Abstract
Background
To determine if the APOE e4 allele influences both the functional activation and connectivity of the medial temporal lobes (MTL) during successful memory encoding in young adults.
Methods
Twenty-four healthy young adults, twelve carriers and twelve non-carriers of the APOE e4 allele, were scanned in a subsequent memory paradigm, using event-related functional magnetic resonance imaging (fMRI). The neuroanatomical correlates of successful encoding were measured as greater neural activity for subsequently remembered versus forgotten task items, or in short, encoding success activity (ESA). Group differences in ESA within the MTL, as well as whole brain functional connectivity with the MTL, were assessed.
Results
In the absence of demographic or performance differences, APOE e4 allele carriers exhibited greater bilateral MTL activity relative to the non-carriers to accomplish the same encoding task. Additionally, while e4 carriers demonstrated greater functional connectivity of ESA-related MTL activity with the posterior cingulate (PCC) and other peri-limbic regions, overall connectivity reductions were found across anterior and posterior cortices.
Conclusions
These results suggest that the APOE e4 allele may influence not only functional activations within the MTL, but functional connectivity of the MTL to other regions implicated in memory encoding. Enhanced functional connectivity of the MTL with the PCC in young adult e4 carriers suggests that APOE may be expressed early in brain regions known to be involved in Alzheimer's disease long before late-onset dementia is a practical risk or consideration. It is also possible that these functional connectivity differences reflect pleiotropic effects of APOE during early development.
Keywords: fMRI, Memory, Genetics, Alzheimer's disease, Functional Connectivity, APOE
Introduction
The apolipoprotein (APOE) e4 allele has long been associated with an increased risk for mild cognitive impairment (MCI) and late-onset Alzheimer's disease (AD) (Petersen, Waring, Smith, Tangalos and Thibodeau, 1996; Saunders, Strittmatter, Schmechel, George-Hyslop, Pericak-Vance, Joo et al., 1993; Tervo, Kivipelto, Hanninen, Vanhanen, Hallikainen, Mannermaa et al., 2004; Tschanz, Welsh-Bohmer, Lyketsos, Corcoran, Green, Hayden et al., 2006). In recent years, functional magnetic resonance imaging (fMRI) studies have observed that older healthy adult e4 carriers, compared to non-carriers, exhibit greater magnitude and extent of neural activity when performing memory tasks (Bondi, Houston, Eyler and Brown, 2005; Bookheimer, Strojwas, Cohen, Saunders, Pericak-Vance, Mazziotta et al., 2000; Dickerson, Goncharova, Sullivan, Forchetti, Wilson, Bennett et al., 2001) (but see Trivedi, Schmitz, Ries, Torgerson, Sager, Hermann et al., 2006), suggesting that the presence of the APOE e4 allele is associated with alterations in neural activity. Similar enhancements in signal magnitude during memory performance have been observed in MCI patients (Kircher, Weis, Freymann, Erb, Jessen, Grodd et al., 2007) (but see Machulda, Ward, Borowski, Gunter, Cha, O'Brien et al., 2003), suggesting that the APOE e4 carrier-associated changes observed in normal elderly may reflect latent compensatory neural activity (Bookheimer, Strojwas, Cohen, Saunders, Pericak-Vance, Mazziotta et al., 2000) and/or increased cognitive effort (Bondi, Houston, Eyler and Brown, 2005) when performing episodic memory tasks.
Regarding genotype effects in young adults, the results remains unclear as to what neural or cognitive effects may be associated with APOE e4 carrier status earlier in the lifespan. Bondi and colleagues have suggested that APOE e4 plays a antagonistic pleiotropic (Williams, 1957) role for cognition in early adulthood, but serves as a risk factor for dementia in ongoing years (Han and Bondi, 2008). Limited support for their suggestion of APOE-related antagonistic pleiotropism comes from a recent study of examining the role of APOE genotype in recovery of function following traumatic brain injury in young adults (mean age = 23.91 yrs). The study found that e4 carriers outperformed non-carriers on a range of tests assessing attention, executive function, and episodic memory encoding (Han, Drake, Cessante, Jak, Houston, Delis et al., 2007). The authors suggest these results suggest a pleiotropic advantage of the e4 genotype in developing and/or utilizing neurocognitive compensatory mechanisms. Expounding on this idea, the authors also suggest that healthy older adults with the e4 allele are able to buffer against episodic memory declines by recruiting additional and compensatory cognitive resources in order to perform cognitive tasks at a level comparable to non-carriers (Han and Bondi, 2008). Moreover, Bondi and colleagues (Han and Bondi, 2008) argue that “buffering” may begin early in the lifespan with e4 carriers cognitively outperforming non-carriers, and recruiting increased cognitive and neural mechanisms to accomplish said levels.
Recent work by Shaw and colleagues (2007) may provide a neuroanatomical basis for the cognitive “buffering” proposed by Bondi and colleagues, as they discovered a linear decrease in anterior MTL grey matter volume in healthy adolescents as a function of APOE allele carrier status (i.e., APOE e2 > e3 > e4 carriers). Shaw et al.'s (2007) results reinforce findings from other researchers that APOE, in concert with low density lipoprotein receptors, may play a fundamental role in cerebral development (Herz and Chen, 2006; Qiu, Korwek and Weeber, 2006), and that carriers of the e4 allele may inherently have thinner entorhinal cortices, establishing a necessary condition for the cognitive “buffering” described by Bondi and colleagues. In essence, any antagonistic pleiotrophy of APOE e4 that might be argued based upon middle aged neuropsychological performance outcomes (Han and Bondi, 2008), may arise only as a by-product of possible negative effects on cortical development much earlier in the lifespan.
While the aforementioned studies suggest a rational for APOE-associated activation differences in young adults, the studies themselves did not test functional differences between APOE genotypes. In fact, only a limited number of studies have attempted to identify at what developmental stage these genotypically-driven brain activity changes are first observed (Filbey, Slack, Sunderland and Cohen, 2006; Filippini, Macintosh, Hough, Goodwin, Frisoni, Smith et al., 2009; Mondadori, de Quervain, Buchmann, Mustovic, Wollmer, Schmidt et al., 2007; Scarmeas, Habeck, Hilton, Anderson, Flynn, Park et al., 2005). And, none have determined if there is an e4 allele carrier effect in the fMRI activity of healthy young adults or if APOE-associated differences in neural activity are related specifically to successful memory performance. The current study used the subsequent memory paradigm (Paller and Wagner, 2002), which identifies brain regions showing greater study phase activity for items that are remembered than for those that are forgotten in a subsequent memory test, in order to determine if the presence of the e4 allele was associated with differences in neural correlates governing memory success in healthy young adults.
Studies of functional, structural, and behavioral differences associated with variants of the APOE genotype have focused on the medial temporal lobe (MTL) based on abundant evidence identifying the MTL as the earliest site of AD-associated pathology (Braak, Braak and Bohl, 1993). The MTL has been shown to be the first region in the development of the neurofibrillary tangles that are one of the hallmarks for the staging of AD (Braak and Braak, 1998) and one of the first structures to show volumetric changes in AD (e.g., Jack, Petersen, Xu, Waring, O'Brien, Tangalos et al., 1997). These MTL-associated histopathological and volumetric changes have also been associated with decreased performance on a wide range of memory tasks in older adults (e.g., (Mori, Yoneda, Yamashita, Hirono, Ikeda and Yamadori, 1997; Remy, Mirrashed, Campbell and Richter, 2005; Stout, Bondi, Jernigan, Archibald, Delis and Salmon, 1999). Based upon the foregoing evidence linking the MTL to the earliest site AD-associated pathology (Braak and Braak, 1996) and recent work suggesting that APOE-related structural differences in the MTL may exist as early as late childhood (Shaw, Lerch, Pruessner, Taylor, Rose, Greenstein et al., 2007), as well as the known role of the MTL in episodic memory abilities (Dobbins and Davachi, 2006), our analyses focused primarily on group differences with this region.
Based upon this research, we focused our analyses of APOE effects on successful memory activity to that of the MTL. Specifically we sought to identify genotyped differences in encoding success activity (ESA) in the MTL, as well as differences in functional connectivity with these region, also associated with successful encoding processing. In doing so we also sought to test Bondi's compensation/pleiotropic theory of APOE by examining whether e4 carriers would exhibit increased ESA and functional connectivity in the MTL associated with enhanced behavioral performance.
Methods and Materials
Participants
We studied 24 right-handed healthy young adults, genotyped for APOE (12 APOE e4 carriers and 12 non-carriers). Participants with a history of neurological difficulties or psychiatric illness, alcoholism, drug abuse, and learning disabilities were excluded from the study. The participants were matched on age, years of education, and a battery of neuropsychological tests taken from the Cambridge Neuropsychological Test Automated Battery (CANTABeclipse, version 2.0; Cambridge Cognition Ltd; see Table 1). All participants provided written informed consent and received financial compensation for their participation. All experimental procedures were approved by the Duke University Institutional Review Board for the ethical treatment of human participants.
Table 1.
Demographic and behavioral characteristics
| e4 carriers (n = 12) |
non-carriers (n = 12) |
p-value a | |
|---|---|---|---|
| APOE Genotype Distribution | 11 (3/4) | 4 (2/3) | |
| 1 (4/4) | 8 (3/3) | ||
| Gender (F) | 7 | 9 | |
| Mean age, y (SD) | 23.2 (3.3) | 22.8 (2.6) | 0.73 |
| Neuropsychological Assessment Battery Performances b | |||
| Spatial Span length (SD) | 7.2 (1.5) | 7.3 (1.3) | 0.89 |
| Intra-Extra Dimensional Set Shifting errors (SD) | 3.3 (3.2) | 5.2 (8.5) | 0.49 |
| Paired Associate Learning errors (SD) | 2.7 (3.0) | 3.7 (4.8) | 0.51 |
| Pattern Recognition Memory % correct (SD) | 91.5 (15.3) | 89.2 (10.0) | 0.67 |
| Choice Reaction Time ms, (SD) | 340.7 (37.0) | 316.9 (45.9) | 0.18 |
| Spatial Recognition Memory % correct (SD) | 87.9 (10.5) | 84.6 (10.3) | 0.44 |
| Rapid Visual Information Processing hit probability (SD) | 0.8 (0.2) | 0.8 (0.2) | 0.93 |
| fMRI Procedure Performances | |||
| Animacy Decision accuracy (SD) | 97 (0.1) | 100 (0.0) | 0.20 |
| Animacy Decision Reaction Time ms, (SD) | 1010 (210.0) | 1090 (250.0) | 0.40 |
| Subsequent Item Memory Hit Rate (SD) | 0.5 (0.2) | 0.4 (0.2) | 0.44 |
| Subsequent Item Memory False Alarm Rate (SD) | 0.3 (0.2) | 0.3 (0.1) | 0.48 |
Independent, two-sample t-test comparisons.
Cambridge Neuropsychological Test Automated Battery (CANTABeclipse, version 2.0; Cambridge Cognition, Ltd.) subtests.
Genotyping
Taqman-based allelic discrimination assay (Applied Biosystems) was used for genotype determination. Two separate genotype assays were used to establish the APOE status of a subject: (1) rs429358 334 T/C (ABI assay ID: C_3084793_20), and (2) rs7412 472T/C (ABI assay ID: C_904973_10). Assays were conducted per manufacturer's protocol, using 10 ng of DNA from each subject per assay. Fluorescence outputs were quantified in real time using a 7900HT Fast Real Time PCR System and the data were analyzed using SDS software v.2.2.2 (Applied Biosystems). APOE genotype assignments were defined as described by Koch and colleagues (Koch, Ehrenhaft, Griesser, Pfeufer, Muller, Schomig et al., 2002).
Stimuli and procedure
Participants encoded 120 emotionally neutral pictures taken from the Snodgrass and Vanderwart visual object stimuli set (Snodgrass and Vanderwart, 1980)- normed for name agreement, image agreement, familiarity, and visual complexity. Participants completed three runs, each of which included 5 on(task) blocks (46sec) and 5 off(rest) blocks (44 sec). During each on-block participants were presented with 8 pictures serially, for 3 seconds each, during which time they were asked to make an animacy decision regarding the image (i.e., living /non-living). Button responses and response time were recorded using a magnetically shielded 4-button box held in the participant's right hand. Stimuli were separated with a jittered ISI which ranged from 0 to 6 seconds to allow the hemodynamic response to facilitate deconvolution of the hemodynamic response (Dale, 1999). Off-block stimuli consist of a centered crosshair figure, which is also used for memory task on-block ISI periods. Participants were not told that they would be asked to perform a subsequent memory test at a later time, and no corrective feedback was given during the task blocks.
Twenty-four hours following the scanning session participants were brought back to the lab and performed a surprise recognition test. Participants were presented with 200 words serially and asked to make an old/new decision about each word. One hundred twenty words represented the set of pictures that were presented during the scanning session and 80 words represented new, not previously viewed, items. After making each memory decision, participants rated their response confidence on a 6-point scale. It should be noted that the retention delay in the current study (24 hours) was longer than most studies examining subsequent memory effects. This was done to equate memory performance between young, healthy older adults and patients with MCI (results to be presented in a subsequent publication).
Image acquisition & processing
Images were collected using a 4-T GE scanner. Stimuli were presented using liquid crystal display goggles (Resonance Technology, Northridge, CA), and behavioral responses were recorded using a four-button fiber-optic response box (Resonance Technology). Scanner noise was reduced with earplugs, and head motion was minimized using foam pads and a headband. Anatomical scanning started with a T2-weighted sagittal localizer series. The anterior (AC) and posterior commissures (PC) were identified in the midsagittal slice, and 34 contiguous oblique slices were prescribed parallel to the AC–PC plane. High-resolution T1-weighted structural images were collected with a 5.3-msec echo time (TE), a 12-msec repetition time (TR), a 24-cm field of view (FOV), a 2562 matrix, 68 slices, and a slice thickness of 1.9 mm. Functional images were acquired using an inverse spiral sequence with a 1500-msec TR, a 6-msec TE, a 24-cm FOV, a 642 matrix, and a 60° flip angle. Thirty-four contiguous slices were acquired with the same slice prescription as the anatomical images. Slice thickness was 3.75 mm, resulting in cubic 3.75 mm3 isotropic voxels. Head motion was assessed prior to preprocessing. No individual moved more than 3 mm in any direction, in any run.
Data were preprocessed and analyzed using Statistical Parameter Mapping (SPM2; Wellcome Department of Cognitive Neurology) implemented in MATLAB. Time-series data were corrected for differences in slice acquisition time, motion and field inhomogeneity. After realigning the full brain anatomical images and individually checking subject-wise error, we co-registered the functional images to the target structurals. Structural images were then spatially normalized to a standard stereotaxic space using the Montreal Neurological Institute (MNI) templates implemented in SPM2, after which the resulting transformation matrices were applied to the functional data to warp them to MNI space. Functional volumes were then spatially smoothed using a 10-mm isotropic Gaussian kernel.
Statistical analysis
Individual trial activity was assessed within the context of a mixed model to control for error variance and BOLD signal drift in the MTL. The onset of each trial (i.e., subsequently remembered and subsequently forgotten items – defined below) were modeled with a stick function at each stimulus onset and convolving the neural response with two hemodynamic response functions (HRFs) – the first being the canonical HRF and the second, an HRF shifted forward 1TR, with the latter orthogonalized to the first so as to attribute any shared variance to the earlier function (Woodruff, Uncapher and Rugg, 2006). In accord with the typical mixed model, task blocks were also modeled and added with head motion and scanner drift as control factors, all of which were treated as regressors of no interest.
Encoding success activity (ESA) is neural activity modulated by memory performance. ESA is operationalized as activity associated with subsequently remembered > subsequently forgotten items. Subsequent hits were categorized as encoding trials that correctly led to an “old” response combined with a confidence response of 4-6. Subsequent misses were defined as encoding trials classified as “new” or classified as “old” but with low confidence. This approach has been used in other subsequent memory fMRI studies as a means to evaluate the neural correlates of successful encoding under high recollective strength (Dennis, Hayes, Prince, Huettel, Madden and Cabeza, 2008; Sperling, Chua, Cocchiarella, Rand-Giovannetti, Poldrack, Schacter et al., 2003).
Based upon our a priori hypotheses focusing on group differences in the MTL we constructed and applied an anatomically defined mask of the region of interest (ROI) to the group analyses, using a standard anatomical library (Tzourio-Mazoyer, Landeau, Papathanassiou, Crivello, Etard, Delcroix et al., 2002) available within SPM2. Within each group, ESA was assessed at p < .05, with minimum cluster extent threshold of 10 contiguous voxels within the focal MTL ROI. These results were subsequently used as an inclusive mask for identifying group effects at p <.05 and a minimum cluster size of 10 contiguous voxels. Thus, significant activations were required to pass a two thresholds process: (1) a significant ESA effect (subsequent Hit > subsequent Miss) within one of the groups (p < .05 & >=10 voxels); and (2) a group difference in ESA (p < .05 & >=10 voxels). The conjoint probability following inclusive masking approached p < .0025 (Fisher, 1950; Forman, Cohen, Fitzgerald, Eddy, Mintun and Noll, 1995; Lazar, Luna, Sweeney and Eddy, 2002), but this estimate should be taken with caution given that the contrasts were not completely independent. As an added methodological check on the ESA paradigm, we also conducted an omnibus, whole brain ESA analysis including all participants at p<.005 and minimum cluster size of 10 voxels. Results are shown in Supplementary data (Figure 3; Table 4) and reveal the expected pattern of ESA activity (i.e., left PFC, left MTL, parietal cortex, and bilateral occiptotemporal cortex), reinforcing that any differences observed in the a priori MTL ROIs are not due to any systematic bias in ESA task engagement between groups. Finally, whole brain differences in ESA were also examined at a threshold of p <.001 and extent of 10 contiguous voxels.
In order to follow-up on the foregoing ESA within the MTL, we also investigated group differences in functional connectivity of the MTL associated with successful encoding task activity. We used the MTL regions that exhibited group differences in ESA as seed regions and examined correlations of these MTL activations with whole brain activity1. A new analytical model was created in which each task trial was uniquely coded as a separate event. This allows for the activity in an MTL seed region to be correlated with activity in all other voxels in the brain. The validity and utility of this technique to interrogate functional brain networks has been confirmed in previous studies (Daselaar, Fleck, Dobbins, Madden and Cabeza, 2006; Dennis, Hayes, Prince, Huettel, Madden and Cabeza, 2008; Rissman, Gazzaley and D'Esposito, 2004). Because the goal of this analysis was to assess group differences in connectivity for ESA, the analysis was constrained to subsequently remembered trials. Moreoever, As a second step, group averages (of MTL connectivity on remembered trials) were calculated by employing a one-sample t-test on the resulting correlation maps across all group members. Group differences were again calculated using a multiple contrast approach. The between group two-sample t-test was conducted at p < .05 with a minimum cluster size of 20 voxels, inclusively masking for effects of interest within each group (p < .05 and a minimal cluster size of 20 voxels). Thus, like the previous analyses, the resulting activity showing group differences in the functional connectivity with the MTL also had to be confirmed by differences observed in individual groups.
Finally, voxel-based morphometry (VBM) analyses were conducted in SPM2, in accordance with established methodologies (Ashburner and Friston, 2000; Good, Johnsrude, Ashburner, Henson, Friston and Frackowiak, 2001), to examine for gray and white matter volume differences between APOE e4 carrier groups. Modulated grey and white matter images were smoothed with a 9mm kernel, and individually assessed for regional grey and white matter volume differences between groups at p<.001 and 10 voxels.
Results
Behavioral results
Neuropsychological assessment performances were equivalent between groups on a range of tasks tapping memory skills, information processing speed, attention, and executive skills. Accuracy and response time to making the in-scanner animacy decision were equivalent between groups. Additionally, no differences were observed in the hit and false alarm rates for the subsequent memory task (see Table 1).
Neuroimaging results
Medial temporal lobe differences in ESA
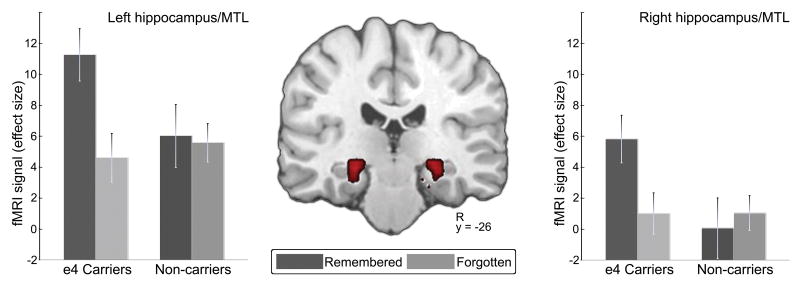
Carriers of the e4 allele exhibited greater ESA in bilateral hippocampus & parahippocampal cortex compared to non-carriers (see Figure 1). No MTL region showed greater ESA for non-carriers.
Figure 1.
Group differences in medial temporal lobe activity predictive of successful subsequent memory (e4 carriers > non-carriers). Activation bars represent effect sizes extracted from peak voxels within the activated region.
Outside of the MTL, carriers of the e4 allele did not exhibit greater ESA in any region, while non-carriers exhibited greater ESA only in right middle temporal gyrus (48x, -19y, -9z voxels=18).
Group differences in medial temporal lobe connectivity
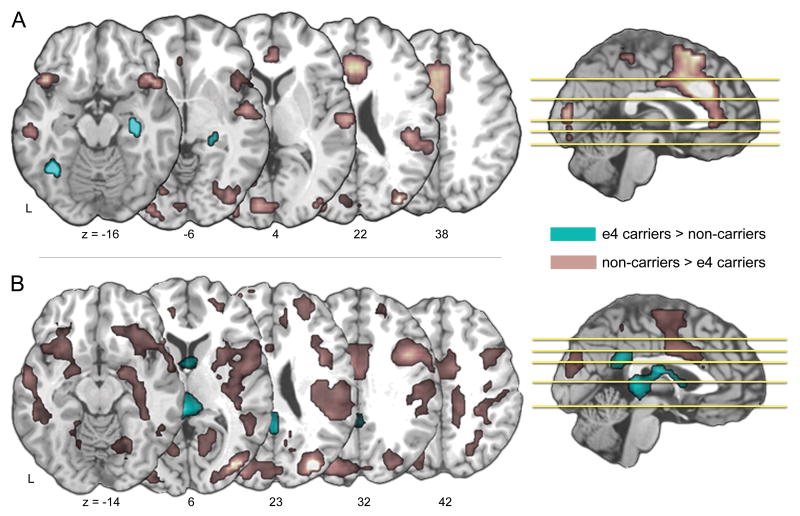
Carriers of the e4 allele also exhibited greater functional connectivity with the MTL only within a very limited set of brain regions, but notably regions that have been implicated in PET imaging studies of APOE effects in normal adults across the age span (Reiman, Chen, Alexander, Caselli, Bandy, Osborne et al., 2004; Reiman, Chen, Alexander, Caselli, Bandy, Osborne et al., 2005). For left MTL connectivity these included right hippocampus and left fusiform gyrus/middle temporal gyrus, and for right MTL connectivity, retrosplenial cortex and bilateral caudate. Non-carriers, on the other hand, exhibited much greater functional connectivity with both left and right MTL – including regions in bilateral PFC, visual cortex, dorsal PCC, and ACC (see Figure 2 & Table 3).
Figure 2.
Whole brain differences in medial temporal lobe functional connectivity (seed regions based upon ROIs showing group differences in ESA).
A = Functional connectivity to left MTL seed region (Table 2; -22x, -26y, -8z locus)
B = Functional connectivity to right MTL seed region (Table 2; 26x, -40y, 2z locus)
Table 3.
Whole brain functional connectivity to medial temporal regions showing increased e4 carrier effect.
| Connectivity Contrastsa | Activation Locus (Brodmann Areas)b |
SPM [t] |
kE | Local Maxima | ||||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Right MTL seed connectivity c | ||||||||
| e4 carriers > non-carriers | ||||||||
| L. Hemisphere | Retrosplenial / PHG (BA 30) | 3.49 | 1368 | -7 | -36 | 9 | ||
| L/R Hemisphere | Ventral PCC (BA 23) | 3.58 | 266 | 0 | -46 | 23 | ||
| e4 carriers < non-carriers | ||||||||
| L. Hemisphere | Middle temporal gyrus (BA 21) | 4.39 | 1702 | -59 | -8 | -9 | ||
| Cuneus (BA 18) | 4.13 | 1500 | -15 | -68 | 17 | |||
| ACC (BA 24) | 2.87 | 446 | -4 | 1 | 28 | |||
| Occipital gyrus (BA 19) | 2.42 | 116 | -41 | -80 | 4 | |||
| R. Hemisphere | Middle frontal gyrus (BA 11) | 4.61 | 285 | 26 | 25 | -17 | ||
| Dorsal PCC (BA 31) | 4.03 | 3499 | 7 | -42 | 44 | |||
| Superior temporal gyrus (BA 38) | 3.67 | 431 | 59 | 7 | -10 | |||
| Insula (BA 13) | 3.46 | 712 | 41 | -32 | 19 | |||
| Inferior frontal gyrus (BA 9) | 3.30 | 218 | 37 | 9 | 28 | |||
| Superior frontal gyrus (BA 10) | 3.11 | 334 | 30 | 48 | 22 | |||
| Left MTL seed connectivity d | ||||||||
| e4 carriers > non-carriers | ||||||||
| L. Hemisphere | Inferior temporal gyrus (BA 37) | 3.96 | 1102 | -48 | -69 | 0 | ||
| Superior parietal lobule (BA 7) | 3.23 | 158 | -22 | -52 | 59 | |||
| R. Hemisphere | Fusiform gyrus (BA 37) | 3.10 | 206 | 48 | -59 | -10 | ||
| Retrosplenial / PHG (BA 30) | 2.89 | 116 | 19 | -40 | -4 | |||
| e4 carriers < non-carriers | ||||||||
| L. Hemisphere | Fusiform gyrus (BA 20) | 3.88 | 945 | -45 | -23 | -18 | ||
| Middle frontal gyrus (BA 6) | 3.63 | 446 | -30 | 14 | 52 | |||
| Superior frontal gyrus (BA 10) | 3.60 | 375 | -33 | 55 | 15 | |||
| Superior temporal gyrus (BA 38) | 3.41 | 124 | -45 | 13 | -23 | |||
| Angular gyrus (BA39) | 2.62 | 146 | -48 | -64 | 35 | |||
| Superior temporal gyrus (BA 38) | 2.48 | 248 | -45 | 11 | -7 | |||
| R. Hemisphere | Superior frontal gyrus (BA9) | 3.76 | 3390 | 19 | 52 | 29 | ||
| Inferior frontal gyrus (BA 45) | 3.56 | 319 | 52 | 19 | 6 | |||
| Middle temporal gyrus (BA 21) | 3.52 | 930 | 52 | -33 | -5 | |||
| Dorsal PCC (BA 31) | 3.25 | 116 | 26 | -58 | 13 | |||
| Dorsal entorhinal cortex (BA 34) | 2.78 | 562 | 19 | 6 | -19 | |||
ACC = anterior cingulate cortex; BA = Brodmann area; PCC = posterior cingulate cortex; PHG = parahippocampal gyrus.
Statistical parametric mapping (SPM) t-test contrast of ESA.
Anatomical and Brodmann area labels based upon Talairach coordinate nearest grey matter search of the Talairach Daemon Database (31).
Connectivity to e4 carrier > non-carrier ESA right PHG locus (Table 2; coordinate 26x, -40y, 2z).
Connectivity to e4 carrier > non-carrier ESA left PHG locus (Table 2; coordinate -22x, -26y, - 8z).
Structural imaging comparisons
No group differences in proportionally scaled, modulated regional grey or white matter volumes were detected in the MTL or remaining cortex, suggesting that both groups had fairly equitable measures of brain parenchyma in regions showing functional activity and connectivity differences.
Discussion
The current study yielded two main findings. Despite exhibiting no significant differences in memory performance or grey matter volume, young healthy adult APOE e4 carriers exhibited significantly greater ESA in bilateral hippocampus/PHG compared to non-carriers. Additionally, while exhibiting greater overall ESA in these MTL regions, e4 carriers exhibited globally reduced connectivity to other task-associated cortical regions relative to non-carriers (see Table 3). Notably, exceptions to the reduced functional connectivity in the e4 carriers were in regions known to evince some of the earliest functional activation changes in e4 adults [i.e., PCC; (Chen, Reiman, Alexander, Caselli, Gerkin, Bandy et al., 2007; Reiman, Chen, Alexander, Caselli, Bandy, Osborne et al., 2004; Reiman, Chen, Alexander, Caselli, Bandy, Osborne et al., 2005)]. These results argue against a positive (or anagonistic) pleiotropic role of the APOE e4 allele in successful memory encoding in young adulthood. It is still possible that e4 carriers may derive an early beneficial effect, but results suggest that any window for benefit may be much earlier in the developmental process.
Focusing on our first finding, young adult APOE e4 carriers, compared to non-carriers, exhibited greater ESA activation within the MTL (see Figure 1B), specifically within the hippocampus and PHG. However, both groups exhibited equitable memory performance and were found to have comparable volumes of grey matter within the MTL, indicating that these functional differences cannot be explained by either differences in memory performance or MTL atrophy. Results are consistent with previous studies in older adults which also found increased MTL activity for APOE e4 carriers compared to noncarriers (Bondi, Houston, Eyler and Brown, 2005; Dickerson, Salat, Bates, Atiya, Killiany, Greve et al., 2004; Han, Houston, Jak, Eyler, Nagel, Fleisher et al., 2007; Wishart, Saykin, Rabin, Santulli, Flashman, Guerin et al., 2006). These studies concluded that increased activations (in the absence of behavioral and volumetric differences) reflect a compensatory mechanism in APOE e4 carriers. Specifically, that e4 carriers required additional activation, perhaps reflecting additional cognitive effort in memory-related tasks in order to maintain similar performance to noncarriers.
The increased MTL activation in e4 carriers observed in the current study may reflect an innate difference in functionality of this region compared to non-carriers, which, in turn, may be indicative of premorbid functional weakness or generally non-contributory hyperactive processing in regions known to be sensitive to late-onset AD pathology (Morishima-Kawashima, Oshima, Ogata, Yamaguchi, Yoshimura, Sugihara et al., 2000; Selkoe, 2002). The prospect of APOE-driven innate differences support work from Shaw et al. (2007) who found a linear decrease in anterior MTL grey matter volume in healthy adolescents as a function of APOE allele carrier status (i.e., APOE e2 > e3 > e4 carriers). Differences between our results and Shaw's in regards to MTL volume may be based on differences in methodology (e.g., separation of genotyped groups).
In addition to these group differences in ESA, our second finding focused on observed group differences in functional connectivity with the MTL. Despite exhibiting greater ESA in bilateral hippocampus, the APOE e4 carriers exhibited reduced functional connectivity with these regions compared to non-carriers. Taken together results suggest that APOE e4 non-carriers may be better able to integrate processing mediated by the MTL with that of other brain regions, regions also involved in ESA. The reduced functional connectivity exhibited by the APOE e4 carriers may be indicative of the fact that activation in this region is modulated relatively independently of other brain regions. APOE e4 carriers did exhibit increased connectivity with the ventral PCC, a region exhibiting reduced metabolism and altered activations in those genetically at risk for late-onset AD (Reiman, Chen, Alexander, Caselli, Bandy, Osborne et al., 2004). In accordance with the abovementioned hyperactivation of the MTL by APOE e4 carriers, results may reflect a change in the underlying physiology of this region that is present at an early age. Whether these changes in MTL activation and functional connectivity reflect early changes associated with the disease state or are a causal factor in developing said impairments is a research question that needs to be answered.
Again, whether these functional changes represent a compensatory mechanism of the MTL network, or a more fundamental difference in functional brain organization associated with functional variants of the APOE genotype has yet to be determined. Bondi and colleagues (Han and Bondi, 2008) have argued that the APOE gene may have pleiotropic (beneficial) effects in young adults, which serve to enhance cognitive performance. Likewise, Mondadori and colleagues (Mondadori, de Quervain, Buchmann, Mustovic, Wollmer, Schmidt et al., 2007) posit that their young adult cohort exhibited beneficially increased neural activity. The current results do not appear to support the notion of pleiotropic effects in memory function by young adulthood, as increased MTL activity and connectivity to the PCC in our e4 carriers did not result in enhanced, but equitable memory performance compared to non-carriers. Furthermore, results support recent finding from Filippini and colleagues (2009) who concluded that the presence of the APOE e4 allele was associated in greater MTL activity during encoding in healthy young adults (25-30 year olds; 18 carriers vs. 18 non-carriers) in the absence of behavioral or structural differences between the two groups.
Rather, the current findings fit better with either an inefficiency or a compensatory account of cognitive function. That is, based on the lack of behavioral differences between genotyped groups, increased MTL activity in e4 carriers may be interpreted as either a) inefficient processing associated with dysfunction in the MTL or b) compensatory processing necessary to achieve similar cognitive output to the seen in non-carriers who are able to rely on more efficient MTL functioning. As noted, these two ideas are not incompatible. The fact that, in e4 carriers, these task-induced increases in MTL activation are, in turn, associated with reductions in functional connectivity with this region suggest that this enhanced processing isn't reflected in other ESA regions. Rather, the observed changes are limited to the MTL and the PCC, another region affected early in AD pathology (Lustig, Snyder, Bhakta, O'Brien, McAvoy, Raichle et al., 2003; Petrella, Prince, Wang, Hellegers and Doraiswamy, 2007; Prince, Woo, Doraiswamy and Petrella, 2008), indicating that they may reflect regional-specific neural alterations associated with the e4 allele. While the basis for these differences require future research, the results suggest that investigation should not be limited to individuals in older cohorts alone.
Furthermore, it should also be noted that our compensatory theory (defined above as increased processing necessary to achieve similar cognitive function) differs from the that of Han and Bondi (Han and Bondi, 2008) in that their account is, in actuality, one of enhancement, rather than compensation in young e4 carriers. That is, Han and Bondi propose that “Young e4 participants perform better on memory and other neurocognitive tasks” (p. 252). They further suggest that increased neural recruitment in older e4 carriers would help compensate for cognitive declines. Their theory does not outline whether enhanced (not comparable) performance in young carriers is accompanied by increased or comparable neural recruitment compared to non-carriers. The current results observed increased MTL recruitment and equal performance in e4 carriers compared to non-carriers, suggesting (as describe above) either the increased activation is inefficient in some way or that young e4 carriers require increased neural processing to accomplish similar performance to non-carriers. Further research investigating other cognitive processing associated with MTL function (e.g., retrieval, novelty, source memory) will be necessary to fully elucidate the validity of the compensation theory. In doing so it will be crucial to consider not only group differences in activation levels and performance, but also whether cognitive processes may differ between groups. Additionally, it should be noted that the current study employed a sample size of 24 participants. Future studies should additionally focus on larger samples in order to validate current findings.
Taken together our findings suggest that the APOE e4 allele influences brain activity earlier in the lifespan than previously reported. Moreover, the presence of the APOE e4 allele appears to alter not only functional activations within the MTL, but also the functional connectivity of the MTL, during the memory encoding process. Brain regions implicated in this investigation are known to incur structural and functional changes in MCI and AD and have been observed to be altered in healthy middle aged adults and seniors with the e4 allele. While these commonalities exist with our project participant sample, it is unclear whether the brain activation differences observed in the young adult APOE e4 carriers reflects an adaptive response to an underlying weakness in the episodic memory network or a more fundamental, APOE-driven pleiotropism in functional brain organization. Results from our functional connectivity analyses suggest the former, as the APOE e4 carriers appear to be less able to integrate processing mediated by the MTL with other ESA regions.
Supplementary Material
Figure 3 (Supplemental). Common ESA activity across both groups.
Figure 4 (Supplemental). Whole brain differences in medial temporal lobe functional connectivity (seed regions based upon ROIs showing similar ESA).
A = Functional connectivity to left anterior MTL seed region (Table 4; -15x, -4y, -16z locus)
B = Functional connectivity to left posterior MTL seed region (Table 4; -26x, -37y, -8z locus)
Table 2.
Medial temporal lobe encoding success activity (ESA) contrasts
| Difference ESA Contrastsa | Activation Locus (Brodmann Areas)b |
SPM [t] |
kE | Local Maximac | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| (e4 carriers > non-carriers) | ||||||
| R. Hemisphere | PHG (BA 28/35) / Hippocampus | 3.11 | 221 | 26 | -40 | 2 |
| L. Hemisphere | PHG (BA 28/35) / Hippocampus | 2.42 | 98 | -22 | -26 | -8 |
| (e4 carriers < non-carriers) | ||||||
| None | ||||||
BA = Brodmann Area; MTL = medial temporal lobe; PHG = parahippocampal gyrus
Statistical parametric mapping (SPM), independent samples t-test contrast of MTL ESA.
Anatomical and Brodmann area labels based upon coordinate nearest grey matter search of the Talairach Daemon Database.
Talairach & Tourneau coordinates.
Acknowledgments
The authors wish to thank Sander Daselaar and Mathias Fleck for help in task development, Jim Kragel, Simon Davis and Micah Adams for their assistance in data collection and preparation, and the Goldstein Lab at Duke University for help in participant selection and recruitment. This research was supported, in part, by NIH/NIA grants P30-AG028377 and R01-AG019731.
Footnotes
Disclosure: The authors report no conflicts of interest.
A similar connectivity analysis was conducted by choosing peak voxels from the common ESA regions within the MTL as seeds (see coordinates identified in Supplementary Table 4 & Figure 4 legend). The pattern of connectivity differences between groups closely matched that which was found in the main connectivity analysis. (Please see Supplementary Figure 4.) Results indicate that group differences in MTL functional connectivity are not isolated to regions exhibiting overall differences in activation between groups, but are indicative of overall MTL function within the ESA network.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Houston WS, Eyler LT, Brown GG. fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology. 2005;64:501–8. doi: 10.1212/01.WNL.0000150885.00929.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, et al. Patterns of brain activation in people at risk for Alzheimer's disease. N Engl J Med. 2000;343:450–6. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Evolution of the neuropathology of Alzheimer's disease. Acta Neurol Scand Suppl. 1996;165:3–12. doi: 10.1111/j.1600-0404.1996.tb05866.x. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Evolution of neuronal changes in the course of Alzheimer's disease. J Neural Transm Suppl. 1998;53:127–40. doi: 10.1007/978-3-7091-6467-9_11. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E, Bohl J. Staging of Alzheimer-related cortical destruction. Eur Neurol. 1993;33:403–8. doi: 10.1159/000116984. [DOI] [PubMed] [Google Scholar]
- Chen K, Reiman EM, Alexander GE, Caselli RJ, Gerkin R, Bandy D, et al. Correlations between apolipoprotein E epsilon4 gene dose and whole brain atrophy rates. Am J Psychiatry. 2007;164:916–21. doi: 10.1176/ajp.2007.164.6.916. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R. Effects of healthy aging on hippocampal and rhinal memory functions: an event-related fMRI study. Cerebral Cortex. 2006;16:1771–82. doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Hayes SM, Prince SE, Huettel SA, Madden DJ, Cabeza R. Effects of aging on the neural correlates of successful item and source memory encoding. Journal of Experimental Psychology: Learning, Memory and Cognition. 2008;34:791–808. doi: 10.1037/0278-7393.34.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Goncharova I, Sullivan MP, Forchetti C, Wilson RS, Bennett DA, et al. MRI-derived entorhinal and hippocampal atrophy in incipient and very mild Alzheimer's disease. Neurobiology of Aging. 2001;22:747–754. doi: 10.1016/s0197-4580(01)00271-8. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Bates JF, Atiya M, Killiany RJ, Greve DN, et al. Medial temporal lobe function and structure in mild cognitive impairment. Ann Neurol. 2004;56:27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins IG, Davachi L. Functional Neuroimaging of Episodic Memory. In: Cabeza R, Kingstone A, editors. Handbook of Functional Neuroimaging of Cognition. 2nd. Cambridge, MA: MIT Press; 2006. pp. 229–268. Vol. [Google Scholar]
- Filbey FM, Slack KJ, Sunderland TP, Cohen RM. Functional magnetic resonance imaging and magnetoencephalography differences associated with APOEepsilon4 in young healthy adults. Neuroreport. 2006;17:1585–90. doi: 10.1097/01.wnr.0000234745.27571.d1. [DOI] [PubMed] [Google Scholar]
- Filippini N, Macintosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, et al. Distinct patterns of brain activity in young carriers of the APOE-{varepsilon}4 allele. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RA. Statistical methods for Research Workers. London: Oliver and Boyd; 1950. [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Han SD, Bondi MW. Revision of the apolipoprotein E compensatory mechanism recruitment hypothesis. Alzheimers Dement. 2008;4:251–4. doi: 10.1016/j.jalz.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Han SD, Drake AI, Cessante LM, Jak AJ, Houston WS, Delis DC, et al. Apolipoprotein E and traumatic brain injury in a military population: evidence of a neuropsychological compensatory mechanism? J Neurol Neurosurg Psychiatry. 2007;78:1103–8. doi: 10.1136/jnnp.2006.108183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SD, Houston WS, Jak AJ, Eyler LT, Nagel BJ, Fleisher AS, et al. Verbal paired-associate learning by APOE genotype in non-demented older adults: fMRI evidence of a right hemispheric compensatory response. Neurobiol Aging. 2007;28:238–47. doi: 10.1016/j.neurobiolaging.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz J, Chen Y. Reelin, lipoprotein receptors and synaptic plasticity. Nat Rev Neurosci. 2006;7:850–9. doi: 10.1038/nrn2009. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Petersen RC, Xu YC, Waring SC, O'Brien PC, Tangalos EG, et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease. Neurology. 1997;49:786–94. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher TT, Weis S, Freymann K, Erb M, Jessen F, Grodd W, et al. Hippocampal activation in patients with mild cognitive impairment is necessary for successful memory encoding. J Neurol Neurosurg Psychiatry. 2007;78:812–8. doi: 10.1136/jnnp.2006.104877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch W, Ehrenhaft A, Griesser K, Pfeufer A, Muller J, Schomig A, et al. TaqMan systems for genotyping of disease-related polymorphisms present in the gene encoding apolipoprotein E. Clin Chem Lab Med. 2002;40:1123–31. doi: 10.1515/CCLM.2002.197. [DOI] [PubMed] [Google Scholar]
- Lazar NA, Luna B, Sweeney JA, Eddy WF. Combining brains: a survey of methods for statistical pooling of information. Neuroimage. 2002;16:538–50. doi: 10.1006/nimg.2002.1107. [DOI] [PubMed] [Google Scholar]
- Lustig C, Snyder AZ, Bhakta M, O'Brien KC, McAvoy M, Raichle ME, et al. Functional deactivations: Change with age and dementia of the Alzheimer type. PNAS. 2003;100:14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machulda MM, Ward HA, Borowski B, Gunter JL, Cha RH, O'Brien PC, et al. Comparison of memory fMRI response among normal, MCI, and Alzheimer's patients. Neurology. 2003;61:500–6. doi: 10.1212/01.wnl.0000079052.01016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondadori CR, de Quervain DJ, Buchmann A, Mustovic H, Wollmer MA, Schmidt CF, et al. Better memory and neural efficiency in young apolipoprotein E epsilon4 carriers. Cereb Cortex. 2007;17:1934–47. doi: 10.1093/cercor/bhl103. [DOI] [PubMed] [Google Scholar]
- Mori E, Yoneda Y, Yamashita H, Hirono N, Ikeda M, Yamadori A. Medial temporal structures relate to memory impairment in Alzheimer's disease: an MRI volumetric study. J Neurol Neurosurg Psychiatry. 1997;63:214–21. doi: 10.1136/jnnp.63.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima-Kawashima M, Oshima N, Ogata H, Yamaguchi H, Yoshimura M, Sugihara S, et al. Effect of apolipoprotein E allele epsilon4 on the initial phase of amyloid beta-protein accumulation in the human brain. Am J Pathol. 2000;157:2093–9. doi: 10.1016/s0002-9440(10)64847-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paller KA, Wagner AD. Observing the transformation of experience into memory. Trends in Cognitive Sciences. 2002;6:93–102. doi: 10.1016/s1364-6613(00)01845-3. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Waring SC, Smith GE, Tangalos EG, Thibodeau SN. Predictive value of APOE genotyping in incipient Alzheimer's disease. Ann N Y Acad Sci. 1996;802:58–69. doi: 10.1111/j.1749-6632.1996.tb32599.x. [DOI] [PubMed] [Google Scholar]
- Petrella JR, Prince SE, Wang L, Hellegers C, Doraiswamy PM. Prognostic value of posteromedial cortex deactivation in mild cognitive impairment. PLoS ONE. 2007;2:e1104. doi: 10.1371/journal.pone.0001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince SE, Woo S, Doraiswamy PM, Petrella JR. Functional MRI in the early diagnosis of Alzheimer's disease: is it time to refocus? Expert Rev Neurother. 2008;8:169–75. doi: 10.1586/14737175.8.2.169. [DOI] [PubMed] [Google Scholar]
- Qiu S, Korwek KM, Weeber EJ. A fresh look at an ancient receptor family: emerging roles for low density lipoprotein receptors in synaptic plasticity and memory formation. Neurobiol Learn Mem. 2006;85:16–29. doi: 10.1016/j.nlm.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer's dementia. PNAS. 2004;101:284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, et al. Correlations between apolipoprotein E epsilon4 gene dose and brain-imaging measurements of regional hypometabolism. Proc Natl Acad Sci U S A. 2005;102:8299–302. doi: 10.1073/pnas.0500579102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy F, Mirrashed F, Campbell B, Richter W. Verbal episodic memory impairment in Alzheimer's disease: a combined structural and functional MRI study. Neuroimage. 2005;25:253–66. doi: 10.1016/j.neuroimage.2004.10.045. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D'Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage. 2004;23:752–63. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer's disease. Neurology. 1993;43:1467–72. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- Scarmeas N, Habeck CG, Hilton J, Anderson KE, Flynn J, Park A, et al. APOE related alterations in cerebral activation even at college age. J Neurol Neurosurg Psychiatry. 2005;76:1440–4. doi: 10.1136/jnnp.2004.053645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's Disease Is a Synaptic Failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Shaw P, Lerch JP, Pruessner JC, Taylor KN, Rose AB, Greenstein D, et al. Cortical morphology in children and adolescents with different apolipoprotein E gene polymorphisms: an observational study. Lancet Neurol. 2007;6:494–500. doi: 10.1016/S1474-4422(07)70106-0. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J Exp Psychol [Hum Learn] 1980;6:174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Sperling R, Chua E, Cocchiarella A, Rand-Giovannetti E, Poldrack R, Schacter DL, et al. Putting names to faces: successful encoding of associative memories activates the anterior hippocampal formation. Neuroimage. 2003;20:1400–10. doi: 10.1016/S1053-8119(03)00391-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout JC, Bondi MW, Jernigan TL, Archibald SL, Delis DC, Salmon DP. Regional cerebral volume loss associated with verbal learning and memory in dementia of the Alzheimer type. Neuropsychology. 1999;13:188–97. doi: 10.1037//0894-4105.13.2.188. [DOI] [PubMed] [Google Scholar]
- Tervo S, Kivipelto M, Hanninen T, Vanhanen M, Hallikainen M, Mannermaa A, et al. Incidence and risk factors for mild cognitive impairment: a population-based three-year follow-up study of cognitively healthy elderly subjects. Dement Geriatr Cogn Disord. 2004;17:196–203. doi: 10.1159/000076356. [DOI] [PubMed] [Google Scholar]
- Trivedi MA, Schmitz TW, Ries ML, Torgerson BM, Sager MA, Hermann BP, et al. Reduced hippocampal activation during episodic encoding in middle-aged individuals at genetic risk of Alzheimer's disease: a cross-sectional study. BMC Med. 2006;4:1. doi: 10.1186/1741-7015-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschanz JT, Welsh-Bohmer KA, Lyketsos CG, Corcoran C, Green RC, Hayden K, et al. Conversion to dementia from mild cognitive disorder: the Cache County Study. Neurology. 2006;67:229–34. doi: 10.1212/01.wnl.0000224748.48011.84. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]
- Wishart HA, Saykin AJ, Rabin LA, Santulli RB, Flashman LA, Guerin SJ, et al. Increased brain activation during working memory in cognitively intact adults with the APOE epsilon4 allele. Am J Psychiatry. 2006;163:1603–10. doi: 10.1176/ajp.2006.163.9.1603. [DOI] [PubMed] [Google Scholar]
- Woodruff CC, Uncapher MR, Rugg MD. Neural correlates of differential retrieval orientation: Sustained and item-related components. Neuropsychologia. 2006;44:3000–10. doi: 10.1016/j.neuropsychologia.2006.06.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 3 (Supplemental). Common ESA activity across both groups.
Figure 4 (Supplemental). Whole brain differences in medial temporal lobe functional connectivity (seed regions based upon ROIs showing similar ESA).
A = Functional connectivity to left anterior MTL seed region (Table 4; -15x, -4y, -16z locus)
B = Functional connectivity to left posterior MTL seed region (Table 4; -26x, -37y, -8z locus)