Abstract
Mesothelin is a potential new target for cancer immunotherapy because it is present at relatively low levels only in mesothelial cells of pleura, peritoneum and pericardium of healthy people but is significantly elevated in a number of tumors, including mesothelioma, ovarian, pancreatic and lung cancers. However, all currently available antibodies against mesothelin are either murine or chimeric which could limit their use because of increased likelihood of immunogenicity compared to fully human antibodies. Here we report the identification and characterization of a novel fully human monoclonal antibody, m912, which was isolated from a human Fab library by panning against recombinant mesothelin. This antibody in scFv, Fab and IgG1 formats bound specifically and with high affinity (equilibrium dissociation constant in the nM range) to cell surface-associated human mesothelin and to recombinant mesothelin. It specifically lysed cancer cells engineered to express mesothelin in the presence of peripheral blood mononuclear cells isolated from healthy donors most likely by antibody-dependent cellular cytotoxicity (ADCC). M912 is the first reported fully human monoclonal antibody to mesothelin, which has potential for cancer treatment and diagnosis
Keywords: mesothelin, mesothelioma, ovarian cancer, lung cancer, therapeutic antibody
Introduction
Mesothelin was identified as antigen to an antibody Mab K1, which specifically recognized several ovarian cancers (1). It is encoded as a 628-amino acids glycoprotein and cleaved by furin into a membrane-attached 40 kD form, mesothelin, and a smaller form released from cells (2). Mesothelin is attached to cell surface via glycosyl-phosphatidyl inositol link to its carboxyl terminus. Presently, limited knowledge about its function is available. Mice with both copies of mesothelin genes inactivated seem to have normal growth and reproduction ability (3). It has been reported that mesothelin interacts to CA125 (or MUC16), an ovarian cancer antigen, and the interaction may play a role in metastasis of ovarian cancers to the peritoneal cavity (4, 5). The downstream signals activated by the interaction of CA125 are still not clear.
The unique distribution pattern of mesothelin in human bodies suggests its potential as a cancer target. In healthy people, mesothelin expression is limited to mesothelial cells lining the pleura, peritoneum and pericardium. Other normal tissues tested do not express mesothelin protein (1). However, mesothelin is over-expressed in a high percentage of ovarian cancers, pancreatic cancers, non-small lung cancers and mesothelioma (6–8). It has been reported that a majority of serous carcinomas of the ovary and adenocarcinomas of the pancreas express high levels of mesothelin (9). In addition, high levels of mesothelin have been detected in >55% of lung cancers and >70% ovarian cancers (7, 10) . In mesothelioma patients, mesothelin protein is not only readily detectable on tumors, but it is also present in patient serum (11). Furthermore, the mesothelin-positive lung cancer cells die upon exposure to a recombinant immunotoxin targeted to mesothelin (10). Because of its limited distribution in normal tissues and elevated expression in cancers, mesothelin has been considered as an excellent target for cancer therapy. Various methods have been employed to deliver cytotoxic drugs to mesothelin-positive cells or elicit cell-mediated and humoral responses to mesothelin and in turn eliminate tumors. DNA vaccines against mesothelin have been shown to inhibit tumor growth in a mouse model (12, 13). A fusion protein of mesothelin-specific single chain and immunotoxin (SS1P) is currently in phase I trial (14). A chimeric monoclonal antibody specific to mesothelin, MORAb-009, is being tested in a phase I trial. In a xenograft model, MORAb-009 synergizes with chemotherapy drugs taxol and gemcitabine, even though it has little effect when used alone in these models (15). Given the potential of targeting mesothelin as an effective treatment for mesothelin-positive tumors, a fully-human therapeutic antibody could provide additional options in terms of immunogenicity and better tolerance. Here we describe a high-affinity fully human mesothelin antibody with a potential as a cancer therapeutic.
Materials and Methods
Cell cultures
A431 cells, human epidermoid carcinoma cells, were maintained in RPMI1640 supplemented with 10% FBS and penicillin/streptomycin (complete growth medium). A431 cells do not express mesothelin. H9 cells were stable clone cells established from A431 cells that have been transfected with a vector carrying full-length mesothelin cDNA. H9 cells were maintained in complete RPMI1640 growth medium supplemented with 0.75 mg/ml G418. OVCAR-3 cells were purchased from ATCC, and maintained in RPMI1640 complete growth medium.
Expression of recombinant mesothelin protein
Human mesothelin fragment including amino acids 296~600 (the numbers are based on sequence in AY743922 in the NCBI database) was cloned from pcDNA3.2 to a baculovirus transfer vector pAcGP67 via Sma I and Not I sites. The recombinant product had extra residues ADPG on the N-terminus and 6 histidines on the C-terminus. It was co-transfected with BaculoGold viral DNA into SF9 insect cells according to the manufacturer’s instruction. Mesothelin protein was purified from conditioned medium with a nickel-chelating column, and further polished with a Superdex75 gel filtration column in PBS. Purity of mesothelin was examined with SDS-PAGE.
Antibody selection by phage display
Purified mesothelin was labeled with biotin first and used for panning of a human naïve Fab phage library (16). Briefly, amplified phage (~1012 pfu) pre-absorbed with MyOne streptavidin T1 beads (Invitrogen) was incubated with 4 µg of biotin-mesothelin for 2 hr. Specific phages were captured by fresh streptavidin beads. After extensive washes of the beads with PBS+0.05% Tween 20, phage was rescued by exponentially growing TG1 bacteria and helper phage. Pannings were repeated for three more times with more stringent washes at the latter two rounds. Three hundred colonies were picked from the last two rounds of panning and rescued with helper phage for screening. Two unique clones were selected, of which m912 had higher affinity and was selected for further characterization.
Antibody expression and purification
The Fab fragment was expressed in HB2151 cells as described previously (17). A single chain form of m912 was made by cloning VH and VL from Fab, connected by linker 3(GGGGS), into pComb3x (18). Expression and purification of scFv were similar to that of the Fab. Fab was converted into IgG1 by subcloning the heavy chain variable region and the light chain into pDR12. 293 Free Style cells were transfected with pDR12-m912, and IgG was secreted into medium. Fab and IgG were purified with a protein G column, and scFv was purified with a nickel-chelating column. All preparations were dialysed against PBS.
ELISA binding assay
Antigen (mesothelin) diluted in PBS is coated on narrow-well 96 well plate at 50 ng/well overnight at 4°C. Wells are blocked with 100 µl of 4% milk/PBS (MPBS) for 1 hr at 37°C. For Fab binding kinetics, Fab is titrated from 3000 nM to 0.038 nM (1: 5 serial dilutions). 50 µl of diluted Fab is added to duplicate wells. In competition ELISA, designated concentration of competing IgG was included in all Fab solutions. After 2 hr incubation at 37°C, the wells are washed with PBST (PBS+0.05% Tween 20) for 4 times. Bound Fab was detected with anti-FLAG-HRP mAb (1:1000) (Sigma) for 1 hr at 37°C. Wells are washed again with PBST. Substrate ABTS is added (50 µl/well), and the reaction is read at 405 nm. For ELISA with IgG, a goat anti-human Fc IgG conjugated with HRP was used at 1:1000.
Flow cytometry
A413 cells and H9 cells were detached by cell dissociation buffer and rinsed in PBS. Aliquots of cells were incubated with primary antibody (m912, or isotype controls) at indicated concentrations in 250 µl of RPMI+10% FBS for 1 hr on ice. Unbound antibodies were washed away with medium. Secondary antibody goat anti-human IgG conjugated with FITC (Sigma) was incubated with cells at 8 µl/ml for 30 min. For detection of Fab or scFv, 1.6 µg/ml of anti-His6 monoclonal antibody (Qiagen) and 8 ul/ml of goat anti-mouse IgG-FITC (Sigma) were incubated with cells. Cells were washed and resuspended in PBS+0.5% BSA for flow cytometry on FACSCalibur (Beckton Dickinson).
Cell lysis by ADCC (antibody-dependent cell-mediated cytotoxicity)
Peripheral blood mononuclear cells (PBMC) were isolated from healthy donors with Ficoll-Paque Plus (GE Healthcare). Collections of blood from donors were approved by NCI-Frederick Research Donor Program. The viability of isolated cells was >95%. PBMC were seeded in a 96 well plate in RPMI+10% FBS at 500,000 cells/well. Cells were incubated at 37°C and allowed to attach to the plate for 3 hr. Unattached cells were rinsed off by two washes of warm PBS, cells attached in the wells were used as the effector cells. Target cells, A431 or H9 cells, were trypsinized and resuspended into single cell suspensions. The target cells were incubated with various concentrations of antibody at room temperature for 30 min then added to effector cells at 10,000 cells/well. The ratio of effector and target cells is 50:1. The plate was centrifuged at 300g × 5 min and incubated at 37°C for 24 hr. Supernatant (100 µl) was transferred to an all-white plate and 100 µl of CytoTox-ONE reagent (Promega) was added to each well. The lactate dehydrogenase (LDH) released from lysed cells converted CytoTox substrate to fluorescent resazurin, which was measured in fluorometer (Ex 560 nm/Em 590 nm). The percentage of specific lysis was calculated as following: (experimental treatment-effector cell control)/(high control-target cell control) ×100%. Measurement of target cells alone treated with 1% Triton X-100 was used as high control. Each treatment was carried out in 6 duplicated wells. Each assay plate included control wells.
Western blot
H9 and OVCAR-3 cells were lysed in RIPA buffer (50 mM Tris, pH 7.4, 1% Triton X-100, 0.1% SDS, 1% deoxycholate, 150 mM NaCl, 5 mM NaF, 5 mM EDTA). After centrifugation at 20,000 g × 20 minutes, the clear supernatant was resolved on 4~12% NuPAGE and transferred to PVDF membrane. Primary antibodies used for blotting were MORAb-009 at 0.5 ug/ml (for mesothelin) and goat polyclonal antibody against actin (clone I-19, Santa Cruz Biotechnology, for monitoring equal loading) at 1 ug/ml, respectively. Corresponding secondary antibodies were goat anti-human Fc-HRP and donkey anti-goat IgG-HRP at 1 ug/ml for detection.
Results
Expression of recombinant human mesothelin in insect cells and its purification
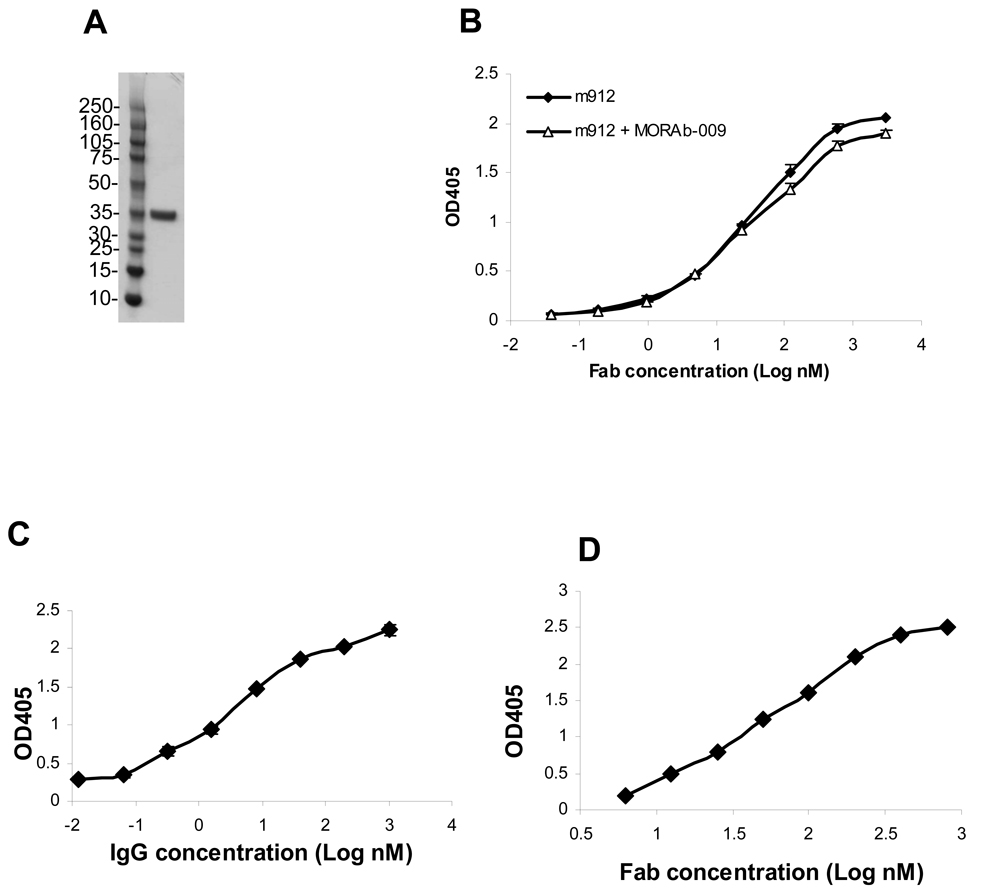
To efficiently select antibodies from phage libraries a purified recombinant antigen, in this case mesothelin, is needed. In order to include as much as possible of the mesothelin molecule, we expressed its entire extracellular domain, including the GPI linkage site serine-598 (19). To achieve relatively high yields and glycosylation in eukaryotic cells, mesothelin was cloned in a baculovirus expression vector. The transfer vector pAcGP67 has a signal peptide that directs secretion of recombinant protein into medium. Indeed, recombinant mesothelin (296–600 aa) expressed in SF9 insect cells was secreted into the culture medium. The mesothelin purified by a two-step procedure was >95% in purity and migrated at ~35 kD (Figure 1A) on polyacrylamide gel. It migrated slightly faster than the same fragment that was expressed in mammalian cells (data not shown), reflecting differences in post-translational modifications, such as glycosylation. The purified mesothelin was recognized by MORAb-009 (20), a mouse/human chimeric IgG, and was used for panning of a naïve human Fab phage display library.
Figure 1. High affinity binding of Fab m912 and IgG1 m912 to recombinant mesothelin.
A, A sample of recombinant human mesothelin purified from insect cell supernatant was run on 4~12% NuPAGE Bis-Tris gel. Molecular weight standards are in kD. B, Binding of Fab m912 to recombinant mesothelin coated on ELISA plate (-◆-). In competition ELISA, 3 nM MORAb-009 was added to all Fab m912 solutions (-Δ-) C, Binding of IgG1 m912 to recombinant mesothelin on ELISA. D. Binding of Fab m912 to mouse recombinant mesothelin on ELISA.
High-affinity binding of m912 to recombinant mesothelin
Mesothelin produced in insect cells was labeled with biotin and used for panning of our large naïve Fab library as described in Materials and Methods. After the third and fourth rounds of panning, three hundred clones were screened and two positive clones with different sequences were identified. Clone m912 bound with higher affinity to mesothelin than the other clone and was selected for further characterization. Fab m912 was converted to single chain and IgG1 formats. Fab m912 bound to mesothelin with an EC50 of 20 nM as measured by ELISA (Figure 1B). Including of MORAb-009 at a concentration higher than its IC50 in Fab m912 ELISA did not reduce binding signals, suggesting that the two antibodies have different epitopes on mesothelin (Figure 1B). The IgG1 exhibited higher effective binding affinity (avidity) of ~1.5 nM as measured by ELISA (Figure 1C). Deglycosylation of mesothelin by PNGase F reduced its size by at least 3 kD, but it did not change the binding ability of m912 (data not shown), indicating that the m912 epitope is independent of mesothelin glycosylation. Fab m912 also recognized mouse mesothelin with about the same affinity (Figure 1D).
Specific binding of m912 to cell surface-associated mesothelin
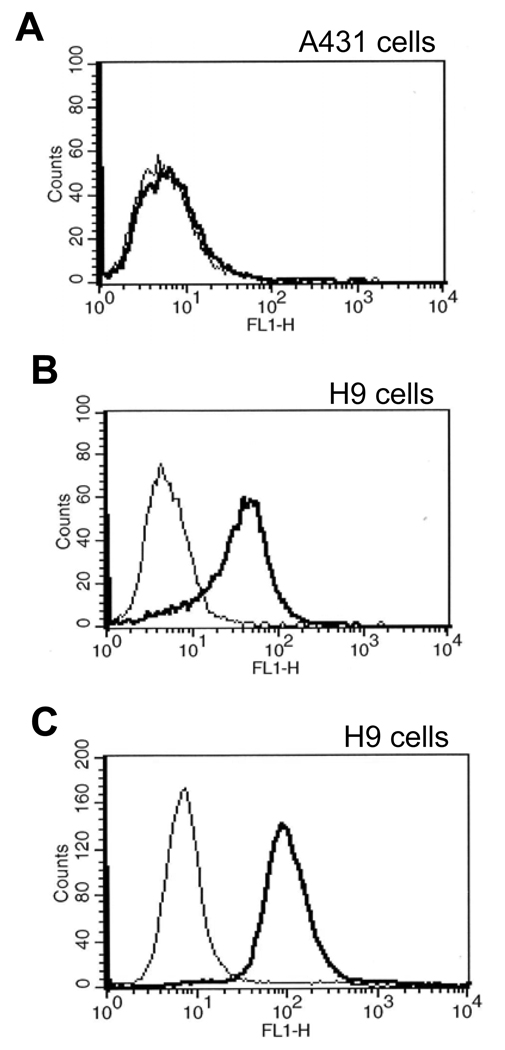
It is essential that a therapeutic or diagnostic antibody recognize the native protein. Therefore, Fab m912 was tested for binding to native mesothelin associated with cell surfaces. We used H9 cells which are derived from parental cells (A431), which do not express mesothelin, and stably express mesothelin protein. Fab m912 bound to H9 cells but not to A431 cells (Figure 2B), indicating that it is highly specific and does not recognize other membrane proteins on these cells.
Figure 2. Specific, high-affinity binding of Fab m912 and scFv m912 to cell surface-associated mesothelin.
A, Lack of Fab m912 binding to mesothelin-negative (A431) cells. B, Binding of Fab m912 but not of a control Fab to mesothelin-positive H9 cells. C, Binding of scFv m912 but not of a control scFv to mesothelin-positive H9 cells. In all three panels, thick lines represent binding of m912 in Fab or scFv formats and thin lines are for control Fab and scFv. The concentrations of all Fabs and scFv are 40 nM and 100 nM, respectively.
Various clinical applications require different sizes and valences of antibodies for best effect. For example, small sizes are preferred for targeting and imaging, whereas full-size antibodies (IgGs) have much longer half–life in circulation, and some are able to mediate effecter functions. Therefore, Fab m912 was converted to scFv and IgG formats, and tested for binding to cells by using flow cytometry. We found that m912 in both formats, scFv and IgG1, can bind specifically to cell surface-associated mesothelin (Figure 2C and Figure 3A).
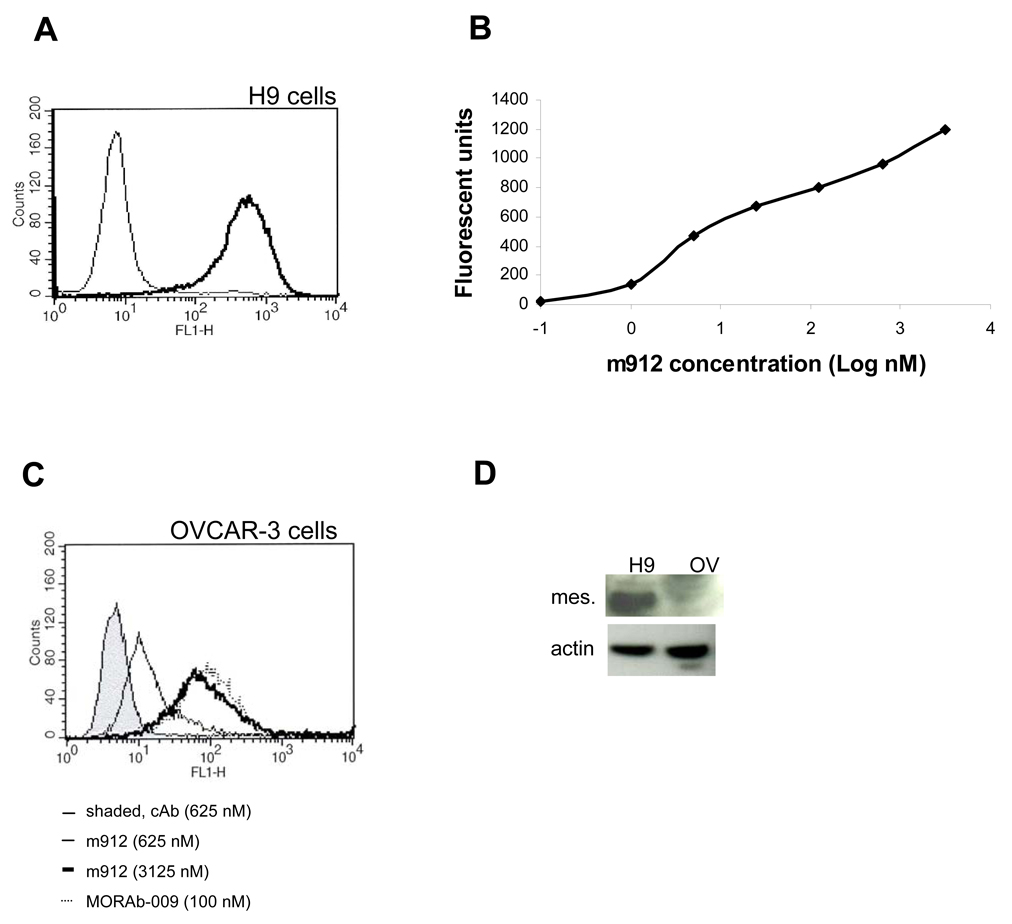
Figure 3. High avidity binding of IgG1 m912 to cell surface-associated mesothelin.
A. IgG1 m912 at 5 nM was incubated with mesothelin-positive H9 cells, the cells were washed and further incubated with goat anti-human IgG conjugated with FITC, then washed and analyzed by flow cytometry. An isotype control IgG (thin line) at 100 nM was used as negative control. B. The same flow cytometry analyses were performed at different concentrations of IgG1m912, and medium fluorescent units plotted as function of the antibody concentration. C. IgG1 m912 at 625 nM (thin line without shade) and 3125 nM (thick line) was incubated with OVCAR-3 cancer cells, as performed similarly in panel A. MORAb-009 was used at 100 nM (dotted line). An isotype control human IgG was used at 625 nM (thin line, shaded area). D. Levels of mesothelin proteins in H9 and OVCAR-3 cells as shown by Western blot. Equal amount of lysates were loaded as shown by actin blot.
To estimate the binding ability (avidity) of IgG1 m912 to native cell surface-associated mesothelin, we used antibody concentrations ranging from 0.1 nM to 3125 nM and H9 cells in a flow cytometry experiment (Figure 3B). Even at the lowest concentration of 0.1 nM IgG m912 exhibited significant binding to H9 cells. Based on the medium fluorescent units bound to H9 cells at each m912 concentration, a 50% binding of m912 was estimated to be at about 5~10 nM for these cells.
The binding of m912 was further tested in non-transfected cancer cells, ovarian cancer cell line, OVCAR-3. These cells have been reported to be positive with cell surface mesothelin. In flow cytometry, m912 showed specific binding in a dose-dependent fashion (Figure 3C); however, higher concentrations were required to reach the same level of binding seen with H9 cells. This was partially due to the much lower levels of mesothelin protein expressed by the OVCAR-3 cells than by H9 cells as demonstrated by the Western blot (Figure 3D). MORAb-009 still showed high avidity on OVCAR-3 cells.
Specific lysis of mesothelin-positive cells by m912 in presence of PBMC
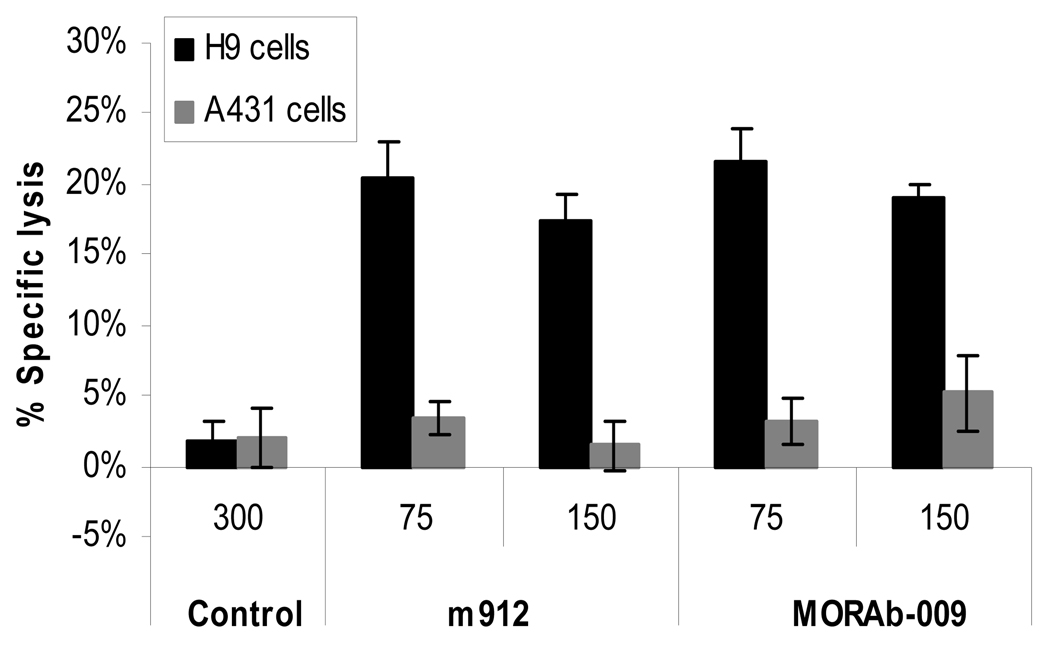
Therapeutic antibodies can kill cancer cells via ADCC mediated by the Fc portion of the IgG. To test whether IgG1 m912 has such activity we used a cell growth assay based on A431 and H9 cells. Incubation of IgG1 m912 alone with these cells did not affect their growth (data not shown). However, in the presence of PBMCs isolated from peripheral blood of healthy donors, IgG1 m912 specifically lysed mesothelin-positive (H9) cells likely by ADCC but not mesothelin-negative (A431) cells. The mouse-human chimeric anti-mesothelin antibody MORAb-009 (15) used as a positive control exhibited similar activity, while a control isotype antibody had a baseline lysis activity (Figure 4). These results indicate that IgG1 m912 lysed the H9 cells by ADCC through specific binding to cell surface-associated mesothelin.
Figure 4. IgG1 m912 induced ADCC in mesothelin-positive cells, but not in mesothelin-negative cells.
Freshly isolated PBMCs were incubated with target cells (H9 or A431) at ratio of 50:1, in the presence of IgG1 m912 or MORAb-009 at two different concentrations, 75 or 150 nM, or an isotype control IgG1 at 300 nM. ADCC was detected with CytoTox-ONE reagent, which measures the lactate dehydrogenase released by lysed target cells. The percentage (%) of specific lysis was calculated as described in the Materials and Methods.
Discussion
Mesothelin is an attractive target for cancer therapy because of its differential cell surface-associated expression in normal and tumor cells. It is differentially expressed not only in mesothelioma but also in ovarian, pancreatic and lung cancers (6–8). Although the cellular function of mesothelin is not clear at present, mesothelin-targeted therapies have shown benefits in a number of studies in animal models (12, 13) and several early clinical trials are ongoing (14, 15). Among thirty-three patients (with mesothelioma, ovarian and pancreatic cancers) treated with SS1P, a recombinant anti-mesothelin immunotoxin, at least two patients had complete resolution of ascites, and 50% of the patients had stable disease (14). In a separate clinical study, pancreatic cancer patients vaccinated with autologous tumor cells have demonstrated increased mesothelin-specific CD8+ T cell response, including CD8+ T cells that are able to lyse mesothelin-expressing tumor cells (21). These encouraging clinical results support the notion of targeting certain types of cancer cells through mesothelin and justify further studies on mesothelin therapeutics. A fully human mesothelin antibody seems to be a promising addition to mesothelin-related therapeutics currently under studies. In addition, it has been reported that mesothelin may also be released into serum through deletion at its carboxyl terminus or by proteolytical cleavage from its membrane bound form (19). An increase in the soluble form of mesothelin could be detected several years before malignant mesotheliomas occur among workers exposed to asbestos (22). Patients with ovarian, pancreatic, and lung cancers also have elevated soluble mesothelin in serum (11, 23, 24). Thus, mesothelin could also be a valuable biomarker for diagnosis and prognosis. New antibodies to mesothelin, therefore, could be also used for detection of mesothelin.
The antibody we have developed, m912, is fully human and in Fab, scFv and IgG formats was able to specifically recognize cell surface-associated human mesothelin. It also bound to mouse mesothelin which would facilitate its use in mouse models of cancer. We have not localized its epitope. However, m912 and MORAb-009 do not compete for binding to mesothelin (Figure 1B) and MORAb-009 does not recognize mouse mesothelin (15), suggesting that m912 binds to an epitope that does not overlap the MORAb-009 epitope. In addition, the cross-reactivity of m912 to human and mouse mesothelin provides an advantage compared to MORAb-009 because it could be used in mouse models.
MORAb-009, which was derived from a mouse immune library and further improved by in vitro maturation, binds with high avidity even to non-transfected cells, such as OVCAR-3, which express relatively low levels of mesothelin (15). M912 was selected from a non-immune human library and was not further matured in vitro. This is why it was not unexpected that its binding to OVCAR-3 cells requires higher concentrations than MORAb-009 to reach the same antibody surface concentration (Fig. 3C). Currently, we are improving the m912 affinity by in vitro maturation. As mentioned above MORAb-009 does not bind to mouse mesothelin and may not be used in mouse models of cancer. In addition, it binds to an epitope which does not overlap the m912 epitope. Thus the two antibodies may have different areas of application and could be used in combination.
The m912 was selected from a naïve library and its sequence does not deviate significantly from the germ line sequences. Its VL and JL regions together have only one mutation compared with the closest germ line gene IGKV1-39 (accession number X59315) and IGKJ4 (J00242). The heavy chain V gene of m912 is identical to the germ line gene IGHV4-61 (M29811) and its J gene is identical to IGHJ3*02 (X86355). The D gene region has two mutations and an N2 insertion compared with IGHD3-16*01 (X93614).
In the presence of PBMC, IgG1 m912 specifically lysed mesothelin-positive cells likely by ADCC but did not affect mesothelin-negative cells. Thus, even non-conjugated m912 could be a potential candidate therapeutic for treatment of mesothelin-positive tumors. Its tumor killing activity could be further improved by conjugation to toxins or small molecule drugs. The antibody could also be utilized for guiding liposomes or other carriers of anti-cancer drugs or imaging agents to cancer cells, and as an agent for detection of soluble mesothelin in serum of cancer patients.
Acknowledgments
We thank members of our group for helpful discussions. This project was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research, and by federal funds from the National Cancer Institute, NIH, under contract N01-CO-12400.
Abbreviations
- ADCC
antibody-dependent cell-mediated cytotoxicity
- Fab
antigen-binding fragment
- scFv
single chain variable fragment
- kD
kilodalton
- ELISA
enzyme-linked immunosorbent assay
References
- 1.Chang K, Pastan I, Willingham MC. Isolation and characterization of a monoclonal antibody, K1, reactive with ovarian cancers and normal mesothelium. Int J Cancer. 1992;50:373–381. doi: 10.1002/ijc.2910500308. [DOI] [PubMed] [Google Scholar]
- 2.Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc Natl Acad Sci U S A. 1996;93:136–140. doi: 10.1073/pnas.93.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bera TK, Pastan I. Mesothelin is not required for normal mouse development or reproduction. Mol Cell Biol. 2000;20:2902–2906. doi: 10.1128/mcb.20.8.2902-2906.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rump A, Morikawa Y, Tanaka M, et al. Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J Biol Chem. 2004;279:9190–9198. doi: 10.1074/jbc.M312372200. [DOI] [PubMed] [Google Scholar]
- 5.Gubbels JA, Belisle J, Onda M, et al. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol Cancer. 2006;5:50. doi: 10.1186/1476-4598-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Argani P, Iacobuzio-Donahue C, Ryu B, et al. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE) Clin Cancer Res. 2001;7:3862–3868. [PubMed] [Google Scholar]
- 7.Hassan R, Kreitman RJ, Pastan I, Willingham MC. Localization of mesothelin in epithelial ovarian cancer. Appl Immunohistochem Mol Morphol. 2005;13:243–247. doi: 10.1097/01.pai.00000141545.36485.d6. [DOI] [PubMed] [Google Scholar]
- 8.Li M, Bharadwaj U, Zhang R, et al. Mesothelin is a malignant factor and therapeutic vaccine target for pancreatic cancer. Mol Cancer Ther. 2008;7:286–296. doi: 10.1158/1535-7163.MCT-07-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yen MJ, Hsu CY, Mao TL, et al. Diffuse mesothelin expression correlates with prolonged patient survival in ovarian serous carcinoma. Clin Cancer Res. 2006;12:827–831. doi: 10.1158/1078-0432.CCR-05-1397. [DOI] [PubMed] [Google Scholar]
- 10.Ho M, Bera TK, Willingham MC, et al. Mesothelin expression in human lung cancer. Clin Cancer Res. 2007;13:1571–1575. doi: 10.1158/1078-0432.CCR-06-2161. [DOI] [PubMed] [Google Scholar]
- 11.Cristaudo A, Foddis R, Vivaldi A, et al. Clinical significance of serum mesothelin in patients with mesothelioma and lung cancer. Clin Cancer Res. 2007;13:5076–5081. doi: 10.1158/1078-0432.CCR-07-0629. [DOI] [PubMed] [Google Scholar]
- 12.Chowdhury PS, Pastan I. Analysis of cloned Fvs from a phage display library indicates that DNA immunization can mimic antibody response generated by cell immunizations. J Immunol Methods. 1999;231:83–91. doi: 10.1016/s0022-1759(99)00142-8. [DOI] [PubMed] [Google Scholar]
- 13.Chang CL, Wu TC, Hung CF. Control of human mesothelin-expressing tumors by DNA vaccines. Gene Ther. 2007;14:1189–1198. doi: 10.1038/sj.gt.3302974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hassan R, Bullock S, Premkumar A, et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res. 2007;13:5144–5149. doi: 10.1158/1078-0432.CCR-07-0869. [DOI] [PubMed] [Google Scholar]
- 15.Hassan R, Ebel W, Routhier EL, et al. Preclinical evaluation of MORAb-009, a chimeric antibody targeting tumor-associated mesothelin. Cancer Immun. 2007;7:20. [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu ZY, Dimitrov DS. Construction of a large naïve human phage-displayed Fab library through one-step cloning. Meth Mol Biol. in press doi: 10.1007/978-1-59745-554-1_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng Y, Zhu Z, Xiao X, Choudhry V, Barrett JC, Dimitrov DS. Novel human monoclonal antibodies to insulin-like growth factor (IGF)-II that potently inhibit the IGF receptor type I signal transduction function. Mol Cancer Ther. 2006;5:114–120. doi: 10.1158/1535-7163.MCT-05-0252. [DOI] [PubMed] [Google Scholar]
- 18.Scott JK, Barbas CF., III . Phage-display vector. In: Barbas CF III, editor. Phage display: a laboratory manual. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- 19.Hassan R, Bera T, Pastan I. Mesothelin: a new target for immunotherapy. Clin Cancer Res. 2004;10:3937–3942. doi: 10.1158/1078-0432.CCR-03-0801. [DOI] [PubMed] [Google Scholar]
- 20.Chowdhury PS, Viner JL, Beers R, Pastan I. Isolation of a high-affinity stable single-chain Fv specific for mesothelin from DNA-immunized mice by phage display and construction of a recombinant immunotoxin with anti-tumor activity. Proc Natl Acad Sci U S A. 1998;95:669–674. doi: 10.1073/pnas.95.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas AM, Santarsiero LM, Lutz ER, et al. Mesothelin-specific CD8(+) T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients. J Exp Med. 2004;200:297–306. doi: 10.1084/jem.20031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Creaney J, Robinson BW. Detection of malignant mesothelioma in asbestosexposed individuals: the potential role of soluble mesothelin-related protein. Hematol Oncol Clin North Am. 2005;19:1025–1040. doi: 10.1016/j.hoc.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Hassan R, Remaley AT, Sampson ML, et al. Detection and quantitation of serum mesothelin, a tumor marker for patients with mesothelioma and ovarian cancer. Clin Cancer Res. 2006;12:447–453. doi: 10.1158/1078-0432.CCR-05-1477. [DOI] [PubMed] [Google Scholar]
- 24.Corso CD, Stubbs DD, Lee SH, Goggins M, Hruban RH, Hunt WD. Real-time detection of mesothelin in pancreatic cancer cell line supernatant using an acoustic wave immunosensor. Cancer Detect Prev. 2006;30:180–187. doi: 10.1016/j.cdp.2006.03.004. [DOI] [PubMed] [Google Scholar]