Abstract
Neonatal sepsis continues to take a devastating toll globally. Although adequate to protect against invasive infection in most newborns, the distinct function of neonatal innate host defense coupled with impairments in adaptive immune responses, increases the likelihood of acquiring infection early in life with subsequent rapid dissemination and death. Unique differences exist between neonates and older populations with respect to the capacity, quantity, and quality of innate host responses to pathogens. Recent characterization of the age-dependent maturation of neonatal innate immune function has identified novel translational approaches that may lead to improved diagnostic, prophylactic and therapeutic modalities.
Keywords: Neonate, early onset sepsis, innate immunity, antimicrobial proteins and peptides, neutrophil, monocyte, acute phase response, complement, pattern-recognition receptors, TLR
Introduction
Infection claims the lives of nearly three thousand neonates worldwide every day1. Preterm newborns are the most affected, as they exhibit the highest sepsis-related morbidity and mortality among pediatric patients2. The distinct neonatal immune system, although adequate to protect against infection in most neonates, contributes to the newborn’s enhanced susceptibility to infection3-6. The innate immune system represents the first line of prevention against microbial invasion and of defense once infection has occurred. Initial immunomodulatory efforts directed at improving neonatal sepsis survival through enhancement of innate immune function (also reviewed in Chapter XXXX of this issue), including IVIg, GM-CSF, and G-CSF, have thus far not yielded major benefit3. Dramatic progress in molecular characterization of innate immunity has been made over the past decade, paving the way to new prophylactic and therapeutic approaches. While the roles of innate immunity in adult sepsis have been examined in great detail7-12, the distinct neonatal innate immune response remains incompletely characterized. In the context of increasing incidence of prematurity and the potential for neurodevelopmental impact in sepsis survivors13, there is an unmet medical need for novel approaches to prevention and therapy.
Early onset sepsis
Early onset neonatal sepsis (EONS) typically occurs during the first 24 hours of life, and is a fulminant multisystem infection acquired by vertical transmission from the mother14. Maternal factors that increase the risk of EONS include preterm labor and delivery, colonization with group B streptococcus (GBS), prolonged rupture of membranes, chorioamnionitis, and intra-partum fever15. The neonate can present with signs of respiratory distress including apnea, temperature instability, hypotension, bradycardia, tachycardia, lethargy or irritability, and abdominal distension or feeding intolerance requiring prompt evaluation and appropriate treatment from the physician. Once overtly symptomatic, mortality is unacceptably high16.
The incidence of EONS in term neonates is 1-2/1000 live births with a mortality of ~3%17, 18. Responsible pathogens are dominated by two individual bacteria, GBS (41%) and Escherichia coli (17%)17. GBS emerged as the leading pathogen of EONS in the 1970s with case fatality rates as high as 55%19. A significant decrease in EONS due to GBS occurred, especially in neonates ≥ 34 weeks gestation, following the national guidelines for the use of intra-partum antibiotic prophylaxis (IAP) to prevent neonatal GBS infection20, 21. IAP has decreased overall GBS rates, but it is also associated with an increase in the incidence of EONS due to Escherichia coli16, and an increase in newborn exposure to antibiotics that may have adverse consequences22.
Preterm neonates, especially the very low birth weight (VLBW) neonate, suffer attack rates >10 times higher than those born at term with associated mortality in over one-third23. Morbidities include increased respiratory distress, chronic lung disease (CLD), white-matter damage, neurodevelopmental impairment, and increased risk of death, especially with Gram-negative infections23, 24. Compared to term neonates, VLBW neonates demonstrate an increased percentage of EONS caused by Gram-negative pathogens25. EONS associated with Gram-negative infection is more likely to result in death within 72 hours of birth (29%) than Gram-positive infection (6%)25. Extremely low birth weight neonates (<1000g, ELBW) are even more vulnerable as EONS accounts for over 50% of deaths that occur within the first 48 hours of life26.
An adaptive immune response, including the selection and amplification of specific clones of lymphocytes (B-cells and T-cells) that results in immunologic memory, generally requires 5-7 days to develop. Moreover, the neonatal adaptive immune response is functionally distinct from the adult response at multiple levels5. As a result, the neonate is thought to largely depend on the function of innate immunity for protection from infection during the first days of life. The innate response, defined as that which is present at birth prior to microbial exposure, consists of a preformed immune response mediated by barriers, sentinel immune cells, pathogen recognition systems, inflammatory response proteins, host defense proteins and peptides, as well as passively acquired immunoglobulin from the mother.
An overview of the current state of knowledge regarding innate immunity of the newborn must take into consideration the research approaches that have thus far been employed to study this area. In vitro studies have largely focused on cord blood, though in some cases similar patterns and/or age-dependent maturation/normalization have been documented in newborn peripheral blood. In vivo studies of non-human vertebrates have largely focused on newborn mice. The neonatal mouse model has two potential limitations: (a) mice are not humans, and the innate immune system is particularly divergent27, and (b) the post-natal age at which the mice are studied is a matter of variation and debate. Although all approaches have limitations, they provide valuable information towards characterizing in vivo function. Herein, we will discuss neonatal innate host defense systems and their relationship to susceptibility to and progression of EONS.
Innate host defense systems
Maternal Innate defenses
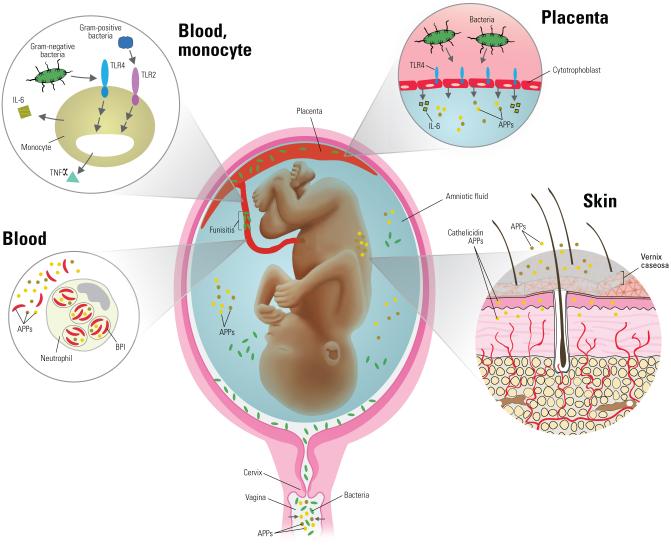
In utero infection is a significant risk factor for the development of EONS. In particular, ~80% of preterm deliveries at less than 30 weeks gestation have evidence of intrauterine infection28. Ascending infection induces maternal immune responses in utero that may also influence mobilization of fetal neutrophils (polymorphonuclear cells-PMNs) found in infected amniotic fluid29 and the development of the fetal and neonatal inflammatory response30. Immune responses to intra-amniotic infection likely begin with Toll-Like receptors (TLRs) expressed on maternal trophoblast cells28 [Figure1]. Human trophoblast cells express all ten TLRs and, upon stimulation, produce inflammatory cytokines found in infected amniotic fluid28, 31. In particular, elevated amniotic fluid IL-6 concentrations are commonly found during intra-amniotic infection31 and are associated with rapid parturition32 and acceleration of fetal lung maturation33. In addition to the TLR-mediated innate immune sensing capabilities of trophoblasts, the amnion, chorion, placenta, amniotic fluid, cervical mucosa, and vagina are replete with antimicrobial proteins and peptides (APPs) that possess key host defense functions and are up-regulated with infection34-36. Thus, ascending bacterial infection that underlies EONS triggers early innate immune activation of both maternal and fetal tissues that mobilize host defense effectors to the amniotic fluid.
Figure 1. Role of innate immunity in responses to in utero infection.
Early onset sepsis is typically caused by an ascending maternal lower genital tract infection. Antimicrobial proteins and peptides (APPs) within the vagina and amniotic fluid help to reduce bacterial burden and are up-regulated during infection. Pathogen detection begins with placental trophoblast cell Toll-like receptors (TLR) that up-regulate APP and inflammatory cytokine production. Neonatal monocytes are simultaneously stimulated via TLR-mediated pathogen detection with subsequent cytokine production that in turn activates innate immune function (neutrophils, macrophages) and induces the hepatic production of acute phase reactants. Innate immunity within the skin and vernix (APPs) facilitates appropriate commensal microbial colonization associated with erythema toxicum.
Epithelial and Mucosal Barriers
Host barriers provide the first means of protection from microbial invasion. There are two critical barrier regions: the mucosa (respiratory and intestinal) and the skin. While in utero, the fetus is protected within the normally sterile environment provided by the amnion and amniotic fluid that is replete with APPs4. At birth, vernix enhances skin barrier function for the late-preterm and term neonate but is largely absent in preterm neonates born before 28 weeks gestation. Vernix is a complex material comprised of water (80.5%), lipids (10.3%), and proteins (9.1%) produced by fetal sebaceous glands during the last trimester37. The vernix provides a barrier to water loss, improves temperature control, and serves as a shield containing antioxidants and innate immune factors such as APPs38. Furthermore, vernix is important for maintenance of the pH balance of the skin and thus sets the stage for appropriate colonization with commensal organisms instead of pathogens38.
The outermost layer of the skin, the stratum corneum, prevents microbial invasion, maintains temperature, and reduces the risk of dehydration through prevention of transcutaneous water loss39. The immature and incompletely developed stratum corneum of preterm newborns takes at least 1-2 weeks after birth to become fully functional40 and may take up to 8 weeks to develop in the extremely preterm neonate significantly increasing the risk for barrier dysfunction41. Neonates have an increased density of hair follicles as compared to adults, which provides a larger reservoir for skin commensal organisms such as Staphylococcus epidermidis42, 43. Additionally, the risk for a microbial breech of the cutaneous barrier rises in the presence of intravenous catheters essential for critical care. The response of the dermal innate immune system to host commensal organisms is exemplified by the common newborn rash erythema toxicum. Recent evidence suggests that this common newborn condition is an immune-mediated manifestation of the bacterial colonization of the skin42, 43. Because the skin is arid, it is a more formidable barrier for microbial invasion than that of the moist mucosal surfaces. However in extremely preterm infants, humidification systems are used to decrease insensible water loss and these may delay cornification of the skin and thus predispose to microbial growth.
Mucosal barriers contain multiple components that serve to prevent infection including acidic pH, mucus, cilia, destructive enzymes, APPs, opsonins such as surfactant proteins, sentinel immune cells such as macrophages, dendritic cells (DC), PMN, and T cells, as well as commensal organisms44. After birth, the gut is quickly colonized and contains a significant repository of microorganisms45. Although not believed to contribute significantly to the development of EONS, intestinal barrier integrity is paramount for prevention of spread of microorganisms out of the intestinal compartment and likely plays a role in the development of necrotizing enterocolitis46. Factors known to disrupt the neonatal intestinal barrier are antibiotic treatment, hypoxia, or remote infection as has been recently reviewed3, 47.
Ascending infection, with or without chorioamnionitis, or acquisition of microbes during passage through the birth canal represent the common origins of EONS14. The portal of pathogen entry into the neonate is primarily through the respiratory tract. In utero, amniotic fluid and pulmonary APPs, surfactant proteins A and D, alveolar macrophages, PMNs, and trophoblast-based TLRs serve as the first line of defense [Figure 1]. After birth, respiratory mucosal function can be impaired by surfactant deficiency, altered mucus production, intubation and mechanical ventilation that is associated with decreased mucociliary clearance, and airway irritation. The surface and submucosal gland epithelium of the conducting airways is a constitutive primary participant in innate immunity through the production of mucus and mucociliary clearance of pathogens and debris48. Premature neonates have relatively more goblet cells than more mature neonates leading to a decrease in mucociliary clearance. Additional insult to the neonatal respiratory system may result from physical damage to lung parenchyma via atelectrauma, barotrauma, or chemical injury (e.g. oxygen or aspiration). Intubation is also associated with the progressive accumulation of colonizing bacteria and bacterial endotoxin in respiratory fluids with concomitant mobilization to the airway of endotoxin-modulating host defense proteins49. Neonates with surfactant deficiency lack host defense proteins with valuable immune function such as surfactant proteins A and D that are absent in commercially available exogenous surfactants due to destruction during preparation50. There is an age-dependent maturation in the ability of respiratory epithelium to elaborate APPs (cathelicidin [LL-37] and β-defensins) such that respiratory epithelium of preterm newborns mounts a deficient antimicrobial peptide response51. These deficiencies as well as those related to cellular function in combination with invasive procedures lead to a reduction in respiratory barrier function that increases the risk for early infection.
Pathogen recognition systems
The development of an immune response by local immune sentinel cells, including macrophages, endothelium, epithelium, PMNs, and DCs, is dependent on the identification of invading pathogens or the presence of tissue damage that result in elaboration of exogenous or endogenous danger signals. Evolution of pattern recognition receptors (PRRs) capable of recognizing damage/danger associated molecular patterns (DAMPs) [cytokines, intracellular proteins, and/or mediators released by dying or damaged cells] in addition to pathogen associated molecular patterns (PAMPs) [e.g., bacterial cell wall components, flagellin, nucleic acids] allows for specificity in the innate immune response. PRRs are present on the cell surface, within intracellular vesicles, and in the cytoplasm of multiple cell types. Examples include the TLRs, nucleotide-binding oligomerization domain (NOD)-like receptors, retinoic-acid-inducible protein I (RIG-I)-like receptors, and integrins52, 53. As described below, with respect to multiple microbial stimuli, engagement of neonatal PRRs on neonatal blood leukocytes often leads to a pattern of response that is distinct from that in older individuals with impaired neonatal production of pro-inflammatory/TH1-polarizing cytokines that may increase the risk for development and progression of infection.
Activation of TLRs by microbial or synthetic agonists results in downstream production of cytokines, chemokines, and complement and coagulation proteins, as well as initiation of antimicrobial effector mechanisms including enhanced phagocytic function54. There are 10 TLRs in humans that respond to specific molecular triggers54, 55. Molecular identification of particular pathogens and subsequent signaling can occur via simultaneous stimulation of multiple TLRs, allowing for tremendous diversity in specific pathogen detection and response55, 56. As TLRs play an essential role in recognition and response to pathogens, alterations in their expression, structure, and signaling pathways can impair host defense and increase host vulnerability to infection53.
Basal expression and cellular distribution of TLRs is similar in monocytes derived from term umbilical cord blood and peripheral blood monocytes obtained from adults57. Cord blood monocyte TLR4 expression has been found to increase with gestational age58. In a study of patients with EONS, peripheral blood cells from both preterm and full-term neonates demonstrated up-regulation of TLR2 and TLR4 mRNA during Gram-positive and Gram-negative bacteremia, respectively59.
Hypomorphic mutations or decreased expression of proteins that enhance TLR activation may increase the risk for progression of early neonatal infection60-62. Examples of ancillary proteins needed for optimal TLR-mediated pathogen recognition include lipopolysaccharide-binding protein (LBP) that extracts LPS monomers from Gram-negative bacteria and delivers them to the endotoxin receptor (CD14/MD2/TLR4) on the monocyte surface and CD14, both of which are normally up-regulated during neonatal sepsis and are required for LPS-mediated signaling through TLR463-65.
In addition to TLRs and their co-receptors, other intracellular signaling mechanisms are important for the detection of pathogens. Examples of cytosolic receptors include the nucleotide oligomerization domain leucine-rich repeat containing family or NOD-like receptors (NLRs), which detect peptidoglycan in the cytosol, as well as the retinoic-acid-inducible protein I (RIG-I)-like receptors (RLRs) which sense double-stranded RNA of viral origin and induce production of type I interferons (IFNs)53. Intracellular bacteria, including Listeria monocytogenes, can be recognized by NLRs66. Polymorphisms in NLR domains are associated with dysregulated inflammatory pathology including Neonatal-Onset Multisystem Inflammatory Disease (cryopyrin)67, but NLR alleles have not yet demonstrated any correlation with sepsis susceptibility68. Much remains to be learned about the functional expression of NLRs in neonates.
Recognition of a PAMP or DAMP by PRRs activates a complex series of intracellular cascades that trigger gene activation53. Polymorphisms or mutations in TLRs and downstream signaling molecules such as MyD88 (Myeloid differentiation factor 88), NEMO (NF-κB essential modulator), and IRAK-4 (IL-1-receptor-associated kinase 4) are associated with increased risk for infection in adults69-74 and in children75-78. Children with IRAK-4 deficiency manifest decreasing susceptibility to pyogenic infection with age, suggesting that the TLR pathway is of greatest importance early in life, in line with findings in murine models79, 80. In contrast to adults, specific characterization of neonatal PRR intracellular signaling intermediates, their regulation, and response to infection, have been incompletely characterized81. Of note, newborn umbilical cord PMNs were found to have lower MyD88 expression and reduced p38 phosphorylation following ex vivo stimulation with endotoxin, potentially contributing to diminished responses82. Decreased MyD88, IRF5, and p38 phosphorylation was noted in endotoxin-stimulated monocytes isolated from both umbilical cord and peripheral venous blood that exhibited gestational age-specific diminution83, 84. Deficient expression of TLR second messenger proteins in preterm newborns likely contributes to the increased risk of infection as compared to more mature neonates.
Another important neonatal PRR is the β2-integrin complement receptor 3 (CR3) that functions as a pathogen sensor on the surface of phagocytes in addition to its role in binding complement. CR3 (also known as MAC-1 and CD11b/CD18) binds LPS, in cooperation with or independent of CD14, as well as other microbial surface components and triggers up-regulation of inducible nitric oxide synthase (iNOS) and nitric oxide (NO) production85. Engagement of CR3 on PMN activates both reactive oxygen intermediate (ROI) production and phagocytosis. Accordingly, decreased expression of L-selectin and CR3 on stimulated neonatal PMN impairs neonatal PMN and monocyte activation and accumulation at sites of inflammation86, 87. This decreased expression persists for at least the first month of life in term infants possibly contributing to an increased risk for infection88. The expression of CR3 (CD11b) may be reduced further in preterm neonates as compared to term neonates89. In umbilical cord blood from neonates less than 30 weeks gestation, PMN CR3 content was similar to levels found in those with type 1 leukocyte adhesion deficiency (failure to express CD18)86, 87. Thus, decreased leukocyte CR3 surface expression increases the likelihood of suboptimal pathogen detection and cellular activation, particularly in the preterm neonate.
Innate cellular immunity
Antigen presenting cells (APCs)
After compromising barriers, invading pathogens come into contact with sentinel immune cells including monocytes, macrophages, and DC (see Figure 1, chapter xxxxxx). Following detection of microorganisms via the PRRs, these APCs amplify cellular recruitment through production of inflammatory mediators (complement, cytokines, coagulation factors, and extracellular matrix proteins90), phagocytose and kill pathogens, and present foreign antigens to cells of the adaptive immune system. Initial pathogen detection and innate response by APCs are critical for the development of an effective host immune response.
Responses following stimulation of PRRs in neonatal blood monocytes are generally reduced as compared to adults57, 91. In response to many stimuli, including most TLR agonists, human neonatal cord blood mononuclear cells tested in vitro exhibit a marked polarization, with impaired production of TH1-polarizing cytokines such as TNF-α, IFN-γ, and IL-12p70 and increased production of TH2/TH17 and anti-inflammatory cytokines such as IL-6, IL-10, IL-17, and IL-234, 92. Elevated concentrations in neonatal cord blood mononuclear cells of intracellular cAMP, a secondary messenger that inhibits production of TH1-polarizing cytokines, appears to contribute to this skewed cytokine pattern93. Proposed benefits of this skew are immune tolerance to avoid potentially harmful allo-immune reactions between fetus and mother, avoidance of excessive inflammation upon initial microbial colonization, and induction of epithelial APPs to avoid microbial penetration and infection4, 94. However, a consequence of impairment in TH1-polarizing cytokine production by neonatal mononuclear cells is a reduced ability to defend against infection with microorganisms; particularly intracellular pathogens such as Listeria spp.95, Mycobacteria spp.96, and Herpes simplex virus97.
Presentation of foreign epitopes by antigen presenting cells (APC) including monocytes, macrophages, and DC to cells of the adaptive immune system is less efficient in neonates as compared to adults. Neonatal monocytes and DCs demonstrate impaired expression of MHC-II 98, 99 and of co-stimulatory molecules including CD40 and CD86100 resulting in reduced antigen presentation to naïve CD4+ T cells. Neonatal DCs require increased stimulation for activation101 and demonstrate poor stimulation of T-cell proliferation as well as a predilection for the induction of immune tolerance via impaired LPS-induced IL-12p70 production102. Still, neonatal cord blood DCs stimulated with LPS can effectively induce cytotoxic lymphocyte responses103. A reduction in APC function impairs development of effective adaptive immunity, placing further burden on innate immune function for pathogen clearance.
In addition to altered cytokine production and reduced antigen presentation, monocyte phagocytic and chemotactic function are also reduced during neonatal sepsis relative to adults and neonatal baseline values104, 105. The number of peripheral monocytes decreases during sepsis (between 60-120 hours after presentation), likely secondary to extravasation and differentiation into macrophages and DCs. Located just below epithelial borders, macrophages encounter pathogens immediately after entry. Like monocytes, macrophages play an important role in the amplification of the immune response through the production of cytokines and chemokines, phagocytosis and killing, and antigen presentation to naïve CD4+ T cells. Neonatal macrophages are poorly responsive to several TLR agonists106 and IFN-γ94. During infection, neonatal macrophages exhibit decreased production of reactive nitrogen intermediates107.
In summary, neonatal APCs, including monocytes, macrophages, and DCs, exhibit deficits in pathogen recognition, activation following stimulation, phagocytic function, bactericidal function, and amplification of the immune response that may increase the risk for the development and progression of EONS. Of note, however, certain stimuli, such as GBS, Mycobacterium bovis (Bacille Calmette Guerin), and TLR8 agonists can effectively activate neonatal APCs in vitro108-110. The molecular rules that govern which stimuli do or do not effectively activate human neonatal APCs is a topic of active research with important translational implications for neonatal adjuvant development.
Neutrophils
Neutrophils or polymorphonuclear leukocytes (PMN) are the primary mediators of neonatal innate cellular defense. Antimicrobial mechanisms employed by PMNs have been reviewed in detail111. Many quantitative and qualitative PMN deficits have been described for neonates as compared to adults94, 112. PMN are activated and increased in number with onset of early during sepsis in term and preterm neonates113, 114. Mature neonatal bone marrow PMN reserves are rapidly depleted during infection115, which results in a release of immature “band” forms (described as a left shift); the proportion of which can be used to aid in assessing the probability of sepsis116. Neonates who develop sepsis-associated neutropenia are more likely to have Gram-negative sepsis and exhibit a higher mortality114, 117, 118. Conflicting reports exist regarding the risk of EONS in VLBW newborns with neutropenia associated with other causes such as maternal preeclampsia118-121. ELBW newborns experience the highest frequency of neutropenia among all neonatal groups. However, neutropenia among ELBW newborns is most often not secondary to sepsis. Furthermore, ELBW newborns with neutropenia do not suffer increased subsequent mortality due to sepsis as compared with non-neutropenic ELBW newborns122.
In addition to the quantitative PMN deficits, multiple qualitative defects of neonatal PMN function are noted. The process of PMN recruitment and extravasation to sites of inflammation is less efficient in neonates contributes to their increased susceptibility to early infection. Specifically, PMNs from compared to older age groups and likely preterm and term neonates exhibit reduced basal chemotaxis and random migration123, that is exacerbated in septic and post-operative newborns124. Impaired signaling downstream of chemokine-receptor binding may be partially responsible for this finding125. The propensity towards very high production of IL-6, a cytokine with anti-inflammatory properties that reduces PMN migration Pto inflammatory sites, may also contribute to the inability of newborns to mount an adequate PMN response in the context of sepsis92, 126. In septic neonates, bacterial evasion mechanisms may also contribute to poor chemotaxis127. Emerging data suggests that IL-8 priming of PMNs that occurs during labor significantly improves PMN chemotaxis over that seen with caesarean delivery and even adult controls128. Modifications in PRRs following labor may also contribute to the improvement in cellular chemotaxis. TLR4 is up-regulated on monocytes and PMN from term neonates after labor and PMN migration is improved following exposure to a TLR4 agonist129-131. Decreased neonatal expression or upregulation of surface adhesion molecules on PMNs (LFA-1[preterm neonates], CR3[term and preterm neonates], L-selectin[term and preterm neonates]) during sepsis limits rolling and subsequent diapedesis86, 87. Basal PMN deformation is reduced, and this impairment is further exacerbated in the immature band forms found commonly during infection, which reduces the ability to migrate between endothelial cells to sites of infection132. Aggregation defects lead to vascular accumulation of newborn PMNs following stimulation and contribute to decreased extravasation, rapid depletion of bone marrow reserves, vascular crowding132, and an increased likelihood of microvascular ischemia112, 133.
Similar basal degranulation capabilities are present in PMNs from premature and term neonates, but content differs compared to adults. Compared to other innate immune cells, PMNs represent the most abundant and reliable source of APPs. PMNs of term neonates contain similar amounts of myeloperoxidase and defensins, but reduced lactoferrin, elastase, and bactericidal/permeability-increasing protein (BPI) relative to adult PMNs134, 135. Neonatal PMN respiratory burst activity is also distinct as compared to adult PMN function. For example, hydroxyl radical production by term PMNs is reduced, particularly under stressed or septic conditions112, 134. In contrast, superoxide production may actually exceed that of adult PMNs, but is suppressed during sepsis, contributing to poor microbicidal activity136-138.
When the target is fully opsonized, ex vivo phagocytosis of bacteria by PMN from late-preterm and term neonates is equivalent to adult controls whereas that from VLBW function remains depressed112, 139, 140. Neonatal PMN exhibit delayed apoptosis, sustained capacity for activation (CD11b up-regulation) and cytotoxic function (ROI production), as well as extracellular release of destructive enzymes or ROI112. These mechanisms contribute to local tissue and endothelial damage with resultant increases in inflammatory cytokine production141-144. Novel PMN bactericidal mechanisms have been recently identified in studies of adult cells. TLR4-mediated activation of platelets by Gram-negative bacterial endotoxin or lipopolysaccharide (LPS) induces platelet-PMN binding with subsequent extracellular release of neutrophil extracellular traps (NETs) that contain DNA145. NETs also contain APP and hydrolytic enzymes that result in bacterial killing even after PMN death146, 147. Formation of NETs following stimulation was absent in preterm (≤ 30 weeks) and nearly absent in term neonates148. Overall, the constellation of neonatal PMN functional deficits may increase the risk for development and rapid dissemination of infection.
Mast Cells
Mast cells may possess diverse immune functions including production of vasoactive substances, cytokines, phagocytosis of pathogens, and antigen presentation149. These capabilities suggest the potential for mast cells to make a significant contribution to neonatal innate host defenses. Mast cells likely participate in the initial response to pathogens and may contribute to the neonate’s relative predilection for immune tolerance through the actions of histamine. Of note, neonatal mast cells secrete significantly more histamine following stimulation as compared to adults150. Production of histamine is well known to facilitate vasodilation, but also is capable of modification of subsequent immune responses. Specifically, neonatal mast cell histamine production alters DC cytokine production (increased IL-10 and decreased IL-12) and subsequent T cell polarizing activity (TH2 phenotype)151. Recently mast cell involvement was demonstrated in the common newborn rash, erythema toxicum, where mast cell recruitment, degranulation, and tryptase expression was noted152.
Inflammatory response proteins
Complement
Non-cellular elements of the innate immune response include inflammatory response proteins such as complement, acute phase reactants, cytokines, chemokines, coagulation proteins, and vasoactive substances. Actions of complement include opsonization and killing of pathogens, alteration of vascular tone to facilitate recruitment and activation of leukocytes, initiation of the coagulation cascade, and proinflammatory cytokine production7. Neonates exhibit gestational age-related decreases in complement proteins, assays of hemolytic function, and complement-mediated opsonic capabilities as compared to adults [Figure 2a]153-156. In particular, neonates have very low levels of C9157, which is critical for the formation of the membrane attack complex (MAC) and is associated with increased susceptibility to Neisseria infection in older populations. Neonatal Neisseria infections are most commonly ophthalmologic and rarely become systemic despite this deficiency. Moreover, human neonatal cord blood complement levels are sufficient to enhance GBS-induced TNF production in vitro, (via alternative complement pathway activation and engagement of monocyte CR3) 108-110. Nevertheless, reduced complement levels may contribute to the rapid proliferation of bacteria when assayed in human cord blood in vitro158.
Figure 2. Comparison of neonatal and adult levels of opsonins and antimicrobial proteins and peptides.
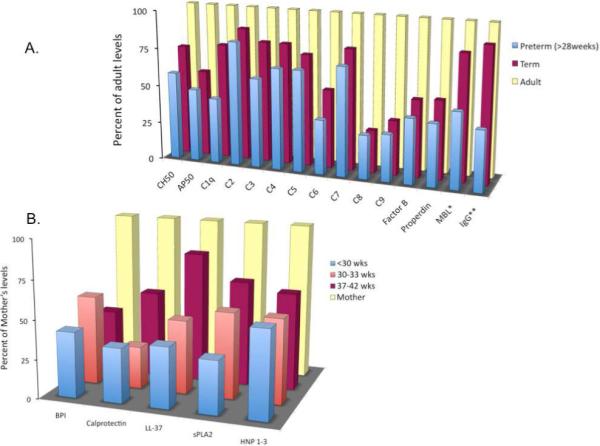
A. Complement functional assays and complement proteins, mannose-binding lectin, and IgG concentrations in preterm neonates, term neonates, and adults. (Reproduced with permission from Lewis DB and Wilson CB. “Developmental Immunology and Role of Host Defenses in Fetal and Neonatal Susceptibility to Infection. Pgs 87-210, In ”Infectious Diseases of the Fetus and Newborn Infant“: 2006 Elsevier Saunders, Philadelphia. Remington, Klein, Wilson, and Baker.) *- Reproduced with permission from Lau YL, et al. Mannose-binding protein in preterm infants: developmental profile and clinical significance. Clin Exp Immunol 102:649, 1995, **-Reproduced with permission from Fanaroff AA, et al. Incidence, presenting features, risk factors and significance of late onset septicemia in very low birth weight infants. The National Institute of Child Health and Human Development Neonatal Research Network. Pediatr Infect Dis J 17:593, 1998. B. Serum antimicrobial protein and peptide levels in preterm neonates, term neonates, and maternal levels. (Reproduced with permission from Strunk T, et al. Reduced levels of antimicrobial proteins and peptides in human cord blood plasma. Arch Dis Child Fetal Neonatal Ed 94:F230, 2009.). MBL-mannose-binding lectin, BPI-bactericidal/permeability-increasing protein, sPLA2-Secretory phospholipase 2, HNP-human neutrophil peptide.
During bacterial infection the alternative complement pathway is the primary route of complement activation in both preterm and term neonates159, 160. Significant increases in alternative pathway components have been found in the plasma from newborns with sepsis including Factor B, C3a desArg, C3bBbP (C3 convertase), and sC5b-9 (MAC), with C3a desArg reaching levels seen in infected adults159. In these studies, markers of classical pathway activation were not elevated during sepsis and suggest that antibody-mediated complement activation does not play a significant role in neonatal sepsis even in term neonates that have received ample passive immunization via placental antibody transfer160. As mannose-binding lectin (MBL) is capable of activating the alternative pathway, and decreased MBL levels are associated with an increased risk of sepsis during the first month of life (term and preterm), it is likely that MBL plays an important role in complement activation and thus innate host defense [Figure 2a]161, 162.
Multiple aspects of innate cellular recruitment, activation, and function are attributed to the actions of complement-receptor binding163-166. For example, neonatal leukocyte expression of complement receptors (CR1, CR3) increases during sepsis resulting in enhanced pathogen recognition, phagocytosis, and production of ROI, as well as enhanced endothelial adhesion, rolling, and migration. Additional effects of complement protein-receptor binding include aggregation of platelets and endothelial activation (production of chemokines, cytokines, vasoactive substances). Blunted up-regulation of CR3 and deficiencies of C5aR on neonatal PMNs following stimulation likely contributes to poor complement-mediated chemotaxis and transmigration compared to adult PMNs167, 168
Cytokines and chemokines
Activation and amplification of host immune cells during infection is mediated in part by production of cytokines and chemokines following stimulation of PRRs. Interleukin (IL)-1β, IL-6, IL-12, IL-18, interferon gamma (IFN-γ), and tumor necrosis factor-alpha (TNF-α) are among the elevated pro-inflammatory cytokines commonly found during neonatal sepsis169. Important aspects of the cytokine response following TLR stimulation in neonates are diminished. In particular, decreased production of TH1-polarizing cytokines (IL-1β, TNF-α, IFN-γ, and IL-1257, 170-172) with enhanced production of TH2-polarizing cytokines relative to adults likely contribute to neonatal susceptibility to infection8, 57, 79, 80, 83, 170. Decreased neonatal cytokine production during infection as compared to adults is likely related to decreased intracellular mediators of TLR signaling83. Plasma adenosine, an endogenous plasma metabolite that rises with hypoxia and stress, is elevated in newborn blood plasma and inhibits production of TNF-α by monocytes with preservation of IL-6 synthesis93. Adenosine binds the G-protein-coupled adenosine 3 receptor (A3R) expressed on neonatal leukocytes, thereby triggering intracellular accumulation of cAMP, a second messenger that inhibits production of TH1-polarizing cytokines while preserving that of T 2-polarizing cytokines, including IL-64, 93. The skewed polarization of neonatal cytokine responses against TH1 polarizing cytokines may reduce the risk of immune reactions that could trigger preterm birth or excessive post-natal inflammation during colonization with commensal flora, but may also contribute to neonatal susceptibility to infection with intracellular pathogens.
Notable exceptions to the impairment in TLR-mediated neonatal production of TH1-polarizing cytokines are agonists of TLR8. Synthetic TLR8 agonists, including imidazoquinolines and single-stranded RNAs induce marked neonatal production of TNF-α to adult levels revealing a signaling pathway that is fully functional at birth109. These observations raise the possibility that TLR8 agonists may have unique efficacy as stand alone immune response modifiers in newborns and/or as neonatal vaccine adjuvants, a major unmet medical need173.
In addition to altered signaling capacity and the effects of adenosine on cytokine production following TLR stimulation, alterations in the genes coding for cytokines and their receptors have the potential to contribute to the neonate’s increased susceptibility to EONS. Following the discovery of specific cytokine or cytokine receptor polymorphisms and their association with an increased risk for infection in older populations, neonates have been evaluated with mixed results174-180. Large, population-based studies will be necessary to more completely characterize the impact of genetic variation in cytokines and their receptors on the risk for development of neonatal infection.
Development of an effective inflammatory response is necessary to successfully respond to an infectious challenge. However, uncontrolled proinflammatory responses may lead to host tissue injury through excessive cellular activation. A recent evaluation in mice given a large intraperitoneal dose (10mg/kg) of endotoxin revealed an increase (>3 fold) in the relative systemic inflammatory response (serum TNF-α, MCP-1, and IL-6) in neonatal mice as compared to adult mice181. Another murine evaluation reported reduced IL-6 (3 fold) and elevated TNF-α (3 fold) in 1 day old neonates compared to adults in vivo two hours after subcutaneous LPS exposure182. The finding of elevated endotoxin-induced serum TNF-α in neonatal mice relative to adult mice is in contrast to previous reports in humans57, 170-172 and mice79, 80, may reflect specific features of the model or the timing of cytokine sampling, and will require further investigation.
Acute phase reactants
The production of inflammatory cytokines early during infection is associated with increased hepatic production of innate immune proteins known as acute phase reactants (APR). Predominantly induced by IL-6, the primary function of these components is to reduce host bacterial load through improved cellular recruitment, opsonin function, and direct antimicrobial activity. Examples include MBL, LBP, pentraxins (C-reactive protein [CRP] and serum amyloid A [SAA]), and fibronectin4, 63, 64, 169, 183-186. Of note, APRs have been studied in neonates with sepsis primarily to assess for diagnostic utility, rather than immunologic function. In particular, elevated plasma concentrations of CRP and LBP are often associated with EONS64, 187. Despite APR increases and the presence of maternally-derived immunoglobulin, neonates exhibit impaired opsonizing activity compared to adults which likely increases the risk for progression of infection188.
The coagulation cascade is intimately tied to inflammation, including complement activation, and is important in preventing microbial spread beyond the local environment. A microvascular pro-coagulant state develops via stimulation of monocytes, PMNs, platelets, and endothelium resulting in expression of tissue factor189, 190. Tissue factor-mediated activation of the coagulation cascade results in activation of thrombin-antithrombin complex, plasminogen activator inhibitor, and plasmin-a2-antiplasmin complex191, as well as inactivation of protein S and depletion of anticoagulant proteins including antithrombin III and protein C192, 193. Activated platelets may be consumed in clot formation and/or may also be removed from the circulation by the liver194 potentially resulting in thrombocytopenia, particularly during Gram-negative and fungal infections195-197. Systemic activation of coagulation is associated with consumption of clotting factors and increased risk of bleeding, inflammation, and disseminated intravascular coagulation (DIC)198. Inflammatory dysregulation and its effects on the coagulation system are demonstrated by the association of altered ratios of serum pro- and anti-inflammatory cytokines and the development of DIC in neonates170.
Passive immunity
Neonates have passively acquired antibodies via placental transfer with a significant increase beginning around 20 weeks gestation. As a result, preterm neonates have lower IgG levels as compared to term neonates [Figure 2a]; particularly IgG1 and IgG2 subclasses199. Examination of the impact of low immunoglobulin in preterm neonates 24-32 weeks gestational age (serum levels <400mg/dL total IgG at birth) revealed an increased risk for development of late-onset infection but not mortality compared to those with levels >400mg/dL after controlling for gestational age. There was a 15% increase in LONS risk in these infants for every 100mg/dL decrease in serum IgG below baseline that was not reduced with IVIg infusion200, 201. In the child or adult with deficient serum IgG1 (<100mg/dL)202, 203, there are also significant infectious consequences204. Interestingly, despite functional immune system limitations of both innate and adaptive immunity, most premature neonates do not develop overwhelming bacterial sepsis, even in the absence of significant levels of passively acquired immunoglobulins. Reliance on other means of innate immune defense likely provides the premature neonate with some degree of microbial control mechanisms.
Antimicrobial proteins and peptides
APPs represent the most phylogenetically ancient means of innate immune defense against microbial invasion. Present in nearly every organism including bacteria, plants, insects, non-mammalian vertebrates, and mammals, these small, often cationic peptides are capable of killing microbes of multiple types including viruses, bacteria, parasites, and fungi largely by disruption of the pathogen membrane205. Constitutive expression of APPs occurs in humans on barrier areas with consistent microbial exposure such as skin and mucosa [Figure 1]. Following microbial stimulation, both release of pre-formed APPs as well as inducible expression are thought to contribute to early host defense206. Importantly, there is no evidence to date for the development of microbial resistance to APPs that target fundamental components of the microbial cell wall. Some APPs can bind and neutralize microbial components such as endotoxin, precluding engagement with TLRs and other PRRs and thereby reducing inflammation.
Bactericidal/permeability-increasing protein (BPI) is a 55kDa protein present present in the respiratory tract, PMN primary granules, and blood plasma [Figure 1]. BPI exerts selective cytotoxic, anti-endotoxic, and opsonic activity against Gram-negative bacteria158. Lactoferrin is the major whey protein in mammalian milk (in particularly high concentrations in colostrum) and is important in innate immune host defenses. An 80kDa protein, lactoferrin is also present in tears and saliva and has antimicrobial activity both via binding iron (depriving microorganisms of this key nutrient) and by direct membrane perturbing activity. Lysozyme is present in tears, tracheal aspirates, skin, and in PMN primary and secondary granules and contributes to degradation of peptidoglycan of bacterial cell walls. Secretory phospholipase 2 (sPLA2) can destroy Gram-positive bacteria through hydrolysis of their membrane lipids207. PMN elastase is a serine protease released by activated PMNs with microbicidal function and is believed to play a role in the inflammatory damage seen with PMN recruitment, particularly in the lung89, 208. Cathelicidin and the defensins are other APPs that possess antimicrobial properties209. LL-37, the only known human cathelicidin, is present in the amniotic fluid, vernix, skin, saliva, respiratory tract, and leukocytes (PMNs, B cells, T cells, monocytes, and macrophages) [Figure 2B]. α-defensins are cysteine-rich 4 kDa peptides expressed in the amniotic fluid, vernix, spleen, cornea, thymus, paneth cells, and leukocytes (PMNs, monocytes, macrophages, and lymphocytes). β-defensins are found in skin, GI tract (salivary gland, tonsil, gastric antrum, stomach, liver, pancreas, small intestine, colon), reproductive organs and urinary tract (placenta, uterus, testes, kidney), respiratory tract, breast milk and mammary gland, and thymus. In addition to microbicidal action, APPs possess a wide range of immunomodulatory functions on multiple cell types from both the innate and adaptive immune system206, 210, 211. Immunomodulatory effects on macrophages, DC, monocytes, mast cells, PMNs, and epithelia attributed to APPs include altered cytokine and chemokine production, improved cellular chemotaxis and recruitment, improved cell function (maturation, activation, phagocytosis, ROI production), enhancement of wound healing (neovascularization, mitogenesis), and decreased apoptosis.
Plasma and intracellular contributions
The cytosolic granules of PMN are rich in APPs that can be released upon stimulation into the extracellular space including α-defensins, lactoferrin, lysozyme, LL-37, sPLA2, and BPI. Following release into the phagolysosome following phagocytosis or extracellularly into the surrounding tissue or blood, APPs contribute to microbial killing and binding of bacterial toxins such as LPS. Many APPs (LL-37, α/β-defensins, and BPI) can potentially reduce the intensity of the inflammatory response associated with the presence of bacterial toxins. Recently, gestational age-related decreases in the cord blood concentration of several APPs (LL-37, BPI, Calprotectin, sPLA2, α-defensins) were described in comparison to maternal serum levels [Figure 2b]212. Plasma APP deficiencies may contribute to the increased risk of infection associated with prematurity, and their absence may increase the risk of excessive levels of bacterial toxins. Up-regulation of APPs (defensins) occurs in blood of infected adults213 and children (defensins, lactoferrin)214. The effect of sepsis on the production of plasma APPs in neonates has not been investigated in detail. PMNs from term neonates produce similar concentrations of defensins but reduced BPI and elastase as compared to adults112, 134, 135. While term neonates demonstrate up-regulation of plasma BPI during infection, premature neonates showed a decreased ability to mobilize BPI upon stimulation215.,which may contribute to their risk for infection with Gram-negative bacteria. Polymorphisms in BPI increase the risk for Gram-negative sepsis in children, but the impact of these polymorphisms in neonates is unknown216.
Skin
APPs present in amniotic fluid, vernix, and on newborn skin including lysozyme, LL-37, ubiquitin, α/β-defensins, and psoriasin have antimicrobial efficacy against common neonatal pathogens such as Staphylococcus aureus, E. coli, Klebsiella spp, and GBS217-219. Expression of LL-37 and β-defensin 2 are elevated on neonatal skin as compared to adult skin220. A role for β-defensins in defense against vertical HIV transmission has been suggested by a recent single-nucleotide polymorphism study221. The common benign newborn skin lesions of erythema toxicum are associated with induction of LL-37 production42 demonstrating up-regulation following colonization of the skin with commensal flora including coagulase-negative Staphylococci42, 43. The increased concentration of APPs on neonatal skin as compared to adults and their up-regulation in response to colonization likely serves to improve immune protection for the fragile skin barrier [Figure 1]217, 222.
Airway
Airway protection is conferred in utero in part via amniotic APPs. In the absence of microbial invasion of the amniotic cavity, levels of calprotectin and BPI exhibit gestational age-dependent expression with much lower concentrations of amniotic fluid BPI levels in mid-trimester (14-18 week) fetuses223. Significant increases (>10 fold) in amniotic fluid APPs (BPI, α-defensins, and calprotectin) occur in the presence of intra-amniotic microbial infection and preterm labor 223 [Figure 1].
β-defensins 1-3, LL-37, lactoferrin, lysozyme, and BPI have been measured in neonatal tracheal aspirates. Reduced basal β-defensin concentrations in tracheal aspirates from preterm neonates may contribute to the risk of early pulmonary infection51. Mobilization of APPs is evident by increases in airway fluid concentrations of BPI, LL-37, and β-defensins that occur with mechanical ventilation, pneumonia, or systemic infection49, 206, 224. Reduced lactoferrin and lysozyme concentrations have been described in tracheal aspirates of neonates with BPD and may contribute to chronic cellular inflammation due to poor clearance of bacteria225.
GI tract
APPs are present in meconium and are constitutively secreted by GI epithelium (predominantly Paneth cells). Up-regulation of APPs occurs upon stimulation in neonatal intestine following microbial colonization226 and with the development of necrotizing enterocolitis (NEC)227. Premature neonates have fewer Paneth cells and express less APPs as compared to more mature neonates that may increase their risk for poor intestinal barrier function or the development of NEC206. Human milk is an important post-natal source of APPs, including lactoferrin, defensins (α and β), and LL-37228 that inhibit the growth of bacteria and may be inactivated by human milk fortifier229. No newborn formula preparation contains human-derived, bovine-derived, or synthetic APPs at this time. Although unlikely to have a significant impact on EONS because onset precedes significant oral intake, human milk-derived APPs likely participate in the reduction of late-onset infection seen in neonates fed human milk230.
Efforts to reduce EONS mortality and enhance innate defenses
Successful obstetric interventions directed at reducing the incidence and impact of EONS have included antimicrobial treatment of mothers with GBS colonization231, premature rupture of membranes232, 233, preterm premature rupture of membranes234, 235, and intra-amniotic infection236. For a complete review on attempts to enhance neonatal immunity, the reader is referred to prior publications3, 237 and to chapter XXX in this issue on neonatal adjuvants for prophylaxis and treatment of sepsis.
Activated protein C (aPC) administration demonstrated a modest reduction in mortality when given to adults with sepsis238. However, despite reduced plasma levels of aPC in septic neonates239, evaluations of aPC in children and infants with sepsis revealed no difference in mortality as compared to placebo but identified an increase in the risk for bleeding in infants < 60 days of age240.
Based on suboptimal neonatal PMN function, the presence of limited bone marrow PMN reserves, and the poor outcomes associated with septic neutropenia, cytokine therapy and granulocyte transfusions have been evaluated as prophylaxis to reduce the development of sepsis and as treatment to enhance neonatal immunity and sepsis survival. However, despite being well tolerated and increasing the number of effective circulating PMNs, granulocyte transfusions do not improve sepsis survival241. However, treatment with colony stimulating factor therapy (G-CSF, GM-CSF) in a subgroup (97 neonates) with documented culture-positive sepsis (largely due to Gram-negatives and GBS) and neutropenia (ANC<1700/μL) significantly reduced the risk of death (RR 0.34 [95% CI 0.12, 0.92]). Therefore colony stimulating factors may be beneficial under these specific circumstances although further studies focused on this sub-population and outcome are needed242, 243.
Future directions and translational opportunities
Biomarkers
Recent discoveries in neonatal immunology have brought to light some new diagnostic opportunities. New proteomic approaches can enhance detection of subclinical intra-amniotic infection, chorioamnionitis, and EONS through identification of specific inflammatory factors including IL-6 and select defensins and have helped to more completely characterize the immune response to in utero infection244, 245. Multiple biomarkers of inflammation including cytokines (IL-1β, IL-6, IL-8 [chemokine], IL-10, IL-18), APRs (CRP, procalcitonin, SAA, LBP), cell surface markers and molecules (E-selectin, P-selectin, VCAM-1, CD11b, CD64, HLA-DR, CD69), receptors (IL-2 soluble receptor, urokinase plasminogen-activated receptor), and enzymes (neutrophil elastase, urokinase plasminogen activator) have been explored to enhance diagnostic accuracy169, 187, 208, 246. A review of existing and novel diagnostic laboratory tests to aid in identification of septic neonates is covered in Chapters XXXX yyyyy and zzzzzzz within this issue.
Novel anti-infectives
The characterization of the properties of APPs have prompted significant biopharmaceutical development and investigation247. None of these therapies have been examined or are approved for use in neonates at present but hold promise for improvement of innate immune function. For example, development and use of skin preparations that contain APPs (synthetic or natural) may provide fragile neonatal skin with improved barrier function and capacity to protect against early microbial invasion with a resultant decrease in infectious risk. Administration of recombinant BPI congeners (e.g., rBPI21) with endotoxin neutralizing activity may reduce the deleterious actions of LPS via neutralization, reduce pro-inflammatory cytokines, and improve bacterial clearance via direct microbicidal activity158. Other APPs may also have potential to reduce sepsis mortality when administered systemically, and are under evaluation248. Pretreatment with LL-37 was recently evaluated in neonatal rats given systemic LPS249. Low dose systemic pretreatment with LL-37 reduced inflammation (decreased CRP levels) and mortality compared to sham but higher doses led to increased mortality. As with all new investigational agents, safety concerns will need to be thoroughly investigated in preclinical neonatal animal models prior to systemic administration to human neonates. Recently, administration of enteral bovine lactoferrin to preterm human newborns was associated with a reduction in the incidence of late-onset sepsis (bacterial and fungal)250.
Timely abrogation of the inflammatory response helps to prevent the spread of inflammation beyond the local environment to a systemic level where dangerous consequences can occur12. Simultaneous increases in anti-inflammatory cytokines such as IL-4, IL-10, IL-11, and IL-13 occur during sepsis and counter the actions of the pro-inflammatory cytokines169, 251-256. Cytokine and receptor antagonists also prevent ligand-receptor coupling and reduce the effects of inflammatory cytokines. Examples of these regulatory molecules elevated during sepsis include TNFR2, IL-6sR, sIL2, and IL-1ra197, 253, 257. Of note, despite apparent benefit in preclinical adult models of sepsis, anti-cytokine therapies aimed at reducing proinflammatory cytokines have been evaluated in infected human adults with minimal benefit and potential harm, likely reflecting the importance of TNF and other inflammatory cytokines to host defense 258. In neonates, reduction of excessive or sustained inflammation could theoretically decrease the frequency and intensity of devastating post-sepsis sequelae including chronic lung disease259, retinopathy of prematurity260, and white matter damage13.
Pulmonary infection and or intubation and subsequent ventilator-associated lung injury increase the likelihood of respiratory mucosal damage and loss of barrier integrity. Of note, intubated preterm newborns demonstrate accumulation of endotoxin in airway fluids corresponding to duration of intubation, raising the possibility that endotoxin contributes to pulmonary inflammation and its consequences49. In this context, improvement of pulmonary mucosal immune function through the administration of anti-infective proteins such as surfactant proteins A and D, β-defensins, or endotoxin antagonists may help to reduce local and systemic inflammation associated with pneumonia. Administration of recombinant surfactant protein D reduced pulmonary and systemic inflammation following administration of intra-tracheal LPS in a preterm animal model261. Caution is indicated as recent data showed that some APPs may bind SP-D, reducing its benefit, and may increase the risk for infection262.
Immune adjuvants
Sepsis survival benefit following innate immune priming via specific TLR agonists has been demonstrated in a neonatal preclinical model80. Priming with select TLR agonists resulted in improved cellular recruitment and function as well as decreased bacteremia and significantly improved sepsis survival over sham-primed septic neonates. Further evaluation, including assessment of safety, will be necessary before such positive immunomodulation reaches phase I trials in humans. Of note, vaccination with BCG, a live attenuated strain of Mycobacterium bovis with TLR2/4 agonist activity, was associated with an improvement in survival in low birth weight neonates (not related to tuberculosis infection) compared to those that did not receive the vaccine and may represent an existing example of the benefits and safety of innate immune priming263.
Summary
Neonatal EONS continues to take a devastating global toll. The innate immune system is distinct at birth and although usually adequate, does leave the newborn at increased risk of infection. Unique differences exist between newborns and older individuals with respect to capacity, quantity, and quality of innate host responses to pathogens. Characterization of the age-dependent maturation of neonatal innate immune function has identified novel translational approaches that may lead to improved diagnostic, prophylactic and therapeutic approaches.
Acknowledgements
We thank Patrick Bibbins at Children’s Hospital Boston for creating Fig. 1. OL acknowledges the mentorship of Drs. Michael Wessels, Peter Elsbach, Jerrold Weiss, Phillip Pizzo, Eva Guinan, and Raif Geha. OL’s laboratory is funded by NIH RO1 AI067353 and by the Bill & Melinda Gates Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lawn JE, Cousens S, Zupan J. 4 million neonatal deaths: when? Where? Why? Lancet. 2005 Mar 5-11;365(9462):891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 2.Martinot A, Leclerc F, Cremer R, Leteurtre S, Fourier C, Hue V. Sepsis in neonates and children: definitions, epidemiology, and outcome. Pediatr Emerg Care. 1997 Aug;13(4):277–281. doi: 10.1097/00006565-199708000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Wynn JL, Neu J, Moldawer LL, Levy O. Potential of immunomodulatory agents for prevention and treatment of neonatal sepsis. J Perinatol. 2009 Feb;29(2):79–88. doi: 10.1038/jp.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007 May;7(5):379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 5.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004 Jul;4(7):553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 6.Kollmann TR, Crabtree J, Rein-Weston A, et al. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol. 2009 Dec 1;183(11):7150–7160. doi: 10.4049/jimmunol.0901481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rittirsch D, Flierl MA, Ward PA. Harmful molecular mechanisms in sepsis. Nat Rev Immunol. 2008 Oct;8(10):776–787. doi: 10.1038/nri2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003 Jan 9;348(2):138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 9.Abraham E, Singer M. Mechanisms of sepsis-induced organ dysfunction. Crit Care Med. 2007 Oct;35(10):2408–2416. doi: 10.1097/01.ccm.0000282072.56245.91. [DOI] [PubMed] [Google Scholar]
- 10.Cinel I, Dellinger RP. Advances in pathogenesis and management of sepsis. Curr Opin Infect Dis. 2007 Aug;20(4):345–352. doi: 10.1097/QCO.0b013e32818be70a. [DOI] [PubMed] [Google Scholar]
- 11.Jean-Baptiste E. Cellular mechanisms in sepsis. J Intensive Care Med. 2007 Mar-Apr;22(2):63–72. doi: 10.1177/0885066606297123. [DOI] [PubMed] [Google Scholar]
- 12.Sriskandan S, Altmann DM. The immunology of sepsis. J Pathol. 2008 Jan;214(2):211–223. doi: 10.1002/path.2274. [DOI] [PubMed] [Google Scholar]
- 13.Shah DK, Doyle LW, Anderson PJ, et al. Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. J Pediatr. 2008 Aug;153(2):170–175. doi: 10.1016/j.jpeds.2008.02.033. 175 e171. [DOI] [PubMed] [Google Scholar]
- 14.Palazzi D, Klein J, Baker C. Bacterial Sepsis and Meningitis. In: Remington, Klein, Wilson, Baker, editors. Infectious Diseases of the Fetus and Newborn Infant. 6th ed. Elsevier Saunders; Philadelphia: 2006. [Google Scholar]
- 15.Benitz WE, Gould JB, Druzin ML. Risk factors for early-onset group B streptococcal sepsis: estimation of odds ratios by critical literature review. Pediatrics. 1999 Jun;103(6):e77. doi: 10.1542/peds.103.6.e77. [DOI] [PubMed] [Google Scholar]
- 16.Bizzarro MJ, Dembry LM, Baltimore RS, Gallagher PG. Changing patterns in neonatal Escherichia coli sepsis and ampicillin resistance in the era of intrapartum antibiotic prophylaxis. Pediatrics. 2008 Apr;121(4):689–696. doi: 10.1542/peds.2007-2171. [DOI] [PubMed] [Google Scholar]
- 17.Hyde TB, Hilger TM, Reingold A, Farley MM, O’Brien KL, Schuchat A. Trends in incidence and antimicrobial resistance of early-onset sepsis: population-based surveillance in San Francisco and Atlanta. Pediatrics. 2002 Oct;110(4):690–695. doi: 10.1542/peds.110.4.690. [DOI] [PubMed] [Google Scholar]
- 18.Moore MR, Schrag SJ, Schuchat A. Effects of intrapartum antimicrobial prophylaxis for prevention of group-B-streptococcal disease on the incidence and ecology of early-onset neonatal sepsis. Lancet Infect Dis. 2003 Apr;3(4):201–213. doi: 10.1016/s1473-3099(03)00577-2. [DOI] [PubMed] [Google Scholar]
- 19.Anthony BF, Okada DM. The emergence of group B streptococci in infections of the newborn infant. Annu Rev Med. 1977;28:355–369. doi: 10.1146/annurev.me.28.020177.002035. [DOI] [PubMed] [Google Scholar]
- 20.American Academy of Pediatrics Committee on Infectious Diseases and Committee on Fetus and Newborn: Guidelines for prevention of group B streptococcal (GBS) infection by chemoprophylaxis. Pediatrics. 1992 Nov;90(5):775–778. [PubMed] [Google Scholar]
- 21.Trends in perinatal group B streptococcal disease - United States, 2000-2006. MMWR Morb Mortal Wkly Rep. 2009 Feb 13;58(5):109–112. [PubMed] [Google Scholar]
- 22.Cotten CM, Taylor S, Stoll B, et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. 2009 Jan;123(1):58–66. doi: 10.1542/peds.2007-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoll BJ, Hansen NI, Higgins RD, et al. Very low birth weight preterm infants with early onset neonatal sepsis: the predominance of gram-negative infections continues in the National Institute of Child Health and Human Development Neonatal Research Network, 2002-2003. Pediatr Infect Dis J. 2005 Jul;24(7):635–639. doi: 10.1097/01.inf.0000168749.82105.64. [DOI] [PubMed] [Google Scholar]
- 24.Stoll BJ, Hansen N. Infections in VLBW infants: studies from the NICHD Neonatal Research Network. Semin Perinatol. 2003 Aug;27(4):293–301. doi: 10.1016/s0146-0005(03)00046-6. [DOI] [PubMed] [Google Scholar]
- 25.Stoll BJ, Hansen N, Fanaroff AA, et al. Changes in pathogens causing early-onset sepsis in very-low-birth-weight infants. N Engl J Med. 2002 Jul 25;347(4):240–247. doi: 10.1056/NEJMoa012657. [DOI] [PubMed] [Google Scholar]
- 26.Barton L, Hodgman JE, Pavlova Z. Causes of death in the extremely low birth weight infant. Pediatrics. 1999 Feb;103(2):446–451. doi: 10.1542/peds.103.2.446. [DOI] [PubMed] [Google Scholar]
- 27.Holladay SD, Smialowicz RJ. Development of the murine and human immune system: differential effects of immunotoxicants depend on time of exposure. Environ Health Perspect. 2000 Jun;108(Suppl 3):463–473. doi: 10.1289/ehp.00108s3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koga K, Aldo PB, Mor G. Toll-like receptors and pregnancy: trophoblast as modulators of the immune response. J Obstet Gynaecol Res. 2009 Apr;35(2):191–202. doi: 10.1111/j.1447-0756.2008.00963.x. [DOI] [PubMed] [Google Scholar]
- 29.Sampson JE, Theve RP, Blatman RN, et al. Fetal origin of amniotic fluid polymorphonuclear leukocytes. Am J Obstet Gynecol. 1997 Jan;176(1 Pt 1):77–81. doi: 10.1016/s0002-9378(97)80015-4. [DOI] [PubMed] [Google Scholar]
- 30.Gotsch F, Romero R, Kusanovic JP, et al. The fetal inflammatory response syndrome. Clin Obstet Gynecol. 2007 Sep;50(3):652–683. doi: 10.1097/GRF.0b013e31811ebef6. [DOI] [PubMed] [Google Scholar]
- 31.Saji F, Samejima Y, Kamiura S, Sawai K, Shimoya K, Kimura T. Cytokine production in chorioamnionitis. J Reprod Immunol. 2000 Jul;47(2):185–196. doi: 10.1016/s0165-0378(00)00064-4. [DOI] [PubMed] [Google Scholar]
- 32.Kemp B, Winkler M, Maas A, et al. Cytokine concentrations in the amniotic fluid during parturition at term: correlation to lower uterine segment values and to labor. Acta Obstet Gynecol Scand. 2002 Oct;81(10):938–942. doi: 10.1034/j.1600-0412.2002.811007.x. [DOI] [PubMed] [Google Scholar]
- 33.Kallapur SG, Willet KE, Jobe AH, Ikegami M, Bachurski CJ. Intra-amniotic endotoxin: chorioamnionitis precedes lung maturation in preterm lambs. Am J Physiol Lung Cell Mol Physiol. 2001 Mar;280(3):L527–536. doi: 10.1152/ajplung.2001.280.3.L527. [DOI] [PubMed] [Google Scholar]
- 34.Erez O, Romero R, Tarca AL, et al. Differential expression pattern of genes encoding for anti-microbial peptides in the fetal membranes of patients with spontaneous preterm labor and intact membranes and those with preterm prelabor rupture of the membranes. J Matern Fetal Neonatal Med. 2009 Jul 8;:1–13. doi: 10.3109/14767050902994796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leth-Larsen R, Floridon C, Nielsen O, Holmskov U. Surfactant protein D in the female genital tract. Mol Hum Reprod. 2004 Mar;10(3):149–154. doi: 10.1093/molehr/gah022. [DOI] [PubMed] [Google Scholar]
- 36.King AE, Kelly RW, Sallenave JM, Bocking AD, Challis JR. Innate immune defences in the human uterus during pregnancy. Placenta. 2007 Nov-Dec;28(11-12):1099–1106. doi: 10.1016/j.placenta.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 37.Tollin M, Bergsson G, Kai-Larsen Y, et al. Vernix caseosa as a multi-component defence system based on polypeptides, lipids and their interactions. Cell Mol Life Sci. 2005 Oct;62(19-20):2390–2399. doi: 10.1007/s00018-005-5260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Visscher MO, Narendran V, Pickens WL, et al. Vernix caseosa in neonatal adaptation. J Perinatol. 2005 Jul;25(7):440–446. doi: 10.1038/sj.jp.7211305. [DOI] [PubMed] [Google Scholar]
- 39.Rutter N. Clinical consequences of an immature barrier. Semin Neonatol. 2000 Nov;5(4):281–287. doi: 10.1053/siny.2000.0014. [DOI] [PubMed] [Google Scholar]
- 40.Evans NJ, Rutter N. Development of the epidermis in the newborn. Biol Neonate. 1986;49(2):74–80. doi: 10.1159/000242513. [DOI] [PubMed] [Google Scholar]
- 41.Kalia YN, Nonato LB, Lund CH, Guy RH. Development of skin barrier function in premature infants. J Invest Dermatol. 1998 Aug;111(2):320–326. doi: 10.1046/j.1523-1747.1998.00289.x. [DOI] [PubMed] [Google Scholar]
- 42.Marchini G, Lindow S, Brismar H, et al. The newborn infant is protected by an innate antimicrobial barrier: peptide antibiotics are present in the skin and vernix caseosa. Br J Dermatol. 2002 Dec;147(6):1127–1134. doi: 10.1046/j.1365-2133.2002.05014.x. [DOI] [PubMed] [Google Scholar]
- 43.Marchini G, Nelson A, Edner J, Lonne-Rahm S, Stavreus-Evers A, Hultenby K. Erythema toxicum neonatorum is an innate immune response to commensal microbes penetrated into the skin of the newborn infant. Pediatr Res. 2005 Sep;58(3):613–616. doi: 10.1203/01.pdr.0000176836.27156.32. [DOI] [PubMed] [Google Scholar]
- 44.Lewis D, Wilson C. Developmental Immunology and Role of Host Defenses in Fetal and Neonatal Susceptibility to Infection. In: Remington, Klein, Wilson, Baker, editors. Infectious Diseases of the Fetus and Newborn Infant. 6th ed. Elsevier Saunders; Philadelphia: 2006. [Google Scholar]
- 45.Martin CR, Walker WA. Probiotics: role in pathophysiology and prevention in necrotizing enterocolitis. Semin Perinatol. 2008 Apr;32(2):127–137. doi: 10.1053/j.semperi.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 46.Sharma R, Tepas JJ, 3rd, Hudak ML, et al. Neonatal gut barrier and multiple organ failure: role of endotoxin and proinflammatory cytokines in sepsis and necrotizing enterocolitis. J Pediatr Surg. 2007 Mar;42(3):454–461. doi: 10.1016/j.jpedsurg.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 47.Neu J. Gastrointestinal maturation and implications for infant feeding. Early Hum Dev. 2007 Dec;83(12):767–775. doi: 10.1016/j.earlhumdev.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 48.Bartlett JA, Fischer AJ, McCray PB., Jr. Innate immune functions of the airway epithelium. Contrib Microbiol. 2008;15:147–163. doi: 10.1159/000136349. [DOI] [PubMed] [Google Scholar]
- 49.Nathe KE, Parad R, Van Marter LJ, et al. Endotoxin-directed innate immunity in tracheal aspirates of mechanically ventilated human neonates. Pediatr Res. 2009 Aug;66(2):191–196. doi: 10.1203/PDR.0b013e3181aa33d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pfister RH, Soll RF. New synthetic surfactants: the next generation? Biol Neonate. 2005;87(4):338–344. doi: 10.1159/000084882. [DOI] [PubMed] [Google Scholar]
- 51.Starner TD, Agerberth B, Gudmundsson GH, McCray PB., Jr. Expression and activity of beta-defensins and LL-37 in the developing human lung. J Immunol. 2005 Feb 1;174(3):1608–1615. doi: 10.4049/jimmunol.174.3.1608. [DOI] [PubMed] [Google Scholar]
- 52.Brown GD. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat Rev Immunol. 2006 Jan;6(1):33–43. doi: 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- 53.Kawai T, Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol. 2009 Apr;21(4):317–337. doi: 10.1093/intimm/dxp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kumagai Y, Takeuchi O, Akira S. Pathogen recognition by innate receptors. J Infect Chemother. 2008 Apr;14(2):86–92. doi: 10.1007/s10156-008-0596-1. [DOI] [PubMed] [Google Scholar]
- 55.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007 Mar;7(3):179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 56.Krumbiegel D, Zepp F, Meyer CU. Combined Toll-like receptor agonists synergistically increase production of inflammatory cytokines in human neonatal dendritic cells. Hum Immunol. 2007 Oct;68(10):813–822. doi: 10.1016/j.humimm.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 57.Levy O, Zarember KA, Roy RM, Cywes C, Godowski PJ, Wessels MR. Selective impairment of TLR-mediated innate immunity in human newborns: neonatal blood plasma reduces monocyte TNF-alpha induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod, but preserves the response to R-848. J Immunol. 2004 Oct 1;173(7):4627–4634. doi: 10.4049/jimmunol.173.7.4627. [DOI] [PubMed] [Google Scholar]
- 58.Forster-Waldl E, Sadeghi K, Tamandl D, et al. Monocyte toll-like receptor 4 expression and LPS-induced cytokine production increase during gestational aging. Pediatr Res. 2005 Jul;58(1):121–124. doi: 10.1203/01.PDR.0000163397.53466.0F. [DOI] [PubMed] [Google Scholar]
- 59.Zhang JP, Chen C, Yang Y. Changes and clinical significance of Toll-like receptor 2 and 4 expression in neonatal infections. Zhonghua Er Ke Za Zhi. 2007 Feb;45(2):130–133. [PubMed] [Google Scholar]
- 60.Hartel C, Rupp J, Hoegemann A, et al. 159C>T CD14 genotype--functional effects on innate immune responses in term neonates. Hum Immunol. 2008 Jun;69(6):338–343. doi: 10.1016/j.humimm.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 61.Mollen KP, Gribar SC, Anand RJ, et al. Increased expression and internalization of the endotoxin coreceptor CD14 in enterocytes occur as an early event in the development of experimental necrotizing enterocolitis. J Pediatr Surg. 2008 Jun;43(6):1175–1181. doi: 10.1016/j.jpedsurg.2008.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hubacek JA, Stuber F, Frohlich D, et al. Gene variants of the bactericidal/permeability increasing protein and lipopolysaccharide binding protein in sepsis patients: gender-specific genetic predisposition to sepsis. Crit Care Med. 2001 Mar;29(3):557–561. doi: 10.1097/00003246-200103000-00015. [DOI] [PubMed] [Google Scholar]
- 63.Behrendt D, Dembinski J, Heep A, Bartmann P. Lipopolysaccharide binding protein in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2004 Nov;89(6):F551–554. doi: 10.1136/adc.2003.030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Berner R, Furll B, Stelter F, Drose J, Muller HP, Schutt C. Elevated levels of lipopolysaccharide-binding protein and soluble CD14 in plasma in neonatal early-onset sepsis. Clin Diagn Lab Immunol. 2002 Mar;9(2):440–445. doi: 10.1128/CDLI.9.2.440-445.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blanco A, Solis G, Arranz E, Coto GD, Ramos A, Telleria J. Serum levels of CD14 in neonatal sepsis by Gram-positive and Gram-negative bacteria. Acta Paediatr. 1996 Jun;85(6):728–732. doi: 10.1111/j.1651-2227.1996.tb14135.x. [DOI] [PubMed] [Google Scholar]
- 66.Warren SE, Mao DP, Rodriguez AE, Miao EA, Aderem A. Multiple Nod-like receptors activate caspase 1 during Listeria monocytogenes infection. J Immunol. 2008 Jun 1;180(11):7558–7564. doi: 10.4049/jimmunol.180.11.7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goldbach-Mansky R, Dailey NJ, Canna SW, et al. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. N Engl J Med. 2006 Aug 10;355(6):581–592. doi: 10.1056/NEJMoa055137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Szebeni B, Szekeres R, Rusai K, et al. Genetic polymorphisms of CD14, toll-like receptor 4, and caspase-recruitment domain 15 are not associated with necrotizing enterocolitis in very low birth weight infants. J Pediatr Gastroenterol Nutr. 2006 Jan;42(1):27–31. doi: 10.1097/01.mpg.0000192246.47959.b2. [DOI] [PubMed] [Google Scholar]
- 69.Bochud PY, Chien JW, Marr KA, et al. Toll-like receptor 4 polymorphisms and aspergillosis in stem-cell transplantation. N Engl J Med. 2008 Oct 23;359(17):1766–1777. doi: 10.1056/NEJMoa0802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wurfel MM, Gordon AC, Holden TD, et al. Toll-like receptor 1 polymorphisms affect innate immune responses and outcomes in sepsis. Am J Respir Crit Care Med. 2008 Oct 1;178(7):710–720. doi: 10.1164/rccm.200803-462OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Agnese DM, Calvano JE, Hahm SJ, et al. Human toll-like receptor 4 mutations but not CD14 polymorphisms are associated with an increased risk of gram-negative infections. J Infect Dis. 2002 Nov 15;186(10):1522–1525. doi: 10.1086/344893. [DOI] [PubMed] [Google Scholar]
- 72.Lorenz E, Mira JP, Cornish KL, Arbour NC, Schwartz DA. A novel polymorphism in the toll-like receptor 2 gene and its potential association with staphylococcal infection. Infect Immun. 2000 Nov;68(11):6398–6401. doi: 10.1128/iai.68.11.6398-6401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.von Bernuth H, Picard C, Jin Z, et al. Pyogenic bacterial infections in humans with MyD88 deficiency. Science. 2008 Aug 1;321(5889):691–696. doi: 10.1126/science.1158298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Picard C, Puel A, Bonnet M, et al. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science. 2003 Mar 28;299(5615):2076–2079. doi: 10.1126/science.1081902. [DOI] [PubMed] [Google Scholar]
- 75.Zhang SY, Jouanguy E, Ugolini S, et al. TLR3 deficiency in patients with herpes simplex encephalitis. Science. 2007 Sep 14;317(5844):1522–1527. doi: 10.1126/science.1139522. [DOI] [PubMed] [Google Scholar]
- 76.Mockenhaupt FP, Cramer JP, Hamann L, et al. Toll-like receptor (TLR) polymorphisms in African children: common TLR-4 variants predispose to severe malaria. J Commun Dis. 2006 Mar;38(3):230–245. [PubMed] [Google Scholar]
- 77.Faber J, Meyer CU, Gemmer C, et al. Human toll-like receptor 4 mutations are associated with susceptibility to invasive meningococcal disease in infancy. Pediatr Infect Dis J. 2006 Jan;25(1):80–81. doi: 10.1097/01.inf.0000195595.22547.fe. [DOI] [PubMed] [Google Scholar]
- 78.Ku CL, von Bernuth H, Picard C, et al. Selective predisposition to bacterial infections in IRAK-4-deficient children: IRAK-4-dependent TLRs are otherwise redundant in protective immunity. J Exp Med. 2007 Oct 1;204(10):2407–2422. doi: 10.1084/jem.20070628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wynn JL, Scumpia PO, Delano MJ, et al. Increased mortality and altered immunity in neonatal sepsis produced by generalized peritonitis. Shock. 2007 Dec;28(6):675–683. doi: 10.1097/SHK.0b013e3180556d09. [DOI] [PubMed] [Google Scholar]
- 80.Wynn JL, Scumpia PO, Winfield RD, et al. Defective innate immunity predisposes murine neonates to poor sepsis outcome but is reversed by TLR agonists. Blood. 2008 Sep 1;112(5):1750–1758. doi: 10.1182/blood-2008-01-130500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Salomao R, Brunialti MK, Gomes NE, et al. Toll-like receptor pathway signaling is differently regulated in neutrophils and peripheral mononuclear cells of patients with sepsis, severe sepsis, and septic shock. Crit Care Med. 2009 Jan;37(1):132–139. doi: 10.1097/CCM.0b013e318192fbaf. [DOI] [PubMed] [Google Scholar]
- 82.Al-Hertani W, Yan SR, Byers DM, Bortolussi R. Human newborn polymorphonuclear neutrophils exhibit decreased levels of MyD88 and attenuated p38 phosphorylation in response to lipopolysaccharide. Clin Invest Med. 2007;30(2):E44–53. doi: 10.25011/cim.v30i2.979. [DOI] [PubMed] [Google Scholar]
- 83.Sadeghi K, Berger A, Langgartner M, et al. Immaturity of infection control in preterm and term newborns is associated with impaired toll-like receptor signaling. J Infect Dis. 2007 Jan 15;195(2):296–302. doi: 10.1086/509892. [DOI] [PubMed] [Google Scholar]
- 84.Yan SR, Qing G, Byers DM, Stadnyk AW, Al-Hertani W, Bortolussi R. Role of MyD88 in diminished tumor necrosis factor alpha production by newborn mononuclear cells in response to lipopolysaccharide. Infect Immun. 2004 Mar;72(3):1223–1229. doi: 10.1128/IAI.72.3.1223-1229.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ehlers MR. CR3: a general purpose adhesion-recognition receptor essential for innate immunity. Microbes Infect. 2000 Mar;2(3):289–294. doi: 10.1016/s1286-4579(00)00299-9. [DOI] [PubMed] [Google Scholar]
- 86.McEvoy LT, Zakem-Cloud H, Tosi MF. Total cell content of CR3 (CD11b/CD18) and LFA-1 (CD11a/CD18) in neonatal neutrophils: relationship to gestational age. Blood. 1996 May 1;87(9):3929–3933. [PubMed] [Google Scholar]
- 87.Buhrer C, Graulich J, Stibenz D, Dudenhausen JW, Obladen M. L-selectin is down-regulated in umbilical cord blood granulocytes and monocytes of newborn infants with acute bacterial infection. Pediatr Res. 1994 Dec;36(6):799–804. doi: 10.1203/00006450-199412000-00020. [DOI] [PubMed] [Google Scholar]
- 88.Kim SK, Keeney SE, Alpard SK, Schmalstieg FC. Comparison of L-selectin and CD11b on neutrophils of adults and neonates during the first month of life. Pediatr Res. 2003 Jan;53(1):132–136. doi: 10.1203/00006450-200301000-00022. [DOI] [PubMed] [Google Scholar]
- 89.Nupponen I, Pesonen E, Andersson S, et al. Neutrophil activation in preterm infants who have respiratory distress syndrome. Pediatrics. 2002 Jul;110(1 Pt 1):36–41. doi: 10.1542/peds.110.1.36. [DOI] [PubMed] [Google Scholar]
- 90.Nathan CF. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Willems F, Vollstedt S, Suter M. Phenotype and function of neonatal DC. Eur J Immunol. 2009 Jan;39(1):26–35. doi: 10.1002/eji.200838391. [DOI] [PubMed] [Google Scholar]
- 92.Angelone DF, Wessels MR, Coughlin M, et al. Innate immunity of the human newborn is polarized toward a high ratio of IL-6/TNF-alpha production in vitro and in vivo. Pediatr Res. 2006 Aug;60(2):205–209. doi: 10.1203/01.pdr.0000228319.10481.ea. [DOI] [PubMed] [Google Scholar]
- 93.Levy O, Coughlin M, Cronstein BN, Roy RM, Desai A, Wessels MR. The adenosine system selectively inhibits TLR-mediated TNF-alpha production in the human newborn. J Immunol. 2006 Aug 1;177(3):1956–1966. doi: 10.4049/jimmunol.177.3.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Marodi L. Innate cellular immune responses in newborns. Clin Immunol. 2006 Feb-Mar;118(2-3):137–144. doi: 10.1016/j.clim.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 95.Marodi L. Down-regulation of Th1 responses in human neonates. Clin Exp Immunol. 2002 Apr;128(1):1–2. doi: 10.1046/j.1365-2249.2002.01873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ottenhoff TH, De Boer T, van Dissel JT, Verreck FA. Human deficiencies in type-1 cytokine receptors reveal the essential role of type-1 cytokines in immunity to intracellular bacteria. Adv Exp Med Biol. 2003;531:279–294. doi: 10.1007/978-1-4615-0059-9_24. [DOI] [PubMed] [Google Scholar]
- 97.Whitley R, Arvin A, Prober C, et al. The National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group Predictors of morbidity and mortality in neonates with herpes simplex virus infections. N Engl J Med. 1991 Feb 14;324(7):450–454. doi: 10.1056/NEJM199102143240704. [DOI] [PubMed] [Google Scholar]
- 98.Jones CA, Holloway JA, Warner JO. Phenotype of fetal monocytes and B lymphocytes during the third trimester of pregnancy. J Reprod Immunol. 2002 Jul-Aug;56(1-2):45–60. doi: 10.1016/s0165-0378(02)00022-0. [DOI] [PubMed] [Google Scholar]
- 99.Hunt DW, Huppertz HI, Jiang HJ, Petty RE. Studies of human cord blood dendritic cells: evidence for functional immaturity. Blood. 1994 Dec 15;84(12):4333–4343. [PubMed] [Google Scholar]
- 100.Velilla PA, Rugeles MT, Chougnet CA. Defective antigen-presenting cell function in human neonates. Clin Immunol. 2006 Dec;121(3):251–259. doi: 10.1016/j.clim.2006.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Krumbiegel D, Rohr J, Schmidtke P, Knuf M, Zepp F, Meyer CU. Efficient maturation and cytokine production of neonatal DCs requires combined proinflammatory signals. Clin Dev Immunol. 2005 Jun;12(2):99–105. doi: 10.1080/17402520500116772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wong OH, Huang FP, Chiang AK. Differential responses of cord and adult blood-derived dendritic cells to dying cells. Immunology. 2005 Sep;116(1):13–20. doi: 10.1111/j.1365-2567.2005.02191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Salio M, Dulphy N, Renneson J, et al. Efficient priming of antigen-specific cytotoxic T lymphocytes by human cord blood dendritic cells. Int Immunol. 2003 Oct;15(10):1265–1273. doi: 10.1093/intimm/dxg123. [DOI] [PubMed] [Google Scholar]
- 104.Hallwirth U, Pomberger G, Zaknun D, et al. Monocyte phagocytosis as a reliable parameter for predicting early-onset sepsis in very low birthweight infants. Early Hum Dev. 2002 Apr;67(1-2):1–9. doi: 10.1016/s0378-3782(01)00245-6. [DOI] [PubMed] [Google Scholar]
- 105.Klein RB, Fischer TJ, Gard SE, Biberstein M, Rich KC, Stiehm ER. Decreased mononuclear and polymorphonuclear chemotaxis in human newborns, infants, and young children. Pediatrics. 1977 Oct;60(4):467–472. [PubMed] [Google Scholar]
- 106.Chelvarajan L, Popa D, Liu Y, Getchell TV, Stromberg AJ, Bondada S. Molecular mechanisms underlying anti-inflammatory phenotype of neonatal splenic macrophages. J Leukoc Biol. 2007 Aug;82(2):403–416. doi: 10.1189/jlb.0107071. [DOI] [PubMed] [Google Scholar]
- 107.Aikio O, Vuopala K, Pokela ML, Hallman M. Diminished inducible nitric oxide synthase expression in fulminant early-onset neonatal pneumonia. Pediatrics. 2000 May;105(5):1013–1019. doi: 10.1542/peds.105.5.1013. [DOI] [PubMed] [Google Scholar]
- 108.Levy O, Jean-Jacques RM, Cywes C, et al. Critical role of the complement system in group B streptococcus-induced tumor necrosis factor alpha release. Infect Immun. 2003 Nov;71(11):6344–6353. doi: 10.1128/IAI.71.11.6344-6353.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Levy O, Suter EE, Miller RL, Wessels MR. Unique efficacy of Toll-like receptor 8 agonists in activating human neonatal antigen-presenting cells. Blood. 2006 Aug 15;108(4):1284–1290. doi: 10.1182/blood-2005-12-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vekemans J, Amedei A, Ota MO, et al. Neonatal bacillus Calmette-Guerin vaccination induces adult-like IFN-gamma production by CD4+ T lymphocytes. Eur J Immunol. 2001 May;31(5):1531–1535. doi: 10.1002/1521-4141(200105)31:5<1531::AID-IMMU1531>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 111.Dale DC, Boxer L, Liles WC. The phagocytes: neutrophils and monocytes. Blood. 2008 Aug 15;112(4):935–945. doi: 10.1182/blood-2007-12-077917. [DOI] [PubMed] [Google Scholar]
- 112.Urlichs F. Neutrophil function in preterm and term infants. NeoReviews. 2004;5:e417–e430. SC. [Google Scholar]
- 113.Weinschenk NP, Farina A, Bianchi DW. Premature infants respond to early-onset and late-onset sepsis with leukocyte activation. J Pediatr. 2000 Sep;137(3):345–350. doi: 10.1067/mpd.2000.107846. [DOI] [PubMed] [Google Scholar]
- 114.Engle WA, McGuire WA, Schreiner RL, Yu PL. Neutrophil storage pool depletion in neonates with sepsis and neutropenia. J Pediatr. 1988 Oct;113(4):747–749. doi: 10.1016/s0022-3476(88)80394-9. [DOI] [PubMed] [Google Scholar]
- 115.Christensen RD, Rothstein G. Exhaustion of mature marrow neutrophils in neonates with sepsis. J Pediatr. 1980 Feb;96(2):316–318. doi: 10.1016/s0022-3476(80)80837-7. [DOI] [PubMed] [Google Scholar]
- 116.Christensen RD, Bradley PP, Rothstein G. The leukocyte left shift in clinical and experimental neonatal sepsis. J Pediatr. 1981 Jan;98(1):101–105. doi: 10.1016/s0022-3476(81)80553-7. [DOI] [PubMed] [Google Scholar]
- 117.Squire E, Favara B, Todd J. Diagnosis of neonatal bacterial infection: hematologic and pathologic findings in fatal and nonfatal cases. Pediatrics. 1979 Jul;64(1):60–64. [PubMed] [Google Scholar]
- 118.Doron MW, Makhlouf RA, Katz VL, Lawson EE, Stiles AD. Increased incidence of sepsis at birth in neutropenic infants of mothers with preeclampsia. J Pediatr. 1994 Sep;125(3):452–458. doi: 10.1016/s0022-3476(05)83294-9. [DOI] [PubMed] [Google Scholar]
- 119.Gray PH, Rodwell RL. Neonatal neutropenia associated with maternal hypertension poses a risk for nosocomial infection. Eur J Pediatr. 1999 Jan;158(1):71–73. doi: 10.1007/s004310051013. [DOI] [PubMed] [Google Scholar]
- 120.Paul DA, Leef KH, Sciscione A, Tuttle DJ, Stefano JL. Preeclampsia does not increase the risk for culture proven sepsis in very low birth weight infants. Am J Perinatol. 1999;16(7):365–372. doi: 10.1055/s-2007-993886. [DOI] [PubMed] [Google Scholar]
- 121.Teng RJ, Wu TJ, Garrison RD, Sharma R, Hudak ML. Early neutropenia is not associated with an increased rate of nosocomial infection in very low-birth-weight infants. J Perinatol. 2009 Mar;29(3):219–224. doi: 10.1038/jp.2008.202. [DOI] [PubMed] [Google Scholar]
- 122.Christensen RD, Henry E, Wiedmeier SE, Stoddard RA, Lambert DK. Low blood neutrophil concentrations among extremely low birth weight neonates: data from a multihospital health-care system. J Perinatol. 2006 Nov;26(11):682–687. doi: 10.1038/sj.jp.7211603. [DOI] [PubMed] [Google Scholar]
- 123.Turkmen M, Satar M, Atici A. Neutrophil chemotaxis and random migration in preterm and term infants with sepsis. Am J Perinatol. 2000;17(2):107–112. doi: 10.1055/s-2000-9507. [DOI] [PubMed] [Google Scholar]
- 124.Merry C, Puri P, Reen DJ. Defective neutrophil actin polymerisation and chemotaxis in stressed newborns. J Pediatr Surg. 1996 Apr;31(4):481–485. doi: 10.1016/s0022-3468(96)90479-0. [DOI] [PubMed] [Google Scholar]
- 125.Meade VM, Barese CN, Kim C, et al. Rac2 concentrations in umbilical cord neutrophils. Biol Neonate. 2006;90(3):156–159. doi: 10.1159/000092451. [DOI] [PubMed] [Google Scholar]
- 126.Xing Z, Gauldie J, Cox G, et al. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest. 1998 Jan 15;101(2):311–320. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hickey MJ, Kubes P. Intravascular immunity: the host-pathogen encounter in blood vessels. Nat Rev Immunol. 2009 May;9(5):364–375. doi: 10.1038/nri2532. [DOI] [PubMed] [Google Scholar]
- 128.Yektaei-Karin E, Moshfegh A, Lundahl J, Berggren V, Hansson LO, Marchini G. The stress of birth enhances in vitro spontaneous and IL-8-induced neutrophil chemotaxis in the human newborn. Pediatr Allergy Immunol. 2007 Dec;18(8):643–651. doi: 10.1111/j.1399-3038.2007.00578.x. [DOI] [PubMed] [Google Scholar]
- 129.Zentay Z, Sharaf M, Qadir M, Drafta D, Davidson D. Mechanism for dexamethasone inhibition of neutrophil migration upon exposure to lipopolysaccharide in vitro: role of neutrophil interleukin-8 release. Pediatr Res. 1999 Oct;46(4):406–410. doi: 10.1203/00006450-199910000-00008. [DOI] [PubMed] [Google Scholar]
- 130.Molloy EJ, O’Neill AJ, Grantham JJ, et al. Labor promotes neonatal neutrophil survival and lipopolysaccharide responsiveness. Pediatr Res. 2004 Jul;56(1):99–103. doi: 10.1203/01.PDR.0000130473.30874.B6. [DOI] [PubMed] [Google Scholar]
- 131.Shen CM, Lin SC, Niu DM, Kou YR. Labour increases the surface expression of two Toll-like receptors in the cord blood monocytes of healthy term newborns. Acta Paediatr. 2009 Jun;98(6):959–962. doi: 10.1111/j.1651-2227.2009.01280.x. [DOI] [PubMed] [Google Scholar]
- 132.Linderkamp O, Ruef P, Brenner B, Gulbins E, Lang F. Passive deformability of mature, immature, and active neutrophils in healthy and septicemic neonates. Pediatr Res. 1998 Dec;44(6):946–950. doi: 10.1203/00006450-199812000-00021. [DOI] [PubMed] [Google Scholar]
- 133.Mease AD, Burgess DP, Thomas PJ. Irreversible neutrophil aggregation. A mechanism of decreased newborn neutrophil chemotactic response. Am J Pathol. 1981 Jul;104(1):98–102. [PMC free article] [PubMed] [Google Scholar]
- 134.Levy O, Martin S, Eichenwald E, et al. Impaired innate immunity in the newborn: newborn neutrophils are deficient in bactericidal/permeability-increasing protein. Pediatrics. 1999 Dec;104(6):1327–1333. doi: 10.1542/peds.104.6.1327. [DOI] [PubMed] [Google Scholar]
- 135.Kjeldsen L, Sengelov H, Lollike K, Borregaard N. Granules and secretory vesicles in human neonatal neutrophils. Pediatr Res. 1996 Jul;40(1):120–129. doi: 10.1203/00006450-199607000-00021. [DOI] [PubMed] [Google Scholar]
- 136.Drossou V, Kanakoudi F, Tzimouli V, et al. Impact of prematurity, stress and sepsis on the neutrophil respiratory burst activity of neonates. Biol Neonate. 1997;72(4):201–209. doi: 10.1159/000244485. [DOI] [PubMed] [Google Scholar]
- 137.Shigeoka AO, Santos JI, Hill HR. Functional analysis of neutrophil granulocytes from healthy, infected, and stressed neonates. J Pediatr. 1979 Sep;95(3):454–460. doi: 10.1016/s0022-3476(79)80535-1. [DOI] [PubMed] [Google Scholar]
- 138.Wright WC, Jr., Ank BJ, Herbert J, Stiehm ER. Decreased bactericidal activity of leukocytes of stressed newborn infants. Pediatrics. 1975 Oct;56(4):579–584. [PubMed] [Google Scholar]
- 139.Bialek R, Bartmann P. Is there an effect of immunoglobulins and G-CSF on neutrophil phagocytic activity in preterm infants? Infection. 1998 Nov-Dec;26(6):375–378. doi: 10.1007/BF02770839. [DOI] [PubMed] [Google Scholar]
- 140.Falconer AE, Carr R, Edwards SW. Impaired neutrophil phagocytosis in preterm neonates: lack of correlation with expression of immunoglobulin or complement receptors. Biol Neonate. 1995;68(4):264–269. doi: 10.1159/000244245. [DOI] [PubMed] [Google Scholar]
- 141.Allgaier B, Shi M, Luo D, Koenig JM. Spontaneous and Fas-mediated apoptosis are diminished in umbilical cord blood neutrophils compared with adult neutrophils. J Leukoc Biol. 1998 Sep;64(3):331–336. doi: 10.1002/jlb.64.3.331. [DOI] [PubMed] [Google Scholar]
- 142.Hanna N, Vasquez P, Pham P, et al. Mechanisms underlying reduced apoptosis in neonatal neutrophils. Pediatr Res. 2005 Jan;57(1):56–62. doi: 10.1203/01.PDR.0000147568.14392.F0. [DOI] [PubMed] [Google Scholar]
- 143.Koenig JM, Stegner JJ, Schmeck AC, Saxonhouse MA, Kenigsberg LE. Neonatal neutrophils with prolonged survival exhibit enhanced inflammatory and cytotoxic responsiveness. Pediatr Res. 2005 Mar;57(3):424–429. doi: 10.1203/01.PDR.0000153945.49022.96. [DOI] [PubMed] [Google Scholar]
- 144.Ohman L, Tullus K, Katouli M, Burman LG, Stendahl O. Correlation between susceptibility of infants to infections and interaction with neutrophils of Escherichia coli strains causing neonatal and infantile septicemia. J Infect Dis. 1995 Jan;171(1):128–133. doi: 10.1093/infdis/171.1.128. [DOI] [PubMed] [Google Scholar]
- 145.Clark SR, Ma AC, Tavener SA, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007 Apr;13(4):463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 146.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004 Mar 5;303(5663):1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 147.Fuchs TA, Abed U, Goosmann C, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007 Jan 15;176(2):231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yost CC, Cody MJ, Harris ES, et al. Impaired neutrophil extracellular trap (NET) formation: a novel innate immune deficiency of human neonates. Blood. 2009 Jun 18;113(25):6419–6427. doi: 10.1182/blood-2008-07-171629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Marshall JS, Jawdat DM. Mast cells in innate immunity. J Allergy Clin Immunol. 2004 Jul;114(1):21–27. doi: 10.1016/j.jaci.2004.04.045. [DOI] [PubMed] [Google Scholar]
- 150.Damsgaard TE, Nielsen BW, Henriques U, Hansen B, Herlin T, Schiotz PO. Histamine releasing cells of the newborn. Mast cells from the umbilical cord matrix and basophils from cord blood. Pediatr Allergy Immunol. 1996 May;7(2):83–90. doi: 10.1111/j.1399-3038.1996.tb00111.x. [DOI] [PubMed] [Google Scholar]
- 151.Mazzoni A, Young HA, Spitzer JH, Visintin A, Segal DM. Histamine regulates cytokine production in maturing dendritic cells, resulting in altered T cell polarization. J Clin Invest. 2001 Dec;108(12):1865–1873. doi: 10.1172/JCI13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nelson A, Ulfgren AK, Edner J, et al. Urticaria Neonatorum: accumulation of tryptase-expressing mast cells in the skin lesions of newborns with Erythema Toxicum. Pediatr Allergy Immunol. 2007 Dec;18(8):652–658. doi: 10.1111/j.1399-3038.2007.00568.x. [DOI] [PubMed] [Google Scholar]
- 153.Drossou V, Kanakoudi F, Diamanti E, et al. Concentrations of main serum opsonins in early infancy. Arch Dis Child Fetal Neonatal Ed. 1995 May;72(3):F172–175. doi: 10.1136/fn.72.3.f172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Miller ME, Stiehm ER. Phagocytic, opsonic and immunoglobulin studies in newborns. Calif Med. 1973 Aug;119(2):43–63. [PMC free article] [PubMed] [Google Scholar]
- 155.Wolach B, Dolfin T, Regev R, Gilboa S, Schlesinger M. The development of the complement system after 28 weeks’ gestation. Acta Paediatr. 1997 May;86(5):523–527. doi: 10.1111/j.1651-2227.1997.tb08924.x. [DOI] [PubMed] [Google Scholar]
- 156.Notarangelo LD, Chirico G, Chiara A, et al. Activity of classical and alternative pathways of complement in preterm and small for gestational age infants. Pediatr Res. 1984 Mar;18(3):281–285. doi: 10.1203/00006450-198403000-00014. [DOI] [PubMed] [Google Scholar]
- 157.Lassiter HA, Watson SW, Seifring ML, Tanner JE. Complement factor 9 deficiency in serum of human neonates. J Infect Dis. 1992 Jul;166(1):53–57. doi: 10.1093/infdis/166.1.53. [DOI] [PubMed] [Google Scholar]
- 158.Levy O, Sisson RB, Kenyon J, Eichenwald E, Macone AB, Goldmann D. Enhancement of neonatal innate defense: effects of adding an N-terminal recombinant fragment of bactericidal/permeability-increasing protein on growth and tumor necrosis factor-inducing activity of gram-negative bacteria tested in neonatal cord blood ex vivo. Infect Immun. 2000 Sep;68(9):5120–5125. doi: 10.1128/iai.68.9.5120-5125.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zilow EP, Hauck W, Linderkamp O, Zilow G. Alternative pathway activation of the complement system in preterm infants with early onset infection. Pediatr Res. 1997 Mar;41(3):334–339. doi: 10.1203/00006450-199703000-00005. [DOI] [PubMed] [Google Scholar]
- 160.Zilow G, Zilow EP, Burger R, Linderkamp O. Complement activation in newborn infants with early onset infection. Pediatr Res. 1993 Aug;34(2):199–203. doi: 10.1203/00006450-199308000-00020. [DOI] [PubMed] [Google Scholar]
- 161.Frakking FN, Brouwer N, van Eijkelenburg NK, et al. Low mannose-binding lectin (MBL) levels in neonates with pneumonia and sepsis. Clin Exp Immunol. 2007 Nov;150(2):255–262. doi: 10.1111/j.1365-2249.2007.03479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dzwonek AB, Neth OW, Thiebaut R, et al. The role of mannose-binding lectin in susceptibility to infection in preterm neonates. Pediatr Res. 2008 Jun;63(6):680–685. doi: 10.1203/PDR.0b013e31816fdbff. [DOI] [PubMed] [Google Scholar]
- 163.Nupponen I, Andersson S, Jarvenpaa AL, Kautiainen H, Repo H. Neutrophil CD11b expression and circulating interleukin-8 as diagnostic markers for early-onset neonatal sepsis. Pediatrics. 2001 Jul;108(1):E12. doi: 10.1542/peds.108.1.e12. [DOI] [PubMed] [Google Scholar]
- 164.Berger M, O’Shea J, Cross AS, et al. Human neutrophils increase expression of C3bi as well as C3b receptors upon activation. J Clin Invest. 1984 Nov;74(5):1566–1571. doi: 10.1172/JCI111572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Snyderman R, Goetzl EJ. Molecular and cellular mechanisms of leukocyte chemotaxis. Science. 1981 Aug 21;213(4510):830–837. doi: 10.1126/science.6266014. [DOI] [PubMed] [Google Scholar]
- 166.Vogt W. Anaphylatoxins: possible roles in disease. Complement. 1986;3(3):177–188. doi: 10.1159/000467894. [DOI] [PubMed] [Google Scholar]
- 167.Anderson DC, Rothlein R, Marlin SD, Krater SS, Smith CW. Impaired transendothelial migration by neonatal neutrophils: abnormalities of Mac-1 (CD11b/CD18)-dependent adherence reactions. Blood. 1990 Dec 15;76(12):2613–2621. [PubMed] [Google Scholar]
- 168.Nybo M, Sorensen O, Leslie R, Wang P. Reduced expression of C5a receptors on neutrophils from cord blood. Arch Dis Child Fetal Neonatal Ed. 1998 Mar;78(2):F129–132. doi: 10.1136/fn.78.2.f129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ng PC. Diagnostic markers of infection in neonates. Arch Dis Child Fetal Neonatal Ed. 2004 May;89(3):F229–235. doi: 10.1136/adc.2002.023838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ng PC, Li K, Wong RP, et al. Proinflammatory and anti-inflammatory cytokine responses in preterm infants with systemic infections. Arch Dis Child Fetal Neonatal Ed. 2003 May;88(3):F209–213. doi: 10.1136/fn.88.3.F209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bozza FA, Salluh JI, Japiassu AM, et al. Cytokine profiles as markers of disease severity in sepsis: a multiplex analysis. Crit Care. 2007;11(2):R49. doi: 10.1186/cc5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hodge G, Hodge S, Haslam R, et al. Rapid simultaneous measurement of multiple cytokines using 100 microl sample volumes--association with neonatal sepsis. Clin Exp Immunol. 2004 Aug;137(2):402–407. doi: 10.1111/j.1365-2249.2004.02529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Demirjian A, Levy O. Safety and efficacy of neonatal vaccination. Eur J Immunol. 2009 Jan;39(1):36–46. doi: 10.1002/eji.200838620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ahrens P, Kattner E, Kohler B, et al. Mutations of genes involved in the innate immune system as predictors of sepsis in very low birth weight infants. Pediatr Res. 2004 Apr;55(4):652–656. doi: 10.1203/01.PDR.0000112100.61253.85. [DOI] [PubMed] [Google Scholar]
- 175.Reiman M, Kujari H, Ekholm E, Lapinleimu H, Lehtonen L, Haataja L. Interleukin-6 polymorphism is associated with chorioamnionitis and neonatal infections in preterm infants. J Pediatr. 2008 Jul;153(1):19–24. doi: 10.1016/j.jpeds.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 176.Gopel W, Hartel C, Ahrens P, et al. Interleukin-6-174-genotype, sepsis and cerebral injury in very low birth weight infants. Genes Immun. 2006 Jan;7(1):65–68. doi: 10.1038/sj.gene.6364264. [DOI] [PubMed] [Google Scholar]
- 177.Baier RJ, Loggins J, Yanamandra K. IL-10, IL-6 and CD14 polymorphisms and sepsis outcome in ventilated very low birth weight infants. BMC Med. 2006;4:10. doi: 10.1186/1741-7015-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Schueller AC, Heep A, Kattner E, et al. Prevalence of two tumor necrosis factor gene polymorphisms in premature infants with early onset sepsis. Biol Neonate. 2006;90(4):229–232. doi: 10.1159/000093605. [DOI] [PubMed] [Google Scholar]
- 179.Treszl A, Kocsis I, Szathmari M, et al. Genetic variants of TNF-[FC12]a, IL-1beta, IL-4 receptor [FC12]a-chain, IL-6 and IL-10 genes are not risk factors for sepsis in low-birth-weight infants. Biol Neonate. 2003;83(4):241–245. doi: 10.1159/000069484. [DOI] [PubMed] [Google Scholar]
- 180.Chauhan M, McGuire W. Interleukin-6 (-174C) polymorphism and the risk of sepsis in very low birth weight infants: meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2008 Nov;93(6):F427–429. doi: 10.1136/adc.2007.134205. [DOI] [PubMed] [Google Scholar]
- 181.Zhao J, Kim KD, Yang X, Auh S, Fu YX, Tang H. Hyper innate responses in neonates lead to increased morbidity and mortality after infection. Proc Natl Acad Sci U S A. 2008 May 27;105(21):7528–7533. doi: 10.1073/pnas.0800152105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cusumano V, Mancuso G, Genovese F, et al. Neonatal hypersusceptibility to endotoxin correlates with increased tumor necrosis factor production in mice. J Infect Dis. 1997 Jul;176(1):168–176. doi: 10.1086/514019. [DOI] [PubMed] [Google Scholar]
- 183.Wong HR, Doughty LA, Wedel N, et al. Plasma bactericidal/permeability-increasing protein concentrations in critically ill children with the sepsis syndrome. Pediatr Infect Dis J. 1995 Dec;14(12):1087–1091. doi: 10.1097/00006454-199512000-00011. [DOI] [PubMed] [Google Scholar]
- 184.Romeo MG, Tina LG, Sciacca A, et al. Decreased plasma fibronectin (pFN) level in preterm infants with infections. Pediatr Med Chir. 1995 Nov-Dec;17(6):563–566. [PubMed] [Google Scholar]
- 185.Kalayci AG, Adam B, Yilmazer F, Uysal S, Gurses N. The value of immunoglobulin and complement levels in the early diagnosis of neonatal sepsis. Acta Paediatr. 1997 Sep;86(9):999–1002. doi: 10.1111/j.1651-2227.1997.tb15187.x. [DOI] [PubMed] [Google Scholar]
- 186.Dyke MP, Forsyth KD. Decreased plasma fibronectin concentrations in preterm infants with septicaemia. Arch Dis Child. 1993 May;68(5):557–560. doi: 10.1136/adc.68.5_spec_no.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Benitz WE, Han MY, Madan A, Ramachandra P. Serial serum C-reactive protein levels in the diagnosis of neonatal infection. Pediatrics. 1998 Oct;102(4):E41. doi: 10.1542/peds.102.4.e41. [DOI] [PubMed] [Google Scholar]
- 188.Madden NP, Levinsky RJ, Bayston R, Harvey B, Turner MW, Spitz L. Surgery, sepsis, and nonspecific immune function in neonates. J Pediatr Surg. 1989 Jun;24(6):562–566. doi: 10.1016/s0022-3468(89)80506-8. [DOI] [PubMed] [Google Scholar]
- 189.Rivers RP, Cattermole HE, Wright I. The expression of surface tissue factor apoprotein by blood monocytes in the course of infections in early infancy. Pediatr Res. 1992 Jun;31(6):567–573. doi: 10.1203/00006450-199206000-00006. [DOI] [PubMed] [Google Scholar]
- 190.Markiewski MM, Nilsson B, Ekdahl KN, Mollnes TE, Lambris JD. Complement and coagulation: strangers or partners in crime? Trends Immunol. 2007 Apr;28(4):184–192. doi: 10.1016/j.it.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 191.Aronis S, Platokouki H, Photopoulos S, Adamtziki E, Xanthou M. Indications of coagulation and/or fibrinolytic system activation in healthy and sick very-low-birth-weight neonates. Biol Neonate. 1998 Nov;74(5):337–344. doi: 10.1159/000014051. [DOI] [PubMed] [Google Scholar]
- 192.Roman J, Velasco F, Fernandez F, et al. Coagulation, fibrinolytic and kallikrein systems in neonates with uncomplicated sepsis and septic shock. Haemostasis. 1993 May-Jun;23(3):142–148. doi: 10.1159/000216867. [DOI] [PubMed] [Google Scholar]
- 193.Lauterbach R, Pawlik D, Radziszewska R, Wozniak J, Rytlewski K. Plasma antithrombin III and protein C levels in early recognition of late-onset sepsis in newborns. Eur J Pediatr. 2006 Sep;165(9):585–589. doi: 10.1007/s00431-006-0139-7. [DOI] [PubMed] [Google Scholar]
- 194.Grewal PK, Uchiyama S, Ditto D, et al. The Ashwell receptor mitigates the lethal coagulopathy of sepsis. Nat Med. 2008 Jun;14(6):648–655. doi: 10.1038/nm1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Guida JD, Kunig AM, Leef KH, McKenzie SE, Paul DA. Platelet count and sepsis in very low birth weight neonates: is there an organism-specific response? Pediatrics. 2003 Jun;111(6 Pt 1):1411–1415. doi: 10.1542/peds.111.6.1411. [DOI] [PubMed] [Google Scholar]
- 196.Sola MC, Del Vecchio A, Rimsza LM. Evaluation and treatment of thrombocytopenia in the neonatal intensive care unit. Clin Perinatol. 2000 Sep;27(3):655–679. doi: 10.1016/s0095-5108(05)70044-0. [DOI] [PubMed] [Google Scholar]
- 197.Spear ML, Stefano JL, Fawcett P, Proujansky R. Soluble interleukin-2 receptor as a predictor of neonatal sepsis. J Pediatr. 1995 Jun;126(6):982–985. doi: 10.1016/s0022-3476(95)70228-8. [DOI] [PubMed] [Google Scholar]
- 198.Hathaway WE, Mull MM, Pechet GS. Disseminated intravascular coagulation in the newborn. Pediatrics. 1969 Feb;43(2):233–240. [PubMed] [Google Scholar]
- 199.Malek A, Sager R, Schneider H. Maternal-fetal transport of immunoglobulin G and its subclasses during the third trimester of human pregnancy. Am J Reprod Immunol. 1994 Aug;32(1):8–14. doi: 10.1111/j.1600-0897.1994.tb00873.x. [DOI] [PubMed] [Google Scholar]
- 200.Fanaroff AA, Korones SB, Wright LL, et al. National Institute of Child Health and Human Development Neonatal Research Network A controlled trial of intravenous immune globulin to reduce nosocomial infections in very-low-birth-weight infants. N Engl J Med. 1994 Apr 21;330(16):1107–1113. doi: 10.1056/NEJM199404213301602. [DOI] [PubMed] [Google Scholar]
- 201.Sandberg K, Fasth A, Berger A, et al. Preterm infants with low immunoglobulin G levels have increased risk of neonatal sepsis but do not benefit from prophylactic immunoglobulin G. J Pediatr. 2000 Nov;137(5):623–628. doi: 10.1067/mpd.2000.109791. [DOI] [PubMed] [Google Scholar]
- 202.Ballow M, Cates KL, Rowe JC, Goetz C, Desbonnet C. Development of the immune system in very low birth weight (less than 1500 g) premature infants: concentrations of plasma immunoglobulins and patterns of infections. Pediatr Res. 1986 Sep;20(9):899–904. doi: 10.1203/00006450-198609000-00019. [DOI] [PubMed] [Google Scholar]
- 203.Conway SP, Dear PR, Smith I. Immunoglobulin profile of the preterm baby. Arch Dis Child. 1985 Mar;60(3):208–212. doi: 10.1136/adc.60.3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bonagura VR, Marchlewski R, Cox A, Rosenthal DW. Biologic IgG level in primary immunodeficiency disease: the IgG level that protects against recurrent infection. J Allergy Clin Immunol. 2008 Jul;122(1):210–212. doi: 10.1016/j.jaci.2008.04.044. [DOI] [PubMed] [Google Scholar]
- 205.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003 Sep;3(9):710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 206.Yoshio H, Lagercrantz H, Gudmundsson GH, Agerberth B. First line of defense in early human life. Semin Perinatol. 2004 Aug;28(4):304–311. doi: 10.1053/j.semperi.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 207.Schrama AJ, de Beaufort AJ, Poorthuis BJ, Berger HM, Walther FJ. Secretory phospholipase A(2) in newborn infants with sepsis. J Perinatol. 2008 Apr;28(4):291–296. doi: 10.1038/sj.jp.7211929. [DOI] [PubMed] [Google Scholar]
- 208.Kingsmore SF, Kennedy N, Halliday HL, et al. Identification of diagnostic biomarkers for infection in premature neonates. Mol Cell Proteomics. 2008 Oct;7(10):1863–1875. doi: 10.1074/mcp.M800175-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ayabe T, Ashida T, Kohgo Y, Kono T. The role of Paneth cells and their antimicrobial peptides in innate host defense. Trends Microbiol. 2004 Aug;12(8):394–398. doi: 10.1016/j.tim.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 210.Holzl MA, Hofer J, Steinberger P, Pfistershammer K, Zlabinger GJ. Host antimicrobial proteins as endogenous immunomodulators. Immunol Lett. 2008 Aug 15;119(1-2):4–11. doi: 10.1016/j.imlet.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 211.Selsted ME, Ouellette AJ. Mammalian defensins in the antimicrobial immune response. Nat Immunol. 2005 Jun;6(6):551–557. doi: 10.1038/ni1206. [DOI] [PubMed] [Google Scholar]
- 212.Strunk T, Doherty D, Richmond P, et al. Reduced levels of antimicrobial proteins and peptides in human cord blood plasma. Arch Dis Child Fetal Neonatal Ed. 2009 May;94(3):F230–231. doi: 10.1136/adc.2008.143438. [DOI] [PubMed] [Google Scholar]
- 213.Ihi T, Nakazato M, Mukae H, Matsukura S. Elevated concentrations of human neutrophil peptides in plasma, blood, and body fluids from patients with infections. Clin Infect Dis. 1997 Nov;25(5):1134–1140. doi: 10.1086/516075. [DOI] [PubMed] [Google Scholar]
- 214.Thomas NJ, Carcillo JA, Doughty LA, Sasser H, Heine RP. Plasma concentrations of defensins and lactoferrin in children with severe sepsis. Pediatr Infect Dis J. 2002 Jan;21(1):34–38. doi: 10.1097/00006454-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 215.Nupponen I, Turunen R, Nevalainen T, et al. Extracellular release of bactericidal/permeability-increasing protein in newborn infants. Pediatr Res. 2002 Jun;51(6):670–674. doi: 10.1203/00006450-200206000-00002. [DOI] [PubMed] [Google Scholar]
- 216.Michalek J, Svetlikova P, Fedora M, et al. Bactericidal permeability increasing protein gene variants in children with sepsis. Intensive Care Med. 2007 Dec;33(12):2158–2164. doi: 10.1007/s00134-007-0860-3. [DOI] [PubMed] [Google Scholar]
- 217.Dorschner RA, Lin KH, Murakami M, Gallo RL. Neonatal skin in mice and humans expresses increased levels of antimicrobial peptides: innate immunity during development of the adaptive response. Pediatr Res. 2003 Apr;53(4):566–572. doi: 10.1203/01.PDR.0000057205.64451.B7. [DOI] [PubMed] [Google Scholar]
- 218.Yoshio H, Tollin M, Gudmundsson GH, et al. Antimicrobial polypeptides of human vernix caseosa and amniotic fluid: implications for newborn innate defense. Pediatr Res. 2003 Feb;53(2):211–216. doi: 10.1203/01.PDR.0000047471.47777.B0. [DOI] [PubMed] [Google Scholar]
- 219.Baker SM, Balo NN, Abdel Aziz FT. Is vernix caseosa a protective material to the newborn? A biochemical approach. Indian J Pediatr. 1995 Mar-Apr;62(2):237–239. doi: 10.1007/BF02752334. [DOI] [PubMed] [Google Scholar]
- 220.Larson AA, Dinulos JG. Cutaneous bacterial infections in the newborn. Curr Opin Pediatr. 2005 Aug;17(4):481–485. doi: 10.1097/01.mop.0000171321.68806.bd. [DOI] [PubMed] [Google Scholar]
- 221.Ricci E, Malacrida S, Zanchetta M, Montagna M, Giaquinto C, De Rossi A. Role of beta-defensin-1 polymorphisms in mother-to-child transmission of HIV-1. J Acquir Immune Defic Syndr. 2009 May 1;51(1):13–19. doi: 10.1097/QAI.0b013e31819df249. [DOI] [PubMed] [Google Scholar]
- 222.Walker VP, Akinbi HT, Meinzen-Derr J, Narendran V, Visscher M, Hoath SB. Host defense proteins on the surface of neonatal skin: implications for innate immunity. J Pediatr. 2008 Jun;152(6):777–781. doi: 10.1016/j.jpeds.2007.11.030. [DOI] [PubMed] [Google Scholar]
- 223.Espinoza J, Chaiworapongsa T, Romero R, et al. Antimicrobial peptides in amniotic fluid: defensins, calprotectin and bacterial/permeability-increasing protein in patients with microbial invasion of the amniotic cavity, intra-amniotic inflammation, preterm labor and premature rupture of membranes. J Matern Fetal Neonatal Med. 2003 Jan;13(1):2–21. doi: 10.1080/jmf.13.1.2.21. [DOI] [PubMed] [Google Scholar]
- 224.Schaller-Bals S, Schulze A, Bals R. Increased levels of antimicrobial peptides in tracheal aspirates of newborn infants during infection. Am J Respir Crit Care Med. 2002 Apr 1;165(7):992–995. doi: 10.1164/ajrccm.165.7.200110-020. [DOI] [PubMed] [Google Scholar]
- 225.Revenis ME, Kaliner MA. Lactoferrin and lysozyme deficiency in airway secretions: association with the development of bronchopulmonary dysplasia. J Pediatr. 1992 Aug;121(2):262–270. doi: 10.1016/s0022-3476(05)81201-6. [DOI] [PubMed] [Google Scholar]
- 226.Kai-Larsen Y, Bergsson G, Gudmundsson GH, et al. Antimicrobial components of the neonatal gut affected upon colonization. Pediatr Res. 2007 May;61(5 Pt 1):530–536. doi: 10.1203/pdr.0b013e318045be83. [DOI] [PubMed] [Google Scholar]
- 227.Salzman NH, Polin RA, Harris MC, et al. Enteric defensin expression in necrotizing enterocolitis. Pediatr Res. 1998 Jul;44(1):20–26. doi: 10.1203/00006450-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 228.Armogida SA, Yannaras NM, Melton AL, Srivastava MD. Identification and quantification of innate immune system mediators in human breast milk. Allergy Asthma Proc. 2004 Sep-Oct;25(5):297–304. [PubMed] [Google Scholar]
- 229.Chan GM, Lee ML, Rechtman DJ. Effects of a human milk-derived human milk fortifier on the antibacterial actions of human milk. Breastfeed Med. 2007 Dec;2(4):205–208. doi: 10.1089/bfm.2007.0015. [DOI] [PubMed] [Google Scholar]
- 230.Newburg DS, Walker WA. Protection of the neonate by the innate immune system of developing gut and of human milk. Pediatr Res. 2007 Jan;61(1):2–8. doi: 10.1203/01.pdr.0000250274.68571.18. [DOI] [PubMed] [Google Scholar]
- 231.Schrag SJ, Zell ER, Lynfield R, et al. A population-based comparison of strategies to prevent early-onset group B streptococcal disease in neonates. N Engl J Med. 2002 Jul 25;347(4):233–239. doi: 10.1056/NEJMoa020205. [DOI] [PubMed] [Google Scholar]
- 232.Cararach V, Botet F, Sentis J, Almirall R, Perez-Picanol E, Collaborative Group on PROM Administration of antibiotics to patients with rupture of membranes at term: a prospective, randomized, multicentric study. Acta Obstet Gynecol Scand. 1998 Mar;77(3):298–302. [PubMed] [Google Scholar]
- 233.Pylipow M, Gaddis M, Kinney JS. Selective intrapartum prophylaxis for group B streptococcus colonization: management and outcome of newborns. Pediatrics. 1994 Apr;93(4):631–635. [PubMed] [Google Scholar]
- 234.Segel SY, Miles AM, Clothier B, Parry S, Macones GA. Duration of antibiotic therapy after preterm premature rupture of fetal membranes. Am J Obstet Gynecol. 2003 Sep;189(3):799–802. doi: 10.1067/s0002-9378(03)00765-8. [DOI] [PubMed] [Google Scholar]
- 235.Johnston MM, Sanchez-Ramos L, Vaughn AJ, Todd MW, Benrubi GI. Antibiotic therapy in preterm premature rupture of membranes: a randomized, prospective, double-blind trial. Am J Obstet Gynecol. 1990 Sep;163(3):743–747. doi: 10.1016/0002-9378(90)91060-p. [DOI] [PubMed] [Google Scholar]
- 236.Hopkins L, Smaill F. Antibiotic regimens for management of intraamniotic infection. Cochrane Database Syst Rev. 2002;(3) doi: 10.1002/14651858.CD003254. CD003254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Cohen-Wolkowiez M, Benjamin DK, Jr., Capparelli E. Immunotherapy in neonatal sepsis: advances in treatment and prophylaxis. Curr Opin Pediatr. 2009 Apr;21(2):177–181. doi: 10.1097/MOP.0b013e32832925e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 2008 Jan;34(1):17–60. doi: 10.1007/s00134-007-0934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Venkataseshan S, Dutta S, Ahluwalia J, Narang A. Low plasma protein C values predict mortality in low birth weight neonates with septicemia. Pediatr Infect Dis J. 2007 Aug;26(8):684–688. doi: 10.1097/INF.0b013e3180f616f0. [DOI] [PubMed] [Google Scholar]
- 240.Nadel S, Goldstein B, Williams MD, et al. Drotrecogin alfa (activated) in children with severe sepsis: a multicentre phase III randomised controlled trial. Lancet. 2007 Mar 10;369(9564):836–843. doi: 10.1016/S0140-6736(07)60411-5. [DOI] [PubMed] [Google Scholar]
- 241.Mohan P, Brocklehurst P. Granulocyte transfusions for neonates with confirmed or suspected sepsis and neutropaenia. Cochrane Database Syst Rev. 2003;(4) doi: 10.1002/14651858.CD003956. CD003956. [DOI] [PubMed] [Google Scholar]
- 242.Carr R, Modi N, Dore C. G-CSF and GM-CSF for treating or preventing neonatal infections. Cochrane Database Syst Rev. 2003;(3) doi: 10.1002/14651858.CD003066. CD003066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Carr R, Brocklehurst P, Dore CJ, Modi N. Granulocyte-macrophage colony stimulating factor administered as prophylaxis for reduction of sepsis in extremely preterm, small for gestational age neonates (the PROGRAMS trial): a single-blind, multicentre, randomised controlled trial. Lancet. 2009 Jan 17;373(9659):226–233. doi: 10.1016/S0140-6736(09)60071-4. [DOI] [PubMed] [Google Scholar]
- 244.Buhimschi CS, Bhandari V, Hamar BD, et al. Proteomic profiling of the amniotic fluid to detect inflammation, infection, and neonatal sepsis. PLoS Med. 2007 Jan;4(1):e18. doi: 10.1371/journal.pmed.0040018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Buhimschi CS, Bhandari V, Han YW, et al. Using proteomics in perinatal and neonatal sepsis: hopes and challenges for the future. Curr Opin Infect Dis. 2009 Jun;22(3):235–243. doi: 10.1097/QCO.0b013e32832a5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Ng PC, Li G, Chui KM, et al. Quantitative measurement of monocyte HLA-DR expression in the identification of early-onset neonatal infection. Biol Neonate. 2006;89(2):75–81. doi: 10.1159/000088288. [DOI] [PubMed] [Google Scholar]
- 247.Hancock RE, Sahl HG. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol. 2006 Dec;24(12):1551–1557. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- 248.Hirsch T, Metzig M, Niederbichler A, Steinau HU, Eriksson E, Steinstraesser L. Role of host defense peptides of the innate immune response in sepsis. Shock. 2008 Aug;30(2):117–126. doi: 10.1097/shk.0b013e318160de11. [DOI] [PubMed] [Google Scholar]
- 249.Fukumoto K, Nagaoka I, Yamataka A, et al. Effect of antibacterial cathelicidin peptide CAP18/LL-37 on sepsis in neonatal rats. Pediatr Surg Int. 2005 Jan;21(1):20–24. doi: 10.1007/s00383-004-1256-x. [DOI] [PubMed] [Google Scholar]
- 250.Manzoni P, Rinaldi M, Cattani S, et al. Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: a randomized trial. JAMA. 2009 Oct 7;302(13):1421–1428. doi: 10.1001/jama.2009.1403. [DOI] [PubMed] [Google Scholar]
- 251.Brubaker JO, Montaner LJ. Role of interleukin-13 in innate and adaptive immunity. Cell Mol Biol (Noisy-le-grand) 2001 Jun;47(4):637–651. [PubMed] [Google Scholar]
- 252.Koj A. Termination of acute-phase response: role of some cytokines and anti-inflammatory drugs. Gen Pharmacol. 1998 Jul;31(1):9–18. doi: 10.1016/s0306-3623(97)00435-7. [DOI] [PubMed] [Google Scholar]
- 253.Sikora JP, Chlebna-Sokol D, Krzyzanska-Oberbek A. (IL-6, IL-8), cytokine inhibitors (IL-6sR, sTNFRII) Proinflammatory cytokines and anti-inflammatory cytokines (IL-10, IL-13) in the pathogenesis of sepsis in newborns and infants. Arch Immunol Ther Exp (Warsz) 2001;49(5):399–404. [PubMed] [Google Scholar]
- 254.Opal SM, Esmon CT. Bench-to-bedside review: functional relationships between coagulation and the innate immune response and their respective roles in the pathogenesis of sepsis. Crit Care. 2003 Feb;7(1):23–38. doi: 10.1186/cc1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Trepicchio WL, Bozza M, Pedneault G, Dorner AJ. Recombinant human IL-11 attenuates the inflammatory response through down-regulation of proinflammatory cytokine release and nitric oxide production. J Immunol. 1996 Oct 15;157(8):3627–3634. [PubMed] [Google Scholar]
- 256.Wang P, Wu P, Siegel MI, Egan RW, Billah MM. Interleukin (IL)-10 inhibits nuclear factor kappa B (NF kappa B) activation in human monocytes. IL-10 and IL-4 suppress cytokine synthesis by different mechanisms. J Biol Chem. 1995 Apr 21;270(16):9558–9563. doi: 10.1074/jbc.270.16.9558. [DOI] [PubMed] [Google Scholar]
- 257.Dollner H, Vatten L, Linnebo I, Zanussi GF, Laerdal A, Austgulen R. Inflammatory mediators in umbilical plasma from neonates who develop early-onset sepsis. Biol Neonate. 2001 Jul;80(1):41–47. doi: 10.1159/000047118. [DOI] [PubMed] [Google Scholar]
- 258.Cunnington A, Nadel S. New therapies for sepsis. Curr Top Med Chem. 2008;8(7):603–614. doi: 10.2174/156802608783955601. [DOI] [PubMed] [Google Scholar]
- 259.Bancalari E. Changes in the pathogenesis and prevention of chronic lung disease of prematurity. Am J Perinatol. 2001;18(1):1–9. doi: 10.1055/s-2001-12940. [DOI] [PubMed] [Google Scholar]
- 260.Chawla D, Agarwal R, Deorari AK, Paul VK. Retinopathy of prematurity. Indian J Pediatr. 2008 Jan;75(1):73–76. doi: 10.1007/s12098-008-0011-z. [DOI] [PubMed] [Google Scholar]
- 261.Ikegami M, Carter K, Bishop K, et al. Intratracheal recombinant surfactant protein d prevents endotoxin shock in the newborn preterm lamb. Am J Respir Crit Care Med. 2006 Jun 15;173(12):1342–1347. doi: 10.1164/rccm.200509-1485OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Doss M, White MR, Tecle T, et al. Interactions of alpha-, beta-, and theta-defensins with influenza A virus and surfactant protein D. J Immunol. 2009 Jun 15;182(12):7878–7887. doi: 10.4049/jimmunol.0804049. [DOI] [PubMed] [Google Scholar]
- 263.Roth A, Jensen H, Garly ML, et al. Low birth weight infants and Calmette-Guerin bacillus vaccination at birth: community study from Guinea-Bissau. Pediatr Infect Dis J. 2004 Jun;23(6):544–550. doi: 10.1097/01.inf.0000129693.81082.a0. [DOI] [PubMed] [Google Scholar]