Abstract
Introduction
Obstructive sleep apnea (OSA) and hyperaldosteronism are very common in subjects with resistant hypertension. We hypothesized that aldosterone mediated chronic fluid retention may influence OSA severity in patients with resistant hypertension. We tested this in an open label evaluation by assessing the changes in the severity of OSA in patients with resistant hypertension following treatment with spironolactone.
Methods
Subjects with resistant hypertension [clinic blood pressure (BP) ≥140/90 mm Hg on ≥3 antihypertensive medications, including a thiazide diuretic and OSA [defined as an apneahypopnea index (AHI) ≥ 15] had full diagnostic, polysomnography before and 8 weeks after spironolactone (25–50 mg/day) was added to their ongoing antihypertensive therapy.
Results
Twelve patients (mean age 56 years and body mass index 36.8 kg/m2) were evaluated. Following treatment with spironolactone, the AHI (39.8±19.5 vs. 22.0±6.8 events/hr; p < 0.05) and hypoxic index (13.6±10.8 vs. 6.7±6.6 events/hr; p < 0.05), weight, clinic and ambulatory BP were significantly reduced. Plasma renin activity and serum creatinine were significantly higher.
Conclusion
This study provides preliminary evidence that treatment with a mineralocorticoid receptor antagonist substantially reduces the severity of OSA. If confirmed in a randomized assessment it will support aldosterone-mediated chronic fluid retention as an important mediator of OSA severity in patients with resistant hypertension.
Keywords: Hyperaldosteronism, Resistant hypertension, Obstructive sleep apnea, Spironolactone
Introduction
Obstructive sleep apnea (OSA) is very common in patients with resistant hypertension.1,2 In a prospective evaluation of 42 subjects with resistant hypertension, Logan et al. found that 83% of the subjects had unsuspected OSA based on an apnea-hypopnea index (AHI) of ≥10 events/hr.1 The pathophysiologic mechanism of this high prevalence of OSA in patients with resistant hypertension has not been explained.
We and others have identified primary aldosteronism as being common in patients with resistant hypertension, with a prevalence of approximately 20%.3–6 Aldosterone, through its classical effects at the level of the distal nephron, induces sodium and fluid retention. Beyond hyperaldosteronism, we reported that resistant hypertension is in general characterized by significant fluid retention, even in subjects without demonstrable aldosterone excess. This fluid retention persists in spite of long term thiazide diuretic use.7
Recent studies link acute changes in intravascular volume to upper airway resistance and OSA severity.8,9 These studies suggest that peripharyngeal fluid accumulation predisposes to upper airway obstruction, particularly when in the supine position, as with sleep. In finding both a high prevalence of OSA and broad evidence of intravascular volume overload in patients with resistant hypertension, we hypothesized that chronic excess fluid retention may underlie the high prevalence of OSA in patients with resistant hypertension. To test this hypothesis we administered spironolactone for 8 weeks in patients with resistant hypertension and OSA and assessed changes in OSA variables.
Methods
Subjects
Consecutive subjects referred to University of Alabama at Birmingham (UAB) Hypertension Clinic for resistant hypertension (defined as uncontrolled hypertension at 2 clinic visits, in spite of use of 3 antihypertensive medications including a thiazide diuretic at pharmacologically effective doses) were enrolled in the study. Secondary causes of hypertension other than hyperaldosteronism, including renovascular hypertension, pheochromocytoma or Cushing’s syndrome were excluded by laboratory analysis and/or radiological imaging as clinically indicated. Subjects with current use of continuous positive airway pressure (CPAP) for OSA or with recent myocardial infarction or stroke (within 6 months prior to study), congestive heart failure, chronic kidney disease (creatinine clearance < 60 ml/min/1.73m2) and long term use of systemic steroids were excluded. This study was approved by the UAB Institutional Review Board, and all subjects provided a written consent prior to study enrollment.
Clinical evaluation
Seated clinic blood pressure (BP) was measured using a sphygmomanometer with an appropriately sized cuff at each clinic visit after 5 minutes of rest. The mean of 2 readings was recorded. Twenty four hour ambulatory blood pressure measurements were done using Suntech monitor (Suntech Medical, Morrisville, North Carolina, USA). The monitor recorded systolic and diastolic BP every 20 minutes during the daytime (6 AM to 10 PM) and every 30 minutes at night (10 PM to 6 AM). The ambulatory data were included in the analysis if the monitoring period was >20 hours and there were no periods of >2 hours without measurements.
Biochemical evaluation
Biochemical evaluation was done in all subjects on an outpatient basis. Early morning ambulatory plasma aldosterone concentration, plasma renin activity (PRA), brain natriuretic peptide (BNP), serum potassium, serum creatinine, 24-hour urinary collection for aldosterone, sodium and creatinine were obtained at baseline during consumption of the subject’s routine diet. Antihypertensive treatment was maintained constant for at least 4 weeks prior to baseline evaluation. Potassium sparing diuretics including spironolactone, eplerenone or amiloride were discontinued at least 6 weeks prior to the study. Serum potassium was corrected to above 3.5 mEq/L prior to biochemical evaluation.
Polysomnography
All subjects underwent full-night, attended, diagnostic polysomnography. Polysomnographic evaluation included airflow monitoring with thermocouple and/or nasal pressure, respiratory effort using piezo belts at the chest and abdominal positions, oxygen saturation using pulse oximetry, heart rate using single lead ECG, EEG (C4-A1, C3-A2, O2-A1, O1-A2), submental and tibial electromyograms and bilateral electro-oculograms. Apnea was defined as cessation in airflow for ≥ 10s. Hypopnea was defined as a reduction in the amplitude of airflow of at least 30% for ≥ 10s, followed by either decrease in oxygen saturation of 4% or signs of physiologic arousal (at least 3s of α activity). The AHI was calculated as the total number of apneas plus hypopneas divided by the hours of sleep. Sleep was staged according to criteria of Rechtschaffen and Kales.10 The percentage of sleep time spent with an oxygen saturation < 90%, the hypoxic index (HI), was also determined. Supine AHI (AHI during supine position) and REM AHI (AHI during rapid eye movement sleep) were also determined and compared between baseline and follow up studies, in order to control for the variability in position and duration of various stages of sleep (OSA tends to be more severe during REM supine sleep). Sleep stage and nocturnal events were continuously supervised by a registered polysomnographic technologist. The scoring of all studies was confirmed by an American Board of Sleep Medicine Diplomat who was blinded to the whether studies were baseline or follow-up evaluations.
Treatment and Follow up
Following baseline evaluation, subjects with moderate-severe OSA (AHI ≥15 events/hr) were started on spironolactone 25 mg once daily and force titrated to 50 mg once daily at 4 weeks. Other antihypertensive medications were discontinued if needed because of low BP (<110/70 mm Hg and/or symptoms). Withdrawn medications were limited to centrally acting agents or vasodilators followed by β blockers and calcium channel blockers such that all patients remained on their baseline thiazide diuretic and renin angiotensin system blocker (angiotensin converting enzyme inhibitor (ACEI) and/or angiotensin receptor blocker (ARB)) throughout the treatment period. Clinic BP, ambulatory BP, overnight polysomnography, and biochemical evaluation for serum potassium, creatinine, BNP and PRA were repeated after 8 weeks of spironolactone intervention.
Analysis
Values are expressed as mean ± SD. Due to small sample size, differences between baseline and follow up measures are compared using Wilcoxon Signed Rank Test. As measures within subjects are highly correlated, no adjustments were made for multiple testing, p-values are provided for descriptive purposes and p <0.05 was deemed as meaningful change. Data analysis was carried out using statistical software (JMP, version 6; SAS Institute Inc; Cary, North Carolina).
Results
Twelve subjects, including 58% males and 50% whites, were evaluated (Table 1). Subjects were receiving an average of 4.3 antihypertensive medications including, in all subjects, a thiazide diuretic and either an ACEI or an ARB. After eight weeks of addition of spironolactone, body weight, clinic systolic and diastolic BP, 24-hr ambulatory systolic BP, daytime systolic BP, and nighttime systolic and nighttime diastolic BP were all significantly decreased (Table 2). Ambulatory daytime and 24-hr diastolic BP tended to be lower but did not reach statistical significance. Severity of OSA assessed by AHI, hypoxic index (HI), supine AHI, and AHI during rapid eye movement (REM) sleep was significantly lower with spironolactone treatment (Figure). Reduction in total AHI, supine AHI and REM AHI was noted in all 12 subjects irrespective of aldosterone status. Serum creatinine and PRA were significantly increased with treatment. In addition, neck circumference, and BNP tended to be lower and serum potassium tended to be higher but did not achieve statistical significance (Table 2).
Table 1.
Baseline Characteristics
| Characteristics | Mean ± SD |
|---|---|
| N | 12 |
| Age, yrs | 56.5 ± 6.5 |
| Body mass index, kg/m2 | 36.8 ± 6.8 |
| Males/Females | 7/5 |
| Blacks/Whites | 6/6 |
| Plasma aldosterone, ng/dl | 12.4 ± 6.3 |
| Plasma renin activity, ng/ml/hr | 1.4 ± 2.1 |
| 24-h Urinary aldosterone, mcg/24h | 14.3 ± 7.7 |
| 24-h Urinary sodium, mEq/24h | 190 ± 46 |
Table 2.
Characteristics before and after spironolactone treatment
| Characteristics | Baseline | 8 Weeks | p-value |
|---|---|---|---|
| Weight, lbs | 243.0 ± 32.4 | 239.9 ± 29.4 | 0.03 |
| Neck, cm | 42.1 ± 3.5 | 41.2 ± 3.4 | 0.195 |
| Clinic SBP, mm Hg | 145 ± 18 | 124 ± 16 | <0.001 |
| Clinic DBP, mm Hg | 81 ± 16 | 72 ± 9 | 0.04 |
| Ambulatory daytime SBP, mm Hg† | 150 ± 14 | 134 ± 18 | 0.04 |
| Ambulatory daytime DBP, mm Hg† | 85 ± 15 | 75 ± 11 | 0.06 |
| Ambulatory nighttime SBP, mm Hg† | 142 ± 16 | 120 ± 23 | 0.02 |
| Ambulatory nighttime DBP, mm Hg† | 77 ± 12 | 64 ± 13 | 0.016 |
| 24-h systolic blood pressure, mm Hg† | 147 ± 13 | 130 ± 19 | 0.025 |
| 24-h diastolic blood pressure, mm Hg† | 82 ± 14 | 72 ± 11 | 0.051 |
| No. of antihypertensive medications | 4.3 ± 1.1 | 4.5 ± 1.0 | 0.76 |
| Serum creatinine, | 1.2 ± 0.3 | 1.3 ± 0.2 | 0.035 |
| Serum potassium, mEq/L | 4.0 ± 0.3 | 4.4 ± 0.5 | 0.05 |
| Plasma renin activity, ng/mL/h | 1.4 ± 2.1 | 14.3 ± 13.8 | 0.005 |
| BNP, pg/mL | 17.3 ± 12.7 | 12.6 ± 18.8 | 0.24 |
| AHI, events/h | 39.8 ± 19.5 | 22.0 ± 6.8 | <0.001 |
| Hypoxic index, % | 13.6 ± 10.8 | 6.7 ± 6.6 | 0.04 |
| Supine AHI, events/h | 63.2 ± 28.7 | 40.8 ± 19.3 | 0.007 |
| REM AHI, events/h† | 55.7 ± 27.9 | 33.9 ± 19.7 | 0.003 |
Values, mean ± SD. SBP, systolic blood pressure; DBP, diastolic blood pressure; BNP, brain natriuretic peptide; AHI, apnea-hypopnea index; REM, rapid eye movement sleep.
n = 11 due to one subject having no REM sleep during the baseline study.
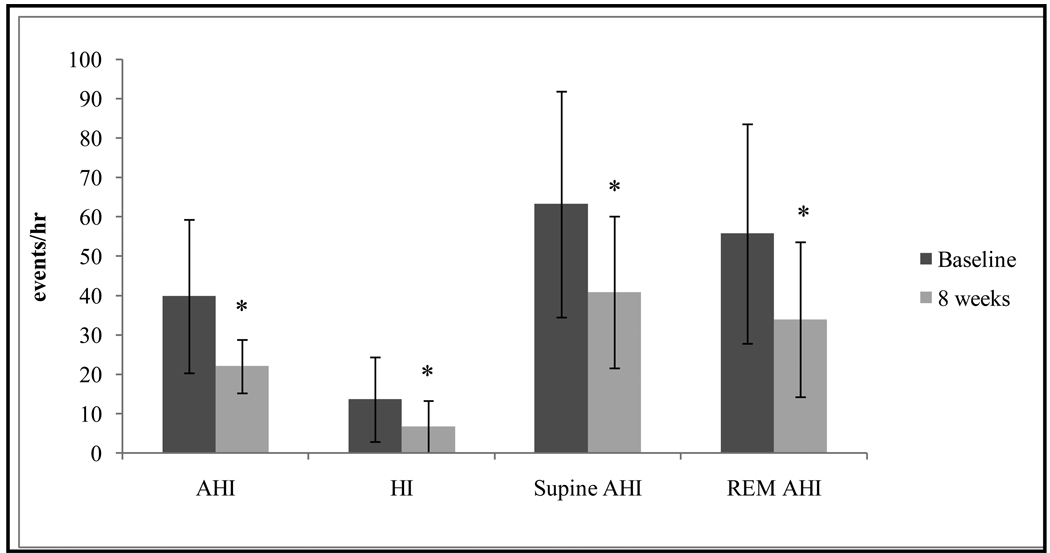
Figure.
Changes in apnea-hypopnea index (AHI) (39.8 ± 19.5 vs. 22.0 ± 6.8); hypoxic index (HI) (13.6 ± 10.8 vs. 6.7 ± 6.6); supine AHI (63.2 ± 28.7 vs. 40.8 ± 19.3); rapid eye movement sleep (REM) AHI (55.7 ± 27.9 vs. 33.9 ± 19.7) at 8 weeks (light grey bars) compared to baseline (dark grey bars). Values, mean ± SD. *Different compared to baseline, P < .05.
Discussion
This is the first study demonstrating that treatment with a mineralocorticoid receptor antagonist substantially reduces OSA severity in patients with resistant hypertension. Overall, the OSA severity as indexed by the AHI and HI was reduced by almost 50%. This benefit occurred in the setting of increased diuresis suggesting that the effect may be secondary to decreases in pharyngeal edema and consequent reductions in upper airway resistance. These results provide preliminary evidence linking aldosterone-induced fluid retention to severity of OSA and if confirmed in a randomized comparison, would explain in part, the high prevalence of OSA in patients with resistant hypertension.
OSA is extraordinarily common in patients with resistant hypertension. In 2 separate prospective evaluations, OSA was found to have a prevalence of 80–85% of subjects with resistant hypertension.1,2 The interaction between OSA and hypertension is not clear. Studies have implicated systemic inflammation, oxidative stress, endogenous vasoactive factors, endothelial dysfunction, increased sympathetic activation and metabolic dysregulation as potential mechanisms of hypertension related to OSA11. Several studies have shown that continuous positive airway pressure (CPAP) treatment in patients with OSA reduces sympathetic activity12, decreases production of free radicals and other inflammatory products13 and reverses the endothelial dysfunction14. However, only modest improvement in BP has been shown with CPAP treatment. 15
Primary aldosteronism has also been found to be very common in patients with resistant hypertension. In a prospective evaluation done by our laboratory, 20% of subjects with resistant hypertension were confirmed to have primary aldosteronism.3 A similarly high prevalence of primary aldosteronism in subjects with resistant hypertension has been confirmed by other laboratories.4–6 Recognizing that OSA and primary aldosteronism are both prevalent in patients with resistant hypertension, we hypothesized that the 2 entities may be causally related, that is, one contributing to the other. Two earlier studies provided preliminary support for the hypothesis that primary aldosteronism contributes to development and/or severity of OSA.16,2 In the first study16 we reported that risk of having OSA, based on the Berlin Questionnaire, a validated survey for identifying subjects at risk of having OSA,17 was significantly higher in subjects with resistant hypertension and primary aldosteronism compared to subjects without evidence of aldosterone excess. In the second study,2 we reported that aldosterone levels positively correlated with OSA severity in patients with resistant hypertension, but not in control patients with similar age, body mass index and OSA severity but without resistant hypertension. Combined, these 2 studies suggested that in subjects with resistant hypertension, aldosterone may contribute to OSA severity.
In the current study we directly tested this hypothesis by evaluating the effects of mineralocorticoid blockade on OSA severity in subjects with resistant hypertension. After 8 weeks of treatment, OSA severity was substantially reduced. These results provide support of a possible role of aldosterone in contributing to OSA risk in patients with resistant hypertension. This reduction in OSA severity was associated with evidence of more effective diuresis as indicated by a significant increase in PRA and serum creatinine, decrease in body weight and a tendency toward lower plasma BNP, consistent with reduction in intravascular volume. Although not tested in this evaluation, we speculate that improved diuresis with accompanying reduction in peripharyngeal edema and improvement in upper airway resistance represents the pathophysiologic mechanism of the strong association between aldosterone excess and OSA.
Support of peripharyngeal edema as a cause of upper airway obstruction is provided by acute studies relating changes in intravascular volume to upper airway resistance and severity of OSA.8,9 In the first case, acute increases in venous return with application of lower body positive pressure resulted in increases in neck circumference and upper airway resistance in healthy volunteers.8 This suggests that increases in venous pressure and fluid accumulation in the neck predisposes to pharyngeal obstruction. In the second case, acute diuresis, including with spironolactone, improved severity of OSA in patients admitted with exacerbation of congestive heart failure secondary to diastolic dysfunction.9 This improvement in OSA occurred in association with reduction in oropharyngeal cross-sectional area (as indexed by acoustic pharyngometry) also suggesting a role of peripharyngeal fluid accumulation. Fluid overload as a cause of OSA is further supported by studies demonstrating improvements in OSA after dialysis in patients with end-stage-renal disease.18,19
The above studies suggest that volume overload can acutely worsen OSA by increasing pharyngeal obstruction with peripharyngeal fluid accumulation. The results of the current study extend these observations by suggesting that similar effects may occur chronically in patients with resistant hypertension secondary to aldosterone-induced fluid retention. We hypothesize that spironolactone, by reversing chronic fluid retention, reduces peripharyngeal fluid accumulation and thereby lowering risk of upper airway obstruction. Importantly, in the current analysis, the benefit of spironolactone occurred when added to chronic thiazide diuretic use. This suggests that thaizides, at least at conventional doses, are not sufficient to overcome this chronic fluid retention. It may be that more effective diuresis with use of higher doses of the thiazide diuretic or perhaps addition of a loop diuretic would also facilitate comparable improvements in OSA, but this needs further evaluation in comparison to spironolactone.
The present study is strengthened by its prospective design, use of full-night diagnostic polysomnograms to quantify OSA severity and blinding of the scoring physician to study status. Important study limitations include evaluation of a relatively small number of subjects and lack of an active comparator. Comparison to an active control, such as another class of diuretic, will be necessary to establish that the effects are unique to mineralocorticoid receptor blockade.
Whether the findings in the present study are limited to patients with resistant hypertension or could be extended to a more general hypertensive population with OSA remains to be established. Resistant hypertension is known to be characterized by increased aldosterone levels and chronic fluid retention7 and so the effects of mineralocorticoid blockade are likely to be most pronounced in this subgroup of hypertensive patients. However, primary aldosteronism is being recognized as being generally much more common than thought historically20,21 such that its role in contributing to OSA may not be limited to just patients with resistant hypertension.
Clinical implications
The current findings implicate aldosterone-induced fluid retention as an important cause of OSA in patients with resistant hypertension. If confirmed, these results would explain at least in part the high prevalence of OSA in patients with resistant hypertension. These results emphasize the importance of screening for OSA in patients with resistant hypertension and highlight the potential benefit of spironolactone as an adjunct to continuous positive airway pressure in this group of patients. In addition, it is well recognized that OSA and primary aldosteronism are separately associated with increased risk of cardiovascular disease, including myocardial infarction, stroke, arrythmias, and congestive heart failure.22–24 The current findings provide explanation of these shared risks in suggesting that aldosterone excess may predispose to the development of OSA.
Summary Table
What is known about this topic?
Obstructive sleep apnea (OSA) and primary aldosteronism are very common in patients with resistant hypertension.
The pathophysiologic mechanism of this high prevalence of OSA in patients with resistant hypertension has not been explained.
Beyond hyperaldosteronism, we reported that resistant hypertension is in general characterized by significant fluid retention, even in subjects without demonstrable aldosterone excess. This fluid retention persists in spite of long term thiazide diuretic use.
What this study adds?
This is the first study demonstrating that treatment with a mineralocorticoid receptor antagonist substantially reduces OSA severity in patients with resistant hypertension.
This study provides preliminary evidence linking aldosterone-induced fluid retention to severity of OSA and if confirmed in a randomized comparison, would explain in part, the high prevalence of OSA in patients with resistant hypertension.
It is well recognized that OSA and primary aldosteronism are separately associated with increased risk of cardiovascular disease, including myocardial infarction, stroke, arrythmias, and congestive heart failure. The current findings provide explanation of these shared risks in suggesting that aldosterone excess may predispose to the development of OSA.
Acknowledgments
This study was supported by NHLBI SCCOR P50 HL077100 (DAC), RO1-HL79040 (DAC), T32 HL007457 (KG) and GCRC Grant M01-RR00032.
We thank David Moore, RPSGT, Melissa Butler, RPSGT, and Louise Dover, RPSGT, for assistance in conducting the overnight sleep studies.
Abbreviation list
- OSA
Obstructive sleep apnea
- BP
blood pressure
- AHI
apnea-hypopnea Index
- HI
hypoxic index
- REM
rapid eye movement sleep
- PRA
plasma renin activity
- BNP
brain natriuretic peptide
- ACEI
angiotensin converting enzyme inhibitor
- ARB
angiotensin receptor blocker
References
- 1.Logan AG, Perlikowski SM, Mente A, Tisler A, Tkacova R, Niroumand M, Leung RS, Bradley TD. High prevalence of unrecognized sleep apnea in drug resistant hypertension. J Hypertens. 2001;19:2271–2277. doi: 10.1097/00004872-200112000-00022. [DOI] [PubMed] [Google Scholar]
- 2.Pratt-Ubunama, Nishizaka MK, Boedefeld RL, Cofield SS, Harding SM, Calhoun DA. Plasma aldosterone is related to severity of obstructive sleep apnea in subjects with resistant hypertension. Chest. 2007;131:453–459. doi: 10.1378/chest.06-1442. [DOI] [PubMed] [Google Scholar]
- 3.Calhoun DA, Nishizaka MK, Zaman MA, Thakkar RB, Weismann P. Hyperaldosteronism among black and white subjects with resistant hypertension. Hypertension. 2002;40:892–896. doi: 10.1161/01.hyp.0000040261.30455.b6. [DOI] [PubMed] [Google Scholar]
- 4.Gallay BJ, Ahmad S, Xu L, Toivola B, Davidson RC. Screening for primary aldosteronism without discontinuing hypertensive medications: plasma aldosterone-renin ratio. Am J Kidney Dis. 2001;37:699–705. doi: 10.1016/s0272-6386(01)80117-7. [DOI] [PubMed] [Google Scholar]
- 5.Strauch B, Zelinka T, Hampf M, Bernhardt R, Widimsky J., Jr Prevalence of primary hyperaldosteronism in moderate to severe hypertension in the Central Europe region. J Hum Hypertens. 2003;17:349–352. doi: 10.1038/sj.jhh.1001554. [DOI] [PubMed] [Google Scholar]
- 6.Eide IK, Torjesen PA, Drolsum A, Babovic A, Lilledahl NP. Low-renin status in therapy-resistant hypertension: a clue to efficient treatment. J Hypertens. 2004;22:2217–2226. doi: 10.1097/00004872-200411000-00026. [DOI] [PubMed] [Google Scholar]
- 7.Gaddam KK, Nishizaka MK, Pratt-Ubunama MN, Pimenta E, Aban I, Oparil S, Calhoun DA. Characterization of Resistant Hypertension - Association between Resistant Hypertension, Aldosterone and Persistant Intravscular Volume Expansion. Arch Int Med. 2008;168:1159–1164. doi: 10.1001/archinte.168.11.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiota S, Ryan CM, Chiu KL, Ruttanaumpawan P, Haight J, Arzt M, Floras JS, Chan C, Bradley TD. Alterations in upper airway cross-sectional area in response to lower body positive pressure in healthy subjects. Thorax. 2007;62:868–872. doi: 10.1136/thx.2006.071183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bucca CB, Brssino L, Battisti A, Mutani R, Rolla G, Mangiardi L, Cicolin A. Diuretics in obstructive sleep apnea with diastolic heart failure. Chest. 2007;132:440–446. doi: 10.1378/chest.07-0311. [DOI] [PubMed] [Google Scholar]
- 10.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques, and scoring system for sleep stages of human sleep. Los Angeles, CA: Brain Information Service/ Brain Research Institute, UCLA; 1968. [Google Scholar]
- 11.Kapa S, Sert Kuniyoshi FH, Somers VK. Sleep apnea and hypertension: Interaction and implications for management. Hypertension. 2008;51:605–608. doi: 10.1161/HYPERTENSIONAHA.106.076190. [DOI] [PubMed] [Google Scholar]
- 12.Hedner J, Darpo B, Ejnell H. Reduction in sympathetic activity after long-term CPAP treatment in sleep apnea: cardiovascular implications. Eur Respir J. 1995;8:222–229. doi: 10.1183/09031936.95.08020222. [DOI] [PubMed] [Google Scholar]
- 13.Schulz R, Mahmoudi S, Hattar K. Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea: impact of continuous positive airway pressure therapy. Am J Respir Crit Care Med. 2000;162:566–570. doi: 10.1164/ajrccm.162.2.9908091. [DOI] [PubMed] [Google Scholar]
- 14.IP MS, Lam B, Chan LY. Circulating nitric oxide is suppressed in obstructive sleep apnea and is reversed by nasal continuous positive airway pressure. Am J Respir Crit Care Med. 2000;162:2166–2171. doi: 10.1164/ajrccm.162.6.2002126. [DOI] [PubMed] [Google Scholar]
- 15.Demaika TA, Kinasewitz GT, Tawk MM. Effects of nocturnal continuous positive airway pressure therapy in patients with resistant hypertension and obstructive sleep apnea. J Clin Sleep Med. 2009;5:103–107. [PMC free article] [PubMed] [Google Scholar]
- 16.Calhoun DA, Nishizaka MK, Zaman MA, et al. Aldosterone excretion among subjects with resistant hypertension and symptoms of sleep apnea. Chest. 2004;125:112–117. doi: 10.1378/chest.125.1.112. [DOI] [PubMed] [Google Scholar]
- 17.Netzer NC, Stoohs RA, Netzer CM, et al. Using the Berlin Questionare to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–491. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 18.Hanly PJ, Perratos A. Improvement of sleep apnea in patients with chronic renal failure who undergo nocturnal hemodialysis. N Engl J Med. 2001;344:02–107. doi: 10.1056/NEJM200101113440204. [DOI] [PubMed] [Google Scholar]
- 19.Tang SC, Lam B, Ku PP, et al. Alleviation of sleep apnea in patients with chronic renal failure by nocturnal cycler-assisted peritoneal dialysis compared with conventional continuous ambulatory peritoneal dialysis. J Am Soc Nephrol. 2006;17:2607–2616. doi: 10.1681/ASN.2005090936. [DOI] [PubMed] [Google Scholar]
- 20.Mosso L, Carvajal C, Gonzalez A, et al. Primary aldosteronism and hypertensive disease. Hypertension. 2003;42:161–165. doi: 10.1161/01.HYP.0000079505.25750.11. [DOI] [PubMed] [Google Scholar]
- 21.Rossi GP, Bernini G, Calliumi C, et al. PAPY Study Investigators. A prospective study of the prevalence of primary aldosteronism in 1125 hypertensive patients. J Am Coll Cardiol. 2006;48:2293–2300. doi: 10.1016/j.jacc.2006.07.059. [DOI] [PubMed] [Google Scholar]
- 22.Shahar E, Whitnet CW, Redline S. Sleep disordered breathing and cardiovascular disease cross sectional results of the Sleep Heart health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 23.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 24.Marin JM, Carrizo SJ, Vicente E, Agusti AGN. Long-term cardiovascular outcomes in men with obstructive sleep apnea-hypopnea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]