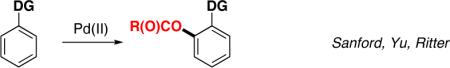
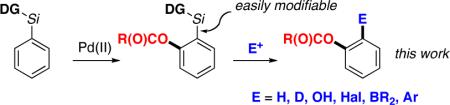
Palladium-catalyzed, ligand-directed C-H functionalization has emerged as a powerful tool for direct conversion of arenes into a variety of valuable products.1 Among these transformations, the Pd-catalyzed directed acetoxylation reactions of aromatic C-H bonds2,3 are particularly attractive as they allow for direct formation of oxygenated arenes.4 A variety of directing groups, such as pyridine,3a,c,g pyrimidine,3e,3j pyrazole,3e oxazoline,3d,e amide,3f,k and oxime ether,3d have been shown to be efficient in these reactions. However, despite the achieved high yields and selectivities, the acyloxylation reactions somewhat lack their generality, as the majority of the directing groups, necessary for the selective transformation, often are not easily removable from the products, thus limiting these methodologies to particular types of substrates (eq 1). Herein we wish to report a pyridyl-diisopropylsilyl (PyDipSi) as a new silicon-tethered directing group, which allows for efficient acetoxylation/pivaloxylation of aromatic C-H bonds. Most importantly, this directing group can efficiently be removed, or converted into a variety of other functional groups (eq 2).
 |
(1) |
 |
(2) |
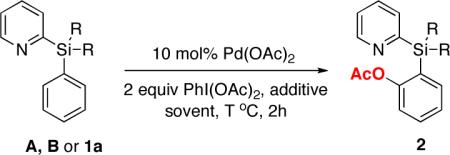
Our approach on the design of the removable directing group is based on the employment of a temporary silicon tether to connect a good metal-coordinating group for C-H activation with an aromatic substrate of interest. We thought that employment of a silicon-tethered directing group5,6 would bring a certain advantage, since it is easily removable from the products of C-H functionalization. We chose pyridine as a platform for our new group design, as it is known to be superior in coordinating palladium in directed C-H activation processes.1j To this end, we aimed at establishing a suitable silicon tether for the pyridine group. Accordingly, substrate A (Fig. 1), containing a pyridyldimethylsilyl directing group, previously developed by Yoshida5 for highly regio- and stereoselective Pd-catalyzed Heck arylation of vinylsilanes,5b was tested first. However, the reaction with PhI(OAc)2 in the presence of 10 mol% Pd(OAc)2 in PrCN at 80 °C3a resulted in total decomposition of the starting arylsilane A with no formation of the desired product (Table 1, entry 1). To further optimize the silicon tether, the silacyclopentane (B) and diisopropylsilyl (1a) derivatives were synthesized (Fig. 1). Similarly to A, compound B appeared to be not stable, as well (Table 1, entry 2). However, we were pleased to find that diisopropylsilyl derivative 1a was more stable toward PhI(OAc)2 at 80 °C, producing the desired product 2a in 15% yield (entry 3). Increasing the temperature to 100 °C led to the slight improvement of the reaction outcome (entry 4). Addition of stoichiometric amount of Cu(OAc)2 gave only traces of product (entry 5). Surprisingly, employment of 1 equivalent AgOAc7 resulted in a dramatic improvement of the reaction, affording the desired 2a in 70% yield. Furthermore, performing the reaction at slightly higher temperature (100 °C) resulted in a better yield of 2a (80%, entry 7). Finally, switching solvent to dichloroethane provided the highest yield (85%) of the product at lower (80 °C) temperature (entry 8). It should be mentioned that a synthesis of arylsilanes, containing PyDipSi directing group is straightforward (Scheme 1). First, pyridyldiisopropylsilane is obtained in excellent yield from commercially available 2-bromopyridine and diisopropylsilylchloride. Next, the hydride-substitution reaction in pyridyldiisopropylsilane with in situ generated aryllithium reagents affords arylsilanes 1 in high yields (Scheme 1).
Figure 1.
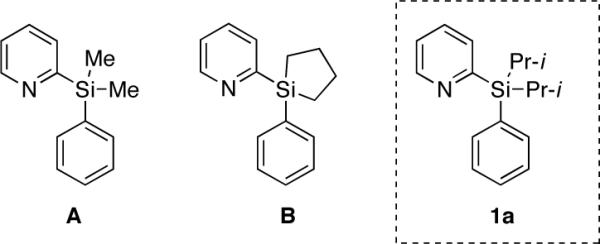
Substrates for optimization of silicon tether.
Table 1.
Optimization of ortho-Acetoxylation Reaction Conditions
| entry | substrate | additive (equiv) | solvent | T, °C | yield, %a |
|---|---|---|---|---|---|
| 1 | A | none | PrCN | 80 | -b |
| 2 | B | none | PrCN | 80 | -b |
| 3 | 1a | none | PrCN | 80 | 15 (2a)b |
| 4 | 1a | none | PrCN | 100 | 30 (2a)b |
| 5 | 1a | Cu(OAc)2 (1) | PrCN | 100 | trace (2a) |
| 6 | 1a | AgOAc (1) | PrCN | 80 | 70 (2a) |
| 7 | 1a | AgOAc (1) | PrCN | 100 | 80 (2a) |
| 8 | 1a | AgOAc (1) | DCE | 80 | 85 (2a) |
NMR yield.
Decomposition of starting arylsilane was observed.
Scheme 1.
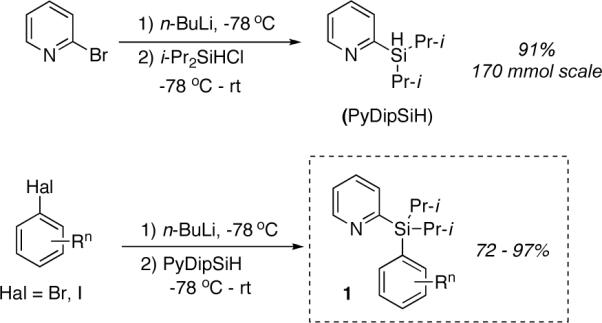
Synthesis and Installation of PyDipSi Directing Group
With the optimized conditions in hand, the generality of the Pd-catalyzed acyloxylation reaction of PyDipSi-containing arenes 1 was examined (Table 2). To our delight, it was found that this transformation is highly efficient for the exclusive mono-acyloxylation of a wide range of substrates. Thus, arylsilanes 2a–x possessing acetoxy-8 and even more synthetically valuable pivaloxy- group9 were synthesized in good to excellent yields. A variety of functional groups, such as OMe (entries 2c–e), F (entries 2q, 2t), Cl (entries 2p, 2r, 2u), Br (entries 2o, 2s), pinacol-protected aldehyde (entry 2v), CO2Et (entry 2w), and CON(i-Pr)2 (entry 2x), were perfectly tolerated under these reaction conditions. Notably, substrates containing meta substituents displayed excellent regio-selectivity in both acetoxylation and pivaloxylation reactions, producing the corresponding oxygenated products as single regioisomers in high yields (2c, 2f–h, 2n, 2o, 2t, 2u). Remarkable site selectivity was also observed in acetoxylation and pivaloxylation of 2-naphtyl derivatives, yielding the desired compounds as sole isomers (entries 2l and 2m).
Table 2.

|
Isolated yields.
See Supporting Information for details.
Our initial mechanistic studies indicated that electron-rich arenes were acyloxylated faster than electron-deficient ones.10,11 In addition, the substantial value of kH/kD (6.7) was observed in intramolecular KIE studies of the pivaloxylation reaction of 1a-d1 (Scheme 2).12 It is believed that the present Pd-catalyzed acyloxylation reaction follows the C-H activation pathway.3b,e,h,i,,13
Scheme 2.
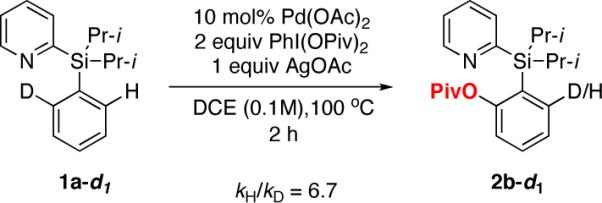
Intramolecular Kinetic Isotope Effect Studies
Naturally, after the development of the efficient directed C-H acyloxylation of arylsilanes, we explored further transformations of the PyDipSi group of the product 2g (Scheme 3). First, it was found that the reaction of 2g with AgF14 in methanol resulted in efficient deprotection of the directing group, affording tolylpivalate 3 in 92% yield. Moreover, treatment of 2g with AgF in THF/D2O produced the deuterated arylpivalate 3-d1 in 95% yield. Remarkably, a combination of AgF/NIS allowed for a quantitative conversion of the PyDipSi group into iodide functionality. The latter transformation, taken together with the installation and pivaloxylation steps, represents a formal 3-step ortho-oxygenation of 3-iodotoluene.15 Furthermore, 2g was converted into a synthetically valuable arylboronate 516 in 94% yield via a one-pot borodesilylation with BCl3/protection with pinacol sequence.17 In addition, borodesilylation of 2g, followed by oxidation in the presence of H2O2/NaOH, produced substituted catechol 6 in excellent yield. Finally, it was found that the acetoxy-derivative 2a underwent an efficient Hiyama-Denmark cross-coupling18 with phenyl iodide and subsequent hydrolysis of acetoxy-group, providing 2-phenylphenol 7 in 93% yield (Scheme 3).
Scheme 3.
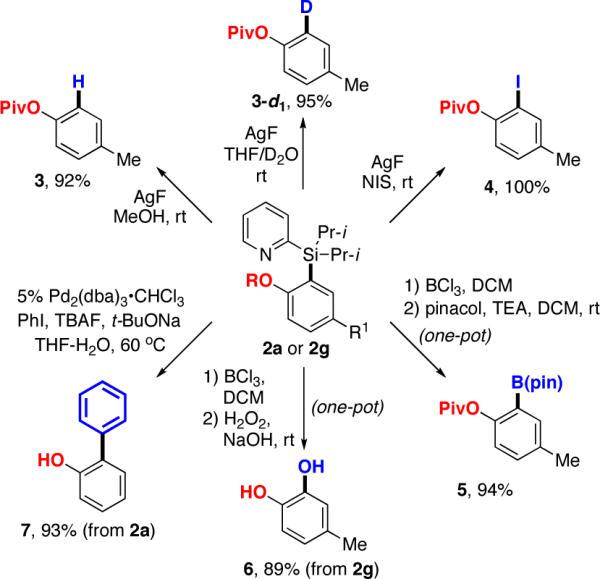
Further Transformations of PyDipSi Group
In summary, we have shown that PyDipSi group can serve as new, general directing group for the Pd-catalyzed acyloxylation of arenes, providing access to a variety of acetoxylated and pivaloxylated aromatic compounds in good yields. Most importantly, it was shown that this newly designed directing group could efficiently be cleaved, as well as converted into other valuable functional groups, such as iodide and boronate. Finally, borodesilylation of this directing group under oxidative conditions allows for preparation of substituted catechol, whereas the Hiyama-Denmark coupling provides direct access to ortho-hydroxybiphenyl.
Supplementary Material
Acknowledgement
This work is dedicated to the memory of Prof. Alexey Andreev. We thank the National Institute of Health (Grant GM-64444) for financial support of this work.
Footnotes
Supporting Information Available: Detailed experimental procedures and characterization data for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).For reviews on transition metal-catalyzed C-H activation of arenes, see: Dick AR, Sanford MS. Tetrahedron. 2006;62:2439.. Alberico D, Scott ME, Lautens M. Chem. Rev. 2007;107:174. doi: 10.1021/cr0509760.. Beccalli EM, Broggini G, Martinelli M, Sottocornola S. Chem. Rev. 2007;107:5318. doi: 10.1021/cr068006f.. Campeau L-C, Stuart DR, Fagnou K. Aldrichim. Acta. 2007;40:35.. Seregin IV, Gevorgyan V. Chem. Soc. Rev. 2007;36:1173. doi: 10.1039/b606984n.. Ackermann L, Vicente R, Kapdi AR. Angew. Chem., Int. Ed. 2009;48:9792. doi: 10.1002/anie.200902996.. Chen X, Engle KM, Wang D-H, Yu J-Q. Angew. Chem., Int. Ed. 2009;48:5094. doi: 10.1002/anie.200806273.. Colby DA, Bergman RG, Ellman JA. Chem. Rev. 2009;110:624. doi: 10.1021/cr900005n.. Daugulis O, Do H-Q, Shabashov D. Acc. Chem. Res. 2009;42:1074. doi: 10.1021/ar9000058.. Lyons TW, Sanford MS. Chem. Rev. 2010;110:1147. doi: 10.1021/cr900184e.. Sun C-L, Li B-J, Shi Z-J. Chem. Commun. 2010;46:677. doi: 10.1039/b908581e..
- (2).For early examples on Pd-catalyzed C-H acetoxylation of arenes, see: Henry PM. J. Org. Chem. 1971;36:1886.. Yoneyama T, Crabtree RH. J. Mol. Catal. A: Chem. 1996;108:35..
- (3).For recent representative examples on Pd-catalyzed C-H acetoxylation of arenes, see: Dick AR, Hull KL, Sanford MS. J. Am. Chem. Soc. 2004;126:2300. doi: 10.1021/ja031543m.. Dick AR, Kampf JW, Sanford MS. J. Am. Chem. Soc. 2005;127:12790. doi: 10.1021/ja0541940.. Kalyani D, Sanford MS. Org. Lett. 2005;7:4149. doi: 10.1021/ol051486x.. Desai LV, Malik HA, Sanford MS. Org. Lett. 2006;8:1141. doi: 10.1021/ol0530272.. Desai LV, Stowers KJ, Sanford MS. J. Am. Chem. Soc. 2008;130:13285. doi: 10.1021/ja8045519.. Wang G-W, Yuan T-T, Wu X-L. J. Org. Chem. 2008;73:4717. doi: 10.1021/jo8003088.. Kim SH, Lee HS, Kim SH, Kim JN. Tetrahedron Lett. 2008;49:5863.. Racowski JM, Dick AR, Sanford MS. J. Am. Chem. Soc. 2009;131:10974. doi: 10.1021/ja9014474.. Stowers KJ, Sanford MS. Org. Lett. 2009;11:4584. doi: 10.1021/ol901820w.. Gu S, Chen C, Chen W. J. Org. Chem. 2009;74:7203. doi: 10.1021/jo901316b.. Gou F-R, Wang X-C, Huo P-F, Bi H-P, Guan Z-H, Liang Y-M. Org. Lett. 2009;11:5726. doi: 10.1021/ol902497k..
- (4).For a recent review on transition metal-catalyzed synthesis of hydroxylated arenes, see: Alonso DA, Nájera C, Pastor IM, Yus M. Chem. Eur. J. 2010;16:5274. doi: 10.1002/chem.201000470..
- (5).For employment of pyridyldimethylsilyl directing group in Heck arylations, see: Itami K, Mitsudo K, Kamei T, Koike T, Nokami T, Yoshida J-I. J. Am. Chem. Soc. 2000;122:12013.. Itami K, Nokami T, Yoshida J-I. J. Am. Chem. Soc. 2001;123:5600. doi: 10.1021/ja015655u.. Itami K, Ushiogi Y, Nokami T, Ohashi Y, Yoshida J-I. Org. Lett. 2004;6:3695. doi: 10.1021/ol048620i..
- (6).For employment of dimethylhydrosilyl directing group in Ir-catalyzed C–H borylations, see: Robbins DW, Boebel TA, Hartwig JF. J. Am. Chem. Soc. 2010;132:4068. doi: 10.1021/ja1006405.. Boebel TA, Hartwig JF. J. Am. Chem. Soc. 2008;130:7534. doi: 10.1021/ja8015878..
- (7).Role of AgOAc is currently unclear. We believe it helps maintaing effective concetration of reactive Pd(OAc)2 species.
- (8).For recent example on acetate cross-coupling reaction, see: Guan B-T, Wang Y, Li B-J, Yu D-G, Shi Z-J. J. Am. Chem. Soc. 2008;130:14468. doi: 10.1021/ja8056503..
- (9).For representative examples on pivalate cross-coupling reactions, see: Li B-J, Li Y-Z, Lu X-Y, Liu J, Guan B-T, Shi Z-J. Angew. Chem., Int. Ed. 2008;47:10124. doi: 10.1002/anie.200803814.. Quasdorf KW, Tian X, Garg NK. J. Am. Chem. Soc. 2008;130:14422. doi: 10.1021/ja806244b.. Shimasaki T, Tobisu M, Chatani N. Angew. Chem., Int. Ed. 2010;49:2929. doi: 10.1002/anie.200907287.. For review, see: Gooβen LJ, Gooβen K, Stanciu C. Angew. Chem., Int. Ed. 2009;48:3569. doi: 10.1002/anie.200900329.. For recent example on pivalate directed C-H arylation, see: Xiao B, Fu Y, Xu J, Gong T-J, Dai J-J, Yi J, Liu L. J. Am. Chem. Soc. 2009;132:468. doi: 10.1021/ja909818n..
- (10).See Supporting Information for details.
- (11).For another recent example on formation of palladacycles in C-H activation processes, see: Giri R, Lam JK, Yu J-Q. J. Am. Chem. Soc. 2009;132:686. doi: 10.1021/ja9077705..
- (12).For the reported high (5.1) kH/kD value of intramolecular KIE in acetoxylation reaction of 2-arylpyridines, see: Powers DC, Geibel MAL, Klein JEMN, Ritter T. J. Am. Chem. Soc. 2009;131:17050. doi: 10.1021/ja906935c.. Also, see: Powers DC, Ritter T. Nat. Chem. 2009;1:302. doi: 10.1038/nchem.246.. Chen X, Goodhue CE, Yu J-Q. J. Am. Chem. Soc. 2006;128:12634. doi: 10.1021/ja0646747..
- (13).Presumably, this reaction proceeds via formation of a palladacycle. See Supporting Information for details.
- (14).(a) Yanagisawa A, Kageyama H, Nakatsuka Y, Asakawa K, Matsumoto Y, Yamamoto H. Angew. Chem., Int. Ed. 1999;38:3701. doi: 10.1002/(sici)1521-3773(19991216)38:24<3701::aid-anie3701>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]; (b) Itami K, Mineno M, Kamei T, Yoshida J-I. Org. Lett. 2002;4:3635. doi: 10.1021/ol026573t. [DOI] [PubMed] [Google Scholar]
- (15).For example of ortho-hydroxylation of haloarenes, see: de Rege FMG, Buchwald SL. Tetrahedron. 1995;51:4291..
- (16).For a review on C-B bond formation via C-H activation, see: Mkhalid IAI, Barnard JH, Marder TB, Murphy JM, Hartwig JF. Chem. Rev. 2009;110:890. doi: 10.1021/cr900206p.. For selected examples on synthetic utility of arylboronates, see: Cho J-Y, Tse MK, Holmes D, Maleczka RE, Jr., Smith MR., III Science. 2002;295:305. doi: 10.1126/science.1067074.. Maleczka RE, Shi F, Holmes D, Smith MR., III J. Am. Chem. Soc. 2003;125:7792. doi: 10.1021/ja0349857.. Holmes D, Chotana GA, Maleczka RE, Smith MR., III Org. Lett. 2006;8:1407. doi: 10.1021/ol060205y.. Paul S, Chotana GA, Holmes D, Reichle RC, Maleczka RE, Smith MR., III J. Am. Chem. Soc. 2006;128:15552. doi: 10.1021/ja0631652.. Shi F, Smith MR, III, Maleczka RE. Org. Lett. 2006;8:1411. doi: 10.1021/ol060207i.. Murphy JM, Liao X, Hartwig JF. J. Am. Chem. Soc. 2007;129:15434. doi: 10.1021/ja076498n.. Murphy JM, Tzschucke CC, Hartwig JF. Org. Lett. 2007;9:757. doi: 10.1021/ol062903o.. Tzschucke CC, Murphy JM, Hartwig JF. Org. Lett. 2007;9:761. doi: 10.1021/ol062902w..
- (17).(a) Itami K, Kamei T, Yoshida J-I. J. Am. Chem. Soc. 2003;125:14670. doi: 10.1021/ja037566i. [DOI] [PubMed] [Google Scholar]; (b) Kamei T, Itami K, Yoshida J-I. Adv. Synth. Catal. 2004;346:1824. [Google Scholar]; (c) Zhao Z, Snieckus V. Org. Lett. 2005;7:2523. doi: 10.1021/ol0506563. [DOI] [PubMed] [Google Scholar]; (d) Rottländer M, Palmer N, Knochel P. Synlett. 1996:573. [Google Scholar]
- (18).(a) Denmark SE, Regens CS. Acc. Chem. Res. 2008;41:1486. doi: 10.1021/ar800037p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Anderson JC, Anguille S, Bailey R. Chem. Commun. 2002:2018. doi: 10.1039/b205765d. [DOI] [PubMed] [Google Scholar]; (c) Denmark SE, Smith RC, Chang W-TT, Muhuhi JM. J. Am. Chem. Soc. 2009;131:3104. doi: 10.1021/ja8091449. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Denmark SE, Baird JD. Org. Lett. 2004;6:3649. doi: 10.1021/ol048328a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



