Summary
Background
Activated protein C in complex with endothelial protein C receptor (EPCR) can reverse barrier disruptive and cytotoxic effects of proinflammatory cytokines by cleaving protease-activated receptor 1 (PAR-1). Recently, it was reported that the PAR-1-dependent vascular barrier-protective effect of APC is mediated through transactivation of the angiopoietin (Ang)/Tie2 signaling pathway. The antagonist of this pathway, Ang2, is stored in Weibel-Palade bodies within endothelial cells.
Objectives
The objective of this study was to determine whether the occupancy of EPCR by its ligand can switch the PAR-1-dependent signaling specificity of thrombin through the Ang/Tie2 axis.
Methods
We activated endothelial cells with thrombin before and after treating them with the catalytically inactive Ser-195 to Ala substitution mutant of protein C. The expression levels of Ang1, Ang2 and Tie2 were measured by both an ELISA and cell permeability assay in response to thrombin in the absence and presence of siRNA and a blocking antibody to Tie2.
Results
We discovered that thrombin up-regulates the expression of both Ang1 and Tie2, but down-regulates Ang2 when EPCR is occupied by its ligand. The Ang1/Tie2-dependent protective effect of thrombin was initiated through protein C inhibiting the rapid mobilization of Ang2 from Weibel-Palade bodies. Interestingly, the protein C mutant also inhibited the thrombin mobilization of P-selectin.
Conclusions
These results suggest a physiological role for the low concentration of thrombin in maintaining the integrity of the EPCR-containing vasculature through the PAR-1-dependent inhibition of Ang2 and P-selectin release from Weibel-Palade bodies.
Keywords: EPCR, thrombin, APC, PAR-1, angiopoietin, P-selectin, signaling
Introduction
Several recent studies have indicated that protective anti-permeability and anti-inflammatory effects of activated protein C (APC) are mediated through the protease binding to endothelial protein C receptor (EPCR) and activating the G-protein coupled receptor, protease-activated receptor 1 (PAR-1), on the surface of endothelial cells [1,2]. Further studies have revealed that the PAR-1-dependent barrier-protective effect of APC requires transactivation of another G-protein coupled receptor, sphingosine 1-phosphate receptor (S1P1) [3–5]. The activation of S1P1 elicits a Gi-protein mediated signaling response which culminates in the activation of the phosphatidylinositol 3- kinase (PI3K)/Akt survival pathway [3,6,7]. Interestingly, a recent study showed that EPCR and PAR-1-dependent barrier-protective effects of APC also require transactivation of the angiopoietin (Ang)/Tie2 signaling pathway [8]. Tie2 is an endothelial cell specific tyrosine kinase receptor, which together with its two known ligands, Ang1 and Ang2, plays an essential role in the regulation of endothelial cell survival and maintenance of vascular integrity [9–12]. This signaling pathway has been reported to also be responsible for the quiescent phenotype of endothelial cells under normal physiological conditions [9]. It is thought that Ang1 functions as an agonist of this pathway and Ang2 functions as an antagonist of Ang1 by binding to overlapping sites on Tie2, thereby reversing the vascular protection conferred by Ang1 [9].
Unlike APC, it has been demonstrated that PAR-1 cleavage by thrombin elicits a proinflammatory response, thereby increasing the ratio of Ang2:Ang1 and down-regulating the Ang1/Tie2 signaling pathway in endothelial cells [8,12]. The mechanism by which the cleavage of PAR-1 by either thrombin or APC initiates paradoxical protective and disruptive cellular responses is not fully understood, but the evidence for that has been primarily based on in vitro studies utilizing cultured human umbilical vein endothelial cells (HUVEC). To investigate this question further and determine whether the PAR-1 cleavage-dependent Ang1/Tie2 signaling specificity of endothelial cells can be influenced by the occupancy of EPCR, we pretreated HUVECs with protein C-S195A before activating cells with thrombin. We discovered that when EPCR is occupied by protein C, a physiologically relevant concentration of thrombin up-regulates the Ang1/Tie2 signaling pathway by promoting the expression of both Ang1 and Tie2 and inhibiting the expression of Ang2 by the PAR-1-cleavage dependent activation of the PI3K pathway. Interestingly, further studies revealed that the occupancy of EPCR by protein C potently inhibits the rapid thrombin mobilization of both P-selectin and Ang2 from Weibel-Palade bodies of endothelial cells, suggesting that thrombin can play an antiinflammatory role under normal physiological conditions.
Materials and methods
Regents
Thrombin, the PI3K inhibitor, LY-294002, and the cholesterol depleting drug methyl-β-cyclodextrin (MβCD) were obtained from Sigma (St. Louis, MO, USA). Blocking and non-blocking monoclonal anti-PAR-1, anti-Tie2 and anti-P-selectin antibodies were purchased from Santa Cruz Biologics (Santa Cruz, CA). The function-blocking anti-EPCR antibody (Clone RCR-252) was purchased from Cell Sciences (Canton, MA). Expression and purification of the Ser-195 to Ala substitution mutant of PC (PC-S195A), APC and Gla-domainless APC (GD-APC) has been described [13,14].
Cell culture
Immortalized human umbilical vein endothelial cells (EA.hy926) was kindly provided by Dr. C. Edgell (University of North Carolina at Chapel Hill, USA) and maintained as descried [13]. Primary HUVECs were obtained from Cambrex Bio Science Inc. (Charles City, IA) and maintained as described [13]. Cells were cultured at 37 °C in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum and antibiotics (penicillin G and streptomycin).
ELISA for Ang1, Ang2, and Tie2
Commercially available ELISA kits for Ang1 (Dang 10), Ang2 (Dang 20) and Tie2 (DTE 200) were purchased from R&D Systems (Minneapolis, MN, USA) and used according to the manufacturer’s instruction. Briefly, conditioned cells grown in 6-well plates were lysed by the cell lysis buffer and incubated with Ang1, Ang2, and Tie2, conjugates followed by washing and addition of the substrate for 30 min. The optical density was measured with a microplate reader at 450 nm. These measurements were also conducted in the presence of MβCD (10 mM for 1h) before stimulation with thrombin (2 nM ± 50 nM PC-S195A).
Cell-based ELISA measuring Tie2 phosphorylation
The commercially available ELISA kit for measuring phospho-Tie2 (KCB 2720) was purchased from R&D Systems and used according to the manufacturer’s instruction. Briefly, cells in 96-well plates were treated with APC (20 nM) or thrombin (2 nM) for 0–8h. Cells were fixed with 4% formaldehyde, and Tie2 phosphorylation was measured using two primary antibodies recognizing either total or phospho-Tie2. Cells were then incubated with horseradish-peroxidase or alkaline phosphatase labeled secondary antibodies followed by the addition of two spectrally distinct fluorogenic substrates. The extent of Tie2 phosphorylation was measured by a fluorescence plate reader at 450 nm (total Tie2) and 600 nm (phopho-Tie2) according to the manufacturer’s instruction.
Cell surface ELISA for P-selectin and Ang2
The mobilization of P-selectin, Ang1 and Ang2 to cell surface was determined by a whole-cell-based ELISA [5]. Briefly, conditioned cells were grown in 96-well plates. After medium was removed, cells were washed with PBS, and fixed by adding 50 μL of 1% paraformaldehyde. After washing, 100 μL of mouse anti-human monoclonal antibodies for P-selectin, Ang1 and Ang2 (1:50 each) were added. Cells were washed three times and peroxidase-conjugated anti-mouse IgG antibodies was added. Cells were washed again and developed using o-phenylenediamene substrate at 490 nm.
Permeability assay
Cell permeability in response to thrombin (5 nM for 10min) was quantitated by spectrophotometric measurements of the flux of Evans blue-bound albumin across cell monolayers using a modified 2-compartment chamber model [13]. In experiments in the presence of PC-S195A, cells were pretreated with the zymogen (50 nM) for 30min prior to stimulation by thrombin (2 nM for 0–24h).
RNA Interference
Cell permeability and the expression of Ang1, Ang2 and Tie2 in response to thrombin before and after treatment of cells with PC-S195A (50 nM, 30min) was evaluated following the knockdown of the expression of S1P1 and Tie2 by specific siRNA to each receptor according to the manufacturer’s instruction (Santa Cruz Biotechnology, Santa Cruz, CA).
Statistical Analysis
Results are expressed as means ± standard deviation (S.D.) of at least three independent experiments. The statistical significance of differences between test groups was used for statistical comparison (SPSS, version 14.0, SPSS Science, Chicago, Il, USA). Statistical relevance was determined using analysis of variance (ANOVA) with p-values less than 0.05.
Results
Effect of thrombin on the regulation of the Ang/Tie2 signaling pathway in endothelial cells
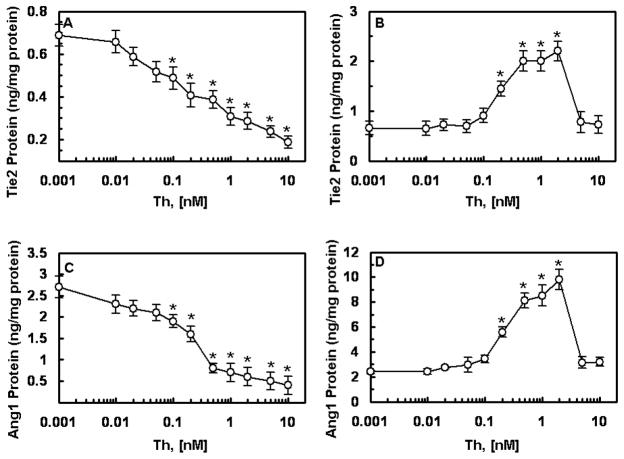
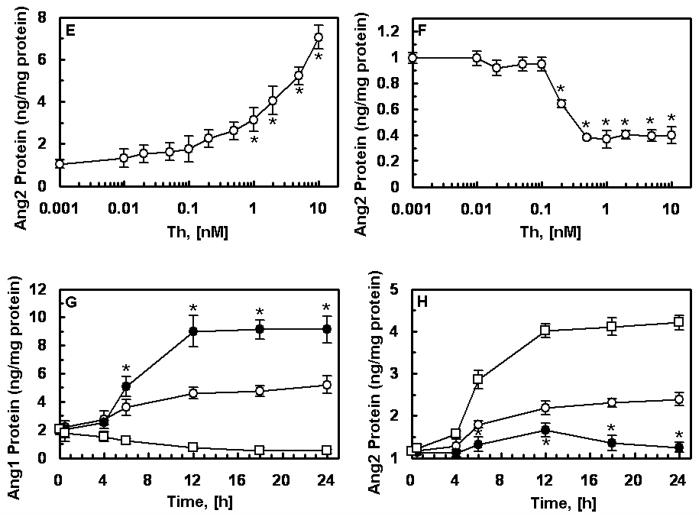
It was recently demonstrated that APC up-regulates the Ang/Tie2 pathway in endothelial cells by promoting the expression of both Ang1 and Tie2 and inhibiting the expression of Ang2, thus increasing the Ang1:Ang2 ratio and favoring the receptor occupancy by Ang1 [8]. Unlike APC, thrombin down-regulates the Ang/Tie2 axis in endothelial cells [8,12]. Both thrombin and APC activate cells by cleaving PAR-1 [1,2], though the efficiency of cleavage by the former exceeds the latter by 3–4 orders of magnitude [15]. To determine whether the cleavage of PAR-1 on cells by the two proteases initiates opposite responses, or if the occupancy of EPCR by Gla-domain of protein C/APC is responsible for the differential regulation of the Ang/Tie2 pathway by two proteases, we pretreated transformed HUVECs (EA.hy926 cells) with PC-S195A and then activated them with increasing concentrations of thrombin. Interestingly, when EPCR was occupied, thrombin up-regulated the Ang/Tie2 axis by promoting the expression of both Tie2 (panels A and B) and Ang1 (panels C and D) and inhibiting the expression of Ang2 (panels E and F). Time course analysis indicated a robust expression level for Ang1 after 6h with thrombin (Fig. 1G). The Ang2 protein level in the cell lysate did not increase even after 24h stimulation with thrombin if cells were pretreated with PC-S195A (Fig. 1H). These results suggest that the occupancy of EPCR by its ligand is responsible for the up-regulation of the Ang/Tie2 axis by both proteases. It should be noted that some up-regulation of both Ang1 (Fig. 1G) and Ang2 (Fig. 1H) was observed in cells pretreated with PC-S195A alone after 6h, possibly suggesting alterations in the gene expression during the incubation time.
Figure 1.
Effect of thrombin on the expression of Ang/Tie2 signaling molecules. EA.hy926 cells were incubated with indicated concentrations of thrombin for 12h either before (panels A, C, and E) or after (panels B, D, and F) treating monolayers with PC-S195A (50 nM, 30 min). Protein levels in cell lysates for Tie2 (A and B), Ang1 (C and D) and Ang2 (E and F) were measured by ELISA. * p< 0.05 as compared to 0 nM Th. Time course of the thrombin-mediated expression of Ang1 (G) and Ang2 (H) in the PC-S195A-pretreated cells were measured after incubation with 2 nM thrombin followed by measuring protein levels in cell lysates by ELISA. Symbols are: ○, PC-S195A; ●, thrombin + PC-S195A; □, thrombin alone. *p< 0.05 as compared to 0 nM Th.
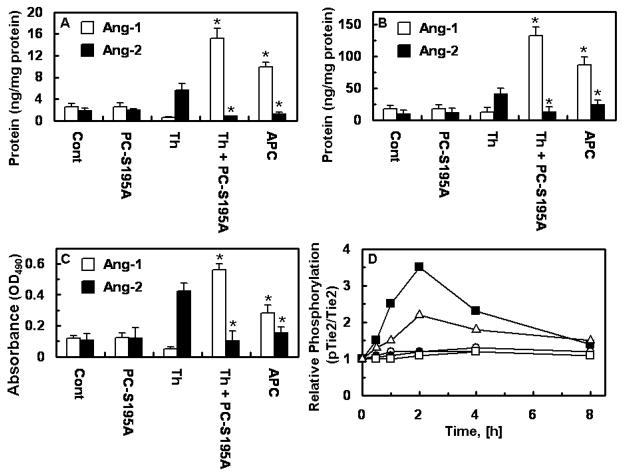
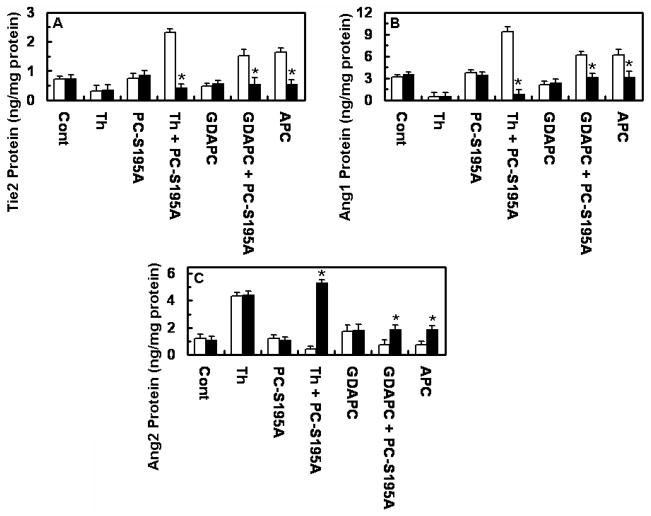
The regulation of the Ang/Tie2 pathway by either APC or thrombin was compared side by side using primary HUVECs. Results presented in Fig. 2 support the conclusion that when EPCR is occupied by its ligand thrombin up-regulates the pathway with a higher efficiency relative to APC as evidenced by the higher expression level of Ang1 in supernatants, cell surface and cell lysates. Further studies revealed that both APC and thrombin mediate the phosphorylation of Tie2, and consistent with the data presented above, thrombin was more efficient than APC in initiating this pathway (Fig. 2D).
Figure 2.
The occupancy of EPCR by its ligand switches the PAR-1-dependent effect of thrombin on the expression of Tie2, Ang1, and Ang2. Primary HUVECs were incubated with APC (20 nM) or thrombin (2 nM) for 16h before or after treating cells with PC-S195A followed by measuring Ang1 and Ang2 levels in supernatant (A) and cell lysate (B) by a regular ELISA and cell surface (C) by a cell-based ELISA. *p< 0.05 as compared to thrombin. (D) Time course of phosphorylation of Tie2 in lysates of primary HUVECs was analyzed by ELISA after treating cells with APC (20 nM) or thrombin (2 nM) ± PC-S195A (50 nM, 30 min) for 16h. The ratio of phosphor-Tie2 to total Tie2 is shown in y-axis. Symbols are: ○, control buffer; ●, thrombin alone; □, PC-S195A alone; ■, thrombin + PC-S195A; △, APC.
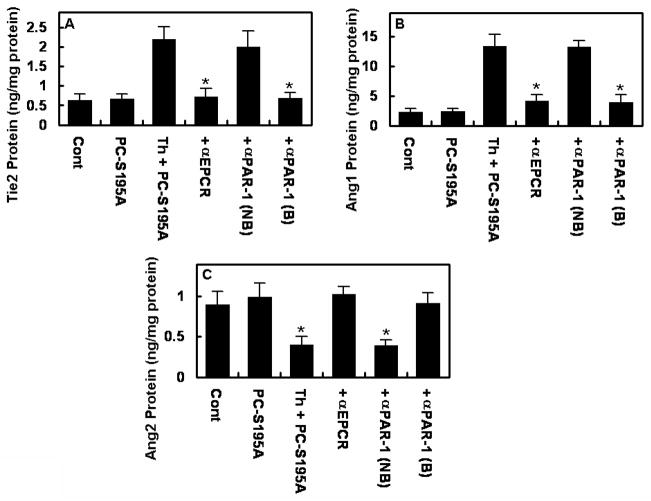
To demonstrate that the occupancy of EPCR is responsible for the PAR-1-dependent up-regulation of the Ang/Tie2 signaling cascade by thrombin, we measured expression levels of all three proteins in cells pretreated with antibodies against either EPCR or PAR-1 prior to their incubation with PC-S195A and stimulation by thrombin. Results presented in Fig. 3 demonstrate that function-blocking antibodies to both EPCR and PAR-1 can inhibit the thrombin-mediated expression of both Tie2 and Ang1. As expected, both function-blocking antibodies also led to thrombin up-regulation of the Ang2 expression in cells pretreated with PC-S195A (Fig. 3C). The Non-blocking anti-PAR-1 antibody had no effect on the expression of signaling molecules.
Figure 3.
EPCR- and PAR-1-dependent effect of thrombin on the expression of Tie2, Ang1, and Ang2. EA.hy926 cells were preincubated with blocking antibodies to EPCR and PAR-1 (25 μg/mL, 30min) followed by treatment with PC-S195A (50 nM, 30min) and stimulation by thrombin (2 nM for 12h). Protein levels of Tie2 (A) Ang1 (B) and Ang2 (C) were measured by ELISA. *p< 0.05 as compared to 2 nM Th with PC-S195A (A and B) or compared to PC-S195A alone (C). Cont, not treated control; B, blocking; NB, nonblocking
EPCR and PAR-1-dpendent up-regulation of the Ang/Tie2 pathway requires lipid-raft localization and is independent of the protease cleaving the receptor
To provide further support for the hypothesis that the occupancy of EPCR governs the specificity of the Ang/Tie2 response, but not the protease type cleaving PAR-1, protein levels of Tie2, Ang1, and Ang2 in cell lysates were measured after stimulating cells with Gla-domainless APC (GD-APC), which can activate PAR-1 with similar efficiency as APC, but the mutant protease cannot elicit a protective signaling response [14]. Similar to thrombin, GD-APC down-regulated the Ang/Tie2 axis by inhibiting the expression of both Tie2 and Ang1 and promoting the expression of Ang2 in endothelial cells (Fig. 4). However, when cells were pretreated with PC-S195A, GD-APC up-regulated the Ang/Tie2 pathway, thus promoting the expression of both Tie2 and Ang1 and inhibiting the expression of Ang2 (Fig. 4). These results confirm our hypothesis that the EPCR occupancy determines the specificity of the Tie2 signaling by coagulation proteases.
Figure 4.
Effects of different proteases on the expression of Tie2, Ang1, and Ang2 proteins. Primary HUVECs were incubated with indicated proteases (2 nM thrombin, 20 nM APC or GD-APC) for 16h with (black bars) or without (white bars) sequential treatment with MβCD (10 mM, 1h) and PC-S195A (50 nM, 30 min) followed by measuring expression levels of Tie2 (A) Ang1 (B) and Ang2 (C) by ELISA. *p< 0.05 as compared to without MβCD.
We previously demonstrated that EPCR and PAR-1 are co-localized within lipid-rafts of endothelial cells and that disruption of lipid-rafts by MβCD abrogates the EPCR-dependent barrier-protective effects of both APC and thrombin in endothelial cells [5,14]. To determine whether lipid-raft localization is required for the EPCR-dependent up-regulation of the Ang/Tie2 signaling pathway, expression levels of proteins were evaluated in the presence of MβCD. Results presented in Fig. 4 demonstrate that MβCD eliminates the EPCR-dependent protective effects of thrombin, APC and GD-APC, suggesting that signaling via Tie2 occurs in lipid-rafts of endothelial cells.
Thrombin up-regulation of the Ang/Tie2 axis requires the activation of S1P1 and PI3K pathways
Previous results have indicated that the EPCR and PAR-1-dependent protective activity of APC is mediated through the transactivation of S1P1 [3–5]. To investigate this question for thrombin, we transfected endothelial cells (both primary and transformed HUVECs) with the siRNA for S1P1 prior to treatment with PC-S195A and stimulation by thrombin. Results presented in Fig. 5 (panels A–C) demonstrate that the S1P1 siRNA abrogates the ability of thrombin to up-regulate the Ang/Tie2 pathway, thus inhibiting the expression of both Ang1 and Tie2 and promoting the expression of Ang2.
Figure 5.
S1P1- and PI3K-dependent thrombin regulation of the expression of Tie2, Ang1, and Ang2. The PC-S195A pretreated primary HUVECs were incubated with thrombin (2 nM, 12h) before or after transfection with control or specific siRNA for S1P1 (10 nM, 24h) or treatment with LY294002 (10 μM, 1h). Expression levels of Tie2 (A) Ang1 (B) and Ang2 (C) were measured by ELISA. .*p< 0.05 as compared to 2 nM Th + PC-S195A. (D) Primary HUVECs were preincubated with blocking antibodies to EPCR, PAR-1, or Tie2 (25 μg/mL, 30min) or transfected with siRNA for Tie2 (10 nM, 24h) followed by incubation with PC-S195A and stimulation by thrombin (2 nM for 12h). The cell permeability was measured as described under “Materials and Methods”. *p< 0.05 as compared to PC-S195A alone. NS, non-specific
Previous results have demonstrated that the protective activity of APC is mediated via the PI3K pathway [3,6,7]. To determine whether this pathway is also involved in the thrombin-mediated up-regulation of the Ang/Tie2 signaling cascade, cells were preincubated with the PI3K inhibitor, LY-294002. Results presented in Fig. 5 (Panels A–C) demonstrate that the protective effect of thrombin in the PC-S195A-treated cells is suppressed by LY-294002, suggesting that the PI3K pathway is also involved in the EPCR-dependent up-regulation of the Ang/Tie2 signaling cascade by thrombin.
Tie2 transactivation is required for the EPCR-dependent barrier-protective effect of thrombin
It was recently shown that the barrier-protective effect of APC requires the Ang1-mediated activation of Tie2 [8]. It is known that thrombin can markedly increase the permeability of endothelial cells [1,3,12,16]. However, we previously demonstrated that the occupancy of EPCR by protein C switches the signaling specificity of thrombin from a barrier-disruptive to a barrier-protective response [5,13]. The results presented in Fig. 5D (shown for the primary HUVECs only) demonstrate that the EPCR and PAR-1-dependent barrier-protective effect of thrombin also requires transactivation of the Tie2 signaling cascade and that this response is blocked by antibodies to all three receptors. Further support for this hypothesis was provided by the observation that the Tie2 specific siRNA, but not the non-specific control siRNA, significantly increased the cell permeability in response to thrombin even if cells were pretreated with PC-S195A (Fig. 5D).
EPCR occupancy by PC-S195A inhibits Ang2 and P-selectin release by thrombin from Weibel-Palade bodies (WPBs)
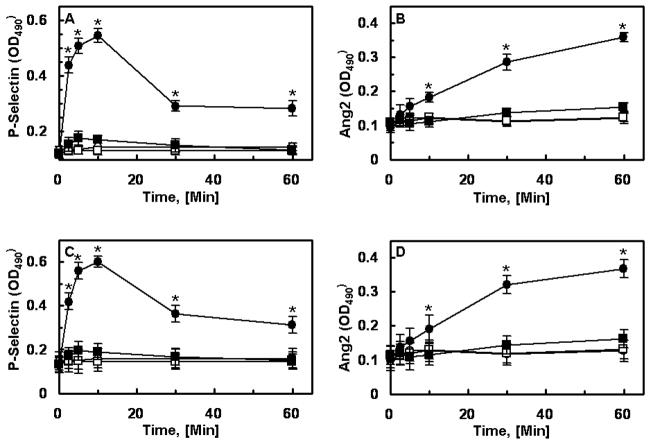
P-selectin is stored in the pre-formed state in WPBs in endothelial cells [17,18]. The stimulation of endothelial cells by thrombin and proinflammatory cytokines mobilizes P-selectin to the cell surface within minutes, thereby facilitating the recruitment and transmigration of leukocytes across the activated endothelium [17,18]. Recent results have indicated that Ang2 is also stored in the pre-synthesized form within WPBs [9]. To determine whether thrombin down-regulates the Ang/Tie2 pathway by mediating the rapid release of Ang2 from these storage compartments, and whether the occupancy of EPCR by protein C inhibits this process, the time course of the thrombin release of P-selectin and Ang2 were studied in endothelial cells by a cell-based ELISA with and without treatment with PC-S195A. Interestingly, the thrombin mobilization of P-selectin from WPBs was completely inhibited if cells were pretreated with PC-S195A (Fig. 6, panel A for transformed and panel C for primary HUVECs). Since upon release, Ang2 can tightly bind to Tie2, we monitored the Ang2 level bound to the cell surface after stimulation by thrombin. As shown in Fig. 6 (panel B for transformed and panel D for primary HUVECs), Ang2 was mobilized onto the cell surface with similar kinetics as P-selectin and PC-S195A potently inhibited the thrombin mobilization of Ang2. This EPCR-dependent protective effect of thrombin was eliminated if cells were treated with MβCD (data not presented). A slower binding curve for Ang2 may reflect the fact that, unlike the integral membrane protein P-selectin, Ang2 is a soluble protein that reversibly binds to Tie2. Thus, some of the protein, most likely, is released to the supernatant which cannot be detected by the cell-based ELISA.
Figure 6.
PC-S195A inhibits the mobilization of P-selectin and Ang2 onto endothelial cell surface. EA.hy926 cells (A and B) or primary HUVECs (C and D) were incubated with thrombin (2 nM) with or without pretreatment of cells with PC-S195A. The cell surface P-selectin (A and C) or Ang2 (B and D) was measured by an ELISA described under “Materials and Methods”. Symbols are: ○, control buffer; ●, thrombin alone; □, PC-S195A alone; ■, thrombin + PC-S195A. *p< 0.05 as compared to Th + PC-S195A.
Discussion
We have demonstrated in this study that the occupancy of EPCR by its ligand, protein C, confers a PAR-1-dependent protective cellular property for thrombin. This is derived from the observation that thrombin up-regulated the Ang/Tie2 signaling cascade by promoting the expression of the Tie2 agonist, Ang1, and inhibiting the rapid release of the Tie2 antagonist, Ang2, from WPBs of endothelial cells. It has been reported that Ang1; constitutively expressed by endothelial cells, pericytes and many other cell types; plays a protective role by maintaining the vascular quiescence through low-level Tie2 phosphorylation in the adult vasculature [9]. On the other hand, Agn2 is exclusively expressed by endothelial cells and has a proinflammatory role, thus its storage in WPB and its rapid release by proinflammatory cytokines results in rapid destabilization and enhanced permeability of endothelium [9]. It is traditionally thought that the cleavage of PAR-1 by thrombin initiates proinflammatory responses in endothelial cells, thus rapidly converting the non-thrombogenic and non-adhesive phenotype of the vasculature to one that can support clotting, adhesion and transmigration of activated leukocytes [1,3,12,16). A classic example is the observation that the treatment of endothelial cells with a low concentration of thrombin rapidly mobilizes P-selectin in seconds to minutes from WPBs onto the surface of endothelial cells to facilitate the initial rolling and subsequent tight adhesion of neutrophils and other immune cells to activated endothelium (17,18). However, our results in this study clearly suggest that this conclusion may only be applicable to in vitro cell culture systems and that the activation of PAR-1 by either thrombin or APC would elicit similar protective responses if EPCR is occupied by the physiologically relevant concentrations of the ligand protein C.
We previously demonstrated that the occupancy of EPCR by protein C switches the PAR-1-dependent signaling specificity of thrombin from a hyperpermeability to a barrier-protective response [13]. In this study now, for the first time, we demonstrate that the cleavage of PAR-1 by thrombin also inhibits the release of proinflammatory molecules, P-selectin and Ang2 from WPBs, suggesting that thrombin activation of PAR-1 on the healthy vasculature expressing EPCR would only initiate protective anti-inflammatory and anticoagulant responses under normal physiological conditions. However, the PAR-1-dependent protective effect of thrombin can be reversed if the vascular bed is lacking EPCR or if the receptor is down-regulated by injury or inflammation, in which case the cleavage of PAR-1 by thrombin is expected to initiate a proinflammatory response. In this case, thrombin can rapidly release Ang2 from WPBs, thereby increasing the ratio of Ang2:Ang1 and terminating the constitutive protective Tie2 signaling pathway that is thought to be regulated by the pericyte-derived Ang1 in the quiescent vascular endothelial cells [9]. Thus, the type of signaling response that the cleavage of PAR-1 produces is not dependent on the type of the coagulation protease that is cleaving the receptor, but rather it is the occupancy of EPCR that governs the specificity of PAR-1 signaling. Further support for this hypothesis was provided by the observation that GD-APC also down-regulated the Ang/Tie2 pathway in endothelial cells, but the pre-incubation of cells with PC-S195A reverted the signaling specificity of GD-APC, thus initiating a protective response that was similar, in both duration and magnitude, to that of APC (Fig. 4). It was interesting to note that the potency of thrombin in eliciting a protective Ang1/Tie2 response in endothelial cells pretreated with PC-S195A was significantly higher than that of APC both in its magnitude and duration. This may be due to thrombin activating PAR-1 with a 3–4 orders of magnitude higher catalytic efficiency than APC [15]. It should be noted that high concentrations of thrombin can cleave PAR-4 to initiate barrier disruptive responses even if EPCR is occupied by protein C [13].
The EPCR-dependent cytoprotective and anti-inflammatory activity of APC is mediated through the activation of the PI3K/Akt survival pathway which leads to the phosphorylation of the Gi-protein coupled receptor, S1P1 [3–6]. The observation that both the specific PI3K inhibitor and the siRNA for S1P1 blocked the EPCR-dependent up-regulation of the Ang/Tie2 signaling pathway by thrombin suggests that the PAR-1-dependent protective activity of thrombin is also mediated through the activation of the same pathway. Thus, the protective effect of thrombin requires coordinated signaling through at least four receptors, EPCR, PAR-1, Tie2 and S1P1 (Fig. 7). The observation that MβCD abrogated the up-regulation of the Ang/Tie2 pathway further suggests that all four receptors are colocalized within the lipid-rafts. In support of this hypothesis, we previously showed that EPCR and PAR-1 are associated with caveolin-1 in lipid-rafts of endothelial cells and that that the occupancy of EPCR by protein C leads to dissociation of EPCR from caveolin-1, possibly accounting for the PAR-1-dependent switch in the signaling specificity of thrombin (Fig. 7) [5,13]. The sensitivity of the Ang1/Tie2 response to MβCD suggests that Tie2 is also localized within lipid-rafts of endothelial cells, nevertheless, there is also a report indicating that Tie2 translocates to lipid-rafts only after it binds to its agonist ligand Ang1 [19]. Thus, further studies will be required to understand the exact relationship between the four receptors within lipid-rafts and the mechanisms by which their crosstalk in response to various stimuli regulate the phenotype of the vasculature under different pathophysiological conditions.
Figure 7.
Hypothetical model of crosstalks between EPCR, PAR-1, S1P1, and Tie2. EPCR is associated with caveolin-1 (Cav-1) in lipid-rafts of endothelial cells when the receptor is not occupied by Gla-domain of protein C/APC. Thrombin cleavage of PAR-1 elicits disruptive responses through signaling via Gq and/or G12/13, thereby activating the NF-κB pathway. However, the occupancy of EPCR by protein C (PC) results in the dissociation of EPCR from caveolin-1, thereby switching the specificity of PAR-1 signaling by coupling it to the Gi-protein and/or transactivation of the Gi-protein coupled receptor, S1P1, and mediating the phosphorylation of the receptor by the PI3K/Akt pathway. The EPCR-dependent PAR-1 cleavage by thrombin also increases the expression levels of Ang1 and Tie2, thus initiating/amplifying the PI3K/Akt-dependent protective pathway through the Ang1-mediated phosphorylation of Tie2.
Acknowledgments
We would like to thank Audrey Rezaie for proofreading the manuscript. The research discussed herein was supported by National Research Foundation (NRF) of Korea funded by the Ministry of Education, Science and Technology (2009-0065105) to JSB and by grants awarded by the National Heart, Lung, and Blood Institute of the National Institute of Health HL 68571 and HL 62565 to ARR.
References
- 1.Mosnier LO, Griffin JH. The cytoprotective protein C pathway. Blood. 2007;109:3161–72. doi: 10.1182/blood-2006-09-003004. [DOI] [PubMed] [Google Scholar]
- 2.Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science. 2002;296:1880–2. doi: 10.1126/science.1071699. [DOI] [PubMed] [Google Scholar]
- 3.Finigan JH, Dudek SM, Singleton PA, Chiang ET, Jacobson JR, Camp SM, Ye SQ, Garcia JG. Activated protein C mediates novel lung endothelial barrier enhancement: role of sphingosine 1-phosphate receptor transactivation. J Biol Chem. 2005;280:17286–93. doi: 10.1074/jbc.M412427200. [DOI] [PubMed] [Google Scholar]
- 4.Feistritzer C, Riewald M. Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine 1-phosphate receptor-1 crossactivation. Blood. 2005;105:3178–84. doi: 10.1182/blood-2004-10-3985. [DOI] [PubMed] [Google Scholar]
- 5.Bae JS, Rezaie AR. Protease activated receptor 1 (PAR-1) activation by thrombin is protective in human pulmonary artery endothelial cells if endothelial protein C receptor is occupied by its natural ligand. Thromb Haemost. 2008;100:101–9. doi: 10.1160/TH08-02-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bae JS, Kim YU, Park MK, Rezaie AR. Concentration dependent dual effect of thrombin in endothelial cells via Par-1 and Pi3 Kinase. J Cell Physiol. 2009;219:744–51. doi: 10.1002/jcp.21718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uchiba M, Okajima K, Oike Y, Ito Y, Fukudome K, Isobe H, Suda T. Activated protein C induces endothelial cell proliferation by mitogen-activated protein kinase activation in vitro and angiogenesis in vivo. Circ Res. 2004;95:34–41. doi: 10.1161/01.RES.0000133680.87668.FA. [DOI] [PubMed] [Google Scholar]
- 8.Minhas N, Xue M, Fukudome K, Jackson CJ. Activated protein C utilizes the angiopoietin/Tie2 axis to promote endothelial barrier function. FASEB J. 2009 Oct 26; doi: 10.1096/fj.09-134445. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Fiedler U, Augustin HG. Angiopoietin: a link between angiogenesis and inflammation. Trends Immunol. 2006;27:552–8. doi: 10.1016/j.it.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Jones N, Iljin K, Dumont DJ, Alitalo K. Tie receptors: new modulators of angiogenic and lymphangiogenic responses. Nat Rev Mol Cell Biol. 2001;2:257–67. doi: 10.1038/35067005. [DOI] [PubMed] [Google Scholar]
- 11.Gamble JR, Drew J, Trezise L, Underwood A, Parsons M, Kasminkas L, Rudge J, Yancopoulos G, Vadas MA. Angiopoietin-1 is an antipermeability and anti-inflammatory agent in vitro and targets cell junctions. Circ Res. 2000;87:603–7. doi: 10.1161/01.res.87.7.603. [DOI] [PubMed] [Google Scholar]
- 12.Li X, Hahn CN, Parsons M, Drew J, Vadas MA, Gamble JR. Role of protein kinase Cζ in thrombin-induced endothelial permeability changes: inhibition by angiopoietin-1. Blood. 2004;104:1716–24. doi: 10.1182/blood-2003-11-3744. [DOI] [PubMed] [Google Scholar]
- 13.Bae J-S, Yang L, Manithody C, Rezaie AR. The ligand occupancy of endothelial protein C receptor switches the PAR-1-dependent signaling specificity of thrombin from a permeability-enhancing to a barrier-protective response in endothelial cells. Blood. 2007;110:3909–16. doi: 10.1182/blood-2007-06-096651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bae JS, Yang L, Rezaie AR. Receptors of the protein C activation and activated protein C signaling pathways are colocalized in lipid rafts of endothelial cells. Proc Natl Acad Sci USA. 2007;104:2867–72. doi: 10.1073/pnas.0611493104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ludeman MJ, Kataoka H, Srinivasan Y, Esmon NL, Esmon CT, Coughlin SR. PAR1 cleavage and signaling in response to activated protein C and thrombin. J Biol Chem. 2005;280:13122–8. doi: 10.1074/jbc.M410381200. [DOI] [PubMed] [Google Scholar]
- 16.Coughlin SR. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost. 2005;3:1800–14. doi: 10.1111/j.1538-7836.2005.01377.x. [DOI] [PubMed] [Google Scholar]
- 17.McEver RP. Selectins: novel receptors that mediate leukocyte adhesion during inflammation. Thromb Haemost. 1991;65:223–8. [PubMed] [Google Scholar]
- 18.Mayadas TN, Johnson RC, Rayburn H, Hynes RO, Wagner DD. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell. 1993;74:541–554. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- 19.Katoh S-Y, Kamimoto T, Yamakawa D, Takakura N. Lipid rafts serve as signaling platforms for Tie2 receptor tyrosine kinase in vascular endothelial cells. Exp Cell Res. 2009;315:2818–23. doi: 10.1016/j.yexcr.2009.07.008. [DOI] [PubMed] [Google Scholar]