Abstract
Human activities often involve hand‐motor responses following external auditory–verbal commands. It has been believed that hand movements are predominantly driven by the contralateral primary sensorimotor cortex, whereas auditory–verbal information is processed in both superior temporal gyri. It remains unknown whether cortical activation in the superior temporal gyrus during an auditory–motor task is affected by laterality of hand‐motor responses. Here, event‐related γ‐oscillations were intracranially recorded as quantitative measures of cortical activation; we determined how cortical structures were activated by auditory‐cued movement using each hand in 15 patients with focal epilepsy. Auditory–verbal stimuli elicited augmentation of γ‐oscillations in a posterior portion of the superior temporal gyrus, whereas hand‐motor responses elicited γ‐augmentation in the pre‐ and postcentral gyri. The magnitudes of such γ‐augmentation in the superior temporal, precentral, and postcentral gyri were significantly larger when the hand contralateral to the recorded hemisphere was required to be used for motor responses, compared with when the ipsilateral hand was. The superior temporal gyrus in each hemisphere might play a greater pivotal role when the contralateral hand needs to be used for motor responses, compared with when the ipsilateral hand does. Hum Brain Mapp, 2010. © 2010 Wiley‐Liss, Inc.
Keywords: in‐vivo animation movie, pediatric epilepsy surgery, event‐related synchronization (ERS), cross‐modal spatial attention, electroencephalography (EEG)
INTRODUCTION
Humans are often required to listen to simple auditory–verbal commands, understand the meaning of these commands, make a relevant decision, and respond by moving their fingers. Examples include a junior surgeon operating under supervision of a senior surgeon or an individual sharing an elevator with others. Previous studies using functional magnetic resonance imaging (fMRI) have suggested that external auditory–verbal stimuli are processed in bilateral superior temporal gyri but predominantly on the left side in approximately 95% of right‐handed healthy individuals [Knecht et al.,2000; Pujol et al.,1999; Springer et al.,1999] and approximately 80% of left‐handed [Pujol et al.,1999; Szaflarski et al.,2002]. It has been also suggested that hand movements are predominantly driven by the contralateral primary sensorimotor area [Crone et al.,1998; Penfield and Boldrey,1937; Yousry et al.,1997].
Let us imagine the situation when an individual is required to provide responses using the right hand. It would be plausible in most cases to assume that simple auditory–verbal commands are initially processed by the superior temporal gyri predominantly on the left side [Calvert et al.,1997; Démonet et al.,1992; Poldrack et al.,2001; Price et al.,1996] and relevant signals are transferred to the left primary sensorimotor area [Abler et al.,2006; Crone et al.,1998; Miller et al.,2007b] in an intrahemispheric manner [Barrick et al.,2007; Gazzaniga,2000]. If the same individual is required to provide responses using the left hand, can we still assume that auditory–verbal commands are processed predominantly in the left superior temporal gyrus and relevant signals are somehow transferred to the right primary sensorimotor area in an interhemispheric manner, for example, via the corpus callosum [Gazzaniga,2000; Josse et al.,2008]? Or, would it be possible that the right superior temporal gyrus plays a greater role and that relevant signals are transferred to the right primary sensorimotor area through shorter neural circuits in an intrahemispheric manner? It has been hypothesized that such a signal transfer occurs more efficiently via an intra‐ than interhemispheric pathway [Gazzaniga,2000]. Thus, we have wondered whether cortical activation in the superior temporal gyrus during an auditory–motor task is affected by laterality of motor responses, regardless of the side of language dominance.
In this study using electrocorticography (ECoG) recording, we hypothesize that (i) sequential cortical activation elicited by an auditory–motor task includes the superior temporal gyrus and the pre‐ and postcentral gyri in the recorded hemisphere and (ii) the magnitude of cortical activation in the gyri of interest (superior temporal, precentral, and postcentral gyri) is larger when the hand contralateral to the recorded hemisphere needs to be used for motor responses, compared with when the ipsilateral hand does. We tested the above‐mentioned hypotheses, using event‐related γ‐oscillations as quantitative measures of cortical activation [Pfurtscheller and Lopes da Silva,1999]. In short, augmentation of γ‐oscillations was considered to represent cortical activation [Crone et al.,1998; Ray et al.,2008a; Tallon‐Baudry and Bertrand,1999], whereas attenuation of γ‐oscillations was considered to represent cortical deactivation [Asano et al.,2009; Towle et al.,2008]. Benefits of ECoG recording include: (i) less artifacts from cranial muscles [Crone et al.,1998] and (ii) a better signal‐to‐noise ratio compared to scalp electroencephalography (EEG) and magnetoencephalography (MEG), which record cortical signals from outside of the scalp [Dalal et al.,2009; Gaetz et al.,2008; Pfurtscheller and Cooper,1975].
MATERIALS AND METHODS
Patients
The inclusion criteria of this study consisted of (i) age 4 years or above, (ii) patients with focal epilepsy undergoing chronic subdural ECoG recording as a part of presurgical evaluation in Children's Hospital of Michigan, Detroit, between April 2007 and March 2009, (iii) measurement of γ‐oscillations modulated by auditory–motor tasks described below, and (iv) subdural electrodes chronically implanted on both pre‐ and postcentral gyri at least 4 cm above the Sylvian fissure as well as a posterior portion of the superior temporal gyrus at least 5 cm posterior to the temporal pole. The exclusion criteria consisted of the following: (i) the presence of massive brain malformations (such as large porencephaly, perisylvian polymicrogyria or hemimegalencephaly) which are known to confound the anatomical landmarks for the central sulcus, (ii) history of previous epilepsy surgery, and (iii) the presence of epilepsia partialis continua. The study has been approved by the Institutional Review Board at Wayne State University, and written informed consent was obtained from the parents or guardians of all subjects.
Subdural Electrode placement
For chronic extraoperative ECoG recording and subsequent functional cortical mapping, platinum grid electrodes (10 mm intercontact distance, 4 mm diameter; Ad‐tech, Racine, WI) were surgically implanted [Brown et al.,2008]. The total number of electrode contacts in each subject ranged from 100 to 150. All electrode plates were stitched to adjacent plates and/or the edge of dura mater, to avoid movement of subdural electrodes after placement. In addition, intraoperative pictures were taken with a digital camera before dural closure, to confirm the spatial accuracy of electrode display on the three‐dimensional brain surface reconstructed from MRI.
Coregistration of Subdural Electrodes to the Individual Three‐Dimensional MRI
MRI including a T1‐weighted spoiled gradient echo image as well as fluid‐attenuated inversion recovery image was preoperatively obtained. The spoiled gradient sequence generates 164 contiguous 1.2‐mm sections of the entire head, performed in the sagittal plane, using a (TR/TE/TI = 5/3/450 ms) pulse sequence, flip angle of 12°, matrix size of 256 × 256, and field of view of 220 × 220 mm. Planar X‐ray images (lateral and anteroposterior) were acquired with the subdural electrodes in place for electrode localization on the brain surface; three metallic fiducial markers were placed at anatomically well‐defined locations on the patient's head for coregistration of the X‐ray image with the MRI. A three‐dimensional surface image was created with the location of electrodes directly defined on the brain surface [Juhasz et al., 2009; Muzik et al.,2007; von Stockhausen et al.,1997]. The accuracy of this procedure was reported previously as 1.24 ± 0.66 mm with a maximal misregistration of 2.7 mm [von Stockhausen et al.,1997], and was confirmed by intraoperative digital photographs showing in situ locations of the subdural electrodes [Asano et al.,2005; Wellmer et al.,2002]. The central sulcus, the precentral gyrus and postcentral gyrus were identified according to anatomical MRI landmarks [Berger et al.,1990; Fukuda et al.,2008; Yousry et al.,1997].
Extraoperative Video‐ECoG Recording
Extraoperative video‐ECoG recordings were obtained for 3 to 10 days, using a 192‐channel Nihon Kohden Neurofax 1100A Digital System (Nihon Kohden America Inc, Foothill Ranch, CA), which has an input impedance of 200 Megaohm, a common mode rejection ratio greater than 110 dB, an A/D conversion of 16 bits, and a sampling frequency selectable from 200 to 10,000 Hz. For evaluation of interictal and ictal activities as well as event‐related γ‐oscillations, the sampling rate was set at 1,000 Hz with the amplifier band pass at 0.08–300 Hz. The averaged voltage of ECoG signals derived from the fifth and sixth electrodes of the ECoG amplifier (system reference potential) was used as the original reference. ECoG signals were then remontaged to a common average reference [Fukuda et al.,2008; Miller et al.,2007b; Towle et al.,2008]. Advantage and limitation of usage of a common average reference for measurement of event‐related γ‐oscillations were previously discussed [Asano et al.,2009; Crone et al.,2001]. Channels contaminated with large interictal epileptiform discharges or artifacts were excluded from the average reference [Fukuda et al.,2008]. No notch filter was used for further analysis in any subjects. Antiepileptic medications were discontinued or reduced during ECoG monitoring until a sufficient number of habitual seizures were captured.
Auditory–Motor Tasks
None of the patients had a seizure within two hours prior to the auditory–motor tasks. The tasks were employed in a sound‐attenuated room, and each patient was awake, unsedated, and comfortably seated on the bed during the tasks. Subjects held a button in one hand and placed the hand on the thigh (Supporting Information Fig. S1 on the journal website). Before the formal auditory–motor tasks started, each subject was provided with a practice period. Thereby, auditory–verbal commands saying “Press” as well as “Do not press” were given, and the intensity of auditory‐syllable was adjusted to a comfortable hearing level [Towle et al.,2008]. Each subject was instructed to press the button using the thumb when a prerecorded verbal command saying “Press” was given and not to press the button when a verbal command saying “Do not press” was given. Subjects practiced how to press the button using each hand following auditory–verbal commands, until they felt comfortable.
Subsequently, each subject completed two formal auditory–motor tasks (one for each hand). An auditory–motor task contained 40 trials; 20 auditory–verbal commands saying “Press”, and 20 commands saying “Do not press” were given in a pseudorandom sequence during each task. Auditory–verbal commands used in this study are available on the journal website (Supporting Information Audio Files S1–S8). Auditory–verbal commands were given, using Presentation Software (Neurobehavioral Systems Inc, Albany, CA), with an approximate intensity of 65–70 dB, via two open‐field speakers, and with inter‐stimulus intervals randomized between 2.5 and 3.0 s.
The auditory–verbal commands were recorded using a Digital Voice Recorder (WS‐300M, Olympus America Inc, Hauppauge, NY) concurrently with ECoG recording, and the amplified audio waveform was integrated into the Digital ECoG Recording System [Brown et al.,2008]. Cool Edit Pro version 2.00 (Syntrillium Software Corp., Phoenix, AZ) was used to visually and audibly aid in the manual determination of the onset of verbal command as needed [Brown et al.,2008]. The onset (i.e., button‐press) and offset (i.e., button‐release) of motor responses were also integrated into the Digital ECoG Recording System via its DC input (Supporting Information Fig. S2 on the journal website). The reaction time was defined as the period between the onset of auditory–verbal command saying “Press” and the onset of button‐press. We determined whether the mean or standard deviation (SD) of reaction time differed between the right and left hands or between the contralateral and ipsilateral hands (Wilcoxon‐Signed Ranks Test). We also determined whether the reaction time was correlated with the age of subjects (Spearman's Rank Test).
Measurement of ECoG Amplitude Modulations Elicited by Auditory–Motor Tasks
Analysis of γ‐oscillations relative to ‘the onset of auditory–verbal command’
This time‐frequency analysis was designed to evaluate initial cortical activation associated with the onset of auditory–verbal commands and to identify the auditory‐related area presumably located in the superior temporal gyrus on the recorded hemisphere. Since the duration of auditory–verbal commands was not uniform across trials, this analytic method was not designed to evaluate sequential cortical activation associated with subsequent finger‐motor responses, as suggested in our previous study [Brown et al.,2008]. ECoG amplitude modulations elicited by perception of auditory–verbal commands were evaluated using the trigger point set at the onset of all types of verbal commands. In this study, “event‐related” ECoG oscillations were defined as oscillatory responses consisting of both phase‐locked (i.e., a component present after averaging; also often known as “evoked” oscillations) and nonphase‐locked (i.e, a component absent after averaging; also often known as “induced” oscillations) components [Crone et al.,2001; Pfurtscheller and Lopes da Silva,1999; Towle et al.,2008].
The inclusion criteria defining ECoG epochs suitable for this time‐frequency analysis included: (i) at least 600‐ms of silence (i.e., no auditory noise such as coughing) occurred prior to the onset of auditory–verbal command and (ii) either auditory–verbal command saying “Press” or “Do not press” was given. The exclusion criteria were: (i) ECoG trace was affected by movement artifacts on visual assessment; (ii) ECoG trace was affected by electrographic seizures during the task; and (iii) ECoG trace derived from the superior temporal gyrus was affected by runs of interictal epileptiform discharges during the task. In this study, ECoG traces were visually inspected with a low‐frequency filter at 53 Hz and a sensitivity of 20 μV/mm, and very high frequency signals synchronized with facial muscle activities detected on electrooculography electrodes [Asano et al.,2009] were treated as movement artifacts [Otsubo et al.,2008]. Exclusion of ECoG epochs was performed by an investigator (T.N.) while being blinded to the final results of time‐frequency analysis. All ECoG epochs (up to 80 ECoG epochs derived from two auditory–motor tasks) which satisfied all of the inclusion and exclusion criteria were utilized for the time‐frequency ECoG analysis described below.
Time‐frequency analysis was performed using BESA® EEG V.5.1.8 software (MEGIS Software GmbH, Gräfelfing, Germany); each suitable ECoG trial was transformed into the time‐frequency domain using complex demodulation technique [Asano et al.,2009; Brown et al.,2008; Fan et al.,2007; Hoechstetter et al.,2004; Papp and Ktonas,1977]. In that technique, the time‐frequency transform was obtained by multiplication of the time‐domain signal with a complex exponential, followed by a low‐pass filter. The low‐pass filter used here was a finite impulse response filter of Gaussian shape, making the complex demodulation effectively equivalent to a Gabor transform. Details on the complex demodulation technique for time‐frequency transformation are described elsewhere [Hoechstetter et al.,2004; Papp and Ktonas,1977]. As a result of this transformation, the signal was assigned a specific amplitude and phase as a function of frequency and time (relative to the onset of verbal command). In this study, only the amplitude (also known as “square root of power”) averaged across all trials, was used for further analysis. Time‐frequency transformation was performed for frequencies between 30 and 200 Hz and latencies between −600 ms and +2,000 ms relative to the onset of verbal command, in steps of 5 Hz and 10 ms. This corresponded to a time‐frequency resolution of ±9.9 Hz and ±22.2 ms (defined as the 50% amplitude drop of the finite impulse response filter).
At each time‐frequency bin we analyzed the percentage change in amplitude (averaged across trials) relative to the mean amplitude in a reference period, defined as the resting state of 400 ms in duration between −600 and −200 ms relative to the onset of auditory–verbal command. This parameter is commonly termed “event‐related synchronization and desynchronization” [Pfurtscheller and Lopes da Silva,1999] or “temporal spectral evolution” (TSE) [Salmelin and Hari,1994].
To test for statistical significance for each obtained TSE value, two‐step statistics was performed using the BESA software. First, statistics based on bootstrapping approach [Davidson and Hinkley, 1999] was applied to obtain an uncorrected P‐value at each time‐frequency bin. In a second step, correction for multiple testing was performed (each electrode was analyzed at 7,700 time‐frequency bins, with TSE values at neighboring bins being partially dependent). A modification of the correction developed by Simes [1986] was used as suggested for time‐frequency analysis by Auranen [2002]: P values of one frequency bin and channel were sorted in ascending order (P i, I = 1, …, N). The maximum index m in the sorted array for which P i < α*i/N was determined. All uncorrected P‐values with i < m were accepted as significant. The corrected significance level α was set to 0.05. This approach is less conservative than the classic Bonferroni correction and is specifically suited for partially dependent multiple testing [Auranen,2002; Simes,1986]. In all figures, blue color indicates a significant attenuation of amplitude, and red color a significant augmentation of amplitude in the corresponding time‐frequency bin relative to the reference period.
As described in our previous studies [Asano et al.,2009; Brown et al.,2008], an additional correction for testing in multiple electrodes (the number of subdural electrodes ranged from 100 to 150 across subjects) was employed. TSE values in a given electrode were declared to be statistically significant only if a minimum of eight time‐frequency bins in the γ‐band range were arranged in a continuous array spanning (i) at least 20 Hz in width and (ii) at least 20 ms in duration. Such correction provides a very small probability of Type‐I error in determination of cortical activation or deactivation. We recognize that this approach may potentially underestimate γ‐modulations with a restricted frequency band (less than 20 Hz in width) or that with a short duration (less than 20 ms). Some previous studies using scalp EEG recording showed augmentation of a narrow‐range γ‐band oscillations around 40 Hz [Tallon‐Baudry et al.,1996], whereas event‐related γ‐modulations observed in studies using intracranial ECoG recording commonly involved wide‐range frequency bands ranging at least 20 Hz in width [Asano et al.,2009; Brown et al.,2008; Fukuda et al.,2008; Tallon‐Baudry et al.,2005].
Analysis of γ‐Oscillations Relative to “the Onset of Motor Response”
ECoG amplitude modulations were also evaluated using the trigger point set at the onset of motor response (defined as the onset of button press in this study). This analytic method was designed to evaluate sequential cortical activation consisting of: (i) comprehension of given verbal commands, (ii) relevant decision making, and (iii) execution of button‐press [Brown et al.,2008]. Since the duration between the onset and offset of button‐press was not uniform across trials, this analytic method was not designed to evaluate cortical activation associated with the offset of button‐press (i.e., the onset of button‐release).
The inclusion criteria defining ECoG epochs suitable for this time‐frequency analysis included: (i) the patient provided a correct motor response; (ii) the variability of delay between the onset of auditory–verbal commands and the onset of button‐press must be within 1,000‐ms across trials; (iii) the duration between the onset and offset of button‐press must be not longer than 1,000‐ms; and (iv) a period of silence lasting 400‐ms must be available as a reference period between +1,600 ms to +2,000 ms after the onset of button‐press. The exclusion criteria were: (i) ECoG trace was affected by movement artifacts; (ii) ECoG trace was affected by electrographic seizures; and (iii) ECoG trace from the pre‐ or postcentral gyri was affected by runs of interictal epileptiform discharges. All 3,000 ms ECoG epochs (starting 1,000 ms prior to and ending 2,000 ms after the onset of motor responses) which satisfied all of the inclusion and the exclusion criteria were utilized for the time‐frequency ECoG analysis; inclusion of ECoG epochs was employed independent of the analysis relative to “the onset of auditory–verbal command”. Alteration of ECoG amplitude was determined using the statistical approach as described above.
Delineation of ECoG Data on Three‐Dimensional MRI
ECoG data for each electrode channel were exported to the given electrode site on the individual three‐dimensional brain surface in two different ways [Asano et al.,2009; Brown et al.,2008]. To delineate “when”, “where”, and “at what frequency band” significant alteration of spectral amplitude occurred, time‐frequency plot matrixes created above were placed onto a three‐dimensional MRI at the cortical sites corresponding to their respective subdural electrode positions (Fig. 1). To animate “when”, “where”, and “how many fold” γ‐oscillations were increased or decreased, “γ‐range amplitude” (defined as the spectral amplitude averaged across 50 to 150 Hz frequency bands and normalized to that of the reference period) was sequentially delineated on the individual three‐dimensional MRI (Figs. 1 and 2; Supporting Information Video 1 on the journal website) [Akiyama et al.,2006; Asano et al.,2009; Brown et al.,2008]. “Gamma‐range amplitude” was calculated without a frequency band at 60 Hz if visual inspection revealed a 60‐Hz artifact peak on the amplitude spectral curve for all subdural electrodes. “Gamma‐range amplitude” (unit: %) for each electrode channel at each 10‐ms epoch was registered into Insight II software (Persyst, Prescott, AZ), and the interpolated topography map of “γ‐range amplitude” at each 10‐ms epoch was accurately superimposed to the individual three‐dimensional MRI. This procedure yielded a movie file showing a sequential alteration of γ‐oscillations elicited by the auditory–motor task.
Figure 1.
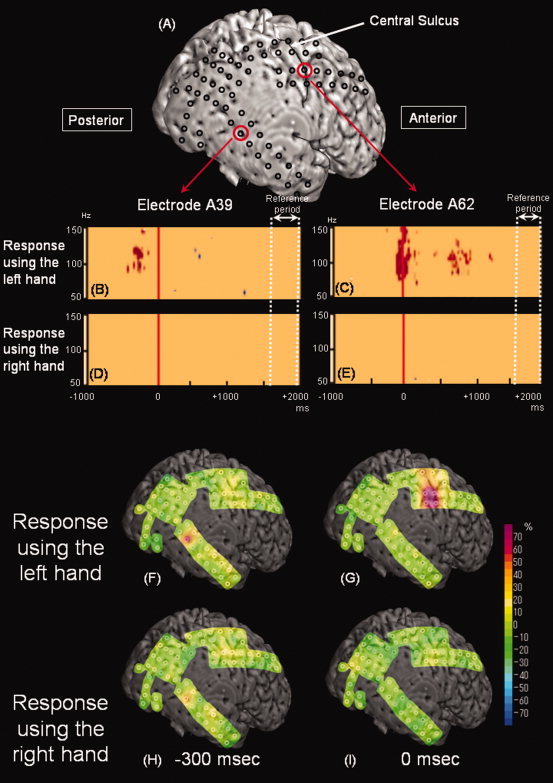
Time‐frequency analysis of γ‐oscillations relative to “the onset of button‐press” in patient 13. (A) Subdural electrodes were placed on the right hemisphere. (B and C) In the auditory–motor task when the subject was required to provide responses using the left hand, significant γ‐augmentation (red) involving 90–130 Hz was noted at electrode A39 located in the right superior temporal gyrus at 360–210 ms prior to the onset of button‐press. Subsequently, significant γ‐augmentation involving 75–165 Hz was noted at electrode A62 located over the right central sulcus between −110 ms and +100 ms relative to the onset of button‐press. Furthermore, significant γ‐augmentation involving 80–135 Hz was noted in the same site between 660 and 990 ms following the onset of button press; this late γ‐augmentation may be associated with button‐release movement. Neurostimulation of this electrode site elicited movement of the left‐sided fingers. (D and E) In the task with the right hand used for motor responses, no significant γ‐augmentation was noted at electrode A39 or A62. (F and G) In the task for the left hand, “γ‐range amplitude” was increased by 77% at electrode A39 in the right superior temporal gyrus at −300 ms relative to the onset of button‐press and increased by at least 70% at multiple sites overlying the central sulcus and postcentral gyrus at the onset of button press. (H and I) In the task for the right hand, “γ‐range amplitude” was increased by 43% at electrode A39 at −300 ms relative to the onset of button press but the magnitude of γ‐augmentation did not reach significance defined in this study. No significant γ‐augmentation was noted in the precentral or postcentral gyrus. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 2.
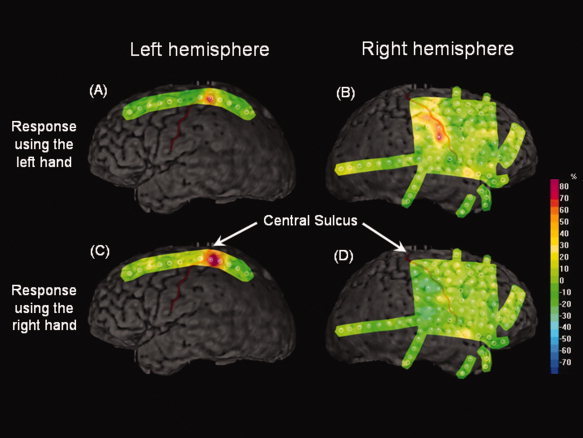
Time‐frequency analysis of γ‐oscillations relative to “the onset of button‐press” in Patient 10 with a diagnosis of MR nonlesional focal epilepsy. “Gamma‐range amplitudes” at the onset of button‐press using the left hand (A and B) and the right hand (C and D) are shown. “Gamma‐range amplitudes” were increased in each Rolandic area, to a greater extent, when the hand contralateral to the recorded hemisphere was used for motor‐responses. It also seems as if the left‐hand movement elicited rather bilateral γ‐augmentation, whereas the right‐hand movement elicited unilateral γ‐augmentation confined to the left Rolandic area. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Functional Cortical Mapping Using Neurostimulation
Functional cortical mapping by electrical neurostimulation was performed during extraoperative ECoG recording [Asano et al.,2009; Brown et al.,2008; Fukuda et al.,2008]. A pulse‐train of electrical stimuli was delivered using the Grass S88 constant‐current stimulator (Astro‐Med, Inc, West Warwick, RI); clinical responses associated with stimulations were observed by at least two investigators. Subdural electrode pairs were stimulated by an electrical pulse‐train of 5‐s maximum duration using pulses of 300‐μs duration. Initially, stimulus intensity was set to 3 mA and stimulus frequency was set to 50 Hz. Stimulus intensity was increased from 3 to 9 mA in a stepwise manner by 3 mA until a clinical response or after‐discharge was observed. Brain regions at which stimulation consistently elicited a clinical response were declared “eloquent for that function”. When after‐discharge without a clinical response or when neither clinical response nor after‐discharge was elicited by the maximally intense stimuli, the brain region was declared “not proven eloquent”.
Functional Correlate of Event‐Related γ‐Augmentation
The presumed eloquent areas determined by event‐related γ‐augmentation were correlated with the results of neurostimulation. In time‐frequency ECoG analysis relative to the onset of auditory–verbal commands, we initially localized the electrode site showing the largest “γ‐range amplitude” elicited by auditory–verbal commands. Thereby, a site showing the largest “γ‐range amplitude” was defined as “the center of auditory event‐related γ‐augmentation” in this study. We subsequently determined whether neurostimulation of “the center of auditory event‐related γ‐augmentation” elicited positive auditory symptoms or receptive language impairment more frequently than did that of the other sites (Chi‐square test).
Similarly, in time‐frequency ECoG analysis relative to the onset of motor‐responses, we localized the site showing the largest “γ‐range amplitude” elicited by motor responses using the contralateral hand. This electrode site was defined as “the center of movement‐related γ‐augmentation” of each recorded hemisphere. Subsequently, we determined whether neurostimulation of “the center of movement‐related γ‐augmentation” elicited contralateral hand movement more frequently than did that of the other sites (Chi‐square test).
We are aware that sensitivity of electrical neurostimulation is not as good in children as in adults and that failure to elicit a clinical symptom using neurostimulation does not prove the absence of eloquent function in the stimulated site [Haseeb et al.,2007; Ojemann et al.,2003; Schevon et al.,2007]. Nevertheless, statistically significant concordance between the results of time‐frequency ECoG analysis and electrical neurostimulation, if present, can provide validation to our methodology for localization of the auditory‐language related and sensorimotor areas in children.
Assessment of Cortical Activation in the Superior Temporal Gyrus
We determined whether the magnitude of cortical activation in “the center of auditory event‐related γ‐augmentation” was different between when the hand contralateral to the recorded hemisphere was used for motor‐responses and when the ipsilateral hand was used. The maximum “γ‐range amplitude” occurring prior to the onset of button‐press was measured in “the center of auditory event‐related γ‐augmentation” in each auditory–motor task in each subject, using ECoG traces time‐locked to the onset of button press. The maximum “γ‐range amplitude” was then compared between the contralateral and ipsilateral hands (Wilcoxon‐Signed Ranks Test).
Assessment of Cortical Activation in the Rolandic Area
We determined whether the magnitude of cortical activation in “the center of movement‐related γ‐augmentation” was different between when the hand contralateral to the recorded hemisphere was used for motor‐responses and when the ipsilateral hand was used. The maximum “γ‐range amplitude” around the onset of button‐press was measured in “the center of movement‐related γ‐augmentation” of each hemisphere, using ECoG traces time‐locked to the onset of button‐press. The maximum “γ‐range amplitude” in “the center of movement‐related γ‐augmentation” was then compared between the contralateral and ipsilateral hands (Wilcoxon‐Signed Ranks Test).
RESULTS
A total of 17 patients met the inclusion criteria, but two patients were excluded due to a history of previous epilepsy surgery. Thus, we studied 15 right‐handed patients with a diagnosis of medically‐uncontrolled focal seizures (age range, 6–18 years; nine females) (Supporting Information Table S1 on the journal website). Locations of seizure onset zones or interictal epileptiform activity are described in Table I; the seizure onset zone involved a portion of the pre‐ and postcentral gyri in Patients 6 and 10 and involved a portion of the superior temporal gyrus in Patient 10.
Table I.
Patient profile
| Patient | Gender | Age (years) | Antiepileptic medications | Electrode placement | Seizure onset zones on ECoG | Resected areas | Histology |
|---|---|---|---|---|---|---|---|
| 1 | M | 6 | LEV, OXC, ZNS | Rt FPTO | Rt PO | Rt POT | Tumor |
| 2 | F | 8 | LEV, OXC | Rt FPTO | Not captureda | Rt FP | Gliosis |
| 3 | M | 8 | OXC | Lt FPTO | Not capturedb | Lt T | Tumor |
| 4 | F | 10 | OXC, TPM | Lt FPTO | Lt TP | Lt T | Tumor |
| 5 | M | 10 | LEV, OXC, TPM | Rt FPTO | Rt O | Rt OPT | Dysplasia |
| 6 | F | 11 | LEV, OXC, VPA | Rt FPTO | Rt FP | Rt FP | Dysplasia |
| 7 | F | 11 | LEV, OXC | Rt FPTO | Rt T | Rt TF | Dysplasia |
| 8 | M | 14 | LEV, OXC, TPM | Rt FPTO | Rt PT | Rt PT | Dysplasia |
| 9 | F | 16 | CBZ | Rt FPTO | Not capturedc | Not applicable | Not Available |
| 10 | F | 16 | CZP, PHT, TPM | Rt FPTO Lt FP | Rt FPT | Rt FPT | Gliosis |
| 11 | M | 16 | OXC, TPM | Rt FPTO | Rt T | Rt T | Dysplasia; Hippocampal Sclerosis |
| 12 | F | 17 | LTG | Lt FPTO | Lt F | Lt F | Gliosis |
| 13 | F | 17 | LEV, OXC | Rt FPTO | Rt O | Rt O | Gliosis |
| 14 | M | 17 | OXC | Lt FPTO | Lt T | Lt T | Tumor |
| 15 | F | 18 | LTG, OXC, ZNS | Rt FPTO | Rt PTO | Rt PTO | Ulegyria |
Frequent interictal spikes were noted in the right inferior frontal‐parietal regions.
Occasional generalized spike‐wave discharges were noted; lesionectomy of the tumor in the left temporal region was performed.
Habitual seizures failed to be captured during chronic ECoG recording; no resective surgery was performed. However, habitual seizures characterized by forced head‐deviation toward the left side were captured during preoperative scalp EEG recording; thereby, delayed ictal discharges were noted over the right hemisphere but further localization of the presumed epileptogenic zone was not tenable in Patient 9.
F, female; M, male; Lt, left; Rt, right; CBZ, carbamazepine; CZP, clonazepam; LEV, levetiracetam; LTG, lamotrigine; OXC, oxcarbazepine; PHT, phenytoin; TPM, topiramate; VPA, valproic acid; ZNS, zonisamide; F, frontal; P, parietal; T, temporal; O, occipital.
Behavioral Data
According to the group analysis across the 15 patients, the mean number of trials per task satisfying both inclusion and exclusion criteria was 19.3 when the right hand was used for motor responses, 19.0 for the left hand, 19.3 for the contralateral hand, and 18.9 for the ipsilateral hand. There was no difference in the mean number of included trials between the right and left hands or between the contra‐ and ipsilateral hands (P > 0.3 on the Wilcoxon‐Signed Ranks Test). The mean reaction time was 818 ms (SD 170 ms) when the right hand was used for motor‐responses, 847 ms (SD 188 ms) for the left hand, 813 ms (SD 167 ms) for the contralateral hand, and 851 ms (SD 191 ms) for the ipsilateral hand. There was no difference in the mean or SD of reaction time between the right and left hands or between the contralateral and ipsilateral hands (P > 0.4 on the Wilcoxon‐Signed Ranks Test). The Spearman's Rank Test revealed a significant negative correlation between the age of subjects and the reaction time (rho: −0.65 when the right hand was used, −0.62 for the left hand, −0.52 for the contralateral hand, and −0.70 for the ipsilateral hand; P < 0.05), suggesting that older children may have been more motivated, more attentive or less exhausted during the auditory–motor tasks compared to younger ones.
Gamma‐Augmentation in the Superior Temporal Gyrus
Auditory–verbal commands elicited significant gamma augmentation in the superior temporal gyrus in the recorded hemisphere in all 15 patients. The mean number of electrode sites showing γ‐augmentation in the temporal neocortex following auditory–verbal commands was 6.7 per subject (SD: 2.7) without a significant difference between the left and right hemispheres (P = 0.9 on the Mann‐Whitney Test). There was no correlation between the age and the number of temporal‐lobe sites showing γ‐augmentation (P = 0.9 on the Spearman's Rank Test). The site showing the largest “γ‐range amplitude” (i.e., “the center of auditory event‐related γ‐augmentation”) was located in the superior temporal gyrus at 6.3 cm (SD, 0.8 cm) posterior from the temporal pole on average across the 15 patients, and localized in the seizure onset zone in Patient 10. ECoG analysis relative to “the onset of auditory–verbal commands” showed that the maximum “γ‐range amplitude” in “the center of auditory event‐related γ‐augmentation” was 100% on average (SD, 51%) and that the peak latency of “γ‐range amplitude” was +222 ms (SD, 124 ms) on average; neither measures were significantly correlated with the age of subjects (P ≥ 0.1 on the Spearman's Rank test).
Electrical neurostimulation of an electrode pair including “the center of auditory event‐related γ‐augmentation” was performed in 12 patients (all but Patients 1, 6, and 8). Among these 12 patients, neurostimulation of 6 out of the 12 pairs including “the center of auditory event‐related γ‐augmentation” elicited congruent clinical symptoms. Positive auditory symptoms were elicited in Patients 4, 7, 9, and 15, whereas receptive language impairment was elicited in patients 3 and 12. Among the same 12 patients, neurostimulation of 6 out of the total of 475 pairs other than “the center of auditory event‐related γ‐augmentation” elicited congruent clinical symptoms (auditory symptoms: three pairs in Patient 4 and a pair in Patient 7; receptive language impairment: a pair in Patients 3 and 14). The chi‐square test revealed that neurostimulation of pairs including “the center of auditory event‐related γ‐augmentation” elicited congruent clinical symptoms more frequently than that of other pairs (P < 0.0001).
ECoG analysis relative to “the onset of motor response” assessed how “γ‐range amplitude” was modulated in “the center of auditory event‐related γ‐augmentation” prior to the onset of button‐press; we found that the maximum “γ‐range amplitude” in “the center of auditory event‐related γ‐augmentation” was larger when the contralateral hand was used for motor responses, compared to when the ipsilateral hand was used (mean, 82% [SD: 35%] vs. 63% [SD, 38%]; P = 0.01 on the Wilcoxon‐Signed Ranks Test; Fig. 3). Exclusion of patient 10, whose seizure onset zone involved “the center of auditory event‐related γ‐augmentation”, from this analysis yielded a similar result (mean, 85% [SD: 34%] vs. 64% [SD: 39%]; P = 0.005). Additional group analysis demonstrated no difference in the maximum “γ‐range amplitude” in “the center of auditory event‐related γ‐augmentation” between the right and left hands (mean, 71% [SD: 38%] vs. 75% [SD, 37%]; P = 0.5). Again, exclusion of Patient 10 from this analysis yielded a similar result (mean, 72% [SD, 39%] vs, 77% [SD, 37%]; P = 0.4).
Figure 3.
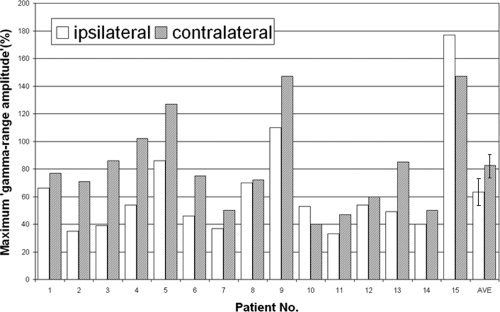
The magnitude of γ‐augmentation elicited in the superior temporal gyrus. The maximum “γ‐range amplitude” (unit: %) in “the center of auditory event‐related γ‐augmentation” is shown by a bar graph. ECoG analysis relative to the onset of motor responses showed that the maximum “γ‐range amplitude” was 82% on average when the hand contralateral to the recorded hemisphere was used for motor responses and 63% on average when the ipsilateral hand was used. AVE, average across the 15 hemispheres (error bars shown).
Gamma‐Augmentation in the Rolandic Area
Motor‐responses using the contralateral hand elicited significant gamma augmentation in the pre‐ or postcentral gyri in all 16 hemispheres of the 15 patients, regardless of the age of patient, location of seizure focus, or location of structural lesion. In Patient 10 with subdural electrodes placed on both hemispheres (Fig. 2), γ‐oscillations were differentially augmented in each Rolandic area when the contralateral hand was used. Motor‐responses using the contralateral hand elicited significant γ‐augmentation in 5.1 Rolandic sites per hemisphere on average (SD, 2.0), whereas those using the ipsilateral hand did in 0.6 sites per hemisphere (SD, 1.0). The Wilcoxon‐Signed Ranks Test suggested that the number of Rolandic sites showing significant γ‐augmentation was larger when the contralateral hand was used compared to when the ipsilateral hand was (P < 0.001).
”The center of movement‐related γ‐augmentation” was located in the precentral gyrus in two hemispheres, in the postcentral gyrus in 10 hemispheres and on the central sulcus in four hemispheres. The seizure onset zone involved “the center of movement‐related γ‐augmentation” in Patients 6 and 10. The maximum “γ‐range amplitude” elicited by the contralateral hand movement in “the center of movement‐related γ‐augmentation” was 178% (SD, 71%) on average across the 16 hemispheres, and the peak latency of “γ‐range amplitude” was +50 ms (SD, 77 ms) following the onset of button‐press; neither measures were correlated with the age of subjects (P > 0.7 on the Spearman's Rank Test).
Electrical neurostimulation of a pair including “the center of movement‐related γ‐augmentation” was performed in all 15 patients. Neurostimulation was employed only in the left hemisphere for Patient 10. Contralateral hand movement was elicited by neurostimulation of 11 out of the 15 pairs including “the center of movement‐related γ‐augmentation” (all but patients 2, 4, 8, and 14). Neurostimulation of 50 out of the 538 pairs other than “the center of movement‐related γ‐augmentation” elicited contralateral hand movement. The chi‐square test revealed that neurostimulation of pairs including “the center of movement‐related γ‐augmentation” elicited contralateral hand movement more frequently than that of other electrode pairs (P < 0.0001).
Gamma‐Augmentation in the Rolandic Area Ipsilateral to the Movement
In “the center of movement‐related γ‐augmentation”, the maximum “γ‐range amplitude” elicited by the ipsilateral finger movement was 42% on average across the 16 hemispheres (SD, 31%), showing no correlation with the age of subjects (P > 0.3 on the Spearman's Rank Test). Group analysis showed that the magnitude of γ‐augmentation elicited by the ipsilateral finger movement was smaller than that elicited by the contralateral finger movement (P < 0.001 on the Wilcoxon‐Signed Ranks Test; Fig. 4) but was significantly larger than zero (P < 0.001). Additional group analysis demonstrated no difference in the maximum “γ‐range amplitude” in “the center of movement‐related γ‐augmentation” between the right and left hands (mean, 96% [SD, 103%] vs. 123% [SD, 70%]; P = 0.4). Exclusion of Patients 6 and 10, whose seizure onset zone involved “the center of movement‐related γ‐augmentation” from the above analyses yielded similar results (Fig. 4).
Figure 4.
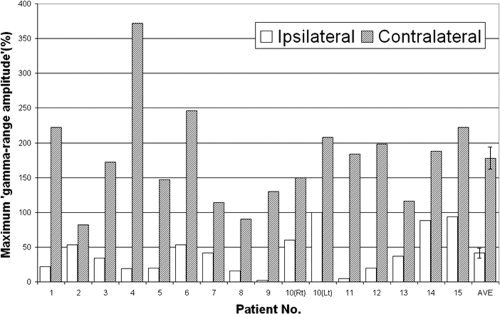
The magnitude of γ‐augmentation elicited in the Rolandic area. The maximum “γ‐range amplitude” (unit, %) in “the center of movement‐related γ‐augmentation” is shown by a bar graph; thereby, “the center of movement‐related γ‐augmentation” was defined as the site showing the largest “γ‐range amplitude” elicited by the contralateral hand movement. The maximum “γ‐range amplitude” was 178% on average when the hand contralateral to the recorded hemisphere was used for motor responses and 42% on average when the ipsilateral hand was used. The maximum “γ‐range amplitude” elicited by the ipsilateral hand movement was noted in a site different from “the center of movement‐related γ‐augmentation” in two patients (Patients 5 and 11). “The center of movement‐related γ‐augmentation” was localized in the postcentral gyrus in Patient 5 and in the site over the central sulcus in Patient 11; the maximum “γ‐range amplitude” elicited by the ipsilateral hand movement was noted in the precentral gyrus in both patients. Difference in the maximum “γ‐range amplitude” between the ipsi‐ and contra‐lateral hand responses was larger in the Rolandic area, compared to that in the superior temporal gyrus (see also Fig. 3). AVE, average across the 16 hemispheres (error bars shown); Lt, left; Rt, right.
DISCUSSION
The auditory–motor tasks elicited sequential γ‐augmentation in the superior temporal gyrus and the Rolandic area. Neurostimulation of the site showing the largest event‐related γ‐augmentation frequently elicited congruent clinical symptoms. The magnitudes of γ‐augmentation in the superior temporal gyrus and the Rolandic area on the recorded hemisphere were larger when the hand contralateral to the recorded hemisphere was required to be used for motor responses, compared with when the ipsilateral hand was.
Significance of γ‐Augmentation in the Superior Temporal Gyrus
Our observation of γ‐augmentation initially elicited in the superior temporal gyrus is consistent with previous ECoG studies. It was demonstrated that nonlinguistic auditory tones elicited γ‐augmentation at 40–160 Hz in the superior temporal gyrus on either hemisphere [Bidet‐Caulet et al.,2007; Crone et al.,2001; Edwards et al.,2005; Ray et al.,2008b; Towle et al.,2008]. It was also reported that γ‐augmentation at 50–100 Hz was elicited in the same site within the left superior temporal gyrus commonly by auditory tones and simple vocal sounds [Brosch et al.,2002; Chandrasekaran and Ghazanfar,2009; Crone et al.,2001]. Other studies demonstrated that γ‐augmentation at 50–150 Hz was elicited initially in the left superior temporal gyrus by auditory semantic verbal words [Brown et al.,2008; Lachaux et al.,2007; Towle et al.,2008].
Our observation of γ‐augmentation in the superior temporal gyrus was also consistent with previous fMRI studies. Studies of healthy adults have localized overlapping regions for processing of both speech and nonspeech sounds in the posterior portions of superior temporal gyri bilaterally [Belin et al.,2000; Binder et al.,2000; Burton and Small,2006; Callan et al.,2004; Jäncke et al.,2002; Joanisse and Gati,2003; Rimol et al.,2006; Warren et al.,2002]. It was reported that the magnitude of cortical activation in the superior temporal gyri was greater when semantic auditory verbal stimuli were given compared with when vocal syllables or auditory tones were given [Binder et al.,2000; Jäncke et al.,2002; Joanisse and Gati,2003; Rimol et al.,2006; Vouloumanos et al.,2001]. Thus, cortical activation represented by γ‐augmentation in the superior temporal gyrus may represent neural processing for auditory information at least at acoustic and phonetic levels and possibly at a semantic level. One cannot exclude the possibility that some of our subjects may have completed the auditory–motor tasks without fully understanding the semantic meaning of the auditory–verbal commands, since two types of commands used in this study (i.e., “Press” and “Do not press”) had different phonetic features and length.
Gamma‐augmentation in the superior temporal gyrus seen in our study might also represent neural processing for decision making occurring before relevant motor‐responses. A previous study using fMRI reported that the amount of blood oxygen level‐dependent (BOLD) in the superior temporal gyri predicted accuracy in responses on a sound‐identification task, and suggested that the superior temporal gyri play a role in decision making in addition to auditory–sensory processing [Binder et al.,2004]. Other studies using fMRI and positron emission tomography (PET) also demonstrated that the superior temporal gyri were activated by tasks involving decision making following perception of auditory or visual stimuli [Abler et al.,2006; Braver et al.,2001; Carreiras et al.,2007; Holcomb et al.,1998; Lee et al.,2008; Rissman et al.,2003].
The Effect of the Laterality of Movements on Activation in the Superior Temporal Gyrus
The magnitude of γ‐augmentation in the superior temporal gyrus on the recorded hemisphere was larger by 30% on average when the contralateral hand was used, compared with when the ipsilateral hand was. Increased attention or movement preparation given to the assigned hand might have resulted in increased functional connectivity between the contralateral Rolandic and superior temporal gyri via an intrahemispheric pathway. Our novel observation is consistent with the hypothesis that local and short neural circuitries are more predominantly utilized for proper coordination and integration of information, compared to distant and long circuitries in humans [Gazanniga, 2000].
Our observation may have an important implication for fMRI studies to determine language dominant hemisphere, taking into account the tight correlation between γ‐augmentation on ECoG and increased BOLD signals on fMRI [Niessing et al.,2005]. In general, fMRI is susceptible to motion artifacts, and language tasks involving overt speech are not easy to apply to even healthy adults and adolescents, due to inevitable motion during speech [Pulvermüller et al.,2006]. Therefore, subjects are often instructed to provide relevant responses by a finger in some fMRI studies [Springer et al.,1999; Szaflarski et al.,2002]. Our observation suggests that laterality of finger‐motor responses may potentially confound BOLD signals in the superior temporal gyri in fMRI studies to determine language dominant hemisphere. A similar study using fMRI may be warranted to directly address this issue.
Intracranial electrodes were placed on the left hemisphere in four patients and primarily on the right hemisphere in the remaining 11 patients. One may be concerned that such unbalanced ECoG sampling may have a potential effect on the results. It has been reported that two tasks performed close together in time often elicit response delays [i.e., dual‐task interference phenomena, reviewed in Pashler,1994]. Previous studies of healthy adults and children showed that a concurrent reading task slowed down the speed of right‐finger movement compared to that of left‐finger movement [Kosaka et al.,1993; Van Hoof and Van Strien,1997; Waldie and Mosley,2000]. Yet, group analysis in this study demonstrated no significant difference in the mean or SD of reaction time between the left and right hands, or between the contralateral and ipsilateral hands. Thus, there is no strong evidence suggesting that our observations can be explained primarily by the dual‐task interference phenomena.
Significance of γ‐Augmentation in the Rolandic Area
This study of children demonstrated that γ‐augmentation was elicited in the Rolandic area around the onset of button‐press, and larger γ‐augmentation was elicited by the contralateral finger movement than the ipsilateral one. Movement of the contralateral hand was frequently elicited by neurostimulation of the site showing the largest movement‐related γ‐augmentation. Our observations are consistent with previous ECoG studies primarily focusing on adults with focal epilepsy. It was demonstrated that γ‐augmentation at 40–200 Hz was elicited in both pre‐ and postcentral gyri by movement of the contralateral hand [Crone et al.,1998; Miller et al.,2007a,b; Ohara et al.,2000; Pfurtscheller et al.,2003; Szurhaj et al.,2005; Schalk et al.,2008; Wisneski et al.,2008; Zanos et al.,2008]. A study of five adults with focal epilepsy suggested that γ‐augmentation was elicited over the Rolandic area strictly during contralateral movement but not during ipsilateral movement [Crone et al.,1998]. Another study of a 27‐year‐old male with focal epilepsy showed that slight γ‐augmentation was elicited over the Rolandic area during ipsilateral thumb movement [Zanos et al.,2008]. Group analysis employed in our study demonstrated the presence of weak but significant γ‐augmentation elicited in the ipsilateral Rolandic area. Our individual‐based analyses also demonstrated the presence of movement‐related γ‐augmentation over the ipsilateral Rolandic area in 6 out of the 15 children. Previous studies using several diagnostic modalities have suggested that hand‐movement‐related cortical activation in the contralateral Rolandic area may represent processing for motor execution, whereas such cortical activation in the ipsilateral Rolandic area may represent planning or selection of movement [Catalan et al.,1998; Haaland et al.,2004; Kawashima et al.,1998; Rao et al.,1993; Wisneski et al.,2008].
Movement‐related γ‐augmentation in the postcentral gyrus does not necessarily represent pure somatosensory processing. Previous human studies showed that neurostimulation of the postcentral gyrus frequently resulted in movement of the contralateral extremities [Fukuda et al.,2008; Nii et al.,1996], regardless of the age, the presence of dysplastic lesion, or seizure onset involving the frontal lobe [Haseeb et al.,2007]. Another human study suggested that resection of the postcentral gyrus resulted in more pronounced deficits of the contralateral extremities compared to those after resection of the precentral gyrus [Polkey,2000]. Previous studies of healthy adults using transcranial magnetic stimulation (TMS) demonstrated that the TMS‐related current flowing across the central sulcus not in an “anterior‐to‐posterior” but a “posterior‐to‐anterior” direction optimally activated the motor cortex [Brasil‐Neto et al.,1992; Werhahn et al.,1994]. A study of healthy adults using diffusion tensor images showed that the cortico‐spinal tracts were connected to the postcentral gyrus in addition to the precentral gyrus [Hua et al.,2008]. Furthermore, statistical analyses in this study suggested that neurostimulation of the sites showing movement‐related γ‐augmentation frequently elicited hand movement.
Methodological Limitations
Inevitable limitations of ECoG recording include: sampling limitation, antiepileptic drugs, and inability to study healthy volunteers. In our study, most of patients had subdural electrodes placed only on the cortical surface of the presumed epileptogenic hemisphere; we were not able to evaluate the other hemisphere or subcortical structures. It has been reported that interictal epileptiform activities in animals and patients with focal epilepsy include paroxysmal γ‐band oscillations (also known as “ripples”) [Bagshaw et al.,2009; Buzsáki et al.,1992], which may potentially influence the measurements of event‐related γ‐oscillations. Such ripples are observed most frequently during non‐REM sleep and rather rarely during wakefulness [Bagshaw et al.,2009]. In this study, visual assessment failed to observe interictal spike discharges originating from the Rolandic area during the tasks. All subjects included in this study had a diagnosis of focal epilepsy and the sampled hemispheres were suspected to be dysfunctional; thus, interpretation and generalization of our results must be made cautiously along with the observations in studies of healthy humans using noninvasive diagnostic modalities such as fMRI. We found that the locations of eloquent cortices suggested by ECoG analyses in this study were concordant with those reported in previous fMRI studies of healthy adults [Belin et al.,2000; Binder et al.,2000; Yousry et al.,1997]. Our observation of the magnitudes of event‐related γ‐augmentation being larger when the contralateral hand was used would be difficult to explain by the effect of underlying disease process alone. Even if localization of eloquent cortices may have been altered in some of our subjects, it would be plausible to assume that the sequential order of eloquent areas participating in the auditory–motor process (i.e., perception of externally presented auditory–verbal commands, comprehension of given verbal commands, relevant decision making, and execution of button‐press) would be similar between epileptic patients and healthy individuals.
Antiepileptic drugs may affect the findings of neurostimulation and time‐frequency ECoG analysis. Here, phenytoin was loaded intravenously prior to neurostimulation to minimize the risk of stimulation‐related seizures. It was reported that phenytoin elevated motor thresholds to TMS but had no effect on motor‐evoked potential amplitudes [Chen et al.,1997]. We recognize that failure to elicit clinical symptoms by neurostimulation could be partially attributed to the acute effect of phenytoin given prior to neurostimulation. Studies of healthy volunteers using other diagnostic modalities should be taken into account for interpretation of the results of our study.
Time‐frequency ECoG analyses in this study satisfactorily determined “the centers of auditory‐ and movement‐related γ‐augmentation” in all 15 subjects. Yet, we recognize that the number of trials in each auditory–motor task could have influenced the results of ECoG analyses. More electrode sites potentially could have shown γ‐augmentation reaching significance, if a larger number of trials were administered to our children and if the whole tasks were successfully completed. Because of the limited time frame with each patient room being quiet and due to the limited attention span in some of our children, we recognized that a larger number of trials were difficult to assign to all of our subjects.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information Figure 1
Supporting Information Figure 2
Supporting Information Sound S1
Supporting Information Sound S2
Supporting Information Sound S3
Supporting Information Sound S4
Supporting Information Sound S5
Supporting Information Sound S6
Supporting Information Sound S7
Supporting Information Sound S8
Table S1: Patient Profile
Supporting Information Video S1
Acknowledgements
The authors are grateful to Harry T. Chugani, M.D., Lunliya Thampratankul, M.D., Carol Pawlak, R.EEG/EP.T, Ruth Roeder, R.N., M.S. and the staff of the Division of Electroneurodiagnostics at Children's Hospital of Michigan, Wayne State University for the collaboration and assistance in performing the studies described above.
REFERENCES
- Abler B, Roebroeck A, Goebel R, Höse A, Schönfeldt‐Lecuona C, Hole G, Walter H ( 2006): Investigating directed influences between activated brain areas in a motor‐response task using fMRI. Magn Reson Imaging 24: 181–185. [DOI] [PubMed] [Google Scholar]
- Akiyama T, Otsubo H, Ochi A, Galicia EZ, Weiss SK, Donner EJ, Rutka JT, Snead OC III ( 2006): Topographic movie of ictal high‐frequency oscillations on the brain surface using subdural EEG in neocortical epilepsy. Epilepsia 47: 1953–1957. [DOI] [PubMed] [Google Scholar]
- Asano E, Juhász C, Shah A, Muzik O, Chugani DC, Shah J, Sood S, Chugani HT ( 2005): Origin and propagation of epileptic spasms delineated on electrocorticography. Epilepsia 46: 1086–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano E, Nishida M, Fukuda M, Rothermel R, Juhász C, Sood S ( 2009): Differential visually‐induced gamma‐oscillations in human cerebral cortex. Neuroimage 45: 477–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auranen T ( 2002): Nonparametric statistical analysis of time‐frequency representations of magnetoencephalographic data. Master's Thesis, Helsinki University of Technology, Department of Electrical and Communications Engineering, Espoo, Finland.
- Bagshaw AP, Jacobs J, LeVan P, Dubeau F, Gotman J ( 2009): Effect of sleep stage on interictal high‐frequency oscillations recorded from depth macroelectrodes in patients with focal epilepsy. Epilepsia 50: 617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrick TR, Lawes IN, Mackay CE, Clark CA ( 2007): White matter pathway asymmetry underlies functional lateralization. Cereb Cortex 17: 591–598. [DOI] [PubMed] [Google Scholar]
- Belin P, Zatorre RJ, Lafaille P, Ahad P, Pike B ( 2000): Voice‐selective areas in human auditory cortex. Nature 403: 309–312. [DOI] [PubMed] [Google Scholar]
- Berger MS, Cohen WA, Ojemann GA ( 1990): Correlation of motor cortex brain mapping data with magnetic resonance imaging. J Neurosurg 72: 383–387. [DOI] [PubMed] [Google Scholar]
- Bidet‐Caulet A, Fischer C, Bauchet F, Aguera PE, Bertrand O ( 2007): Neural substrate of concurrent sound perception: Direct electrophysiological recordings from human auditory cortex. Front Hum Neurosci 1: 5. (e‐publication). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Springer JA, Kaufman JN, Possing ET ( 2000): Human temporal lobe activation by speech and nonspeech sounds. Cereb Cortex 10: 512–528. [DOI] [PubMed] [Google Scholar]
- Binder JR, Liebenthal E, Possing ET, Medler DA, Ward BD ( 2004): Neural correlates of sensory and decision processes in auditory object identification. Nat Neurosci 7: 295–301. [DOI] [PubMed] [Google Scholar]
- Brasil‐Neto JP, McShane LM, Fuhr P, Hallett M, Cohen LG ( 1992): Topographic mapping of the human motor cortex with magnetic stimulation: Factors affecting accuracy and reproducibility. Electroencephalogr Clin Neurophysiol 85: 9–16. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A ( 2001): Anterior cingulate cortex and response conflict: Effects of frequency, inhibition and errors. Cereb Cortex 11: 825–836. [DOI] [PubMed] [Google Scholar]
- Brosch M, Budinger E, Scheich H ( 2002): Stimulus‐related gamma oscillations in primate auditory cortex. J Neurophysiol 87: 2715–2725. [DOI] [PubMed] [Google Scholar]
- Brown EC, Rothermel R, Nishida M, Juhász C, Muzik O, Hoechstetter K, Sood S, Chugani HT, Asano E ( 2008): In vivo animation of auditory‐language‐induced gamma‐oscillations in children with intractable focal epilepsy. Neuroimage 41: 1120–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton MW, Small SL ( 2006): Functional neuroanatomy of segmenting speech and nonspeech. Cortex 42: 644–651. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Horváth Z, Urioste R, Hetke J, Wise K ( 1992): High‐frequency network oscillation in the hippocampus. Science 256: 1025–1027. [DOI] [PubMed] [Google Scholar]
- Callan DE, Jones JA, Callan AM, Akahane‐Yamada R ( 2004): Phonetic perceptual identification by native‐ and second‐language speakers differentially activates brain regions involved with acoustic phonetic processing and those involved with articulatory‐auditory/orosensory internal models. Neuroimage 22: 1182–1194. [DOI] [PubMed] [Google Scholar]
- Calvert GA, Bullmore ET, Brammer MJ, Campbell R, Williams SC, McGuire PK, Woodruff PW, Iversen SD, David AS ( 1997): Activation of auditory cortex during silent lipreading. Science 276: 593–596. [DOI] [PubMed] [Google Scholar]
- Carreiras M, Mechelli A, Estévez A, Price CJ ( 2007): Brain activation for lexical decision and reading aloud: Two sides of the same coin? J Cogn Neurosci 19: 433–444. [DOI] [PubMed] [Google Scholar]
- Catalan MJ, Honda M, Weeks RA, Cohen LG, Hallett M ( 1998): The functional neuroanatomy of simple and complex sequential finger movements: A PET study. Brain 121: 253–264. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran CF, Ghazanfar AA ( 2009): Different neural frequency bands integrate faces and voices differently in the superior temporal sulcus. J Neurophysiol 101: 773–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Samii A, Canos M, Wassermann EM, Hallett M ( 1997): Effects of phenytoin on cortical excitability in humans. Neurology 49: 881–883. [DOI] [PubMed] [Google Scholar]
- Crone NE, Miglioretti DL, Gordon B, Lesser RP ( 1998): Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event‐related synchronization in the gamma band. Brain 121: 2301–2315. [DOI] [PubMed] [Google Scholar]
- Crone NE, Boatman D, Gordon B, Hao L ( 2001): Induced electrocorticographic gamma activity during auditory perception. Brazier Award‐winning article, 2001. Clin Neurophysiol 112: 565–582. [DOI] [PubMed] [Google Scholar]
- Davison AC, Hinkley DV ( 1999): Bootstrap Methods and their Application, Chapter 4.4.1: Studentized Bootstrap Method. Cambridge: Cambridge University Press; pp 161–175. [Google Scholar]
- Dalal SS, Baillet S, Adam C, Ducorps A, Schwartz D, Jerbi K, Bertrand O, Garnero L, Martinerie J, Lachaux JP ( 2009): Simultaneous MEG and intracranial EEG recordings during attentive reading. Neuroimage 45: 1289–1304. [DOI] [PubMed] [Google Scholar]
- Démonet JF, Chollet F, Ramsay S, Cardebat D, Nespoulous JL, Wise R, Rascol A, Frackowiak R ( 1992): The anatomy of phonological and semantic processing in normal subjects. Brain 115: 1753–1768. [DOI] [PubMed] [Google Scholar]
- Edwards E, Soltani M, Deouell LY, Berger MS, Knight RT ( 2005): High gamma activity in response to deviant auditory stimuli recorded directly from human cortex. J Neurophysiol 94: 4269–4280. [DOI] [PubMed] [Google Scholar]
- Fan J, Byrne J, Worden MS, Guise KG, McCandliss BD, Fossella J, Posner MI ( 2007): The relation of brain oscillations to attentional networks. J Neurosci 27: 6197–6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Nishida M, Juhász C, Muzik O, Sood S, Chugani HT, Asano E ( 2008): Short‐latency median‐nerve somatosensory‐evoked potentials and induced gamma‐oscillations in humans. Brain 131: 1793–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetz W, Otsubo H, Pang EW ( 2008): Magnetoencephalography for clinical pediatrics: The effect of head positioning on measurement of somatosensory‐evoked fields. Clin Neurophysiol 119: 1923–1933. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS ( 2000): Cerebral specialization and interhemispheric communication: Does the corpus callosum enable the human condition? Brain 123: 1293–1326. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Elsinger CL, Mayer AR, Durgerian S, Rao SM ( 2004): Motor sequence complexity and performing hand produce differential patterns of hemispheric lateralization. J Cogn Neurosci 16: 621–636. [DOI] [PubMed] [Google Scholar]
- Haseeb A, Asano E, Juhasz C, Shah A, Sood S, Chugani HT ( 2007): Young patients with focal seizures may have the primary motor area for the hand in the postcentral gyrus. Epilepsy Res 76: 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoechstetter K, Bornfleth H, Weckesser D, Ille N, Berg P, Scherg M ( 2004): BESA source coherence: A new method to study cortical oscillatory coupling. Brain Topography 16: 233–238. [DOI] [PubMed] [Google Scholar]
- Holcomb HH, Medoff DR, Caudill PJ, Zhao Z, Lahti AC, Dannals RF, Tamminga CA ( 1998): Cerebral blood flow relationships associated with a difficult tone recognition task in trained normal volunteers. Cereb Cortex 8: 534–542. [DOI] [PubMed] [Google Scholar]
- Hua K, Oishi K, Zhang J, Wakana S, Yoshioka T, Zhang W, Akhter KD, Li X, Huang H, Jiang H, van Zijl P, Mori S ( 2008): Mapping of functional areas in the human cortex based on connectivity through association fibers. Cereb Cortex doi:10.1093/cercor/bhn215. (e‐publication). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäncke L, Wüstenberg T, Scheich H, Heinze HJ ( 2002): Phonetic perception and the temporal cortex. Neuroimage 15: 733–746. [DOI] [PubMed] [Google Scholar]
- Joanisse MF, Gati JS ( 2003): Overlapping neural regions for processing rapid temporal cues in speech and nonspeech signals. Neuroimage 19: 64–79. [DOI] [PubMed] [Google Scholar]
- Josse G, Seghier ML, Kherif F, Price CJ ( 2008): Explaining function with anatomy: Language lateralization and corpus callosum size. J Neurosci 28: 14132–14139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhász C, Asano E, Shah A, Chugani DC, Batista CE, Muzik O, Sood S, Chugani HT ( 2009): Focal decreases of cortical GABAA receptor binding remote from the primary seizure focus: What do they indicate? Epilepsia 50: 240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima R, Matsumura M, Sadato N, Naito E, Waki A, Nakamura S, Matsunami K, Fukuda H, Yonekura Y ( 1998): Regional cerebral blood flow changes in human brain related to ipsilateral and contralateral complex hand movements‐a PET study. Eur J Neurosci 10: 2254–2260. [DOI] [PubMed] [Google Scholar]
- Knecht S, Deppe M, Dräger B, Bobe L, Lohmann H, Ringelstein E, Henningsen H ( 2000): Language lateralization in healthy right‐handers. Brain 123: 74–81. [DOI] [PubMed] [Google Scholar]
- Kosaka B, Hiscock M, Strauss E, Wada JA, Purves S ( 1993): Dual task performance by patients with left or right speech dominance as determined by carotid amytal tests. Neuropsychologia 31: 127–136. [DOI] [PubMed] [Google Scholar]
- Lachaux JP, Jerbi K, Bertrand O, Minotti L, Hoffmann D, Schoendorff B, Kahane P ( 2007): A blueprint for real‐time functional mapping via human intracranial recordings. PLoS ONE 2: e1094. (e‐publication). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TW, Dolan RJ, Critchley HD ( 2008): Controlling emotional expression: Behavioral and neural correlates of nonimitative emotional responses. Cereb Cortex 18: 104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KJ, denNijs M, Shenoy P, Miller JW, Rao RP, Ojemann JG ( 2007a): Real‐time functional brain mapping using electrocorticography. Neuroimage 37: 504–507. [DOI] [PubMed] [Google Scholar]
- Miller KJ, Leuthardt EC, Schalk G, Rao RP, Anderson NR, Moran DW, Miller JW, Ojemann JG ( 2007b): Spectral changes in cortical surface potentials during motor movement. J Neurosci 27: 2424–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzik O, Chugani DC, Zou G, Hua J, Lu Y, Lu S, Asano E, Chugani HT ( 2007): Multimodality data integration in epilepsy. Int J Biomed Imaging doi:10.1155/2007/13963. (e‐publication). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessing J, Ebisch B, Schmidt KE, Niessing M, Singer W, Galuske RA ( 2005): Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science 309: 948–951. [DOI] [PubMed] [Google Scholar]
- Nii Y, Uematsu S, Lesser RP, Gordon B ( 1996): Does the central sulcus divide motor and sensory functions? Cortical mapping of human hand areas as revealed by electrical stimulation through subdural grid electrodes. Neurology 46: 360–367. [DOI] [PubMed] [Google Scholar]
- Ohara S, Ikeda A, Kunieda T, Yazawa S, Baba K, Nagamine T, Taki W, Hashimoto N, Mihara T, Shibasaki H ( 2000): Movement‐related change of electrocorticographic activity in human supplementary motor area proper. Brain 123: 1203–1215. [DOI] [PubMed] [Google Scholar]
- Ojemann SG, Berger MS, Lettich E, Ojemann GA ( 2003): Localization of language function in children: Results of electrical stimulation mapping. J Neurosurg 98: 465–470. [DOI] [PubMed] [Google Scholar]
- Otsubo H, Ochi A, Imai K, Akiyama T, Fujimoto A, Go C, Dirks P, Donner EJ ( 2008): High‐frequency oscillations of ictal muscle activity and epileptogenic discharges on intracranial EEG in a temporal lobe epilepsy patient. Clin Neurophysiol 119: 862–868. [DOI] [PubMed] [Google Scholar]
- Papp N, Ktonas P ( 1977): Critical evaluation of complex demodulation techniques for the quantification of bioelectrical activity. Biomed Sci Instrum 13: 135–145. [PubMed] [Google Scholar]
- Pashler H ( 1994): Dual‐task interference in simple tasks: data and theory. Psychol Bull 116: 220–244. [DOI] [PubMed] [Google Scholar]
- Penfield W, Boldrey E ( 1937): Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain 60: 389–443. [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH ( 1999): Event‐related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol 110: 1842–1857. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Cooper R ( 1975): Frequency dependence of the transmission of the EEG from cortex to scalp. Electroencephalogr Clin Neurophysiol 38: 93–96. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Graimann B, Huggins JE, Levine SP, Schuh LA ( 2003): Spatiotemporal patterns of beta desynchronization and gamma synchronization in corticographic data during self‐paced movement. Clin Neurophysiol 114: 1226–1236. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Temple E, Protopapas A, Nagarajan S, Tallal P, Merzenich M, Gabrieli JD ( 2001): Relations between the neural bases of dynamic auditory processing and phonological processing: Evidence from fMRI. J Cogn Neurosci 13: 687–697. [DOI] [PubMed] [Google Scholar]
- Polkey CE ( 2000): Physical complications of epilepsy surgery In: Oxbury J, Polkey C, Duchowny M, editors. Intractable Focal Epilepsy. London: W.B. Saunders; pp 784–794. [Google Scholar]
- Price CJ, Wise RJ, Warburton EA, Moore CJ, Howard D, Patterson K, Frackowiak RS, Friston KJ ( 1996): Hearing and saying. The functional neuro‐anatomy of auditory word processing. Brain 119: 919–931. [DOI] [PubMed] [Google Scholar]
- Pujol J, Deus J, Losilla JM, Capdevila A ( 1999): Cerebral lateralization of language in normal left‐handed people studied by functional MRI. Neurology 52: 1038–1043. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F, Huss M, Kherif F, Moscoso del Prado Martin F, Hauk O, Shtyrov Y ( 2006): Motor cortex maps articulatory features of speech sounds. Proc Natl Acad Sci USA 103: 7865–7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SM, Binder JR, Bandettini PA, Hammeke TA, Yetkin FZ, Jesmanowicz A, Lisk LM, Morris GL, Mueller WM, Estkowski LD, Hyde JS ( 1993): Functional magnetic resonance imaging of complex human movements. Neurology 43: 2311–2138. [DOI] [PubMed] [Google Scholar]
- Ray S, Crone NE, Niebur E, Franaszczuk PJ, Hsiao SS ( 2008a): Neural correlates of high‐gamma oscillations (60–200 Hz) in macaque local field potentials and their potential implications in electrocorticography. J Neurosci 28: 11526–11536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Niebur E, Hsiao SS, Sinai A, Crone NE ( 2008b): High‐frequency gamma activity (80–150Hz) is increased in human cortex during selective attention. Clin Neurophysiol 119: 116–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimol LM, Specht K, Hugdahl K ( 2006): Controlling for individual differences in fMRI brain activation to tones, syllables, and words. Neuroimage 30: 554–562. [DOI] [PubMed] [Google Scholar]
- Rissman J, Eliassen JC, Blumstein SE ( 2003): An event‐related FMRI investigation of implicit semantic priming. J Cogn Neurosci 15: 1160–1175. [DOI] [PubMed] [Google Scholar]
- Salmelin R, Hari R ( 1994): Spatiotemporal characteristics of sensorimotor MEG rhythms related to thumb movement. Neuroscience 60: 537–550. [DOI] [PubMed] [Google Scholar]
- Schalk G, Leuthardt EC, Brunner P, Ojemann JG, Gerhardt LA, Wolpaw JR ( 2008): Real‐time detection of event‐related brain activity. Neuroimage 43: 245–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schevon CA, Carlson C, Zaroff CM, Weiner HJ, Doyle WK, Miles D, Lajoie J, Kuzniecky R, Pacia S, Vazquez B, Luciano D, Najjar S, Devinsky O ( 2007): Pediatric language mapping: sensitivity of neurostimulation and wada testing in epilepsy surgery. Epilepsia 48: 539–545. [DOI] [PubMed] [Google Scholar]
- Simes RJ ( 1986): An improved Bonferroni procedure for multiple tests of significance. Biometrika 73: 751–754. [Google Scholar]
- Springer JA, Binder JR, Hammeke TA, Swanson SJ, Frost JA, Bellgowan PS, Brewer CC, Perry HM, Morris GL, Mueller WM ( 1999): Language dominance in neurologically normal and epilepsy subjects: A functional MRI study. Brain 122: 2033–2046. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Binder JR, Possing ET, McKiernan KA, Ward BD, Hammeke TA ( 2002): Language lateralization in left‐handed and ambidextrous people: fMRI data. Neurology 59: 238–244. [DOI] [PubMed] [Google Scholar]
- Szurhaj W, Bourriez JL, Kahane P, Chauvel P, Mauguière F, Derambure P ( 2005): Intracerebral study of gamma rhythm reactivity in the sensorimotor cortex. Eur J Neurosci 21: 1223–1235. [DOI] [PubMed] [Google Scholar]
- Tallon‐Baudry C, Bertrand O, Delpuech C, Pernier J ( 1996): Stimulus specificity of phase‐locked and non‐phase‐locked 40 Hz visual responses in human. J Neurosci 16: 4240–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon‐Baudry C, Bertrand O ( 1999): Oscillatory gamma activity in humans and its role in object representation. Trends Cogn Sci 3: 151–162. [DOI] [PubMed] [Google Scholar]
- Tallon‐Baudry C, Bertrand O, Hénaff MA, Isnard J, Fischer C ( 2005): Attention modulates gamma‐band oscillations differently in the human lateral occipital cortex and fusiform gyrus. Cereb Cortex 15: 654–662. [DOI] [PubMed] [Google Scholar]
- Towle VL, Yoon HA, Castelle M, Edgar JC, Biassou NM, Frim DM, Spire JP, Kohrman MH ( 2008): ECoG gamma activity during a language task: Differentiating expressive and receptive speech areas. Brain 131: 2013–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoof K, Van Strien JW ( 1997): Verbal‐to‐manual and manual‐to‐verbal dual‐task interference in left‐handed and right‐handed adults. Percept Mot Skills 85: 739–746. [DOI] [PubMed] [Google Scholar]
- von Stockhausen HM, Thiel A, Herholz K, Pietrzyk U ( 1997): A convenient method for topographical localization of intracranial electrodes with MRI and a conventional radiograph. Neuroimage 5: S514. [Google Scholar]
- Vouloumanos A, Kiehl KA, Werker JF, Liddle PF ( 2001): Detection of sounds in the auditory stream: Event‐related fMRI evidence for differential activation to speech and nonspeech. J Cogn Neurosci 13: 994–1005. [DOI] [PubMed] [Google Scholar]
- Waldie KE, Mosley JL ( 2000): Hemispheric specialization for reading. Brain Lang 75: 108–122. [DOI] [PubMed] [Google Scholar]
- Warren JD, Zielinski BA, Green GG, Rauschecker JP, Griffiths TD ( 2002): Perception of sound‐source motion by the human brain. Neuron 34: 139–148. [DOI] [PubMed] [Google Scholar]
- Wellmer J, von Oertzen J, Schaller C, Urbach H, König R, Widman G, Van Roost D, Elger CE ( 2002): Digital photography and 3D MRI‐based multimodal imaging for individualized planning of resective neocortical epilepsy surgery. Epilepsia 43: 1543–1550. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Fong JK, Meyer BU, Priori A, Rothwell JC, Day BL, Thompson PD ( 1994): The effect of magnetic coil orientation on the latency of surface EMG and single motor unit responses in the first dorsal interosseous muscle. Electroencephalogr Clin Neurophysiol 93: 138–146. [DOI] [PubMed] [Google Scholar]
- Wisneski KJ, Anderson N, Schalk G, Smyth M, Moran D, Leuthardt EC ( 2008): Unique cortical physiology associated with ipsilateral hand movements and neuroprosthetic implications. Stroke 39: 3351–3359. [DOI] [PubMed] [Google Scholar]
- Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, Winkler P ( 1997): Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain 120: 141–157. [DOI] [PubMed] [Google Scholar]
- Zanos S, Miller KJ, Ojemann JG ( 2008): Electrocorticographic spectral changes associated with ipsilateral individual finger and whole hand movement. Conf Proc IEEE Eng Med Biol Soc 2008: 5939–5942. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information Figure 1
Supporting Information Figure 2
Supporting Information Sound S1
Supporting Information Sound S2
Supporting Information Sound S3
Supporting Information Sound S4
Supporting Information Sound S5
Supporting Information Sound S6
Supporting Information Sound S7
Supporting Information Sound S8
Table S1: Patient Profile
Supporting Information Video S1


