Abstract
Objective
Purging disorder (PD), a recently recognized eating disorder syndrome, is differentiated from bulimia nervosa (BN) based on the absence of objectively large binge episodes. BN has been associated with low serum leptin levels. This study examined whether PD is also characterized by low serum leptin.
Method
Participants included women with PD (n=20) or BN (n=37), and non-eating disorder controls (n=33). Blood samples for measurement of leptin and total ghrelin were obtained after overnight fast.
Results
In comparison to control values, leptin levels were significantly decreased in PD (p<.01), as well as in BN (p<.02). Plasma ghrelin levels did not differ significantly across groups.
Conclusion
These results provide the first evidence that PD is associated with alteration in a neurobiological pathway influencing eating patterns and body weight. Further research is needed to assess whether low leptin levels in PD and BN are associated with restrained eating and weight suppression.
Keywords: eating disorders, bulimia nervosa, leptin, ghrelin, appetite regulation, eating behavior
The provisional syndrome known as purging disorder, a prevalent subtype of “eating disorder not otherwise specified,” shares several psychological and behavioral characteristics with bulimia nervosa, including preoccupation with body weight and shape, and recurrent purging episodes (1-3). Purging disorder is symptomatically distinct from bulimia nervosa as reflected in the absence of objectively large binge episodes.
An important focus of current research is to identify psychobiological factors that contribute to the onset, severity, and perpetuation of this syndrome. Thus, a recent study showed that individuals with purging disorder have normal release of the gut peptide cholecystokinin (CCK) following a standardized test-meal, in contrast to significantly blunted CCK response in bulimia nervosa (4). The purging disorder group reported less hunger before the test meal, and less hunger and greater fullness following the test meal, compared to the bulimia nervosa and control groups.
Leptin, a circulating adipokine derived primarily from white adipose tissue, acts in the central nervous system to decrease food intake and increase energy metabolism (5, 6). Most studies in bulimia nervosa have demonstrated low serum leptin levels in comparison to values for matched controls (7-11). These alterations may be related to symptom severity (12), a factor which may contribute to some variability in findings across studies (13-16). Additionally, circulating leptin levels are strongly influenced by nutritional patterns and can be substantially reduced by dieting and weight loss (17, 18). Thus, leptin levels are markedly reduced in anorexia nervosa (10, 19, 20).
Although the physiological basis for reduced leptin levels in normal weight patients with bulimia nervosa has not been identified, recent theories have focused on patients’ restriction of energy intake motivated by a desire to maintain a body weight which is below their physiological weight set point (9, 21). In that purging disorder resembles bulimia nervosa with respect to significantly elevated ratings of dietary restraint (4), we hypothesized that individuals with purging disorder might also exhibit decreased serum leptin levels.
Recent studies on the neurobiology of eating disorders have also focused on ghrelin, a gut-related peptide which acts in the hypothalamus to stimulate food intake (22, 23). Ghrelin levels increase in response to fasting (24), and decrease postprandially in association with reduction in hunger ratings (25, 26). While the majority of studies report that fasting plasma ghrelin levels in bulimia nervosa are similar to control values (16, 27-30), there are also reports of increased levels in patients with the binge-purge subtype of the disorder (11, 31-33). On an exploratory basis, plasma ghrelin levels were compared across study groups.
METHODS
Participants
Blood samples for analysis of leptin and ghrelin levels were obtained in conjunction with a study of behavioral characteristics and test meal responses in purging disorder (4). Inclusion criteria for the purging disorder group (n=20, mean age 21.2 ± 4.4 years, mean body mass index (BMI) 22.1 ± 2.2 kg/m2) were recurrent purging episodes occurring on average at least twice a week over three months in the absence of objectively large binge episodes. Individuals with a lifetime history of bulimia nervosa or binge eating disorder were excluded. Average weekly symptom frequency was 5.5 ± 4.6 for self-induced vomiting and 6.7 ± 4.9 for total purging episodes. For the bulimia nervosa group (n=37, mean age 21.1 ± 3.4 years, mean BMI 22.3 ± 1.8 kg/m2), participants met DSM-IV criteria for the purging subtype of the disorder (1). Average weekly symptom frequency was 4.7 ± 4.0 for objective binge episodes, 5.6 ± 4.4 for self-induced vomiting, and 7.0 ± 5.0 for total purging episodes. Individuals with a history of anorexia nervosa (3 in the purging disorder and 5 in the bulimia nervosa group) had been weight recovered for at least one year. The controls (n=33, mean age 22.2 ± 4.3 years, mean BMI 22.3 ± 1.5 kg/m2) had no lifetime history of an eating disorder, had not engaged in dieting for weight loss during the previous eight weeks, and scored less than 10 on the Cognitive Restraint Scale of the Three Factor Eating Questionnaire (34). All participants were women, between 18 and 45 years old, in a healthy weight range (BMI of 18.5 to 26.5 kg/m2), and free of psychotropic medications for at least 8 weeks. After complete description of the study to the participants, written informed consent was obtained as approved by the institutional review board.
Methods
Research diagnostic interviews conducted during the first study visit included the Eating Disorders Examination (35) and Module H of the Structured Clinical Interview for DSM-IV Axis I Disorders (36). Data collected included the frequency of “objective” binges as defined in DSM-IV, as well as the frequency of “subjective” binges (binge-like episodes that do not include an abnormally large amount of food). Participants followed an overnight fast prior to the second study visit, which was scheduled at approximately 8:00 AM on a General Clinical Research Center. Baseline blood samples and self-ratings were obtained, including assessments of “hungry,” “full” and “satiated (satisfied)” assessed using 100 mm visual analog scales, as previously described in more detail (4).
Serum samples for leptin assay were obtained for all participants. Plasma samples containing aprotinin for peptide measurements were obtained for participants who participated during the second half of the study, including 14 with purging disorder, 17 with bulimia nervosa, and 14 controls. Samples were stored at −70 °C until assayed for leptin and total ghrelin by radioimmunoassay using commercially available kits (Linco/Millipore, St. Charles, Missouri).
Data Analysis
Group values are reported as mean ± standard deviation (mean ± standard error of the mean in the figure). Age and BMI were compared across groups by Kruskal-Wallis test and analysis of variance, respectively. Leptin levels were not normally distributed in the patient groups (Shapiro-Wilk test) and were log-transformed prior to statistical analyses to evaluate the relationship of leptin to BMI by Pearson correlation coefficient, and to evaluate group differences in leptin levels by analysis of covariance. Untransformed leptin levels are reported throughout the results to facilitate interpretation of the findings. BMI was included as a covariate in group comparisons when BMI was significantly associated with the dependent variable. We employed Bonferroni-corrected significance levels to evaluate post-hoc, pair-wise comparisons of groups. Relationships between biological measures (leptin and ghrelin concentrations) and behavioral measures (symptom patterns and self-ratings) were assessed by Spearman correlation coefficients. Statistical significance was set at alpha = 0.05, two-tailed. Analyses were performed using SPSS® 16 software (SPSS Inc., Chicago, IL).
RESULTS
Leptin
Mean age and BMI did not differ significantly across study groups, as previously reported (4). Serum leptin was not significantly correlated with age, but was correlated with BMI in the purging disorder group (BMI=22.1 ± 2.2 kg/m2; r=.74, p<.001), in the bulimia nervosa group (BMI=22.3 ± 1.8; r=.39, p<.02), and in the controls (BMI=22.3 ± 1.5; r=.62, p<.001).
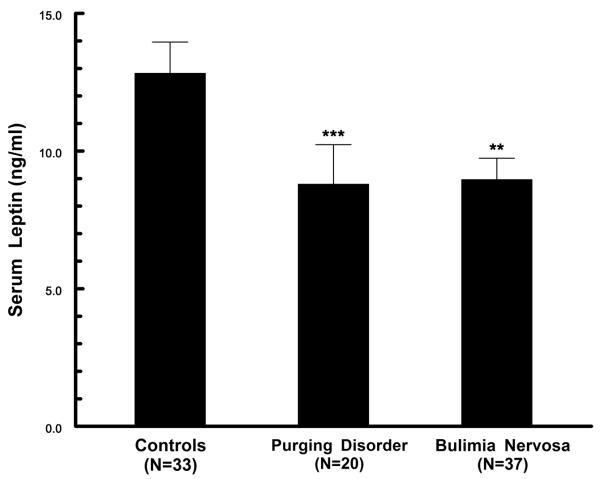
Serum leptin levels, adjusted for BMI by analysis of co-variance, differed significantly across study groups (F=18.77, df=3,86, p<.0001) (Figure 1). Leptin levels for the purging disorder group were significantly lower than for the controls (p<.01). Levels for bulimia nervosa were also lower than for controls (p<.02), and were not significantly different from purging disorder values. When these analyses were repeated excluding eight individuals with a history of anorexia nervosa, results again demonstrated significantly reduced leptin in both purging disorder (9.6 ± 6.6 ng/ml, n=17, p<.05) and bulimia nervosa (8.3 ± 4.1, n=32, p<.005) in comparison to controls.
Figure 1.
Serum leptin levels in purging disorder were not significantly correlated with frequency of self-induced vomiting; total purging episodes per week; or frequency or size of subjective binge episodes. Similarly, in participants with bulimia nervosa leptin levels were not significantly correlated with frequency of self-induced vomiting; total purging episodes per week; or frequency or size of objective binge episodes. Leptin levels were not significantly correlated with baseline ratings of fullness, satiety, or hunger.
Ghrelin
Plasma total ghrelin levels were not significantly correlated with age, BMI or leptin levels. Ghrelin levels in purging disorder (1.8 ± 0.6 ng/ml, n=14) did not differ significantly from values for controls (1.8 ± 0.6 ng/ml, n=14) or bulimia nervosa (2.0 ± 0.8 ng/ml, n=17) (F=0.29, df=2,42, p=ns). Ghrelin levels were not significantly correlated with frequency of self-induced vomiting in the purging disorder group (rho=0.18, p=ns), although a significant correlation was observed in bulimia nervosa (rho=0.52, p<.05). Ghrelin levels were not significantly correlated with frequency or size of subjective binge episodes in purging disorder, or with frequency or size of objective binge episodes in bulimia nervosa.
Although ghrelin concentrations were not significantly correlated with baseline ratings of hunger in the purging disorder or control groups, a significant positive correlation was noted in bulimia nervosa (rho=0.57, p<.02). Ghrelin levels were not significantly correlated with fullness or satiety ratings.
DISCUSSION
The central finding in this study was the demonstration that serum leptin levels were lower in women with purging disorder than in non-eating disorder female controls who were in a similar BMI range. To our knowledge, this represents the first report of a neurobiological alteration differentiating individuals with purging disorder from healthy controls. Although leptin and ghrelin levels did not differ between bulimia nervosa and purging disorder, previous studies have demonstrated that purging disorder differs significantly from bulimia nervosa in other ways, as reflected in psychological symptoms and test meal responses (2, 4). Further studies are needed to identify specific factors contributing to reduced serum leptin levels in purging disorder, including assessment of body composition and historical information on participants’ previous weight patterns. It is a limitation of the current study that body composition was not assessed in order to confirm that the subject groups did not differ significantly in percent body fat. Longitudinal studies will be helpful in identifying the influence of body weight fluctuations and recent dietary patterns (18). The relationship to dietary restraint, to weight suppression, and to family history of obesity may be of particular importance (9, 37-39). Conversely, additional research is needed to assess whether decreased leptin levels contribute to behavioral or physiological symptoms of the disorder (40).
In this initial study, fasting plasma ghrelin levels in purging disorder did not differ significantly from values for bulimia nervosa and controls. Test-meal studies showing blunted postprandial suppression of ghrelin levels have provided the most consistent evidence for abnormal regulation of the peptide in bulimia nervosa (16, 27, 30, 33). Thus, follow-up studies with larger sample size and comprehensive test-meal assessments will be needed to provide a clearer characterization of ghrelin regulation in purging disorder.
Observation of similar ghrelin values in bulimia nervosa and controls is in agreement with most previous reports (16, 27-29, 41). The significant positive correlation between plasma ghrelin concentration and frequency of self-induced vomiting is of note, given previous reports suggesting that ghrelin levels may be elevated in the purging subtype of bulimia nervosa (31-33). While absence of a significant association between ghrelin levels and hunger ratings in the controls is consistent with previous findings (42), the significant positive correlation observed in the bulimia nervosa group is of interest. Follow-up studies with a larger sample size are needed to replicate the correlations observed in the bulimia nervosa group, and to assess whether a pattern of persistent dietary restraint or wide variations in meal anticipation may have contributed to these findings (26, 43, 44).
In summary, this study contributes new physiological data to an emerging body of research on purging disorder. The finding of low serum leptin levels in purging disorder provides the first evidence of a neurobiological alteration associated with this recently characterized eating disorder syndrome.
Acknowledgments
This study was supported by US Public Health Service, National Institutes of Health Grants R01 MH61836, M01-RR-59 and M01-RR-0132, and the Bernice S. Weisman Fund. This study was presented in part at the 2007 Annual Meeting of the Eating Disorders Research Society, Pittsburgh, PA, October 25-27, 2007.
Footnotes
This study was presented in part at the Annual Meeting of the Eating Disorders Research Society, Pittsburgh, PA, October 25-27, 2007.
References
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. American Psychiatric Association; Washington, DC: 2000. Text Revision. [Google Scholar]
- 2.Keel PK, Wolfe BE, Gravener JA, Jimerson DC. Co-morbidity and disorder-related distress and impairment in purging disorder. Psychol Med. 2008;38:1435–42. doi: 10.1017/S0033291707001390. [DOI] [PubMed] [Google Scholar]
- 3.Wade TD, Bergin JL, Tiggemann M, Bulik CM, Fairburn CG. Prevalence and long-term course of lifetime eating disorders in an adult Australian twin cohort. Aust N Z J Psychiatry. 2006;40:121–8. doi: 10.1080/j.1440-1614.2006.01758.x. [DOI] [PubMed] [Google Scholar]
- 4.Keel PK, Wolfe BE, Liddle RA, De Young KP, Jimerson DC. Clinical features and physiological response to a test meal in purging disorder and bulimia nervosa. Arch Gen Psychiatry. 2007;64:1058–66. doi: 10.1001/archpsyc.64.9.1058. [DOI] [PubMed] [Google Scholar]
- 5.Friedman JM. Obesity in the new millennium. Nature. 2000;404:632–4. doi: 10.1038/35007504. [DOI] [PubMed] [Google Scholar]
- 6.Coll AP, Farooqi IS, O’Rahilly S. The hormonal control of food intake. Cell. 2007;129:251–62. doi: 10.1016/j.cell.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brewerton TD, Lesem MD, Kennedy A, Garvey WT. Reduced plasma leptin concentrations in bulimia nervosa. Psychoneuroendocrinology. 2000;25:649–58. doi: 10.1016/s0306-4530(00)00016-0. [DOI] [PubMed] [Google Scholar]
- 8.Monteleone P, Bortolotti F, Fabrazzo M, La Rocca A, Fuschino A, Maj M. Plasma leptin response to acute fasting and refeeding in untreated women with bulimia nervosa. J Clin Endocrinol Metab. 2000;85:2499–503. doi: 10.1210/jcem.85.7.6673. [DOI] [PubMed] [Google Scholar]
- 9.Jimerson DC, Mantzoros C, Wolfe BE, Metzger ED. Decreased serum leptin in bulimia nervosa. J Clin Endocrinol Metab. 2000;85:4511–4. doi: 10.1210/jcem.85.12.7051. [DOI] [PubMed] [Google Scholar]
- 10.Monteleone P, DiLieto A, Castaldo E, Maj M. Leptin functioning in eating disorders. CNS Spectr. 2004;9:523–9. doi: 10.1017/s1092852900009615. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka M, Nakahara T, Muranaga T, Kojima S, Yasuhara D, Ueno H, et al. Ghrelin concentrations and cardiac vagal tone are decreased after pharmacologic and cognitive-behavioral treatment in patients with bulimia nervosa. Horm Behav. 2006;50:261–5. doi: 10.1016/j.yhbeh.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Monteleone P, Martiadis V, Colurcio B, Maj M. Leptin secretion is related to chronicity and severity of the illness in bulimia nervosa. Psychosom Med. 2002;64:874–9. doi: 10.1097/01.psy.0000024239.11538.a5. [DOI] [PubMed] [Google Scholar]
- 13.Calandra C, Musso F, Musso R. The role of leptin in the etiopathogenesis of anorexia nervosa and bulimia. Eating and Weight Disorders. 2003;8:130–7. doi: 10.1007/BF03325002. [DOI] [PubMed] [Google Scholar]
- 14.Tagami T, Satoh N, Usui T, Yamada K, Shimatsu A, Kuzuya H. Adiponectin in anorexia nervosa and bulimia nervosa. J Clin Endocrinol Metab. 2004;89:1833–7. doi: 10.1210/jc.2003-031260. [DOI] [PubMed] [Google Scholar]
- 15.Housova J, Anderlova K, Krizova J, Haluzikova D, Kremen J, Kumstyrova T, et al. Serum adiponectin and resistin concentrations in patients with restrictive and binge/purge form of anorexia nervosa and bulimia nervosa. J Clin Endocrinol Metab. 2005;90:1366–70. doi: 10.1210/jc.2004-1364. [DOI] [PubMed] [Google Scholar]
- 16.Dynesen AW, Bardow A, Astrup A, Petersson B, Holst JJ, Nauntofte B. Meal-induced compositional changes in blood and saliva in persons with bulimia nervosa. Am J Clin Nutr. 2008;87:12–22. doi: 10.1093/ajcn/87.1.12. [DOI] [PubMed] [Google Scholar]
- 17.Chin-Chance C, Polonsky KS, Schoeller DA. Twenty-four-hour leptin levels respond to cumulative short-term energy imbalance and predict subsequent intake. J Clin Endocrinol Metab. 2000;85:2685–91. doi: 10.1210/jcem.85.8.6755. [DOI] [PubMed] [Google Scholar]
- 18.Wolfe BE, Jimerson DC, Orlova C, Mantzoros CS. Effect of dieting on plasma leptin, soluble leptin receptor, adiponectin and resistin levels in healthy volunteers. Clin Endocrinol (Oxf) 2004;61:332–8. doi: 10.1111/j.1365-2265.2004.02101.x. [DOI] [PubMed] [Google Scholar]
- 19.Mantzoros C, Flier JS, Lesem MD, Brewerton TD, Jimerson DC. Cerebrospinal fluid leptin in anorexia nervosa: correlation with nutritional status and potential role in resistance to weight gain. J Clin Endocrinol Metab. 1997;82:1845–51. doi: 10.1210/jcem.82.6.4006. [DOI] [PubMed] [Google Scholar]
- 20.Hebebrand J, Muller TD, Holtkamp K, Herpertz-Dahlmann B. The role of leptin in anorexia nervosa: clinical implications. Mol Psychiatry. 2007;12:23–35. doi: 10.1038/sj.mp.4001909. [DOI] [PubMed] [Google Scholar]
- 21.Obarzanek E, Lesem MD, Goldstein DS, Jimerson DC. Reduced resting metabolic rate in patients with bulimia nervosa. Arch Gen Psychiatry. 1991;48:456–62. doi: 10.1001/archpsyc.1991.01810290068013. [DOI] [PubMed] [Google Scholar]
- 22.Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992–5. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- 23.Wren AM, Bloom SR. Gut hormones and appetite control. Gastroenterology. 2007;132:2116–30. doi: 10.1053/j.gastro.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 24.Leidy HJ, Gardner JK, Frye BR, Snook ML, Schuchert MK, Richard EL, Williams NI. Circulating ghrelin is sensitive to changes in body weight during a diet and exercise program in normal-weight young women. J Clin Endocrinol Metab. 2004;89:2659–64. doi: 10.1210/jc.2003-031471. [DOI] [PubMed] [Google Scholar]
- 25.Tschöp M, Wawarta R, Riepl RL, Friedrich S, Bidlingmaier M, Landgraf R, Folwaczny C. Post-prandial decrease of circulating human ghrelin levels. J Endocrinol Invest. 2001;24:RC19–RC21. doi: 10.1007/BF03351037. [DOI] [PubMed] [Google Scholar]
- 26.Cummings DE, Frayo RS, Marmonier C, Aubert R, Chapelot D. Plasma ghrelin levels and hunger scores in humans initiating meals voluntarily without time- and food-related cues. Am J Physiol Endocrinol Metab. 2004;287:E297–E304. doi: 10.1152/ajpendo.00582.2003. [DOI] [PubMed] [Google Scholar]
- 27.Monteleone P, Martiadis V, Fabrazzo M, Serritella C, Maj M. Ghrelin and leptin responses to food ingestion in bulimia nervosa: implications for binge-eating and compensatory behaviours. Psychol Med. 2003;33:1387–94. doi: 10.1017/s0033291703008316. [DOI] [PubMed] [Google Scholar]
- 28.Nakazato M, Hashimoto K, Shiina A, Koizumi H, Mitsumoti M, Imai M, et al. No changes in serum ghrelin levels in female patients with bulimia nervosa. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:1181–4. doi: 10.1016/j.pnpbp.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 29.Troisi A, Di LG, Lega I, Tesauro M, Bertoli A, Leo R, et al. Plasma ghrelin in anorexia, bulimia, and binge-eating disorder: relations with eating patterns and circulating concentrations of cortisol and thyroid hormones. Neuroendocrinology. 2005;81:259–66. doi: 10.1159/000087923. [DOI] [PubMed] [Google Scholar]
- 30.Monteleone P, Martiadis V, Rigamonti AE, Fabrazzo M, Giordani C, Muller EE, Maj M. Investigation of peptide YY and ghrelin responses to a test meal in bulimia nervosa. Biol Psychiatry. 2005;57:926–31. doi: 10.1016/j.biopsych.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka M, Naruo T, Nagai N, Kuroki N, Shiiya T, Nakazato M, et al. Habitual binge/purge behavior influences circulating ghrelin levels in eating disorders. J Psychiatr Res. 2003;37:17–22. doi: 10.1016/s0022-3956(02)00067-5. [DOI] [PubMed] [Google Scholar]
- 32.Fassino S, Daga GA, Mondelli V, Piero A, Broglio F, Picu A, et al. Hormonal and metabolic responses to acute ghrelin administration in patients with bulimia nervosa. Psychoneuroendocrinology. 2005;30:534–40. doi: 10.1016/j.psyneuen.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 33.Kojima S, Nakahara T, Nagai N, Muranaga T, Tanaka M, Yasuhara D, et al. Altered ghrelin and peptide YY responses to meals in bulimia nervosa. Clin Endocrinol (Oxf) 2005;62:74–8. doi: 10.1111/j.1365-2265.2004.02176.x. [DOI] [PubMed] [Google Scholar]
- 34.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 35.Fairburn CG, Cooper Z. The Eating Disorder Examination. In: Fairburn CG, Cooper Z, Wilson GT, editors. Binge Eating: Nature, Assessment, and Treatment. 12th Edition Guilford Press; New York: 1993. pp. 317–31. [Google Scholar]
- 36.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders--Patient Edition (SCID/P) Biometrics Research Department, New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- 37.Fairburn CG, Welch SL, Doll HA, Davies BA, O’Connor ME. Risk factors for bulimia nervosa. A community-based case-control study. Arch Gen Psychiatry. 1997;54:509–17. doi: 10.1001/archpsyc.1997.01830180015003. [DOI] [PubMed] [Google Scholar]
- 38.von Prittwitz S, Blum WF, Ziegler A, Scharmann S, Remschmidt H, Hebebrand J. Restrained eating is associated with low leptin levels in underweight females. Mol Psychiatry. 1997;2:420–2. doi: 10.1038/sj.mp.4000300. [DOI] [PubMed] [Google Scholar]
- 39.Butryn ML, Lowe MR, Safer DL, Agras WS. Weight suppression is a robust predictor of outcome in the cognitive-behavioral treatment of bulimia nervosa. J Abnorm Psychol. 2006;115:62–7. doi: 10.1037/0021-843X.115.1.62. [DOI] [PubMed] [Google Scholar]
- 40.Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest. 2005;115:3579–86. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monteleone P, Fabrazzo M, Tortorella A, Martiadis V, Serritella C, Maj M. Circulating ghrelin is decreased in non-obese and obese women with binge eating disorder as well as in obese non-binge eating women, but not in patients with bulimia nervosa. Psychoneuroendocrinology. 2005;30:243–50. doi: 10.1016/j.psyneuen.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 42.Doucet É , Laviolette M, Imbeault P, Strychar I, Rabasa-Lhoret R, Prud’homme D. Total peptide YY is a correlate of postprandial energy expenditure but not of appetite or energy intake in healthy women. Metabolism. 2008;57:1458–64. doi: 10.1016/j.metabol.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 43.Frecka JM, Mattes RD. Possible entrainment of ghrelin to habitual meal patterns in humans. Am J Physiol Gastrointest Liver Physiol. 2008;294:G699–G707. doi: 10.1152/ajpgi.00448.2007. [DOI] [PubMed] [Google Scholar]
- 44.Olszewski PK, Schioth HB, Levine AS. Ghrelin in the CNS: from hunger to a rewarding and memorable meal? Brain Res Rev. 2008;58:160–70. doi: 10.1016/j.brainresrev.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]