Abstract
Deviation in growth rates of the follicles of the ovulatory wave begins at the end of a common growth phase and is characterized by continued growth of the developing dominant follicle (F1) and regression of the largest subordinate follicle (F2). Follicle diameters during an interovulatory interval were compared between 30 mares and 30 women, using similar methods for collecting and analyzing data. Follicles were tracked and measured daily by ultrasonography. Diameter at follicle emergence (mares, 13 mm; women, 6 mm) and the required minimal attained diameter for assessment of follicles (mares, 17 mm; women, 8 mm) were chosen to simulate the reported ratio between the two species in mean diameter of F1 at the beginning of deviation (mares, 22.5 mm; women, 10.5 mm). F1 emerged before F2 (P < 0.02) in each species, and the interval between emergence of the two follicles was similar (not significantly different) between species. Growth rate for F1 and F2 during the common growth phase was similar within species, and the percentage of diameter increase was similar between species. Proportionality between species in diameter of F1 at deviation (2.2 times larger for mares than for women) and at maximum preovulatory diameter (2.1 times larger) indicated that relative growth of F1 after deviation was similar between species. A predeviation follicle was identified in 33% of mares and 40% of women and was characterized by growth to a diameter similar to F1 at deviation but with regression beginning an average of 1 day before the beginning of deviation. The incidence of a major anovulatory wave preceding the ovulatory wave was not different between species (combined, 25%). Results indicated that mares and women have comparable follicle interrelationships during the ovulatory wave, including 1) emergence of F1 before F2, 2) similar length of intervals between sequential emergence of follicles within a wave, 3) similar percentage growth of follicles during the common growth phase, and 4) similar relative diameter of F1 from the beginning of deviation to ovulation. Similar follicle dynamics between mares and women indicate the mare may be a useful experimental model for study of folliculogenesis in women, with the advantage of larger follicle size.
Keywords: follicle, follicular development, follicular waves, mares, ovary, ovulation, ovulatory cycle, women
INTRODUCTION
The development of follicle dominance (selection) in monovular farm animals (cattle, mares) is highlighted by diameter deviation (for a review, see [1]). Deviation begins at the end of a common growth phase for the follicles of the wave and is characterized by continued growth of the developing dominant follicle and regression of the subordinate follicles. In mares, the end of the common growth phase and the beginning of deviation occurs when the future dominant follicle has a mean diameter of 22.5 mm. In women, follicle interrelationships during the common growth phase have not been described and deviation has not been documented, specifically. However, selection has been defined to occur when the largest follicle reaches 10 mm and exceeds the diameter of the next largest follicle by 2 mm [2].
Both mares [3, 4] and women [2, 5] develop major ovulatory follicular waves and major and minor anovulatory waves during an estrous cycle and menstrual cycle, respectively. In major follicular waves, deviation occurs with development of a dominant follicle. The dominant follicle of major anovulatory waves generally does not reach a mean diameter comparable with the maximal diameter of ovulatory follicles. The incidence of major anovulatory waves during an interovulatory interval has been reported as 24% in Quarter Horses [3] and, recently, as 22% in women [2]. Minor waves also develop in both species and are characterized by a largest follicle that does not reach the diameter of a dominant follicle. Reports of minor waves in individual mares were based on a statistical increase in diameters of the six largest follicles [3] and in women were based on observed increases in number of follicles that did not reach 10 mm [2, 5]. In both species, the only wave that develops consistently is the major wave that emerges during the mid-interovulatory interval and gives origin to the ovulatory follicle. In contrast, cattle, another monovulatory species, consistently develop one or two major anovulatory waves during an estrous cycle; the anovulatory follicle of the first major wave attains a diameter similar to the diameter of the ovulating dominant follicle of the last major wave [6]. The preovulatory diameter of the dominant follicle of the ovulatory wave is considerably larger in mares (e.g., 45 mm) than in women (e.g., 20 mm). In contrast, the preovulatory follicle is smaller in heifers (e.g., 16 mm) than in women.
The purpose of this study was to compare and contrast between mares and women the characteristics of follicle development within the ovulatory follicular wave. In addition, similarities between the two species in the patterns and incidence of major and minor follicular waves were compared throughout an interovulatory interval. An incentive for this study was the potential for using the mare as a model for studying the mechanisms of follicle dynamics in women.
MATERIALS AND METHODS
Similar methods were used for mares and women to facilitate and enhance the species comparisons. Follicles were measured by ultrasonography, using the transrectal approach in mares and the transvaginal approach in women. The ultrasonography equipment and scanning techniques are described for mares [7, 8] and women [2, 5]. Examinations were done daily throughout an interovulatory interval and included a few days before the first ovulation and after the second ovulation.
Definitions
Follicle emergence was defined as the last day the follicle was 13 mm in mares and 6 mm in women followed by a progressive increase in diameter. Diameters were derived so that the quotient between species (13/6 = 2.17) was similar to the quotient for reported mean diameters at the beginning of deviation in mares (22.5 mm [1]) and apparent selection in women (10.5 mm [2]; 22.5/10.5 = 2.14). A follicular wave (major or minor) included all follicles that emerged within 2 days of the emergence of another follicle, as previously defined in mares [9], but follicles emerging on or after the beginning of deviation were not included as a component of the major wave. When only one follicle was detected, the single follicle was assumed to represent a wave; in the previous study in women [2], single ≥8-mm follicles were associated with other follicles that did not reach 8 mm. The protocol precluded identifying minor waves in which all follicles were <17 mm in mares and <8 mm in women. Ovulatory waves were excluded from the analyses of the relationships among follicles of the wave when no subordinate follicles meeting the definition of being a member of the wave were detected.
The beginning of observed deviation (Day 0) was indicated by the day a developing dominant follicle (F1) continued to grow at an apparently constant rate and the largest subordinate follicle (F2) began to grow at a comparatively reduced rate or began to regress, as indicated by a decrease in diameter. Thus, F1 and F2 were designated after their destiny became clear, and the identity extended, retrospectively, to emergence. A follicle smaller than F2 that began to regress on the same day as F2 was defined as F3. These definitions are similar to those used previously for cattle and horses [1], except that a follicle was not designated as F3 in the present study unless it began to regress on the same day as F2. Follicles of the ovulatory wave that began to regress before or after the beginning of deviation were designated as early and late regressing follicles, respectively.
A follicle that met the definition of being part of the ovulatory wave and reached a diameter within the range of an F1 at the beginning of deviation (19–27 mm in mares and 8–13 mm in women) but began to regress before the beginning of deviation between F1 and F2 was defined as a predeviation follicle. Growth rates were calculated for the 3 days before deviation for F1 and F2 and the 3 days before the predeviation follicle reached maximum diameter. Percentage growth rate between Days −3 and 0 was used to permit a comparison between species. Regression rates and their percentages were calculated for the 2 days after the beginning of deviation (F2) or maximum diameter (predeviation follicle).
Double dominant follicles were defined as two follicles that reached >28 mm in mares and >13 mm in women. The >28-mm criterion for mares and >13-mm criterion for women were chosen to exceed the maximum diameter of a predeviation follicle and the maximum diameter of the future dominant follicle at the beginning of deviation. These diameters are in the same ratio (similar quotient) between the two species as the diameters at the beginning of deviation, as described above for developing the criterion for diameter at emergence. The >28-mm criterion for mares has been used previously to indicate a second dominant follicle [3]. When double-dominant follicles were present, F3 was used as the largest subordinate follicle and to determine the day of deviation that would be comparable with the day of deviation in individuals with one dominant follicle.
A major anovulatory wave was indicated by the development of diameter deviation and thus contained a dominant follicle as for a major ovulatory wave. A minor wave did not manifest deviation and did not contain a dominant follicle. These wave definitions have been used previously for mares [3] and women [2].
Follicle Monitoring
An identity method was used for detecting the emergence of follicles throughout the interovulatory interval. The identity method involved day-to-day tracking of each follicle that reached ≥17 mm in mares and ≥8 mm in women. The 8-mm criterion was used previously in women [2, 5] and the 17-mm criterion for mares was calculated from the ratio of the mean F1 diameters at the beginning of deviation between mares and women, as described above. Tracking was done retrospectively from daily sketches of ovaries showing diameter and location as described for mares [7, 8] and women [2, 5]. Briefly, the relative position among follicles and between a follicle and the corpus luteum, ovarian attachment, ovarian poles and surfaces, and stroma that did not contain follicles were used as guides. The sonographers operated without knowledge of expected outcome.
Mares
Mares were managed according to the Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching. Follicle data were obtained from 30 mares during the October–March ovulatory season in Brazil (latitude, 21°S). Nonpregnant, nonlactating, small draft-type, crossbred Breton mares between 3 and 13 yr of age and weighing 390–550 kg were used. Mares were kept on pasture under natural light.
Women
The protocol was approved by the Institutional Review Board of the University of Saskatchewan [5]. Follicle data sets for 30 women were selected randomly from 50 sets that were used in previous studies [2, 5]. Thirty women were used so that the number of individuals was consistent for women and mares. Duplication of previously reported findings was minimal. The women were nonpregnant, nonlactating, and 19–43 yr of age; the selection criteria have been previously described [5].
Statistics
Comparisons of quantitative end points between follicle types within species were made by paired t-tests. Comparisons between species were made by unpaired t-tests when two groups were compared and by one-way ANOVA when more than two groups were compared. Frequencies were compared by chi-square tests. A probability of P ≤ 0.05 was used to indicate a significant difference.
RESULTS
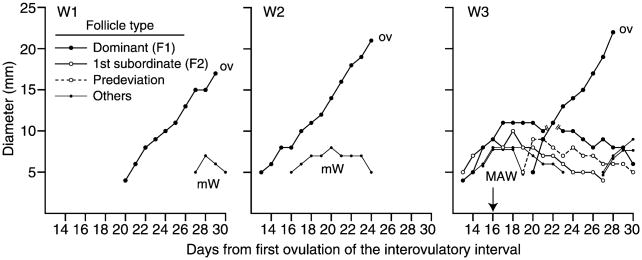
Subordinate follicles of the ovulatory wave were not detected in three mares and five women. These individuals were omitted from the analyses of the follicle profiles of the ovulatory wave because the beginning of deviation could not be determined. The follicle profiles of three of the omitted women are shown (Fig. 1, W1–W3) to illustrate the absence of a subordinate follicle within the definition of the composition of the ovulatory wave. The incidences for the types of follicles during the ovulatory wave are shown (Table 1). There were no differences between species in the number of individuals with multiple subordinate follicles, a predeviation follicle, or an early regressing follicle. However, the number of individuals with late regressing follicles and double dominant follicles was greater in mares. Number of follicles reaching 17 mm in mares and 8 mm in women during the interovulatory interval (Table 1) and during the ovulatory wave (Table 2) was greater for mares than for women.
FIG. 1.
Follicle profiles for the ovulatory wave for three (W1, W2, W3) of the five women that were omitted from the analyses because a subordinate follicle was not detected. mW = minor wave. MAW = major anovulatory wave. OV = ovulation. Arrow indicates the apparent day of the beginning of deviation for MAW. In W1 and W2, the second follicle emerged more than 2 days after the first follicle and therefore was not considered part of the ovulatory wave. In W3, the ovulatory wave consisted of only an apparent predeviation follicle and the ovulatory follicle.
TABLE 1.
Number of follicles during interovulatory intervals, number of intervals with major anovulatory follicular waves and minor waves, and number of ovulatory waves with follicles of various types.a
| End point | Mares | Women | Probability |
|---|---|---|---|
| Interovulatory intervals | |||
| Length (days) | 20.9 ± 0.5 | 27.4 ± 0.5 | <0.001 |
| Follicles (number) | 9.0 ± 0.7 | 6.8 ± 0.2 | <0.03 |
| Major waves (number of IOIs) | |||
| Ovulatory | 30 | 30 | — |
| Anovulatory | 6/30 (20%) | 9/30 (30%) | NS |
| Minor waves (number of IOIs) | 29/30 (97%) | 17/30 (57%) | <0.0002 |
| Follicle types in ovulatory wave (number of waves) | |||
| Largest subordinate follicle | 27/30 (90%) | 25/30 (83%) | NS |
| Second-largest subordinate follicle | 14/27 (52%) | 9/25 (36%) | NS |
| Predeviation follicle | 10/27 (37%) | 12/25 (48%) | NS |
| Early regressing follicles | 9/27 (33%) | 9/25 (36%) | NS |
| Late regressing follicles | 8/27 (30%) | 2/25 (8%) | <0.05 |
| Double dominant follicles | 6/30 (20%) | 0/30 (0%) | <0.02 |
IOI, Interovulatory interval; NS, not significant.
TABLE 2.
Characteristics of the ovulatory follicular wave in mares and women.a
| End point | Mares | Women |
|---|---|---|
| Follicles/wave (number) | 5.9 ± 0.6 (30) | 3.9 ± 0.4 (30) |
| Intervals (days) | ||
| First ovulation to emergence of | ||
| 7.8 ± 1.1 (10b | 14.7 ± 0.7 (12)b | |
| F1 paired with PDF | 9.1 ± 1.0 (10) | 15.8 ± 0.7 (12) |
| F1 | 7.0 ± 1.0 (27)b | 15.8 ± 0.5 (25)b |
| F2 | 7.8 ± 0.9 (27) | 16.8 ± 0.6 (25) |
| Emergence of F1 to deviationc | 3.9 ± 0.3 (27) | 4.0 ± 0.3 (25) |
| Emergence of F1 to ovulation | 14.0 ± 0.6 (27) | 11.5 ± 0.3 (25) |
| Deviation to ovulation | 10.1 ± 0.6 (27) | 7.4 ± 0.4 (25) |
| PDF, emergence to maximumc | 4.3 ± 0.5 (10) | 3.5 ± 0.4 (12) |
| Maximum to deviationc | 1.2 ± 0.4 (10) | 1.0 ± 0.3 (12) |
| Diameters (mm) | ||
| At deviation | ||
| F1 | 22.7 ± 0.5 (27) | 10.3 ± 0.3 (25) |
| F2 | 20.3 ± 0.6 (27) | 9.0 ± 0.2 (25) |
| F3 | 19.4 ± 0.7 (14) | 8.3 ± 0.2 (9) |
| PDF, maximum | 23.6 ± 0.6 (10) | 10.0 ± 0.4 (12) |
| Preovulatory follicle, maximum | 44.8 ± 0.8 (29) | 21.8 ± 0.5 (30) |
| Growth rate (mm/day) | ||
| PDF, Days −4 to −1d | 3.0 ± 0.7 (10) | 1.1 ± 0.1 (12) |
| F1, Days −3 to 0 | 2.7 ± 0.2 (27) | 1.1 ± 0.1 (25)b |
| Days 0 to 3 | 2.7 ± 0.1 (27) | 1.8 ± 0.1 (25) |
| F2, Days −3 to 0 | 2.6 ± 0.2 (27) | 1.0 ± 0.1 (25) |
| Regression rate (mm/day) | ||
| PDF, Days −1 to 1c | 0.5 ± 0.3 (10) | 0.7 ± 0.2 (12) |
| F2, Days 0 to 2c | 1.0 ± 0.3 (27) | 0.8 ± 0.2 (25) |
Mean ± SEM. PDF = predeviation follicle.
Mean is different (P < 0.05) from the mean below it. Number in parentheses is number of ovulatory waves available for determining the mean.
End points with no significant difference (P > 0.05) between species.
Day 0 = day of the beginning of observed deviation.
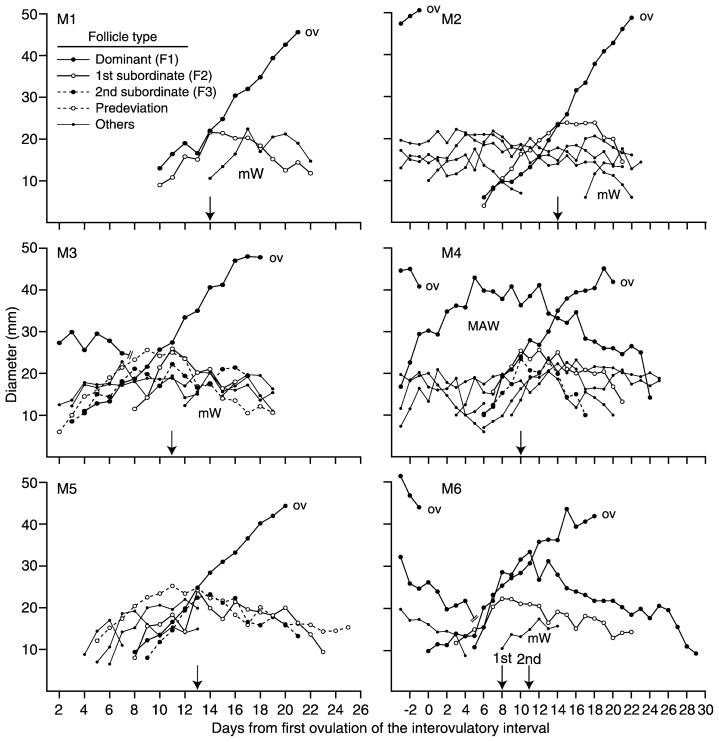
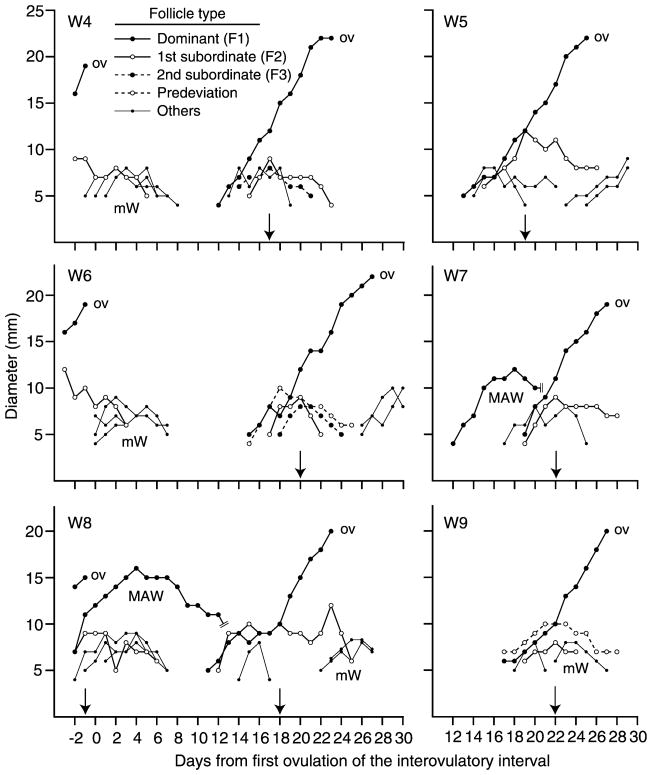
Individual follicle profiles are shown for six of the 27 mares (Fig. 2) and six of the 25 women (Fig. 3) to illustrate the types of follicles of the ovulatory wave and the presence of major anovulatory waves and minor waves. Day of deviation and diameter profile for the dominant follicle (F1) and largest subordinate follicle (F2) are shown for each ovulatory wave. Predeviation follicles (Fig. 1, W3; Fig. 2, M3 and M5; Fig. 3, W6 and W9), second-largest subordinate follicles (F3; M3, M4, W4, W6), early regressing follicles (W5, W7, W9), and late regressing follicles (M4) are illustrated. An apparent transient plateau in the growth of F1 and F2 during 13–18 days after ovulation is shown (Fig. 3, W8). A similar plateau in transiently arrested growth of F1 or F1 and F2 for a period of 3–5 days near the beginning of deviation occurred in three mares and four women. The beginning of regression of F2 occurred abruptly at the beginning of deviation in 15 of 27 mares (56%; Fig. 2, M3 and M5) and in 21 of 25 women (84%; Fig. 3, W4–W9); the difference between species was significant (P < 0.03). In the remaining individuals, F2 increased in diameter at a reduced rate after deviation for 1 or 2 days or plateaued before regressing. Double dominant follicles were detected only in mares (Table 1; Fig. 2, M6).
FIG. 2.
Follicle profiles in mares for three ovulatory waves (M1, M3, M5) and three interovulatory intervals (M2, M4, M6). Emerging follicles were not detected earlier in the interovulatory interval in M1, M3, and M5. MAW = major anovulatory wave. mW = minor wave. OV = ovulation. An arrow indicates the beginning of deviation. Follicles of the ovulatory wave intermingled with regressing follicles from a previous ovulatory wave (M3, M6), a major anovulatory wave (M4), and static follicles from a previous wave (M2). A predeviation follicle (M3, M5) and a late regressing follicle (M4) are shown.
FIG. 3.
Follicle profiles in women for three interovulatory intervals (W4, W6, W8) and three ovulatory waves (W5, W7, W9). Emerging follicles were not detected before 12 days postovulation in W5, W7, and W9. MAW = major anovulatory wave. mW = minor wave. OV = ovulation. An arrow indicates the beginning of deviation. Note the predeviation follicle (W6, W9), a second-largest subordinate follicle (W4, W6), and the transiently arrested growth of the follicles during 13–18 days postovulation (W8).
Discrete data for each species for the predeviation follicle, F1, F2, and F3 of the ovulatory wave are shown (Table 2). The predeviation follicle emerged (interval from ovulation to emergence) earlier than F1 in mares (P < 0.04) and women (P < 0.02), and F1 emerged earlier than F2 in mares (P < 0.02) and women (P < 0.007). The intervals between emergence of the predeviation follicle and F1 and between F1 and F2 were not significantly different within or between species. Emergence of F1 occurred later (P < 0.04) in mares with a predeviation follicle (9.1 ± 1.0 days after ovulation) than in mares without a predeviation follicle (5.5 ± 1.4 days). This difference was not significant in women. The interval from emergence of F1 to the beginning of deviation was not significantly different from the interval from emergence to maximum diameter of the predeviation follicle within or between species. The diameter of F1 at the beginning of deviation and the maximum diameter of the predeviation follicle were not significantly different within either species. There were no significant differences in growth rates among F1 and F2 before deviation and the predeviation follicle before maximum diameter in either species. The growth rate of F1 after deviation was greater (P < 0.0001) than before deviation in women but not in mares. The regression rate (decrease in diameter) was not different between F2 over Days 0–2 and the predeviation follicle over Days −1 to 1 within each species. In mares, the regression rate for F2 after deviation was less (P < 0.0001) than the growth rate before deviation, whereas in women, the corresponding regression rate was not significantly different from the growth rate.
On a percentage basis, the increase in diameter of F1 per day between Days −3 and 0 was not different between mares (19.8% ± 2.1%) and women (18.7% ± 2.1%). The percentage increase in diameter of the predeviation follicle between Days −4 and −1 also was not different between mares (23.0% ± 4.2%) and women (19.4% ± 3.5%). Between Days 0 and 3, the percentage diameter increase for F1 was greater (P < 0.0001) for women (17.6% ± 1.0%) than for mares (12.0% ± 0.9%). However, the percentage decrease for F2 was greater (P < 0.03) in women (9.5% ± 1.9%) than in mares (4.7% ± 1.6%). Similarly, the decrease for the predeviation follicle was greater (P < 0.05) in women (7.4% ± 2.4%) than in mares (2.3% ± 1.4%).
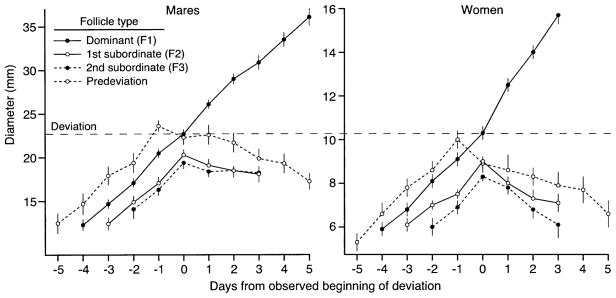
Mean follicle diameters before and after deviation for four follicle types are shown (Fig. 4) for each species. The observed beginning of deviation between the dominant follicle (F1) and the largest subordinate follicle (F2) was designated Day 0 and was used as the centralization reference for F1 and F2. The second-largest subordinate follicle (F3) was included in the centralization for individuals who had an F3, which, by definition, began to deviate on the same day as F2. In both species, the predeviation follicles began to regress on average on Day −1 relative to the beginning of deviation and were centralized accordingly. The relationships among the follicle types illustrated in Figure 4 are given in Table 2, including the results of statistical analyses. The ovulatory follicle (F1) reached its maximum diameter on the day before ovulation in 18 of 29 mares and 29 of 30 women (P < 0.002). In the 11 exceptions in mares, maximum diameter was reached 2 days (n = 8), 3 days (n = 2), or 4 days (n = 1) before ovulation.
FIG. 4.
Means (± SEM) for diameters of four follicle types during ovulatory waves in 27 mares and 25 women. Numbers of waves for mares and women, respectively, with various follicle types were: dominant and first subordinate follicles (27 and 25), second subordinate follicles (14 and 9), and predeviation follicles (10 and 12). Dominant and subordinate follicles are centralized among individuals to the beginning of deviation (Day 0), and the predeviation follicle is centralized to the mean day at the beginning of its regression (Day −1). Statistical analyses for the follicle types are given in Table 2.
Major anovulatory waves are illustrated for mares (Fig. 2, M4) and women (Fig. 1, W3; Fig. 3, W7 and W8). The outcome of waves (major or minor) beginning near the end of the interovulatory interval could not be determined in some individuals (e.g., W3, W5, W6). The frequency of major anovulatory waves during the interovulatory interval did not differ significantly between mares and women (Table 1; combined frequency, 25% of interovulatory intervals). The maximum diameter of the dominant follicle of the anovulatory waves was 35.7 ± 3.9 mm (range, 28.9–51.9 mm) in mares and 12.9 ± 0.8 mm (range, 10–17 mm) in women. The future dominant follicle (F1) of major anovulatory waves in mares emerged at 13 mm 2–4 days before the first ovulation of the interovulatory interval (four mares) or 4 and 7 days before emergence of F1 of the ovulatory wave (two mares). The dominant follicle of the anovulatory wave overlapped the ovulatory wave and, during the overlapping, was larger than all follicles in four of six mares, except the ovulatory follicle. The anovulatory F1 in six women emerged at 6 mm just before or near the first ovulation of the interovulatory interval. These waves did not overlap the ovulatory wave except for one wave (Fig. 3, W8). The remaining three waves began a few days before emergence of the first follicle of the ovulatory wave, and the follicles of the anovulatory wave intermingled with the follicles of the ovulatory wave (Fig. 1, W3; Fig. 3, W7). In addition, one of the nine women had two major anovulatory waves, and the second wave intermingled with the follicles of the ovulatory wave.
In addition to the overlapping of the ovulatory waves by major anovulatory waves in both species, overlapping occurred in mares apparently by the regressing dominant follicle in mares that had both an ovulatory and anovulatory dominant follicle in the previous ovulatory wave (Fig. 2, M3 and M6). In some mares (26%), the ovulatory wave was partly obscured by follicles from a previous wave that were in an apparent static phase (Fig. 2, M2). This apparent phenomenon was not observed in women.
Minor waves are illustrated for mares (Fig. 2, M1, M2, M3, M6) and women (Fig. 1, W1 and W2; Fig. 3, W4, W6, W8, W9). The number of interovulatory intervals with minor waves was greater in mares than in women (Table 1). There was a total of 40 waves in mares and 22 in women during the 30 interovulatory intervals. More than one follicle was detected in the minor waves in mares (40%) and women (60%). The minor waves seemed to occur throughout the interovulatory interval, except for the absence, by definition, during the ovulatory wave from 2 days before emergence to the beginning of deviation. The maximum diameter of the largest follicle of minor waves was 20.2 ± 0.5 mm (range, 16–25 mm) in mares and 8.1 ± 0.1 mm (range, 7–9 mm) in women.
DISCUSSION
Comparing follicle dynamics between mares and women required reference points that were similar in relative value between species. For this purpose, the following four events during the ovulatory wave were used: 1) follicle emergence (derived from reported findings for deviation or selection), 2) beginning of deviation (observed herein), 3) minimal diameter that a follicle must attain to be included for assessment (derived), and 4) maximum preovulatory diameter (observed herein). The present means for the beginning of deviation (mares, 22.7 mm; women, 10.3 mm) were close to the reported means (22.5 [1] and 10.5 mm [2]) that were used in developing the protocol for defining emergence and minimal diameter. Support for the use of reported ratios between species in setting comparable values between species was as follows: 1) although not realized until the study was completed, the derived diameters representing follicle emergence (mare, 13 mm; women, 6 mm) were at the reported peak of the wave-stimulating FSH surge in mares [7] and approximately at the peak in women [2]; 2) similarity between species in the present study in the percentage growth of follicles for the 3 days before deviation; and 3) similarities in the present study in the ratios between species in the diameters of F1 at the beginning of deviation (2.2 times larger for mares than for women) and at maximum preovulatory diameter (2.1 times larger for mares than for women). It is concluded that the chosen reference values were reasonable and that the relative growth of follicles was similar between the two species, with diameters of the follicles being slightly more than two times larger in mares throughout the ovulatory wave.
The incidence of predeviation follicles during the ovulatory wave was similar between species (combined, 42%). Although predeviation follicles have not been described previously in any species, the follicles had the following average features in both species: 1) earlier emergence than for F1, 2) a growth rate similar to F1 during the common growth phase, 3) maximum diameter similar to diameter of F1 at the beginning of deviation, and 4) maximum diameter occurring 1 day before the beginning of deviation. The day of observed beginning of deviation was obscured in these individuals until the predeviation follicle was recognized as a separate follicle type. Confirmation that the predeviation follicle is a specific follicle type will require elucidation of a mechanism that accounts for its regression before the beginning of deviation. Speculatively, the predeviation follicle may represent early cessation of growth from reaching a diameter similar to F1 at deviation before a deviation mechanism is in place. A delay in development of the deviation mechanism may also account for the transient 3- to 5-day plateaus in growth of F1 before deviation in a few individuals in each species.
The follicles of the ovulatory wave emerged in the following average sequence in both species: predeviation follicle, F1, and F2, with a mean sequential interval between emergence of follicles of 0.8–1.0 days in the two species. In agreement, a mean interval between emergence of F1 and F2 of 1 day has been reported for mares in which a two-follicle model was used (two follicles retained and all others ablated as they developed [7]). In a study in 50 women that included 30 of the same data sets used in the present study, it was concluded that the two largest future subordinate follicles emerged before the future dominant follicle [2]. Two considerations seem to account for the conflicting conclusions between the two studies. The subordinate follicles in the previous study but not in the present study included predeviation follicles and, in some individuals, may have included follicles from a previous adjacent wave. In regard to the latter consideration, the present study imposed a limitation of no more than a 1-day lapse between sequential emergence of follicles for a follicle to be included as part of the same wave. It is concluded, that by day of emergence at 13 mm in mares and 6 mm in women, the future dominant follicle had a mean diameter advantage over the largest future subordinate follicle in both species. The similar average growth rate between the two follicles during the common growth phase in both species in the present study resulted in a larger F1 than F2 at the beginning of deviation. It has been proposed that this advantage in diameter in farm species represents an advantage in time and allows F1 to establish its destiny as the dominant follicle before F2 reaches a similar diameter (for a review, see [1]).
The relative or percentage growth rate of follicles during the common growth phase was similar among follicles and between species. Approximately parallel growth of F1 and F2 before deviation has been reported previously for the two follicles of a two-follicle model in mares [7] However, the interrelationships among follicles during the common growth phase have not been reported previously for spontaneous wave development in mares and have not been reported for women. A finding in women but not in mares was the greater growth rate of F1 after deviation than before deviation. The underlying mechanism that would account for the more rapid increase in F1 diameter after deviation in women but not in mares is not known. Although relative percentage growth rates were similar between species before deviation, the regression rates for the predeviation follicle and F2 were not; a greater 2-day percentage regression of the predeviation follicle and F2 occurred in women than in mares. In this regard, regression of F2 occurred abruptly in more women (84%) than in mares (56%), so that the day of the beginning deviation was more pronounced in women. The factors accounting for the more rapid regression of the predeviation and subordinate follicles in women are not known. Another difference between species was the more frequent occurrence of maximum diameter of the preovulatory follicle before the day preceding ovulation in mares.
The equine ovulatory waves also differed in that late-regressing follicles and double-dominant follicles were more numerous. Late-regressing follicles satisfied the definition of being a component of the wave but began to regress after the beginning of deviation. The mechanisms accounting for their characteristics are not known, but the low incidence in women (8% of waves) compared with mares (30%) may be at least partly related to the lesser follicle population in the ovulatory waves of women. Double-dominant follicles (either anovulatory or ovulatory) did not occur in women during the assessed ovulatory wave (end of interovulatory interval), but ovulation of two follicles did occur in two women at the beginning of the interovulatory interval. Double-dominant follicles occurred in six mares with ovulation of either one follicle (five mares; e.g., Fig. 2, M6) or both follicles (one mare). The regressing anovulatory follicle contributed to the complexity of the next ovulatory wave in mares.
The development of major anovulatory waves before emergence of the ovulatory waves occurred with similar frequency between species (combined incidence, 25%). The incidence and features for the major anovulatory waves were consistent with previous reports for mares [3] and women [2]. The largest anovulatory follicle of major waves met the definition of a dominant follicle (>28 mm) for all waves in mares. However, in women, only 3 of the 10 waves had a follicle that could be defined as dominant on the basis of exceeding a comparable diameter of 13 mm. The seven waves with a largest follicle of 10–13 mm were nevertheless considered to be major waves because of the presence of apparent deviation. In some instances, deviation was less obvious and could have been considered equivocal (e.g., Fig. 1, W3). No ovulations were detected from the major waves in either species.
The intermingling of follicles from a previous wave with the follicles of the ovulatory wave occurred in both species, but was more common in mares. As noted above, the intermingling follicles originated from a previous anovulatory wave in both species and from nonovulatory follicles of a previous ovulatory wave in mares. In addition, apparent static follicles from earlier waves persisted during the ovulatory wave in 25% of mares. Also, the greater number of follicles reaching 17 mm in mares than for follicles reaching 8 mm in women during the interovulatory interval and ovulatory wave added to the relatively greater follicle complexity in mares. Greater complexity among follicles in mares than in women was also indicated by more individuals with minor waves, despite the shorter interovulatory intervals.
The striking similarities between mares and women in the dynamics of follicles during the interovulatory interval and during the ovulatory follicular wave provide encouragement for the potential use of the mare as a relevant experimental model for study of folliculogenesis in women. Although mares have added complexity from the intermingling of follicles from previous waves with follicles of the ovulatory wave, this aspect can be experimentally managed by ablating all follicles and studying the new follicular wave [7]. Furthermore, the number of follicles in the new wave can be modified by selective ablations to simplify follicle tracking from examination to examination. The equine model allows hypothesis testing using invasive technologies that cannot be used in women and thereby may provide additional information that can be considered in human clinical medicine. The large size of equine follicles provides superb intrafollicular experimental access for in vivo experimental manipulations, such as intrafollicular treatment [10], sequential sampling of follicular fluid [10], and monitoring predeviation vascular changes by color Doppler ultrasonography [11]. The present results also encourage comparative study between the two species on the similarities and differences in the systemic and local physiologic changes associated with the morphologic follicle dynamics.
In conclusion, the differences between mares and women in dynamics of follicle development during an interovulatory interval and during the ovulatory follicular wave included 1) a more complex ovulatory follicular wave in mares than in women, due to more follicles per wave and more intermingling of follicles from previous waves; 2) greater growth rate of the dominant follicle after deviation than before deviation in women but not in mares; and 3) greater regression rate for nondominant follicles in women. The similarities between mares and women included 1) emergence of the future dominant follicle before the future largest subordinate follicle, 2) similar length of intervals between sequential emergence of follicles, 3) similar percentage growth of follicles during the common growth phase, 4) maintenance of the mare:women ratio in diameter of the dominant follicle (about two times larger in mares) from the beginning of deviation to ovulation, 5) similar incidence of predeviation follicles during ovulatory waves, and 6) similar incidence of major anovulatory waves during the interovulatory interval.
Acknowledgments
The authors thank S. Jensen for statistical assistance and preparation of figures.
Footnotes
Supported by the Eutheria Foundation (Cross Plains, WI), Federal University of Viçosa (Viçosa, Brazil), and a grant from the Canadian Institutes of Health Research, project W1-OG-04. E.L.G. and M.O.G. are on leave from the Departments of Veterinary and Animal Science, respectively, Federal University of Viçosa, Viçosa, Brazil.
References
- 1.Ginther OJ, Beg MA, Donadeu FX, Bergfelt DR. Mechanism of follicle deviation in monovular farm species. Anim Reprod Sci. 2003;78:239–257. doi: 10.1016/s0378-4320(03)00093-9. [DOI] [PubMed] [Google Scholar]
- 2.Baerwald AR, Adams GP, Pierson RA. Characterization of ovarian follicular wave dynamics in women. Biol Reprod. 2003;69:1023–1031. doi: 10.1095/biolreprod.103.017772. [DOI] [PubMed] [Google Scholar]
- 3.Ginther OJ. Major and minor follicular waves during the equine estrous cycle. J Equine Vet Sci. 1993;13:18–25. [Google Scholar]
- 4.Ginther OJ. Reproductive Biology of the Mare, Basic and Applied Aspects. 2. Cross Plains, WI USA: Equiservices Publishing; 1992. [Google Scholar]
- 5.Baerwald AR, Adams GP, Pierson RA. A new model for ovarian follicular development during the human menstrual cycle. Fertil Steril. 2003;80:116–122. doi: 10.1016/s0015-0282(03)00544-2. [DOI] [PubMed] [Google Scholar]
- 6.Ginther OJ, Knopf L, Kastelic JP. Temporal associations among ovarian events in cattle during oestrous cycles with two and three follicular waves. J Reprod Fertil. 1989;87:223–230. doi: 10.1530/jrf.0.0870223. [DOI] [PubMed] [Google Scholar]
- 7.Gastal EL, Gastal MO, Bergfelt DR, Ginther OJ. Role of diameter differences among follicles in selection of a future dominant follicle in mares. Biol Reprod. 1997;57:1320–1327. doi: 10.1095/biolreprod57.6.1320. [DOI] [PubMed] [Google Scholar]
- 8.Ginther OJ. Ultrasonic Imaging and Animal Reproduction: Book 2, Horses. Cross Plains, WI USA: Equiservices Publishing; 1995. [Google Scholar]
- 9.Gastal EL, Gastal MO, Nogueira GP, Bergfelt DR, Ginther OJ. Temporal interrelationships among luteolysis, FSH and LH concentrations and follicle deviation in mares. Theriogenology. 2000;53:925–940. doi: 10.1016/S0093-691X(00)00240-5. [DOI] [PubMed] [Google Scholar]
- 10.Ginther OJ, Gastal EL, Gastal MO, Checura CM, Beg MA. Dose-response study of intrafollicular injection of insulin-like growth factor-1 on follicular-fluid factors and follicle dominance in mares. Biol Reprod. 2003;70:1063–1069. doi: 10.1095/biolreprod.103.024844. [DOI] [PubMed] [Google Scholar]
- 11.Acosta TJ, Gastal EL, Gastal MO, Beg MA, Ginther OJ. Differential blood flow changes between the future dominant and subordinate follicles precede diameter changes during follicle selection in mares. Biol Reprod. 2004;71:502–507. doi: 10.1095/biolreprod.104.027896. [DOI] [PubMed] [Google Scholar]