Abstract
Cognitive and physical decline are important predictors of functional independence in Alzheimer’s disease (AD). However, little is known about AD-related neural change leading to decreased independence. We hypothesized that regional gray matter atrophy, including the medial frontal cortex, would be related to cognition, physical function, and functional independence. Individuals without dementia (n = 56) and subjects with early-stage AD (n = 58) underwent MRI and a comprehensive cognitive and physical function evaluation. The relationship of cognitive and physical function measures and independence performing complex daily activities was explored using correlation and mediation analysis. These results suggest that cognition had both a strong direct effect and mediated the influence of physical function on independence for those with AD. We followed this with a voxel-based morphometric global conjunction analysis of imaging data within each group to identify neural substrates common to our function measures. Imaging evidence supported our mediation analysis results. Imaging evidence revealed that in AD, regional gray matter atrophy measures in medial frontal and temporo-parietal areas were related to decreased cognition, physical function, and independence. Loss of independence in early AD is closely related to impaired cognition associated with performing complex behaviors. People with early AD may have decreased gray matter volume in the medial frontal and temporal-parietal cortices that is associated with loss of independence in activities of daily living. These results are the first to identify regionally specific brain volume changes that may be related to functional dependence seen in early AD.
Keywords: Activities of daily living, cognition, physical function, voxel-based morphometry
INTRODUCTION
The unprecedented growth of the elderly population has been accompanied by an increased prevalence of Alzheimer’s disease (AD), now affecting nearly 1 in 10 adults over the age of 71 [1]. In addition to the commonly acknowledged problem of episodic memory and cognitive dysfunction, decline in physical function, self-care, and level of independence are frequent characteristics of disease progression [2]. Loss of independence in daily function is a key feature of AD, with great cost to an individual’s quality of life [3]. However, little is known about AD-related neural change leading to decreased independence. Identification of therapeutics to support independence first requires a more complete understanding of mechanisms behind loss of functional independence.
Gray matter loss in AD includes many areas that mediate functional, goal-oriented daily activity including temporal, frontal, occipital, and limbic regions [4]. Importantly, early degeneration of fronto-parietal circuits necessary for movement planning in those with AD have been blamed for poor execution of behaviors crucial for performing daily self-care [5]. A key frontal region involved in daily activity is the medial frontal cortex [6] which plays a role in “cognitive control” [7], vigilance and attention to stimuli requiring a response [8], and motivating performance adjustment [9]. However, individuals with AD also experience decreased physical capacity [2,10], complicating the relationship of cognition to functional independence. Together, cognitive and physical health are important components of independent living [11–13]. To this point, the relationship between global cognitive function (multimodal information processing measured by a cognitive test battery) and physical function (sensorimotor performance measured by a series of basic physical tasks) in AD remains imprecisely defined, particularly with regard to functional independence (independent performance of fundamental daily activities) [14,15].
Thus, our aim in the present investigation was to explore how cognitive and physical function is related to independence in daily activities and identify underlying brain changes associated with dependence in AD. Previous work has linked total cortical gray matter volume and functional independence [16]. However, the literature currently lacks a regional examination of local brain structure changes with AD in the context of functional independence. Our hypothesis was that both measures of cognition and physical functioning would contribute to activities of daily living in both AD and nondemented groups, and that this relationship would correlate with differences in gray matter volume, specifically in the medial frontal cortex.
We have previously used newvoxel-based morphometry image analysis (VBM) techniques to examine regional differences in gray matter volume between subjects with early AD and cognitively healthy elderly subjects [17]. For the present study, we first sought to characterize the relationship between cognition, physical function, and independence in complex functional behaviors (i.e., instrumental activities of daily living, IADLs) using mediation analysis [18]. Mediation analysis allows for an assessment of the extent to which a mediating variable accounts for the relation between two other variables [19]. Guided by the results of the mediation analysis, we then employed VBM to examine regional gray matter volume associated with measures of physical function, cognitive function, and functional independence.
MATERIALS AND METHODS
Demographics
All participants were assessed by a board certified neurologist with specialized training in the evaluation of dementia. The presence or absence of dementia and its severitywas determined using the Clinical Dementia Rating (CDR) [20,21]. Individuals diagnosed with AD met standard criteria for a clinical diagnosis of AD[22]. Nondemented (ND) individuals and those with early AD between the ages of 60 and 85 were enrolled in the University of Kansas Brain Aging Project (BAP), an ongoing longitudinal study of lifestyle, brain aging, and AD progression. Institutionally approved, informed consent was obtained from participants or their legal representatives as appropriate. All participants enrolled with an informant knowledgeable about the participant’s daily life. BAP exclusions are neurological disease other than AD, active ischemic heart disease, history of significant mental illness, diabetes mellitus, or other systemic illness that might impair completion of the study. Physical and neurological characteristics of these participants have been presented previously [23]. Those who had satisfactorily completed all baseline BAP assessments relevant to this report were included for this cross-sectional analysis (ND n = 56, CDR 0; AD n = 58, CDR 0.5 or 1).
Function measures
Our primary measure of interest was functional independence which we indexed using the Alzheimer’s Disease Cooperative Study Activities of Daily Living Scale for Mild Cognitive Impairment (ADCS-ADL) with information collected from the informant. The ADCS-ADL is a well characterized measure of independence in activities of daily living [24]. The 18-item measure is heavily weighted towards independence in IADLs such as meal preparation, travel outside the home, shopping, and performing household chores. Tasks are scored by increasing level of independence with greater scores reflecting more independence in IADLs. The range of possible scores is 0–53.
Our primary measure of physical function was a short battery of physical tasks, the modified Physical Performance Test (PPT) [23,25]. Scores are given based on time to complete each task. The modified PPT includes writing a sentence, simulated eating, lifting a book and placing it on a shelf above shoulder height, putting on and removing a jacket, picking up a penny from the floor, turning 360 degrees and ambulating 50 feet. We modified the PPT to test the ability to transfer from sit-to-stand (e.g., 5 consecutive chair rises) and balance ability (e.g., progressive Romberg test). The range of possible scores is 0–36.
Because no one test can readily characterize dementia associated with AD, we administered a global cognitive battery. The tests included common measures of memory (Wechsler Memory Scale [WMS] – Revised Logical Memory IA and IIA [26], Free and Cued Selective Reminding Task [27]), language (Boston Naming Test–15 item [28]), working memory (Wechsler Adult Intelligence Scale [WAIS] letter – number sequencing [29], WMS III Digit Span Forwards and Backwards [26]), executive function (Trailmaking A and B [30], Verbal Fluency [31] [animals and vegetables], and Stroop Color-Word Interference Test [32]), and vi-suospatial ability (WAIS Block Design [29]). Each score in the cognitive battery was standardized to the mean and standard deviation of a larger nondemented cohort from the BAP (n = 84), including all ND participants in the present study, who had completed cognitive testing. The mean of each participant’s z-scores was used as an index of global cognitive performance (COG) [23]. The Mini-Mental State Exam (MMSE) [33] was administered to facilitate comparison to other reports.
Imaging
High-resolution T1 weighted anatomical images were acquired on a Siemens 3.0 Tesla Allegra MRI Scanner (magnetization-prepared rapid gradient echo [MPRAGE]; 1 × 1 × 1 mm3 voxels, repetition time [TR]=2500 ms, echo time [TE]=4.38 ms, inversion time [TI]=1100 ms, field of view 256×256 with 18% oversample, flip angle=8 degrees). Scans were visually inspected and processed for voxel-based analysis. Data analysis was performed using the VBM5 toolbox (http://dbm.neuro.uni-jena.de), an extension of the SPM5 algorithms (Wellcome Department of Cognitive Neurology, London, UK) running under MATLAB 7.1 (The Math Works, Natick, MA, USA) on Linux.
Voxel-based morphometry is a method for detecting group differences in the density or volume of brain matter that is sensitive to detecting small disease-related variations in brain volume [34]. Our structural image processing method for VBM is detailed elsewhere [17]. Briefly, we chose the VBM5 toolbox because it extends and enhances the unified segmentation approach implemented in SPM5 [35] by using a generative model that integrates tissue classification, image registration and MRI inhomogeneity bias correction. We used the Hidden Markov Field (HMRF) model on the estimated tissue maps (3 × 3 × 3). Based on recommendations [35] for aging and diseased populations, estimated tissue probability maps were written without making use of the ICBM tissue priors to avoid a segmentation bias, as these priors are derived from young healthy controls [36]. Images were then modulated and saved using affine registration plus non-linear spatial normalization [37]. The resulting gray matter volume maps were smoothed with a 10 mm FWHM Gaussian kernel before statistical analysis [38,39]. This kernel size was chosen as a balance between maximizing localization accuracy of smaller smoothing kernels and reducing false positives associated with larger smoothing kernels.
We sought to identify neural correlates of our functional measures, and focused on gray matter volume for this analysis. Gray matter thinning and atrophy is a more established measure of AD-related change [40, 41] and has been correlated with cognitive and functional measures relevant to this study [4,16].
Demographic and behavioral statistics
Demographic and functional measures were compared between groups using ANOVA. Gender distribution between groups was compared using a Chi-square test. We then assessed the relationship between our variables separately for each group, using partial Pearson correlations adjusted for age and gender. Based on previous literature suggesting cognitive and motor interdependence in AD, and our partial correlation results, we performed a follow-up mediation analysis to assess the relationship between cognition (COG), physical function (PPT) and functional independence (ADCS-ADL) measures (SPSS 16.0, S PSS Inc. Chicago, IL). This procedure supports the investigation of both direct effects between variables as well as indirect effects mediated through additional variables. The method and associated SPSS macro are available online [18].
For the present investigation, we defined ADCS-ADL as the dependent variable a priori. As with partial Pearson correlations, mediation analyses were performed separately for AD and ND groups. Gender and age were treated as covariates in the mediation model. The literature provides no rationale for selecting cognition or physical function as the mediating variable a priori, therefore we investigated models in which each served as the mediator. A bootstrapping procedure was employed (5000 resamples) to describe the confidence interval (CI) of the indirect effect. A 95% CI not containing zero was considered a significant indirect effect. All other descriptive analyses outside imaging space were tested at α = 0.05.
Imaging statistics
We used a multiple regression model with COG,PPT, and ADCS-ADL scores as primary regressors, age and gender as covariates of no interest, no grand mean scaling, and covariates centered on the overall mean. The absolute threshold masking was set at 0.10 to restrict each analysis to gray matter. This multiple regression model was run on AD and ND separately in order to look at the effects of these measures within diagnosis groups. We used a global conjunction model to test the hypothesis that each of the primary regressors (COG, PPT, and ADCS-ADL) would correlate with an overlapping region of brain volume, localizing these functions to a neural location or locations. The global conjunction model asks where in the brain the regressors covary together [42]. SPM5 tests for the null global conjunction using the minimum T statistic and looks for consistent effects within variables of interest on a brain measure of interest, in this case gray matter volume [43]. Resulting clusters from the global conjunction analysis were considered significant at p < 0.001 uncorrected.
Finally we sought to test whether COG, PPT, and ADCS-ADL correlated together with medial frontal gray matter volume in AD and ND. This a priori hypothesis requires statistical correction. The small volume corrected region of interest (ROI) was derived from the Wake Forest University Pickatlas (http://www.fmri.wfubmc.edu) [44] and selected based on the hypothesis that deficits in cognition and function may be due to change in the prefrontal cortices [45]. The ROI was constructed from the Anatomic Automatic Labeling (AAL) masks of bilateral frontal gyrus, superior medial portion and anterior cingulate gyrus. To correct for multiple comparisons in ROI analyses, results were considered significant at p < 0.05, Family-wise Error adjusted (FWE). Location of peak voxels within significant clusters are reported with reference to the Montreal Neurological Institute (MNI) standard space within SPM5 using the Pickatlas for AAL and Talairach Daemon Brodmann Area (BA) estimates.
RESULTS
Group measures
Characteristics of the groups are summarized in Table 1. Groups were similar in age and gender distribution. The groups differed slightly in years of education; both groups averaged at least three years of postsecondary education. One hundred eight individuals, self-identified their primary race as white, non Hispanic (ND=56; AD=52). Five individuals with AD identified themselves as African-American and one as Native American. One nondemented individual identified as white and of Hispanic ethnicity.
Table 1.
Demographics of nondemented and early Alzheimer’s disease groups
| Nondemented (n = 56) | Alzheimer’s disease (n = 58) | p | |
|---|---|---|---|
| Age (yrs) | 73 (6.2) | 74.2 (6.3) | 0.58 |
| % Female | 57.1 | 64.4 | 0.43 |
| Education (yrs) | 16.4 (2.2) | 15.2 (3.0) | 0.02 |
| Mini-Mental State Exam (MMSE) | 29.5 (0.8) | 26.7 (3.1) | < 0.001 |
| Physical Performance Test (PPT) | 30.4 (3.3) | 27.6 (4.1) | 0.001 |
| Activities of Daily Living Scale (ADCS-ADL ) | 48.6 (3.3) | 39.8 (8.2) | < 0.001 |
| Global Cognitive Battery (COG) | 0 (0.5) | −1.7 (1.2) | < 0.001 |
All values are means (SD) except gender (% females in cohort). Negative values of global cognitive function for the AD group indicate lower cognitive function than the mean performance of nondemented individuals. Lower scores on the remaining items (MMSE, PPT, AND ADCS-ADL) are associated with worse performance on the measure. P values of group comparisons are provided.
As expected, the AD group had significantly lower global cognitive function (COG), functional independence (ADCS-ADL), and physical function (PPT) measures. We then explored the relationship of cognition and physical function to independence in IADLs, separately for each group using partial correlations to account for age and gender. For both groups, functional independence was positively related to physical function (PPT: ND r = 0.29, p = 0.031; AD r = 0.32, p = 0.015) and global cognitive function (COG: ND r = 0.27, p = 0.05; AD r = 0.5,p < 0.001).
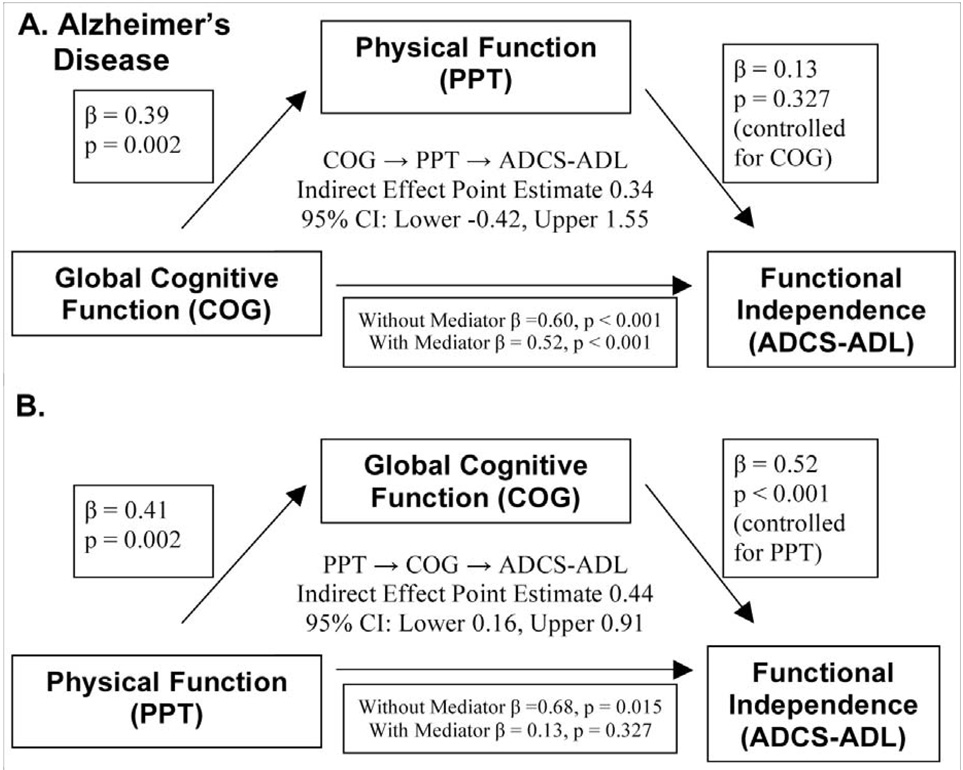
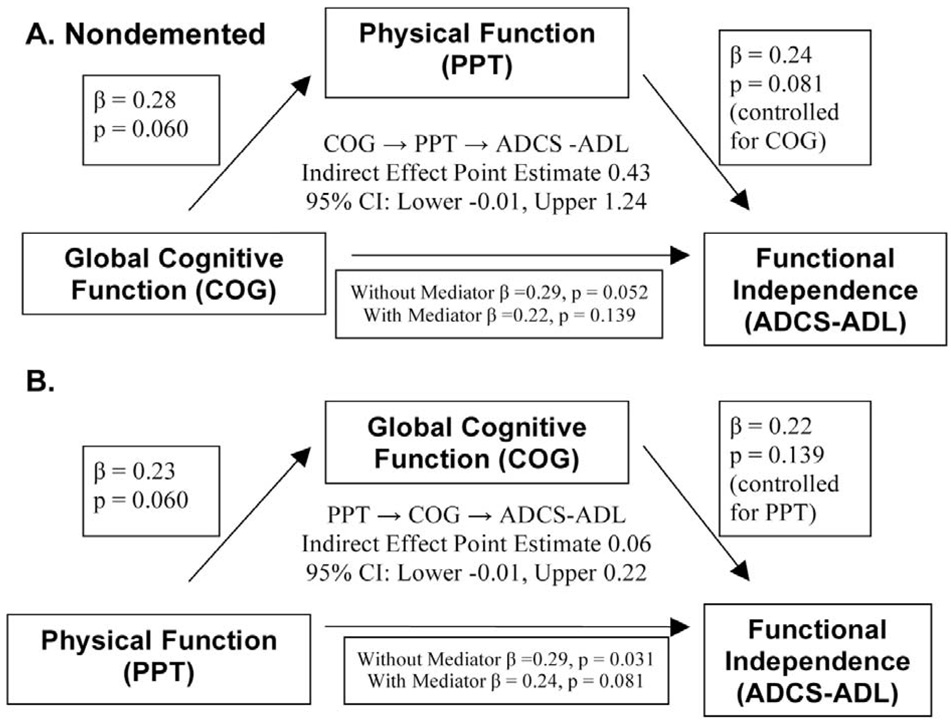
To further characterize the relationships between physical function, cognition and functional independence we subjected PPT, COG, and ADCS-ADL to mediation analysis. Figure 1 and Figure 2 graphically summarize the mediation analyses for the AD and ND groups, respectively. Pictured are the direct and indirect effects of each analysis; first treating COG as the independent variable and PPT as the mediating variable (panel A of Fig 1 and Fig 2), then the results from the converse mediation analysis (panel B). The results suggest that cognition strongly influenced independence for individuals with AD through two points of evidence (Fig. 1B). First, a significant direct effect of COG on ADCS-ADL (standardized β = 0.52, p < 0.001) was present. Second, COG mediated the relationship between PPT and ADCS-ADL as demonstrated by a significant point estimate of the indirect effect. (Point Estimate 0.44; Lower CI=0.16). No significant mediation effects were detected for the ND cohort. PPT was not a significant mediator for either group.
Fig. 1.
Graphic depiction of mediation analysis results for the cohort with early Alzheimer’s disease. A) The COG → PPT → ADCS-ADL model tests the mediating effect of physical function on the relationship between global cognitive function and functional independence. Physical function does not have a mediating effect. B) The PPT→COG→ADCS-ADL model tests the mediating effect of cognition on the relationship between physical function and functional independence. Both a direct effect of global cognitive function on independence as well as an indirect effect mediating the relationship between physical function and independence are evident. Standardized coefficients are presented along with the associated p-value.
Fig. 2.
Graphic depiction of mediation analysis results for the nondemented group. A) The COG → PPT → ADCS-ADL model tests the mediating effect of physical function on the relationship between global cognitive function and functional independence. B) The PPT → COG →ADCS-ADL model tests the mediating effect of cognition on the relationship between physical function and functional independence. Standardized coefficients are presented along with the associated p-value. The indirect effect point estimated is centered within the chart. Mediating effects were not significant for the nondemented group.
Imaging measures
To explore possible neural substrates for the cognition-mediated relationship between physical function and independence in IADLs, we performed voxel-wise examination of the gray matter volumes of both cohorts. All clusters for the voxel-wise analysis (k > 100 and Z > 3.8, p < 0.001 uncorrected) are listed in Table 2.
Table 2.
Regions of significant gray matter atrophy associated with global cognitive function, physical function and functional independence
| Alzheimer’s disease | ||||||
|---|---|---|---|---|---|---|
| MNI Peak | k | x | y | z | T | Z |
| R Precuneus/BA 7 | 656 | 10 | −58 | 42 | 2.23 | 4.51 |
| R Supramarginal Gyrus/BA 40 | 328 | 54 | −49 | 34 | 1.74 | 3.76 |
| R Hippocampus/BA 27 | 480 | 24 | −32 | −4 | 1.78 | 3.83 |
| L Rolandic Operculum/BA 40 | 137 | −62 | −22 | 14 | 1.69 | 3.68 |
| R Superior Temporal Gyrus/BA 22 | 481 | 66 | −19 | 3 | 2.10 | 4.30 |
| L Parahippocampal Gyrus/BA 28 | 382 | −24 | −15 | −26 | 1.93 | 4.04 |
| L Insula/BA 13 | 123 | −38 | 13 | 2 | 1.51 | 3.41 |
| R Inferior Frontal Gyrus (orbital)/BA 47 | 206 | 51 | 30 | −5 | 1.86 | 3.94 |
| L Inferior Frontal Gyrus (triangle)/BA 46 | 1837 | −53 | 33 | 15 | 2.24 | 4.52 |
| L Superior Frontal Gyrus (medial)/BA 9 | 1213 | −6 | 40 | 28 | 2.08 | 4.27 |
| R Middle Frontal Gyrus/BA 46 | 167 | 49 | 46 | 5 | 1.77 | 3.81 |
| R Middle Frontal Gyrus/BA 10 | 141 | 37 | 51 | 18 | 1.59 | 3.53 |
| Nondemented | ||||||
| MNI Peak | k | x | y | z | T | Z |
| L Precentral Gyrus/BA 6 | 119 | −35 | −8 | 63 | 1.86 | 3.94 |
| R Precentral Gyrus/BA 6 | 208 | 46 | 1 | 46 | 2.15 | 4.37 |
| R Middle Frontal Gyrus/BA 8 | 419 | 38 | 13 | 52 | 1.91 | 4.02 |
Summary of the results obtained by voxel-wise statistical analysis of the gray matter maps of the cohort with AD. Significant clusters detected at p <= 0.001 uncorrected, cluster size k >100. Only the peak voxels are listed.
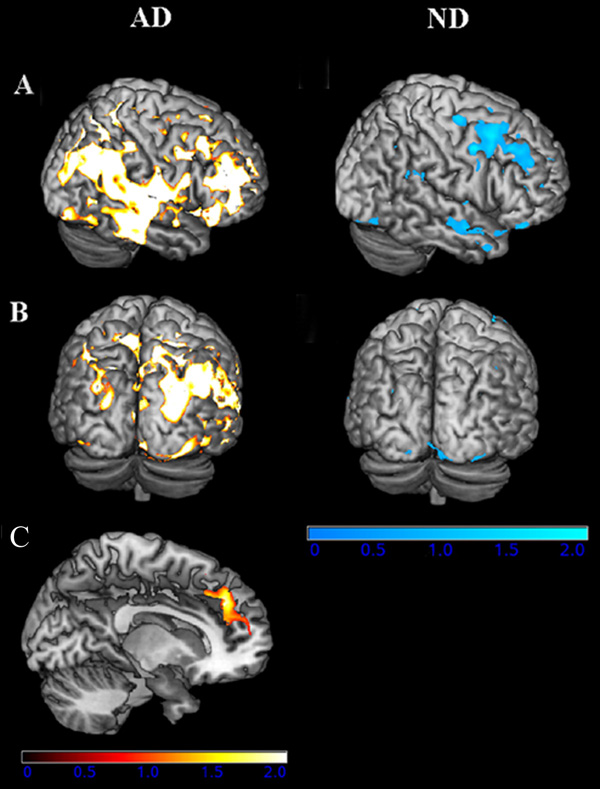
In subjects with early AD a common neural substrate for PPT, COG, and independence in IADLs (ADCS-ADL) was detected in several locations across the brain. The largest clusters of gray matter volume that positively and significantly covaried with all three measures were in the right precuneus, (BA 7; Z = 4.51, cluster size (k)= 656); the left superior frontal gyrus, medial portion (BA 9; Z = 4.27, k = 1213); and the left inferior frontal gyrus, triangular region, (BA 46; Z = 4.52, k = 1837). Other regions of commonality include the left parahippocampus, right hippocampus, right superior temporal gyrus, and right supramarginal gyrus. Figure 3A and B, left column depicts these regions of structural/behavioral association for the AD group. The ROI analysis revealed that poor performance on cognitive, physical and independence measures was associated with lower medial frontal cortex gray matter volume (BA 9) in subjects with early AD (p = 0.03 FWE corrected, Fig. 3C).
Fig. 3.
Statistical maps of the global conjunction analysis showing gray matter volume reductions that covary with our three primary measures, global cognition, physical function and functional independence in nondemented participants (ND, cool hues, right column) and those with AD (warm hues, left column) (at a threshold of p <= 0.001). Thresholded images have been transformed from MNI space into Talairach space and converted to T-scores, which were projected onto the pial surface of a representative standard surface. The lateral surface is in the top row of each section (A), and the posterior surface is shown below the lateral (B). (C) Small volume corrected analysis restricted to the medial frontal cortex yielded a region of gray matter atrophy significantly related to global cognitive function, physical function and functional independence. This region was significant only in individuals with AD. T-scores are projected on to a representative sagittal slice and index gray matter volume reductions in the bilateral BA 9, centered in the left hemisphere (p < 0.05 FWE). (Colours are visible in the electronic version of the article at www.iospress.nl.)
In the ND subjects, the global conjunction analysis revealed a common neural substrate between physical, cognitive, and IADL measures in the middle frontal (BA 8) and precentral (BA 6) cortex (Fig. 3A and B, right column). The ROI analysis of medial frontal cortex volume in the ND subjects did not reveal any significant relationship between our three measures of functional independence and gray matter volume change in this region.
DISCUSSION
Our goal in the present investigation was to use novel imaging analysis to clarify how cognitive and physical function are related to independence in daily activities. Specifically, we sought to identify underlying brain changes associated with functional decline in AD. We found 1) a relationship between ADCS-ADL, COG), and PPT in nondemented older adults and individuals with early AD; and 2) identified a common neural underpinning of that relationship in the medial frontal and temporo-parietal cortices unique to subjects with AD.
There are limitations inherent to the study that should be considered. Our imaging analysis was not designed to directly test group (AD vs. nondemented) interactions within the conjunction models. We have previously identified regional decreases in gray matter volume in this early AD group compared to nondemented participants [17]. This previous study identified AD-related decreases in gray matter volume, with frontal cortex decreases in gray matter volume primarily in the medial frontal gyrus (BA 9, 10), bilateral middle frontal gyrus (BA 6), insula (BA 13), and dorsolateral prefrontal cortex. In the present study, there was not a significant relationship between medial frontal atrophy and our measures of cognition, physical function and independence in the ND group, even when using a small-volume corrected, medial frontal ROI. These converging lines of evidence suggest a disease-specific pattern underlying functional change.
Convergent behavioral and imaging results
Our expectation was that both cognition and physical function would contribute to independent performance of complex behaviors, specifically those that are central to function in the community (IADLs). This was tested first using partial correlation and mediation analysis. We found that cognitive and physical function were both related to functional independence. Specifically, our composite measure of global cognitive function was strongly related to functional independence in the AD group as evidenced by both a direct effect on ADCS-ADL and a mediating indirect influence between PPT and ADCS-ADL.
Concomitant with the findings from our mediation analyses, our imaging data demonstrate for the first time, patterns of gray matter differences in the medial frontal and temporo-parietal regions that are associated with declining physical function, cognition, and independence specific to AD. Global conjunction analysis of our imaging data revealed that gray matter volume in the middle frontal gyrus was associated collectively with our measures of physical function, cognition, and functional independence in the nondemented group. Individuals with early AD not only displayed reduced frontal gray matter co-varying with measures of cognition, physical function and independence (inferior orbitofrontal gyrus, superior medial frontal gyrus, middle frontal gyrus), but caudal regions (precuneus, supramarginal gyrus, postcentral gyrus) were associated with these measures as well.
We identified several foci in the temporo-parietal area (right supramarginal gyrus and right superior temporal gyrus) and precuneus that were collectively related to our functional measures. These findings are particularly relevant in light of previous reports of AD-related atrophy in these regions and their purported roles in behavior [46–50]. The precuneus plays a role in self-referential [51] visuomotor control and attention [52], whereas temporal lobe change has previously been linked to aberrant behavior and confusion in elderly psychiatric patients [53], including dependence in ADLs [54]. The supramarginal gyrus appears to be related to object-oriented sensory integration [52,55], apraxia [56], and object manipulation [57].
In addition, the ROI analysis yielded a common neuroanatomical relationship between PPT, COG, and ADCS-ADL and decreased medial frontal cortex volume (BA 9) in AD subjects, such that decreased performance on measures of physical function, independence, and cognition correlated with decreased gray matter. Previous imaging studies have reported structural [58] and functional [59,60] involvement of the prefrontal cortex in cognition, attention, and motor processing. These frontal regions participate in complex executive functioning and working memory [61] that are required for IADLs [62–65], as well as play a role in encoding the motivational value of external events and physical performance [9].
Cognitive aspects of motor activities necessary for independence
Changes in cognitive function, including the capacity for self-environment interaction and goal-directed behavior, can manifest themselves even before AD diagnosis [66]. For example, those with mild cognitive impairment demonstrate slower performance on instrumental activities of daily living as a possible consequence of decreased processing speed [67]. Our results confirm that preserved cognitive function is critical to translating successful performance of simple behaviors such as those in the PPT to complex behaviors such as the IADLs indexed by the ADCS-ADL. Further, the present data support the notion that early AD is associated with atrophy in brain regions, the medial frontal and temporo-parietal cortex, responsible for the cognitive activity of motor function [5,68].
Conclusion
In summary, we found converging evidence for a disease-related impairment of cognitive function associated with activities of daily living. In early AD, impaired cognitive function may be a result of reduced gray matter volume in the medial frontal and temporo-parietal cortex, which likely affects independent performance of IADLs in part through decline in associated executive aspects of function. Our work is the first to characterize the relationship between regional brain volume and functional independence through both physical performance and cognitive function in early AD. These functional and imaging data provide a window into the cognitive deficits that affect independent function, including basic and complex motor behaviors, in the daily activities of those with early AD.
ACKNOWLEDGMENTS
This study was supported by grants R03 AG026374 and R21 AG029615 from the National Institutes of Aging and K23 NS058252 from the National Institute on Neurological Disorders and Stroke. The University of Kansas General Clinical Research Center (M01RR023940) provided essential space, expertise, and nursing support. The Hoglund Brain Imaging Center is supported by grant C76 HF00201. We appreciate the assistance and commitment of the Alzheimer and Memory Program Team and the participants.
Footnotes
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=123).
REFERENCES
- 1.Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, Burke JR, Hurd MD, Potter GG, Rodgers WL, Steffens DC, Willis RJ, Wallace RB. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29:125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krenz C, Larson EB, Buchner DM, Canfield CG. Characterizing patient dysfunction in Alzheimer’s-type dementia. Medical Care. 1988;26:453–461. doi: 10.1097/00005650-198805000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Andersen CK, Wittrup-Jensen KU, Lolk A, Andersen K, Kragh-Sorensen P. Ability to perform activities of daily living is the main factor affecting quality of life in patients with dementia. Health Qual Life Outcomes. 2004;2:52. doi: 10.1186/1477-7525-2-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson PM, Hayashi KM, de Zubicaray G, Janke AL, Rose SE, Semple J, Herman D, Hong MS, Dittmer SS, Doddrell DM, Toga AW. Dynamics of gray matter loss in Alzheimer’s disease. J Neurosci. 2003;23:994–1005. doi: 10.1523/JNEUROSCI.23-03-00994.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camarda R, Camarda C, Monastero R, Grimaldi S, Camarda LK, Pipia C, Caltagirone C, Gangitano M. Movements execution in amnestic mild cognitive impairment and Alzheimer’s disease. Behav Neurol. 2007;18:135–142. doi: 10.1155/2007/845914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rushworth MF. Intention, choice, and the medial frontal cortex. Ann N Y Acad Sci. 2008;1124:181–207. doi: 10.1196/annals.1440.014. [DOI] [PubMed] [Google Scholar]
- 7.Gehring WJ, Willoughby AR. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295:2279–2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- 8.Burgess PW, Gilbert SJ, Dumontheil I. Function and localization within rostral prefrontal cortex (area 10) Philos Trans R Soc Lond B Biol Sci. 2007;362:887–899. doi: 10.1098/rstb.2007.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc Natl Acad Sci U S A. 2002;99:523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchman AS, Schneider JA, Leurgans S, Bennett DA. Physical frailty in older persons is associated with Alzheimer disease pathology. Neurology. 2008;71:499–504. doi: 10.1212/01.wnl.0000324864.81179.6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spirduso WW, Cronin DL. Exercise dose-response effects on quality of life and independent living in older adults. Med Sci Sports Exerc. 2001;33:S598–S608. doi: 10.1097/00005768-200106001-00028. discussion S609-510. [DOI] [PubMed] [Google Scholar]
- 12.MacNeill SE, Lichtenberg PA. Home alone: the role of cognition in return to independent living. Arch Phys Med Rehabil. 1997;78:755–758. doi: 10.1016/s0003-9993(97)90085-x. [DOI] [PubMed] [Google Scholar]
- 13.Gill TM, Williams CS, Richardson ED, Tinetti ME. Impairments in physical performance and cognitive status as predisposing factors for functional dependence among nondisabled older persons. J Gerontol A Biol Sci Med Sci. 1996;51:M283–M288. doi: 10.1093/gerona/51a.6.m283. [DOI] [PubMed] [Google Scholar]
- 14.Shettleworth S. Cognition, Evolution and Behavior. New York: Oxford University Press; 1998. Cognition, Evolution and the Study of Behavior; p. 5. [Google Scholar]
- 15.Jette AM, Cleary PD. Functional disability assessment. Phys Ther. 1987;67:1854–1859. doi: 10.1093/ptj/67.12.1854. [DOI] [PubMed] [Google Scholar]
- 16.Cahn-Weiner DA, Farias ST, Julian L, Harvey DJ, Kramer JH, Reed BR, Mungas D, Wetzel M, Chui H. Cognitive and neuroimaging predictors of instrumental activities of daily living. J Int Neuropsychol Soc. 2007;13:747–757. doi: 10.1017/S1355617707070853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Honea RA, Thomas GP, Harsha A, Anderson HS, Donnelly JE, Brooks WM, Burns JM. Cardiorespiratory fitness and preserved medial temporal lobe volume in Alzheimer’s Disease. Alzheimer Dis Assoc Disord. 2009 doi: 10.1097/WAD.0b013e31819cb8a2. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 19.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- 20.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412b–2414b. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 21.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 22.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 23.Burns JM, Cronk BB, Anderson HS, Donnelly JE, Thomas GP, Harsha A, Brooks WM, Swerdlow RH. Cardiorespiratory fitness and brain atrophy in early Alzheimer disease. Neurology. 2008;71:210–216. doi: 10.1212/01.wnl.0000317094.86209.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galasko D, Bennett D, Sano M, Ernesto C, Thomas R, Grundman M, Ferris S. An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. Alz Dis Assoc Disord Suppl 2:S33-9, 1997. 1997;11:S33–S39. [PubMed] [Google Scholar]
- 25.Reuben DB, Siu AL. An objective measure of physical function of elderly outpatients. The Physical Performance Test. J Am Geriatr Soc. 1990;38:1105–1112. doi: 10.1111/j.1532-5415.1990.tb01373.x. [DOI] [PubMed] [Google Scholar]
- 26.Wechsler D, Stone CP. Manual: Wechsler Memory Scale. New York: Psychological Corporation; 1973. [Google Scholar]
- 27.Grober E, Buschke H, Crystal H, Bang S, Dresner R. Screening for dementia by memory testing. Neurology. 1988;38:900–903. doi: 10.1212/wnl.38.6.900. [DOI] [PubMed] [Google Scholar]
- 28.Goodglass H, Kaplan E. Boston Naming Test scoring booklet. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 29.Wechsler D. Manual: Wechsler Adult Intelligence Scale. New York: Psychological Corporation; 1955. [Google Scholar]
- 30.Armitage SG. An analysis of certain psychological tests used in the evaluation of brain injury. Psychological Monographs. 1946;60:1–48. [Google Scholar]
- 31.Hänninen T, Reinikainen KJ, Helkala EL, Koivisto K, Mykk, nen L, Laakso M, Py”r, l, K, Riekkinen P. Subjective memory complaints and personality traits in normal elderly subjects. J Am Geriatr Soc. 1994;42:1–4. doi: 10.1111/j.1532-5415.1994.tb06064.x. [DOI] [PubMed] [Google Scholar]
- 32.Stroop J. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–662. [Google Scholar]
- 33.Folstein MF, Folstein SE, McHugh PR. Mini-mental State: A practical method for grading the cognitive state of patients for the clinicians. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 34.Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry. 2005;162:2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- 35.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 36.Gaser C, Altaye M, Wilke M, Holland SK. Unified segmentation without tissue priors. Neuroimage. 2007;36:S68. [Google Scholar]
- 37.Wilke M, Holland SK, Altaye M, Gaser C. Template-O-Matic: a toolbox for creating customized pediatric templates. Neuroimage. 2008;41:903–913. doi: 10.1016/j.neuroimage.2008.02.056. [DOI] [PubMed] [Google Scholar]
- 38.Buckholtz JW, Meyer-Lindenberg A, Honea RA, Straub RE, Pezawas L, Egan MF, Vakkalanka R, Kolachana B, Verchinski BA, Sust S, Mattay VS, Weinberger DR, Callicott JH. Allelic variation in RGS4 impacts functional and structural connectivity in the human brain. J Neurosci. 2007;27:1584–1593. doi: 10.1523/JNEUROSCI.5112-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boyke J, Driemeyer J, Gaser C, Buchel C, May A. Training-induced brain structure changes in the elderly. J Neurosci. 2008;28:7031–7035. doi: 10.1523/JNEUROSCI.0742-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bakkour A, Morris JC, Dickerson BC. The cortical signature of prodromal AD. Regional thinning predicts mild AD dementia. Neurology. 2009;72:1048–1055. doi: 10.1212/01.wnl.0000340981.97664.2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Apostolova LG, Steiner CA, Akopyan GG, Dutton RA, Hayashi KM, Toga AW, Cummings JL, Thompson PM. Three-dimensional gray matter atrophy mapping in mild cognitive impairment and mild Alzheimer disease. Arch Neurol. 2007;64:1489–1495. doi: 10.1001/archneur.64.10.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Friston KJ, Holmes AP, Price CJ, Buchel C, Worsley KJ. Multisubject fMRI studies and conjunction analyses. Neuroimage. 1999;10:385–396. doi: 10.1006/nimg.1999.0484. [DOI] [PubMed] [Google Scholar]
- 43.Friston KJ, Ashburner J. Generative and recognition models for neuroanatomy. Neuroimage. 2004;23:21–24. doi: 10.1016/j.neuroimage.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 44.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 45.Guimaraes HC, Levy R, Teixeira AL, Beato RG, Caramelli P. Neurobiology of apathy in Alzheimer’s disease. Arq Neuropsiquiatr. 2008;66:436–443. doi: 10.1590/s0004-282x2008000300035. [DOI] [PubMed] [Google Scholar]
- 46.Najlerahim A, Bowen DM. Regional weight loss of the cerebral cortex and some subcortical nuclei in senile dementia of the Alzheimer type. Acta Neuropathol. 1988;75:509–512. doi: 10.1007/BF00687139. [DOI] [PubMed] [Google Scholar]
- 47.Harasty JA, Halliday GM, Kril JJ, Code C. Specific temporoparietal gyral atrophy reflects the pattern of language dissolution in Alzheimer’s disease. Brain. 1999;122(Pt 4):675–686. doi: 10.1093/brain/122.4.675. [DOI] [PubMed] [Google Scholar]
- 48.Grignon Y, Duyckaerts C, Bennecib M, Hauw JJ. Cytoarchitectonic alterations in the supramarginal gyrus of late onset Alzheimer’s disease. Acta Neuropathol. 1998;95:395–406. doi: 10.1007/s004010050816. [DOI] [PubMed] [Google Scholar]
- 49.Kinkingnehun S, Sarazin M, Lehericy S, Guichart-Gomez E, Hergueta T, Dubois B. VBM anticipates the rate of progression of Alzheimer disease: a 3-year longitudinal study. Neurology. 2008;70:2201–2211. doi: 10.1212/01.wnl.0000303960.01039.43. [DOI] [PubMed] [Google Scholar]
- 50.Whitwell JL, Shiung MM, Przybelski SA, Weigand SD, Knopman DS, Boeve BF, Petersen RC, Jack CR., Jr MRI patterns of atrophy associated with progression to AD in amnestic mild cognitive impairment. Neurology. 2008;70:512–520. doi: 10.1212/01.wnl.0000280575.77437.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boly M, Balteau E, Schnakers C, Degueldre C, Moonen G, Luxen A, Phillips C, Peigneux P, Maquet P, Laureys S. Baseline brain activity fluctuations predict somatosensory perception in humans. Proc Natl Acad Sci U S A. 2007;104:12187–12192. doi: 10.1073/pnas.0611404104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nickel J, Seitz RJ. Functional clusters in the human parietal cortex as revealed by an observer-independent meta-analysis of functional activation studies. Anat Embryol (Berl) 2005;210:463–472. doi: 10.1007/s00429-005-0037-1. [DOI] [PubMed] [Google Scholar]
- 53.Benes FM, Swigar ME, Rothman SL, Opsahl C, Dowds M. CT scan studies of superficial cerebral regions: frequency and distribution of abnormalities in elderly psychiatric patients. Neurobiol Aging. 1983;4:289–295. doi: 10.1016/0197-4580(83)90005-2. [DOI] [PubMed] [Google Scholar]
- 54.Swigar ME, Benes FM, Rothman SL, Opsahl C, Dowds MM. Behavioral correlates of computed tomographic (CT) scan changes in older psychiatric patients. J Am Geriatr Soc. 1985;33:96–103. doi: 10.1111/j.1532-5415.1985.tb02273.x. [DOI] [PubMed] [Google Scholar]
- 55.Naito E, Ehrsson HH. Somatic sensation of hand-object interactive movement is associated with activity in the left inferior parietal cortex. J Neurosci. 2006;26:3783–3790. doi: 10.1523/JNEUROSCI.4835-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heilman KM, Rothi LJ, Valenstein E. Two forms of ideomotor apraxia. Neurology. 1982;32:342–346. doi: 10.1212/wnl.32.4.342. [DOI] [PubMed] [Google Scholar]
- 57.Ohgami Y, Matsuo K, Uchida N, Nakai T. An fMRI study of tool-use gestures: body part as object and pantomime. Neuroreport. 2004;15:1903–1906. doi: 10.1097/00001756-200408260-00014. [DOI] [PubMed] [Google Scholar]
- 58.Zimmerman ME, Brickman AM, Paul RH, Grieve SM, Tate DF, Gunstad J, Cohen RA, Aloia MS, Williams LM, Clark CR, Whitford TJ, Gordon E. The relationship between frontal gray matter volume and cognition varies across the healthy adult lifespan. Am J Geriatr Psychiatry. 2006;14:823–833. doi: 10.1097/01.JGP.0000238502.40963.ac. [DOI] [PubMed] [Google Scholar]
- 59.Solbakk AK, Alpert GF, Furst AJ, Hale LA, Oga T, Chetty S, Pickard N, Knight RT. Altered prefrontal function with aging: Insights into age-associated performance decline. Brain Res. 2008;1232C:30–47. doi: 10.1016/j.brainres.2008.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johannsen P, Jakobsen J, Bruhn P, Gjedde A. Cortical responses to sustained and divided attention in Alzheimer’s disease. Neuroimage. 1999;10:269–281. doi: 10.1006/nimg.1999.0475. [DOI] [PubMed] [Google Scholar]
- 61.du Boisgueheneuc F, Levy R, Volle E, Seassau M, Duffau H, Kinkingnehun S, Samson Y, Zhang S, Dubois B. Functions of the left superior frontal gyrus in humans: a lesion study. Brain. 2006;129:3315–3328. doi: 10.1093/brain/awl244. [DOI] [PubMed] [Google Scholar]
- 62.Scherder E, Eggermont L, Sergeant J, Boersma F. Physical activity and cognition in Alzheimer’s disease: relationship to vascular risk factors, executive functions and gait. Rev Neurosci. 2007;18:149–158. doi: 10.1515/revneuro.2007.18.2.149. [DOI] [PubMed] [Google Scholar]
- 63.Cahn-Weiner DA, Boyle PA, Malloy PF. Tests of executive function predict instrumental activities of daily living in community-dwelling older individuals. Appl Neuropsychol. 2002;9:187–191. doi: 10.1207/S15324826AN0903_8. [DOI] [PubMed] [Google Scholar]
- 64.Bell-McGinty S, Podell K, Franzen M, Baird AD, Williams MJ. Standard measures of executive function in predicting instrumental activities of daily living in older adults. Int J Geriatr Psychiatry. 2002;17:828–834. doi: 10.1002/gps.646. [DOI] [PubMed] [Google Scholar]
- 65.Royall DR, Chiodo LK, Polk MJ. Correlates of disability among elderly retirees with “subclinical” cognitive impairment. J Gerontol A Biol Sci Med Sci. 2000;55:M541–M546. doi: 10.1093/gerona/55.9.m541. [DOI] [PubMed] [Google Scholar]
- 66.Amieva H, Rouch-Leroyer I, Letenneur L, Dartigues JF, Fabrigoule C. Cognitive slowing and learning of target detection skills in pre-demented subjects. Brain Cogn. 2004;54:212–214. doi: 10.1016/j.bandc.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 67.Wadley VG, Okonkwo O, Crowe M, Ross-Meadows LA. Mild cognitive impairment and everyday function: evidence of reduced speed in performing instrumental activities of daily living. Am J Geriatr Psychiatry. 2008;16:416–424. doi: 10.1097/JGP.0b013e31816b7303. [DOI] [PubMed] [Google Scholar]
- 68.Boxer AL, Garbutt S, Rankin KP, Hellmuth J, Neuhaus J, Miller BL, Lisberger SG. Medial versus lateral frontal lobe contributions to voluntary saccade control as revealed by the study of patients with frontal lobe degeneration. J Neurosci. 2006;26:6354–6363. doi: 10.1523/JNEUROSCI.0549-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]