Abstract
Purpose of the research
The purpose of this study was to describe the occurrence of significant mood disturbance and evaluate for differences in sleep quality among four mood groups (i.e., neither anxiety nor depression, only anxiety, only depression, anxiety and depression) prior to the initiation of radiation therapy (RT).
Methods and sample
Patients (n=179) with breast, prostate, lung, and brain cancer were evaluated prior to the initiation of RT using the Pittsburgh Sleep Quality Index (PSQI), the Center for Epidemiological Studies Depression Scale, and the Spielberger State Anxiety Inventory. Differences in sleep disturbance among the four mood groups were evaluated using analyses of variance.
Key results
While 38% of the patients reported some type of mood disturbance, 57% of the patients reported sleep disturbance. Patients with clinically significant levels of anxiety and depression reported the highest levels of sleep disturbance.
Conclusions
Overall, oncology patients with mood disturbances reported more sleep disturbance than those without mood disturbance. Findings suggest that oncology patients need to be assessed for mood and sleep disturbances.
Keywords: Sleep disturbance, depression, anxiety, oncology patients, radiation therapy
INTRODUCTION
Depression and anxiety are frequently reported problems in patients undergoing cancer treatment (Clark, et al., 2004; Lee, et al., 2004; Payne, et al., 2006). While estimates of the prevalence of depression and anxiety vary widely depending on when oncology patients were evaluated and the methods used to collect data, in studies of patients about to undergo radiation therapy (RT), 2% to 24% reported clinically significant levels of depression (Kelly, et al., 2007; Kilbride, et al., 2007; Stone, et al., 2001) and 15% to 22% reported clinically significant levels of anxiety (Kelly, et al., 2007; Kilbride, et al., 2007; Stone, et al., 2001). The high rates of depression and anxiety in patients prior to the initiation of RT may be related to concerns about the cancer diagnosis and its impact on the patient's survival, as well as to concerns about the effectiveness of and adverse effects associated with RT.
Less is known about the prevalence of sleep disturbance in oncology patients at the initiation of RT. Data from a number of reviews on sleep disturbance in oncology patients (Clark, et al., 2004; Lee, et al., 2004; O'Donnell, 2004; Savard & Morin, 2001) suggest that 30% to 88% of patients report a variety of sleep disturbances. In the limited number of studies of sleep disturbance in patients undergoing RT (Beszterczey & Lipowski, 1977; Faithfull, 1991; Faithfull & Brada, 1998; Miaskowski & Lee, 1999; Reyes-Gibby, et al., 2007), 10% to 80% of patients reported sleep problems and daytime sleepiness. Like depression and anxiety, these high rates of sleep disturbance may be precipitated by the stress associated with the cancer diagnosis as well as by the adverse effects of the disease and its treatments. As noted by Savard and Morin (2001), cancer and its treatment can be characterized as a series of successive stressors that can precipitate sleep disturbance at any point in the patient's disease trajectory.
While the relationships among depression, anxiety, and sleep disturbance have been examined in the general population (Johnson, et al., 2006; Taylor, et al., 2005) and in patients with psychiatric disorders (for reviews see (Mellman, 2008; Peterson & Benca, 2008)), little is known about the relationships among these three symptoms in patients at the initiation of cancer treatment. Of note, findings from one large epidemiologic study (n=772), that compared individuals with and without insomnia (Taylor, et al., 2005), found that individuals with insomnia had higher levels of anxiety and depression. In addition, individuals with insomnia were 9.8 more likely to have clinically significant levels of depression and 17.4 times more likely to have clinically significant levels of anxiety than individuals without insomnia. These results suggest that close relationships exist between insomnia, depression, and anxiety. However, only three studies were found that examined the relationships among these three symptoms in patients during RT.
In one of the first pilot studies of insomnia in cancer patients undergoing RT, Beszterczey and Lipowski (Beszterczey & Lipowski, 1977) noted that insomnia occurred more frequently in cancer patients than in the general population and that insomnia was positively correlated with depression and anxiety (correlation coefficients not reported). In another study of women who underwent RT for breast cancer (Mock, et al., 1997), patients with higher levels of anxiety and depression reported significantly higher levels of sleep disturbance at the end of RT (i.e., r = 0.59 and r = 0.51, respectively, both p<0.0001). In a third study (Savard, et al., 2005), patients who had undergone radical prostatectomy within the previous 10 years were recruited during an appointment with their surgeon or radiation oncologist. Approximately 32% of these patients (n=327), reported nonspecific sleep difficulties and 18% met the criteria for insomnia syndrome. In addition, 20.4% of the patients with sleep difficulties reported both depression and anxiety.
Based on a limited number of largely cross-sectional studies, depression, anxiety, and sleep disturbances appear to be prevalent problems in oncology patients during RT. In addition, a limited amount of evidence suggests that these symptoms can co-occur in these patients. However, no studies were found that evaluated the relationships between depression and anxiety or their co-occurrence and sleep quality in oncology patients prior to the initiation of RT. Data on the association among these three symptoms, at the initiation of RT, is important so that clinicians can identify high risk patients and initiate appropriate referrals and interventions. Given the paucity of research in this area, the purposes of this study, in a sample of oncology patients who were about to undergo primary or adjuvant RT, were: to describe the occurrence of clinically significant mood disturbance (i.e., neither depression nor anxiety, only depression, only anxiety, or both depression and anxiety) and to evaluate for differences in self-reported sleep quality among these four mood groups. We hypothesized that patients with neither depression nor anxiety would report the best sleep quality compared to the other three mood groups and that patients with clinically meaningful levels of both depression and anxiety would report the worst sleep outcomes.
METHODS
Patients and Settings
This descriptive, correlational study is part of a larger, longitudinal study that evaluated multiple symptoms in patients who underwent primary or adjuvant RT. The theoretical framework that was used to guide this study is the Theory of Symptom Management (TSM) developed by faculty members at the University of California, San Francisco (Dodd, et al., 2001; Humphreys, et al., 2008). The TSM contains three dimensions, namely the symptom experience, symptom management strategies, and symptom outcomes. The symptom experience dimension was the focus of this analysis. This dimension involves the interactions of the patient's perception of the symptom, evaluation of the meaning of the symptom, and responses to the symptom. In this analysis, mood disturbance (i.e., depression and anxiety) was the symptom of interest and its relationship to patient's ability to sleep was evaluated.
The patients were recruited from two RT departments located in a Comprehensive Cancer Center and a community-based oncology program. The study was approved by the Committee on Human Research at the University of California, San Francisco and at the second site.
Patients were eligible to participate if they: were ≥18 years of age; were scheduled to receive primary or adjuvant RT for one of four cancer diagnoses (i.e. breast, prostate, lung, brain); were able to read, write, and understand English; gave written informed consent; and had a Karnofsky Performance Status (KPS) score of ≥ 60. Patients were excluded if they had: metastatic disease; more than one cancer diagnosis; or a diagnosed sleep disorder.
Of the 472 patients approached, 185 consented to participate (i.e., 39.2% response rate). The major reasons for refusal were being too overwhelmed with their cancer or too busy. No differences were found in any demographic or clinical characteristics between patients who did and did not choose to participate.
Study Procedures
Approximately one week prior to the initiation of RT (i.e., at the time of the simulation visit), patients were approached by a research nurse to discuss study participation. The details of the study were explained and written informed consent was obtained from each patient. Baseline questionnaires were completed at the time of the enrollment visit.
Instruments
At baseline, patients completed a demographic questionnaire, the KPS scale (Karnofsky, 1977), the Center for Epidemiologic Studies-Depression Scale (CES-D) (Radloff, 1977), the Spielberger State Trait Anxiety Inventories (STAI-S and STAI-T) (Speilberger, et al., 1983), and the Pittsburgh Sleep Quality Index (PSQI) (Buysse, et al., 1989). In addition, medical records were reviewed for disease and treatment information.
The demographic questionnaire obtained information on age, gender, marital status, education, ethnicity, employment status, length of time since cancer diagnosis, and the presence of a number of co-morbid conditions.
The CES-D, which consists of 20 items, was designed to measure the current level of depressive symptoms in a community population (Radloff, 1977). Scores can range from 0 to 60, with scores ≥16 indicating the need for participants to seek a clinical evaluation for major depression. The CES-D has well-established concurrent and construct validity (Carpenter, et al., 1998; Sheehan, et al., 1995). In this study, its Cronbach's alpha was 0.89.
The STAI-T and STAI-S each consist of 20 items and scores for each scale can range from 20 to 80. A higher score indicates greater anxiety. The STAI-T measures an individual's predisposition to anxiety and estimates how a person feels generally. The STAI-S measures an individual's transitory emotional response to a stressful situation. It evaluates the emotional responses of worry, nervousness, tension, and feelings of apprehension related to how people feel “right now” in a stressful situation. The STAI-T and STAI-S have well-established criterion and construct validity and internal consistency reliability coefficients (Bieling, et al., 1998; Kennedy, et al., 2001; Stanley, et al., 2001). In this study, the Cronbach's alphas for the STAI-T and the STAI-S were 0.92 and 0.95, respectively.
The PSQI consists of 19 items designed to assess the quality of sleep in the past month. The global PSQI score is the sum of the seven component scores (i.e., subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, daytime dysfunction). Each component score ranges from 0 (no difficulty) to 3 (severe difficulty) and the global PSQI score ranges from 0 (no difficulty) to 21 (severe difficulties in all areas). Higher global and component scores indicate higher levels of sleep disturbance. A global PSQI score of >5 indicates a significant level of sleep disturbance (Buysse, et al., 1989). The PSQI has established internal consistency, test-retest reliability, and construct validity (Beck, et al., 2004; Buysse, et al., 1989; Carpenter & Andrykowski, 1998). In this study, the Cronbach's alpha for the global PSQI score was 0.72.
Data Analysis
Data were analyzed using SPSS version 15. Descriptive statistics and frequency distributions were generated for sample characteristics. In order to create the four mood groups, CES-D (i.e, ≥ 16) and STAI-S (i.e., ≥ 33.36) cutpoints were chosen based on published reports of clinically meaningful differences (Fletcher, et al., 2008; Radloff, 1977; Speilberger, et al., 1983). The STAI-S score was used to create the mood status groups because it was thought to be more reflective of the patients' perceptions of anxiety associated with RT than the trait anxiety score. Based on these cutpoints, patients were categorized as being in one of four mood groups: neither depression nor anxiety (NEITHER), only depression (DEP), only anxiety (ANX), or both depression and anxiety (BOTH).
Differences in demographic and clinical characteristics among the four mood groups were evaluated using one-way analyses of variance (ANOVAs) or Chi Square analyses. Based on these initial analyses, significant differences were found in the percentages of men and women in the four mood groups. Based on reported gender differences in depression, anxiety, and sleep quality (Aass, et al., 1997; Ohayon, 2007; Ohayon & Roth, 2003; Taylor, et al., 2005), gender was added along with mood group as fixed factors in the subsequent analyses of symptom severity scores, using a two-way ANOVA. The two-way ANOVA tested the significance of the main effect of mood group, the main effect of gender, and the mood group by gender interaction.
All calculations used actual values. Adjustments were not made for missing data. Therefore, the cohort for each analysis was dependent on the largest set of available data across groups. If the overall ANOVA indicated significant differences among the four mood groups (p< 0.05), pairwise contrasts were done to determine where the differences were. The Bonferroni procedure was used to distribute a family alpha of 0.05 across the six possible pairwise contrasts (i.e., 0.008; 0.05/6) so that any one pairwise contrast's p-value needed to be <0.008 to be considered significant.
RESULTS
Distribution of Mood Groups
Of the 185 patients who consented to participate, data on mood status was available for 179 patients. As shown in Table 1, of these 179 patients, 62.0% (n=111) of the sample was categorized as NEITHER, 3.3% (n=6) as DEP, 16.8% (n=30) as ANX, and 17.9% (n=32) as BOTH.
Table 1.
Demographic Characteristics of the Total Sample and Differences in Demographic Characteristics Among the Four Mood Groups
| Characteristic | Total sample (n=179) Mean (SD) |
(1) Neither depression nor anxiety 62.0% (n=111) Mean (SD) |
(2) Only depression 3.3% (n=6) Mean (SD) |
(3) Only anxiety 16.8% (n=30) Mean (SD) |
(4) Both depression and anxiety 17.9% (n=32) Mean (SD) |
Statistics | |
|---|---|---|---|---|---|---|---|
| Age (years) | Male | 66.2 (8.7) | 67.0 (8.8) | 59.0 (14.3) | 64.7 (7.5) | 65.4 (7.8) | Main effect of gender F(1,171)=12.8; p< 0.0001 |
| Female | 54.3 (11.8) | 55.3 (12.5) | 58.5 (10.8) | 51.9 (13.1) | 53.6 (9.8) | ||
| Education (in years) | 16.0 (2.9) | 16.0 (2.9) | 14.8 (4.2) | 15.6 (2.9) | 16.7 (2.6) | N.S. | |
| % (n) | % (n) | % (n) | % (n) | % (n) | |||
| Gender | Male | 52.5 (94) | 61.3 (68) | 50.0 (3) | 46.7 (14) | 28.1 (9) | X2 = 11.5; p= 0.009a |
| Female | 47.5 (85) | 38.7 (43) | 50.0 (3) | 53.3 (16) | 71.9 (23) | ||
| Ethnicity | Non-white | 28.2 (50) | 22.7 (25) | 66.7 (4) | 37.9 (11) | 31.3 (10) | N.S. |
| White | 71.8 (127) | 77.3 (85) | 33.3 (2) | 62.1 (18) | 68.8 (22) | ||
| Marital Status | Married | 56.8 (100) | 60.9 (67) | 50.0 (3) | 48.3 (14) | 51.6 (16) | N.S |
| Non-married | 43.2 (76) | 39.1 (43) | 50.0 (3) | 51.7 (15) | 48.4 (15) | ||
| Lives alone | Yes | 30.7 (55) | 29.7 (33) | 33.3 (2) | 26.7 (8) | 37.5 (12) | N.S. |
| No | 69.3 (124) | 70.3 (78) | 66.7 (4) | 73.3 (22) | 62.5 (20) | ||
| Employed | Yes | 44.8 (78) | 47.7 (52) | 50.0 (3) | 46.7 (14) | 31.0 (9) | N.S. |
| No | 55.2 (96) | 52.3 (57) | 50.0 (3) | 53.3 (16) | 69.0 (20) | ||
| Children at home | Yes | 17.7 (28) | 15.2 (15) | 40.0 (2) | 19.2 (5) | 21.4 (6) | N.S. |
| No | 82.3 (130) | 84.8 (84) | 60.0 (3) | 80.8 (21) | 78.6 (22) | ||
N.S. = not significant, SD = standard deviation
Group 4 versus Group 1 = females>males (p<0.008)
Demographic Characteristics
As shown in Table 1, the majority of the sample was white (71.8%), male (52.5%), married/partnered (56.8%), well educated (16.0 years), with an average age of 60.1 years. No differences were found among the four mood groups on any demographic characteristics, except gender (X2=11.5, p=0.009). In this study, a significantly higher percentage of women were in the BOTH group (71.9%) compared to the NEITHER group (38.7%; p<0.05). In terms of age, the ANOVA revealed a significant main effect of gender (F(1,171)=12.8, p<0.0001), but no main effect of mood group (F(3,171)=0.7, p=0.56) or gender by mood group interaction (F(3,171)=0.6, p=0.60). Regardless of mood group, women were significantly younger on average (54.3 ± 11.8 years) than men (66.2 ± 8.7 years; p<0.0001).
Clinical Characteristics
As shown in Table 2, patients had an average KPS score of 90.6, an average of 4.9 comorbidities, and were diagnosed with cancer 6.8 months prior to the initiation of RT. The majority of the sample was diagnosed with either breast (41.9%) or prostate (45.3%) cancer. All of the patients with breast and brain cancer had undergone surgery prior to RT. Only 9.8% of the patients with prostate cancer and 8.3% of the patients with lung cancer had undergone surgery prior to RT. Approximately 56% of the patients with breast cancer had received CTX prior to RT and 53% of the patients with prostate cancer had received hormonal therapy prior to RT.
Table 2.
Clinical Characteristics of the Total Sample and the Differences in Clinical Characteristics Among the Four Mood Groups
| Characteristic | Total sample (n=179) Mean (SD) |
(1) Neither depression nor anxiety 62.0% (n=111) Mean (SD) |
(2) Only depression 3.3% (n=6) Mean (SD) |
(3) Only anxiety 16.8% (n=30) Mean (SD) |
(4) Both depression and anxiety 17.9% (n=32) Mean (SD) |
Statistics | |
|---|---|---|---|---|---|---|---|
| KPS score | 90.6 (11.7) | 93.7 (8.9) | 80.0 (21.0) | 90.3 (13.0) | 81.7 (12.1) | F(3,167)= 8.1; p<0.0001 1 >2 and 4*; 3 > 4* |
|
| Months since diagnosis | 6.8 (9.5) | 7.4 (10.8) | 5.8 (5.6) | 7.3 (9.5) | 4.6 (3.0) | N.S. | |
| Number of comorbidities | 4.9 (2.5) | 4.7 (2.4) | 5.8 (2.0) | 5.0 (2.4) | 5.2 (2.8) | N.S. | |
| % (n) | % (n) | % (n) | % (n) | % (n) | |||
| Diagnosis | Breast | 41.9 (75) | 35.1 (39) | 50.0 (3) | 46.7 (14) | 59.4 (19) | X2=35.5; p<0.0001 |
| Prostate | 45.3 (81) | 58.6 (65) | 16.7 (1) | 33.3 (10) | 15.6 (5) | ||
| Brain | 6.7 (12) | 5.4 (6) | 0.0 (0) | 6.7 (2) | 12.5 (4) | ||
| Lung | 6.1 (11) | 0.9 (1) | 33.3 (2) | 13.3 (4) | 12.5 (4) | ||
| CES-D score | 9.6 (8.7) | 4.9 (4.3) | 18.8 (1.8) | 10.0 (4.5) | 24.0 (5.6) | F(3,171)=130.7, p<0.0001 1 < 2, 3, and 4*; 2 < 3*, 3 < 4* |
|
| STAI-T score | 34.3 (10.2) | 28.8 (6.6) | 40.3 (5.5) | 38.6 (7.4) | 48.0 (7.7) | F(3,170)=56.1, p<0.0001 1 < 2, 3, and 4*; 3 < 4* |
|
| STAI-S score | 31.5 (11.2) | 24.5 (4.2) | 29.7 (4.8) | 40.0 (5.2) | 48.3 (9.4) | F(3,171)=146.8; p<0.0001 1 < 3 and 4*; 2 and 3 < 4* |
|
CES-D = Center for Epidemiologic Studies Depression Scale, KPS = Karnofsky Performance Status, N.S. = not significant, SD = standard deviation, STAI-T = Speilberger Trait Anxiety Inventory, STAI-S = Spielberger State Anxiety Inventory
p<0.008
No differences were found among the four mood groups, or between the genders, on number of co-morbidities. Differences among the four mood groups were found for KPS score and diagnosis. In terms of KPS scores, the two-way ANOVA demonstrated a significant main effect of mood group (F(3,167)=8.1, p<0.0001). Regardless of gender, the NEITHER group reported significantly higher KPS scores (93.7) than the DEP (80.0) and BOTH (81.7; both p<0.008) groups. In addition, the ANX group reported significantly higher KPS scores (90.3) than the BOTH group (81.7; p<0.008). In terms of cancer diagnosis, the Chi Square analysis revealed significant between group differences (X2=35.5, p<0.0001). The NEITHER group consisted predominantly of men with prostate cancer (58.6%), while the BOTH group consisted predominantly of women with breast cancer (59.4%). These differences in cancer diagnosis among the four mood groups are similar to differences in the proportions of males and females among the four mood groups.
Depression and Anxiety Scores
The CES-D, STAI-T, and STAI-S scores for the entire sample and for each of the four mood groups are summarized in Table 2. The mean CES-D score for the entire sample was 9.6 ± 8.7. Approximately 21.0% of the sample (n=38) reported a CES-D score ≥16. The mean STAI-T and STAI-S scores for the entire sample were 34.3 ± 10.2 and 31.5 ± 11.2, respectively. Approximately 35% of the sample (n=62) reported a state anxiety score ≥33.36.
Differences in Sleep Disturbances Among the Four Mood Status Groups
For all of the PSQI subscale scores, as well as for the total PSQI score, no main effects of gender or gender × mood group interactions were identified using the two-way ANOVA. The findings for the main effects of mood group and the post hoc contrasts are summarized below and in Table 3.
Table 3.
Pittsburgh Sleep Quality Index Subscale Scores for the Total Sample and Differences in the Pittsburgh Sleep Quality Index Subscale Scores Among the Four Mood Groups
| Subscales+ | Total Sample (n=179) Mean (SD) |
(1) Neither depression nor anxiety 62.0% (n=111) Mean (SD) |
(2) Only depression 3.3% (n=6) Mean (SD) |
(3) Only anxiety 16.8% (n=30) Mean (SD) |
(4) Both depression and anxiety 17.9% (n=32) Mean (SD) |
Statistics |
|---|---|---|---|---|---|---|
| Subjective sleep quality | 1.0 (0.7) | 0.8 (0.7) | 1.3 (0.8) | 1.0 (0.6) | 1.5 (0.8) | F(3,170)=8.3; p<0.0001 4>1 and 3* |
| Sleep latency | 1.0 (1.0) | 0.8 (0.9) | 1.2 (1.0) | 1.3 (0.9) | 1.6 (0.9) | F(3,170)=5.3; p=0.002 3 and 4>1* |
| Sleep duration | 1.0 (0.9) | 0.8 (0.8) | 2.0 (1.3) | 1.0 (0.9) | 1.4 (0.8) | F(3,167)=6.8; p<0.0001 2 and 4>1* |
| Habitual sleep efficiency | 0.7 (1.0) | 0.5 (0.8) | 1.7 (1.2) | 0.9 (1.1) | 1.1 (1.1) | F(3,166)=4.3; p=0.006 2 and 4>1* |
| Sleep disturbances | 1.4 (0.6) | 1.4 (0.5) | 1.8 (0.8) | 1.4 (0.6) | 1.7 (0.5) | F(3,170)=3.8; p=0.012 4>1* |
| Use of sleeping medication | 0.7 (1.2) | 0.5 (1.1) | 0.5 (1.2) | 0.7 (1.0) | 1.6 (1.3) | F(3,168)=4.5; p=0.004 4 > 1 and 3* |
| Daytime dysfunction | 0.8 (0.7) | 0.6 (0.5) | 1.5 (1.0) | 1.0 (0.6) | 1.4 (0.8) | F(3,170)=16.0, p<0.0001 2, 3, and 4 > 1* |
N.S. = not significant, S.D. = standard deviation
All subscale scores range from 0 to 3 with higher scores indicating worse sleep quality
p≤0.008
Global PSQI score
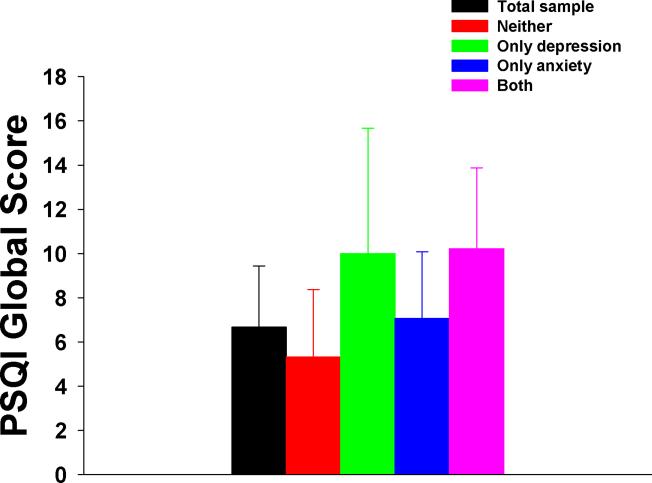
As shown in Figure 1, the mean global PSQI score for the entire sample was 6.7 ± 3.8. Approximately 57% of the sample (n=102) had a total PSQI score of > 5. The two-way ANOVA demonstrated a significant main effect of mood group (F(3,170)=15.7, p<0.0001). Post hoc contrasts revealed that the NEITHER group had a lower mean global PSQI score than the other three mood groups (all, p≤ 0.008). However, the mean global PSQI scores for the DEP group (10.0) and the ANX group (7.1) were not significantly different from each other. Finally, the mean global PSQI score for the ANX group (7.1) was significantly lower than for the BOTH group (10.2), but higher than for the NEITHER group (5.3; all p≤0.008).
Figure 1.
Differences in mean global Pittsburgh Sleep Quality Index (PSQI) scores for the total sample and the four mood groups. Values are plotted as means ± standard deviations. Post hoc contrasts demonstrated that the NEITHER group had significantly lower scores than the other three mood groups and that the ANX group had significantly lower scores than the BOTH group but significantly higher than the NEITHER group (all p<0.008).
PSQI subjective sleep quality
The mean PSQI subjective sleep quality score for the entire sample was 1.0 ± 0.7. The two-way ANOVA demonstrated a significant main effect of mood group (F(3,170)=8.3, p<0.0001). Post hoc contrasts revealed that the BOTH group reported significantly poorer subjective sleep quality scores (1.5) than the NEITHER (0.8) and the ANX (1.0; both p≤0.008) groups.
PSQI sleep latency
The mean PSQI sleep latency score for the entire sample was 1.0 ± 1.0. The two-way ANOVA demonstrated a significant main effect of mood group (F(3,170)=5.3, p=0.002). Post hoc contrasts demonstrated that the ANX (1.3) and the BOTH (1.6; both p≤0.008) groups had significantly higher sleep latency scores than the NEITHER group (0.8).
PSQI sleep duration
The mean PSQI sleep duration score for the entire sample was 1.0 ± 0.9. The two-way ANOVA revealed a significant main effect of mood group (F(3,167)=6.8, p<0.0001). Post hoc contrasts demonstrated that the DEP (2.0) and the BOTH (1.4; both p≤0.008) groups had significantly worse sleep duration scores than the NEITHER group (0.8).
PSQI habitual sleep efficiency
The mean PSQI habitual sleep efficiency score for the entire sample was 0.7 ± 1.0. The two-way ANOVA revealed a significant main effect of mood group (F(3,166)=4.3, p=0.006). Post hoc contrasts revealed that the DEP (1.7) and the BOTH (1.1; both p≤0.008) groups had poorer sleep efficiency scores than the NEITHER group (0.5).
PSQI sleep disturbances
The mean PSQI sleep disturbances score for the entire sample was 1.4 ± 0.6. The two-way ANOVA demonstrated a significant main effect of mood group (F(3,170)=3.8, p=0.012). Post hoc contrasts revealed that the BOTH group had significantly higher sleep disturbance scores (1.7) than the NEITHER group (1.4; p<0.008).
PSQI use of sleeping medication
The mean PSQI use of sleeping medication score for the entire sample was 0.7 ± 1.2. The two-way ANOVA demonstrated a significant main effect of mood group (F(3,168)=4.5, p=0.004). Post hoc contrasts demonstrated that the BOTH group had significantly higher use of sleeping medication scores (1.6) than the NEITHER (0.5) and the ANX (0.7; both p≤0.008) groups.
PSQI daytime dysfunction
The mean PSQI daytime dysfunction score for the entire sample was 0.8 ± 0.7. The two-way ANOVA demonstrated a significant main effect of mood group (F(3,170)=16.0, p<0.0001). Post hoc contrasts revealed that the NEITHER group had a lower mean daytime dysfunction score than the other three mood groups (all p≤0.008).
DISCUSSION
To our knowledge, this study is the first to evaluate for differences in various components of sleep quality in a sample of oncology patients, prior to the initiation of RT, who were categorized into one of four mood disturbance groups based on clinically meaningful cutpoint scores. Consistent with our a priori hypothesis, patients who were categorized in the neither depression nor anxiety group reported the least amount of sleep disturbance. However, even within this group, 40.9% of the patients reported a global PSQI score that was above the cutoff of > 5 (Buysse, et al., 1989). Overall, 57% of the patients in this study experienced a significant amount of sleep disturbance. This finding is above the 39% reported for a heterogeneous sample of patients who underwent RT for bone metastasis (Lee, et al., 2004), but is similar to the 61% found in a sample of women who underwent RT for breast cancer (Fortner, et al., 2002). In addition and consistent with previous reports (Beck, et al., 2004; Carpenter & Andrykowski, 1998; Fortner, et al., 2002), all of the patients in this study reported mild to moderate levels of difficulty with all seven components of sleep that are evaluated on the PSQI. Taken together and consistent with the early work of Beszterczey and Lipowski (1977), these findings suggest that sleep disturbance is a common problem in oncology patients at the initiation of RT. Clinicians need to perform appropriate assessments so that high risk patients can be identified and appropriate referrals and interventions can be initiated.
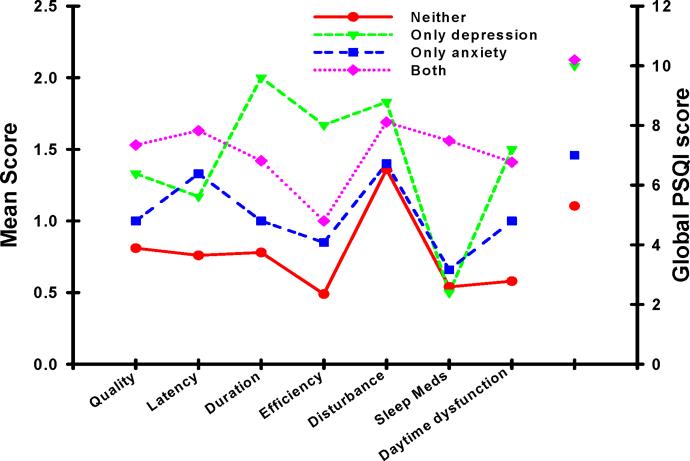
In order to evaluate for distinctive component and global PSQI profiles among the four mood groups, we used an approach similar to the one described by Buysse and colleagues (Buysse, et al., 1989) and plotted the various PSQI scores for each mood group. As illustrated in Figure 2, compared to the other three mood groups, patients in the NEITHER group reported the lowest scores on the majority of the PSQI subscales, as well as the lowest global PSQI score. Of note, this mood group's subscale and total PSQI scores were only slightly higher than those reported by healthy controls (Buysse, et al., 1989).
Figure 2.
Profiles of the mean subscale and global scores from the Pittsburgh Sleep Quality Index (PSQI) for each of the mood groups. Higher PSQI scores indicate higher levels of sleep disturbance.
In contrast and consistent with our a priori hypothesis, patients with both anxiety and depression reported the highest global PSQI scores, as well as the highest subscale scores for subjective sleep quality and sleep latency. This profile suggests that these patients had more of a problem with the initiation of sleep than with sleep maintenance. However, no studies were found that characterized the types of sleep disturbance that occur in oncology patients with both depression and anxiety. Therefore, additional research is warranted, using both subjective and objective measures of sleep quality, to confirm this finding and to determine the specific type of insomnia that oncology patients with the co-occurrence of depression and anxiety experience. This type of information is needed to guide the development and testing of interventions to improve sleep and reduce psychological distress in these vulnerable patients.
Finally, patients in the DEP group demonstrated a different pattern of PSQI scores than the other three mood status groups. Consistent with previous reports (Hubain, et al., 2006; Taylor, et al., 2005), patients in the DEP group reported higher scores on the duration, efficiency, and sleep disturbance subscales of the PSQI which suggests that maintenance of sleep is more of a problem in these patients. However, these findings need to be interpreted with caution due to the small number of patients in this group and warrant replication in future studies.
While the occurrence of depressive symptoms (21.2%) was consistent with previous reports (Massie, 2004; Pirl, 2004), 34.7% of this sample reported clinically significant levels of anxiety which is higher than the 15% to 22% found in previous studies (Kilbride, et al., 2007; Stone, et al., 2001; Takahashi, et al., 2008). Reasons for these inconsistencies may be related to differences in instruments used to assess and categorize depression and anxiety, as well as differences in patients' cancer diagnoses or stage of disease. However, these findings suggest that both of these symptoms are highly prevalent in patients at the initiation of RT. Additional research is warranted to determine the specific etiologies for these high rates of psychological distress.
Of note, approximately 18% of the patients in this study reported clinically significant levels of both depression and anxiety. In addition, women (23%) were more likely to be in this group compared to men (9%). While no studies were found that investigated the co-occurrence of depression and anxiety in outpatients prior to the initiation of RT, in two studies of heterogeneous samples of oncology patients undergoing a variety of cancer treatments the co-occurrence of depression and anxiety ranged from 5% (Aass, et al., 1997) to 9.5% (Frick, Tyroller, & Panzer, 2007). Again, the reason for these inconsistent results may relate to the methods used to categorize the mood disturbances or the distribution of cancer diagnoses across studies. However, these findings in oncology patients warrant additional investigation because studies in the general population suggest that when these two mood disturbances do co-occur, they have a negative impact on individual's health status and QOL (Phillips, et al., 2009; Pirkola, et al., 2009; Stewart, et al., 2008). In addition, because of the relatively small number of patients in each of the mood status groups, future research needs to determine the relative contribution of gender versus cancer diagnosis to the co-occurrence of depression and anxiety.
Despite reporting the highest use of sleep medications compared to the other three mood groups, patients with clinically meaningful levels of depression and anxiety reported the worst PSQI global, subjective sleep quality, and sleep latency scores. While data on the sleep medication prescriptions are not available, these findings suggest that these medications were not effective. Future studies need to evaluate the effectiveness of both pharmacologic and nonpharmacologic approaches to improve sleep quality and reduce psychological distress in patients during cancer treatment.
Several limitations of this study need to be acknowledged. The causal relationships among depression and anxiety and the various types of sleep disturbance could not be determined because of the study's cross-sectional design. Second, while valid and reliable self report measures were used to assess for anxiety, depression, and sleep disturbance, definitive diagnoses of specific psychiatric conditions and sleep disorders were not possible. Third, data were not available on pre-existing psychiatric comorbidities. Fourth, while the overall sample size was relatively large, the size of each mood group was small. Therefore, these findings warrant replication with larger samples. Finally, data were not available on specific sleep, anxiety, and depression medications used by these patients.
Despite these limitations, findings from this study suggest that sleep disturbance is a significant problem in oncology patients prior to the initiation of RT. Moreover, patients' level of anxiety and depression appear to be related to various aspects of sleep quality. Longitudinal studies are warranted to disentangle the cause and effect relationships among these three symptoms as well as to evaluate for patterns of change in sleep disturbance in relationship to patients' level of depression and anxiety. Information from these types of descriptive studies can be used to design and test targeted interventions for oncology patients with mood and sleep disturbances.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aass N, Fossa SD, Dahl AA, Moe TJ. Prevalence of anxiety and depression in cancer patients seen at the Norwegian Radium Hospital. Eur J Cancer. 1997;33(10):1597–1604. doi: 10.1016/s0959-8049(97)00054-3. [DOI] [PubMed] [Google Scholar]
- Beck SL, Schwartz AL, Towsley G, Dudley W, Barsevick A. Psychometric evaluation of the Pittsburgh Sleep Quality Index in cancer patients. J Pain Symptom Manage. 2004;27(2):140–148. doi: 10.1016/j.jpainsymman.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Beszterczey A, Lipowski ZJ. Insomnia in cancer patients. Can Med Assoc J. 1977;116(4):355. [PMC free article] [PubMed] [Google Scholar]
- Bieling PJ, Antony MM, Swinson RP. The State-Trait Anxiety Inventory, Trait version: structure and content re-examined. Behav Res Ther. 1998;36(7–8):777–788. doi: 10.1016/s0005-7967(98)00023-0. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh Sleep Quality Index. J Psychosom Res. 1998;45(1 Spec No):5–13. doi: 10.1016/s0022-3999(97)00298-5. [DOI] [PubMed] [Google Scholar]
- Carpenter JS, Andrykowski MA, Wilson J, Hall LA, Rayens MK, Sachs B, Cunningham LL. Psychometrics for two short forms of the Center for Epidemiologic Studies-Depression Scale. Issues Ment Health Nurs. 1998;19(5):481–494. doi: 10.1080/016128498248917. [DOI] [PubMed] [Google Scholar]
- Clark J, Cunningham M, McMillan S, Vena C, Parker K. Sleep-wake disturbances in people with cancer part II: evaluating the evidence for clinical decision making. Oncol Nurs Forum. 2004;31(4):747–771. doi: 10.1188/04.ONF.747-771. [DOI] [PubMed] [Google Scholar]
- Dodd M, Janson S, Facione N, Faucett J, Froelicher ES, Humphreys J, Lee K, Miaskowski C, Puntillo K, Rankin S, Taylor D. Advancing the science of symptom management. J Adv Nurs. 2001;33(5):668–676. doi: 10.1046/j.1365-2648.2001.01697.x. [DOI] [PubMed] [Google Scholar]
- Faithfull S. Patients' experiences following cranial radiotherapy: a study of the somnolence syndrome. J Adv Nurs. 1991;16(8):939–946. doi: 10.1111/j.1365-2648.1991.tb01798.x. [DOI] [PubMed] [Google Scholar]
- Faithfull S, Brada M. Somnolence syndrome in adults following cranial irradiation for primary brain tumours. Clin Oncol (R Coll Radiol) 1998;10(4):250–254. doi: 10.1016/s0936-6555(98)80011-3. [DOI] [PubMed] [Google Scholar]
- Fletcher BS, Paul SM, Dodd MJ, Schumacher K, West C, Cooper B, Lee K, Aouizerat B, Swift P, Wara W, Miaskowski C. Prevalence, severity, and impact of symptoms on female family caregivers of patients at the initiation of radiation therapy for prostate cancer. J Clin Oncol. 2008;26(4):599–605. doi: 10.1200/JCO.2007.12.2838. [DOI] [PubMed] [Google Scholar]
- Fortner BV, Stepanski EJ, Wang SC, Kasprowicz S, Durrence HH. Sleep and quality of life in breast cancer patients. J Pain Symptom Manage. 2002;24(5):471–480. doi: 10.1016/s0885-3924(02)00500-6. [DOI] [PubMed] [Google Scholar]
- Frick E, Tyroller M, Panzer M. Anxiety, depression and quality of life of cancer patients undergoing radiation therapy: a cross-sectional study in a community hospital outpatient centre. Eur J Cancer Care (Engl) 2007;16(2):130–136. doi: 10.1111/j.1365-2354.2006.00720.x. [DOI] [PubMed] [Google Scholar]
- Hubain P, Le Bon O, Vandenhende F, Van Wijnendaele R, Linkowski P. Major depression in males: effects of age, severity and adaptation on sleep variables. Psychiatry Res. 2006;145(2–3):169–177. doi: 10.1016/j.psychres.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Humphreys J, Lee KA, Carrieri-Kohlman V, Puntillo K, Faucett J, Janson S, Aouizerat B, Donesky-Cuenco D, UCSF Symptom Management Faculty Group . Theory of Symptom Management. In: Smith MJ, Liehr PR, editors. Middle Range Theory for Nursing. Second ed. Springer Publishing Company; New York: 2008. pp. 145–158. [Google Scholar]
- Johnson EO, Roth T, Breslau N. The association of insomnia with anxiety disorders and depression: exploration of the direction of risk. J Psychiatr Res. 2006;40(8):700–708. doi: 10.1016/j.jpsychires.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Karnofsky D. Performance Scale. In: Kennealy GT, Mitchell MS, editors. Factors that Influence the Therapeutic Repsone in Cancer: A Comprehensive Treatise. Plenum Press; New York: 1977. pp. 97–101. [Google Scholar]
- Kelly C, Paleri V, Downs C, Shah R. Deterioration in quality of life and depressive symptoms during radiation therapy for head and neck cancer. Otolaryngol Head Neck Surg. 2007;136(1):108–111. doi: 10.1016/j.otohns.2006.06.1278. [DOI] [PubMed] [Google Scholar]
- Kennedy BL, Schwab JJ, Morris RL, Beldia G. Assessment of state and trait anxiety in subjects with anxiety and depressive disorders. Psychiatr Q. 2001;72(3):263–276. doi: 10.1023/a:1010305200087. [DOI] [PubMed] [Google Scholar]
- Kilbride L, Smith G, Grant R. The frequency and cause of anxiety and depression amongst patients with malignant brain tumours between surgery and radiotherapy. J Neurooncol. 2007;84(3):297–304. doi: 10.1007/s11060-007-9374-7. [DOI] [PubMed] [Google Scholar]
- Lee BN, Dantzer R, Langley KE, Bennett GJ, Dougherty PM, Dunn AJ, Meyers CA, Miller AH, Payne R, Reuben JM, Wang XS, Cleeland CS. A cytokine-based neuroimmunologic mechanism of cancer-related symptoms. Neuroimmunomodulation. 2004;11(5):279–292. doi: 10.1159/000079408. [DOI] [PubMed] [Google Scholar]
- Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004;32:57–71. doi: 10.1093/jncimonographs/lgh014. [DOI] [PubMed] [Google Scholar]
- Mellman TA. Sleep and anxiety disorders. Sleep Med Clin. 2008;3:261–268. doi: 10.1016/j.jsmc.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miaskowski C, Lee KA. Pain, fatigue, and sleep disturbances in oncology outpatients receiving radiation therapy for bone metastasis: a pilot study. J Pain Symptom Manage. 1999;17(5):320–332. doi: 10.1016/s0885-3924(99)00008-1. [DOI] [PubMed] [Google Scholar]
- Mock V, Dow KH, Meares CJ, Grimm PM, Dienemann JA, Haisfield-Wolfe ME, Quitasol W, Mitcheel S, Chakravarthy A, Gage I. Effects of exercise on fatigue, physical functioning, and emotional distress during radiation therapy for breast cancer. Oncol Nurs Forum. 1997;24(6):991–1000. [PubMed] [Google Scholar]
- O'Donnell JF. Insomnia in cancer patients. Clin Cornerstone. 2004;6(Suppl 1D):S6–14. doi: 10.1016/s1098-3597(05)80002-x. [DOI] [PubMed] [Google Scholar]
- Ohayon MM. Epidemiology of depression and its treatment in the general population. J Psychiatr Res. 2007;41(3–4):207–213. doi: 10.1016/j.jpsychires.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Roth T. Place of chronic insomnia in the course of depressive and anxiety disorders. J Psychiatr Res. 2003;37(1):9–15. doi: 10.1016/s0022-3956(02)00052-3. [DOI] [PubMed] [Google Scholar]
- Payne J, Piper B, Rabinowitz I, Zimmerman B. Biomarkers, fatigue, sleep, and depressive symptoms in women with breast cancer: a pilot study. Oncol Nurs Forum. 2006;33(4):775–783. doi: 10.1188/06.ONF.775-783. [DOI] [PubMed] [Google Scholar]
- Peterson MJ, Benca RM. Sleep in mood disorders. Sleep Med Clin. 2008;3:231–249. [Google Scholar]
- Phillips AC, Batty GD, Gale CR, Deary IJ, Osborn D, MacIntyre K, Carroll D. Generalized anxiety disorder, major depressive disorder, and their comorbidity as predictors of all-cause and cardiovascular mortality: the Vietnam experience study. Psychosom Med. 2009;71(4):395–403. doi: 10.1097/PSY.0b013e31819e6706. [DOI] [PubMed] [Google Scholar]
- Pirkola S, Saarni S, Suvisaari J, Elovainio M, Partonen T, Aalto AM, Honkonen T, Perla J, Lonnquist J. General health and quality-of-life measures in active, recent, and comorbid mental disorders: a population-based health 2000 study. Compr Psychiatry. 2009;50(2):108–114. doi: 10.1016/j.comppsych.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Pirl WF. Evidence report on the occurrence, assessment, and treatment of depression in cancer patients. J Natl Cancer Inst Monogr. 2004;32:32–39. doi: 10.1093/jncimonographs/lgh026. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Reyes-Gibby CC, Chan W, Abbruzzese JL, Xiong HQ, Ho L, Evans DB, Varadhachary G, Bhat S, Wolff RA, Crane C. Patterns of self-reported symptoms in pancreatic cancer patients receiving chemoradiation. J Pain Symptom Manage. 2007;34(3):244–252. doi: 10.1016/j.jpainsymman.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savard J, Morin CM. Insomnia in the context of cancer: a review of a neglected problem. J Clin Oncol. 2001;19(3):895–908. doi: 10.1200/JCO.2001.19.3.895. [DOI] [PubMed] [Google Scholar]
- Savard J, Simard S, Hervouet S, Ivers H, Lacombe L, Fradet Y. Insomnia in men treated with radical prostatectomy for prostate cancer. Psychooncology. 2005;14(2):147–156. doi: 10.1002/pon.830. [DOI] [PubMed] [Google Scholar]
- Sheehan TJ, Fifield J, Reisine S, Tennen H. The measurement structure of the Center for Epidemiologic Studies Depression Scale. J Pers Assess. 1995;64(3):507–521. doi: 10.1207/s15327752jpa6403_9. [DOI] [PubMed] [Google Scholar]
- Speilberger CG, Gorsuch RL, Lushene RE, Vaag PR, Jacobs GA. Manual for the State-Anxiety Inventory (Form Y) Self Evaluation Questionnaire. Consulting Psychologists Press; Palo Alto, CA: 1983. [Google Scholar]
- Stanley MA, Novy DM, Bourland SL, Beck JG, Averill PM. Assessing older adults with generalized anxiety: a replication and extension. Behav Res Ther. 2001;39(2):221–235. doi: 10.1016/s0005-7967(00)00030-9. [DOI] [PubMed] [Google Scholar]
- Stewart ST, Woodward RM, Rosen AB, Cutler DM. The impact of symptoms and impairments on overall health in US national health data. Med Care. 2008;46(9):954–962. doi: 10.1097/MLR.0b013e318179199f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone P, Richards M, A'Hern R, Hardy J. Fatigue in patients with cancers of the breast or prostate undergoing radical radiotherapy. J Pain Symptom Manage. 2001;22(6):1007–1015. doi: 10.1016/s0885-3924(01)00361-x. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Hondo M, Nishimura K, Kitani A, Yamano T, Yanagita H, Osada H, Shinbo M, Honda N. Evaluation of quality of life and psychological response in cancer patients treated with radiotherapy. Radiat Med. 2008;26(7):396–401. doi: 10.1007/s11604-008-0248-5. [DOI] [PubMed] [Google Scholar]
- Taylor DJ, Lichstein KL, Durrence HH, Reidel BW, Bush AJ. Epidemiology of insomnia, depression, and anxiety. Sleep. 2005;28(11):1457–1464. doi: 10.1093/sleep/28.11.1457. [DOI] [PubMed] [Google Scholar]