Abstract
The rat retrotrapezoid nucleus (RTN) contains about 2000 Phox2b-expressing glutamatergic neurons (ccRTN neurons; 800 in mice) with a well-understood developmental lineage. ccRTN neuron development fails in mice carrying a Phox2b mutation commonly present in the congenital central hypoventilation syndrome. In adulthood, ccRTN neurons regulate the breathing rate and intensity, and may regulate active expiration along with other neighboring respiratory neurons. Prenatally, ccRTN neurons form an autonomous oscillator (embryonic parafacial group, e-pF) that activates and possibly paces inspiration. The pacemaker properties of the ccRTN neurons probably vanish after birth to be replaced by synaptic drives. The neonatal parafacial respiratory group (pfRG) may represent a transitional phase during which ccRTN neurons lose their group pacemaker properties. ccRTN neurons are activated by acidification via an intrinsic mechanism or via ATP released by glia. In summary, throughout life, ccRTN neurons seem to be a critical hub for the regulation of CO2 via breathing.
1. Introduction
The retrotrapezoid nucleus, RTN, is a rostral and ventral portion of the medullary reticular formation that was originally delineated in adult cats and rats (Connelly et al., 1989; Smith et al., 1989). The function of the RTN has been investigated primarily from the standpoint of its contribution to the homoeostatic regulation of breathing, namely how breathing is unconsciously regulated to maintain the constancy of blood gases (Feldman et al., 2003; Ritucci et al., 2005; Nattie and Li, 2008; Guyenet, 2008). This perspective traces its roots to the 1960s when it was suggested that the ventral surface of the medulla was the principal site of central respiratory chemoreception (Mitchell et al., 1963; Mitchell, 2004). RTN research has been performed in intact adult animals and in tissue slices from rodents older than 7 days.
The research that has led to the concept of parafacial respiratory group (pfRG) and its presumed embryonic precursor the e-pF (embryonic parafacial oscillator) has been carried out in rodent embryos or neonates less than 4 day old (Ballanyi et al., 1999; Onimaru and Homma, 2008; Thoby-Brisson et al., 2009). The physiological experiments were predominantly done in vitro using brainstem slices or brainstem-spinal cord preparations. Finally, the rationale of the research was also somewhat different because it has been carried out by scientists whose overriding interest was to understand how the breathing rhythm is generated and how this function evolves during development.
Both research lines have recently converged on one particular type of interneurons (hereafter called the ccRTN neurons, Figure 1A) that are the main focus of this review (Stornetta et al., 2006; Onimaru et al., 2008; Thoby-Brisson et al., 2009; Lazarenko et al., 2009). The term ccRTN neuron introduced by Lazarenko et al. (2009) refers specifically to the non-cholinergic, non-aminergic, CO2-activated neurons of the RTN region that express vesicular glutamate transporter2 (VGLUT2), transcription factor Phox2b and substance P (NK1) receptors (Mulkey et al., 2004; Lazarenko et al., 2009). These neurons seem to be critical components of the breathing network at all stages of life but the way in which they control breathing changes during development. Interest in the ccRTN neurons has surged in the wake of recent evidence that an abnormality of their prenatal development may cause the severe respiratory deficits that characterize the congenital central hypoventilation syndrome (CCHS) (Dubreuil et al., 2008; Weese-Mayer et al., 2009).
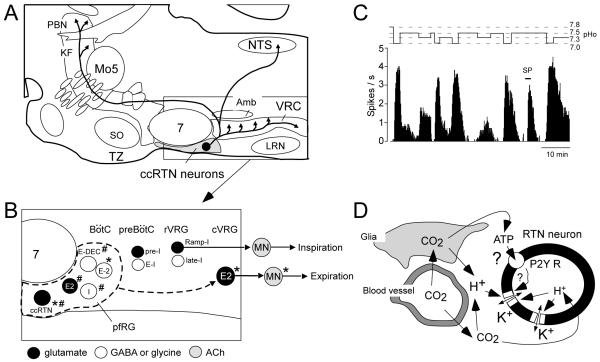
Figure 1.
RTN and pfRG
A. Schematic parasagittal section through the pontomedullary region of the rodent brain showing the location of the ccRTN neurons (grey area) and the known projection of this cell group (Rosin et al., 2006; Abbott et al., 2009). Abbreviations: Amb, compact portion of nucleus ambiguus; KF, Kölliker-Fuse nucleus; LRN, lateral reticular nucleus; Mo5, trigeminal motor nucleus; NTS, nucleus of the solitary tract; PBN, parabrachial nuclei; SO, superior olive; tz, trapezoid body; VRC, ventral respiratory column. B: blow-up of the ventrolateral medulla showing the region that may contain the expiratory oscillator suggested by the experiments of Janczewski and Feldman (2006). Originally, the term parafacial respiratory group, pfRG, referred to neurons with a double pre-I/post-I discharge that were located under and caudal to the facial motor nucleus (Onimaru and Homma, 2003). The asterisk identifies some of the many neurons currently known to exhibit a double pre-I-post-I discharge in the Suzue preparation (neonate brainstem spinal cord in vitro) and / or in hypoxic adult rodent preparations (Onimaru et al., 2008; Fortuna and Guyenet, 2008; Abdala et al., 2009). The pound sign (#) identifies neurons that plausibly contribute inhibitory or excitatory inputs to the expiratory premotor neurons (E2 neurons of the caudal ventral respiratory group, cVRG). This population may include inhibitory neurons such as the expiratory decremental (E-DEC) neurons of the Bötzinger region and the inspiratory (I) neurons of the retrofacial region (Anders et al, 1991; Bainton and Kirkwood, 1979; Ballantyne and Richter, 1986; Iscoe, 1998) and excitatory neurons such as the ccRTN neurons and, perhaps, the newly described E2 neurons (Abbott et al., 2009; Abdala et al., 2009). Under specific circumstances these various neurons may form an expiratory oscillator that functions independently of the inspiratory oscillator. The inspiratory oscillator resides primarily in the pre-Bötzinger complex (pre-BötC) (Feldman and Del Negro, 2006). Pre-I, E-I, Ramp-I and late-I are respiratory-phasic neurons that contribute to the generation of the inspiratory motor outflow according to Smith et al. (2007). rVRG: rostral ventral respiratory group. C: example of one ccRTN neuron recorded at room temperature in a transverse slice obtained from a Phox2b-eGFP 8 day-old mouse (modified from Lazarenko et al., 2009). The record also shows the robust response of this neuron to 0.1 μM substance P. The acid sensitivity of the neuron illustrated in C (~1Hz / 0.1 pH unit) is typical of the ccRTN neurons. The average response to acid doubles at 37 degrees Celsius in the same neonate in vitro preparation and doubles again in vivo in the adult. D: schematic illustration of the hypothetical mechanisms responsible for the acid-sensitivity of ccRTN neurons. In the neonate in vitro, neuronal activation by acid results from the reduction of a background potassium conductance. The proton receptors may be potassium channels that are directly sensitive to intracellular or extracellular acidification. These channels are not identified. In the adult in vivo the acid sensitivity of RTN neurons may be further enhanced by the surrounding glia. The hypothesized mechanisms include the release of ATP and protons by a subset of glial cells depolarized by acid (Erlichman et al., 2008; Gourine et al., 2005).
Many other types of respiratory interneurons reside within or very close to the region of the reticular formation that contains the ccRTN neurons. The well studied Bötzinger region is not far and the reticular formation that borders on the facial motor nucleus, sometimes also called pfRG in a generic sense, may harbor novel or at least insufficiently characterized respiratory interneurons (Janczewski and Feldman, 2006; Abdala et al., 2009). These neurons may regulate active expiration selectively, possibly in cooperation with the ccRTN neurons, and may form the basis of a partially autonomous expiratory oscillator.
The present paper is an attempt to synthesize the literature on the related topics of the RTN and pfRG/e-pF in view of identifying the key issues that need further clarification.
2. Origins of the concepts of RTN and pfRG
The RTN was initially defined in adult cats as a cluster of rostral medullary neurons that were retrogradely labeled following tracer injection into the dorsal or the ventral respiratory groups (Smith et al., 1989). A presumably homologous group of neurons was described soon after in rats (Connelly et al., 1989). These neurons were detected ventral to the facial motor nucleus up to the level of the trapezoid body hence the name “retrotrapezoid” nucleus (Figure 1A). RTN neurons had two potentially interesting characteristics from a respiratory physiology perspective. They were located rostral to the Bötzinger (a.k.a. retrofacial) region of the ventrolateral medulla oblongata (Merrill et al., 1983; Alheid et al., 2002; Alheid and McCrimmon, 2008) which contains the most rostral component of the ventral respiratory group identified at that time. Secondly, RTN neurons were in reasonably good register with area M of the cat, a region of the ventral medullary surface previously associated with respiratory chemosensitivity but very poorly defined from a cytological standpoint (Mitchell et al., 1963; Mitchell, 2004). Because of its location and connections, Smith et al. (1989) suggested that the RTN might contain neurons that regulate breathing, possibly contributing a CO2-modulated drive to the respiratory pattern generator. As detailed later on, the region of the brain originally defined as RTN does indeed contain a cluster of about 2000 acid-sensitive glutamatergic neurons in rats, (800 in mice) that makes an important if not fully understood contribution to breathing (Stornetta et al., 2006; Takakura et al., 2008; Lazarenko et al., 2009) These neurons are identified by a specific combination of biochemical markers and are called here the chemically coded or ccRTN neurons (Lazarenko et al., 2009) to distinguish them from other neurons that may reside within the RTN or in its immediate vicinity.
The concept of the pfRG originates from neurophysiological experiments done by Onimaru and his colleagues since the late ‘80s (Onimaru et al., 1987; Onimaru and Homma, 1987) although the expression “parafacial respiratory group” seems to have been introduced much later (Onimaru and Homma, 2003). The work has been carried out in an isolated brainstem and spinal cord preparation of newborn rat (P0-P4) in which the phrenic and hypoglossal nerves exhibit periodic bursts of respiratory-like activity (Suzue, 1984). This preparation contains neurons with an unusual discharge pattern never observed in anesthetized adult preparations. This pattern consists of twin bursts of action potentials that bracket the phrenic nerve discharge (Onimaru et al., 1987; Onimaru and Homma, 1987). Despite the fact that a majority of their action potentials typically occurs in the post-inspiratory phase, these twin-bursting neurons were named “pre-inspiratory” (pre-I) neurons, a choice evidently motivated by the budding theory that these neurons were somehow involved in the timing of the inspiratory bursts. Pre-I cells are frequently encountered in the vicinity of the facial motor nucleus and this particular subset of pre-I cells is usually called the parafacial respiratory group (pfRG) (Onimaru et al., 1987; Onimaru and Homma, 1987; Ballanyi et al., 1999; Onimaru and Homma, 2003). Further research revealed that these pre-I cells are also neurochemically and functionally heterogeneous but that the most rostral and superficial of them are ccRTN neurons (Onimaru et al., 2008).
More recently, the term pfRG has also been used in a broader sense to refer to all the respiratory neurons that might be present within the portion of the reticular formation located in proximity to the facial motor nucleus (Janczewski and Feldman, 2006). This region is suspected to contain neurons that are selectively implicated in the generation of active expiration (Figure 1B).
3. Towards a biochemical definition of the RTN
Early on, RTN neurons were found to lack serotonergic markers (Connelly et al., 1989) but their biochemical phenotype remained unknown for 15 years. During this period, the anatomical descriptions of the rodent RTN alluded to a region of the reticular formation with rather imprecise borders that encroached to various extents on the medially located paragigantocellular nucleus and the more caudal region claimed as the Rostroventrolateral medulla (RVL) by cardiovascular physiologists and the Bötzinger region by scientists interested in respiration (Andrezik et al., 1981; Morrison et al., 1988; Cream et al., 2002; Nattie and Li, 2006; Alheid and McCrimmon, 2008). Despite its name, the RTN of the rat cannot be identified as a nucleus on mere architectonic grounds. The nearby paragigantocellular nucleus also contains many neurons that innervate the ventral respiratory column or at least neurons within it (Ellenberger and Feldman, 1990; Dobbins and Feldman, 1994). Caudally, the RTN merges with the Bötzinger region which also projects massively to the rest of the ventral respiratory column and to the nucleus of the solitary tract (Ezure et al., 2003).
Weston et al. (2004) used in situ hybridization to first note the presence of extremely superficial neurons that express the vesicular glutamate transporter VGLUT2 in the region of the rat brain previously defined as RTN. They showed that these cells projected to the ventral respiratory column and therefore that they qualified as RTN neurons according to the original definition of the term in rats (Connelly et al., 1989). The next major anatomical clue was that the transcription factor Phox2b is expressed in the RTN region of the adult rat by only two cell types, the C1 adrenergic neurons, and a group of non-aminergic, uniformly glutamatergic neurons (the ccRTN neurons) (Stornetta et al. 2006). The distribution of the ccRTN neurons is in excellent register with the RTN as originally defined by Connelly et al. (1989). The somata of the ccRTN neurons are small and located consistently lateral to the classically defined rostral paragigantocellular nucleus (Andrezik et al., 1981). The ccRTN neurons are detectable up to the trapezoid body where the rostral limit of the RTN had originally been postulated on various grounds, notably the presence of a respiratory response to topical acidification in vivo (Connelly et al., 1989; Cream et al., 2002). In the adult rat, the ccRTN neurons express a low level of substance P receptor (NK1R)-immunoreactivity although substance P is capable of activating these cells vigorously (Mulkey et al., 2007a; Takakura et al., 2008). NK1-R immunoreactivity appears more strongly expressed in the mouse, at least around birth (Dubreuil et al., 2009b). The cluster of ccRTN neurons continues for some distance caudal to the facial motor nucleus ventrolateral to the C1 neurons within an extremely heterogeneous region of the reticular formation (Takakura et al., 2008). The ccRTN neurons number around 2000 in rats and 800 in mice (Takakura et al., 2008; Lazarenko et al., 2009). The ccRTN neurons whose axonal projections have been traced so far were found to innervate exclusively the pontomedullary regions that contain the respiratory pattern generator (Rosin et al., 2006; Abbott et al., 2009). Based on both retrograde and anterograde tract-tracing evidence these neurons do not appear to innervate respiratory motor neurons (Dobbins and Feldman, 1994; Rosin et al., 2006; Abbott et al., 2009) .
In short, the ccRTN neurons are potentially glutamatergic because they contain VGLUT2, they express Phox2b and NK1 receptors but they lack known aminergic markers. These neurons lie at the core of the region previously defined as the RTN on the basis of anatomical and functional studies. The ccRTN neurons appear biochemically homogeneous at the present time but this interpretation may have to be reconsidered in the future. For example, only 50% of the ccRTN neurons express galanin (Stornetta et al., 2009) and a thorough gene expression profile would most likely reveal additional heterogeneity.
4. The ccRTN neurons as a source of CO2-regulated drive to the pattern generator
The RTN region expresses the protooncogene product cFos in rats exposed to hypercapnia (e.g. (Sato et al., 1992) and many of these cells are ccRTN neurons (Fortuna et al., 2009). In vivo acidification of the RTN region increases breathing in both anesthetized and conscious rats (Li et al., 1999). ccRTN neurons are vigorously activated by increases in CNS pCO2 and by peripheral chemoreceptor stimulation (Mulkey et al., 2004; Takakura et al., 2006). Selective photoactivation of channelrhodopsin2-transfected ccRTN neurons in vivo increases the activity of the phrenic nerve (Abbott et al., 2009). In brief, the ccRTN neurons are physiologically activated by both central and peripheral chemoreceptor stimulation and their direct and selective activation increases breathing. This evidence demonstrates that they are a source of CO2-dependent excitatory drive to the respiratory controller.
The ccRTN neurons are also activated by acidification in tissue slices (Figure 1C)(Mulkey et al., 2004; Ritucci et al., 2005; Lazarenko et al., 2009). Their response to acid in vitro persists after blocking glutamatergic, GABAergic, glycinergic and purinergic receptors (Mulkey et al., 2004) and, in adult anesthetized rats in vivo, these neurons maintain their response to CO2 under conditions of reduced synaptic activity (ionotropic glutamate receptor blockade) (Mulkey et al., 2004). In other words, the ccRTN neurons respond to local changes in pH by a mechanism that does not rely on conventional ionotropic synaptic transmission. Whether their response to pH is an intrinsic property or involves neighboring cells via an autocrine process is unsettled.
Results obtained in slices incubated at room temperature suggest that ccRTN neurons detect changes of CO2 via the proxy of pH (Mulkey et al., 2004), possibly via changes in intracellular pH although a change in pHi is not absolutely required for chemosensitivity in other putative chemoreceptor neurons (Ritucci et al., 2005; Hartzler et al., 2008). A pH-sensitive resting potassium conductance contributes to their acid-induced depolarization (Mulkey et al., 2004; Mulkey et al., 2007b) but the responsible potassium channel(s) is (are) unidentified at this time. In theory, these K channels could be downstream effector (s) of the “real” pH sensor. Based on the study of knock-out mice, neither TASK1 nor TASK3 contribute significantly to respiratory chemosensitivity in adults nor to the pH-induced current of ccRTN neurons in slices (Mulkey et al., 2007b). TASK2 is a closely related two-pore potassium channel that also has some pH-sensitivity and is very uncommonly expressed by brain neurons (L’Hoste et al., 2007; Dubreuil et al., 2009a). Intriguingly, this channel is expressed by the ccRTN neurons (Dubreuil et al., 2009a). Whether these channels are an integral part of the pH sensing mechanism of the ccRTN neurons has yet to be tested.
A paracrine mechanism has also been proposed to account for the pH-sensitivity of RTN neurons and, more generally, for central respiratory chemosensitivity (Figure 1D). This theory postulates that a subset of glial cells are depolarized by acidification and activate central chemoreceptors such as the ccRTN neurons via the release of ATP (Spyer et al., 2004; Gourine et al., 2005). This theory is supported by the following evidence. The glial cells of the ventral medullary surface (presumably astrocytes) are depolarized by acidification (Fukuda and Honda, 1975; Fukuda et al., 1978; Ritucci et al., 2005). Astrocytes elsewhere in the brain can release many transmitters including ATP and ATP release is required for the intercellular propagation of calcium waves in glia (Guthrie et al., 1999). ATP is released by hypercapnia at the ventral medullary surface in vivo (Spyer et al., 2004; Gourine et al., 2005). Finally, purinergic receptor antagonists delivered by iontophoresis attenuate the activation by CO2 of various types of respiratory neurons in vivo (Thomas and Spyer, 2000; Gourine et al., 2005).
This evidence is substantial but several important questions or problems remain. The source of the ATP released by hypercapnia at the ventral medullary surface has not been identified and could be cells other than glia. Pia-arachnoid cells, for instance, communicate via ATP very much like glial cells, both between each other and with the underlying superficial glia (Grafstein et al., 2000). However, the major stumbling block is that purinergic P2-receptor antagonists do not change the pH-sensitivity of the ccRTN neurons in slices (Mulkey et al., 2004; Mulkey et al., 2006). The lack of effect of these antagonists could simply mean that glial cells do not release ATP in coronal brain slices. This may be true but it evidently does not explain why the ccRTN neurons are activated by acidification in these slices. Perhaps, ATP release by glia contributes to the pH-sensitivity of the ccRTN neurons, but only in vivo. Two arguments support this possibility to some extent. First, ccRTN neurons are very mildly activated by P2Y receptor agonists in slices (Mulkey et al., 2006). Secondly, the pH-sensitivity of the ccRTN neurons is perhaps 3 times higher in vivo (roughly 0-10 Hz when end-expiratory CO2 is increased from 4 to 8%, a presumed pH change of around 0.2 units)(Mulkey et al., 2004; Guyenet et al., 2005a) than under the best circumstances in vitro (0-12 Hz between pH 7.5 and 6.9 when recorded at 35 degrees Celsius) (Guyenet et al., 2005b). The difference in pH sensitivity (~5 Hz per 0.1 pH unit in vivo vs. 2 Hz per 0.1. pH unit in slices) could have many causes including the age of the animals, a possible convergence of other chemoreceptor inputs on RTN neurons in vivo, lower temperature and partial dendritic pruning in slices. However, ATP release from glial cells could also account for this difference in pH sensitivity. In addition, mature glial cells may also boost extracellular acidification around the ccRTN neurons via the phenomenon called depolarization-induced alkalosis (DIA), whereby an increase in extracellular potassium and or acidification causes glial cell depolarization and the extrusion of protons from these cells (Figure 1D)(Fukuda et al., 1978; Erlichman et al., 2008).
The different types of neurons contributing to central respiratory chemosensitivity and the relative importance of each chemoreceptor candidate population to the overall process has been thoroughly debated recently (Richerson et al., 2005; Nattie and Li, 2008; Guyenet et al., 2008; Branco et al., 2009). In our opinion, the number and hierarchy of central chemoreceptors are issues that are unlikely to be settled until the pH-sensing mechanism is identified and deleted in a cell-specific manner. Prior attempts at testing the importance of a particular type of CNS neuron to central respiratory chemoreception have consisted of deleting (by genetic manipulations or via a toxin) or silencing (via application of an inhibitory substance such as muscimol) the cell type in question and examining whether the chemoreflex is attenuated (orexinergic, noradrenergic, serotonergic, ccRTN neurons) (Li and Nattie, 2006; Penatti et al., 2006; Nattie and Li, 2006; Dubreuil et al., 2008; Hodges et al., 2008; Takakura et al., 2008; Kuwaki, 2008). This approach implicates the targeted neurons in some aspect of the breathing response to CO2 but it does not distinguish between the following three possibilities: the targeted neurons are intrinsically activated by acid in vivo and contribute to the stimulation of breathing by CO2 (i.e. these neurons are true respiratory chemoreceptors), the targeted neurons are not respiratory chemoreceptors but their ongoing activity normally facilitates the brain’s overall respiratory response to CO2 and, the targeted neurons are not respiratory chemoreceptors but they are indirectly activated by hypercapnia and their activation facilitates the breathing response to CO2.
The experiments designed to test the importance of the ccRTN neurons for central chemoreception have not been immune to the experimental design problem evoked above. The acid-sensing mechanism of the ccRTN neurons is unknown and may even partially reside on surrounding glial cells. Thus, deleting it without killing or silencing the ccRTN neurons has not been done. Nonetheless, recent evidence reinforces the impression that the ccRTN neurons are a crucial hub for central chemoreception. For example, the ccRTN neurons appear to target selectively, or at least preferentially, the respiratory pattern generator. A similarly restricted projection pattern has not been shown in the case of the noradrenergic, serotonergic and orexinergic neurons although pH-sensitive subgroups of these neurons could conceivably have such characteristics. ccRTN neurons are necessary to breathe, at least under anesthesia and at birth (Dubreuil et al., 2008; Takakura et al., 2008) which highlights the potential importance of their pH sensitivity for breathing regulation. Finally, the selective deletion of the ccRTN neurons eliminates the pH-dependence of the respiratory-like outflow of the Suzue preparation (Dubreuil et al., 2009b). Because this preparation still generates a periodic outflow, albeit at lower frequency than in controls, the pre-Bötzinger complex and whatever else generates the respiratory outflow in this preparation must be, in the aggregate, insensitive to pH unless the ccRTN neurons are present.
5. The pfRG is anatomically and functionally heterogeneous. The rostral pfRG consists of ccRTN neurons
In the neonate brainstem preparation pfRG neurons are defined by the combination of two criteria: location in the vicinity of the facial motor nucleus and the above described pre-I discharge. Onimaru and colleagues had pointed out a while ago that neurons with a pre-I pattern must be heterogeneous from a transmitter standpoint because some respiratory neurons have membrane potential trajectories consistent with either an excitatory or an inhibitory input from pre-I cells (Onimaru et al., 1992; Arata et al., 1998). These investigators have also provided clear evidence that many pre-I cells reside under the facial motor nucleus in a region that seems in register with the RTN of the adult (Onimaru and Homma, 2003). These particular pre-I neurons are undoubtedly ccRTN neurons because they are non-catecholaminergic, Phox2b-expressing and glutamatergic (Onimaru et al., 2008). Furthermore, like the ccRTN neurons, these particular pfRG neurons are depolarized by acidification and this depolarization is associated with a reduction of membrane conductance consistent with potassium channel closure (Mulkey et al., 2004; Mulkey et al., 2007b; Onimaru et al., 2008). Finally this rostral group of pre-I neurons have the same peculiar dendritic structure as the ccRTN neurons recorded in more mature rodents, namely a profusion of very superficial dendrites oriented in the transverse plane (Mulkey et al., 2004; Mulkey et al., 2007b; Onimaru et al., 2008). On the other hand, the same authors also demonstrated that many neurons with a bona fide pre-I discharge pattern are not ccRTN neurons (Onimaru et al., 2008). These cells are located caudal to the facial motor nucleus and deeper below the medullary surface. They are acid-insensitive, do not express Phox2b and have a dendritic structure that is very different from the Phox2b-positive pre-I neurons. The transmitter phenotype of this second class of pre-I neurons was not identified by Onimaru and colleagues but an educated guess suggests that these cells could be the Bötzinger glycinergic neurons. The first evidence is their location and dendritic morphology (Onimaru et al., 2008). Secondly, although the Bötzinger neurons normally fire in late expiration, they can develop a post-inspiratory inhibition rebound under specific conditions (hypoxia plus hypercarbia) in the adult rodent in vivo (Fortuna et al., 2008). These conditions result in a pre-I post-I double discharge that is pfRG-like (Fortuna et al., 2008). In the adult, this post-expiratory rebound is presumably caused by a reduction in inhibitory input from nearby neurons that have a post-inspiratory discharge pattern (Fortuna et al., 2008). The Bötzinger region of the rodent contains a toggle that consists of mutually inhibitory late-E and post-I neurons (Abdala et al., 2009). Post-inspiratory rebound has not been demonstrated per se in the Suzue preparation but is likely to occur given that many respiratory neurons receive strong inhibitory volleys during inspiration and the preparation contains few if any post-inspiratory cells to stop such a rebound (Ballanyi et al., 1999). The absence of post-inspiratory neurons in the Suzue preparation is consistent with evidence that this sort of discharge pattern requires the integrity of the pons (Smith et al., 2007; Abdala et al., 2009). Finally, the Bötzinger neurons are glycinergic and innervate multiple types of respiratory neurons. These cells are therefore a plausible source for the inhibitory “pre-I” inputs already documented in many respiratory neurons in this preparation.
It is also important to stress that cells with similar pre- and post-I discharge are present in many other regions of the ventrolateral medulla in the Suzue preparation including in caudal regions containing expiratory premotor neurons (e.g.(Ballanyi et al., 2009)).
6. The rostral pfRG may consist of ccRTN neurons in a transitional state between their embryonic and adult forms
As indicated above, a cluster of very superficial pre-I/post-I (i.e. pfRG) neurons located under the facial motor nucleus consist of ccRTN neurons (Onimaru et al., 2008). However, during this critical period (P0 to P4), only a fraction of the ccRTN neurons recorded in the Suzue preparation have a pre-I bursting pattern. Many more do not burst (Onimaru et al., 2008). The latter cells discharge tonically under the influence of pH although they also receive respiratory-related synaptic inputs (Onimaru et al., 2008).
A recent study of the ccRTN neuron developmental lineage indicates that these cells are egr-2 dependent, i.e. originate in rhombomeres 3 or 5, before undergoing a caudal migration alongside the facial motor neurons to their final rostral medullary destination (Thoby-Brisson et al., 2009). Having reached this location, these neurons differentiate to form a bilateral cluster of synchronized bursters that activate and, possibly pace, the more caudally located pre-Bötzinger inspiratory oscillator (Thoby-Brisson et al., 2009). The slow bursting properties of the embryonic ccRTN neurons are attributed to intrinsic membrane properties and their synchronization is likely caused by gap junctions (Thoby-Brisson et al., 2009). By contrast, the pre-I/post-I pattern has not been detected in adult ccRTN neurons in vivo nor has any form of bursting been observed in ccRTN neurons recorded in slices prepared from rodents older than 7 days (Guyenet et al., 2005a; Lazarenko et al., 2009).
These various findings can be interpreted in two ways at least. The first possibility is that the ccRTN neurons initially differentiate into bursters in the embryo and become pacers soon after birth. The Suzue preparation is derived from rodents less than 4 days-old and could therefore be a transitional stage in which a fraction of the ccRTN neurons retain enough of their embryonic characteristics to generate synchronized bursts of action potentials and the rest are silent or tonically active when acidified. A fuller understanding of the molecular changes that shape the intrinsic properties of the ccRTN neurons during the critical period surrounding birth will reveal whether this interpretation is correct. The second possibility is that, throughout development and into adulthood, the ccRTN neurons include at least two distinct subtypes of acid-sensitive cells, “tonic” neurons and rhythmogenic neurons with intrinsic bursting properties. Whether the latter drive expiration or inspiration is unsettled and will be discussed later on.
7. The ccRTN neurons stimulate inspiratory motor activity
The ccRTN neurons seem to be capable of activating the inspiratory motor outflow in all three of their successive developmental avatars (e-pF, rostral pfRG, adult form)(Dubreuil et al., 2008; Onimaru and Homma, 2008; Abbott et al., 2009; Thoby-Brisson et al., 2009). The way in which this activation occurs (rate only, rate plus amplitude and its synaptic mechanisms) is less obvious and presumably evolves during development.
The notion that the rostral pfRG activates inspiration is based on the following evidence (Onimaru et al., 1987; Onimaru and Homma, 1987; Onimaru and Homma, 2003). By definition, the pre-I neurons of the rostral pfRG start bursting before inspiration (Onimaru and Homma, 2003). Lesions of the pfRG region slows the phrenic nerve discharge and some inspiratory neurons receive PSPs consistent with an excitatory input from some form of glutamatergic pre-I neuron (Onimaru et al., 1992). Although the interpretation is plausible and seems generally accepted, we note that the evidence that the pre-I neurons of the rostral pfRG drive inspiration is either correlative (relative timing of discharges, PSPs originating from pre-I cells of uncertain location and phenotype) or derives from the effect produced by brain lesions of undefined specificity (Onimaru and Homma, 2003). Given the possibility that the rostral pfRG contains two subtypes of ccRTN neurons (double bursters a.k.a. pre-I cells and tonic cells)(Onimaru et al., 2008) some uncertainty remains as to which one of the two is responsible for the slowing/acceleration of the inspiratory rhythm caused by lesion/stimulation of this region.
Recent work done at the embryonic stage in mice (Thoby-Brisson et al., 2009) indicates that the ccRTN neurons form a cluster of neurons that have the ability to generate synchronous bursts of activity that are driven by intrinsic properties and synchronized by gap junctions. Physical separation of this embryonic parafacial oscillator produces a slowing of the inspiratory-like outflow of the preparation, again supporting the possibility that the e-pF activates the oscillation rate of the downstream pre-Bötzinger complex (Thoby-Brisson et al., 2009).
Evidence that the ccRTN neurons activate inspiratory motor activity is even more compelling in the adult given that it has been possible to activate these cells selectively in vivo and to demonstrate that increased diaphragmatic activity ensues (Abbott et al., 2009). This evidence refines and extends a large body of work by Nattie and colleagues who had shown that activation of the RTN region by topical acidification or local microinjection of excitatory transmitters increases breathing whereas inhibitory substances produce the converse effect (Li and Nattie, 1997; Cream et al., 1999; Nattie and Li, 2000; Feldman et al., 2003). The literature contains interesting discrepancies as to whether RTN stimulation activates primarily the breathing rate or the breathing amplitude. In the Suzue preparation, the amplitude of the C4 rootlet discharge is essentially invariant and only rate changes are observed including under the influence of acidification. In our personal opinion, this peculiarity may largely explain why the rostral pfRG is often viewed as solely involved in rate control. Under anesthesia in the adult, selective photostimulation of channelrhodopsin-2 transfected ccRTN neurons in vagotomized rats activates both the frequency and the amplitude of the phrenic nerve discharge (Abbott et al., 2009). In similar experiments performed in conscious rats, both the rate and the amplitude of the diaphragmatic EMG were increased by photostimulation of transfected ccRTN neurons (unpublished results of Kanbar and Guyenet). Effects on both rate and amplitude make good physiological sense because ccRTN neurons are not only sensitive to local changes in pH (Lazarenko et al., 2009) but they are also excited by activation of the carotid bodies, probably via a direct projection from the nucleus of the solitary tract (Bodineau et al., 2000a; Takakura et al., 2006). Their activation should therefore partially reproduce the effect of a normoxic hypercapnic stimulus which, in rodents with intact peripheral chemoreceptors, consists of an increase in the rate and the amplitude of breathing (e.g.(Li and Nattie, 2002)). Injections of thyrotropin releasing hormone, a substance that activates the ccRTN neurons also activate both phrenic rate and amplitude (Cream et al., 1997) and injections of muscimol into the RTN region reduce breathing frequency in conscious rats, at least transiently (Nattie and Li, 2000). Yet, RTN acidification in conscious and in anesthetized rats stimulate breathing exclusively through an increase in tidal volume (Li and Nattie, 1997; Li et al., 1999). These discrepancies are not resolved. Anesthetic conditions certainly play a part since, in vagotomized rats, hypercapnia can modulate preferentially frequency (isoflurane) or amplitude (urethane; unpublished observations by Fortuna and Guyenet). It is also possible that the ccRTN neurons include a subset of neurons that control inspiratory amplitude and another that controls the respiratory rate. Also, more conventional forms of activation or inhibition of the RTN region (i.e. with drugs or acidification) may produce their effects by targeting both ccRTN and other neurons. A final confounding factor is that breathing rate and amplitude are not independent variables in the process of CO2 homeostasis. For instance, an imperceptibly gradual increase in breathing rate in man automatically produces a compensatory reduction in tidal volume (Haouzi and Bell, 2009) and the converse phenomenon is not inconceivable. Evidence which suggests that the population of ccRTN neurons could be anatomically and functionally heterogeneous will be presented later.
The physiological evidence that ccRTN neurons, or at least a subset of them, drive inspiration is also supported by the results of neuroanatomical studies. For example, the ccRTN neurons, collectively at least, target every segment of the ventral respiratory column including the region of the pre-Bötzinger complex and the rostral VRG (Abbott et al., 2009)(Figure 1A). The pseudorabies virus experiment of Dobbins and Feldman (1994) showed that at least some RTN neurons are connected oligosynaptically to diaphragmatic motor neurons, possibly via a single intervening neuron. Admittedly, this evidence in itself does not prove that these neurons activate inspiration because the retrogradely labeled cells could conceivably have been synaptically connected to neurons that inhibit diaphragmatic motor neurons during expiration. Furthermore the retrogradely labeled neurons may not have been ccRTN neurons.
8. e-pF, pfRG and RTN as an inspiratory “rhythmogenic” center
As discussed above, the fact that the e-pF/pfRG/RTN activates inspiration and the breathing rhythm is strongly supported by experimental evidence. Whether the e-pF/pfRG/RTN is rhythmogenic is a separate issue. Two theories are currently being pursued. The first one (in chronological order) is that this cell group is the master inspiratory rhythm generator (Onimaru and Homma, 2003). The second view is that this region contains an expiratory oscillator (Janczewski and Feldman, 2006; Feldman et al., 2009) or something in-between (Wittmeier et al., 2008). This section examines the first theory.
The notion that a group of neurons plays a respiratory rhythmogenic role seems intuitively obvious but is in fact difficult to strictly define. To the best of our understanding the term “respiratory rhythmogenic” requires that such neurons be capable of forming an oscillating circuit and that this oscillating circuit is necessary for the generation of a regular phasic motor outflow to the respiratory muscles. So far, only the pre-Bötzinger complex fits fully the definition of a “respiratory rhythmogenic” center since these neurons are capable of generating a regular motor output in isolation from the rest of the brain (Smith et al., 1991; Johnson et al., 2001) and without them, rats cannot breathe (Tan et al., 2008). However, the generation of the respiratory rhythm of intact adult mammals is not reducible to the up or down regulation of the activity of the pre-Bötzinger complex. It is the expression of an oscillating circuit of much greater complexity that incorporates the pre-Bötzinger complex but also includes rhythmically active neurons located in many other brains areas (notably the Bötzinger region and the dorsal pons) (Rybak et al., 2007). In addition, this circuitry requires so-called tonic excitatory drives to be active. The ambiguity of the term respiratory rhythmogenic can be seen in reference to the Bötzinger region. This region contains phasically active E2 and post-I neurons, mostly inhibitory, that interact with a host of other types of respiratory neurons (Rybak et al., 2007). The presence of the Bötzinger region is not required for the pre-Bötzinger complex to form an oscillating circuit, at least in a slice of neonate or embryonic brain and, unlike the pre-Bötzinger complex, the Bötzinger region of the ventral respiratory column probably does not have the ability to form an oscillating circuit in isolation. However, inhibition or stimulation of the Bötzinger cells by local application of transmitters exerts massive effects on the respiratory rate in vivo (e.g.(Monnier et al., 2003)). By all accounts, the E2 and post-I neurons of the Bötzinger region are an integral part of the complete (eupneic) respiratory rhythm-generating circuit and, on that basis, they should qualify as rhythmogenic neurons (Rybak et al., 2007).
In the adult, the respiratory rate can also be greatly altered by increasing or decreasing the activity of the ccRTN neurons in vivo (Abbott et al., 2009). Does this fact also qualify these neurons as rhythmogenic? In our opinion the answer is negative, for the following reasons. The first one is that these neurons, at least in anesthetized rats, have a largely tonic discharge pattern unless the central respiratory drive is very high (Guyenet et al., 2005a). Secondly, the respiratory modulation of the ccRTN neurons is phase-spanning and multiform but a pfRG type of discharge (pre-inspiratory or double-burst pattern) has not been identified so far under conditions when a robust phrenic outflow has been simultaneously recorded (Guyenet et al., 2005a). Recordings made in the putative equivalent of the cat’s RTN could suggest otherwise but the nature of the recorded neurons is uncertain (see below). Thirdly, adult ccRTN neurons produce respiratory effects that decay with a time constant many times longer than the respiratory period (Abbott et al., 2009). This observation suggests that the postsynaptic effect of the ccRTN neurons may be much too slow for these neurons to be able to specify the time of onset of each inspiration. In addition, the activation of ccRTN neurons does not only increase inspiratory rate but inspiratory amplitude and, probably, active expiration (Kanbar and Guyenet, unpublished results). Finally, when the hypothalamus is stimulated to cause an increase in breathing rate and amplitude, ccRTN neurons are activated uniformly throughout the respiratory cycle (Fortuna et al., 2009). This type of stimulation does not cause the phrenic nerve to discharge tonically between bursts nor does it produce a background tonic discharge in the phasically-active respiratory neurons that are assumed to be part of the pattern generator. In sum, the discharge characteristics of the neurons identified to-date as ccRTN in the adult rat in vivo are very different from that of the Bötzinger cells and other neurons assumed to be part of the respiratory rhythm and pattern-generating circuit. The adult properties of the ccRTN neurons seem particularly maladapted to a respiratory rhythmogenic role of any kind. They suggest that these neurons are more likely to be a source of tonic drive to their downstream targets than to be involved in the exact timing of their discharge.
Prenatally and at birth, the evidence that ccRTN neurons have respiratory rhythmogenic properties is much stronger but, in our opinion, far from definitive. The concept is based in large part on the fact that, under the appellation of e-pF or pfRG, these cells form an autonomously active group whose activity anticipates that of the pre-Bötzinger complex. One piece of evidence supporting the rhythm-generating theory is that electrical stimulation of the “pfRG” can reset the inspiratory rhythm in the Suzue preparation (Onimaru et al., 1987). This evidence is only modestly convincing because this procedure does not implicate the ccRTN cells specifically. Not only is the pfRG heterogeneous but electrical stimulation is notoriously unreliable and, as demonstrated only recently, may in fact preferentially activate distantly located neurons with axons passing in the vicinity of the electrode tip (Histed et al., 2009). The second evidence that the e-pF/pfRG is rhythmogenic is that its activity is synchronized with that of the pre-Bötzinger complex. This much is undeniable but there is very little evidence that the synchronization between these two oscillators is caused by the input from the e-pF/pfRG to the pre-Bötzinger complex. The reverse, namely that the synchronization operates via an input from the respiratory pattern generator to the e-pF/pfRG, is much better documented since the e-pF/pfRG receives fast GABAergic synaptic inputs during the inspiratory phase (Onimaru et al., 1990; Onimaru et al., 2008; Thoby-Brisson et al., 2009). In our opinion, this issue is a critical one. The current theory that the e-pF/pfRG is an inspiratory rhythm generator is based on two plausible but unproven assumptions namely that these cells target the pre-Bötzinger complex and that neurotransmission between the e-pF/pfRG neurons and these targets is fast enough for the former to be able to define the onset of the inspiratory burst. The e-pF/pfRG/ccRTN neurons do express the vesicular transporter VGLUT2 but this only means that they have the potential to release glutamate. The orexinergic cells also express vesicular transporters (Rosin et al., 2003) but the bulk of the evidence indicates that the orexin peptides are the workhorses of these neurons (Kukkonen et al., 2002; Kuwaki et al., 2008). The ccRTN neurons could release other transmitters than glutamate, galanin is a known example (Stornetta et al., 2009), and the type of glutamatergic neurotransmission that these neurons use, if any, is not known. Prior evidence from the Suzue preparation does indicate that some respiratory neurons receive conventional EPSPs consistent with an excitatory input from some form of pre-I neurons (Onimaru et al., 1992). This information is insufficient to attribute these PSPs to an input from the ccRTN neurons since the Suzue preparation contains several types of pre-I neurons not only within the pfRG region (Onimaru et al., 2008) but elsewhere in the VRG (Ballanyi et al., 2009). In addition, the interpretation of the function of the e-pF/pfRG in a “rhythmogenic” perspective may be largely influenced by the fact that the properties of this cell group have been examined in preparations in which the amplitude of the inspiratory-like outflow is essentially invariant and only frequency modulation is observed, including under the influence of pH. This is not the case in vivo, at least in the adult, in which ccRTN neuron stimulation increases the breathing rate, inspiration amplitude and probably active expiration as well, as mentioned above.
In summary, neither the concepts of oscillator nor of inspiratory rhythm generator seem to fit the known properties of the ccRTN neurons in their adult form. It is of course conceivable that the rhythmogenic neurons of the adult are a subset of ccRTN neurons that is small and has not yet been detected in vivo. In their embryonic and postnatal version, there is excellent evidence that the ccRTN neurons or a large subset of them form an autonomously active oscillator. There is also excellent evidence that the ongoing activity of this oscillator increases the burst rate of the downstream pre-Bötzinger complex. An interesting speculation is that the excitatory drive from the e-PF is necessary to counteract the respiratory depressant effects of endogenous opiates at birth (Feldman et al., 2009). However, in our opinion, there is still insufficient evidence to conclude that the e-pF/pfRG oscillator is rhythmogenic in the sense of being able to specify the time of onset of each inspiratory burst.
9. RTN, pfRG and active expiration
Expiratory muscles are only recruited for breathing when a high level of lung ventilation is required such as during exercise, hypoxia or exposure to abnormally high levels of CO2. Classically, the respiratory drive of spinal expiratory motor neurons is attributed to the activity of excitatory premotor neurons that are located in the caudal VRG (Iscoe, 1998). These premotor neurons have an expiratory incrementing discharge pattern attributed to the summation of a tonic excitatory drive of still uncertain origin (speculatively some “locomotor center” and /or central chemoreceptors)(Iwamoto et al., 1996) and inhibitory volleys that occur during the inspiratory and post-inspiratory phases (Bainton and Kirkwood, 1979; Ballantyne and Richter, 1986; Arita et al., 1987; Anders et al., 1991). The post-inspiratory input could conceivably originate from neurons located in the immediate vicinity of the expiratory premotor neurons (Ballantyne and Richter, 1986) although inhibitory post-inspiratory interneurons are commonly found throughout the ventral respiratory column, most particularly in the Bötzinger region (Rybak et al., 2007). In the cat, the inhibitory inspiratory input to the cVRG expiratory premotor neurons originates in part from the “retrofacial region” (Anders et al., 1991), presumably what is now called the Bötzinger region. In short, the classic view is that the expiratory incrementing discharge of expiratory premotor neurons is due to the breakthrough of a tonic drive during the relaxation phase of the post-inspiratory volley rather than to an incoming excitatory drive during the late expiratory phase of the respiratory cycle. These assumptions are currently being reevaluated in the light of several challenging experimental results (Feldman and Del Negro, 2006; Feldman et al., 2009).
In juvenile rats (7-13 day-old) treated with the anesthetic ketamine, administration of the opiate agonist fentanyl causes the activity of the diaphragm to skip cycles (“quantal” breathing, (Mellen et al., 2003)) while the activity of the abdominal muscles retain their initial regular periodicity (Janczewski and Feldman, 2006). Also, in this preparation, vagal stimulation suppresses the inspiratory motor outflow while stimulating abdominal muscle contractions (Feldman and Del Negro, 2006; Janczewski and Feldman, 2006). This unexpected dissociation between the activity of inspiratory and expiratory muscles has led to the concept that active expiration may be under the control of an oscillator that is normally coupled with the inspiratory oscillator but can be artificially separated from it in very young rats by the combined administration of ketamine and fentanyl (Feldman and Del Negro, 2006; Janczewski and Feldman, 2006). A phylogenetic argument has also been made for the existence of two mammalian respiratory oscillators, because two rhythm generators are involved in amphibian respiration, one for buccal breathing and the other for lung breathing (Wilson et al., 2002; Duffin, 2004; Feldman and Del Negro, 2006; Feldman et al., 2009). In theory, mammals could therefore have inherited these two rhythm generators in some modified form.
According to Janczewski and Feldman (2006), this hypothetical mammalian expiratory oscillator is located somewhere in the vicinity of the facial motor nucleus because transections of the medulla oblongata midway through this nucleus eliminated the activity of expiratory muscles while preserving the rhythmic activity of the diaphragm (Janczewski and Feldman, 2006). Further experimentation performed in artificially perfused neonate and juvenile rats have indicated that the critical region is within or very close to the RTN (Abdala et al., 2009) and the search is now on for the responsible cells and the “expiratory oscillator”.
One possibility considered by Abdala et al. (2009) is that the excitatory drive from tonically active ccRTN neurons is required for active expiration to break through. These neurons are functionally excitatory, CO2-activated and innervate the cVRG (Guyenet, 2008; Abbott et al., 2009) and therefore could contribute some fraction of the tonic excitatory drive assumed to be required for expiratory premotor neurons to fire under hypercapnia (Bainton and Kirkwood, 1979). In addition, the ccRTN neurons receive excitatory inputs from a region of the hypothalamus which probably mediates some of the cardiorespiratory stimulation associated with exercise (Fortuna et al., 2009). However, this explanation of the role of the RTN in expiration is probably incomplete because Abdala et al. (2009) have also found that the RTN region harbors neurons that are activated by hypercapnia concomitantly and in phase with the abdominal nerves. These late-E neurons need further characterization. It seems most critical to rule out that they are a subset of facial motor neurons with late-expiratory discharges and to test whether they might a subset of ccRTN neurons (Huangfu et al., 1993). Hypothetically, these late-E neurons could be driving the cVRG expiratory premotor neurons (Figure 1B).
Other neurons that are important in shaping the membrane trajectory of cVRG expiratory premotor neurons also probably reside in close proximity to the RTN (Figure 1B). Inspiratory neurons that inhibit cVRG expiratory premotor neurons have been identified within the retrofacial region of cats as indicated above (Anders et al., 1991). Potentially homologous neurons have been described in the adult rat (Sun and Reis, 1996). These neurons were called pre-inspiratory by these authors but their discharge anticipated the phrenic outflow by a mere 10-20 ms. A large proportion of these inspiratory cells could be antidromically activated from the spinal cord and therefore were clearly not motor neurons (Sun and Reis, 1996). The Bötzinger region which overlaps with the RTN also contains an abundance of inhibitory post-I neurons that could, in theory at least, provide an input to the expiratory premotor neurons of the cVRG.
Another link between pfRG and expiratory motor outflow stems from the observation that, in the Suzue preparation, the abdominal nerves display twin bursts of activity coinciding with late expiration and post-inspiration like pfRG neurons (Taccola et al., 2007). However, because the Suzue preparation is replete with twin bursting cells, the twin bursts of the abdominal nerve rootlets are not necessarily driven by the discharge of the rostral pfRG a.k.a. ccRTN neurons. In fact, a simple explanation for the double spiking of the abdominal respiratory outflow could be, as mentioned above, that the expiratory premotor neurons of the cVRG undergo rebound excitation after the inspiratory inhibition in this preparation due to the absence of post-inspiratory inhibition. This speculative interpretation is supported by evidence that the expiratory premotor cells of the cVRG, like the Bötzinger neurons, develop rebound excitation during the post-inspiratory phase under hypoxic/hypercapnic conditions in the adult rat in vivo (Fortuna et al., 2008). It is also supported by evidence that anoxia produces twin preI-postI bursts of activity in the lumbar nerve of the in situ perfused neonate and juvenile rat (Abdala et al., 2009).
In summary, the RTN and the immediately adjacent Bötzinger region contain neurons that could potentially provide tonic drive (the ccRTN neurons) and phasic inhibition (I and post-I neurons) to the expiratory premotor neurons of the cVRG. The RTN region also contains neurons that may contribute a late expiratory excitation to the expiratory premotor neurons of the cVRG. Volleys of late-expiratory EPSPs have not been identified so far in the latter neurons by intracellular recording with sharp electrodes. The recent observations of Abdala et al. (2009) suggest that this possibility should be revisited with improved recording methods. The putative presence within the RTN and its vicinity of many types of interneurons that likely participate in shaping the membrane trajectory of the expiratory premotor neurons, could explain why mechanical damage or non-selective inhibition of this region interferes with expiratory motor activity (Janczewski and Feldman, 2006; Abdala et al., 2009). An interesting issue raised by Janczewski and Feldman (2006) is whether these rostrally located neurons retain the capability to produce a periodic motor outflow in the expiratory muscles when the pre-Bötzinger complex is silenced. Stated another way, the question is whether these parafacial respiratory interneurons can operate as a partially autonomous expiratory oscillator. A small piece of supporting evidence is that opiates interfere only minimally with the activation of ccRTN neurons by CO2 (Fortuna et al., 2009). Therefore, even in the presence of such drugs, the ccRTN neurons could, in theory at least, continue providing an adequate excitatory drive to the expiratory premotor neurons and some of their phasically active inputs.
10. Possible functional heterogeneity of the ccRTN neurons
The ccRTN neurons were identified by location and shared phenotype (Phox2b, VGLUT2, NK1 receptors, lack of catecholamine or serotonergic markers). This phenotype is defined by a limited number of attributes which leaves room for the possibility that it encompasses more than one type of neuron.
In the Suzue preparation, ccRTN neurons include twice-bursting neurons and tonic cells, all of them pH-sensitive. We have evoked the possibility that this distinction might be artificial because, at birth, the entire cell group may be gradually transitioning between the embryonic stage characterized by group pacemaker properties and an adult form in which these cells become solely driven by synaptic inputs and their pH sensitivity. The complete process may span a few days accounting for the co-existence of the two forms of ccRTN neurons between birth and day 4.
About half of the ccRTN neurons express galanin (Stornetta et al., 2009). However, galanin-positive and galanin-negative ccRTN neurons are both CO2 responsive in vivo and in slices. In vivo, galanin-expressing neurons are respiratory modulated and display the same variety of respiratory patterns as those without galanin (Stornetta et al., 2009). Thus, galanin does not co-segregate with any known physiological difference between ccRTN neurons.
ccRTN neurons express several stereotyped respiratory patterns under anesthesia (Guyenet et al., 2005a) indicating that they receive distinct complements of synaptic inputs. Many types of phasically active neurons have also been recorded in the RTN region of the cat along with tonic and respiratory-modulated units (Connelly et al., 1990; Nattie et al., 1993; Bodineau et al., 2000b). Subsets of these cells are likely to have been ccRTN neurons. Input heterogeneity exists even at the embryonic stage since e-pF neurons receive either glutamatergic or GABAergic inputs during the inspiratory phase (Thoby-Brisson et al., 2009). This particular dichotomy may persist in the adult because a substantial portion of the ccRTN neurons have reduced activity during inspiration whereas the rest are most active during this phase (Guyenet et al., 2005a; Fortuna et al., 2009). These input differences suggest the probable existence of subgroups of ccRTN neurons with distinct targets and physiological roles but this interpretation requires more direct experimental support.
Comparing the various estimates of the number of chemically defined RTN neurons of the mouse is also instructive. By our simple anatomical definition, the number of ccRTN neurons (non catecholaminergic, non-cholinergic, VGLUT2- and Phox2b-positive neurons of the parafacial region) is about 780 per brain after Abercrombie correction (Lazarenko et al., 2009). The number of neurons that make up the mouse e-pF was estimated around 540 cells per brain based on histology (Egr2+, Phox2b+, Islet1/2-negative neurons) and on counts of the number of rhythmic cells detected in calcium-loaded preparations (Thoby-Brisson et al., 2009). Finally, the number of mouse RTN neurons defined as Phox2b-, Vglut2-, neurokinin1 receptor- and Atoh1-expressing cells located in the parafacial region was recently evaluated as 1180 per brain (Dubreuil et al., 2009b). The Dubreuil estimate and ours are fairly close given that the Dubreuil numbers were apparently not corrected for stereological errors and therefore could be easily overestimated by 20%. The Thoby-Brisson estimate of the number of e-pF neurons is somewhat lower. This low estimate could have been due to the fact that some e-pF neurons were damaged and therefore were not detected by calcium imaging or that some of the cells of this cell group are not bursting for other reasons. In sum, the discrepancy between the three estimates is relatively small but some room is left for the possibility that the pacemaker population (the e-pF of Thoby-brisson et al.) might be a subset of the population that we have identified as ccRTN neurons and that Dubreuil has outlined on the basis of Atoh-1 expression.
As mentioned above, the RTN region contains a form of late-expiratory neurons that may be driving the activity of expiratory muscles (Abdala et al., 2009). These neurons have not yet been biochemically characterized and could conceivably be a subset of ccRTN neurons. Pushing this speculation a step further, one could imagine that these cells, like other late-expiratory neurons (Fortuna et al., 2008), are prone to developing a post-inspiratory rebound under conditions when inhibitory post-inspiratory neurons are inactive. This line of reasoning leads to the unlikely but not inconceivable possibility that some of the pfRG “pre-I” neurons of the Suzue preparation could in fact be the precursors of the E2 neurons detected by Abdala et al. (2009) and therefore that these cells might drive expiration, not inspiration as proposed by Feldman and colleagues (Feldman et al., 2009).
11. ccRTN neurons and the congenital central hypoventilation syndrome
The cardinal signs of this rare genetic disease (1 in 100,000 births) are a severe hypoventilation during sleep and an extremely reduced respiratory response to central and peripheral chemoreceptors at all times (Weese-Mayer et al., 2009). Autonomic abnormalities, including dysfunction of cardiovascular control are also commonly observed (Gronli et al., 2008; Weese-Mayer et al., 2009). These symptoms are consistent with some kind of brainstem dysfunction. The root cause of CCHS was elucidated in the wake of the discovery of the homeodomain transcription factor Phox2b. Phox2b was found to be critical for the development of multiple pathways involved in autonomic regulation (nucleus of the solitary tract and it major sensory afferents, carotid bodies, brainstem catecholaminergic neurons, sympathetic postganglionic neurons etc.)(Pattyn et al., 1997; Pattyn et al., 2000b; Brunet and Pattyn, 2002). Soon after, the most common cause of CCHS was identified as an abnormal expansion to between 23 and 30 residues of the normal 20 residue polyalanine sequence of Phox2b (Amiel et al., 2003; Weese-Mayer et al., 2003). The severity of CCHS increases with the number of extra alanine residues (Matera et al., 2004). Twenty-seven or more additional alanine residues generally result in the complete inability to breathe while asleep.
In 2006, Stornetta et al. demonstrated that the CO2-sensitive neurons of the RTN region uniformly express Phox2b and noted that Phox2b was expressed by an uninterrupted chain of neurons involved in conveying central and peripheral chemosensory information to the respiratory network. They also noted that this transcription factor was generally absent from the lower brainstem neurons implicated in respiratory rhythm and pattern generation. These observations suggested that the abnormal development of the ccRTN neurons, alone or in combination with their input from the carotid bodies, could produce the respiratory deficits observed in CCHS. The Phox2b27Ala/+ mouse engineered by Dubreuil et al. (2008) reproduces the typical breathing deficits of CCHS at birth (hypoventilation and lack of response to CO2) and lacks entirely the ccRTN neurons but, remarkably, the rest of the Phox2b-expressing neurons appeared intact. Specifically, the catecholaminergic neurons, the nucleus of the solitary tract, the carotid bodies and vagal afferents, all of which depend on Phox2b for their development (Pattyn et al., 2000a; Pattyn et al., 2000b; Dauger et al., 2003), were apparently intact at birth. Another mouse in which the ccRTN neurons are massively and selectively ablated prenatally by genetic manipulations exhibits the same respiratory problems at birth (Dubreuil et al., 2009b). Dubreuil’s observations indicate that the ccRTN neurons are much more vulnerable to the deleterious effects of polyalanine expansion of the Phox2b gene than most other Phox2b-expressing neurons. They also suggest that the selective degeneration of the ccRTN neurons could account for the respiratory symptoms of CCHS, at least at the time of birth. This theory obviously requires histological analysis of human specimens. The first step towards this goal has been completed since a cluster of non-catecholaminergic neurons expressing NK1 receptors and Phox2b has recently been identified in man in the predicted location under the facial motor nucleus (Rudzinski and Kapur, 2009).
The autonomic deficits (gastrointestinal and cardiovascular) experienced by CCHS patients are not adequately explained by the absence of the ccRTN neurons, at least based on current understanding of the function of these neurons. In theory, several of these autonomic problems could be caused by a partial loss of the sympathetic ganglionic neurons, a possibility that Dubreuil et al. (2008) did not seem to have investigated in their mouse model. Other more subtle and still unrecognized defects may be also present in the Phox2b27Ala/+ mouse as well as in the brain of CCHS patients. Evidence of significant forebrain damage has been repeatedly found in CCHS patients who survive into adolescence and adulthood (Macey et al., 2009; Kumar et al., 2009) but this damage could result from the repeated episodes of severe hypoxia experienced by these individuals.
12. Summary and conclusions
Overlapping portions of the rostral ventrolateral medullary reticular formation have received many names (RTN, RVL, C1 region, subretrofacial nucleus, RVLM, Bötzinger region, retrofacial area, caudal paragigantocellularis nucleus, pfRG, etc.). This confusing anatomical nomenclature should be replaced by a catalog of the various types of neurons that reside within the rostral ventrolateral medulla. Ideally, this catalog should rely on cell lineage, defining biochemical markers, connectivity and accurate stereotaxic location. The ccRTN neurons and the C1 neurons are examples of this approach but much more work is needed.
The ccRTN neurons seem relatively homogeneous from a neurochemical standpoint. These non-aminergic neurons express the transcription factor Phox2b throughout life. Collectively, they innervate all the pontomedullary regions known to contain components of the respiratory rhythm and pattern generating circuitry. This anatomical connectivity is in contrast to some of the better described other putative central chemoreceptors (serotonergic, catecholaminergic and orexinergic neurons), as the ccRTN neurons innervate only these well-defined pontomedullary regions. Selective stimulation of the ccRTN neurons activates multiple aspects of breathing including the rate and amplitude of inspiration and, most probably, active expiration. Given the projection pattern of these cells, these effects are probably mediated by actions at multiple levels of the breathing network, including the nucleus of the solitary tract, the ventral respiratory column and the dorsolateral pons. Identifying the specific respiratory neurons that are targeted by the ccRTN neurons is an important research objective.
The ccRTN neurons make the vesicular transporter VGLUT2 suggesting that they use glutamate as one of their transmitters. The postsynaptic effects of these neurons are unknown except for the fact that their kinetics appear to be slow, in the adult at least. One the major current hypotheses concerning the ccRTN neurons is that they contribute a “tonic” CO2-regulated excitatory drive to the respiratory controller. Given that these neurons are respiratory modulated, this notion could only be correct if the postsynaptic effects that they produce is long relative to the average length of the breathing cycle.
There is excellent evidence that the ccRTN neurons or a large subset of them form a cluster of synchronized bursters before birth (the e-pF). This characteristic persists for a few days after birth but it has not been detected in rodents more than 7 days old. Between birth and post-natal day 4 or so, the ccRTN population includes a mixture of bursting and tonic cells suggesting that, during this early postnatal period, the ccRTN neurons could be transitioning between their embryonic pacemaker driven form and their adult form which is regulated primarily by synaptic drives and the surrounding pH.
The ongoing activity of the e-pF and pfRG versions of the ccRTN neurons undoubtedly increases the breathing rate. The fact that their discharge is synchronized with the inspiratory motor outflow and anticipates it slightly (pre-I pattern) remains the principal experimental evidence that the ccRTN neurons could have inspiratory “rhythmogenic” properties during the late embryonic and early postnatal periods. However, it could be that embryonic and postnatal ccRTN neurons have “rhythmogenic” properties only in vitro because the abnormally long cycle period of the pre-Bötzinger complex present under such conditions largely exceeds the T1/2 of the postsynaptic effects produced by the ccRTN neurons. Again, this issue requires a fuller understanding of the postsynaptic effects elicited by these neurons.
Why the ccRTN neurons would form an intrinsically active oscillator prenatally and lose these properties later on is unclear. Conceivably, the RTN derives from neurons that formed an oscillator implicated in some form of branchial activity in fish or in buccal breathing in amphibians. This speculative interpretation could be tested in the future by analyzing the developmental lineage of the putative early vertebrate homolog of the e-pF.
The ccRTN neurons, in all their successive forms (e-pF, pfRG, adult) are acid-activated and presumably contribute to central respiratory chemoreception. At birth, the absence of these neurons eliminates the chemoreflex suggesting they could be the main source of pH-dependent excitatory drive to the respiratory network at this early age. The high pH sensitivity of the adult form of the ccRTN neurons and the fact that selective activation of these neurons increases breathing suggests that they provide an important CO2-dependent excitatory drive to the breathing network. The relative importance of ccRTN vs. other neurons for central chemosensitivity is not known and probably won’t be known until the mechanism by which these neurons detect pH is identified and can be selectively excised or pharmacologically neutralized. Two types of mechanisms are thought to contribute to the activation of the ccRTN neurons by changes in local pH, none of which is definitively proven. A direct action of acid on the ccRTN neurons has been invoked to account for their activation under conditions of reduced synaptic activity. A potassium conductance is implicated in this effect but it is not necessarily the pH sensor. The presumably intrinsic pH response of the ccRTN neurons may not account fully for the exquisite pH-sensitivity of the ccRTN neurons in vivo. An autocrine mechanism involving glial cells as pH detectors and ATP as their gliotransmitter may exist in the adult in vivo but seems inoperative in the neonate in vitro.
The ccRTN neurons could contribute to active expiration in several ways. First, these neurons innervate the cVRG and could provide a tonic CO2- and hypothalamic input-dependent drive to the expiratory premotor neurons that innervate abdominal muscles. Secondly, the ccRTN neurons could provide a tonic drive to several types of phasically active respiratory neurons that are antecedent to the expiratory premotor neurons and define their membrane trajectory during the respiratory cycle. We are referring specifically to inhibitory cells that fire during inspiration (I neurons) or during post-inspiration (E-DEC neurons) and to a newly discovered form of E2 (late expiratory-incrementing) neurons. These three cell types (I, post-I, E2) seem to co-exist at the rostral end of the ventral respiratory column in a region that overlaps with the caudal end of the RTN. The combined presence of these cells may account for the fact that lesions of the RTN region or the introduction of inhibitory substances into this region reduce the expiratory activity of the abdominal muscles with some selectivity. Finally, the newly discovered E2 neurons of the RTN region could be a subset of ccRTN neurons that were not detected previously in anesthetized preparations because they might have been silent.
The ccRTN neurons are the only Phox2b-dependent neurons known to degenerate or to fail to develop in a mouse model of CCHS. Although it is somewhat premature to assign the respiratory deficits of this disease solely to the loss of these neurons, finding out whether these cells selectively degenerate in CCHS patients as they do in the mouse model of the disease is a clear and pressing research objective. Also, if the absence of the ccRTN neurons really accounts for the respiratory deficits of CCHS as postulated, animal experimentation should be able to prove that the contribution of the ccRTN neurons to breathing is especially critical during sleep.
Acknowledgments
This work was supported by research grants from the National Institutes of Health (HL74011 and HL 28785 to PGG) and the University of Connecticut Research Foundation large faculty grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abbott SBG, Stornetta RL, Fortuna MG, Depuy SD, West GH, Harris TE, Guyenet PG. Photostimulation of retrotrapezoid nucleus Phox2b-expressing neurons in vivo produces long-lasting activation of breathing in rats. J. Neurosci. 2009;29:5806–5819. doi: 10.1523/JNEUROSCI.1106-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdala AP, Rybak IA, Smith JC, Paton JF. Abdominal expiratory activity in the rat brainstem-spinal cord in situ: patterns, origins, and implications for respiratory rhythm generation. J Physiol. 2009 doi: 10.1113/jphysiol.2008.167502. DOI:10.113/jphysiol.2008.167502- [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alheid GF, Gray PA, Jiang MC, Feldman JL, McCrimmon DR. Parvalbumin in respiratory neurons of the ventrolateral medulla of the adult rat. J. Neurocytol. 2002;31:693–717. doi: 10.1023/a:1025799830302. [DOI] [PubMed] [Google Scholar]
- Alheid GF, McCrimmon DR. The chemical neuroanatomy of breathing. Respir Physiol Neurobiol. 2008;164:3–11. doi: 10.1016/j.resp.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiel J, Laudier B, Attie-Bitach T, Trang H, de PL, Gener B, Trochet D, Etchevers H, Ray P, Simonneau M, Vekemans M, Munnich A, Gaultier C, Lyonnet S. Polyalanine expansion and frameshift mutations of the paired-like homeobox gene PHOX2B in congenital central hypoventilation syndrome. Nat. Genet. 2003;33:459–461. doi: 10.1038/ng1130. [DOI] [PubMed] [Google Scholar]
- Anders K, Ballantyne D, Bischoff AM, Lalley PM, Richter DW. Inhibition of caudal medullary expiratory neurones by retrofacial inspiratory neurones in the cat. J Physiol. 1991;437:1–25. doi: 10.1113/jphysiol.1991.sp018580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrezik JA, Chan-Palay V, Palay SL. The nucleus paragigantocellularis lateralis in the rat: Conformation and cytology. Anat Embryol. 1981;161:355–371. doi: 10.1007/BF00316048. [DOI] [PubMed] [Google Scholar]
- Arata A, Onimaru H, Homma I. Possible synaptic connections of expiratory neurons in the medulla of newborn rat in vitro. NeuroReport. 1998;9:743–746. doi: 10.1097/00001756-199803090-00033. [DOI] [PubMed] [Google Scholar]
- Arita H, Kogo N, Koshiya N. Morphological and physiological properties of caudal medullary expiratory neurons of the cat. Brain Res. 1987;401(2):258–266. doi: 10.1016/0006-8993(87)91410-7. [DOI] [PubMed] [Google Scholar]
- Bainton CR, Kirkwood PA. The effect of carbon dioxide on the tonic and the rhythmic discharges of expiratory bulbospinal neurones. J. Physiol. 1979;296:291–314. doi: 10.1113/jphysiol.1979.sp013006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballantyne D, Richter DW. The non-uniform character of expiratory synaptic activity in expiratory bulbospinal neurones of the cat. J. Physiol. 1986;370:433–456. doi: 10.1113/jphysiol.1986.sp015943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballanyi K, Onimaru H, Homma I. Respiratory network function in the isolated brainstem-spinal cord of newborn rats. Prog. Neurobiol. 1999;59:583–634. doi: 10.1016/s0301-0082(99)00009-x. [DOI] [PubMed] [Google Scholar]
- Ballanyi K, Ruangkittisakul A, Onimaru H. Opioids prolong and anoxia shortens delay between onset of preinspiratory (pFRG) and inspiratory (preBotC) network bursting in newborn rat brainstems. Pflugers Arch. 2009;458:571–587. doi: 10.1007/s00424-009-0645-3. [DOI] [PubMed] [Google Scholar]
- Bodineau L, Frugière A, Marlot D, Wallois F. Connections between retrotrapezoid nucleus and nucleus tractus solitarii in cat. Neurosci. Lett. 2000a;280:111–114. doi: 10.1016/s0304-3940(00)00770-9. [DOI] [PubMed] [Google Scholar]
- Bodineau L, Frugiere A, Marlot D, Wallois F. Effect of hypoxia on the activity of respiratory and non-respiratory modulated retrotrapezoid neurons of the cat. Auton. Neurosci. 2000b;86:70–77. doi: 10.1016/S1566-0702(00)00237-X. [DOI] [PubMed] [Google Scholar]
- Branco LG, Moreira TS, Guyenet PG, Lalley PM, Kawai A, Putnam RW, Chamberlin NL, Saper CB, Gourine AV, Kanamaru M, Homma I. Commentaries on Viewpoint: Central chemoreception is a complex system function that involves multiple brain stem sites. J Appl Physiol. 2009;106:1467–1470. doi: 10.1152/japplphysiol.00057.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet JF, Pattyn A. Phox2 genes - from patterning to connectivity. Curr. Opin. Genet. Dev. 2002;12:435–440. doi: 10.1016/s0959-437x(02)00322-2. [DOI] [PubMed] [Google Scholar]
- Connelly CA, Ellenberger HH, Feldman JL. Are there serotonergic projections from raphe and retrotrapezoid nuclei to the ventral respiratory group in the rat? Neurosci. Lett. 1989;105:34–40. doi: 10.1016/0304-3940(89)90007-4. [DOI] [PubMed] [Google Scholar]
- Connelly CA, Ellenberger HH, Feldman JL. Respiratory activity in retrotrapezoid nucleus in cat. Am. J. Physiol. 1990;258:L33–L44. doi: 10.1152/ajplung.1990.258.2.L33. [DOI] [PubMed] [Google Scholar]
- Cream C, Li A, Nattie E. The retrotrapezoid nucleus (RTN): local cytoarchitecture and afferent connections. Respir. Physiol. Neurobiol. 2002;130:121–137. doi: 10.1016/s0034-5687(01)00338-3. [DOI] [PubMed] [Google Scholar]
- Cream C, Nattie E, Li A. TRH microdialysis into the RTN of the conscious rat increases breathing, metabolism, and temperature. J. Appl. Physiol. 1999;87:673–682. doi: 10.1152/jappl.1999.87.2.673. [DOI] [PubMed] [Google Scholar]
- Cream CL, Li A, Nattie EE. RTN TRH causes prolonged respiratory stimulation. J. Appl. Physiol. 1997;83:792–799. doi: 10.1152/jappl.1997.83.3.792. [DOI] [PubMed] [Google Scholar]
- Dauger S, Pattyn A, Lofaso F, Gaultier C, Goridis C, Gallego J, Brunet JF. Phox2b controls the development of peripheral chemoreceptors and afferent visceral pathways. Development. 2003;130:6635–6642. doi: 10.1242/dev.00866. [DOI] [PubMed] [Google Scholar]
- Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. J. Comp. Neurol. 1994;347:64–86. doi: 10.1002/cne.903470106. [DOI] [PubMed] [Google Scholar]
- Dubreuil V, Barhanin J, Goridis C, Brunet JF. Breathing with phox2b. Philos. Trans. R. Soc Lond B Biol. Sci. 2009a;364:2477–2483. doi: 10.1098/rstb.2009.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil V, Ramanantsoa N, Trochet D, Vaubourg V, Amiel J, Gallego J, Brunet JF, Goridis C. A human mutation in Phox2b causes lack of CO2 chemosensitivity, fatal central apnoea and specific loss of parafacial neurons. Proc. Natl. Acad. Sci. USA. 2008;105:1067–1072. doi: 10.1073/pnas.0709115105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil V, Thoby-Brisson M, Rallu M, Persson K, Pattyn A, Birchmeier C, Brunet JF, Fortin G, Goridis C. Defective respiratory rhythmogenesis and loss of central chemosensitivity in phox2b mutants targeting retrotrapezoid nucleus neurons. J Neurosci. 2009b;29:14836–14846. doi: 10.1523/JNEUROSCI.2623-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffin J. Functional organization of respiratory neurones: a brief review of current questions and speculations. Exp. Physiol. 2004;89:517–529. doi: 10.1113/expphysiol.2004.028027. [DOI] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL. Brainstem connections of the rostral ventral respiratory group of the rat. Brain Res. 1990;513:35–42. doi: 10.1016/0006-8993(90)91086-v. [DOI] [PubMed] [Google Scholar]
- Erlichman JS, Putnam RW, Leiter JC. Glial modulation of CO2 chemosensory excitability in the retrotrapezoid nucleus of rodents. Adv. Exp Med. Biol. 2008;605:317–321. doi: 10.1007/978-0-387-73693-8_55. [DOI] [PubMed] [Google Scholar]
- Ezure K, Tanaka I, Saito Y. Brainstem and spinal projections of augmenting expiratory neurons in the rat. Neurosci Res. 2003;45:41–51. doi: 10.1016/s0168-0102(02)00197-9. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat. Rev. Neurosci. 2006;7:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Kam K, Janczewski WA. Practice makes perfect, even for breathing. Nat Neurosci. 2009;12:961–963. doi: 10.1038/nn0809-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: Rhythmicity, Plasticity, Chemosensitivity. Annu. Rev. Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortuna MG, Stornetta RL, West GH, Guyenet PG. Activation of the retrotrapezoid nucleus by posterior hypothalamic stimulation. J Physiol. 2009;587:5121–5138. doi: 10.1113/jphysiol.2009.176875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortuna MG, West GH, Stornetta RL, Guyenet PG. Botzinger expiratory-augmenting neurons and the parafacial respiratory group. J. Neurosci. 2008;28:2506–2515. doi: 10.1523/JNEUROSCI.5595-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda Y, Honda Y. pH-sensitive cells at ventro--lateral surface of rat medulla oblongata. Nature. 1975;256:317–318. doi: 10.1038/256317a0. [DOI] [PubMed] [Google Scholar]
- Fukuda Y, Honda Y, Schlafke ME, Loeschcke HH. Effect of H+ on the membrane potential of silent cells in the ventral and dorsal surface layers of the rat medulla in vitro. Pflugers Arch. 1978;376:229–235. doi: 10.1007/BF00584955. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Llaudet E, Dale N, Spyer KM. ATP is a mediator of chemosensory transduction in the central nervous system. Nature. 2005;436:108–111. doi: 10.1038/nature03690. [DOI] [PubMed] [Google Scholar]
- Grafstein B, Liu S, Cotrina ML, Goldman SA, Nedergaard M. Meningeal cells can communicate with astrocytes by calcium signaling. Ann. Neurol. 2000;47:18–25. [PubMed] [Google Scholar]
- Gronli JO, Santucci BA, Leurgans SE, Berry-Kravis EM, Weese-Mayer DE. Congenital central hypoventilation syndrome: PHOX2B genotype determines risk for sudden death. Pediatr Pulmonol. 2008;43:77–86. doi: 10.1002/ppul.20744. [DOI] [PubMed] [Google Scholar]
- Guthrie PB, Knappenberger J, Segal M, Bennett MV, Charles AC, Kater SB. ATP released from astrocytes mediates glial calcium waves. J Neurosci. 1999;19:520–528. doi: 10.1523/JNEUROSCI.19-02-00520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG. The 2008 Carl Ludwig Lecture: retrotrapezoid nucleus, CO2 homeostasis, and breathing automaticity. J. Appl. Physiol. 2008;105:404–416. doi: 10.1152/japplphysiol.90452.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Mulkey DK, Stornetta RL, Bayliss DA. Regulation of ventral surface chemoreceptors by the central respiratory pattern generator. J. Neurosci. 2005a;25:8938–8947. doi: 10.1523/JNEUROSCI.2415-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bayliss DA. Retrotrapezoid nucleus and central chemoreception. J. Physiol. 2008;586:2043–2048. doi: 10.1113/jphysiol.2008.150870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bayliss DA, Mulkey DK. Retrotrapezoid nucleus: a litmus test for the identification of central chemoreceptors. Exp. Physiol. 2005b;90:247–253. doi: 10.1113/expphysiol.2004.029637. [DOI] [PubMed] [Google Scholar]
- Haouzi P, Bell HJ. Control of breathing and volitional respiratory rhythm in humans. J Appl Physiol. 2009;106:904–910. doi: 10.1152/japplphysiol.90675.2008. [DOI] [PubMed] [Google Scholar]
- Hartzler LK, Dean JB, Putnam RW. The chemosensitive response of neurons from the locus coeruleus (LC) to hypercapnic acidosis with clamped intracellular pH. Adv. Exp Med. Biol. 2008;605:333–337. doi: 10.1007/978-0-387-73693-8_58. [DOI] [PubMed] [Google Scholar]
- Histed MH, Bonin V, Reid RC. Direct activation of sparse, distributed populations of cortical neurons by electrical microstimulation. Neuron. 2009;63:508–522. doi: 10.1016/j.neuron.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Tattersall GJ, Harris MB, McEvoy SD, Richerson DN, Deneris ES, Johnson RL, Chen ZF, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J. Neurosci. 2008;28:2495–2505. doi: 10.1523/JNEUROSCI.4729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Koshiya N, Guyenet PG. Central respiratory modulation of facial motoneurons in rats. Neurosci. Lett. 1993;151(2):224–228. doi: 10.1016/0304-3940(93)90025-g. [DOI] [PubMed] [Google Scholar]
- Iscoe S. Control of abdominal muscles. Prog. Neurobiol. 1998;56:433–506. doi: 10.1016/s0301-0082(98)00046-x. [DOI] [PubMed] [Google Scholar]
- Iwamoto GA, Wappel SM, Fox GM, Buetow KA, Waldrop TG. Identification of diencephalic and brainstem cardiorespiratory areas activated during exercise. Brain Res. 1996;726:109–122. [PubMed] [Google Scholar]
- Janczewski WA, Feldman JL. Distinct rhythm generators for inspiration and expiration in the juvenile rat. J. Physiol. 2006;570:407–420. doi: 10.1113/jphysiol.2005.098848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SM, Koshiya N, Smith JC. Isolation of the kernel for respiratory rhythm generation in a novel preparation: The pre-Botzinger complex “island”. J. Neurophysiol. 2001;85:1772–1776. doi: 10.1152/jn.2001.85.4.1772. [DOI] [PubMed] [Google Scholar]
- Kukkonen JP, Holmqvist T, Ammoun S, Åkerman KEO. Functions of the orexinergic/hypocretinergic system. Am. J. Physiol. Cell Physiol. 2002;283:C1567–C1591. doi: 10.1152/ajpcell.00055.2002. [DOI] [PubMed] [Google Scholar]
- Kumar R, Ahdout R, Macey PM, Woo MA, Avedissian C, Thompson PM, Harper RM. Reduced caudate nuclei volumes in patients with congenital central hypoventilation syndrome. Neurosci. 2009;163:1373–1379. doi: 10.1016/j.neuroscience.2009.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwaki T. Orexinergic modulation of breathing across vigilance states. Respir Physiol Neurobiol. 2008;164:204–212. doi: 10.1016/j.resp.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Kuwaki T, Zhang W, Nakamura A, Deng BS. Emotional and state-dependent modification of cardiorespiratory function: role of orexinergic neurons. Auton Neurosci. 2008;142:11–16. doi: 10.1016/j.autneu.2008.03.004. [DOI] [PubMed] [Google Scholar]
- L’Hoste S, Poet M, Duranton C, Belfodil R, Barriere H, Rubera I, Tauc M, Poujeol C, Barhanin J, Poujeol P. Role of TASK2 in the control of apoptotic volume decrease in proximal kidney cells. J Biol. Chem. 2007;282:36692–36703. doi: 10.1074/jbc.M703933200. [DOI] [PubMed] [Google Scholar]
- Lazarenko RM, Milner TA, Depuy SD, Stornetta RL, West GH, Kievits JA, Bayliss DA, Guyenet PG. Acid sensitivity and ultrastructure of the retrotrapezoid nucleus in Phox2b-EGFP transgenic mice. J Comp Neurol. 2009;517:69–86. doi: 10.1002/cne.22136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Nattie E. CO2 dialysis in one chemoreceptor site, the RTN: stimulus intensity and sensitivity in the awake rat. Respir. Physiol. Neurobiol. 2002;133:11–22. doi: 10.1016/s1569-9048(02)00134-9. [DOI] [PubMed] [Google Scholar]
- Li A, Nattie E. Catecholamine neurones in rats modulate sleep, breathing, central chemoreception and breathing variability. J. Physiol. 2006;570:385–396. doi: 10.1113/jphysiol.2005.099325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Nattie EE. Focal central chemoreceptor sensitivity in the RTN studied with a CO2 diffusion pipette in vivo. J. Appl. Physiol. 1997;83:420–428. doi: 10.1152/jappl.1997.83.2.420. [DOI] [PubMed] [Google Scholar]
- Li A, Randall M, Nattie EE. CO(2) microdialysis in retrotrapezoid nucleus of the rat increases breathing in wakefulness but not in sleep. J. Appl. Physiol. 1999;87:910–919. doi: 10.1152/jappl.1999.87.3.910. [DOI] [PubMed] [Google Scholar]
- Macey PM, Richard CA, Kumar R, Woo MA, Ogren JA, Avedissian C, Thompson PM, Harper RM. Hippocampal volume reduction in congenital central hypoventilation syndrome. PLoS One. 2009;4:e6436. doi: 10.1371/journal.pone.0006436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matera I, Bachetti T, Puppo F, Di DM, Morandi F, Casiraghi GM, Cilio MR, Hennekam R, Hofstra R, Schober JG, Ravazzolo R, Ottonello G, Ceccherini I. PHOX2B mutations and polyalanine expansions correlate with the severity of the respiratory phenotype and associated symptoms in both congenital and late onset Central Hypoventilation syndrome. J Med. Genet. 2004;41:373–380. doi: 10.1136/jmg.2003.015412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellen NM, Janczewski WA, Bocchiaro CM, Feldman JL. Opioid-induced quantal slowing reveals dual networks for respiratory rhythm generation. Neuron. 2003;37:821–826. doi: 10.1016/s0896-6273(03)00092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill EG, Lipski J, Kubin L, Fedorko L. Origin of the expiratory inhibition of nucleus tractus solitarius inspiratory neurones. Brain Res. 1983;263:43–50. doi: 10.1016/0006-8993(83)91198-8. [DOI] [PubMed] [Google Scholar]
- Mitchell GS. Back to the future: carbon dioxide chemoreceptors in the mammalian brain. Nat Neurosci. 2004;7:1288–1290. doi: 10.1038/nn1204-1288. [DOI] [PubMed] [Google Scholar]
- Mitchell RA, Loeschcke HH, Massion WH, Severinghaus JW. Respiratory responses mediated through superficial chemosensitive areas on the medulla. J. Appl. Physiol. 1963;18:523–533. doi: 10.1152/jappl.1963.18.3.523. [DOI] [PubMed] [Google Scholar]
- Monnier A, Alheid GF, McCrimmon DR. Defining ventral medullary respiratory compartments with a glutamate receptor agonist in the rat. J. Physiol. 2003;548:859–874. doi: 10.1113/jphysiol.2002.038141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF, Milner TA, Reis DJ. Reticulospinal vasomotor neurons of the rat rostral ventrolateral medulla: relationship to sympathetic nerve activity and the C1 adrenergic cell group. J. Neurosci. 1988;8:1286–1301. doi: 10.1523/JNEUROSCI.08-04-01286.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Mistry AM, Guyenet PG, Bayliss DA. Purinergic P2 receptors modulate excitability but do not mediate pH sensitivity of RTN respiratory chemoreceptors. J. Neurosci. 2006;26:7230–7233. doi: 10.1523/JNEUROSCI.1696-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Rosin DL, West G, Takakura AC, Moreira TS, Bayliss DA, Guyenet PG. Serotonergic neurons activate chemosensitive retrotrapezoid nucleus neurons by a pH-independent mechanism. J. Neurosci. 2007a;27:14128–14138. doi: 10.1523/JNEUROSCI.4167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat. Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Talley EM, Stornetta RL, Siegel AR, West GH, Chen X, Sen N, Mistry AM, Guyenet PG, Bayliss DA. TASK channels determine pH sensitivity in select respiratory neurons but do not contribute to central respiratory chemosensitivity. J. Neurosci. 2007b;27:14049–14058. doi: 10.1523/JNEUROSCI.4254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie E, Li A. Muscimol dialysis in the retrotrapezoid nucleus region inhibits breathing in the awake rat. J. Appl. Physiol. 2000;89:153–162. doi: 10.1152/jappl.2000.89.1.153. [DOI] [PubMed] [Google Scholar]
- Nattie E, Li A. Neurokinin-1 receptor-expressing neurons in the ventral medulla are essential for normal central and peripheral chemoreception in the conscious rat. J. Appl. Physiol. 2006;101:1596–1606. doi: 10.1152/japplphysiol.00347.2006. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Fung ML, Li A, St John WM. Responses of respiratory modulated and tonic units in the retrotrapezoid nucleus to CO2. Resp. Physiol. 1993;94:35–50. doi: 10.1016/0034-5687(93)90055-f. [DOI] [PubMed] [Google Scholar]
- Nattie G, Li A. Multiple central chemoreceptor sites: cell types and function in vivo. Adv. Exp. Med. Biol. 2008;605:343–347. doi: 10.1007/978-0-387-73693-8_60. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Arata A, Homma I. Localization of respiratory rhythm-generating neurons in the medulla of brainstem-spinal cord preparations from newborn rats. Neurosci Lett. 1987;78:151–155. doi: 10.1016/0304-3940(87)90624-0. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Arata A, Homma I. Inhibitory synaptic inputs to the respiratory rhythm generator in the medulla isolated from newborn rats. Pflugers Arch. 1990;417:425–432. doi: 10.1007/BF00370663. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma I. Respiratory rhythm generator neurons in medulla of brainstem-spinal cord preparation from newborn rat. Brain Res. 1987;403:380–384. doi: 10.1016/0006-8993(87)90080-1. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J. Neurosci. 2003;23:1478–1486. doi: 10.1523/JNEUROSCI.23-04-01478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Homma I. Two modes of respiratory rhythm generation in the newborn rat brainstem-spinal cord preparation. Adv. Exp Med. Biol. 2008;605:104–108. doi: 10.1007/978-0-387-73693-8_18. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma I, Iwatsuki K. Excitation of inspiratory neurons by preinspiratory neurons in rat medulla in vitro. Brain Res. Bull. 1992;29:879–882. doi: 10.1016/0361-9230(92)90159-u. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Ikeda K, Kawakami K. CO2-sensitive preinspiratory neurons of the parafacial respiratory group express Phox2b in the neonatal rat. J Neurosci. 2008;28:12845–12850. doi: 10.1523/JNEUROSCI.3625-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattyn A, Goridis C, Brunet JF. Specification of the central noradrenergic phenotype by the homeobox gene Phox2b. Mol. Cell Neurosci. 2000a;15:235–243. doi: 10.1006/mcne.1999.0826. [DOI] [PubMed] [Google Scholar]
- Pattyn A, Hirsch M, Goridis C, Brunet JF. Control of hindbrain motor neuron differentiation by the homeobox gene Phox2b. Development. 2000b;127:1349–1358. doi: 10.1242/dev.127.7.1349. [DOI] [PubMed] [Google Scholar]
- Pattyn A, Morin X, Cremer H, Goridis C, Brunet JF. Expression and interactions of the two closely related homeobox genes Phox2a and Phox2b during neurogenesis. Development. 1997;124:4065–4075. doi: 10.1242/dev.124.20.4065. [DOI] [PubMed] [Google Scholar]
- Penatti EM, Berniker AV, Kereshi B, Cafaro C, Kelly ML, Niblock MM, Gao HG, Kinney HC, Li A, Nattie EE. Ventilatory response to hypercapnia and hypoxia after extensive lesion of medullary serotonergic neurons in newborn conscious piglets. J. Appl. Physiol. 2006;101:1177–1188. doi: 10.1152/japplphysiol.00376.2006. [DOI] [PubMed] [Google Scholar]
- Richerson GB, Wang W, Hodges MR, Dohle CI, Diez-Sampedro A. Homing in on the specific phenotype(s) of central respiratory chemoreceptors. Exp. Physiol. 2005;90:259–266. doi: 10.1113/expphysiol.2005.029843. [DOI] [PubMed] [Google Scholar]
- Ritucci NA, Erlichman JS, Leiter JC, Putnam RW. Response of membrane potential and intracellular pH to hypercapnia in neurons and astrocytes from rat retrotrapezoid nucleus. Am J Physiol Regul Integr Comp Physiol. 2005;289:R851–R861. doi: 10.1152/ajpregu.00132.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin DL, Chang DA, Guyenet PG. Afferent and efferent connections of the rat retrotrapezoid nucleus. J. Comp. Neurol. 2006;499:64–89. doi: 10.1002/cne.21105. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Weston MC, Sevigny CP, Stornetta RL, Guyenet PG. Hypothalamic orexin (hypocretin) neurons express vesicular glutamate transporters VGLUT1 or VGLUT2. J. Comp. Neurol. 2003;465:593–603. doi: 10.1002/cne.10860. [DOI] [PubMed] [Google Scholar]
- Rudzinski E, Kapur RP. PHOX2B Immunolocalization of the Candidate Human Retrotrapezoid Nucleus. Pediatr Dev. Pathol. 2009 doi: 10.2350/09-07-0682-OA.1. [DOI] [PubMed] [Google Scholar]
- Rybak IA, Abdala AP, Markin SN, Paton JF, Smith JC. Spatial organization and state-dependent mechanisms for respiratory rhythm and pattern generation. Prog. Brain Res. 2007;165:201–220. doi: 10.1016/S0079-6123(06)65013-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Severinghaus JW, Basbaum AI. Medullary CO2 chemoreceptor neuron identification by c-fos immunocytochemistry. J. Appl. Physiol. 1992;73:96–100. doi: 10.1152/jappl.1992.73.1.96. [DOI] [PubMed] [Google Scholar]
- Smith JC, Abdala AP, Koizumi H, Rybak IA, Paton JF. Spatial and functional architecture of the mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. J. Neurophysiol. 2007;98:3370–3387. doi: 10.1152/jn.00985.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. PreBotzinger complex--a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Morrison DE, Ellenberger HH, Otto MR, Feldman JL. Brainstem projections to the major respiratory neuron populations in the medulla of the cat. J Comp Neurol. 1989;281:69–96. doi: 10.1002/cne.902810107. [DOI] [PubMed] [Google Scholar]
- Spyer KM, Dale N, Gourine AV. ATP is a key mediator of central and peripheral chemosensory transduction. Exp. Physiol. 2004;89:53–59. doi: 10.1113/expphysiol.2003.002659. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J. Neurosci. 2006;26:10305–10314. doi: 10.1523/JNEUROSCI.2917-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stornetta RL, Spirovski D, Moreira TS, Takakura AC, West GH, Gwilt JM, Pilowsky PM, Guyenet PG. Galanin is a selective marker of the retrotrapezoid nucleus in rats. J Comp Neurol. 2009;512:373–383. doi: 10.1002/cne.21897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun MK, Reis DJ. Excitatory amino acid-mediated chemoreflex excitation of respiratory neurones in rostral ventrolateral medulla in rats. J. Physiol. 1996;492:559–571. doi: 10.1113/jphysiol.1996.sp021329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzue T. Respiratory rhythm generation in the in vitro brain stem-spinal cord preparation of the neonatal rat. J. Physiol. 1984;354:173–183. doi: 10.1113/jphysiol.1984.sp015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taccola G, Secchia L, Ballanyi K. Anoxic persistence of lumbar respiratory bursts and block of lumbar locomotion in newborn rat brainstem spinal cords. J. Physiol. 2007;585:507–524. doi: 10.1113/jphysiol.2007.143594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J. Physiol. 2006;572:503–523. doi: 10.1113/jphysiol.2005.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, Stornetta RL, West GH, Gwilt JM, Guyenet PG. Selective lesion of retrotrapezoid Phox2b-expressing neurons raises the apnoeic threshold in rats. J. Physiol. 2008;586:2975–2991. doi: 10.1113/jphysiol.2008.153163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W, Janczewski WA, Yang P, Shao XM, Callaway EM, Feldman JL. Silencing preBotzinger Complex somatostatin-expressing neurons induces persistent apnea in awake rat. Nat Neurosci. 2008;11:538–540. doi: 10.1038/nn.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoby-Brisson M, Karlen M, Wu N, Charnay P, Champagnat J, Fortin G. Genetic identification of an embryonic parafacial oscillator coupling to the preBotzinger complex. Nat Neurosci. 2009;12:1028–1035. doi: 10.1038/nn.2354. [DOI] [PubMed] [Google Scholar]
- Thomas T, Spyer KM. ATP as a mediator of mammalian central CO2 chemoreception. J. Physiol. 2000;523:441–447. doi: 10.1111/j.1469-7793.2000.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weese-Mayer DE, Berry-Kravis EM, Zhou L, Maher BS, Silvestri JM, Curran ME, Marazita ML. Idiopathic congenital central hypoventilation syndrome: analysis of genes pertinent to early autonomic nervous system embryologic development and identification of mutations in PHOX2b. Am J Med. Genet. A. 2003;123:267–278. doi: 10.1002/ajmg.a.20527. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Rand CM, Berry-Kravis EM, Jennings LJ, Loghmanee DA, Patwari PP, Ceccherini I. Congenital central hypoventilation syndrome from past to future: model for translational and transitional autonomic medicine. Pediatr Pulmonol. 2009;44:521–535. doi: 10.1002/ppul.21045. [DOI] [PubMed] [Google Scholar]
- Weston MC, Stornetta RL, Guyenet PG. Glutamatergic neuronal projections from the marginal layer of the rostral ventral medulla to the respiratory centers in rats. J. Comp. Neurol. 2004;473:73–85. doi: 10.1002/cne.20076. [DOI] [PubMed] [Google Scholar]
- Wilson RJ, Vasilakos K, Harris MB, Straus C, Remmers JE. Evidence that ventilatory rhythmogenesis in the frog involves two distinct neuronal oscillators. J Physiol. 2002;540:557–570. doi: 10.1113/jphysiol.2001.013512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmeier S, Song G, Duffin J, Poon CS. Pacemakers handshake synchronization mechanism of mammalian respiratory rhythmogenesis. Proc. Natl. Acad. Sci. USA. 2008;105:18000–18005. doi: 10.1073/pnas.0809377105. [DOI] [PMC free article] [PubMed] [Google Scholar]