzyme-linked immunosorbent assay (ELISA). The sediment in the original tube was then resuspended and cytocentrifuged (7620 CYTOCENTRIFUGE, Wescor Inc., Logan, Utah). The slides were processed for cytology and molecular analyses.
A minimum of 15 atypical cells was microdissected from the cytospin slides for DNA extraction. Polymerase chain reaction (PCR) was used to detect monoclonality of malignant B-, or rarely, T- cells. The three primer pairs for the malignant B-cells were: the second framework region (FR2A sense 5’-TGG RTC CGM CAG SCV YCN GG-3’ and anti-sense 5’- GGA TGG TAC CAA GCT TTG AGG AGA CGG TGA CCA-3’), the third framework region (FR3A sense 5’-ACA CGG CYS TGT ATT ACT GT-3’ and anti-sense 5’-GGA TGG TAC CAA GCT TTG AGG AGA CGG TGA CCA-3’), and CDR3 (sense 5’-CCG GRA ARR GTC TGG AGT GG-3’ and anti-sense 5’-ATC CTG AGG AGA CGG TGA CC-3’). These primers were used to detect monoclonality within the variable region of the third complementary determining region (CDR3) in the IgH gene of the malignant B-cells. The primer pairs used to detect the gene rearrangement in the TCR gene for the malignant T-cells were TCR-γ (sense 5'-AGG GAT GTG TTG GAA TCA GG-3' and antisense 5'-CGT CGA CAA CAA GTG TTG TTC CAC-3') and TCR-CDR3 (sense 5'-GAA AGG AAT CTG GCA TTC CGT CAG-3' and antisense 5'-GAA GTT ACT ATG AGC YTA GTC CCT T-3').
The data is presented with the mean ± S.E. The chi-square analysis and t-tests were performed with SPSS version 17.0 (SPSS Inc., Chicago, Illinois). Statistical significance was accepted for p values less than 0.05.
Results
Between January 1991 and April 2009, 116 patients were diagnosed with IOL at the NEI, including 101 retinal lymphomas and 15 uveal lymphomas. The 101 retinal lymphomas consisted of 89 PIOLs (88%) and 12 SMRLs (12%). All 12 SMRLs were diagnosed based on the vitreous samples. Among the 12 SMRLs, there are 9 B-cell SMRLs (B-SMRL) and 3 T-cell SMRLs (T-SMRL). Thus, of the IOLs, PIOL was over 7 times more prevalent than SMRL (89/12=7.4). A summary of the demography of these 101 retinal lymphoma cases is in Table 1. The demographic data of the 12 SMRL cases is given in Table 2. Eighty-three percent of the SMRL patients (10/12) were older than 50 years of age at the time of the diagnosis.
Table 1.
Categories of retinal lymphoma
| Category | Case (Percent) | |
|---|---|---|
| PIOL | 89 (100.0%) | |
| B-cell lymphoma | 87 (97.75%) | |
| T-cell lymphoma | 2 (2.25%) | |
| SMRL | 12 (100.0%) | |
| B-cell lymphoma | 9 (75.00%) | |
| T-cell lymphoma | 3 (25.00%) | |
| Total Retinal lymphoma (IOL) | 101 (100.0%) | |
| B-cell lymphoma | 96 (95.05%) | |
| T-cell lymphoma | 5 (4.95%) | |
PIOL, primary intraocular lymphoma; SMRL, systemic metastatic retinal lymphoma; IOL, intraocular lymphoma
Table 2.
Demographics of systemic metastatic retinal lymphoma (SMRL)patients
| Case No. |
Age (year) |
Gender | Type | Primary location of lymphoma |
WHO Classification |
|---|---|---|---|---|---|
| 1 | 81 | Female | B-cell | Skin | Primary cutaneous Diffuse Large B- Cell Lymphoma(DLBCL) – leg type |
| 2 | 63 | Male | B-cell | Retroperitoneum | Follicular Lymphoma |
| 3 | 64 | Female | B-cell | Nasopharynx | DLBCL – paranasal sinus |
| 4 | 38 | Female | B-cell | Breast | DLBCL – breast |
| 5 | 87 | Male | B-cell | Nasopharynx | DLBCL – paranasal sinus |
| 6 | 72 | Male | B-cell | Testis | DLBCL – testis |
| 7 | 70 | Male | B-cell | Testis | DLBCL – testis |
| 8 | 32 | Male | B-cell | Ileo-cecum | Burkitt’s lymphoma |
| 9 | 72 | Female | B-cell | Stomach | Mucosa-associated lymphoid tissue (MALT) lymphoma – gastric |
| 10 (Levy-Clarke et al. 2008) | 78 | Male | T-cell | Skin | Primary cutaneous peripheral T-cell lymphoma (PCPTCL) |
| 11 (Cimino et al. 2009) |
54 | Female | T-cell | Nasopharynx | Extranodal NK/T-cell lymphoma, nasal type |
| 12 | 54 | Female | T-cell | Blood | Adult T-cell lymphoma/leukemia (HTLV 1-positive) |
Cimino L, CC Chan, D Shen, L Masini, F Ilariucci, M Masetti, S Asioli, A Sartori & L Cappuccini (2009): Ocular involvement in nasal natural killer T-cell lymphoma. Int Ophthalmol 29: 275–279.
Levy-Clarke GA, D Greenman, PC Sieving, G Byrnes, D Shen, R Nussenblatt & CC Chan (2008): Ophthalmic manifestations, cytology, immunohistochemistry, and molecular analysis of intraocular metastatic T-cell lymphoma: report of a case and review of the literature. Surv Ophthalmol 53: 285–295.
Of the 96 B-cell retinal lymphomas, 9.4% (9/96) were secondary to systemic B-cell lymphoma. Of the 5 T-cell retinal lymphomas, 60% (3/5) were secondary to systemic T-cell lymphoma. There is a significantly higher chance of metastasis in T-SMRL compared to B-SMRL, given the total occurrences of T-PIOL and B-PIOL (p=0.001, chi-square test). The median time from diagnosis of systemic lymphoma to the onset of ocular involvement was 60.86 ± 18.35 months and 4.62 ± 4.38 months for B-SMRL and T-SMRL, respectively (p= 0.163, independent samples t-test).
Clinical features
Ocular symptoms and manifestations of the SMRL patients were similar to the PIOL patients. All patients complained of blurry vision. Anterior “uveitis” (with neoplastic cells mimicking intraocular inflammation) was seen in 3 patients. On fundus examination, all 12 SMRL patients had features of chronic vitritis. Retinal or subretinal yellowish infiltrations were found in 5 patients. Retinal vasculitis was noted in one patient. Five patients had severe vitritis such that chorioretinal lesions were not visible, and one patient did not have a chorioretinal lesion. There were no abnormal clinical findings in the uvea, conjunctiva or orbit in any of the patients. These patients were first treated with, and did not respond to, anti-inflammatory and/or immunosuppressive therapy including corticosteroids. Diagnostic vitrectomy was performed to rule out malignancy in all 12 patients.
Cytology
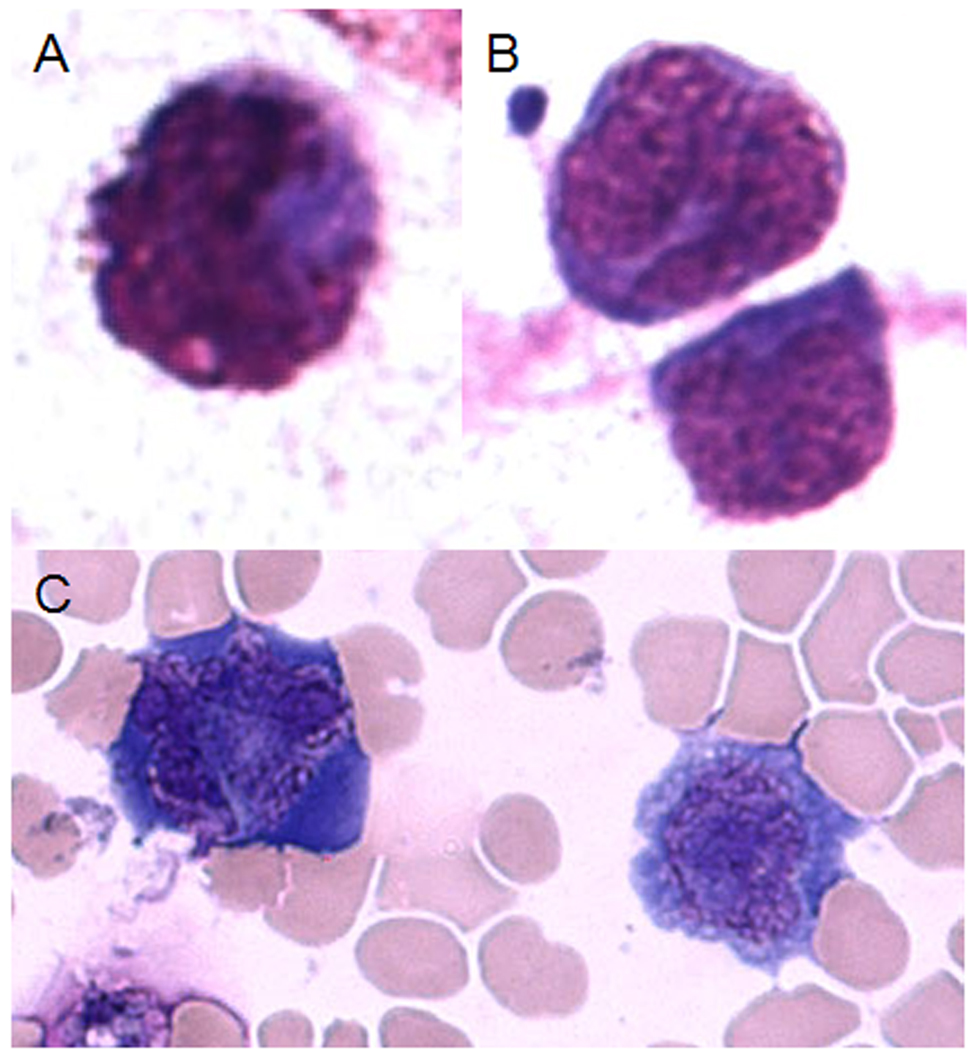
Cytology of vitreous samples illustrated atypical lymphoid cells, which harbored either B- or T- cell monoclonality. These cells have large irregular nuclear, prominent nucleoli, and scanty basophilic cytoplasm. In general, B-SMRL cells were large and T-SMRL cells were variable in size (Figure 1).
Figure 1.
Atypical B-cells and T-cells of systemic metastatic retinal lymphoma (SMRL) in the vitreous. A & B, atypical B-lymphoid cells; C, atypical T-lymphoid cells. (Giemsa, original magnification, ×640)
Vitreal IL-10 and IL-6 levels
Table 3 summarizes cytokine data. Cytokine levels were measured in 10 of the 12 vitreous samples (7 B-SMRLs and 3 T-SMRLs). Among the 7 B-SMRL cases, 4 (57.1%) showed a ratio of IL-10: IL-6 levels greater than 1.00, compared to only 1 of 3 T-SMRL specimens (33%, p=0.48, chi-square test).
Table 3.
Vitreal IL-10 and IL-6 levels of metastatic systemic retinal lymphoma
| Case No. |
Type | IL-10 (pg/ml) |
IL-6 (pg/ml) |
Ratio of IL-10/IL-6 |
|---|---|---|---|---|
| 1 | B-cell | 77 | <15.6 | >1.00 |
| 2 | B-cell | NA | NA | |
| 3 | B-cell | <23.4 | <15.6 | |
| 4 | B-cell | 470 | 15 | >1.00 |
| 5 | B-cell | 380 | 250 | >1.00 |
| 6 | B-cell | NA | NA | |
| 7 | B-cell | 624 | <15.6 | >1.00 |
| 8 | B-cell | <23.4 | <15.6 | |
| 9 | B-cell | <46.8 | <31.2 | |
| 10 | T-cell | 137 | 143 | <1.00 |
| 11 | T-cell | 743 | 311 | >1.00 |
| 12 | T-cell | 203 | 843 | <1.00 |
NA: not measured
Molecular pathology
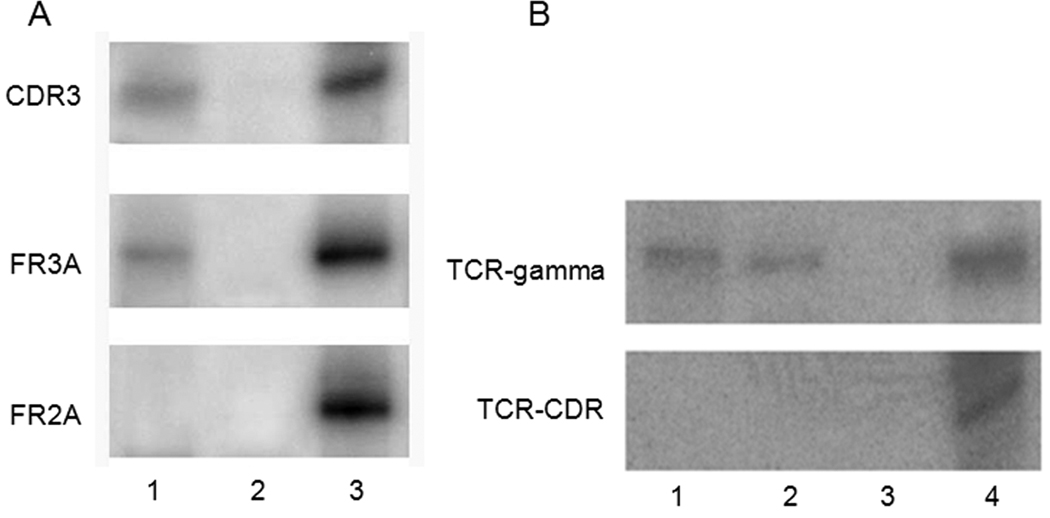
In all 9 B-SMRLs, IgH gene rearrangements were detected, and all 3 T-SMRLs had TCR gene rearrangements (Figure 2). The consistency of lymphoma cell monoclonality in Case 1, Case 2 and Case 6 (B-SMRL), and Case 10 and Case 11 (T-SMRL) were established by PCR results of tumor cells from the original site.
Figure 2.
PCR of gene rearrangement in two cases with SMRL. A, The PCR result of case No. 4 showing IgH gene rearrangement was detected using primer pairs of FR3A and CDR3 and not FR2A; B, The PCR result of case No. 11 showing TCR gene rearrangement was detected using primer pair of TCR-γ and not TCR-CDR. (Lane 1, vitreous specimen; Lane 2, nasopharyngeal lymphoma; Lane 3, negative control; Lane 4, positive control)
Discussion
The incidence of PIOL is estimated to range from 30 to 200 people annually in the United States (Chan 2003; Baehring et al. 2005; Chan 2007). Our retrospective study shows that PIOL has a much higher prevalence than SMRL. Overall, the incidence of SMRL is extremely low (Chan 2003; Baehring et al. 2005; Levy-Clarke et al. 2005). Our center is a tertiary referral center for diagnostic vitrectomy to differentiate between lymphoma and uveitis; therefore, the incidence of SMRL we present is subject to referral bias and is likely skewed when compared to other populations.
Secondary, or metastatic, intraocular lymphoma is usually located in the uvea without involvement of the neurosensory retina; however, rare cases of retinal involvement have been reported (Coupland et al. 1999; Parikh et al. 2005). Multicentricity has been documented in PCNSL/PIOL (Coupland et al. 2005). There is a possibility that the patients may have developed two different lymphomas: one outside the eye and one in the retina. The limited number of malignant cells in the vitreous prevented us from performing clonal analysis of the 12 SMRL cells to rule out this possibility. However, we suggest that these 12 SMRLs are likely from metastatic lymphomas outside the eye based on their clinical presentation and the morphologic and molecular similarities of the primary and secondary lymphoma cells in these cases.
SMRL patients, like PIOL patients, tend to be older. These patients frequently present with clinical features of PIOL, masquerade as uveitis, and elude diagnosis. Often, patients are misdiagnosed as having intractable ocular inflammation and die from dissemination of their disease (Sen et al. 2009). Most commonly, PIOL involves the posterior segment, and patients present with worsening vision due to vitritis (66% of patients), anterior chamber involvement (43%), and/or retinochoroidal involvement (41%) (Freeman et al. 1987; Char et al. 1988; Whitcup et al. 1993; Velez et al. 2000), findings comparable to our current study of SMRL.
Of the 12 SMRLs, 9 SMRLs are of B-cell origin and 3 SMRLs are of T-cell origin. However, there is a significantly higher ratio of T-cell IOLs to T-SMRLs compared with B-cell IOLs to B-SMRLs. T-SMRLs have a much shorter duration from time of the diagnosis of systemic lymphoma to the time of ocular disease. Compared to systemic B-cell lymphoma, systemic T-cell lymphomas appear more aggressive and invade the retina and vitreous (Levy-Clarke et al. 2008). Approximately 85–90% of all non-Hodgkin lymphoma (NHL) in the USA are B-cell lymphomas (Harris et al. 1994). Some B-cell NHLs are indolent, or slow-growing, yet incurable. In contrast, others are very aggressive, may be rapidly fatal, and are often curable. T-cell lymphomas represent a group of malignancies for which there has been remarkably little progress over the past several years. These diseases have a prognosis that is worse than their B-cell counterparts. Because they are extremely rare cancers, there is little consensus regarding management and treatment of the disease because of the difficulties in finding enough patients to enroll in clinical studies. Several informative clinical series of patients have reported a poor prognosis for patients with T-cell neoplasms; the 5-year survival rate is less than 30% with a median survival of less than 2 years (Campo et al. 1998; Lopez-Guillermo et al. 1998). Incredibly, the failure-free survival for patients with high-risk or intermediate-high-risk disease ranges from 0 to less than 10%, with virtually no long-term survivors (Campo et al. 1998; Lopez-Guillermo et al. 1998; Rudiger et al. 2002).
The eye, brain and testis are considered to be immunoprivileged sites (Streilein 2003). In these sites, strong blood-tissue barriers and an altered immune response allow cells, including certain malignant cells expressing non-self antigens, to escape destruction by the immune system (Filippini et al. 2001; Ferguson et al. 2002; Streilein 2003). Immunoprivilege in these specific sites may account for the appearance and metastases of lymphomas to the eye, brain and testis (Wallace et al. 2006). Testicular lymphoma accounts for only 1–8% of all testicular tumors and 1% of all NHLs (Freeman et al. 1972; Doll & Weiss 1986). Primary testicular lymphoma (PTL) is usually a B-cell lymphoma, of which 68% are of the intermediate-grade DLBCL subtype (Woolley et al. 1976; Turner et al. 1981; Ferry et al. 1994). PTL has a proclivity to spread to unusual extranodal sites and generally has a poor prognosis (Doll & Weiss 1986; Shahab & Doll 1999). Of the 12 SMRLs, 2 (17% of total SMRLs and 22% of B-SMRLs) were from testicular metastases.
Nasal lymphomas affecting the nose and nasopharyngeal region occur much more frequently in Asian countries than in Western countries and constitute 7.2% of extranodal lymphomas in Hong Kong (Ho et al. 1984). Sinonasal lymphomas are rare in the USA and Europe and represent only 2.2% of NHL (Murphy et al. 1998; Gaal et al. 2000; Rudiger et al. 2002). Many lymphomas in the sinonasal region are of NK/T-cell origin (Yamanaka et al. 1985). More than 70% of nasal NK/T-cell lymphomas (NKTL) localize in the nasopharyngeal region, although extranasal and disseminated disease can occur. Nasal NKTL is closely associated with Epstein–Barr virus (EBV) positivity. Although the prognosis of sinonasal lymphomas is variable and difficult to evaluate, the clinical course of patients with nasal NKTL is usually a rapid decline (Aozasa et al. 2008). The prognosis is far worse than for their B-cell counterparts (Cheung et al. 1998). Of the 12 SMRLs, 3 (25% of total SMRLs, 22% of B-SMRLs and 33% of T-SMRLs) are from nasopharyngeal lymphoma.
The skin is the most common extranodal site of NHL, with a yearly incidence approaching 3000 (1 case per 100,000 individuals) in the US (Smith et al. 2007). Cutaneous lymphomas represent a broad spectrum of approximately 20 distinct clinical-pathologic entities with multiple clinical presentations, natural histories, and treatment options. Cutaneous T-cell and/or NK-cell lymphomas is the most common category of cutaneous lymphoma, with a yearly incidence of 6 cases per million (Criscione & Weinstock 2007). Cutaneous B-cell lymphomas have a yearly incidence of 4 cases per million, and precursor hematologic neoplasms are extremely rare (Smith et al. 2005). Cutaneous lymphomas rarely metastasize to the eye (Chong DY in press). Of our 12 SMRLs, 2 cases (17% of total SMRLs, 11% of B-SMRLs and 33% of T-SMRLs) are from skin metastasis.
In addition to clinical history, vitreal cytokine levels and molecular pathology of the lymphoma cells are valuable for the diagnosis of SMRL. B lymphoma cells can secrete high levels of IL-10 (Blay et al. 1993), an anti-inflammatory and immunosuppressive cytokine, while inflammatory conditions like uveitis (Murray et al. 1990) are associated with high levels of IL-6 (Bogdan et al. 1991; de Waal Malefyt et al. 1991; D'Andrea et al. 1993), a proinflammatory cytokine (Horn et al. 2000; Hodge et al. 2005). Studies have shown that PIOL can exhibit high IL-10 levels, and an IL-10/IL-6 ratio greater than 1.0 is suggestive of PIOL (Chan et al. 1995; Whitcup et al. 1997; Shen et al. 1998; Whitcup et al. 2000; Wolf et al. 2003; Chan & Wallace 2004; Merle-Beral et al. 2004; Levy-Clarke et al. 2005; Cassoux et al. 2007; Gonzales & Chan 2007). The current study also confirms a high vitreal IL-10/IL-6 ratio in 57% of B-SMRLs and 33% of T-SMRLs. The cytokine levels of IL-10 and IL-6 and the IL-10/IL-6 ratio are by no means diagnostic of SMRL, nor do they differentiate SMRL from PIOL. However, they can be useful adjunctive tests in corroborating a clinical suspicion of malignant lymphocytes in the eye. Elevated serum IL-10 levels have been also reported in NHL (Cortes & Kurzrock 1997) and nasal NKTL (Harabuchi et al. 2009). This study has detected IgH gene rearrangements in all B-SMRLs and TCR gene rearrangements in all T-SMRL. Molecular data is much more reliable than intraocular cytokine levels for the diagnosis of SMRLs.
In summary, SMRLs are extremely rare. Clinical features of SMRL and primary retinal lymphoma are similar, and both diseases may masquerade as uveitis. T-SMRL is more aggressive than B-SMRL. The key to diagnose SMRL is presence of a clinical history of systemic lymphoma (particularly from testis, skin, and nasopharynx) and identification of atypical lymphoid cells in the vitreous or retina. Molecular analysis is also helpful in making the diagnosis of SMRL.
Acknowledgement
The NEI Intramural Research program supported the study. We thank Hema Ramkumar for her assistance in editing.
Reference
- Aozasa K, Takakuwa T, Hongyo T, Yang WI. Nasal NK/T-cell lymphoma: epidemiology and pathogenesis. Int J Hematol. 2008;87:110–117. doi: 10.1007/s12185-008-0021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehring JM, Androudi S, Longtine JJ, Betensky RA, Sklar J, Foster CS, Hochberg FH. Analysis of clonal immunoglobulin heavy chain rearrangements in ocular lymphoma. Cancer. 2005;104:591–597. doi: 10.1002/cncr.21191. [DOI] [PubMed] [Google Scholar]
- Bardenstein DS. Intraocular Lymphoma. Cancer Control. 1998;5:317–325. doi: 10.1177/107327489800500403. [DOI] [PubMed] [Google Scholar]
- Blay JY, Burdin N, Rousset F, Lenoir G, Biron P, Philip T, Banchereau J, Favrot MC. Serum interleukin-10 in non-Hodgkin's lymphoma: a prognostic factor. Blood. 1993;82:2169–2174. [PubMed] [Google Scholar]
- Bogdan C, Vodovotz Y, Nathan C. Macrophage deactivation by interleukin 10. J Exp Med. 1991;174:1549–1555. doi: 10.1084/jem.174.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buggage RR. Ocular manifestations of human T-cell lymphotropic virus type 1 infection. Curr Opin Ophthalmol. 2003;14:420–425. doi: 10.1097/00055735-200312000-00016. [DOI] [PubMed] [Google Scholar]
- Calfa CJ, Lossos IS, Ruiz P, Davis JL. Ocular involvement as the initial manifestation of T-cell chronic lymphocytic leukemia. Am J Ophthalmol. 2007;144:326–329. doi: 10.1016/j.ajo.2007.03.031. [DOI] [PubMed] [Google Scholar]
- Campo E, Gaulard P, Zucca E, Jaffe ES, Harris NL, Diebold J, Schlegelberger B, Feller AC, Delsol G, Gisselbrecht C, Montserrat E. Report of the European Task Force on Lymphomas: workshop on peripheral T-cell lymphomas. Ann Oncol. 1998;9:835–843. doi: 10.1023/a:1008439620513. [DOI] [PubMed] [Google Scholar]
- Cassoux N, Giron A, Bodaghi B, Tran TH, Baudet S, Davy F, Chan CC, Lehoang P, Merle-Beral H. IL-10 measurement in aqueous humor for screening patients with suspicion of primary intraocular lymphoma. Invest Ophthalmol Vis Sci. 2007;48:3253–3259. doi: 10.1167/iovs.06-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CC, Gonzales JA, Hidayat AA. Intraocular lymphoproliferations simulating uveitis. In: Albert DM, Miller JW, editors. Principles and Practice of Ophthalmology. New York: Saunders-Elsevier; 2008. [Google Scholar]
- Chan CC, Gonzales John A. Singapore: World Scientific Publishing Co. Pte. Ltd; Primary intraocular lymphoma. 2007
- Chan CC, Wallace DJ. Intraocular lymphoma: update on diagnosis and management. Cancer Control. 2004;11:285–295. doi: 10.1177/107327480401100502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CC, Whitcup SM, Solomon D, Nussenblatt RB. Interleukin-10 in the vitreous of patients with primary intraocular lymphoma. Am J Ophthalmol. 1995;120:671–673. doi: 10.1016/s0002-9394(14)72217-2. [DOI] [PubMed] [Google Scholar]
- Chan JW. Paraneoplastic retinopathies and optic neuropathies. Surv Ophthalmol. 2003;48:12–38. doi: 10.1016/s0039-6257(02)00416-2. [DOI] [PubMed] [Google Scholar]
- Char DH, Ljung BM, Deschenes J, Miller TR. Intraocular lymphoma: immunological and cytological analysis. Br J Ophthalmol. 1988;72:905–911. doi: 10.1136/bjo.72.12.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung MM, Chan JK, Lau WH, Foo W, Chan PT, Ng CS, Ngan RK. Primary non-Hodgkin's lymphoma of the nose and nasopharynx: clinical features, tumor immunophenotype, and treatment outcome in 113 patients. J Clin Oncol. 1998;16:70–77. doi: 10.1200/JCO.1998.16.1.70. [DOI] [PubMed] [Google Scholar]
- Chong DYJM, Shen D, Chan CC, Callanan DG. Vitreous Metastases of Primary Cutaneous B-cell Lymphoma. Ocul Immunol Inflam. doi: 10.3109/09273940903144705. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes J, Kurzrock R. Interleukin-10 in non-Hodgkin's lymphoma. Leuk Lymphoma. 1997;26:251–259. doi: 10.3109/10428199709051774. [DOI] [PubMed] [Google Scholar]
- Coupland SE, Chan CC, Smith J. Pathophysiology of retinal lymphoma. Ocul Immunol Inflamm. 2009;17:227–237. doi: 10.1080/09273940903168696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupland SE, Damato B. Understanding intraocular lymphomas. Clin Experiment Ophthalmol. 2008;36:564–578. doi: 10.1111/j.1442-9071.2008.01843.x. [DOI] [PubMed] [Google Scholar]
- Coupland SE, Foss HD, Assaf C, Auw-Haedrich C, Anastassiou G, Anagnostopoulos I, Hummel M, Karesh JW, Lee WR, Stein H. T-cell and T/natural killer-cell lymphomas involving ocular and ocular adnexal tissues: a clinicopathologic, immunohistochemical, and molecular study of seven cases. Ophthalmology. 1999;106:2109–2120. doi: 10.1016/S0161-6420(99)90492-X. [DOI] [PubMed] [Google Scholar]
- Coupland SE, Hummel M, Muller HH, Stein H. Molecular analysis of immunoglobulin genes in primary intraocular lymphoma. Invest Ophthalmol Vis Sci. 2005;46:3507–3514. doi: 10.1167/iovs.05-0401. [DOI] [PubMed] [Google Scholar]
- Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973–2002. Arch Dermatol. 2007;143:854–859. doi: 10.1001/archderm.143.7.854. [DOI] [PubMed] [Google Scholar]
- D'Andrea A, Aste-Amezaga M, Valiante NM, Ma X, Kubin M, Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R, Haanen J, Spits H, Roncarolo MG, te Velde A, Figdor C, Johnson K, Kastelein R, Yssel H, de Vries JE. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll DC, Weiss RB. Malignant lymphoma of the testis. Am J Med. 1986;81:515–524. doi: 10.1016/0002-9343(86)90308-6. [DOI] [PubMed] [Google Scholar]
- Ferguson TA, Green DR, Griffith TS. Cell death and immune privilege. Int Rev Immunol. 2002;21:153–172. doi: 10.1080/08830180212058. [DOI] [PubMed] [Google Scholar]
- Ferry JA, Harris NL, Young RH, Coen J, Zietman A, Scully RE. Malignant lymphoma of the testis, epididymis, and spermatic cord. A clinicopathologic study of 69 cases with immunophenotypic analysis. Am J Surg Pathol. 1994;18:376–390. doi: 10.1097/00000478-199404000-00006. [DOI] [PubMed] [Google Scholar]
- Filippini A, Riccioli A, Padula F, Lauretti P, D'Alessio A, De Cesaris P, Gandini L, Lenzi A, Ziparo E. Control and impairment of immune privilege in the testis and in semen. Hum Reprod Update. 2001;7:444–449. doi: 10.1093/humupd/7.5.444. [DOI] [PubMed] [Google Scholar]
- Freeman C, Berg JW, Cutler SJ. Occurrence and prognosis of extranodal lymphomas. Cancer. 1972;29:252–260. doi: 10.1002/1097-0142(197201)29:1<252::aid-cncr2820290138>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Freeman LN, Schachat AP, Knox DL, Michels RG, Green WR. Clinical features, laboratory investigations, and survival in ocular reticulum cell sarcoma. Ophthalmology. 1987;94:1631–1639. doi: 10.1016/s0161-6420(87)33256-7. [DOI] [PubMed] [Google Scholar]
- Gaal K, Sun NC, Hernandez AM, Arber DA. Sinonasal NK/T-cell lymphomas in the United States. Am J Surg Pathol. 2000;24:1511–1517. doi: 10.1097/00000478-200011000-00006. [DOI] [PubMed] [Google Scholar]
- Gonzales JA, Chan CC. Biopsy techniques and yields in diagnosing primary intraocular lymphoma. Int Ophthalmol. 2007;27:241–250. doi: 10.1007/s10792-007-9065-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harabuchi Y, Takahara M, Kishibe K, Moriai S, Nagato T, Ishii H. Nasal natural killer (NK)/T-cell lymphoma: clinical, histological, virological, and genetic features. Int J Clin Oncol. 2009;14:181–190. doi: 10.1007/s10147-009-0882-7. [DOI] [PubMed] [Google Scholar]
- Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, Delsol G, De Wolf-Peeters C, Falini B, Gatter KC, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84:1361–1392. [PubMed] [Google Scholar]
- Ho FC, Todd D, Loke SL, Ng RP, Khoo RK. Clinico-pathological features of malignant lymphomas in 294 Hong Kong Chinese patients, retrospective study covering an eight-year period. Int J Cancer. 1984;34:143–148. doi: 10.1002/ijc.2910340202. [DOI] [PubMed] [Google Scholar]
- Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41:2502–2512. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Horn F, Henze C, Heidrich K. Interleukin-6 signal transduction and lymphocyte function. Immunobiology. 2000;202:151–167. doi: 10.1016/S0171-2985(00)80061-3. [DOI] [PubMed] [Google Scholar]
- Kumar SR, Gill PS, Wagner DG, Dugel PU, Moudgil T, Rao NA. Human T-cell lymphotropic virus type I-associated retinal lymphoma. A clinicopathologic report. Arch Ophthalmol. 1994;112:954–959. doi: 10.1001/archopht.1994.01090190102028. [DOI] [PubMed] [Google Scholar]
- Levy-Clarke GA, Buggage RR, Shen D, Vaughn LO, Chan CC, Davis JL. Human T-cell lymphotropic virus type-1 associated t-cell leukemia/lymphoma masquerading as necrotizing retinal vasculitis. Ophthalmology. 2002;109:1717–1722. doi: 10.1016/s0161-6420(02)01132-6. [DOI] [PubMed] [Google Scholar]
- Levy-Clarke GA, Chan CC, Nussenblatt RB. Diagnosis and management of primary intraocular lymphoma. Hematol Oncol Clin North Am. 2005;19:739–749. doi: 10.1016/j.hoc.2005.05.011. viii. [DOI] [PubMed] [Google Scholar]
- Levy-Clarke GA, Greenman D, Sieving PC, Byrnes G, Shen D, Nussenblatt R, Chan CC. Ophthalmic manifestations, cytology, immunohistochemistry, and molecular analysis of intraocular metastatic T-cell lymphoma: report of a case and review of the literature. Surv Ophthalmol. 2008;53:285–295. doi: 10.1016/j.survophthal.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Guillermo A, Cid J, Salar A, Lopez A, Montalban C, Castrillo JM, Gonzalez M, Ribera JM, Brunet S, Garcia-Conde J, Fernandez de Sevilla A, Bosch F, Montserrat E. Peripheral T-cell lymphomas: initial features, natural history, and prognostic factors in a series of 174 patients diagnosed according to the R.E.A.L. Classification. Ann Oncol. 1998;9:849–855. doi: 10.1023/a:1008418727472. [DOI] [PubMed] [Google Scholar]
- Merle-Beral H, Davi F, Cassoux N, Baudet S, Colin C, Gourdet T, Bodaghi B, LeHoang P. Biological diagnosis of primary intraocular lymphoma. Br J Haematol. 2004;124:469–473. doi: 10.1046/j.1365-2141.2003.04800.x. [DOI] [PubMed] [Google Scholar]
- Mochizuki M, Singh AD. Epidemiology and clinical features of intraocular lymphoma. Ocul Immunol Inflamm. 2009;17:69–72. doi: 10.1080/09273940902957305. [DOI] [PubMed] [Google Scholar]
- Murphy A, O'Keane JC, Blayney A, Powell FC. Cutaneous presentation of nasal lymphoma: a report of two cases. J Am Acad Dermatol. 1998;38:310–313. doi: 10.1016/s0190-9622(98)70571-7. [DOI] [PubMed] [Google Scholar]
- Murray PI, Hoekzema R, van Haren MA, de Hon FD, Kijlstra A. Aqueous humor interleukin-6 levels in uveitis. Invest Ophthalmol Vis Sci. 1990;31:917–920. [PubMed] [Google Scholar]
- Parikh AH, Khan SH, Wright JD, Jr, Oh KT. Systemic non-Hodgkin's lymphoma simulating primary intraocular lymphoma. Am J Ophthalmol. 2005;139:573–574. doi: 10.1016/j.ajo.2004.09.047. [DOI] [PubMed] [Google Scholar]
- Rudiger T, Weisenburger DD, Anderson JR, Armitage JO, Diebold J, MacLennan KA, Nathwani BN, Ullrich F, Muller-Hermelink HK. Peripheral T-cell lymphoma (excluding anaplastic large-cell lymphoma): results from the Non-Hodgkin's Lymphoma Classification Project. Ann Oncol. 2002;13:140–149. doi: 10.1093/annonc/mdf033. [DOI] [PubMed] [Google Scholar]
- Sen HN, Bodaghi B, Hoang PL, Nussenblatt R. Primary intraocular lymphoma: diagnosis and differential diagnosis. Ocul Immunol Inflamm. 2009;17:133–141. doi: 10.1080/09273940903108544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahab N, Doll DC. Testicular lymphoma. Semin Oncol. 1999;26:259–269. [PubMed] [Google Scholar]
- Shen DF, Zhuang Z, LeHoang P, Boni R, Zheng S, Nussenblatt RB, Chan CC. Utility of microdissection and polymerase chain reaction for the detection of immunoglobulin gene rearrangement and translocation in primary intraocular lymphoma. Ophthalmology. 1998;105:1664–1669. doi: 10.1016/S0161-6420(98)99036-4. [DOI] [PubMed] [Google Scholar]
- Shibata K, Shimamoto Y, Nishimura T, Okinami S, Yamada H, Miyahara M. Ocular manifestations in adult T-cell leukemia/lymphoma. Ann Hematol. 1997;74:163–168. doi: 10.1007/s002770050276. [DOI] [PubMed] [Google Scholar]
- Singh AD, Lewis H, Schachat AP, Peereboom D. Lymphoma of the retina and CNS. In: Singh AD, Damato BE, Pe'er J, Murphree AL, Perry JD, editors. Clinical Ophthalmic Oncology. Philadelphia: Saunders-Elsevier; 2007. [Google Scholar]
- Smith BD, Jones G, Wilson LD. Mycosis fungoides. In: Gunderson LLTJ, editor. Clinical Radiation Oncology. Philadelphia, PA: Churchill Livingstone; 2007. [Google Scholar]
- Smith BD, Smith GL, Cooper DL, Wilson LD. The cutaneous B-cell lymphoma prognostic index: a novel prognostic index derived from a population-based registry. J Clin Oncol. 2005;23:3390–3395. doi: 10.1200/JCO.2005.08.137. [DOI] [PubMed] [Google Scholar]
- Streilein JW. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat Rev Immunol. 2003;3:879–889. doi: 10.1038/nri1224. [DOI] [PubMed] [Google Scholar]
- Turner RR, Colby TV, MacKintosh FR. Testicular lymphomas: a clinicopathologic study of 35 cases. Cancer. 1981;48:2095–2102. doi: 10.1002/1097-0142(19811101)48:9<2095::aid-cncr2820480930>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Velez G, de Smet MD, Whitcup SM, Robinson M, Nussenblatt RB, Chan CC. Iris involvement in primary intraocular lymphoma: report of two cases and review of the literature. Surv Ophthalmol. 2000;44:518–526. doi: 10.1016/s0039-6257(00)00118-1. [DOI] [PubMed] [Google Scholar]
- Wallace DJ, Altemare CR, Shen DF, deSmet MD, Buggage RR, Nussenblatt RB, Chan CC. Primary testicular and intraocular lymphomas: two case reports and a review of the literature. Surv Ophthalmol. 2006;51:41–50. doi: 10.1016/j.survophthal.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitcup SM, Chan CC, Buggage RR, Nussenblatt RB, Byrnes GA, Rubin BI. Improving the diagnostic yield of vitrectomy for intraocular lymphoma. Arch Ophthalmol. 2000;118:446. [PubMed] [Google Scholar]
- Whitcup SM, de Smet MD, Rubin BI, Palestine AG, Martin DF, Burnier M, Jr, Chan CC, Nussenblatt RB. Intraocular lymphoma. Clinical and histopathologic diagnosis. Ophthalmology. 1993;100:1399–1406. doi: 10.1016/s0161-6420(93)31469-7. [DOI] [PubMed] [Google Scholar]
- Whitcup SM, Stark-Vancs V, Wittes RE, Solomon D, Podgor MJ, Nussenblatt RB, Chan CC. Association of interleukin 10 in the vitreous and cerebrospinal fluid and primary central nervous system lymphoma. Arch Ophthalmol. 1997;115:1157–1160. doi: 10.1001/archopht.1997.01100160327010. [DOI] [PubMed] [Google Scholar]
- Wolf LA, Reed GF, Buggage RR, Nussenblatt RB, Chan CC. Vitreous cytokine levels. Ophthalmology. 2003;110:1671–1672. doi: 10.1016/S0161-6420(03)00811-X. [DOI] [PubMed] [Google Scholar]
- Woolley PV, 3rd, Osborne CK, Levi JA, Wiernik PH, Canellos GP. Extranodal presentation of non-Hodgkin's lymphomas in the testis. Cancer. 1976;38:1026–1035. doi: 10.1002/1097-0142(197608)38:2<1026::aid-cncr2820380256>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Yamanaka N, Kataura A, Sambe S, Minase T, Ishii Y. Midfacial T cell lymphoma: characterization by monoclonal antibodies. Ann Otol Rhinol Laryngol. 1985;94:207–211. doi: 10.1177/000348948509400223. [DOI] [PubMed] [Google Scholar]