Abstract
Endothelin converting enzyme-2 is a metalloprotease that possesses many properties consistent with it being a neuropeptide processing enzyme. This protease is found primarily in neural tissues with high levels of expression in midbrain, cerebellum, hypothalamus, frontal cortex, and spinal cord, and with moderate levels in hippocampus and striatum. To evaluate its role in neural function, mice have been generated lacking this enzyme. Physical appearance, autonomic reflexes, motor coordination, balance, locomotor activity, and spontaneous emotional responses appear normal in these knockout mice. However, these mutants display deficits in learning and memory as evidenced by marked impairment in the Morris water maze. Knockout mice are deficient also in object recognition memory where they show delays in discerning changes in object location, as well as in recognizing the introduction of a novel object. Here, perseveration appears to interfere with learning and memory. Finally, mutants are impaired in social transmission of food preference where they show poor short-term memory and perturbations in long-term memory; the latter can be ameliorated by reminder cues. As endothelin converting enzyme-2 has been implicated in Alzheimer’s disease, the deficits in learning and memory in the knockout mice may provide unique insights into processes that may contribute to this disease and possible other disorders of cognition.
Keywords: Post-translational processing, neuropeptide biosynthesis, metalloendoproteases, learning, memory, hippocampus, mutant mice
Introduction
Neuroendocrine peptides are intercellular messengers that play critical roles integrating nervous, reproductive, endocrine, cardiovascular, gastrointestinal, pulmonary, and behavioral functions (Strand et al., 1999). Peptides are synthesized as large precursor proteins that undergo multiple posttranslational processing steps to generate bioactive peptides. Typically, neuropeptide processing begins with endoproteolytic cleavage by prohormone convertases at the C-terminal side of mono- or dibasic residues (i.e., -KR, -RR, -RXXR-), considered “classical” sites of cleavage (Docherty & Steiner, 1982).
Some neuropeptides are generated by processing at “non-classical” sites. These peptides are found among bulk-purified peptides from neuroendocrine tissues and are detected in brains of mice lacking specific processing enzymes (Mizuno et al., 1980; Yang et al., 1985; Sigafoos et al., 1993; Che et al., 2001). Additionally, an examination of precursor sequences from some endogenous peptides further supports the idea that “non-classical” processing sites are used for generation of bioactive peptides (Perry et al., 1997; Vilim et al., 1999; Liu et al., 2001).
Members of the metalloprotease family have long been implicated in processing at non-classical sites (Molineaux & Wilk, 1991). Endothelin converting enzyme-1 (ECE-1) and ECE-2 possess substrate specificity consistent with processing at non-classical sites and ECE-2 has additional properties consistent with those of a neuropeptide processing enzyme. For instance, ECE-2 is optimally active at pH 5.5, is localized to intracellular secretory compartments (Emoto & Yanagisawa, 1995; Russell & Davenport, 1999; Davenport & Kuc, 2000), and exhibits restricted neuroendocrine distribution (Nakagomi et al., 2000; Yanagisawa et al., 2000). Recently, a panel of 42 peptides was screened as substrates for ECE-2 (Mzhavia et al., 2003). Processing occurred for 10 of these peptides at acidic pH, a pH consistent with the milieu of secretory vesicles (Moore et al., 2002). Thus, ECE-2 may process a number of peptide intermediates leading to a variety of endogenous ligands. Interestingly, studies characterizing intracellular degradation of β-amyloid peptides have suggested a role for ECE-2 in Aβ clearance in vivo (Eckman et al., 2003). Thus, ECE-2 may play a role in neurodegenerative disorders such as Alzheimer’s disease (Eckman & Eckman, 2005; Weeraratna et al., 2007).
Mice lacking active ECE-2 have been generated by replacing the exon encoding the conserved zinc-binding motif with a neo-cassette using homologous recombination (Yanagisawa, et al., 2000). The mutants are viable and live a normal life-span. As ECE-2 knockout (KO) mice are fertile and show no gross developmental defects, they are ideally suited for evaluation of the role of ECE-2 in neuropeptide processing and behavior. In the present study, we have examined the behavioral phenotype of KO mice and find they possess no gross behavioral abnormalities, but are impaired on various tests of learning and memory.
Materials and methods
Animals
Adult naïve male and female wild type (WT) and KO mice (age 12–20 wks) were used in all experiments. Mice were propagated by heterozygous C57BL6/J-129/SvJ matings and were genotyped by PCR using the following primers: WT ECE-2 allele (sense) 5'-GCCATCTTACAGTAGAGGAG-3' and (antisense) 5'-CTAGAATGGGCCCCTACCTT-3', and KO allele: (sense) 5'-TATTCGGCTATGACTGGGCACAACAG-3' and (antisense) 5'-TTCCACCATGATATTCGGCAAGCAGG-3'. Offspring were weaned after 21 days, segregated by sex and genotype, and housed 2–5 mice/cage. Animals were maintained under a 14:10 hr light/dark cycle in a humidity- and temperature-controlled room with water and laboratory chow supplied ad libitum. All experiments were conducted in accordance with NIH guidelines to minimize potential pain or discomfort and under approved protocols from the Institutional Animal Care and Use Committees at Mount Sinai School of Medicine and Duke University.
RNA isolation and real-time PCR
Mice were euthanized by carbon dioxide asphyxiation and brain regions were dissected. Total RNA was isolated using the RNeasy® Mini kit (Qiagen, Valencia, CA) and reverse transcription was performed using Superscript II® and random hexamer primers (Invitrogen, Carlsbad, CA). The primer sequences for ECE-2 were (sense) 5’-CTTACTACCTTCCAAC-3’ and (antisense) 5’-GTCAGTGACTCATTCT-3’, and (sense) 5’-AATGAAATCGTCTTCC-3’ and (antisense) 5’-GTCAGTGACTCATTC-3’; primers for ECE-1 were (sense) 5’-ATCAGTGGAGTATGAC-3’ and (antisense) 5’-CCTTGATCATCGAAAG-3’; primers for neutral endopeptidase (NEP) were (sense) 5’-CGAAATCAGATAGTCT-3’ and (antisense) 5’-GTCTCCATCTTTATTG-3’; and primers for β-actin (internal control) were (sense) 5’-ACCCACACTGTGCCCATCTA-3’ and (antisense) 5’-TCGGAACCGCTCGTTGCCA-3’. The ECE-2, ECE-1, and NEP PCR products were 150–200 bp and they spanned the Zn+2-binding and catalytic domains. Real-time PCR was performed on a LightCycler II (Roche Applied Science, Indianapolis, IN) using the QuantiTect® SYBR® Green PCR kit (Qiagen, Valencia, CA) where the initial activation step (95°C for 15 min) was followed by 45 cycles of PCR [95°C for 15 sec; 50°C or 47°C (for NEP) for 25 sec; 72°C for 10 sec]. Detection of fluorimetric SYBR Green (i.e., amount of PCR product) was measured at the end of each elongation phase. The specificity of products was documented at the end of each run with a melting curve analysis. The threshold cycle (i.e., cycle number at which fluorescence corresponding to appearance of the amplified PCR product) was used to calculate the starting template amount in each sample. Expression of each gene was normalized to β-actin. Serial 10-fold dilutions (i.e., 100 to 0.0001 pg) of the murine ECE-2 plasmid were used to generate the standard curve to calculate the copy number. The relative expression of ECE-2, ECE-1, and NEP in different samples was determined by the 2−ΔΔCT method (Livak & Schmittger, 2001) using the maximum level of expression (i.e., midbrain for ECE-2, liver for ECE-1 and kidney for NEP as calibrators).
Behavioral assessments
Neurophysiological, emotional, and sensorimotor-gating screen
This screen involved an initial evaluation, as well as, examinations of various behaviors as described (Pillai-Nair et al., 2000; Ribar et al., 2000; Pogorelov et al., 2005; Rodriguiz & Wetsel, 2006; Fukui et al., 2007).
Morris water maze
This test was conducted as outlined (Wolfer et al., 1999; Rodriguiz & Wetsel, 2006) in a 120 cm diameter stainless steel tub filled with opaque water (25°C). A white metal platform (12 cm in diameter) was located approximately 1 cm below the surface of the water and 20 cm from the wall of the maze. Lighting was indirect (~350 lux) and behavior was monitored with a video-camera located 140 cm above the pool. Total swim distance, latency to locate the platform, and swim velocity were calculated from tracking profiles created by Ethovision (Noldus Information Technology, Leesburg, VA).
Object recognition
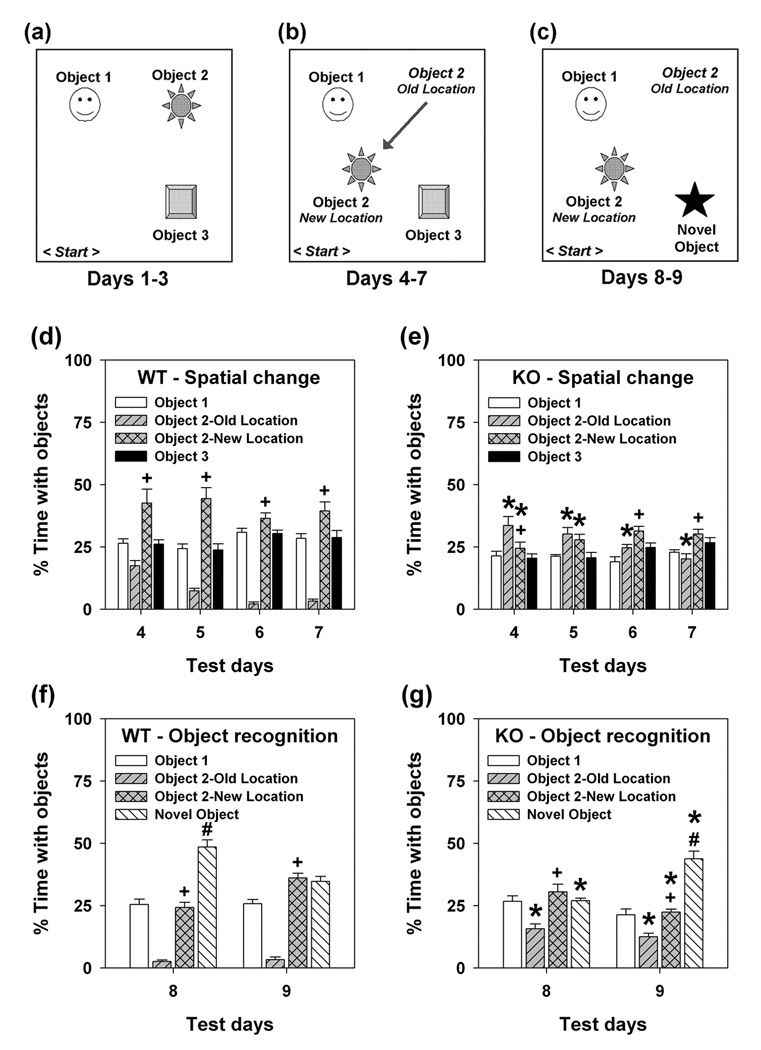
This test was conducted across 9 consecutive days (see Mumby et al., 2002; Pogorelov et al., 2005). On days 1–3 the objects were arranged in a triangular configuration (Fig. 1a). On day 4 the location of object 2 was changed and this was maintained until day 7 (Fig. 1b). On days 8–9 object 3 was replaced with a novel object (Fig. 1c). Object exploration included all instances of sniffing, touching, climbing, rearing near, sitting on and time spent with the object. Additionally, on days 4–9 all instances and times sniffing or rearing in the old location of object 2 were scored. All tests were video-taped and coded with trained observers using Observer (Noldus Information Technologies) who were blind to the genotype of the animals.
Figure 1. Schematic of procedure and object recognition memory for WT and KO mice.
(a) Days 1–3: acclimatization. (b) Days 4–7: spatial change. (c) Days 8–9: novel-object recognition. (d–e) Percent time spent in object contact for WT (d) and KO mice (e) after object 2 had been moved to a new location. An omnibus RMANOVA found main effects of objects [F3,153 = 37.668, P<0.001], and the objects by genotype [F3,153 = 41.226, P<0.001] and the days by objects by genotype interactions to be significant [F9,153 = 3.761, P<0.007]. Bonferroni corrected pair-wise comparisons found that on day 4, WT mice increased exploration of object 2 relative to objects 1 and 3 and to the old location of object 2 (Ps<0.038). This behavior was maintained though day 7 (Ps<0.050) and the percent time spent in the previous location of object 2 decreased significantly on days 5–7 relative to that on day 4 (Ps<0.027). By comparison, percent time spent with object 2 was not increased for KO mice. Instead, mutants spent more time exploring the old location of object 2 than objects 1–3 (Ps<0.021). Exploration of object 2 by mutants increased compared to object 1 (Ps<0.026) on days 5–7 and to object 3 on days 5–6 (Ps<0.051). Unlike WT controls, ECE-2 mice increased exploration of the old location of object 2 on days 4–7 (Ps<0.001) and spent less time exploring object 2 in its new location on days 4–5 (Ps<0.020). (f–g) Percent time in object contact for WT (f) and KO mice (g) after object 3 was substituted with a novel object. An omnibus RMANOVA revealed main effects of objects [F3,51 = 103.112, P<0.001], and the objects by genotype [F3,51 = 25.778, P<0.001] and days by objects by genotype interaction to be significant [F3,51 =17.778, P<0.001]. Bonferroni comparisons showed that on day 8, WT mice increased exploration of the novel relative to objects 1 and 2 (Ps<0.001). However, by day 9 the percent time spent exploring the novel object was no different than that for the two familiar objects. On both days, the percent time spent in the old location was reduced relative to that for object 2 (Ps<0.001). By comparison, KO mice failed to show any increase in the percent time spent exploring the novel object on day 8, but by day 9 there was a marked increase in exploration of the novel compared to the two familiar objects (Ps<0.001). Nonetheless, mutants spent more time in the old location of object 2 (Ps<0.011) on days 8–9 than WT controls. n = 9 WT and 10 KO mice. *P<0.05, WT compared to KO animals; ^P<0.05, percent total time spent with objects on days 2 and 3 compared to day 1, or days 5–7 compared to day 4; +P<0.05, object 2 new location versus its old location; #P<0.05, novel object versus object 1 and object 2 in its new location.
Social transmission of food preference (STFP)
This test has been outlined (Kogan et al., 2000; Rodriguiz & Wetsel, 2006) where learning and memory were evaluated in tester mice at 20 min and 24 hr. The STFP test was run under 2 different protocols. In one, the demonstrator mouse remained in the home-cage with the tester mice. In the other, the demonstrator was removed from the home-cage following the initial 20 min interaction with tester mice (e.g., after the 20 min test). All bowls were weighed before and after each test, and any spillage was recovered. Dietary preference was calculated as the difference in consumption between the two diets relative to total consumption.
Statistics
Statistical analyses were performed with the SPSS-11 statistical programs (SPSS Inc., Chicago, IL) and all data were presented as means and standard errors of the mean. As no sex differences were detected in any behavioral test; data for males and females were combined. For the neurophysiological screen, genotype differences were examined with χ2 or t-tests. For the STFP and object recognition tests, pre-exposure data were evaluated by univariate ANOVA. Prepulse-dependent inhibition was examined with repeated measures ANOVA (RMANOVA) with prepulse intensity as the within-subjects and genotype as the between-subjects factors. Morris water maze data were analyzed by RMANOVA, with test day as the within-subjects and genotype as the between-subjects effects for hidden- and visible-platform testing. Probe test analyses by RMANOVA required two within-subjects effects: test day and maze quadrant, and the between-subjects effect of genotype. For object preference, percent object contact was examined with RMANOVA with test day and object type or location as the within-subjects and genotype as the between-subjects effects. Finally, preference scores for STFP were examined with RMANOVA with time (20 min, 24 hr) as the within-subjects and genotype as the between subjects effects. For ANOVA and RMANOVA, Bonferroni corrected pair-wise comparisons were used. In all cases, P<0.05 was considered significant.
Results
Distribution of ECE-2 suggests a neuroendocrine role
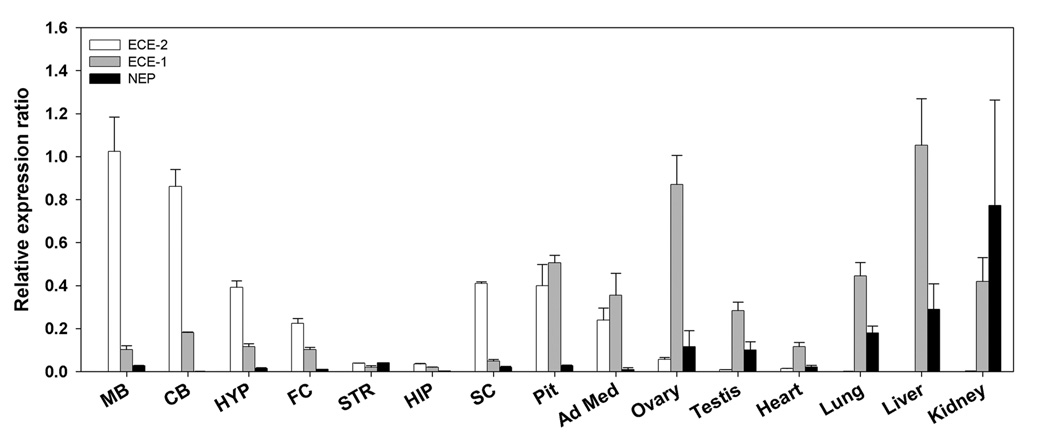
Expression of ECE-2 mRNA was examined in adult WT tissues by quantitative real-time PCR. Consistent with previous reports (Emoto & Yanagisawa, 1995; Yanagisawa, et al., 2000), ECE-2 exhibited primarily a neuroendocrine distribution with very little expression in peripheral tissues (Table 1). Midbrain and cerebellum contained the highest levels in brain, followed by pituitary, hypothalamus, and frontal cortex. Striatum and hippocampus had low levels of ECE-2 mRNA. High levels were detected also in spinal cord and adrenal medulla, with lower levels in ovaries and heart. By contrast, ECE-1 and NEP mRNAs were expressed at higher levels in peripheral tissues; comparable levels of ECE-1 and ECE-2 were found in the pituitary and adrenal medulla (Fig. 2). Together, the distribution pattern of ECE-2 expression in neuroendocrine tissues suggests a role for this enzyme in processing neuropeptides.
Table 1.
Expression of ECE-2 mRNA in brain and peripheral tissues
| Expression of ECE-2 mRNA | |
|---|---|
| Tissue1 | Copy number/µl2 |
| Midbrain | 70,470 ±6,120 |
| Cerebellum | 61,319 ±6,184 |
| Spinal cord | 29,713 ±1,870 |
| Hypothalamus | 27,683 ±8,894 |
| Prefrontal cortex | 16,207 ±971 |
| Hippocampus | 3,299 ±559 |
| Striatum | 3,211 ±612 |
| Pituitary | 35,076 ±18,235 |
| Adrenal medulla | 17,875 ±5,937 |
| Ovaries | 4,385 ±434 |
| Testis | 972 ±261 |
| Heart | 1,869 ±806 |
| Lung | 129 ±129 |
| Kidney | 115 ±71 |
| Muscle | 115 ±71 |
| Liver | 47 ±27 |
Expression of ECE-2 was normalized to that of β-actin and compared to a standard curve of serially diluted plasmids containing known-concentrations of the ECE-2 cDNA.
n=3 mice.
Figure 2. Comparison of expression profiles of ECE-2, ECE-1, and NEP mRNA in various brain regions and peripheral tissues.
The expression profiles are calculated as the ratio of enzyme to β-actin mRNA and normalized to the maximum expression in midbrain for ECE-2, liver for ECE-1, and kidney for NEP. Abbreviations: MB (midbrain), CB (cerebellum), HYP (hypothalamus), FC (frontal cortex), STR (striatum), HIP (hippocampus), SC (spinal cord), Pit (pituitary), Ad Med (adrenal medulla). n = 3 mice/genotype.
Disruption of ECE-2 exerts no effects on gross behavior
No genotype differences were observed in assessments of physical appearance and sensory and motor functioning (Supplemental Table 1). Responses in tail-flick, zero maze, tail suspension, transfer arousal, and prepulse inhibition (PPI) were similar for WT and KO mice. Together, these results show that many behaviors are intact in the mutants.
KO mice show poor memory consolidation
Morris water maze
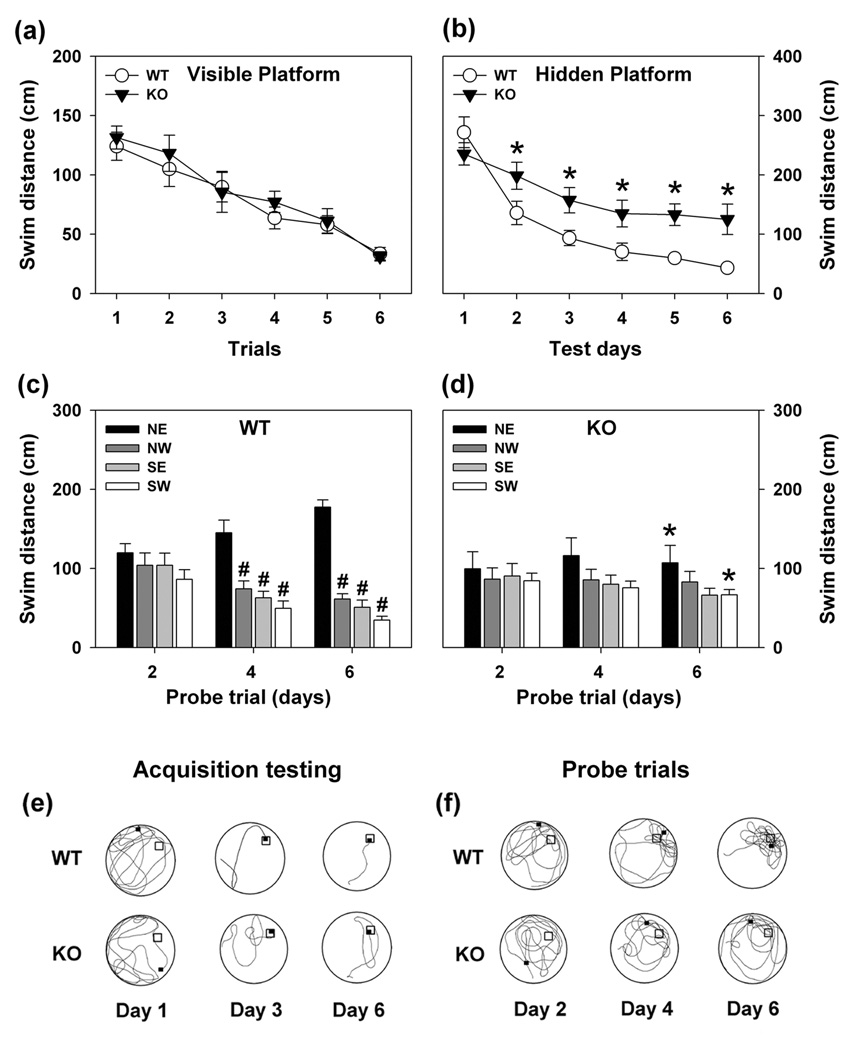
In the visible-platform test, no genotype differences emerged (Fig 3a, Fig S1a, Fig S2a). With the hidden platform version of the test, KO animals were impaired (Fig. 3b). WT mice reduced their swim distances across test days, whereas mutants showed little change over time. Tracings of swim patterns on test days 1, 3, and 6 confirmed KO mice swam further than WT controls (Fig. 3e). Moreover, KO animals swam in repetitive looping patterns. Swim time was also aberrant for KO animals (Fig. S1b). Analyses of swim distances for probe trials on days 4 and 6 confirmed that WT animals preferred the quadrant where the hidden platform had been located (Fig. 3c). By contrast, KO mice showed no preference for any quadrant on any probe trial and, by the last test day, mutants swam less far in the north-east (NE) and further in the south-west quadrant than WT controls (Fig. 3c,d). Swim tracings from each probe trial showed that WT mice preferentially searched in the NE quadrant on trials 4 and 6 (Fig. 3f). KO animals displayed broader search strategies and continued to circle through other quadrants on these trials. Swim times during probe trials reflected similar deficiencies as swim distance in the mutants (Fig. S1c–d). Although swim velocities did not differ between genotypes (Fig. S2b–d), the swim distance and swim time results indicate spatial learning and memory are impaired in KO mice.
Figure 3. Swim distances of WT and KO mice in the Morris water maze.
(a) Swim distances for WT and KO mice during visible platform testing. RMANOVA revealed significant main effects of test trial [F5,90 = 6.392, P<0.001]; the test trial by genotype interaction was not significant. Bonferroni corrected pair-wise comparisons revealed that on trials 3–6 all mice swam significantly shorter distances relative to trial 1 (Ps<0.046). (b) Swim distances for WT and KO mice during 6 days of acquisition testing with the hidden platform. The RMANOVA within-subjects tests demonstrated significant effects of test day [F5,100 = 20.397, P<0.001] and a significant test day by genotype interaction [F5,100 = 6.527, P<0.001]. Bonferroni comparisons showed that although WT and KO animals did not differ on day 1 (P=0.773), mutants had consistently longer swim distances on test days 2–6 (Ps<0.048). In addition, WT mice showed reductions in swim distance on each subsequent test day compared to day 1 (Ps<0.016), whereas no changes over days were observed for KO mice. (c–d) Swim distances during probe trials following acquisition testing on days 2, 4, and 6 for WT (c) and KO mice (d). RMANOVA revealed significant within-subjects effects for probe trial [F2,120 = 6.441, P<0.004] and quadrant [F3,120 = 32.515, P<0.001], and a significant probe trial by quadrant by genotype interaction [F6,120 = 4.117, P<0.006]. Bonferroni comparisons failed to discern differences between WT and mutants on the first probe trial on day 2. However, WT mice swam further in the NE quadrant on days 4 and 6, relative to the other three quadrants (Ps<0.031). By comparison, mutants failed to show any quadrant preferences on any of the three probe trials, and by the final probe trial they swam shorter distances in the NE (P<0.011) and longer distances in the SW quadrant than WT mice (P<0.047). (e) Tracings of representative swim patterns for WT and KO mice on test days 1, 3, and 6 when the hidden platform was located in the NE quadrant. (f) Tracings of representative swim patterns for WT and KO mice during probe trials on days 2, 4, and 6. The apex of the water maze in each tracing is north (N) and the platform location is designated by an open square. The closed square represents where the mouse was located at the end of the trial. n = 10 mice/genotype for visible platform trials, n = 11 mice/genotype for hidden platform and probe trials. *P<0.05, WT compared to KO mice; #P<0.05, NE compared to NW, SE or SW quadrants.
Object recognition testing
During acclimatization, no genotype differences were observed (Fig. S3a, left) and the percent times spent contacting each object were similar (Fig. S3b–c). When object 2 was moved to a new location (day 4), animals spent more time contacting all objects (Fig. S3a, middle). Subsequently, the total time contacting objects decreased for WT, but remained elevated for KO animals. WT mice increased their percent time with the moved object 2 on days 4–7 (Fig. 1d), compared to the other two objects and time spent in the old location of object 2. Although mutants explored the displaced object, the percent time spent in its old location was increased (Fig. 1e). By days 6–7, KO animals spent more time with object 2 than its old location. Nevertheless, exploration of the old location remained high. When object 3 was replaced with a novel object, WT animals increased exploration time for all objects (Fig. S3a, right); mutants continued to have high rates of object exploration. Hence on day 8, WT mice enhanced their percent exploration of the novel object (Fig. 1f), whereas KO mice reduced time spent exploring the old location of object 2 and spent equal amounts of time with all three objects (Fig. 1g). On day 9 WT mice reduced their percent time spent with the novel object (Fig. 1f), whereas mutants markedly increased its exploration (Fig. 1g). Collectively, these data indicate that KO mice are impaired in spatial learning and memory, and they show preservation to the “old” location of object 2. This response may delay the ability of mutants to recognize the introduction of the novel object on day 8.
STFP
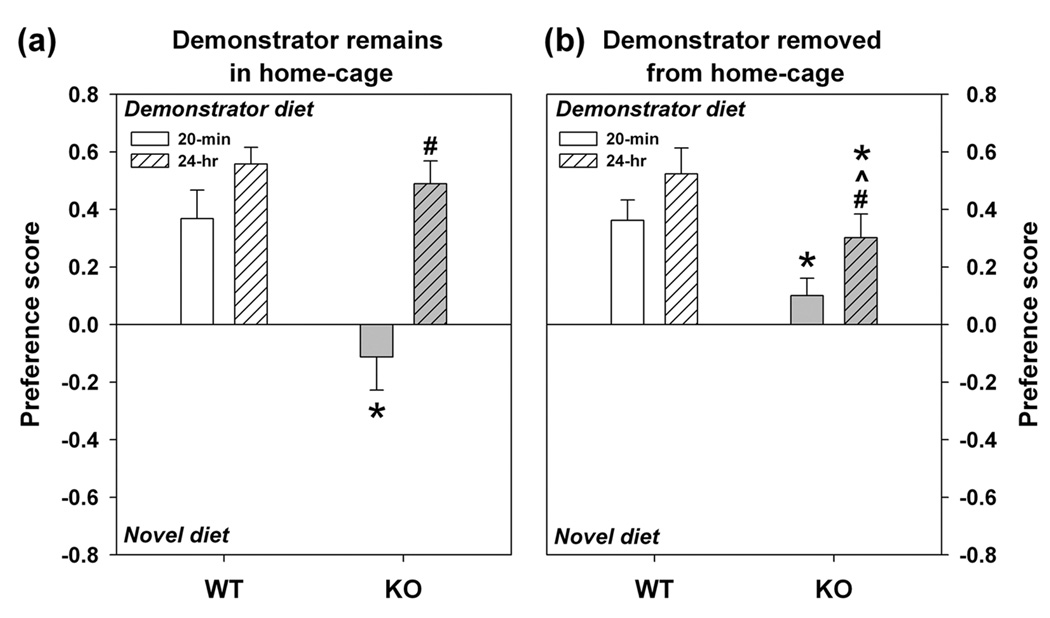
Further studies of learning and memory were instituted with the STFP test. When the demonstrator was housed continuously with tester animals, WT mice showed a preference for the familiar diet at 20 min and maintained this preference 24 hrs later (Fig. 4a). KO animals failed to develop a preference at 20 min, but preferred the familiar diet at 24 hrs -- similar to the WT controls. One reason for the result at 24 hr may be due to the persistence of reminder cues provided by the demonstrator in the home-cage. To evaluate this possibility, the demonstrator was removed at the time of the 20 min test and remained in a separate cage until the end the study. Here, WT mice continued to show strong preferences for the familiar diet at 20 min and 24 hrs (Fig. 1b). KO mice again showed no preference at 20 min, but by 24 hrs they displayed a greatly weakened preference for the familiar diet compared to WT controls. Additionally, regardless of demonstrator presence, no genotype differences were discerned in latencies to contact and eat the diets or in frequencies of bowl contacts at 20 min or 24 hrs in the presence (Fig. S4) or absence of the demonstrator (Fig. S5). Together, these data suggest that acquisition of memory is slower in KO than WT mice and that reminder cues (i.e., demonstrator animal) significantly improve memory consolidation.
Figure 4. Learning and memory in WT and KO mice in the STFP test.
(a) When tester mice have access to demonstrator animals between the 20 min and 24 hr test, WT mice show strong preferences for the demonstrator diet at both the short- and long-term intervals. KO animals fail to exhibit a preference for the demonstrator diet at 20 min, but show a preference for this diet at 24 hrs, similar to the WT controls. (b) When access to demonstrator mice is restricted to 20 min before the short-interval test, WT mice show strong preferences for the familiar demonstrator diet at the 20 min and 24 hr tests. Under these conditions, KO animals show reduced preference for the demonstrator diet at both the 20 min and 24 hr tests. An ominibus RMANOVA revealed a main effect for test interval [F1,33 = 18.708, P<0.001], and significant test interval by genotype [F1,33 = 6.319, P<0.017] and test interval by genotype by paradigm (i.e., presence or absence of demonstrator) interactions [F1,33 = 8.934, P<0.005]. Bonferroni corrected pair-wise showed that, when the demonstrator mouse was housed with the tester mice between tests (panel a), mutants had reduced preference for the familiar diet at 20 min relative to WT controls (P<0.007), but not at the 24 hr test. When the demonstrator mouse was removed immediately before testing (panel b), KO mice also showed reduced preference for the familiar diet at 20 min and at 24 hr relative to WT littermates (Ps<0.030). Strikingly, preference for the familiar diet was lower for mutants at 24 hrs that had been housed without than those housed with the demonstrator (P<0.036). n = 9–10 WT and 10 KO mice/test. *P<0.05, WT compared to KO mice; #P<0.05, preference at 20 min test compared to 24 hr test; ^P<0.05, preference at 24 hr in presence or absence of demonstrator mice.
Discussion
Neuropeptides play important roles in normal brain function. In the present study, ECE2 mRNA is highly expressed in midbrain, cerebellum, hypothalamus, and frontal cortex, with lower levels in striatum and hippocampus. The high expression of ECE-2 in cerebellum and midbrain suggests that mutants may be impaired in balance, motor coordination, and/or motor learning (Houk & Wise, 1995). However, KO mice failed to demonstrate conspicuous disturbances in these behaviors in the neurophysiological screen, rotorod, and open field. Besides these responses, the cerebellum is implicated in encoding long-term memory and recognition memory (Weis et al., 2004). Hence, some of the defects in learning and memory with KO mice may be partially attributed to some undisclosed cerebellar dysfunction.
Besides cerebellum, we found ECE-2 to be present at moderate levels in hippocampus. An in situ hybridization study has shown that ECE-2 mRNA in rat hippocampus is restricted to the granule cell layer of the dentate gyrus (Nakagomi et al., 2000). In mice, ECE-2 mRNA expression in hippocampus is confined also to this cell layer (http://www.brain-map.org). Hence, in our biochemical experiment the use of the entire hippocampus, rather than the dentate gyrus alone, probably underestimates the quantities of ECE-2 in this brain region.
The critical role of the hippocampus and related temporal lobe areas in learning and memory is well-known (Eichenbaum & Cohen, 2001). Loss of ECE-2 in KO hippocampus, along with the correlated anomalies in the Morris water maze, suggests this gene may play a salient role in spatial learning and memory. In our studies, KO mice are impaired on the hidden-platform version of the task, whereas performance with the visible platform is indistinguishable from that of WT controls. These latter findings and those with the neurophysiological screen and PPI experiment indicate that sensorimotor functions are intact in KO animals. Nevertheless, it is noteworthy that rodents with hippocampal lesions (Eichenbaum et al., 1990) or genetic disruption of hippocampally-expressed genes (Nakazawa et al., 2004; Pittenger et al., 2002) retain the ability to swim to a cued location, but are unable to learn the location of the hidden platform. Additionally, these animals typically engage in longer swim paths with more near-misses of the hidden platform than controls. ECE-2 KO mice show a similar proclivity; however, they appear more impaired in the water maze than mere functional disruption of the dentate gyrus suggests (see Niewoehner et al., 2007). Since ECE-2 is expressed in various areas of cerebral cortex and cerebellum (http://www.brain-map.org), it may be the case that ablation of Ece-2 in these additional brain regions may contribute to their impairment (see D’Hooge & De Deyn, 2001).
Besides water maze, mutants are deficient also in object recognition memory. They are slower to recognize shifts in object location, as well as change from a familiar to a novel object. Interestingly, percent exploration time for all objects is increased when the familiar object is moved or when a familiar object is exchanged for a novel one. This increase in percent time with objects suggests that both genotypes recognize the configuration or appearance of objects has changed; however, because mutants spend increased time at the previous location for object 2, preservation appears to interfere with their learning and memory. These findings are consistent with restricted expression of ECE-2 to the dentate gyrus since this hippocampal area is known to play a critical role in forming distinct representations of information in the environment, such as locations or comparisons between contexts (McHugh et al., 2007).
A possible contribution to the learning and memory impairment in KO animals may be due to perseveration. For instance, rats with fornix transections display similar deficiencies in the water maze and have difficulty shifting their search strategies to new locations when the hidden platform is moved to another quadrant (Whishaw & Tomie, 1997). Although we did not change the position of the hidden platform because levels of WT and KO performance were not equivalent at the end of acquisition, responses to the moved object location suggest the mutants have difficulty suppressing previous responses. Nevertheless, perseveration to the previous location suggests that rudimentary place or spatial learning can occur in KO animals, but their ability to solve new problems within the same test context is impaired. In this regard, extensive training of rodents with hippocampal lesions can be sufficient to overcome deficiencies in spatial learning and memory (Eichenbaum et al., 1990); however, when place cues surrounding the maze are moved or the starting location of the animals is varied, lesioned animals resort to perseveration. Thus, our results suggest that KO mice are capable of very simple learning in novel contexts, but once knowledge of context is established, they have difficulty integrating new information with pre-existing memories.
Mice were tested also in STFP; a test that relies upon intact hippocampal functioning (Bunsey & Eichenbaum, 1995). In ablation experiments, animals with hippocampal lesions prefer the familiar diet in the 20 min test, whereas this preference is lost at 24 hrs. This type of deficit has been attributed to impairments in consolidation and recall (Eichenbaum & Cohen, 2001). KO mice are deficient in short-term but long-term memory is relatively intact when the demonstrator remains with the tester mouse. Removal of the demonstrator, results in greatly weakened long-term memory. These results suggest that KO animals are not deficient in formation of long-term memory per se, but processes underlying short-term and/or working memory may be perturbed, leading to delay in memory formation. When reminder cues (i.e., demonstrator animal) are provided, mutants show intact long-term memory suggesting consolidation is protracted. Interestingly, somewhat similar deficits are found in geriatric amnesiac syndromes in humans (Woods et al., 1982) and in rats with surgical lesions of the dentate gyrus (Geinsman et al., 1986). In these cases, there is a selective failure to retain new information while retrieval of older established-memories is preserved. A loss of hippocampal integrity and neural plasticity may be due partially to slowed learning and delay of recall in these patients -- although loss of plasticity in prefrontal cortex may contribute to cognitive decline (Laroche et al., 2000). Nevertheless, temporal aspects of memory consolidation may be detrimentally affected in geriatric dementia patients (Pomara et al., 2004). These findings are interesting in that ECE-1 and ECE-2 have been implicated in limiting the accumulation of β-amyloid peptides in brain and levels of β-amyloid are increased in KO brain (Eckman et al., 2003). Amyloid deposition in mice is known to impair memory consolidation and selectively reduce expression of early immediate genes implicated in synaptic plasticity (Dickey et al., 2004). A recent report has suggested that Alzheimer’s disease and the genes for β-amyloid catabolism and ECE-2 may be linked (Saido & Iwata, 2005). Hence, elucidation of basic mechanisms underlying the deficits in learning and memory of ECE-2 KO mice may provide new insights into processes that contribute to Alzheimer’s disease and possible other disorders of memory function.
Supplementary Material
The following supplementary material is available for this article online from http://www.blackwell-synergy.com/doi/full/10.1111/j.1601-183X.2007.00365.x
Appendix S1 Behavioural assessments, Table S1 (Results from general behavioral assessments of WT and KO mice) and full figure legends.
Figure S1 Swim times of WT and KO mice in the Morris water maze.
Figure S2 Swim velocities of WT and KO mice in the Morris water maze.
Figure S3 Percent time spent with objects for WT and KO mice.
Figure S4 Interactions with the familiar- and novel-flavored diets during the 20 min and 24 hr tests by ECE-2 mice with the demonstrator remaining in the home-cage.
Figure S5 Interactions with the familiar- and novel-flavored diets during the 20 min and 24 hr tests by ECE-2 mice with the demonstrator removed from home-cage.
Acknowledgements
We would like to thank Rosalie Bateson, Sandeep Bhave, Liping Du, John Gilbert, Lien Nguyen, Lindsey Phillips, John Wilkins, and Jiechun Zhou for their assistance in the behavioral tests at Duke. This work was funded in part through unrestricted funds to Dr. William C. Wetsel and by NIH grants (NS026880 and DA019521) to Dr. Lakshmi Devi. This work was supported in part by the Mount Sinai Phenotyping Shared Resource Facility.
References
- Bunsey M, Eichenbaum H. Selective damage to the hippocampal region blocks long-term retention of a natural and nonspatial stimulus-stimulus association. Hippocampus. 1995;5:546–556. doi: 10.1002/hipo.450050606. [DOI] [PubMed] [Google Scholar]
- Che FY, Yan L, Li H, Mzhavia N, Devi LA, Fricker LD. Identification of peptides from brain and pituitary of Cpefat/Cpefat mice. Proc Natl Acad Sci USA. 2001;98:9971–9976. doi: 10.1073/pnas.161542198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport AP, Kuc RE. Cellular expression of isoforms of endothelin-converting enzyme-1 (ECE-1c, ECE-1b and ECE-1a) and endothelin-converting enzyme-2. J Cardiovasc Pharmacol. 2000;36:S12–S14. doi: 10.1097/00005344-200036051-00006. [DOI] [PubMed] [Google Scholar]
- D’Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- Dickey CA, Gordon MN, Mason JE, Wilson NJ, Diamond DM, Guzowski JF, Morgan D. Amyloid suppresses induction of genes critical for memory consolidation in APP + PS1 transgenic mice. J Neurochem. 2004;88:434–442. doi: 10.1111/j.1471-4159.2004.02185.x. [DOI] [PubMed] [Google Scholar]
- Docherty K, Steiner DF. Post-translational proteolysis in polypeptide hormone biosynthesis. Annu Rev Physiol. 1982;44:625–638. doi: 10.1146/annurev.ph.44.030182.003205. [DOI] [PubMed] [Google Scholar]
- Eckman EA, Watson M, Marlow L, Sambamurti K, Eckman CB. Alzheimer’s disease β-amyloid peptide is increased in mice deficient in endothelin-coverting enzyme. J Biol Chem. 2003;278:2081–2084. doi: 10.1074/jbc.C200642200. [DOI] [PubMed] [Google Scholar]
- Eckman EA, Eckman CB. A β-degrading enzymes: modulators of Alzheimer's disease pathogenesis and targets for therapeutic intervention. Biochem Soc Trans. 2005;33:1101–1105. doi: 10.1042/BST20051101. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Stewart C, Morris RGM. Hippocampal representation in spatial learning. J Neurosci. 1990;10:3531–3542. doi: 10.1523/JNEUROSCI.10-11-03531.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. From Conditioning to Conscious Reflection: Memory Systems of the Brain. New York, NY: Oxford University Press; 2001. [Google Scholar]
- Emoto N, Yanagisawa M. Endothelin-converting enzyme-2 is a membrane-bound, phosphoramidon-sensitive metalloprotease with acidic pH optimum. J Biol Chem. 1995;270:15262–15268. doi: 10.1074/jbc.270.25.15262. [DOI] [PubMed] [Google Scholar]
- Fukui M, Rodriguiz RM, Zhou J, Jiang SX, Phillips LE, Caron MG, Wetsel WC. Vmat2 heterozygous mutant mice display a depressive-like phenotype. J Neurosci. 2007;27:10520–10529. doi: 10.1523/JNEUROSCI.4388-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geinsman Y, de Toledo-Morrell L, Morrell F. Loss of perforated synapses in the dentate gyrus: morphological substrate of memory deficit in aged rats. Proc Natl Acad Sci USA. 1986;83:3027–3031. doi: 10.1073/pnas.83.9.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houk JC, Wise SP. Distributed modular architectures linking basal ganglia, cerebellum, and cerebral cortex: Their role in planning and controlling action. Cerebral Cortex. 1995;5:95–110. doi: 10.1093/cercor/5.2.95. [DOI] [PubMed] [Google Scholar]
- Kogan JH, Frankland PW, Silva AJ. Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus. 2000;10:47–56. doi: 10.1002/(SICI)1098-1063(2000)10:1<47::AID-HIPO5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Laroche S, Davis S, Jay TM. Plasticity at hippocampal prefrontal cortex synapses: dual roles in working memory and consolidation. Hippocampus. 2000;10:438–446. doi: 10.1002/1098-1063(2000)10:4<438::AID-HIPO10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Liu Q, Guan XM, Martin WJ, McDonald TP, Clements MK, Jiang Q, Zeng Z, Jacobson M, Williams DL, Jr, Yu H, Bomford D, Figueroa D, Mallee J, Wang R, Evans J, Gould R, Austin CP. Identification and characterization of novel mammalian neuropeptide FF-like peptides that attenuate morphine-induced antinociception. J Biol Chem. 2001;276:36961–36969. doi: 10.1074/jbc.M105308200. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S. Dentate gyrus NMDA receptors mediate rapid separation in the hippocampal network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- Mizuno K, Minamino N, Kangawa K, Matsuo H. A new endogenous opioid peptide from bovine adrenal medulla: isolation and amino acid sequence of a dodecapeptide (BAM-12P) Biochem Biophys Res Commun. 1980;95:1482–1488. doi: 10.1016/s0006-291x(80)80064-7. [DOI] [PubMed] [Google Scholar]
- Molineaux C, Wilk S. Extracellular Processing of Peptides. Boca Raton, FL: CRC Press; 1991. [Google Scholar]
- Moore HP, Andresen JM, Eaton BA, Grabe M, Haugwitz M, Wu MM, Machen TE. Biosynthesis and secretion of pituitary hormones: dynamics and regulation. Arch Physiol Biochem. 2002;110:16–25. doi: 10.1076/apab.110.1.16.903. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn Memory. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mzhavia N, Pan H, Che FY, Fricker LD, Devi LA. Characterization of endothelin-converting enzyme-2. Implication for a role in the nonclassical processing of regulatory peptides. J Biol Chem. 2003;278:14704–14711. doi: 10.1074/jbc.M211242200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, McHugh TJ, Wilson MA, Tonegawa S. NMDA receptors, place cells and hippocampal spatial memory. Nat Rev Neurosci. 2004;5:361–372. doi: 10.1038/nrn1385. [DOI] [PubMed] [Google Scholar]
- Nakagomi S, Kiryu-Seo S, Kiyama H. Endothelin-converting enzymes and endothelin receptor B messenger RNAs are expressed in different neural cell species and these messenger RNAs are coordinately induced in neurons and astrocytes respectively following nerve injury. Neuroscience. 2000;101:441–449. doi: 10.1016/s0306-4522(00)00345-6. [DOI] [PubMed] [Google Scholar]
- Niewoehner B, Single FN, Hvalby Ø, Jensen V, zum Alten Borgloh SM, Seeburg PH, Rawlins JNP, Sprengel R, Bannerman DM. Impaired spatial working memory but spared reference memory following functional loss of NMDA receptors in the dentate gyrus. Eur J Neurosci. 2007;25:837–846. doi: 10.1111/j.1460-9568.2007.05312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry SJ, Yi-Kung Huang E, Cronk D, Bagust J, Sharma R, Walker RJ, Wilson S, Burke JF. A human gene encoding morphine modulating peptides related to NPFF and FMRF amide. FEBS Lett. 1997;409:426–430. doi: 10.1016/s0014-5793(97)00557-7. [DOI] [PubMed] [Google Scholar]
- Pillai-Nair N, Panicker AK, Rodriguiz RM, Miller K, Demyanenko GP, Huang J, Wetsel WC, Maness PF. NCAM-secreting transgenic mice display abnormalities in GABAergic interneurons and alterations in behavior. J Neurosci. 2005;25:4659–4671. doi: 10.1523/JNEUROSCI.0565-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger C, Huang YY, Paletzki RF, Boutchouladze R, Scanlin H, Vronskaya S, Kandel ER. Reversible inhibition of CREB/ATF transcription factors in region CA1 of the dorsal hippocampus disrupts hippocampus-dependent spatial memory. Neuron. 2002;34:447–462. doi: 10.1016/s0896-6273(02)00684-0. [DOI] [PubMed] [Google Scholar]
- Pogorelov VM, Rodriguiz RM, Insco ML, Caron MG, Wetsel WC. Novelty seeking and stereotypic activation of behavior in mice with disruption of the Dat1 gene. Neuropsychopharmacology. 2005;30:1818–1831. doi: 10.1038/sj.npp.1300724. [DOI] [PubMed] [Google Scholar]
- Pomara N, Willoughby LM, Wesnes K, Sidtis JJ. Increased anticholinergic challenge-induced memory impairment associated with APOE-ε4 allele in the elderly: a controlled pilot study. Neuropsychopharmacology. 2004;29:403–409. doi: 10.1038/sj.npp.1300305. [DOI] [PubMed] [Google Scholar]
- Ribar T, Rodriguiz RM, Khiroug L, Wetsel WC, Augustine GJ, Means AR. Cerebellar defects in Ca2+/calmodulin kinase IV deficient mice. J Neurosci. 2000;20(RC107):1–5. doi: 10.1523/JNEUROSCI.20-22-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguiz RM, Wetsel WC. Assessments of cognitive deficits in mutant mice. In: Levin E, Buccafusco JJ, editors. Animal Models of Cognitive Impairment. Boca Raton, FL: CRC Press; 2006. pp. 223–282. [PubMed] [Google Scholar]
- Russell FD, Davenport AP. Evidence for intracellular endothelin-converting enzyme-2 expression in cultured human vascular endothelial cells. Circ Res. 1999;84:891–896. doi: 10.1161/01.res.84.8.891. [DOI] [PubMed] [Google Scholar]
- Saido TC, Iwata N. Metabolism of amyloid β peptide in pathogenesis of Alzheimer’s disease: towards presymptomatic diagnosis, prevention, and therapy. Neurosci Res. 2006;54:235–253. doi: 10.1016/j.neures.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Sigafoos J, Chestnut WG, Merrill BM, Taylor LC, Diliberto EJ, Jr, Viveros OH. Novel peptides from adrenomedullary chromaffin vesicles. J Anat. 1993;183:253–264. [PMC free article] [PubMed] [Google Scholar]
- Strand FL. Neuropeptides: Regulators of Physiological Processes. Cambridge, MA: MIT Press; 1999. [Google Scholar]
- Vilim FS, Aarnisalo AA, Nieminen ML, Lintunen M, Karlstedt K, Kontinen VK, Kalso E, States B, Panula P, Ziff E. Gene for pain modulatory neuropeptide NPFF: induction in spinal cord by noxious stimuli. Mol Pharmacol. 1999;55:804–811. [PubMed] [Google Scholar]
- Weeraratna AT, Kalehua A, Deleon I, Bertak D, Maher G, Wade MS, Lustig A, Becker KG, Wood W, 3rd, Walker DG, Beach TG, Taub DD. Alterations in immunological and neurological gene expression patterns in Alzheimer's disease tissues. Exp Cell Res. 2007;313:450–461. doi: 10.1016/j.yexcr.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis S, Klaver P, Reul J, Elger CE, Fernandez G. Temporal and cerebellar brain regions that support both declarative memory, formation, and retrieval. Cerebral Cortex. 2004;14:256–267. doi: 10.1093/cercor/bhg125. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Tomie J-A. Perseveration on place reversals in spatial swimming pool tasks: further evidence for place learning in hippocampal rats. Hippocampus. 1997;7:361–270. doi: 10.1002/(SICI)1098-1063(1997)7:4<361::AID-HIPO2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Wolfer DP, Stagljar-Bozicevic M, Errington ML, Lipp H-P. Spatial memory and learning in transgenic mice: fact or artifact? News Physiol Sci. 1999;13:118–123. doi: 10.1152/physiologyonline.1998.13.3.118. [DOI] [PubMed] [Google Scholar]
- Woods BT, Schoene W, Kneisley L. Are hippocampal lesions sufficient to cause lasting amnesia? J Neurol Neurosurg Psychiatry. 1982;45:243–247. doi: 10.1136/jnnp.45.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa H, Hammer RE, Richardson JA, Emoto N, Williams SC, Takeda S, Clouthier DE, Yanagisawa M. Disruption of ECE-1 and ECE-2 reveals a role for endothelin-converting enzyme-2 in murine cardiac development. J Clin Invest. 2000;105:1373–1382. doi: 10.1172/JCI7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HY, Fratta W, Majane EA, Costa E. Isolation, sequencing, synthesis, and pharmacological characterization of two brain neuropeptides that modulate the action of morphine. Proc Natl Acad Sci USA. 1985;82:7757–7761. doi: 10.1073/pnas.82.22.7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The following supplementary material is available for this article online from http://www.blackwell-synergy.com/doi/full/10.1111/j.1601-183X.2007.00365.x
Appendix S1 Behavioural assessments, Table S1 (Results from general behavioral assessments of WT and KO mice) and full figure legends.
Figure S1 Swim times of WT and KO mice in the Morris water maze.
Figure S2 Swim velocities of WT and KO mice in the Morris water maze.
Figure S3 Percent time spent with objects for WT and KO mice.
Figure S4 Interactions with the familiar- and novel-flavored diets during the 20 min and 24 hr tests by ECE-2 mice with the demonstrator remaining in the home-cage.
Figure S5 Interactions with the familiar- and novel-flavored diets during the 20 min and 24 hr tests by ECE-2 mice with the demonstrator removed from home-cage.