Abstract
We examined the role of central nervous system (CNS) endogenous melanocortin 3/4 receptors (MC3/4R) activity in controlling cardiovascular and metabolic functions in Sprague Dawley rats fed a high fat diet (HF, n=6) for 10 months compared to rats fed a standard chow (NF, n=8) starting at 3 weeks of age. At 7 months of age, HF rats were heavier (473±3 vs. 424±7 g), consumed more calories with larger, less frequent meals and had reduced respiratory quotient (RQ) compared to NF rats. After 10 months on the diets, arterial and venous catheters were implanted for measurement of mean arterial pressure (MAP) and heart rate (HR) 24-hrs/day and IV infusions, and a 21G steel cannula was placed in the lateral ventricle for intracerebroventricular (ICV) infusions. HF rats were heavier (528±14 vs. 477±11 g) with increased visceral adiposity and significantly higher MAP (108±3 vs. 102±1 mmHg). After a 5-day control period, the rats were infused with a MC3/4R antagonist (SHU-9119, 1 nmol/hr, ICV) for 10 days followed by a 5-day recovery period. SHU-9119 infusion for 10 days increased caloric intake significantly more in HF rats (159±19 vs. 64±8 kcal). Despite increasing caloric intake and rapid weight gain, MC3/4R antagonism reduced MAP more in HF compared to NF rats (−7.9±0.3 vs. −4.7±1.3 mmHg, average reduction of last 4 days of blockade). These observations suggest that a HF diet increases endogenous activity of the CNS MC3/4R and that an intact MC3/4 appears to play an important role in linking increased blood pressure with diet-induced obesity.
Keywords: Obesity, hypertension, proopiomelanocortin, melanocortin receptors, metabolism
INTRODUCTION
Excess weight gain is an important cause of hypertension and cardiovascular disease [1,2]. Although the precise mechanisms by which excess weight gain elevates blood pressure (BP) have not been fully elucidated, we and others have suggested that leptin, an adipocyte derived hormone, may contribute to obesity-induced hypertension by activating proopiomelanocortin (POMC) neurons in the central nervous system (CNS) [3–6]. POMC neurons, in turn, release alpha-melanoycte-stimulating hormone (α-MSH) which then activates melanocortin 3/4 receptors in various regions of the brain [7].
Although activation of POMC neurons has been shown to contribute to leptin’s anorexic and metabolic effects [7], the role of POMC neurons and stimulation of MC3/4R in mediating obesity-induced hypertension is still unclear. We and others have previously shown that chronic activation of CNS MC3/4R increases arterial pressure, despite reduced food intake and weight loss, by activating adrenergic activity [8–15]. Whether MC3/4R activation is actually increased in diet-induced obesity and contributes to elevated blood pressure, however, is unknown.
The present study was designed to determine whether chronic blockade of the endogenous MC3/4R activity specifically in the CNS reduces blood pressure while increasing food intake and appetite to a greater extent in obese rats fed a high fat diet for 10 months compared to lean rats fed a normal chow.
METHODS
The experimental protocols of this study followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center.
Animals
Three-week-old Sprague Dawley (SD; Harlan, Indianapolis, IN) rats were randomly divided into two groups. One group received normal fat chow (13% fat, TD07055 Harlan Teklad, Madison, WS: NF) and a second group was placed on a high fat diet (40% fat, TD07054: HF). The rats were fed these diets for the duration of the experiment which lasted for over 10 months.
Metabolic Monitoring
At 7 months of age, rats (n=6 for each group) were placed individually in a metabolic monitoring system for a continuous five day determination of oxygen consumption (VO2), motor activity, food and water intake, and meal pattern. VO2 was measured every 10 minutes for 2-minute intervals using Zirconia oxygen sensors in the metabolic monitoring (AccuScan Instrument, Columbus, OH). This system also measured carbon dioxide (CO2) production and automatically calculated respiratory quotients (RQ). Motor activity was determined by infrared light beams mounted in the cages in X, Y, and Z axes. Precise measurements of food and water intake were made using a computerized workstation that constantly monitored the weight of the food and water hoppers as well as VO2, CO2 production, RQ, and motor activity. Daily values for each variable were computed from the average of 144 sampling periods.
Surgical Protocol for Implantation of Catheters
After 10 months on the diets, HF (n=6) and NF rats (n=8) were anesthetized with 50 mg/kg sodium pentobarbital (Nembutal) and administered atropine sulfate (0.1 mg/kg) to prevent excess airway secretions. Arterial and venous catheters were implanted for measurements of arterial pressure, 24 hrs/day, and continuous IV infusion as described previously [6]. Using sterile techniques, nonocclusive polyvinyl catheters were inserted into the abdominal aorta and the left femoral vein and exteriorized through a stainless steel button placed subcutaneously in the scapular region between the shoulders.
Intracerebroventricular Cannulation
Immediately after implantation of arterial and venous catheters, rats were placed in a stereotaxic apparatus and a stainless steel cannula (26 gauge, 10 mm long) was implanted into the right lateral cerebral ventricle as described previously [15]. A stainless steel stylet was inserted to seal the cannula until use. Anesthesia was maintained with 1% isofluorane during this procedure.
Seven days after recovery from surgery, the accuracy of the ICV cannula placement was tested by measuring the dipsogenic response to an acute ICV injection of 100 ng of angiotensin II. All rats used in this study drank 5 ml or more of water within 10 minutes after injection of angiotensin II. After the experiments were completed, the rats were killed with an overdose of sodium pentobarbital, and their brains removed and sectioned to confirm accurate placement of the ICV cannula.
Experimental Protocol
MAP and HR were recorded 24 hrs/day using computerized methods as previously described [16]. Urine volume, urinary sodium and potassium excretion, and food and water consumption were recorded daily. After 7–10 days of recovery from surgery and then at least 5 additional days of stable control measurements, osmotic minipumps (Alzet, model 2002) were implanted subcutaneously in the scapular region and connected to the ICV cannula to deliver the MC3/4R antagonist, SHU-9119 (Polypeptide Laboratories) at a dose of 1 nmol/hr. This dose of SHU-9119 was based on previous studies demonstrating a significant increase in food intake and effective MC3/4R blockade when given chronically by ICV infusion to rats as well as on studies by other investigators demonstrating that SHU-9119 completely attenuated the reduction in food intake observed during administration of melanotan II (MTII), a specific agonist of MC3/4R [16, 17]. On day 10 of SHU-9119 infusion, the SHU-9119 infusion was stopped by severing the tubing from the minipump to the ICV cannula and measurements were continued for 5 more days. Rats were then euthanized and visceral adiposity was measured as the combined weight of the retroperitoneal and epididymal fat pads.
Statistical Analyses
The data are expressed as mean ± SEM and analyzed by using 1-way analysis of variance (ANOVA) with repeated measures within each animal group and 2-factor ANOVA with repeated measures between each animal group. The Bonferroni post-hoc test was used for comparisons between groups. Dunnett’s test was performed to compare control versus experimental values within each group. A two-tailed, unpaired t-test was used to compare the data for NF and HF rats collected at 7 months of age as well as the baseline data at 10 months. Statistical significance was accepted at a level of P<0.05.
RESULTS
Food Intake, Energy Expenditure, Body Weight, and Visceral Adiposity
SD rats fed a HF diet for 7 months consumed significantly more calories than rats fed a normal diet (78 kCal/day compared to 66 kCal/day) as shown in Table 1. This increase in caloric intake, and resulting increased body weight, was caused by larger meal sizes despite reduced meal frequency compared to NF (Table 1). HF rats had reduced respiratory quotients and similar VO2 even though HF rats had increased motor activity (Table 1). After 10 months of the HF diet, HF rats had increased body weight compared to NF rats (528 ± 14 vs. 477 ± 11 g, Table 2). The increased body weight of HF rats was associated with a doubling of visceral adiposity (shown in Table 2) compared to NF rats. Urinary volume as well as potassium and sodium excretions were not significantly different between groups.
Table 1.
Effects of feeding rats a normal fat or high fat diet for 7 Months
| Normal Fat (n=6) |
High Fat (n=6) |
|
|---|---|---|
| Body Weight (g) | 424 ± 7 | 473 ± 3* |
| Caloric Intake per day (kcal) | 66 ± 3 | 78 ± 2* |
| Caloric Intake per meal (kcal) | 2.9 ± 0.1 | 4.4 ± 0.1* |
| Number of meals per day | 23.1 ± 1.4 | 17.7 ± 0.6* |
| Motor Activity (cm/hr) | 23048 ± 642 | 27599 ± 859* |
| Oxygen Consumption (ml/kg/min) | 18.5 ± 0.1 | 18.4 ± 0.2 |
| Respiratory Quotient (VCO2/VO2) | 0.923 ± 0.014 | 0.885 ± 0.013* |
Mean ± SEM;
p<0.05 vs. NF control group.
Table 2.
Effects of feeding rats a normal fat or high fat diet for 10 Months
| Normal Fat (n=8) |
High Fat (n=6) |
|
|---|---|---|
| Body Weight (g) | 477 ± 11 | 528 ± 14* |
| Visceral Adiposity (g) | 10.8 ± 1.0 | 20.9 ± 2.4* |
| Mean Arterial Pressure (mmHg) | 102 ± 1 | 108 ± 3* |
| Heart Rate (bpm) | 355 ± 2 | 357 ± 4 |
| Urinary Volume (ml/24 hours) | 15.2 ± 1.7 | 17.2 ± 1.6 |
| Urinary Sodium Excretion (mEq/24 hours) | 1.5 ± 0.2 | 1.6 ± 0.2 |
| Urinary Potassium Excretion (mEq/24 hours) | 1.2 ± 0.1 | 1.4 ± 0.1 |
MAP, HR, Urinary volume, UNa+, and UK+ are the average value for five days. Mean ± SEM;
p<0.05 vs. NF control group.
Effect of High Fat Diet on MAP and HR
After 10 months on the diets, HF rats had significantly higher MAP compared to NF rats (108 ± 3 vs 102 ± 1 mmHg, p<0.05). However, HR was not significantly different in the two groups (357 ± 4 vs 355 ± 2 bpm).
Food Intake Response to MC3/4-R Blockade
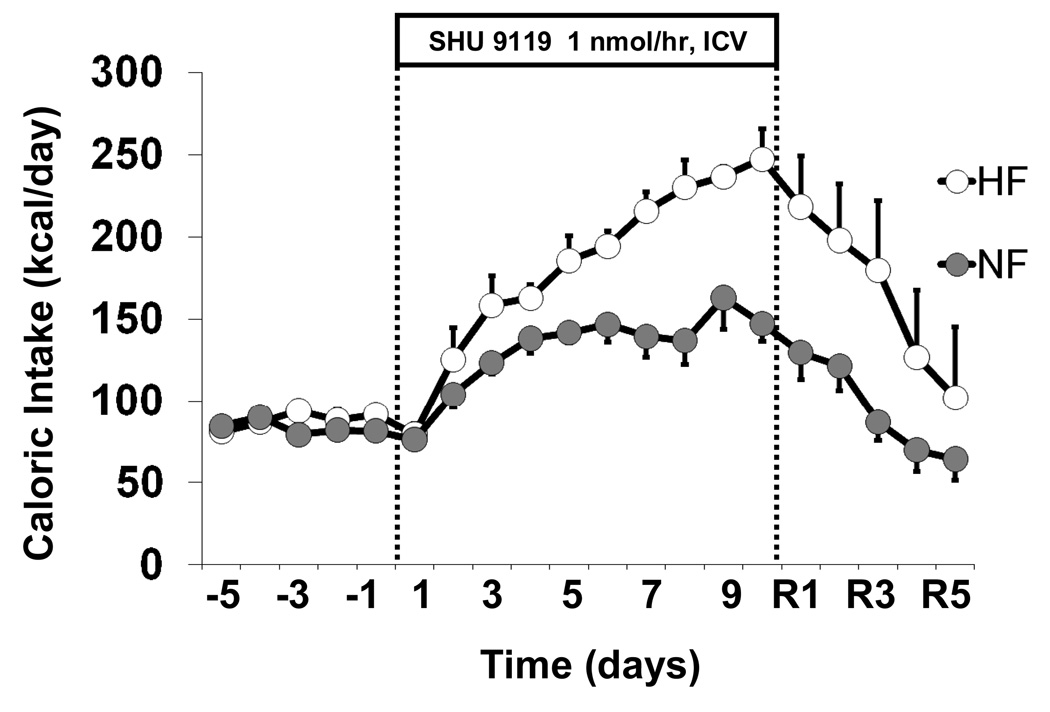
Chronic ICV administration of SHU-9119 markedly increased caloric intake in both groups; however, beginning on day 5 of SHU-9119 treatment caloric intake was significantly higher in HF than in NF rats (Figure 1) and continued to increase during the remaining five days of SHU-9119 infusion to ~240 kcal/day by the last day of treatment. Caloric intake in NF rats remained at around 150 kcal/day for the last 5 days of MC3/4R blockade. After SHU-9119 was stopped, caloric intake gradually returned to control values in NF and HF rats (Figure 1).
Figure 1.
Effects of chronic ICV administration of the MC3/4R antagonist, SHU-9119 (1 nmol/hr) on caloric intake in SD rats fed a NF (n=8) or HF (n=6) diet for 10 months.
MAP and HR Responses to MC3/4-R inhibition
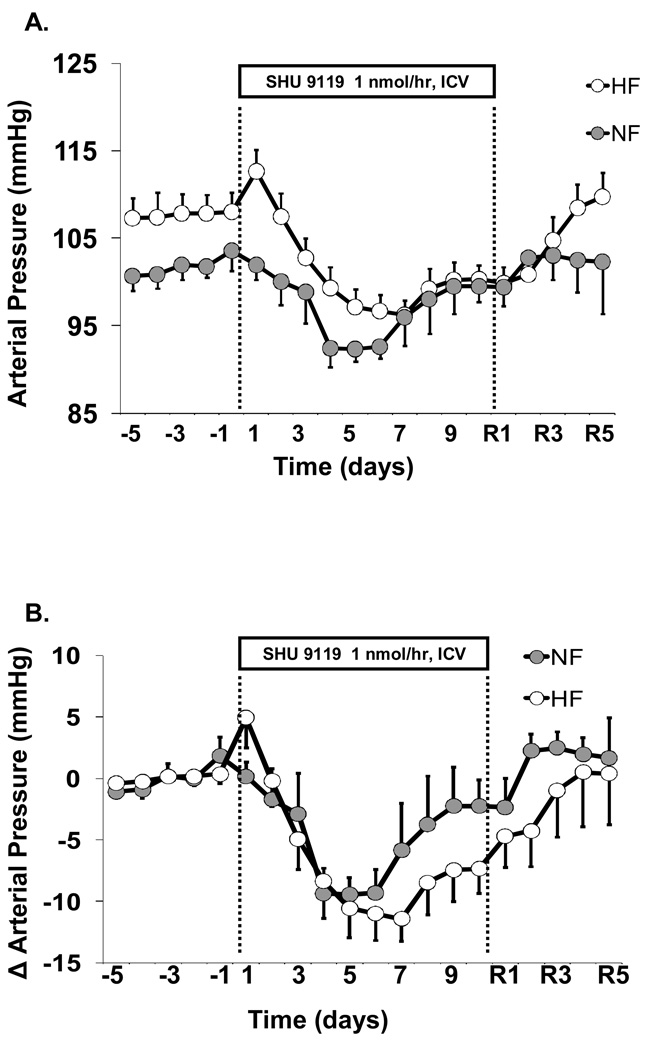
SHU-9119 infusion reduced MAP in both HF and NF rats (Figures 2A and 2B). The fall in MAP was approximately -10 mmHg by day 4 of treatment in both groups (Figure 2B). By day 7 of infusion, blood pressure in NF returned to a level that was not significantly different from control values. However, in HF rats MAP during SHU-9119 infusion remained significantly lower than baseline values until termination of MC3/4R blockade. Heart rate was reduced by ~50 bpm during the 10-day infusion period of SHU-9119 in both groups (Figure 2C). Thus, chronic blockade of MC3/4R caused significantly greater reductions in blood pressure in HF than in NF rats despite greater increases in caloric intake and more weight gain.
Figure 2.
Effects of chronic ICV administration of the MC3/4R antagonist, SHU-9119 (1 nmol/hr), on (A) MAP, (B) change in MAP, and (C) HR in SD rats fed a NF (n=8) or HF (n=6) diet for 10 months.
DISCUSSION
The most important findings of this study are that 1) blockade of endogenous activity of the CNS MC3/4R markedly increased caloric intake in normal and obese rats fed a high fat diet, but lowered blood pressure thereby blocking the usual effects of increased caloric intake and weight gain to raise blood pressure; 2) the effects of MC3/4R antagonism on appetite and blood pressure were enhanced in rats fed a high fat diet for 10 months, compared to rats fed a normal diet. These observations support the hypothesis that intact MC3/4R activation is necessary for increased caloric intake and obesity to raise blood pressure. Furthermore, these results suggest that a chronic high fat diet and obesity increase endogenous activation of MC3/4R, as reflected by greater increases in food intake and more sustained reductions in blood pressure in rats fed a high fat diet compared to rats fed a normal chow.
Previous studies suggest that activation of POMC neurons and subsequent stimulation of MC3/4R play an important role in mediating the acute effects of leptin on food intake and renal sympathetic activity as well as the chronic hypertensive effects of leptin [9,10,14]. In obesity, however, the effects of leptin to reduce food intake appear to be attenuated while the stimulatory effect on renal SNS activity is preserved, a condition that has been called “selective leptin resistance” [11–13, 18]. The observed resistance to the anorexic effects of leptin in obesity does not appear to be caused by resistance to MC3/4R activation. We previously showed that obese rats fed a HF diet have a normal response to chronic activation of MC3/4R during chronic ICV infusion of the specific agonist melanotan (MTII) [15]. The importance of MC3/4 activation in mediating the chronic effects of leptin in obesity, as well as the role of MC3/4R activation in obesity-induced hypertension, however, have not been previous assessed.
Effects of Chronic MC3/4-R Blockade on Food Intake
Chronic ICV infusion of SHU-9119 caused marked increases in caloric intake in NF rats, similar to our previous observations during blockade of the melanocortin system in normal rats [16]. However, the increase in food intake during MC3/4R antagonism was significantly enhanced in HF rats compared to the response observed in NF rats. This observation suggests that MC3/4R tone to reduce feeding may be increased, rather than reduced, in rats fed a HF diet compared to rats fed a normal diet. Thus, our observations provide no evidence that there is resistance to the effects of endogenous CNS MC3/4R activation in rats fed a chronic HF diet.
Our results also suggest that if there is “resistance” to the anorexic effects of leptin in dietary-induced obesity, this reduced sensitivity is not mediated by decreased activation of CNS MC3/4R. In fact, tonic activity of the MC3/4R in regulating food intake appears to actually be enhanced in rats fed a high fat diet for 10 months, rather than reduced. One potential explanation, although not directly tested in the present study, is that MC3/4R activation in obesity is mediated by factors other than leptin activation of POMC neurons. Further studies are needed to test this hypothesis.
Effects of Chronic MC3/4-R Blockade on Arterial Pressure
Our results also indicate that tonic control of blood pressure by endogenous activation of CNS MC3/4R is increased in obesity caused by feeding a HF diet. SHU-9119 infusion caused a more sustained reduction in blood pressure in HF compared to NF rats. Normally, rapid increases in body weight caused by a high fat diet result in increased blood pressure [1,2]. However, after blockade of the CNS MC3/4R large increases in caloric intake and rapid weight gain were associated with reductions in blood pressure. These observations suggest that an intact hypothalamic POMC-MC3/4R system may be necessary for dietary-induced obesity to cause hypertension.
Our findings in dietary induced obesity are consistent with recent observations in humans with loss-of-function mutations in MC4R; these patients are obese but have reduced prevalence of hypertension and lower average blood pressure than subjects with normal MC4R [14,19]. Thus, in experimental animals as well as in humans, an intact MC3/4R system appears to be an important link between obesity and hypertension.
Whether selective blockade of the sympathetic nervous system effects of MC3/4R activation may be beneficial in obesity hypertension is unclear. Although blockade of MC3/4R lowers blood pressure, it also causes hyperphagia and rapid weight gain. However, it is possible that the blood pressure and appetite effects of MC3/4R activation may be controlled by different neuronal populations since MC3/4R have been shown to be expressed in vasomotor neurons of the brainstem as well as in the hypothalamus, the key center for control of appetite [20].
In conclusion, chronic blockade of the melanocortin system lowered arterial pressure and heart rate in both HF and NF groups while markedly increasing caloric intake. In HF rats, however, the orexogenic and blood pressure lowering effects MC3/4R blockade were enhanced in obese rats fed a high fat diet compared to rats fed a normal chow. Thus, obesity caused by a high fat diet does not appear to lead to downregulation or “resistance” to the effects of MC3/4R activation, but instead leads to an increased tone of the endogenous CNS melanocortin system in controlling both food intake and blood pressure.
ACKNOWLEDGEMENTS
The authors’ research was supported by the National Heart, Lung and Blood Institute grant PO1 HL-51971.
Sources of support: NIH
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: none
REFERENCES
- 1.Garrison RJ, Kannel WB, Stokes J, Castelli WP. Incidence and precursors of hypertension in young adults: The Framingham Offspring Study. Prev Med. 1987;16:235–251. doi: 10.1016/0091-7435(87)90087-9. [DOI] [PubMed] [Google Scholar]
- 2.Hall JE. The kidney, hypertension, and obesity. Hypertension. 2003;41:625–633. doi: 10.1161/01.HYP.0000052314.95497.78. [DOI] [PubMed] [Google Scholar]
- 3.Haynes WG, Sivitz WI, Morgan DA, Walsh SA, Mark AL. Sympathetic and cardiorenal actions of leptin. Hypertension. 1997;30:619–623. doi: 10.1161/01.hyp.30.3.619. [DOI] [PubMed] [Google Scholar]
- 4.Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI. Receptor-mediated regional sympathetic nerve activation by leptin. J Clin Invest. 1997;100:270–278. doi: 10.1172/JCI119532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunbar JC, Hu Y, Lu H. Intracerebroventricular leptin increases lumbar and renal sympathetic nerve activity and blood pressure in normal rats. Diabetes. 1997;46:2040–2043. doi: 10.2337/diab.46.12.2040. [DOI] [PubMed] [Google Scholar]
- 6.Shek EW, Brands MW, Hall JE. Chronic leptin infusion increases arterial pressure. Hypertension. 1998;31:409–414. doi: 10.1161/01.hyp.31.1.409. [DOI] [PubMed] [Google Scholar]
- 7.Seeley RJ, Yagaloff KA, Fisher SL, Burn P, Thiele TE, van Dijk G, Baskin DG, Schwartz MW. Melanocortin receptors in leptin effects. Nature. 1997;390:349. doi: 10.1038/37016. [DOI] [PubMed] [Google Scholar]
- 8.Satoh N, Ogawa Y, Katsuura G, Numata Y, Masuzaki H, Yoshimasa Y, Nakao K. Satiety effect and sympathetic activation of leptin are mediated by hypothalamic melanocortin system. Neurosci Lett. 1998;249:107–110. doi: 10.1016/s0304-3940(98)00401-7. [DOI] [PubMed] [Google Scholar]
- 9.Haynes WG, Morgon DA, Djalali A, Sivitz WI, Mark AL. Interaction between the melanocortin system and leptin in control of sympathetic nerve traffic. Hypertension. 1999;33:542–547. doi: 10.1161/01.hyp.33.1.542. [DOI] [PubMed] [Google Scholar]
- 10.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Role of melanocortin-4 receptors in mediating renal sympathoactivation to leptin and insulin. J Neurosci. 2003;23:5998–6004. doi: 10.1523/JNEUROSCI.23-14-05998.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mark AL, Correia ML, Rahmouni K, Haynes WG. Selective leptin resistance: a new concept in leptin physiology with cardiovascular implications. J Hypertens. 2002;20:1245–1250. doi: 10.1097/00004872-200207000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Frederich RC, Hamann A, Anderson S, Lollmann B, Lowell BB, Flier JS. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med. 1995;1:1311–1314. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- 13.Mantzoros CS, Flier JS. Editorial: leptin as a therapeutic agent–trials and tribulations. J Clin Endocrinol Metab. 2000;85:4000–4002. doi: 10.1210/jcem.85.11.7062. [DOI] [PubMed] [Google Scholar]
- 14.Tallam LS, da Silva AA, Hall JE. Melanocortin-4 receptor mediates chronic cardiovascular and metabolic actions of leptin. Hypertension. 2006 Jul;48(1):58–64. doi: 10.1161/01.HYP.0000227966.36744.d9. [DOI] [PubMed] [Google Scholar]
- 15.Silva AA, Kuo JJ, Tallam LS, Liu J, Hall JE. Does obesity induce resistance to the long-term cardiovascular and metabolic actions of melanocortin 3/4 receptor activation? Hypertension. 2006;47:259–264. doi: 10.1161/01.HYP.0000198458.70351.e0. [DOI] [PubMed] [Google Scholar]
- 16.Kuo JJ, Silva AA, Hall JE. Hypothalamic melanocortin receptors and chronic regulation of arterial pressure and renal function. Hypertension. 2003;41:768–774. doi: 10.1161/01.HYP.0000048194.97428.1A. [DOI] [PubMed] [Google Scholar]
- 17.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 18.Jéquier E. Leptin signaling, adiposity, and energy balance. Ann N Y Acad Sci. 2002;967:379–388. doi: 10.1111/j.1749-6632.2002.tb04293.x. [DOI] [PubMed] [Google Scholar]
- 19.Greenfield JR, Miller JW, Keogh JM, Henning E, Satterwhite JH, Cameron GS, et al. Modulation of Blood Pressure by Central Melanocortinergic Pathways. N Engl J Med. 2009;360:44–52. doi: 10.1056/NEJMoa0803085. [DOI] [PubMed] [Google Scholar]
- 20.Cone RD. Studies on the physiological functions of the melanocortin system. Endocr Rev. 2006;27:736–749. doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]