Abstract
Background
Previous studies have suggested that the incidence of retinopathy is lower in preterm infants with exposure to reduced levels of oxygenation than in those exposed to higher levels of oxygenation. However, it is unclear what range of oxygen saturation is appropriate to minimize retinopathy without increasing adverse outcomes.
Methods
We performed a randomized trial with a 2-by-2 factorial design to compare target ranges of oxygen saturation of 85 to 89% or 91 to 95% among 1316 infants who were born between 24 weeks 0 days and 27 weeks 6 days of gestation. The primary outcome was a composite of severe retinopathy of prematurity (defined as the presence of threshold retinopathy, the need for surgical ophthalmologic intervention, or the use of bevacizumab), death before discharge from the hospital, or both. All infants were also randomly assigned to continuous positive airway pressure or intubation and surfactant.
Results
The rates of severe retinopathy or death did not differ significantly between the lower-oxygen-saturation group and the higher-oxygen-saturation group (28.3% and 32.1%, respectively; relative risk with lower oxygen saturation, 0.90; 95% confidence interval [CI], 0.76 to 1.06; P = 0.21). Death before discharge occurred more frequently in the lower-oxygen-saturation group (in 19.9% of infants vs. 16.2%; relative risk, 1.27; 95% CI, 1.01 to 1.60; P = 0.04), whereas severe retinopathy among survivors occurred less often in this group (8.6% vs. 17.9%; relative risk, 0.52; 95% CI, 0.37 to 0.73; P<0.001). There were no significant differences in the rates of other adverse events.
Conclusions
A lower target range of oxygenation (85 to 89%), as compared with a higher range (91 to 95%), did not significantly decrease the composite outcome of severe retinopathy or death, but it resulted in an increase in mortality and a substantial decrease in severe retinopathy among survivors. The increase in mortality is a major concern, since a lower target range of oxygen saturation is increasingly being advocated to prevent retinopathy of prematurity. (ClinicalTrials.gov number, NCT00233324.)
Retinopathy of prematurity is an important cause of blindness and other visual disabilities in preterm infants. The incidence of retinopathy of prematurity was increased with exposure to unrestricted oxygen supplementation in preterm infants in randomized, controlled trials performed in the 1950s.1 In the 1960s, this increase resulted in the practice of restricting the fraction of inspired oxygen (FiO2) to no more than 0.50, which was estimated to result in an excess of 16 deaths per case of blindness prevented.2 More recent data suggest that levels of oxygen saturation previously thought to be at the upper end of the normal range may increase the risk of retinopathy of prematurity as compared with levels at the lower end of the normal range.3-5 Oxygen toxicity may also increase the risk of death,6,7 bronchopulmonary dysplasia,8-10 periventricular leukomalacia,11 cerebral palsy,12 and other conditions. Although a multicenter observational study did not show a significant association between higher values for the partial pressure of arterial oxygen and retinopathy, a single-center cohort study involving transcutaneous oxygen monitoring provided support for an association between an increased risk of retinopathy13 and exposure to arterial oxygen levels of 80 mm Hg or more.14
Pulse oximetry allows clinicians to continuously monitor levels of oxygen saturation and to target levels in a defined range. Associations between lower target levels of oxygen saturation and a lower incidence of retinopathy have been reported.1-5 In a survey of 144 neonatal intensive care units (NICUs), the rate of retinal ablation surgery among very-low-birth-weight infants was increased among infants cared for in NICUs that used higher maximum target levels of oxygen saturation, as compared with infants in NICUs that used lower target levels. The rate of retinal ablation surgery was 3.3% in NICUs using target levels of 92% or higher and 1.4% in NICUs using target levels of less than 92%; the rate was 5.6% in NICUs using target levels of 98% or higher and 3.1% in NICUs using target levels of less than 98%.3 In a retrospective study comparing outcomes at five NICUs, the incidence of severe retinopathy requiring ablation therapy was 27% in NICUs where the target saturation level was 88 to 98% and only 6% in NICUs where the target level was 70 to 90%.3 Rates of death and cerebral palsy did not differ significantly among these NICUs. In three studies with a before-and-after design, the implementation of a policy of target levels of oxygen saturation of approximately 83 to 95% was associated with a substantial reduction in the incidence of retinopathy, as compared with the period before implementation of the policy; however, the actual levels of oxygen saturation achieved, mortality, and neurodevelopmental outcomes were not reported.4,15,16 Although data from these studies suggest that maintenance of oxygenation at ranges lower than those previously used may decrease the incidence of retinopathy of prematurity, the safety of low target levels of oxygen saturation remains a concern.
We conducted the Surfactant, Positive Pressure, and Oxygenation Randomized Trial (SUPPORT), a controlled, multicenter trial with a 2-by-2 factorial design, to compare two target levels of oxygen saturation and two ventilation approaches (continuous positive airway pressure [CPAP] initiated in the delivery room with a protocol-driven strategy of limited ventilation vs. intratracheal administration of surfactant with a protocol-driven strategy of conventional ventilation). The oxygen-saturation component of the trial tested the hypothesis that a lower target range of oxygen saturation (85 to 89%), as compared with a higher target range (91 to 95%), would reduce the incidence of the composite outcome of severe retinopathy of prematurity or death among infants who were born between 24 weeks 0 days of gestation and 27 weeks 6 days of gestation. The ventilation part of this factorial-design trial, which was used to control the ventilation approach and test other hypotheses, is reported elsewhere in this issue of the Journal.17
Methods
Study Design
The study was conducted as part of the Neonatal Research Network of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The study was approved by the institutional review board at each participating site and by RTI International, which is the independent data coordinating center for the Neonatal Research Network. Data collected at the study sites were transmitted to RTI International, which stored, managed, and analyzed the data for this study. Written informed consent was obtained from the parent or guardian of each child before delivery.
Patients
Infants who were born between 24 weeks 0 days of gestation and 27 weeks 6 days of gestation for whom a decision had been made to provide full resuscitation were eligible for enrollment at birth. Infants born in other hospitals and those known to have major congenital anomalies were excluded.
Enrollment and Treatment
Infants were enrolled from February 2005 through February 2009. Permuted-block randomization was used, with stratification according to study center and gestational age (24 weeks 0 days to 25 weeks 6 days or 26 weeks 0 days to 27 weeks 6 days). Using sealed, opaque envelopes, we randomly assigned infants before birth to a target range of oxygen saturation of 85 to 89% (the lower-oxygen-saturation group) or 91 to 95% (the higher-oxygen-saturation group). Infants who were part of multiple births were randomly assigned to the same group.
Blinding was maintained with the use of electronically altered pulse oximeters (Masimo Radical Pulse Oximeter) that showed saturation levels of 88 to 92% for both targets of oxygen saturation, with a maximum variation of 3%. For example, a reading of 90% corresponded to actual levels of oxygen saturation of 87% in the group assigned to lower oxygen saturation (85 to 89%) and 93% in the group assigned to higher oxygen saturation (91 to 95%). A previous trial used a fixed 3% absolute oxygen-saturation variation throughout the entire range of saturation levels to keep caregivers unaware of study-group assignments and to separate levels of oxygen saturation in preterm infants,18 but the algorithm used in the current trial differed, since the oxygen-saturation reading gradually changed and reverted to actual (non-skewed) values when it was less than 84% or higher than 96% in both treatment groups. Limits of 85% and 95% that would trigger an alarm in the delivery system were suggested, but they could be changed for individual patients.
Targeting of levels of oxygen saturation with altered pulse oximetry was initiated within the first 2 hours after birth and was continued until 36 weeks of postmenstrual age or until the infant was breathing ambient air and did not require ventilator support or CPAP for more than 72 hours, whichever occurred first. Infants who were weaned to room air but who subsequently received oxygen supplementation before 36 weeks of postmenstrual age were placed back on the assigned study pulse oximeter. The target ranges were kept unchanged from birth until 36 weeks of postmenstrual age. Adjustments in supplemental oxygen to maintain the target level of oxygen saturation between 88 and 92% were performed by the clinical staff rather than the research staff.
Data on oxygen saturation were electronically sampled every 10 seconds and downloaded by the data center. Readings of levels of oxygen saturation that were pooled (i.e., not separated according to treatment group) were provided quarterly to each center for feedback on compliance. Actual data on oxygen saturation were not provided to the clinicians or researchers but are used exclusively in this article. For the ventilation part of this trial with a 2-by-2 factorial design, participants were randomly assigned to CPAP with a protocol-driven limited ventilation strategy or to prophylactic early administration of surfactant with a protocol-driven conventional ventilation strategy.17
Assessments
Research nurses recorded all data using standardized definitions included in the trial's manual of operations. Data collection, excluding examinations to detect retinopathy of prematurity, was completed at discharge. All surviving infants were followed by ophthalmologists trained in the diagnosis of retinopathy of prematurity. Examinations began by 33 weeks of postmenstrual age and continued until the study outcome was reached or resolution occurred. Resolution was defined as fully vascularized retinas or immature vessels in zone 3 for two consecutive examinations in each eye. Threshold retinopathy of prematurity (called “new type 1 threshold” by the Early Treatment of Retinopathy Cooperative Group19,20) was diagnosed if any of the following findings were present: in zone 1, stage 3 retinopathy of prematurity, even without plus disease (i.e., two or more quadrants of dilated veins and tortuous arteries in the posterior pole), or plus disease with any stage of retinopathy of prematurity; in zone 2, plus disease with stage 2 retinopathy of prematurity or plus disease with stage 3 retinopathy of prematurity. Surgical ophthalmologic intervention was recorded if any of the following occurred: laser therapy, cryotherapy, both laser therapy and cryotherapy, scleral buckling, or vitrectomy. The primary outcome was death before discharge or severe retinopathy as defined by threshold retinopathy, ophthalmologic surgery, or the use of bevacizumab treatment for retinopathy. The original study protocol specified a primary outcome of death before 36 weeks of postmenstrual age, but this was changed to death before discharge before any data analyses were performed. All other outcomes reported were prespecified, including assessment of the need for oxygen at 36 weeks of postmenstrual age21 and safety outcomes.
Statistical Analysis
The analysis for the oxygen-saturation part of this factorial trial compared the percentage of infants in each treatment group in whom the primary outcome of severe retinopathy or death occurred. Analysis of this and all other categorical outcomes was performed with the use of robust Poisson regression in a generalized-estimating-equation model to obtain adjusted relative risks with 95% confidence intervals. Continuous outcomes were analyzed with the use of mixed-effects linear models to obtain adjusted means and standard errors. We performed a post hoc survival analysis with the use of a Cox proportional-hazards model to compare mortality in the two oxygen-saturation groups, assuming that there were no subsequent deaths among the infants who were discharged. In the analysis of all outcomes, the results were adjusted, as prespecified, for stratification according to study center and gestational age, as well as for familial clustering due to random assignment of infants who were part of multiple births to the same treatment group. To compare the actual oxygen-saturation values in the two treatment groups, the median value during oxygen supplementation was determined for each infant. Those values were plotted according to treatment group, and the medians of the resulting distributions were compared with the use of a rank-sum test.
An absolute between-group difference of 10 percentage points in the rate of the composite primary outcome was considered clinically important. The sample-size calculations were based on the rate of death or threshold retinopathy of 47% in the Neonatal Research Network for the year 2000. We increased the sample size by a factor of 1.12 to allow for infants who were part of multiple births to be randomly assigned to the same treatment (since this introduced a clustering effect into the design), and we increased the sample size by an additional 17% to adjust for attrition after hospital discharge. We increased the sample size further to minimize type I error with the use of a conservative 2% level of significance. The result was a target sample of 1310 infants. The study was not powered to detect an interaction effect between the two factorial parts of the study.
Analyses were performed according to the intention-to-treat principle. The denominator that was used to calculate the rate of each outcome was the number of infants for whom that outcome was known. All analyses were conducted at the data center. Two-sided P values of less than 0.05 were considered to indicate statistical significance. Analyses of secondary outcomes did not include adjustment for multiple comparisons; however, for the 46 planned analyses of secondary outcomes according to treatment group, we would expect no more than three tests to have P values of less than 0.05 on the basis of chance alone. Subgroup analyses were conducted within prespecified gestational-age strata for predefined outcomes. Although these tests were not adjusted for multiple comparisons, we would expect no more than two tests per stratum to have P values of less than 0.05 on the basis of chance alone.
An independent data and safety monitoring committee appointed by the director of the National Institute of Child Health and Human Development reviewed the primary outcomes, adverse events, and other interim results at approximately 25%, 50%, and 75% of planned enrollment. In addition, the data and safety monitoring committee, at the request of the investigators, evaluated the data on oxygen saturation to evaluate compliance with the protocol. The Lan–DeMets spending functions with Pocock and O'Brien–Fleming boundaries were used to develop stopping rules for interim safety and efficacy monitoring, respectively. In the final analysis, the nominal level of significance was 0.05. The monitored safety outcomes included death, pneumothorax, intraventricular hemorrhage, and a combination of any of these events.
Results
Characteristics of the Study Sample
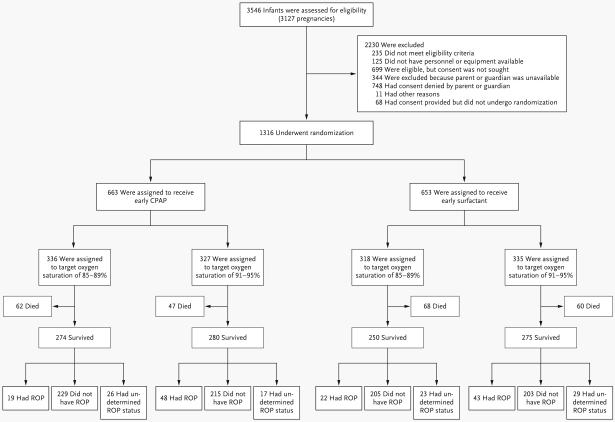
We enrolled 1316 infants in the study (Fig. 1). When 247 infants had been enrolled, enrollment was temporarily suspended on the basis of the recommendation of the data and safety monitoring committee and the decision of the director of the National Institute of Child Health and Human Development because of concern that readings of levels of oxygen saturation often exceeded the target levels. Separation of the oximetry data according to whether patients were breathing ambient air or receiving oxygen supplementation addressed this concern, because infants who did not require supplemental oxygen accounted for a large proportion of the high saturation levels. Resumption of enrollment was approved. The baseline characteristics of the two treatment groups were similar (Table 1).
Figure 1.
The numbers shown exclude infants of women who were screened during pregnancy but whose babies were not subsequently born at a study center between 24 weeks 0 days and 27 weeks 6 days of gestation. The outcome of severe retinopathy of prematurity (ROP) could not be determined in some infants because of loss to follow-up. CPAP denotes continuous positive airway pressure.
Table 1.
Baseline Characteristics of the Patients.
| Characteristic | Lower Oxygen Saturation (N = 654) |
Higher Oxygen Saturation (N = 662) |
|---|---|---|
| Birth weight — g | 836±193 | 825±193 |
| Gestational age — wk | 26±1 | 26±1 |
| Male sex — no./total no. (%) | 341/654 (52.1) | 371/662 (56.0) |
| Race or ethnic group — no./total no. (%)† | ||
| Non-Hispanic white | 242/654 (37.0) | 279/662 (42.1) |
| Non-Hispanic black | 257/654 (39.3) | 232/662 (35.0) |
| Hispanic | 132/654 (20.2) | 127/662 (19.2) |
| Other or unknown | 23/654 (3.5) | 24/662 (3.6) |
| Maternal use of antenatal corticosteroids — no./total no. (%) | ||
| Any | 633/654 (96.8) | 632/661 (95.6) |
| Full course | 477/651 (73.3) | 462/658 (70.2) |
| Apgar score <3 at 5 min — no./total no. (%) | 34/654 (5.2) | 24/662 (3.6) |
| Surfactant treatment — no./total no. (%) | 531/653 (81.3) | 558/660 (84.5) |
| Multiple birth — no./total no. (%) | 161/654 (24.6) | 176/662 (26.6) |
Plus–minus values are means ±SD. P>0.05 for all comparisons.
Race or ethnic group was reported by the mother or guardian of each child.
Primary Outcome
The rate of the composite primary outcome, severe retinopathy or death before discharge, did not differ significantly between the lower-oxygen-saturation group and the higher-oxygen-saturation group (28.3 and 32.1%, respectively; relative risk with lower oxygen saturation, 0.90; 95% confidence interval [CI], 0.76 to 1.06; P = 0.21) (Table 2). Although the trial was not powered to detect an interaction between the level of oxygen saturation and the ventilation intervention, we prospectively planned to evaluate this interaction, and no significant interaction was found (P = 0.57). Death before discharge occurred in 130 of 654 infants in the lower-oxygen-saturation group (19.9%) as compared with 107 of 662 infants in the higher-oxygen-saturation group (16.2%) (relative risk with lower oxygen saturation, 1.27; 95% CI, 1.01 to 1.60; P = 0.04; number needed to harm, 27). The distribution of the major causes of death did not differ significantly between the two groups (see Table 1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). Similar results were observed for both gestational-age strata. Survival analysis with the use of the unadjusted Kaplan–Meier method (Fig. 2) and a Cox proportional-hazards model produced similar results (hazard ratio, 1.28; 95% CI, 0.98 to 1.68; P = 0.07). The rate of severe retinopathy among survivors who were discharged or transferred to another facility or who reached the age of 1 year was lower in the lower-oxygen-saturation group (8.6% vs. 17.9%; relative risk, 0.52; 95% CI, 0.37 to 0.73; P<0.001; number needed to treat, 11). Although use of bevacizumab was among the criteria for this outcome, only three infants received bevacizumab, and these infants also had threshold retinopathy or surgical intervention for retinopathy. Three ophthalmologists adjudicated results for the patients who did not meet the criteria for retinopathy, and the results were materially unchanged (Table 2 in the Supplementary Appendix).
Table 2.
Major Outcomes.*
| Outcome | Lower Oxygen Saturation (N = 654) |
Higher Oxygen Saturation (N = 662) |
Adjusted Relative Risk (95% CI) |
|---|---|---|---|
| no./total no. (%) | |||
| Severe retinopathy of prematurity or death before discharge | 171/605 (28.3) | 198/616 (32.1) | 0.90 (0.76–1.06) |
| Severe retinopathy of prematurity | 41/475 (8.6) | 91/509 (17.9) | 0.52 (0.37–0.73) |
| Death | |||
| Before discharge | 130/654 (19.9) | 107/662 (16.2) | 1.27 (1.01–1.60) |
| By 36 wk postmenstrual age | 114/654 (17.4) | 94/662 (14.2) | 1.27 (0.99–1.63) |
| BPD, defined by use of supplemental oxygen, at 36 wk | 203/540 (37.6) | 265/568 (46.7) | 0.82 (0.72–0.93) |
| BPD, defined by use of supplemental oxygen, or death by 36 wk | 317/654 (48.5) | 359/662 (54.2) | 0.91 (0.83–1.01) |
| BPD, physiological definition, at 36 wk† | 205/540 (38.0) | 237/568 (41.7) | 0.92 (0.81–1.05) |
| BPD, physiological definition, or death by 36 wk† | 319/654 (48.8) | 331/662 (50.0) | 0.99 (0.90–1.10) |
| Intraventricular hemorrhage, grade 3 or 4‡ | 83/630 (13.2) | 81/640 (12.7) | 1.06 (0.80–1.40) |
| Intraventricular hemorrhage, grade 3 or 4, or death‡ | 179/653 (27.4) | 156/661 (23.6) | 1.18 (0.99–1.42) |
| Periventricular leukomalacia | 24/631 (3.8) | 30/641 (4.7) | 0.83 (0.49–1.42) |
| Periventricular leukomalacia or death | 149/654 (22.8) | 132/662 (19.9) | 1.18 (0.96–1.45) |
| Necrotizing enterocolitis, stage ≥2§ | 76/641 (11.9) | 70/649 (10.8) | 1.11 (0.82–1.51) |
| Necrotizing enterocolitis, stage ≥2, or death§ | 176/654 (26.9) | 155/662 (23.4) | 1.18 (0.98–1.43) |
| Pneumothorax | 47/654 (7.2) | 43/662 (6.5) | 1.12 (0.74–1.68) |
| Postnatal corticosteroids for BPD | 61/636 (9.6) | 69/644 (10.7) | 0.91 (0.67–1.24) |
| Death | |||
| By 7 days | 41/654 (6.3) | 38/662 (5.7) | 1.11 (0.72–1.72) |
| By 14 days | 64/654 (9.8) | 56/662 (8.5) | 1.20 (0.84–1.70) |
| Late-onset sepsis | 228/624 (36.5) | 226/634 (35.6) | 1.03 (0.89–1.18) |
| Late-onset sepsis or death | 300/654 (45.9) | 291/662 (44.0) | 1.05 (0.94–1.18) |
| Patent ductus arteriosus | 307/641 (47.9) | 324/648 (50.0) | 0.96 (0.86–1.07) |
| Treatment for patent ductus arteriosus | |||
| Medical | 219/634 (34.5) | 233/645 (36.1) | 0.95 (0.82–1.09) |
| Surgical | 73/641 (11.4) | 68/648 (10.5) | 1.09 (0.80–1.48) |
| Any air leaks in first 14 days | 51/654 (7.8) | 42/662 (6.3) | 1.23 (0.83–1.83) |
Values were adjusted for stratification factors (study center and gestational-age group) as well as for familial clustering. BPD denotes bronchopulmonary dysplasia.
The physiological definition of BPD includes, as a criterion, the receipt of more than 30% oxygen or the need for positive pressure support at 36 weeks or, in the case of infants requiring less than 30% oxygen, the need for any oxygen at 36 weeks after an attempt at oxygen withdrawal.
There are four grades of intraventricular hemorrhage; higher grades indicate more severe bleeding.
There are three stages of necrotizing enterocolitis; higher stages indicate more severe necrotizing enterocolitis.
Figure 2. Kaplan–Meier Estimate of Survival to Hospital Discharge, Transfer, or 1 Year of Life.
Cox proportional-hazards analysis indicated that there was an increased hazard of death in the lower-oxygen-saturation group as compared with the higher-oxygen-saturation group (hazard ratio, 1.28; 95% CI, 0.98 to 1.68; P = 0.07). The analysis assumed that infants who were discharged or transferred from the hospital survived to 1 year of age.
Secondary Outcomes
The rate of oxygen use at 36 weeks was reduced in the lower-oxygen-saturation group as compared with the higher-oxygen-saturation group (P = 0.002), but the rates of bronchopulmonary dysplasia among survivors, as determined by the physiological test of oxygen saturation at 36 weeks, and the composite outcome of bronchopulmonary dysplasia or death by 36 weeks did not differ significantly between the treatment groups. Other prespecified major outcomes also did not differ significantly between the two groups (Table 2).
The median level of oxygen saturation in infants who were receiving oxygen supplementation in the two treatment groups differed substantially but, as expected, there was considerable overlap (Fig. 3). The actual median levels of oxygen saturation were slightly higher than targeted levels in both treatment groups. The duration of oxygen supplementation was shorter in the lower-oxygen-saturation group, but the duration of mechanical ventilation, CPAP, and nasal synchronized intermittent mandatory ventilation did not differ significantly (Table 3 in the Supplementary Appendix). Other measures of resource use also did not differ significantly between the two groups.
Figure 3. Actual Median Oxygen Saturation with Oxygen Supplementation in the Two Treatment Groups.
The medians of the distributions were significantly different on the basis of a rank-sum test (P<0.001). The 80% level of oxygen saturation shown includes all values at or below 80%.
Discussion
In this multicenter, randomized trial, we found no significant difference in the primary outcome — severe retinopathy or death — between infants randomly assigned to a lower target range of oxygen saturation (85 to 89%) and those assigned to a higher target range (91 to 95%). Assessment of the individual components of the primary outcome showed that the lower target range of oxygen saturation increased the risk of in-hospital death, whereas it reduced the risk of severe retinopathy among survivors. These results were observed even though there was substantial overlap of actual levels of oxygen saturation between the two treatment groups. Previous trials of targeting of levels of oxygen saturation have shown similar difficulties in maintaining levels of oxygen saturation within a narrow target range.18,22 Longer follow-up will be required to determine the effects of lower target ranges of oxygen saturation on functional visual and neurodevelopmental outcomes.
Despite the increase in mortality when restrictive oxygen supplementation was used in the 1950s and 1960s and the limited data from observational studies,3-5,15,16 it is becoming common practice to use lower target ranges of oxygen saturation with the goal of reducing the risk of retinopathy of prematurity.23 The results of this large randomized trial to test the effect of lower versus higher target ranges of oxygen saturation, in conjunction with the results of previous studies, add to the concern that oxygen restriction may increase the rate of death among preterm infants. The combined risk difference observed in the trials from the 1950s was an absolute increase in in-hospital mortality of 4.9 percentage points in the oxygen-restricted group,1 which is close to the absolute increase of 3.7 percentage points in the rate of death before discharge in the lower-oxygen-saturation group that was observed in the current trial.
Randomized trials of oxygen restriction in preterm infants at least 2 weeks after birth18 or after moderately severe retinopathy developed22 did not show an increased risk of death or a significantly reduced risk of retinopathy in the lower-oxygen-saturation groups. However, the lower target ranges of oxygen saturation in these trials — 91 to 94% in one trial and 89 to 94% in the other — were closer to the target range in our higher-oxygen-saturation group. The increase in mortality in our trial may be related to the lower target ranges of levels of oxygen saturation, the use of oxygen restriction started soon after birth, or both. A meta-analysis of early restriction of oxygen supplementation based on trials from the 1950s to the 1970s showed a reduction in severe retinopathy (relative risk, 0.19; 95% CI, 0.07 to 0.50) with a nonsignificant trend toward increased mortality.24 These trials were performed by limiting the FiO2 concentration usually to less than 0.50, at a time before the continuous monitoring of arterial oxygen saturation was possible. To our knowledge, no other randomized, controlled trials of different target ranges of oxygen saturation in supplementation initiated soon after birth have been performed since the availability of continuous transcutaneous monitoring of oxygen saturation. Like the meta-analysis24 and most non-randomized studies,3-5,15,16 our trial confirmed that lower target ranges of oxygenation result in a large reduction in the incidence of severe retinopathy among survivors. However, our data suggest that there is one additional death for approximately every two cases of severe retinopathy that are prevented. Several ongoing trials across the world address the same intervention tested in the current trial.25
In summary, a target range of oxygen saturation of 85 to 89%, as compared with a range of 91 to 95%, did not affect the combined outcome of severe retinopathy or death, but it increased mortality while substantially decreasing severe retinopathy among survivors. At the present time, caution should be exercised regarding a strategy of targeting levels of oxygen saturation in the low range for preterm infants, since it may lead to increased mortality.
Supplementary Material
Acknowledgments
Supported by grants (U10 HD21364, U10 HD21373, U10 HD21385, U10 HD21397, U10 HD27851, U10 HD27853, U10 HD27856, U10 HD27880, U10 HD27871, U10 HD27904, U10 HD34216, U10 HD36790, U10 HD40461, U10 HD40492, U10 HD40498, U10 HD40521, U10 HD40689, U10 HD53089, U10 HD53109, U10 HD53119, and U10 HD53124) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, cofunding from the National Heart, Lung, and Blood Institute, and grants (M01 RR30, M01 RR32, M01 RR39, M01 RR44, M01 RR54, M01 RR59, M01 RR64, M01 RR70, M01 RR80, MO1 RR125, M01 RR633, M01 RR750, M01 RR997, M01 RR6022, M01 RR7122, M01 RR8084, M01 RR16587, UL1 RR25008, UL1 RR24139, UL1 RR24979, and UL1 RR25744) from the National Institutes of Health.
We thank our medical and nursing colleagues and the infants and their parents who agreed to take part in this study.
Appendix
The authors are as follows: Waldemar A. Carlo, M.D., Neil N. Finer, M.D., Michele C. Walsh, M.D., Wade Rich, R.R.T., Marie G. Gantz, Ph.D., Abbot R. Laptook, M.D., Bradley A. Yoder, M.D., Roger G. Faix, M.D., Abhik Das, Ph.D., W. Kenneth Poole, Ph.D., Kurt Schibler, M.D., Nancy S. Newman, R.N., Namasivayam Ambalavanan, M.D., Ivan D. Frantz III, M.D., Anthony J. Piazza, M.D., Pablo J. Sánchez, M.D., Brenda H. Morris, M.D., Nirupama Laroia, M.D., Dale L. Phelps, M.D., Brenda B. Poindexter, M.D., C. Michael Cotten, M.D., M.H.S., Krisa P. Van Meurs, M.D., Shahnaz Duara, M.D., Vivek Narendran, M.D., M.R.C.P., Beena G. Sood, M.D., T. Michael O'Shea, M.D., M.P.H., Edward F. Bell, M.D., Richard A. Ehrenkranz, M.D., Kristi L. Watterberg, M.D., and Rosemary D. Higgins, M.D., for the NICHD Neonatal Research Network and the SUPPORT Study Group.
The following are the authors' affiliations: the Division of Neonatology, University of Alabama at Birmingham, Birmingham (W.A.C., N.A.); the University of California at San Diego, San Diego (N.N.F., W.R.); the Department of Pediatrics, Rainbow Babies and Children's Hospital, Case Western Reserve University, Cleveland (M.C.W., N.S.N.); the Statistics and Epidemiology Unit, RTI International, Research Triangle Park (M.G.G., W.K.P.), the Department of Pediatrics, Duke University, Durham (C.M.C.), and Wake Forest University School of Medicine, Winston-Salem (T.M.O.) — all in North Carolina; the Department of Pediatrics, Women and Infants Hospital, Brown University, Providence, RI (A.R.L.); the Department of Pediatrics, Division of Neonatology, University of Utah School of Medicine, Salt Lake City (B.A.Y., R.G.F.); the Statistics and Epidemiology Unit, RTI International, Rockville (A.D.), and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda (R.D.H.) — both in Maryland; the Department of Pediatrics, University of Cincinnati, Cincinnati (K.S., V.N.); the Department of Pediatrics, Division of Newborn Medicine, Floating Hospital for Children, Tufts Medical Center, Boston (I.D.F.); the Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas (P.J.S.); the Department of Pediatrics, Emory University School of Medicine, and Children's Healthcare of Atlanta — both in Atlanta (A.J.P.); the Department of Pediatrics, University of Texas Medical School at Houston, Houston (B.H.M.); the University of Rochester School of Medicine and Dentistry, Rochester, NY (N.L., D.L.P.); the Department of Pediatrics, Indiana University School of Medicine, Indianapolis (B.B.P.); the Department of Pediatrics, Stanford University School of Medicine, Palo Alto, CA (K.P.V.M.); the University of Miami Miller School of Medicine, Miami (S.D.); the Department of Pediatrics, Wayne State University, Detroit (B.G.S.); the Department of Pediatrics, University of Iowa, Iowa City (E.F.B.); the Department of Pediatrics, Yale University School of Medicine, New Haven, CT (R.A.E.); and the University of New Mexico Health Sciences Center, Albuquerque (K.L.W.).
The following investigators, in addition to those listed as authors, participated in this study: Neonatal Research Network Steering Committee Chairs: A.H. Jobe (University of Cincinnati, Cincinnati [2003–2006]), M.S. Caplan (University of Chicago, Pritzker School of Medicine [2006–present]); Alpert Medical School of Brown University and Women and Infants Hospital — both in Providence: W. Oh, A.M. Hensman, D. Gingras, S. Barnett, S. Lillie, K. Francis, D. Andrews, K. Angela; Case Western Reserve University and Rainbow Babies and Children's Hospital — both in Cleveland: A.A. Fanaroff, B.S. Siner, A. Zadell, J. DiFiore; Cincinnati Children's Hospital Medical Center, University of Cincinnati Hospital, and Good Samaritan Hospital — all in Cincinnati: E.F. Donovan, K. Bridges, B. Alexander, C. Grisby, M.W. Mersmann, H.L. Mincey, J. Hessling; Duke University School of Medicine University Hospital, Alamance Regional Medical Center, and Durham Regional Hospital — all in Durham, NC: R.N. Goldberg, K.J. Auten, K.A. Fisher, K.A. Foy, G. Siaw; Emory University, Children's Healthcare of Atlanta, Grady Memorial Hospital, and Emory Crawford Long Hospital — all in Atlanta: B.J. Stoll, S. Buchter, D.P. Carlton, E.C. Hale, A.K. Hutchinson; Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD: S.W. Archer; Indiana University, Indiana University Hospital, Methodist Hospital, Riley Hospital for Children, and Wishard Health Services — all in Indianapolis: J.A. Lemons, F. Hamer, D.E. Herron, L.C. Miller, L.D. Wilson; National Heart, Lung, and Blood Institute, Bethesda, MD: M.A. Berberich, C.J. Blaisdell, D.B. Gail, J.P. Kiley; RTI International, Research Triangle Park, NC: M. Cunningham, B.K. Hastings, A.R. Irene, J. O'D. Auman, C.P. Huitema, J.W. Pickett II, D. Wallace, K.M. Zaterka-Baxter; Stanford University Lucile Packard Children's Hospital, Palo Alto, CA: D.K. Stevenson, M.B. Ball, M.S. Proud; Tufts Medical Center Floating Hospital for Children, Boston: J.M. Fiascone, A. Furey, B.L. MacKinnon, E. Nylen; University of Alabama at Birmingham Health System and Children's Hospital of Alabama — both in Birmingham: M.V. Collins, S.S. Cosby, V.A. Phillips; University of California at San Diego Medical Center and Sharp Mary Birch Hospital for Women — both in San Diego: M.R. Rasmussen, P.R. Wozniak, K. Arnell, R. Bridge, C. Demetrio; University of Iowa Children's Hospital, Iowa City: J.A. Widness, J.M. Klein, K.J. Johnson; University of Miami Holtz Children's Hospital, Miami: R. Everett-Thomas; University of New Mexico Health Sciences Center, Albuquerque: R.K. Ohls, J. Rohr, C.B. Lacy; University of Rochester Medical Center Golisano Children's Hospital, Rochester, NY: G.D. Markowitz, L.J. Reubens, E. Burnell; University of Texas Southwestern Medical Center at Dallas Parkland Health and Hospital System, and Children's Medical Center — all in Dallas: C.R. Rosenfeld, W.A. Salhab, A. Guzman, G. Hensley, M.H. Lepps, N.A. Miller, J. Allen, L. Grau, M. Martin, A. Solis, D.M. Vasil, K. Wilder; University of Texas Health Science Center at Houston Medical School and Children's Memorial Hermann Hospital — both in Houston: K.A. Kennedy, J.E. Tyson, B.F. Harris, A.E. Lis, S. Martin, G.E. McDavid, P.L. Tate, S.L. Wright; University of Utah University Hospital, Intermountain Medical Center, LDS Hospital, and Primary Children's Medical Center — all in Salt Lake City: J. Burnett, J.J. Jensen, K.A. Osborne, C. Spencer, K. Weaver-Lewis; Wake Forest University Baptist Medical Center Brenner Children's Hospital and Forsyth Medical Center — both in Winston-Salem, NC: N.J. Peters; Wayne State University Hutzel Women's Hospital and Children's Hospital of Michigan — both in Detroit: S. Shankaran, R. Bara, E. Billian, M. Johnson; Yale University and Yale–New Haven Children's Hospital, New Haven, and Bridgeport Hospital, Bridgeport — both in Connecticut: V. Bhandari, H.C. Jacobs, P. Cervone, P. Gettner, M. Konstantino, J. Poulsen, J. Taft; Data and Safety Monitoring Committee: G. Avery (chair), Children's National Medical Center, Washington, DC; C.A. Gleason (chair), University of Washington, Seattle; M.C. Allen, Johns Hopkins University School of Medicine, Baltimore; S.I. Bangdiwala, University of North Carolina, Chapel Hill; C.J. Blaisdell, National Heart, Lung, and Blood Institute, Bethesda, MD; R.J. Boyle, University of Virginia Health System, Charlottesville; T. Clemons, EMMES Corporation, Baltimore; M.E. D'Alton, Columbia University, New York; A. Das (ex officio), RTI International, Rockville, MD; D.B. Gail, C. Hunt, National Heart, Lung, and Blood Institute; M. Keszler, Georgetown University Hospital, Washington, DC; W.K. Poole (ex officio), RTI International Research Triangle Park, NC; C.K. Redmond, University of Pittsburgh, Pittsburgh; M.G. Ross, UCLA School of Medicine and Public Health, Los Angeles; M.A. Thomson, Hammersmith Hospital, London; S.J. Weiner, George Washington University, Washington, DC; M. Willinger (ex officio), Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD. Retinopathy of Prematurity Adjudication Committee: G.D. Markowitz, University of Rochester, Rochester, NY; A.K. Hutchinson, Emory University, Atlanta; D.K. Wallace, S.F. Freedman, Duke University, Durham, NC.
Footnotes
Dr. Van Meurs reports receiving reimbursement for travel expenses from Ikaria Holdings. No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Duc G, Sinclair JC. Oxygen administration. In: Sinclair JC, Bracken MB, editors. Effective care of the newborn infant. Oxford University Press; New York: 1992. pp. 178–94. [Google Scholar]
- 2.Bolton DP, Cross KW. Further observations on cost of preventing retrolental fibroplasia. Lancet. 1974;303:445–8. doi: 10.1016/s0140-6736(74)92395-2. [DOI] [PubMed] [Google Scholar]
- 3.Tin W, Milligan DW, Pennefather P, Hey E. Pulse oximetry, severe retinopathy, and outcome at one year in babies of less than 28 weeks gestation. Arch Dis Child Fetal Neonatal Ed. 2001;84:F106–F110. doi: 10.1136/fn.84.2.F106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chow LC, Wright KW, Sola A. Can changes in clinical practice decrease the incidence of severe retinopathy of prematurity in very low birth weight infants? Pediatrics. 2003;111:339–45. doi: 10.1542/peds.111.2.339. [DOI] [PubMed] [Google Scholar]
- 5.Anderson CG, Benitz WE, Madan A. Retinopathy of prematurity and pulse oximetry: a national survey of recent practices. J Perinatol. 2004;24:164–8. doi: 10.1038/sj.jp.7211067. [DOI] [PubMed] [Google Scholar]
- 6.Rabi Y, Rabi D, Yee W. Room air resuscitation of the depressed newborn: a systematic review and meta-analysis. Resuscitation. 2007;72:353–63. doi: 10.1016/j.resuscitation.2006.06.134. [DOI] [PubMed] [Google Scholar]
- 7.Silvers KM, Gibson AT, Russell JM, Powers HJ. Antioxidant activity, packed cell transfusions, and outcome in premature infants. Arch Dis Child Fetal Neonatal Ed. 1998;78:F214–F219. doi: 10.1136/fn.78.3.f214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saugstad OD. Bronchopulmonary dysplasia and oxidative stress: are we closer to an understanding of the pathogenesis of BPD? Acta Paediatr. 1997;86:1277–82. doi: 10.1111/j.1651-2227.1997.tb14897.x. [DOI] [PubMed] [Google Scholar]
- 9.Davis JM. Role of oxidant injury in the pathogenesis of neonatal lung disease. Acta Paediatr Suppl. 2002;91:23–5. doi: 10.1111/j.1651-2227.2002.tb00156.x. [DOI] [PubMed] [Google Scholar]
- 10.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–9. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 11.Haynes RL, Folkerth RD, Keefe RJ, et al. Nitrosative and oxidative injury to premyelinating oligodendrocytes in periventricular leukomalacia. J Neuropathol Exp Neurol. 2003;62:441–50. doi: 10.1093/jnen/62.5.441. [DOI] [PubMed] [Google Scholar]
- 12.Collins MP, Lorenz JM, Jetton JR, Paneth N. Hypocapnia and other ventilation-related risk factors for cerebral palsy in low birth weight infants. Pediatr Res. 2001;50:712–9. doi: 10.1203/00006450-200112000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Kinsey VE, Arnold HJ, Kalina RE, et al. PaO2 levels and retrolental fibroplasia: a report of the cooperative study. Pediatrics. 1977;60:655–68. [PubMed] [Google Scholar]
- 14.Flynn JT, Bancalari E, Snyder ES, et al. A cohort study of transcutaneous oxygen tension and the incidence and severity of retinopathy of prematurity. N Engl J Med. 1992;326:1050–4. doi: 10.1056/NEJM199204163261603. [DOI] [PubMed] [Google Scholar]
- 15.Deulofeut R, Dudell G, Sola A. Treatment-by-gender effect when aiming to avoid hyperoxia in preterm infants in the NICU. Acta Paediatr. 2007;96:990–4. doi: 10.1111/j.1651-2227.2007.00365.x. [DOI] [PubMed] [Google Scholar]
- 16.Wright KW, Thompson SD, Ramanathan R, Joseph R, Farzavandi S. A physiologic reduced oxygen protocol decreases the incidence of threshold retinopathy of prematurity. Trans Am Ophthalmol Soc. 2006;104:78–84. [PMC free article] [PubMed] [Google Scholar]
- 17.SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network Early CPAP versus surfactant in extremely preterm infants. N Engl J Med. 2010;362:1970–9. doi: 10.1056/NEJMoa0911783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Askie LM, Henderson-Smart DJ, Irwing L, Simpson JM. Oxygen-saturation targets and outcomes in extremely preterm infants. N Engl J Med. 2003;349:959–67. doi: 10.1056/NEJMoa023080. [DOI] [PubMed] [Google Scholar]
- 19.Hardy RJ, Good WV, Dobson V, et al. Multicenter trial of early treatment for retinopathy of prematurity: study design. Control Clin Trials. 2004;25:311–25. doi: 10.1016/j.cct.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Early Treatment for Retinopathy of Prematurity Cooperative Group Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121:1684–94. doi: 10.1001/archopht.121.12.1684. [DOI] [PubMed] [Google Scholar]
- 21.Walsh MC, Yao Q, Gettner P, Hale E, Collins M, Hensman A. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics. 2004;114:1305–11. doi: 10.1542/peds.2004-0204. [DOI] [PubMed] [Google Scholar]
- 22.The STOP-ROP Multicenter Study Group Supplemental Therapeutic Oxygen for Prethreshold Retinopathy of Prematurity (STOP-ROP), a randomized controlled trial. I. Primary outcomes. Pediatrics. 2000;105:295–310. doi: 10.1542/peds.105.2.295. [DOI] [PubMed] [Google Scholar]
- 23.Cole CH, Wright KW, Tarnow-Mordi W, Phelps DL. Resolving our uncertainty about oxygen therapy. Pediatrics. 2003;112:1415–9. doi: 10.1542/peds.112.6.1415. [DOI] [PubMed] [Google Scholar]
- 24.Askie LM, Henderson-Smart DJ, Ko H. Restricted versus liberal oxygen exposure for preventing morbidity and mortality in preterm or low birth weight infants. Cochrane Database Syst Rev. 2009;1:CD001077. doi: 10.1002/14651858.CD001077.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tin W, Gupta S. Optimum oxygen therapy in preterm babies. Arch Dis Child Fetal Neonatal Ed. 2007;92:F143–F147. doi: 10.1136/adc.2005.092726. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.