Abstract
Object
Glial cell line–derived neurotrophic factor (GDNF) has potent survival effects on central and peripheral nerve populations. The authors examined the differential effects of GDNF following either a sciatic nerve crush injury in mice that overexpressed GDNF in the central or peripheral nervous systems (glial fibrillary acidic protein [GFAP]–GDNF) or in the muscle target (Myo-GDNF).
Methods
Adult mice (GFAP-GDNF, Myo-GDNF, or wild-type [WT] animals) underwent sciatic nerve crush and were evaluated using histomorphometry and muscle force and power testing. Uninjured WT animals served as controls.
Results
In the sciatic nerve crush, the Myo-GDNF mice demonstrated a higher number of nerve fibers, fiber density, and nerve percentage (p < 0.05) at 2 weeks. The early regenerative response did not result in superlative functional recovery. At 3 weeks, GFAP-GDNF animals exhibit fewer nerve fibers, decreased fiber width, and decreased nerve percentage compared with WT and Myo-GDNF mice (p < 0.05). By 6 weeks, there were no significant differences between groups.
Conclusions
Peripheral delivery of GDNF resulted in earlier regeneration following sciatic nerve crush injuries than that with central GDNF delivery. Treatment with neurotrophic factors such as GDNF may offer new possibilities for the treatment of peripheral nerve injury.
Keywords: glial cell line-derived neurotrophic factor, chromophore, transgenic mouse, sciatic nerve crush, peripheral nerve injury, motor endplate, muscle force, power testing
Surgical treatment of devastating peripheral nerve injuries currently relies on a combination of nerve grafting and nerve transfer procedures. A better understanding of neurotrophic factors and their effects on regeneration is likely to provide the next paradigm shift in the treatment of peripheral nerve injuries. Glial cell line–derived neurotrophic factor is a well-known member of the GDNF family of ligands, which has demonstrated neuroprotective and regenerative properties in a number of PNS and CNS disease and injury models.5,28,33,37,42,55 The receptor complex for the GDNF family of ligands is composed of a signaling component Ret tyrosine kinase and 1 of 4 glycosylphosphatidylinositol-linked high-affinity ligand binding components designated as GFRα1–GFRα4. Glial cell line–derived neurotrophic factor has been extensively studied, especially with regard to its influence on neuronal populations including dopaminergic, central noradrenergic, sensory, and motoneurons.1,4,9,17,19,20,29,35,39,52 Designated as one of the most potent motoneuron survival factors,17 GDNF promotes peripheral nerve regeneration in multiple experimental models.3,16,30,38
To study the in vivo effects of GDNF on different aspects of motoneuron biology in normal and pathological conditions, transgenic mice overexpressing GDNF either in the target, that is, in the skeletal muscle (Myo-GDNF)34 or in the CNS and PNS (astrocytes and Schwann cells) (GFAP-GDNF) were generated.7,55 Using these transgenic animals, it has been shown that both target- and pathway-derived overexpression of GDNF rescues motoneuron subpopulations from developmental programmed cell death.33,35,55 Pathway-derived GDNF promotes complete survival of motoneurons following facial nerve axotomy in neonatal mice and nerve avulsion in adult mice.37,55 However, in a mouse model of amyotrophic lateral sclerosis, a disease isolated to spinal motoneurons, it has been demonstrated that only target-derived GDNF and not pathway-derived GDNF is neuroprotective.28 Furthermore, it has been demonstrated that the expression of endogenous GDNF and its receptor GFRα1 are upregulated both in the distal nerve stump and in skeletal muscle following a nerve injury.6,18,32,46 Moreover, it is known that GDNF is not homogeneously expressed throughout the axon in WT mice, and it is thought that its intracellular trafficking also affects its neuroprotective and regenerative properties.26,36,49
Significant previous work has been published,2,16,38 and based on the existing literature, we hypothesized that GDNF from different transgene sources would have different effects on peripheral nerve regeneration and functional recovery following nerve injury. To test this hypothesis, we performed a sciatic nerve crush injury in mice that overexpress GDNF in the CNS/PNS (GFAP-GDNF) or in skeletal muscle (Myo-GDNF). The present study was designed to further investigate the effects of pathway-derived versus centrally derived GDNF on injured peripheral nerves with respect to the rate of regeneration, myelination, fiber width distribution, and motor endplate reinnervation and their manifestation on muscle force production.
Methods
Animal Population
The GFAP-GDNF mice were generated to facilitate a greater than 175-fold increase in CNS GDNF overexpression and a 20-fold increase in PNS (nonmyelinating Schwann cell) GDNF overexpression as driven by the astrocyte-specific GFAP promoter. 55 To study the effects of GDNF overexpression in the muscle target, we used adult Myo-GDNF mice that overexpressed GDNF in muscle at 2.7 times normal endogenous levels as driven by the myogenin promoter.33 Integration of the GDNF transgene in heterozygous Myo-GDNF and GFAP-GDNF mice was determined by polymerase chain reaction on genomic DNA extracted from mouse tail specimens.
All experimental animals were housed in a central animal facility and were maintained pre- and postoperatively in strict accordance with the National Institutes of Health guidelines and according to protocols approved by the Division of Comparative Medicine at Washington University School of Medicine.
Surgical Procedures
All surgical procedures were performed using aseptic and microsurgical techniques. Mice were anesthetized using subcutaneous injections of ketamine (75 mg/kg) and medetomidine (0.5 mg/kg). The right hind leg was then shaved and depilated prior to antiseptic skin preparation. For the sciatic nerve crush model, animals were positioned prone, and a skin incision was made approximately 1 mm posterior and parallel to the femur. The superior margin of the biceps femoris muscle was bluntly dissected and retracted laterally, to expose the medially coursing sciatic nerve. The sciatic nerve was then crushed 5 mm proximal to its trifurcation with No. 5 jeweler’s forceps for 30 seconds.8 The crush site was marked with an 11-0 epineurial suture. The wound was irrigated, and the skin was closed with interrupted 6-0 nylon. Anesthesia was reversed with atipamezole hydrochloride (1 mg/kg), and the animals recovered on a heated surface.
Light Microscopy and Histomorphometry
Single transgenic Myo-GDNF, GFAP-GDNF, and WT mice were used and nerves (4–7 mice/group) were harvested at 0-, 2-, 3-, 4-, and 6-week time points. Segments of the sciatic nerves were harvested en bloc. Labeled nerve specimens from distinct regions proximal and distal to the site of crush injury were preserved in glutaraldehyde, postfixed in osmium tetroxide, and embedded in Araldite 502, and 1-μm cross-sections were obtained with an LKB III Ultramicrotome. Nerves were qualitatively assessed for the preservation of nerve architecture, quality and quantity of regenerated nerve fibers, extent of myelination, and the presence of ongoing wallerian degeneration. This proprietary semiautomated system used a series of algorithms to distinguish axons, myelin, nerve sheath, and debris from each other.21 Eight-bitplane digital pseudocoloring and our algorithms were used to distinguish axons, myelin, and debris from one another. Processed cross-sections were digitized and assessed for total fascicular area and total fiber number. The mean ± SD was used to present all data in this study. A 2-tailed ANOVA was made to determine the differences between individual groups. Histomorphometric calculations were performed using Statistica (StatSoft, Inc.). If significant, a Student-Newman-Keuls test was performed to compare groups. Statistical significance was established at p < 0.05.
Dynamic Muscle Force Measurement
Functional motor recovery following sciatic nerve crush was assessed in situ by measuring evoked force production in the EDL muscle. The Myo-GDNF, GFAP-GD-NF, and WT mice (2 or 3 mice/group) underwent sciatic nerve crush and were allowed to recover for 2, 4, and 6 weeks postoperatively. At the terminal time point, animals were anesthetized, as previously described, prior to microsurgical isolation of the sciatic nerve and EDL muscle. Specifically, the EDL muscle and distal tendons were exposed through a skin incision extending from the dorsum of the foot to the lateral knee. The distal EDL tendons were then severed, freed from the extensor retinacula, and sutured to a stainless steel S-hook. The sciatic nerve was subsequently exposed and proximally neurolysed prior to severing the tibial and sural nerves just distal to the point of trifurcation.
Prior to analysis, animals were placed on an adjustable warming bath where the target extremity was immobilized by transversely inserting a pin through the femoral condyles. The exposed sciatic nerve was bathed in mineral oil and proximally interfaced using a shielded bipolar silver wire electrode (Harvard Apparatus, Inc.). The distal tendons of the EDL muscle were then connected to the lever arm of a dual-mode lever system (model 300C, Aurora Scientific, Inc.) through the integrated S-hook. Simultaneous stimulation of the sciatic nerve and measurement of evoked active EDL muscle force was accomplished using a custom-built system consisting of a Grass S88× isolated pulse stimulator (Grass Technologies), model 300C dual-mode lever, model 604A interface board, and desktop PC running DMA/DMC software (Aurora Scientific, Inc.).
The amplitude of applied electrical impulses and the length of the EDL muscle required to produce maximum twitch force were determined for each muscle tested to standardize force measurements. The resulting optimal stimulus amplitude and optimal muscle length (Lo) were used for all subsequent measurements. Maximum isometric tetanic force was quantified by applying trains of electrical pulses (pulse width 0.2 msec, burst width 300 msec) of varying frequency (pulse frequency 30–400 Hz) to the sciatic nerve while recording resulting force production in the EDL muscle. Two-minute recovery periods were provided between trials to reduce muscle fatigue and data contamination.
Peak power produced by the EDL muscle was measured during isovelocity shortening contractions facilitated by preprogrammed rotation of the servo lever arm. The optimal fiber length (Lf) was calculated from Lo using an EDL-specific fiber length/muscle length ratio of 0.44.47 The EDL muscles were allowed to contract at increasing velocities (0.5–4.0 Lf/second) through a constant displacement around Lo (105 to 95% Lf) on stimulation of the sciatic nerve by trains of electrical pulses of varying frequency (pulse frequency 100–400 Hz). The average active force during isovelocity contraction was calculated from acquired force traces using custom data analysis software (MATLAB, MathWorks, Inc.). Power was subsequently calculated as the product of the average force and the velocity of contraction.
Maximum isometric tetanic force and peak power measurements were converted to maximum specific tetanic force (sFo) and normalized peak power (nPo), respectively, to better compare measurements acquired from various animals and eliminate variability associated with muscle atrophy. Contralateral uninjured EDL muscles (5–8 mice/group) were quantitatively analyzed in a similar fashion and served as controls. Following analysis, all animals were killed.
Statistical Analysis
Data were analyzed using a Kruskal-Wallis ANOVA test on ranks. All values are displayed as mean ± SD. Histomorphometry and muscle force and power testing were done following a post hoc Dunn test. Confocal imaging analysis was done following a post hoc Student-Newman-Keuls test. A p value < 0.05 was defined as significant for all groups.
Results
Sciatic Nerve Crush
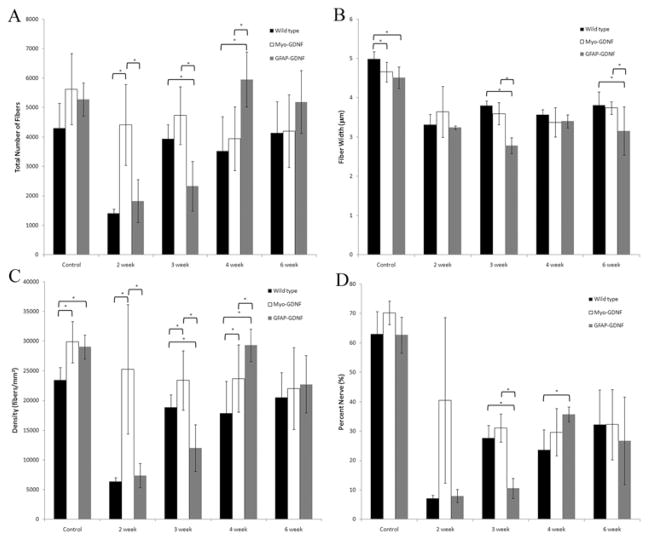
Histomorphometry was performed on harvested sciatic nerves 5 mm distal to the crush site following crush injury (Fig. 1). As depicted in Fig. 1 at the 2- and 3-week time points postcrush, Myo-GDNF mice demonstrated a significantly higher total number of nerve fibers, fiber density, and nerve percentage (p < 0.05) than the other genotypes. The Myo-GDNF mice had 4411 ± 1382 and 4733 ± 981 nerve fibers versus 1404± 157 and 3933 ± 489 fibers for WT and 1616 ± 780 and 2436 ± 795 fibers for GFAP-GDNF mice (Fig. 1A).
Fig. 1.
Bar graphs. Histomorphometry was performed on harvested sciatic nerves following crush injury. Compared with WT and GFAP-GDNF mice, Myo-GDNF demonstrated a statistically significantly higher total number of nerve fibers (A), fiber density (C), and nerve percentage (D) at 2 weeks after sciatic nerve crush injury. At the 3-week time point Myo-GDNF demonstrated a significantly higher total number of nerve fibers, fiber width, fiber density, and nerve percentage compared with GFAP-GDNF mice. The GFAP-GDNF animals demonstrated a delayed regenerative response; at 4 weeks they had significantly more total nerve fibers and fiber density than WT controls. At 6 weeks, there was no statistical difference between any of the groups or measured parameters. Asterisks denote statistical differences (p < 0.05) between measurements.
By the 4-week time point GFAP-GDNF mice had significantly more nerve fibers than either WT or Myo-GDNF animals (p < 0.05) (Fig. 1A). The GFAP-GDNF nerves contained 5954 ± 931 nerve fibers in comparison with 3522 ± 1160 for WT and 3937 ± 1080 for Myo-GDNF animals. The GFAP-GDNF animals had a higher fiber density in comparison with WTs at this time point (p < 0.05).
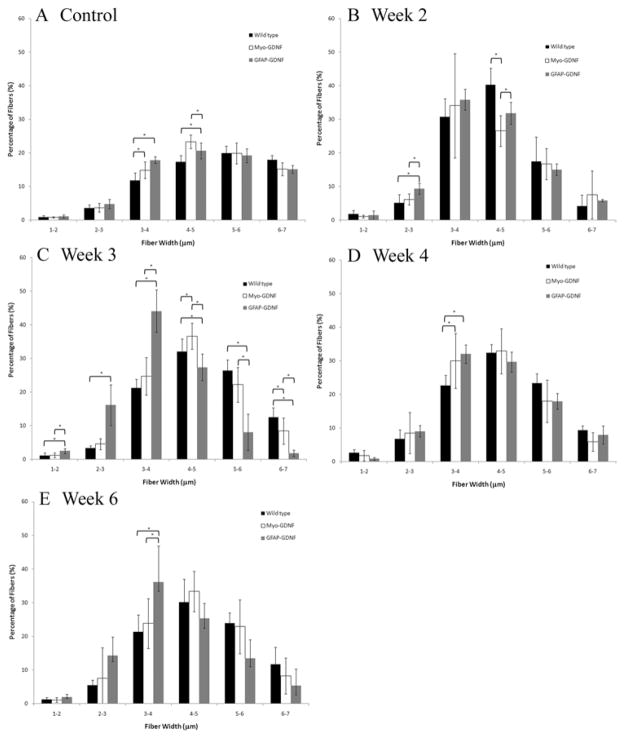
At the 6-week time point, there were no significant differences in total nerve fibers, fiber width, density, or percent nerve (Fig. 1). In addition, the fiber width distribution no longer demonstrated any significant difference regardless of the presence or site of GDNF overexpression (Fig. 2E).
Fig. 2.
Bar graphs showing the distribution of fiber width as a percentage of the total fibers. At each time point following sciatic nerve crush, fiber width data were stratified according to animal genotype. Stratification data are represented as a percentage of the total mean fiber width within each genotype. A: In uninjured control animals, there are statistical differences in fiber width distribution of smaller-diameter fibers. B: At 2 weeks after nerve crush, WT animals show a statistically significant increase in 4–5-μm fibers. C and D: The GFAP-GDNF animals show an increase in small-diameter fibers at 3 weeks (C), with significantly lower numbers of larger-diameter fibers compared with both WT and GFAP-GDNF. E: By 6 weeks, there are no differences between fiber distributions between genotypes. Asterisks denote statistical differences (p < 0.05) between measurements.
Dynamic Muscle Force Measurement of the EDL
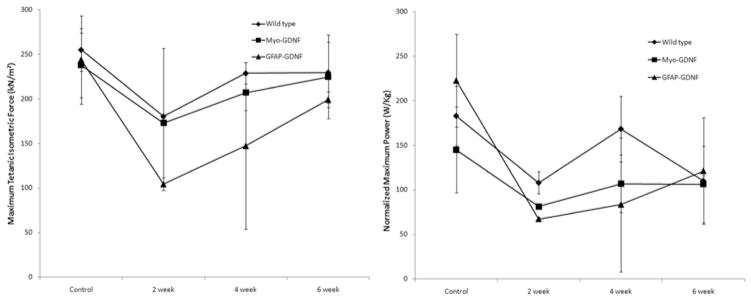
To correlate histological findings with functional motor recovery following sciatic nerve crush, the maximum specific tetanic force (sFo) and normalized peak power (nPo) evoked in the EDL muscle (Fig. 3) were measured in situ. Examination of sFo measurements acquired from Myo-GDNF, GFAP-GDNF, and WT mice revealed progressive, monotonic recovery of EDL function in all groups over the 6-week time course. Two weeks after sciatic nerve crush, EDL muscles of GFAP-GDNF mice demonstrated lower sFo levels (104.45 kN/m2) than those of Myo-GDNF mice (172.87 kN/m2), which were generally equivalent to levels in WT mice (180.22 kN/m2). The GFAP-GDNF mice continued to demonstrate lower average sFo levels at 4 weeks (147.40 kN/m2) than Myo-GDNF and WT mice (206.85 and 228.58 kN/m2). All experimental groups demonstrated sFo values indistinguishable from control measurements at the terminal time point. Further examination of postoperative sFo recovery suggests that all experimental groups recovered motor function at similar rates prior to reaching preinjury levels.
Fig. 3.
Bar graphs. Functional motor recovery following crush injury was assessed through an in situ measurement of maximum specific tetanic force production and normalized peak power production in the EDL muscle. Left: The Myo-GDNF mice demonstrated a greater recovery of specific force production compared with GFAP-GDNF mice at 2 weeks postoperatively, equivalent to WT mice. Right: At a similar time point, recovery of power production in Myo-GDNF and GFAP-GDNF mice was indistinguishable, although both Myo-GDNF and GFAP-GDNF mice demonstrated a lower power production than WT mice.
Examination of nPo measurements acquired from all experimental groups revealed progressive, monotonic recovery of dynamic EDL function in Myo-GDNF and GFAP-GDNF mice. The WT mice did not exhibit this characteristic, progressive recovery due to an unexplained spike in nPo production at the 4-week time point. Assessment of nPo data collected 2 weeks after sciatic nerve crush demonstrated greater power production in EDL muscles of WT mice (107.87 W/kg) compared with Myo-GDNF and GFAP-GDNF mice (81.20 and 67.10 W/kg). Decreased power production in GFAP-GDNF mice at 2 weeks mirrored decreased force production at the same time point. By 6 weeks, all groups had persistent nPo production deficits compared with baseline controls (Fig. 3), suggesting a greater functional deficit than that predicted by sFo measurements.
Discussion
Significant clinical and laboratory research has been devoted to GDNF applications. Glial cell line–derived neurotrophic factor enhances nerve regeneration,2,16,38 is an important trophic factor for motor neuron survival,17,34 and has demonstrated significant potential as a neuroprotective agent.10,11,33,35,55 Despite these advances, a major obstacle in GDNF utilization has been the method and site of delivery that will optimize its benefits.40 Transgenic animals that overexpress GDNF in the CNS and nonmyelinating Schwann cells (GFAP-GDNF) or in peripheral skeletal muscle (Myo-GDNF) have enabled investigators to study site-specific endogenous delivery. In this study, we examined CNS and pathway- versus target-derived GDNF in regard to peripheral nerve regeneration, and evaluated recovery after injury with histological, functional, and imaging outcome measures.
We have shown that Myo-GDNF animals demonstrate an earlier regenerative response 2 weeks after sciatic nerve crush. In contrast, GFAP-GDNF animals demonstrated a delayed neuroregenerative response with more nerve fibers and fiber density occurring by 4 weeks and maintained for at least 6 weeks. The histomorphometric analysis of the harvested sciatic nerves does not distinguish between axonal sprouting or motor and sensory fiber regeneration.14,21 The increased number of fibers noted at the 2- and 3-week time points in the Myo-GDNF group may represent any combination of these variables; however, the high percentage of small-caliber fibers at this early time point relative to uninjured controls (Fig. 2B) is most consistent with increased axonal sprouting. The high percentage of small-diameter fibers in the GFAP-GDNF sciatic nerves at 4 weeks (Fig. 2D) suggests delayed axonal sprouting, sensory nerve regeneration, or small-diameter intrafusal fiber regeneration. This observation supports the claim that GFAP-GDNF animals demonstrate a delayed regenerative response.
It is possible that the increase in fiber counts seen in this study is a result of the experimental model (Myo-GDNF and GFAP-GDNF) rather than the delivery modality of GDNF. Previously, it has been shown that the overexpression of GDNF in the muscle seen in Myo-GDNF mice used in this study results in a larger population of fusimotor neurons or γ-motoneurons during development.50 The increase in this neuron subpopulation correlates with an increase in the percentage of small-diameter γ-motoneuron fibers following development, which can be seen in the fiber distribution plots of our controls (γ-motoneuron fibers are < 3.5 μm in diameter50). It could be argued that the presence of an increased percentage of γ-motoneurons results in an inflated number of nerves observed 2 weeks following injury seen in the Myo-GDNF group in the current study. However, this inflation would then be accompanied by a statistical increase in the percent of γ-motoneuron fibers (< 3.5 μm in diameter), and no such difference is seen between our control and Myo-GDNF groups at any time point except for the 4th week. We believe that the lack of differences in small-diameter fiber distribution between the control and Myo-GDNF groups validates the conclusion that target-derived GDNF delivery increases early nerve fiber regeneration.
Despite the increase in nerve fiber counts, the early regenerative response in Myo-GDNF mice did not directly correlate with superlative functional recovery. Muscle force testing at 2 weeks was equivalent between Myo-GDNF and WT mice. At 4 weeks, GFAP-GDNF mice demonstrated a dramatic increase in total nerve fibers, yet functional data lagged behind with force and power not returning to WT and Myo-GDNF levels until 6 weeks. The discrepancy between estimates of functional recovery based on maximum specific tetanic force (sFo) and normalized peak power (nPo) measurements noted previously,53 specifically demonstrates the complexity of peak power measurements. While maximum specific force values primarily measure functional reinnervation of muscle fibers by regenerating axons, normalized peak power measurements are simultaneously affected by variable reinnervation of muscle fibers, muscle fiber type switching, and muscle fibrosis.23,48 Comparison of sFo and nPo measurements suggests that CNS- and pathway-derived GDNF in GFAP-GDNF mice and target-derived GDNF in Myo-GDNF mice may delay and expedite acute nerve regeneration and functional reinnervation of distal motor targets, respectively. This variable GDNF production has little effect on dynamic muscle function or clinical outcomes in such an acute setting. Furthermore, these findings reinforce the principle that enhanced nerve regeneration alone is unable to overcome the many pathological processes limiting functional motor recovery following peripheral nerve injury and muscle denervation.
The Role of GDNF Gradients in Peripheral Nerve Regeneration
The presence of an enhanced gradient of GDNF expression when regenerating toward denervated muscle tissue may influence axonal regeneration in Myo-GDNF animals. In WT animals, the concentration of GDNF in adult mice is higher in peripheral nerves than in the fore-brain, brainstem, cerebellum, and spinal cord, suggesting that endogenous expression by peripheral Schwann cells and muscle tissue is greater than expression in the CNS.55 This physiological pattern of endogenous GDNF being concentrated in the periphery versus the CNS is preserved and increased in the Myo-GDNF animals.33 In contrast to this, GFAP-GDNF mice, created with the GFAP construct, were optimized for transgene expression to reside predominantly in glial cells, leading to much higher GDNF expression in the CNS. Although these animals have a higher CNS concentration of GDNF relative to peripheral expression, their peripheral expression is still 20-fold higher than that found in WT animals as measured by enzyme-linked immunosorbent assay (ELISA) of pooled sciatic nerve tissues.55 Regardless of this increase, the physiological pattern of endogenous GDNF being higher in the periphery than in the CNS is not preserved in the GFAP-GDNF mice. In light of our results following sciatic nerve crush, these known concentration differences may explain some of our findings.
The GFAP and Myogenin Regulatory Elements
Other factors to consider in the interpretation of our results are the regulatory elements of the transgenic animals studied (GFAP and myogenin). The GFAP is expressed by astrocytes, in addition to nonmyelinating Schwann cells and reactive Muller cells.15,21,22,40 The mechanisms of GFAP regulation in vivo are not fully understood, but it is known that endogenous murine GFAP expression coincides with early neural development and astroglial differentiation, with an increase in GFAP mRNA until 1–2 weeks after birth, followed by a slow decline.7,27,44 Similar to this expression trend in development, it has also been shown that GFAP expression is increased after nerve injury,25 and immunoreactivity of the promoter following facial nerve axotomy in neonates peaks at 1 week and begins to decline after 2 weeks toward normal levels.43 Although the GFAP gene activation mechanism may differ in distinct lesions,45 it is possible that its upregulation and the resulting spike in GDNF concentration in the nerve pathway at 1 and 2 weeks were responsible for the delay seen in sciatic nerve regeneration in GFAP-GDNF animals compared with Myo-GDNF animals at these time points, presumably because of the loss of gradient to the muscle target. Additionally, upregulation of GDNF by Schwann cells in the GFAP-GDNF animals may have induced axonal sprouting and maintenance at the motor endplate, as seen in the EDL confocal images at 1 and 2 weeks.
Myogenin, a helix-loop-helix transcription factor, plays a role in the differentiation of progenitor cells into mature myocytes and is localized in myonuclei after denervation.24 It is transcriptionally repressed by electrical activity provided by motor innervation, and it is rapidly induced following the loss of function, as occurs in denervation.12,31,41,51 In a rat sciatic nerve transection model, myogenin RNA as measured in EDL muscles, has a 50-fold induction 3 days after injury and remains at a 30-fold induction level by 2 weeks.54 In a mouse denervation study, a 40-fold increase of myogenin mRNA is noted in the gastrocnemius muscle with a rapid accumulation of myogenin mRNAs occurring as early as 8 and 16 hours after denervation.13 The rapid on/off of the myogenin regulatory element is one possible explanation for the observed early increase in the total number of nerve fibers in Myo-GDNF animals after sciatic nerve crush, as compared with the other genotypes. Additionally, this early production of GDNF by the Myo-GDNF animals after denervation may have promoted axonal sprouting and maintenance at the motor endplate, as observed on confocal imaging.
Conclusions
In the present study, the end point or target overexpression of GDNF (Myo-GDNF) was characterized by an increase in the number of regenerating axons 2 weeks after injury and a more rapid reinnervation of motor end-plates by terminal axons. Significant differences between these parameters in end point versus pathway GDNF over-expression were not evident at later time points and did not translate to significant differences in muscle isometric force or power production. While findings from this study suggest that the end point overexpression of GDNF may stimulate nerve regeneration, the results must be interpreted in the context of a transgenic mouse model. It will be necessary to both investigate the exogenous peripheral delivery of GDNF and apply our findings to a large animal model. This will enable more sensitive measures of muscle force production following reinnervation and facilitate the evaluation of longer peripheral nerves, which translate to later experimental time points that are more clinically relevant to human peripheral nerve injury.
Acknowledgments
The authors are grateful to the Barnes-Jewish Foundation for confocal microscopy instrument equipment and support. The authors are grateful to Dr. Gregory Borschel for input on this work.
Abbreviations used in this paper
- EDL
extensor digitorum longus
- GDNF
glial cell line–derived neurotrophic factor
- GFAP
glial fibrillary acidic protein
- GFR
GDNF family receptor
- PNS
peripheral nervous system
- WT
wild-type
Footnotes
Disclosure
This study was funded by the National Institutes of Health RO1 Grant No. NS051706 awarded to Dr. Mackinnon, in addition to the American College of Surgeons C. James Carrico Faculty Research Fellowship and the American Association of Plastic Surgeons John E. Hoopes Fellowship awarded to Dr. Myckatyn.
References
- 1.Arenas E, Trupp M, Akerud P, Ibáñez CF. GDNF prevents degeneration and promotes the phenotype of brain noradrenergic neurons in vivo. Neuron. 1995;15:1465–1473. doi: 10.1016/0896-6273(95)90024-1. [DOI] [PubMed] [Google Scholar]
- 2.Barras FM, Pasche P, Bouche N, Aebischer P, Zurn AD. Glial cell line-derived neurotrophic factor released by synthetic guidance channels promotes facial nerve regeneration in the rat. J Neurosci Res. 2002;70:746–755. doi: 10.1002/jnr.10434. [DOI] [PubMed] [Google Scholar]
- 3.Bohn MC. Motoneurons crave glial cell line-derived neurotrophic factor. Exp Neurol. 2004;190:263–275. doi: 10.1016/j.expneurol.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Borgal L, Hong M, Sadi D, Mendez I. Differential effects of glial cell line-derived neurotrophic factor on A9 and A10 dopamine neuron survival in vitro. Neuroscience. 2007;147:712–719. doi: 10.1016/j.neuroscience.2007.03.057. [DOI] [PubMed] [Google Scholar]
- 5.Boyd JG, Gordon T. Glial cell line-derived neurotrophic factor and brain-derived neurotrophic factor sustain the axonal regeneration of chronically axotomized motoneurons in vivo. Exp Neurol. 2003;183:610–619. doi: 10.1016/s0014-4886(03)00183-3. [DOI] [PubMed] [Google Scholar]
- 6.Boyd JG, Gordon T. Neurotrophic factors and their receptors in axonal regeneration and functional recovery after peripheral nerve injury. Mol Neurobiol. 2003;27:277–324. doi: 10.1385/MN:27:3:277. [DOI] [PubMed] [Google Scholar]
- 7.Brenner M, Kisseberth WC, Su Y, Besnard F, Messing A. GFAP promoter directs astrocyte-specific expression in transgenic mice. J Neurosci. 1994;14:1030–1037. doi: 10.1523/JNEUROSCI.14-03-01030.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bridge PM, Ball DJ, Mackinnon SE, Nakao Y, Brandt K, Hunter DA, et al. Nerve crush injuries—a model for axonotmesis. Exp Neurol. 1994;127:284–290. doi: 10.1006/exnr.1994.1104. [DOI] [PubMed] [Google Scholar]
- 9.Cao JP, Wang HJ, Yu JK, Yang H, Xiao CH, Gao DS. Involvement of NCAM in the effects of GDNF on the neurite outgrowth in the dopamine neurons. Neurosci Res. 2008;61:390–397. doi: 10.1016/j.neures.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Cheng H, Huang SS, Lin SM, Lin MJ, Chu YC, Chih CL, et al. The neuroprotective effect of glial cell line-derived neurotrophic factor in fibrin glue against chronic focal cerebral ischemia in conscious rats. Brain Res. 2005;1033:28–33. doi: 10.1016/j.brainres.2004.10.067. [DOI] [PubMed] [Google Scholar]
- 11.Cheng H, Wu JP, Tzeng SF. Neuroprotection of glial cell line-derived neurotrophic factor in damaged spinal cords following contusive injury. J Neurosci Res. 2002;69:397–405. doi: 10.1002/jnr.10303. [DOI] [PubMed] [Google Scholar]
- 12.Edmondson DG, Olson EN. A gene with homology to the myc similarity region of MyoD1 is expressed during myogenesis and is sufficient to activate the muscle differentiation program. Genes Dev. 1989;3:628–640. doi: 10.1101/gad.3.5.628. [DOI] [PubMed] [Google Scholar]
- 13.Eftimie R, Brenner HR, Buonanno A. Myogenin and MyoD join a family of skeletal muscle genes regulated by electrical activity. Proc Natl Acad Sci U S A. 1991;88:1349–1353. doi: 10.1073/pnas.88.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fawcett JW, Keynes RJ. Peripheral nerve regeneration. Annu Rev Neurosci. 1990;13:43–60. doi: 10.1146/annurev.ne.13.030190.000355. [DOI] [PubMed] [Google Scholar]
- 15.Feinstein DL, Weinmaster GA, Milner RJ. Isolation of cDNA clones encoding rat glial fibrillary acidic protein: expression in astrocytes and in Schwann cells. J Neurosci Res. 1992;32:1–14. doi: 10.1002/jnr.490320102. [DOI] [PubMed] [Google Scholar]
- 16.Fine EG, Decosterd I, Papaloïzos M, Zurn AD, Aebischer P. GDNF and NGF released by synthetic guidance channels support sciatic nerve regeneration across a long gap. Eur J Neurosci. 2002;15:589–601. doi: 10.1046/j.1460-9568.2002.01892.x. [DOI] [PubMed] [Google Scholar]
- 17.Henderson CE, Phillips HS, Pollock RA, Davies AM, Lemeulle C, Armanini M, et al. GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science. 1994;266:1062–1064. doi: 10.1126/science.7973664. [DOI] [PubMed] [Google Scholar]
- 18.Höke A, Cheng C, Zochodne DW. Expression of glial cell line-derived neurotrophic factor family of growth factors in peripheral nerve injury in rats. Neuroreport. 2000;11:1651–1654. doi: 10.1097/00001756-200006050-00011. [DOI] [PubMed] [Google Scholar]
- 19.Höke A, Redett R, Hameed H, Jari R, Zhou C, Li ZB, et al. Schwann cells express motor and sensory phenotypes that regulate axon regeneration. J Neurosci. 2006;26:9646–9655. doi: 10.1523/JNEUROSCI.1620-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang L, Guo H, Hellard DT, Katz DM. Glial cell line-derived neurotrophic factor (GDNF) is required for differentiation of pontine noradrenergic neurons and patterning of central respiratory output. Neuroscience. 2005;130:95–105. doi: 10.1016/j.neuroscience.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 21.Hunter DA, Moradzadeh A, Whitlock EL, Brenner MJ, Myckatyn TM, Wei CH, et al. Binary imaging analysis for comprehensive quantitative histomorphometry of peripheral nerve. J Neurosci Methods. 2007;166:116–124. doi: 10.1016/j.jneumeth.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jessen KR, Mirsky R. Nonmyelin-forming Schwann cells co-express surface proteins and intermediate filaments not found in myelin-forming cells: a study of Ran-2, A5E3 antigen and glial fibrillary acidic protein. J Neurocytol. 1984;13:923–934. doi: 10.1007/BF01148594. [DOI] [PubMed] [Google Scholar]
- 23.Kalliainen LKJS, Jejurikar SS, Liang LW, Urbanchek MG, Kuzon WM., Jr A specific force deficit exists in skeletal muscle after partial denervation. Muscle Nerve. 2002;25:31–38. doi: 10.1002/mus.1216. [DOI] [PubMed] [Google Scholar]
- 24.Krempler A, Brenig B. Zinc finger proteins: watchdogs in muscle development. Mol Gen Genet. 1999;261:209–215. doi: 10.1007/s004380050959. [DOI] [PubMed] [Google Scholar]
- 25.Laping NJ, Teter B, Nichols NR, Rozovsky I, Finch CE. Glial fibrillary acidic protein: regulation by hormones, cytokines, and growth factors. Brain Pathol. 1994;4:259–275. doi: 10.1111/j.1750-3639.1994.tb00841.x. [DOI] [PubMed] [Google Scholar]
- 26.Lauffenburger DA. Right on cue. Nat Chem Biol. 2006;2:569–570. doi: 10.1038/nchembio1106-569. [DOI] [PubMed] [Google Scholar]
- 27.Lewis SACN, Cowan NJ. Temporal expression of mouse glial fibrillary acidic protein mRNA studied by a rapid in situ hybridization procedure. J Neurochem. 1985;45:913–919. doi: 10.1111/j.1471-4159.1985.tb04080.x. [DOI] [PubMed] [Google Scholar]
- 28.Li W, Brakefield D, Pan Y, Hunter D, Myckatyn TM, Parsadanian A. Muscle-derived but not centrally derived transgene GDNF is neuroprotective in G93A-SOD1 mouse model of ALS. Exp Neurol. 2007;203:457–471. doi: 10.1016/j.expneurol.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 29.Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F, Lin LF, et al. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 30.Madduri S, Papaloizos M, Gander B. Synergistic effect of GDNF and NGF on axonal branching and elongation in vitro. Neurosci Res. 2009;65:88–97. doi: 10.1016/j.neures.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Merlie JP, Mudd J, Cheng TC, Olson EN. Myogenin and acetylcholine receptor alpha gene promoters mediate transcriptional regulation in response to motor innervation. J Biol Chem. 1994;269:2461–2467. [PubMed] [Google Scholar]
- 32.Naveilhan P, ElShamy WM, Ernfors P. Differential regulation of mRNAs for GDNF and its receptors Ret and GDNFR alpha after sciatic nerve lesion in the mouse. Eur J Neurosci. 1997;9:1450–1460. doi: 10.1111/j.1460-9568.1997.tb01499.x. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen QT, Parsadanian AS, Snider WD, Lichtman JW. Hyperinnervation of neuromuscular junctions caused by GDNF overexpression in muscle. Science. 1998;279:1725–1729. doi: 10.1126/science.279.5357.1725. [DOI] [PubMed] [Google Scholar]
- 34.Oppenheim RW, Houenou LJ, Johnson JE, Lin LF, Li L, Lo AC, et al. Developing motor neurons rescued from programmed and axotomy-induced cell death by GDNF. Nature. 1995;373:344–346. doi: 10.1038/373344a0. [DOI] [PubMed] [Google Scholar]
- 35.Oppenheim RW, Houenou LJ, Parsadanian AS, Prevette D, Snider WD, Shen L. Glial cell line-derived neurotrophic factor and developing mammalian motoneurons: regulation of programmed cell death among motoneuron subtypes. J Neurosci Methods. 2000;20:5001–5011. doi: 10.1523/JNEUROSCI.20-13-05001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paratcha G, Ledda F. GDNF and GFRalpha: a versatile molecular complex for developing neurons. Trends Neurosci. 2008;31:384–391. doi: 10.1016/j.tins.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 37.Parsadanian A, Pan Y, Li W, Myckatyn TM, Brakefield D. Astrocyte-derived transgene GDNF promotes complete and long-term survival of adult facial motoneurons following avulsion and differentially regulates the expression of transcription factors of AP-1 and ATF/CREB families. Exp Neurol. 2006;200:26–37. doi: 10.1016/j.expneurol.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 38.Piquilloud G, Christen T, Pfister LA, Gander B, Papaloïzos MY. Variations in glial cell line-derived neurotrophic factor release from biodegradable nerve conduits modify the rate of functional motor recovery after rat primary nerve repairs. Eur J Neurosci. 2007;26:1109–1117. doi: 10.1111/j.1460-9568.2007.05748.x. [DOI] [PubMed] [Google Scholar]
- 39.Quintero EM, Willis LM, Zaman V, Lee J, Boger HA, Tomac A, et al. Glial cell line-derived neurotrophic factor is essential for neuronal survival in the locus coeruleus-hippocampal noradrenergic pathway. Neuroscience. 2004;124:137–146. doi: 10.1016/j.neuroscience.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 40.Salvatore MF, Ai Y, Fischer B, Zhang AM, Grondin RC, Zhang Z, et al. Point source concentration of GDNF may explain failure of phase II clinical trial. Exp Neurol. 2006;202:497–505. doi: 10.1016/j.expneurol.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 41.Sassoon D, Lyons G, Wright WE, Lin V, Lassar A, Weintraub H, et al. Expression of two myogenic regulatory factors myogenin and MyoDl during mouse embryogenesis. Nature. 1989;341:303–307. doi: 10.1038/341303a0. [DOI] [PubMed] [Google Scholar]
- 42.Tannemaat MR, Eggers R, Hendriks WT, de Ruiter GC, van Heerikhuize JJ, Pool CW, et al. Differential effects of lentiviral vector-mediated overexpression of nerve growth factor and glial cell line-derived neurotrophic factor on regenerating sensory and motor axons in the transected peripheral nerve. Eur J Neurosci. 2008;28:1467–1479. doi: 10.1111/j.1460-9568.2008.06452.x. [DOI] [PubMed] [Google Scholar]
- 43.Tao R, Aldskogius H. Glial cell responses, complement and apolipoprotein J expression following axon injury in the neonatal rat. J Neurocytol. 1999;28:559–570. doi: 10.1023/a:1007067305837. [DOI] [PubMed] [Google Scholar]
- 44.Tardy MFC, Fages C, Riol H, LePrince G, Rataboul P, Charriere-Bertrand C, et al. Developmental expression of the glial fibrillary acidic protein mRNA in the central nervous system and in cultured astrocytes. J Neurochem. 1989;52:162–167. doi: 10.1111/j.1471-4159.1989.tb10911.x. [DOI] [PubMed] [Google Scholar]
- 45.Titeux M, Galou M, Gomes FC, Dormont D, Neto VM, Paulin D. Differential activation of GFAP gene promoter upon infection with prion versus virus infection. Brain Res Mol Brain Res. 2002;109:119–127. doi: 10.1016/s0169-328x(02)00547-8. [DOI] [PubMed] [Google Scholar]
- 46.Trupp M, Belluardo N, Funakoshi H, Ibanez CF. Complementary and overlapping expression of glial cell line-derived neurotrophic factor (GDNF), c-ret proto-oncogene, and GDNF receptor-α indicates multiple mechanisms of trophic actions in the adult rat CNS. J Neurosci Methods. 1997;17:3554–3567. doi: 10.1523/JNEUROSCI.17-10-03554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Urbanchek MS, Chung KC, Asato H, Washington LN, Kuzon WM., Jr Rat walking tracks do not reflect maximal muscle force capacity. J Reconstr Microsurg. 1999;15:143–149. doi: 10.1055/s-2007-1000085. [DOI] [PubMed] [Google Scholar]
- 48.van der Meulen JHUM, Urbanchek MG, Cederna PS, Eguchi T, Kuzon WM., Jr Denervated muscle fibers explain the deficit in specific force following reinnervation of the rat extensor digitorum longus muscle. Plast Reconstr Surg. 2003;112:1336–1346. doi: 10.1097/01.PRS.0000081464.98718.E3. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y, Geng Z, Zhao L, Huang SH, Sheng AL, Chen ZY. GDNF isoform affects intracellular trafficking and secretion of GDNF in neuronal cells. Brain Res. 2008;1226:1–7. doi: 10.1016/j.brainres.2008.05.087. [DOI] [PubMed] [Google Scholar]
- 50.Whitehead J, Keller-Peck C, Kucera J, Tourtellotte WG. Glial cell-line derived neurotrophic factor-dependent fusimotor neuron survival during development. Mech Dev. 2005;122:27–41. doi: 10.1016/j.mod.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 51.Wright WE, Sassoon DA, Lin VK. Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell. 1989;56:607–617. doi: 10.1016/0092-8674(89)90583-7. [DOI] [PubMed] [Google Scholar]
- 52.Yan Q, Matheson C, Lopez OT. In vivo neurotrophic effects of GDNF on neonatal and adult facial motor neurons. Nature. 1995;373:341–344. doi: 10.1038/373341a0. [DOI] [PubMed] [Google Scholar]
- 53.Yoshimura K, Asato H, Cederna PS, Urbanchek MG, Kuzon WM. The effect of reinnervation on force production and power output in skeletal muscle. J Surg Res. 1999;81:201–208. doi: 10.1006/jsre.1998.5498. [DOI] [PubMed] [Google Scholar]
- 54.Zeman R, Zhao J, Zhang Y, Zhao W, Wen X, Wu Y, et al. Differential skeletal muscle gene expression after upper or lower motor neuron transection. Pflugers Arch. 2009;58:525–535. doi: 10.1007/s00424-009-0643-5. [DOI] [PubMed] [Google Scholar]
- 55.Zhao Z, Alam S, Oppenheim RW, Prevette DM, Evenson A, Parsadanian A. Overexpression of glial cell line-derived neurotrophic factor in the CNS rescues motoneurons from programmed cell death and promotes their long-term survival following axotomy. Exp Neurol. 2004;190:356–372. doi: 10.1016/j.expneurol.2004.06.015. [DOI] [PubMed] [Google Scholar]