Abstract
We have previously shown experimental transplantation of living allogeneic bone to be feasible without long-term immunosuppression by development of a recipient-derived neoangiogenic circulation within bone. In this study we study the role of angiogenic cytokine delivery with biodegradable microspheres to enhance this process. Microsurgical femoral allotransplantation was performed from DA to PVG rats. Poly(D,L-lactide-co-glycolide) microspheres loaded with buffer, basic fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), or both were inserted intramedullarly along with a recipient-derived a/v bundle. FK-506 was administered daily for 14 days, then discontinued. At 28 days, bone blood flow was measured using hydrogen washout. Microangiography, histologic and histomorphometric analysis were performed. Capillary density was greater in the FGF+VEGF group (35.1%) than control (13.9%) (p<0.05), and a linear trend was found from control, FGF, VEGF, to FGF+VEGF (p<0.005). Bone formation rates were greater with VEGF (p<0.01) and FGF+VEGF (p<0.05). VEGF or FGF alone increased blood flow more than when combined. Histology rejection grading was low in all grafts. Local administration of vascular and fibroblast growth factors augments angiogenesis, bone formation and bone blood flow from implanted blood vessels of donor origin in vascularized bone allografts after removal of immunosuppression.
Keywords: bone, allotransplantation, microspheres, FGF, VEGF
Introduction
Segmental loss of bone or joint is a difficult problem for which current reconstructive methods often fail. An alternative to current practice is the use of vascularized (living) bone or composite tissue allotransplants (CTAs). Maintaining tissue viability in the face of acute and chronic rejection remains a challenge however, requiring long-term immunosuppression or induction of tolerance with attendant significant risks. We have investigated a novel method to enable transplantation of living bone without long-term immune modulation. This is accomplished by placing vascularized recipient tissue in the form of an arteriovenous (a/v) bundle or fascial flap within the bone at the time of transplantation, together with microvascular repair of nutrient vessels. Only short-term immune modulation is necessary while neoangiogenesis from host tissue occurs. We have demonstrated maintenance of femoral bone viability using this method1, 2, and have evaluated the underlying mechanisms by quantifying angiogenesis and blood flow as well as measuring graft chimerism3. We have shown that this method is independent of donor-specific tolerance induction, and is dependent on transplantation of a vascularized graft, with results comparable to isograft.
The overall goal of the current study is to investigate growth factor enhancement of angiogenesis and new bone formation in our transplantation model. At surgery, we administered biodegradable microspheres encapsulating basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), or both within the transplanted femur adjacent to the a/v bundle. We measured the effect of the growth factor(s) on angiogenesis, bone remodeling and bone blood flow.
Methods
Inbred female Dark Agouti (DA) rats (genetic expression: RT1a, 150-175g) were used as donors in a nonsurvival procedure. Male Piebald Virol Glaxo (PVG) rats (genetic expression: RT1c, 200-250g) were used as the recipient animals. This combination provided a strong histocompatibility mismatch. All animals were obtained from Harlan Sprague Dawley, Madison, WI. Immunosuppression was administered in the form of FK-506 (1mg/kg/day IM) (Tacrolimus, Fujisawa Pharmaceutical Co., Osaka, Japan).
Sterile technique was maintained throughout the procedures. All experiments were performed according to established National Institutes of Health guidelines and under the direction of our Institutional Animal Care and Use Committee. Animals were allowed to move freely in their cages and fed standard rodent feed and water ad libitum.
Animal Model
Microvascular transfer of the rat femur was first described in 1984 based on anatomical dissection and vessel arborization4. The vascular distribution to the bone is provided by proximal and distal nutrient branches arising from the lateral femoral circumflex artery and femoral artery, respectively (Figure 1). The operative steps are shown in Figure 2. The group distribution is shown in Table 1.
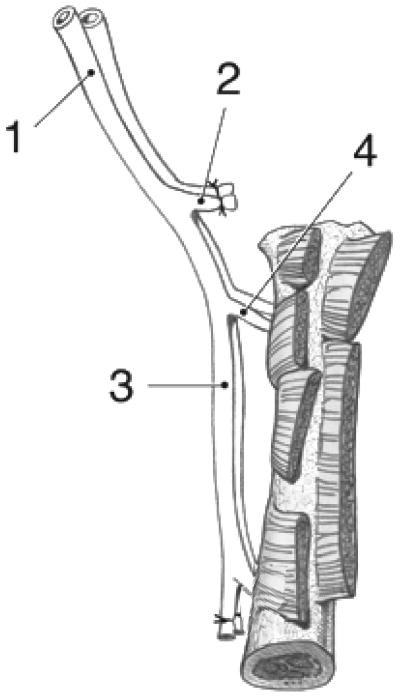
Figure 1.
Drawing of the rat femoral graft with (1) common iliac a. & v., (2) ligated superficial iliac circumflex a. & v., (3) lateral femoral circumflex a. & v. with proximal nutrient branch to femur, (4) femoral a. & v. with distal nutrient branch, (5) ligation at the saphenous a. & v. origin.
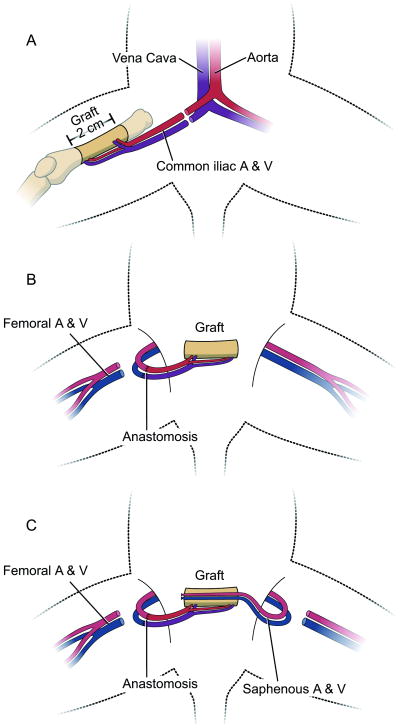
Figure 2.
Diagram showing the surgical technique. (A) Donor procedure; resection of the femur diaphysis based on the bone nutrient vessels. (B) Microvascular anastomosis in the recipient. (C) A/v bundle implantation: saphenous vessels are carefully pulled through the medullar canal.
Table 1.
Group Distributions
| Group | Growth factor | N | Immuno-suppression | AV Bundle | Survival time |
|---|---|---|---|---|---|
| 1 | none | 11 | 2 weeks | Patent | 4 weeks |
| 2 | FGF2 10μg | 10 | 2 weeks | Patent | 4 weeks |
| 3 | VEGF 10μg | 11 | 2 weeks | Patent | 4 weeks |
| 4 | VEGF 10μg + FGF2 10μg | 11 | 2 weeks | Patent | 4 weeks |
Operative Procedure
In a DA donor, the right femur was raised on its nutrient vascular pedicle. The proximal common iliac artery and vein were ligated and cut at their origin, to be used for the microvascular repair. The pedicle was irrigated with heparinized saline, the femoral head and neck and distal femoral condyles were resected and the medullar canal reamed with a 2-mm diameter drill bit. Allograft sizes varied from 20-22 mm in length.
In a PVG recipient, the right femoral artery and vein were ligated at the saphenous origin. There was sufficient collateral flow to the limb to prevent vascular compromise post-operatively. Microvascular anastomosis to the graft vessels was performed in an end-to-end fashion. The use of smaller size donor rats, with a body weight 50g to 70g less than that of the recipient allowed an appropriate match of vessel diameters5. The graft was wrapped with a reinforced silicone membrane to prevent angiogenesis from surrounding tissues and placed in an abdominal skin pocket. The left saphenous artery and vein were isolated along with a small distal fascial flap and implanted as an a/v bundle into the medullar canal to run the entire length of the allograft. Growth factors were delivered as encapsulated poly(D,L-lactide-co-glycolide) (PLGA) microspheres placed on the a/v bundle. The medullar canal was then filled with 20μl of collagen I solution at pH 7.4 and sealed with fibrin glue at each end (Tisseel VH Fibrin sealant, Baxter Healthcare, Westlake Village CA). Closure of the subcutaneous and skin layers was done using interrupted 4-0 absorbable and nylon sutures and staples.
Microsphere manufacture
The fabrication process was done under aseptic conditions. Briefly, 1μl of a 1.0mg/ml growth factor solution (Trevigen Inc., Gaithersburg, MD), was emulsified in 2.5μl dichloromethane per mg PLGA (50:50 lactic to glycolic acid ratio and average molecular weight of 23kD) using a vortex at 3050rpm. Microspheres loaded with buffered saline were used for control groups. The mixture was re-emulsified in 1% w/v aqueous polyvinyl alcohol (PVA) solution to create a double emulsion. The content was then added to 0.3% w/v PVA and 2% w/v isopropanol solution and stirred for one hour. The extraction of the dichloromethane to the external alcoholic phase results in precipitation of the dissolved polymers and the formation of microspheres. The manufacturing parameters were previously determined by our collaborating laboratory6, 7 to provide a zero-order drug release for 28 days after an initial burst, on the condition of embedding in a substrate such as a collagen matrix. Each bone transplant was loaded with 15mg of microspheres containing 0.7μg per mg growth factor (=10μg total).
Assessment of microsphere surface morphology and size distribution
Microsphere morphology was assessed by scanning electron microscopy (SEM) (Hitachi S4700, San Jose, CA). For each group, the diameter of microspheres was measured from each of three micrographs each containing 100-3000 microspheres using image analysis software (Scion Image for Windows 4.0.2; Scion Corporation, Frederick, MD), averaged and compared.
Bone blood flow measurement: Hydrogen washout
We have previously described and validated routine hydrogen washout blood flow measurement in the rat8, 9. Measurements were performed on all grafts, immediately prior to euthanasia. Briefly, a tracheotomy was placed and the graft exposed. A 0.36mm wide hole was drilled into the cortex. A hydrogen sensor (H2-50, Unisense, Aarhus, Denmark) was inserted with a micromanipulator and connected to a picoammeter (Picoammeter2000, Unisense, Aarhus, Denmark). The breathing mixture contained 70% hydrogen from a generator (Whatman 75-32, Haverhill MA). At tissue saturation, 100% oxygen was given. LabVIEW™ software (National Instruments, Austin, TX) was used to calculate the rate of hydrogen washout. This is proportional to the cortical blood flow, expressed as ml/min per 100g of tissue10, 11.
Microangiography
Following euthanasia, the abdominal aorta and vena cava were filled with blue microangiographic solution (Microfil, Flow Tech Inc., Carver, MA) under physiologic pressure. The compound was allowed to polymerize for 24 hours at 4°C. Grafts were then fixed in 10% formalin for 24 hours and a 2-mm trasverse section for histomorphometric analysis was removed by a diamond-coated circular drill. The grafts were then decalcified in 14% ethylenediaminetetraacetic acid for 7 hours in a calibrated laboratory microwave at 750W (Pelco Biowave 3450 Laboratory Microwave, Ted Pella, Inc., Redding, CA). The intraosseous vasculature was visualized by optical bone clearing12 and the capillary density (D) of each specimen was measured using image analysis software (Scion Image for Windows 4.0.2): D = Vessel pixels/Total pixels. Patency of the a/v bundle was documented from the presence of the angiographic polymer after sacrifice.
Histologic grading of rejection
A 2 mm transverse section was cut for histological analysis from the end of each decalcified bone prior to optical clearing. Graft rejection was graded (none, mild, moderate, severe) on hematoxylin-eosin stained specimens based on the amount of inflammation at 400× optical magnification using a 40× lens and 10× eyepiece (Nikon Eclipse 50i, Nikon Instruments, Melville, NY). The grading system was based on previously determined criteria13. To measure bone viability, the number of osteocyte-occupied lacunae versus empty lacunae was counted in three randomly selected fields at 400×. The results were graded: Grade 0, normal histology; Grade 1, less than half empty lacunae and decreased peritrabecular osteoblast lining; Grade 2, more than half empty lacunae and no peritrabecular osteoblast lining; Grade 3, severe necrosis with empty lacunae and fragmentation.
Quantitative histomorphometry for bone remodeling
Calcein and tetracycline hydroxychloride were given by perivascular injection near the tail vein (both 20mg/kg), 2 weeks and 2 days prior to sacrifice, respectively. A semiautomatic image analysis system (Osteomeasure®; Osteometrics, Atlanta, GA) was used for quantitative histomorphometric assessment of new bone formation and resorption14. Ten randomly selected fields were studied. Perimeters of trabecular bone surface, osteoblast-covered surface, eroded surface, and osteoclast-covered surface were measured with this system at 200× optical magnification, allowing calculation of bone formation rates.
Data analysis
Continuous data, including capillary density, bone blood flow, and histomorphometry measures, were reported as means and standard deviations or medians and ranges, as appropriate. Categorical data, including histology, was described as medians and ranges. Capillary density, bone blood flow, and histomorphometry were compared across all four growth factor treatment groups, using analysis of variance or the Kruskal-Wallis test as appropriate. If a significant difference between groups was detected, the two sample t-test or Wilcoxon rank sum test was used to examine where the groups differed. Histology grade within the 4 groups was compared using the exact test for ordered contingency tables15 and cumulative logistic regression16. All statistical tests were two-sided and the threshold of statistical significance was set at α=0.05. Analyses were performed with SAS, version 9.1 (SAS Institute Inc, Cary, NC).
Results
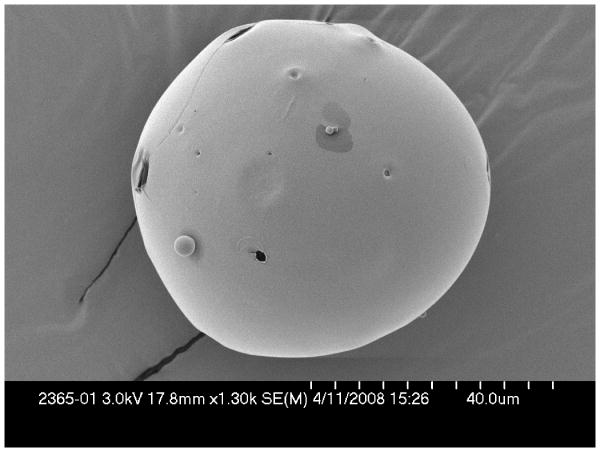
The mean (standard deviation) microsphere size per group was 73.7 (55.0)μm (Group 1, N=29), 66.2 (30.2)μm (Group 2, N=32), 56.8 (29.2)μm (Group 3, N=50) and 50.9 (32.3)μm (Group 4, N=34). There was no significant size variation (p=0.21, Kruskal-Wallis test). Overall morphology was similar for each microsphere type, with uniformly round contours and few perforations (Figure 3).
Figure 3.
Scanning electron microscopy (SEM) of microspheres. (A) Single microsphere containing VEGF and FGF at 1300× magnification showing smooth surface and uniform contour. (B) and (C) 6000× and 25× magnification, respectively, microspheres embedded in a polymerized collagen-I matrix.
Further results are shown in Table 2. All of the saphenous a/v bundles remained patent until sacrifice. There were no wound healing problems and all animals survived until the sacrifice date.
Table 2.
Results. a: blood flow, ml/min/100g mean(range); b: capillary density, % mean(standard deviation); c: inflammation grade median(range); d: rejection grade or level of bone necrosis median(range); e: bone formation rate, μm3/μm2/year mean(range).
| Group | Number | Blood Flowa | CapDensb | Inflamc | Rejecd | BFRe |
|---|---|---|---|---|---|---|
| 1 – Control | 11 | 1.8(0-5.4) | 15.3(13.6) | 3(2-4) | 2(1-3) | 23.5(0-105.2) |
| 2 – FGF2 | 10 | 2.2(0-5.0) | 24.3(17.1) | 3.5(0-4) | 2(1-3) | 90.9(12.2-212.0) |
| 3 – VEGF | 11 | 2.8(0.7-4.8) | 30.3(16.4) | 3(1-4) | 2(0-3) | 96.8(16.4-154.9) |
| 4 – FGF2+VEGF | 11 | 0.7(0-1.3) | 38.4(24.9) | 4(1-4) | 1(1-3) | 93.2(0-211.7) |
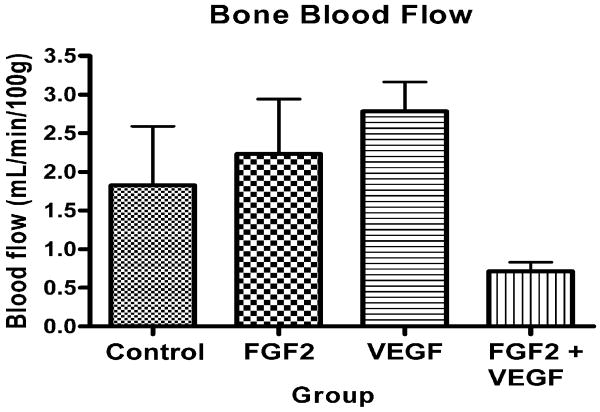
Bone blood flow varied significantly between groups (p=0.05, Kruskal-Wallis test) (Figure 4). Significantly more blood flow was found in Group 3 (VEGF) than Groups 1 (control) (p=0.031), 2 (FGF) (p=0.05) and 4 (FGF+VEGF) (p=0.001, Wilcoxon signed rank test). Group 2 also had more blood flow than Group 4 (p=0.001).
Figure 4.
Bone blood flow as determined by hydrogen washout.
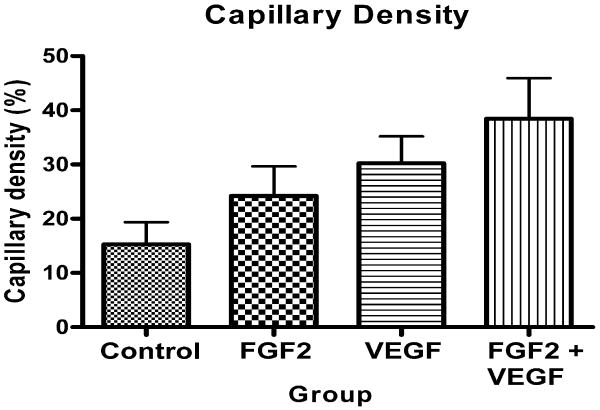
Capillary density varied significantly between groups (p=0.039, analysis of variance). Group 4 showed significantly greater capillary density than Group 1 (p<0.05, Bonferroni multiple comparison test), and there was a significant linear trend (slope 3.78, R=0.43, p=0.0045) from Groups 1 to 4 (Figure 5).
Figure 5.
Capillary density as determined by microangiography.
Inflammation and bone viability did not vary significantly between groups (p<0.001, Cochran-Mantel-Haenszel test).
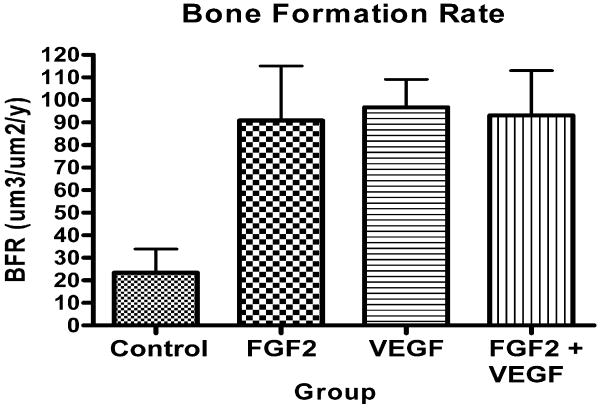
Bone formation varied significantly between groups (p=0.0056, Kruskal-Wallis test). Group 4 had more bone formation than Group 1 (p<0.001), Group 3 more than Groups 1 and 2 (p<0.05), and Group 2 more than Group 1 (p<0.01) (Wilcoxon signed rank tests) (Figures 6 and 7).
Figure 6.
Bone formation rate as determined by quantitative histomorphometry.
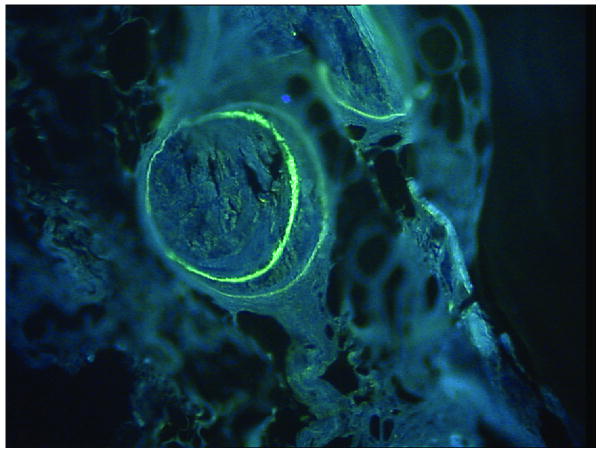
Figure 7.
Representative image from Group 3 showing double label uptake in cortical bone.
Discussion
Local administration of vascular growth factors significantly promotes angiogenesis; a steady-state release is more effective in doing so than a bolus injection17, 18. One method by which continuous administration may be achieved is via PLGA microspheres19. Lower dosages are necessary to achieve a physiologic effect and microspheres protect the growth factor in situ, allowing interaction with target cells. They permit growth factors such as FGF2, whose half life is 50 minutes in vivo, to affect tissues that generally require 24 hours of exposure for response20.
Basic fibroblast growth factor (FGF2) is a powerful stimulator of neoangiogenesis in vivo and a pleiotropic regulator of vascular cell proliferation, migration and differentiation in vitro21. It promotes formation of larger and more complex blood vessels such as arterioles, and in bone simultaneously promotes osteoid formation22. Other angiogenic growth factors, such as VEGF, are specific only to endothelial cells. We have reported VEGF to promote angiogenesis in a/v bundles implanted into necrotic bone, when delivered directly or with endothelial cell viral transfection23, 24. FGF2 has also been used successfully in necrotic bone21 and prefabricated hydroxyapetite molds22. VEGF works synergistically with FGF2 to stimulate angiogenesis in vitro25 and in vivo26. Vascular remodeling in response to these growth factors is further dependant on arterial sufficiency and nitric oxide production27.
We found significantly improved cortical blood flow with the application of VEGF over control, and over FGF2. FGF2 provided increased blood flow, although this was not significant. We did not find the expected synergistic effect of FGF2 and VEGF on blood flow. The apparent disconnect between greater capillary density and lower blood flow seen when FGF2 and VEGF are combined is of interest. We speculate that the large number of capillaries formed in the combined group may have resulted in relatively lower regional flow rates, when a constant total bone blood flow is apportioned between more vessels. Alternatively, capillary formation ending blindly without venous outflow could also result in poor measured regional flow. Capillary density measurements in fact measure the total vascular volume within both cortical and medullary bone, while hydrogen washout measurements are limited to superficial cortical bone. The capillary density was significantly greater in the combined growth factor group, which provides evidence that the two exert a synergistic effect in vivo.
Histologic grading of rejection was similar across all groups, as were levels of bone viability by osteocyte counts. This serves as further control for any deleterious effects of growth factor administration. Bone viability and capillary density did not vary significantly in the control group from previous groups with vascularized allografts and a/v bundle implantation, in which no microspheres were used1 (N=11) This serves as a control for any measurable effect of the presence of microspheres in the medullar canal, or of the waste products after their hydrolysis (a pH decrease from lactic and glycolic acid may have affected a/v bundle patency or the rate of neoangiogenesis and bone formation). Blood flow and viability was uniformly greater than in non-immunosuppressed grafts from previous studies using this experimental model (N=111; N=232).
FGF2 provided more bone formation over control and VEGF more than both control and FGF2 groups. Both factors combined gave similar results to the VEGF group. These results imply that new bone formation is a factor dependent on angiogenesis. If we could manufacture microspheres that sequentially released the two growth factors, or mix microspheres manufactured with different formulations to provide different release characterisitcs28, we may better mimic the in vivo situation where FGF2 is expressed later in bone formation that VEGF29.
Clinical use of vascularized allografts requires a method that maintains long-term tissue viability, including measurable blood flow, osteocyte viability, active bone remodeling and healing response, and maintained biomechanical properties. Safety in such non-life-critical tissues is paramount. Current immune modulation methods carry significant risks, including graft-versus-host (GVH) disease, opportunistic infection and carcinogenesis. Most published research makes use of immunosuppressive drugs and/or efforts to induce a tolerant state. The possibility of eliminating such long-term immune modulation by development of a neoangiogenic host circulation within the transplanted bone is the focus of this research. Here we demonstrate the feasibility of this technique with the enhancement of neoangiogenesis and new bone formation through the local delivery of vasculogenic and angiogenic cytokines.
Acknowledgments
Funding was provided by National Institutes of Health RO1 grant AR49718.
FK-506 was kindly provided by the Fujisawa Pharmaceutical Company, Ltd.
Contributor Information
Mikko Larsen, Department of Plastic and Reconstructive Surgery, VU University Medical Center, PO Box 7057, 1007, MB Amsterdam, The Netherlands
Wouter F. Willems, Department of Orthopedic Surgery, Microvascular Research Laboratory, Mayo Clinic, 200 First Street SW, Rochester, Minnesota 55905
Michael Pelzer, Department of Hand, Plastic and Reconstructive Surgery – Burn Center, BG-Trauma Center Ludwigshafen, Plastic and Hand Surgery, The University of Heidelberg, Ludwigshafen, Germany
Patricia F. Friedrich, Department of Orthopedic Surgery, Microvascular Research Laboratory, Mayo Clinic, 200 First Street SW, Rochester, Minnesota 55905
Michael J. Yaszemski, Tissue Engineering and Biomaterials Laboratory, Mayo Clinic, Rochester, Minnesota; Department of Orthopedic Surgery, Microvascular Research Laboratory, Mayo Clinic, 200 First Street SW, Rochester, Minnesota 55905
Allen T. Bishop, Department of Orthopedic Surgery, Mayo Clinic, 200 First Street SW, Rochester, Minnesota 55905
References
- 1.Pelzer M, Larsen M, Chung YG, et al. Short-term immunosuppression and surgical neoangiogenesis with host vessels maintains long-term viability of vascularized bone allografts. J Orthop Res. 2007;25(3):370–7. doi: 10.1002/jor.20313. [DOI] [PubMed] [Google Scholar]
- 2.Ohno T, Pelzer M, Larsen M, Friedrich PF, Bishop AT. Host-derived angiogenesis maintains bone blood flow after withdrawal of immunosuppression. Microsurgery. 2007;27(8):657–63. doi: 10.1002/micr.20427. [DOI] [PubMed] [Google Scholar]
- 3.Muramatsu K, Bishop AT. Cell repopulation in vascularized bone grafts. Journal of Orthopaedic Research. 2002;20(4):772–8. doi: 10.1016/S0736-0266(01)00173-5. [DOI] [PubMed] [Google Scholar]
- 4.Schoofs M, Cariou JL, Amarante J, Panconi B, Bovet JL, Baudet J. Microvascular free bone transfer: experimental technique on rat's femur. Microsurgery. 1984;5(1):19–23. doi: 10.1002/micr.1920050105. [DOI] [PubMed] [Google Scholar]
- 5.Siemionow M, Ozer K, Porvasnik S, Zins J. Microcirculatory window for early detection of allograft rejection. Annals of Plastic Surgery. 2000;44(6):637–43. doi: 10.1097/00000637-200044060-00010. [DOI] [PubMed] [Google Scholar]
- 6.Kempen DH, Lu L, Zhu X, et al. Development of biodegradable poly(propylene fumarate)/poly(lactic-co-glycolic acid) blend microspheres. I. Preparation and characterization. J Biomed Mater Res A. 2004;70(2):283–92. doi: 10.1002/jbm.a.30079. [DOI] [PubMed] [Google Scholar]
- 7.Kempen DH, Lu L, Zhu X, et al. Development of biodegradable poly(propylene fumarate)/poly(lactic-co-glycolic acid) blend microspheres. II. Controlled drug release and microsphere degradation. J Biomed Mater Res A. 2004;70(2):293–302. doi: 10.1002/jbm.a.30080. [DOI] [PubMed] [Google Scholar]
- 8.Pelzer M, Larsen M, Friedrich PF, Bishop AT. Measurement of bone blood flow using the hydrogen washout Technique-Part I: Quantitative evaluation of tissue perfusion in the laboratory rat. J Orthop Res. 2008;26(6):741–5. doi: 10.1002/jor.20562. [DOI] [PubMed] [Google Scholar]
- 9.Larsen M, Pelzer M, Friedrich PF, Bishop AT. Measurement of bone blood flow using the hydrogen washout technique-part II: Validation by comparison to microsphere entrapment. J Orthop Res. 2008;26(6):746–52. doi: 10.1002/jor.20561. [DOI] [PubMed] [Google Scholar]
- 10.Aukland K, Bower BF, Berliner RW. Measurement of Local Blood Flow with Hydrogen Gas. Circulation Research. 1964;14:164–87. doi: 10.1161/01.res.14.2.164. [DOI] [PubMed] [Google Scholar]
- 11.Whiteside LA, Lesker PA, Simmons DJ. Measurement of regional bone and bone marrow blood flow in the rabbit using the hydrogen washout technique. Clin Orthop. 1977;(122):340–6. [PubMed] [Google Scholar]
- 12.Spalteholz K. Nebst Anhang: Über Knochenfärbung. Leipzig: S. Hirzel; 1914. Über das Durchsichtigmachen von menschlichen und tierischen Präparaten und seine theoretischen Bedingungen. [Google Scholar]
- 13.Buttemeyer R, Jones NF, Min Z, Rao U. Rejection of the component tissues of limb allografts in rats immunosuppressed with FK-506 and cyclosporine. Plast Reconstr Surg. 1996;97(1):139–48. doi: 10.1097/00006534-199601000-00023. [DOI] [PubMed] [Google Scholar]
- 14.Taira H, Moreno J, Ripalda P, Forriol F. Radiological and histological analysis of cortical allografts: an experimental study in sheep femora. Arch Orthop Trauma Surg. 2004;124(5):320–5. doi: 10.1007/s00402-004-0653-x. [DOI] [PubMed] [Google Scholar]
- 15.Mehta CR, Patel NR, Tsiatis AA. Exact significance testing for ordered categorical data. Biometrics. 1984;40:819–25. [PubMed] [Google Scholar]
- 16.Agresti A. Categorical Data Analysis. 2nd. New York: Wiley-Interscience; 2002. [Google Scholar]
- 17.Sato K, Laham RJ, Pearlman JD, et al. Efficacy of intracoronary versus intravenous FGF-2 in a pig model of chronic myocardial ischemia. Ann Thorac Surg. 2000;70(6):2113–8. doi: 10.1016/s0003-4975(00)02018-x. [DOI] [PubMed] [Google Scholar]
- 18.Hiraoka Y, Yamashiro H, Yasuda K, Kimura Y, Inamoto T, Tabata Y. In situ regeneration of adipose tissue in rat fat pad by combining a collagen scaffold with gelatin microspheres containing basic fibroblast growth factor. Tissue Eng. 2006;12(6):1475–87. doi: 10.1089/ten.2006.12.1475. [DOI] [PubMed] [Google Scholar]
- 19.King TW, Patrick CW., Jr Development and in vitro characterization of vascular endothelial growth factor (VEGF)-loaded poly(DL-lactic-co-glycolic acid)/poly(ethylene glycol) microspheres using a solid encapsulation/single emulsion/solvent extraction technique. J Biomed Mater Res. 2000;51(3):383–90. doi: 10.1002/1097-4636(20000905)51:3<383::aid-jbm12>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 20.Sakakibara Y, Tambara K, Sakaguchi G, et al. Toward surgical angiogenesis using slow-released basic fibroblast growth factor. Eur J Cardiothorac Surg. 2003;24(1):105–11. doi: 10.1016/s1010-7940(03)00159-3. discussion 12. [DOI] [PubMed] [Google Scholar]
- 21.Nakamae A, Sunagawa T, Ishida O, et al. Acceleration of surgical angiogenesis in necrotic bone with a single injection of fibroblast growth factor-2. J Orthop Res. 2004;22(3):509–13. doi: 10.1016/j.orthres.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Nakasa T, Ishida O, Sunagawa T, et al. Prefabrication of vascularized bone graft using a combination of fibroblast growth factor-2 and vascular bundle implantation into a novel interconnected porous calcium hydroxyapatite ceramic. J Biomed Mater Res. 2005;75(2):350–5. doi: 10.1002/jbm.a.30435. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki O, Bishop AT, Sunagawa T, Katsube K, Friedrich PF. VEGF-promoted surgical angiogenesis in necrotic bone. Microsurgery. 2004;24(1):85–91. doi: 10.1002/micr.10190. [DOI] [PubMed] [Google Scholar]
- 24.Katsube K, Bishop AT, Simari RD, Yla-Herttuala S, Friedrich PF. Vascular endothelial growth factor (VEGF) gene transfer enhances surgical revascularization of necrotic bone. J Orthop Res. 2005;23(2):469–74. doi: 10.1016/j.orthres.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 25.Pepper MS, Ferrara N, Orci L, Montesano R. Potent synergism between vascular endothelial growth factor and basic fibroblast growth factor in the induction of angiogenesis in vitro. Biochem Biophys Res Commun. 1992;189(2):824–31. doi: 10.1016/0006-291x(92)92277-5. [DOI] [PubMed] [Google Scholar]
- 26.Asahara T, Bauters C, Zheng L. Synergistic effect of vascular endothelial growth factor and basic fibroblast growth factor on angiogenesis in vivo. Circulation. 1995;92(9 Suppl):365–71. doi: 10.1161/01.cir.92.9.365. [DOI] [PubMed] [Google Scholar]
- 27.Yang HT, Yan Z, Abraham JA, Terjung RL. VEGF(121)- and bFGF-induced increase in collateral blood flow requires normal nitric oxide production. Am J Physiol Heart Circ Physiol. 2001;280(3):H1097–104. doi: 10.1152/ajpheart.2001.280.3.H1097. [DOI] [PubMed] [Google Scholar]
- 28.Ungaro F, Biondi M, d'Angelo I, et al. Microsphere-integrated collagen scaffolds for tissue engineering: effect of microsphere formulation and scaffold properties on protein release kinetics. J Control Release. 2006;113(2):128–36. doi: 10.1016/j.jconrel.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 29.Huang Z, Nelson ER, Smith RL, Goodman SB. The sequential expression profiles of growth factors from osteroprogenitors to osteoblasts in vitro. Tissue Eng. 2007;13(9):2311–20. doi: 10.1089/ten.2006.0423. [DOI] [PubMed] [Google Scholar]