Abstract
There has been recent interest in the biologic stimulation of anterior cruciate ligament (ACL) healing. However, the effect of age on the ability of ligaments to heal has not yet been defined. In this study, we hypothesized that skeletal maturity would significantly affect the cellular and vascular repopulation rate of an ACL wound site. Skeletally Immature (open physes), Adolescent (closing physes), and Adult (closed physes) Yucatan minipigs underwent bilateral ACL transection and suture repair using a collagen-platelet composite. The response to repair was evaluated histologically at 1, 2, and 4 weeks. All three groups of animals had completely populated the ACL wound site with fibroblasts at 1 week. The Immature animals had a higher cellular density in the wound site than the Adult animals at weeks 2 and 4. Cells in the Immature ligament wounds were larger and more ovoid than in the Adult wounds. There were no significant differences in the vascular density in the wound site. Animal age had a significant effect on the density of cells populating the ACL wound site. Whether this observed cellular difference has an effect on the later biomechanical function of the repaired ACL requires further study.
Keywords: age, anterior cruciate ligament, wound healing, skeletally immature, platelet
Rupture of the anterior cruciate ligament (ACL) is a common injury, affecting over 175,000 patients each year, including 38,000 high school students.1 Unfortunately, the ACL has long been shown to have poor capacity to heal, and even with suture repair, nonunion rates are reported to be as high as 40%–100%.2–4 The nonunion rate has led to abandonment of suture repair in favor of ACL reconstruction for treatment of ACL tears. Although autograft ACL reconstruction is an excellent operation for restoring the gross stability of the knee, significant problems remain: (1) it requires harvesting of other tissues from the knee, a procedure with its own associated comorbidities; (2) it removes the native ACL tissue and the proprioceptive fibers and function of the ligament5; and (3) it replaces a complex, fan-shaped bundle of 17 different ligament fascicles with one or two bundles of tendon fibers.6 It is especially troublesome that as high as 78% of patients will have radiographic signs of arthritis only 14 years after surgery,7 which for an adolescent with an ACL injury, is a concerning statistic.
A promising answer for ACL ligament injury is enhanced suture repair, where a biologic stimulus is placed in the wound site at the time of suture repair. Renewed enthusiasm for suture repair has developed recently after studies showed that one mechanism behind the failure of the ACL to heal with suture repair may be a lack of a provisional scaffolding in the wound.8–10 Studies have revealed that surgical placement of a substitute provisional scaffold, a collagen-platelet composite (CPC), can promote healing of the ACL after primary repair.11–13 Using a central wound as a model for healing, histologic studies have shown that with placement of a CPC scaffold, the ACL wound is invaded by vessels and fibroblasts and actively remodeled to more closely resemble native tissue. This has also been shown to occur in a complete transection model when studied over a 14-week period.14
However, characteristics of patients who may be good candidates for stimulating healing of the ACL have not yet been defined. Basic science studies of partial ACL transection and patellar tendon healing suggest that the scar formed in skeletally mature animals is actually stronger than that formed in skeletally immature animals.15,16 As patients with open physes stand to have the longest period of disability if premature osteoarthritis occurs, it is clinically important to begin to determine the effect of skeletal maturity on the ability to stimulate functional healing in the ACL. In this work, we evaluated the healing response in skeletally Immature (open physes), Adolescent (closing physes), and Adult animals (closed physes). The hypothesis of the present study is that two of the key mechanisms involved in wound repair, cell proliferation and revascularization, are a function of skeletal maturity.
To test this hypothesis, an in vivo porcine model was used to define the effect of skeletal maturity on the density of cellular repopulation and revascularization of an ACL wound treated with an acellular provisional scaffold.
METHODS
Experimental Design
Institutional Animal Care and Use Committee approvals were obtained prior to initiating this study. Eighteen Yucatan minipigs were utilized from three different age groups (mean ± SD); skeletally Immature (8 ± 2 months, n = 6), Adolescent (16 ± 2 months, n = 6), and Adult (26.1 ± 1 months, n = 6). Their average weights were 33.8 ± 2 kg, 60.2 ± 5 kg, and 79.4 ± 5 kg (mean ± SD), respectively. The statuses of the physes were verified radiographically in all animals prior to tissue procurement. The Immature animals had open physes, the Adolescent animals had closed tibial and femoral physes but an unfused tibial tubercle, and the Adult animals had a physeal scar but no open physes. Each animal had bilateral surgery where the ACL was transected and acutely repaired using suture-repair enhanced with a collagen-platelet composite (CPC). Four ligaments from each age group were harvested at 1, 2, and 4 weeks from injury, and histologic analysis was performed. A total of 36 ACLs were analyzed for cellular proliferation and revascularization.
Surgical Procedure
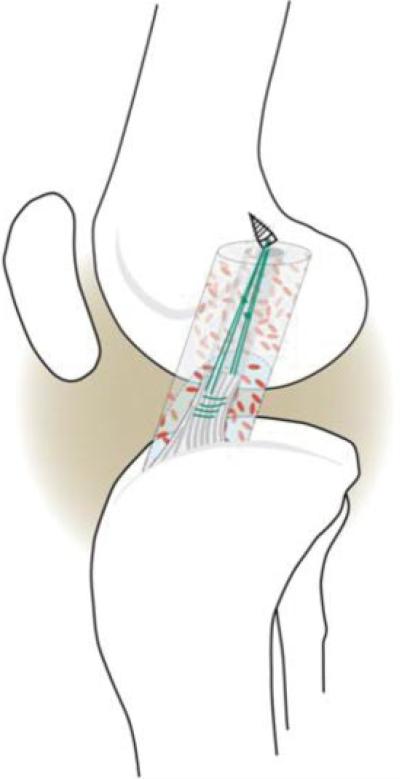
The pigs were premedicated with telazol, xylazine, and atropine, and were intubated. Anesthesia was maintained with 1%–3% isoflurane. An arthrotomy was made and the fat pad partially resected. The ACL was transected at the junction of the proximal and middle thirds of the ligament. Complete transection was verified visually and with a repeat Lachman maneuver that became positive in all knees with no significant end point detected after complete transection. The Lachman exam was conducted with the knee in 30° of flexion as measured from the terminal extension value; therefore, in a knee where terminal extension was 30°, the Lachman was performed at 60° of flexion. An absorbable suture anchor [TwinFix AB 5.0 Suture Anchor with DuraBraid Suture (USP #2); Smith & Nephew, Inc., Andover, MA] was placed into the femoral insertion site of the ACL in the femoral notch; #1 Vicryl sutures (ETHICON, Johnson & Johnson, catalog #J947H, Somerville, NJ) were woven through the distal stump of the ACL using a modified Kessler suture technique. The knee was irrigated with 500 cc of sterile normal saline. A strip of a type I collagen sponge17 was presoaked in 1 cc of a collagen-platelet mixture, described subsequently, and threaded onto sutures and into the region of the proximal ACL stump in the notch. The Durabraid sutures from the anchor were tied individually to the Vicryl sutures in the distal ACL with knees in resting flexion (approximately 70° of flexion) and an additional 2 cc of the collagen-platelet composite were added to fill the intercondylar notch (Fig. 1). The wound was closed in layers. The animals were not restrained postoperatively, and were allowed ab libitum activity. Buprenex 0.01 mg/kg IM once and a Fentanyl patch 1 to 4 μg/kg transdermal was given for postoperative anesthesia.
Figure 1.
Schematic of the augmented suture repair technique. Sutures are placed in the tibial stump, threaded through a collagen scaffold, and tied to sutures attached to a suture anchor in the femur. Collagen-platelet composite (CPC) is added onto the collagen sponge. [Color figure can be viewed in the online issue, which is available at http://www.interscience.wiley.com]
Six animals were subsequently anesthetized at each time point at 1, 2, and 4 weeks postoperatively. Each knee was subsequently retrieved and transferred to neutral-buffered formalin for 4 days before preparation for histology.
Collagen-Platelet Composite (CPC) Preparation
Acid-soluble, type I collagen slurry was made by sterilely harvesting bovine knee capsular tissue which was solubilized in an acidic pepsin solution as previously described.12 Collagen content within the slurry was adjusted to >5 mg/ml and neutralized with 0.1M HEPES (Cellgro, Mediatech, Inc, Herndon, VA), PBS (HyClone Logan, UT), and 7.5% sodium bicarbonate (Cambrex BioScience, Walkersville, MD). Platelet rich plasma (PRP) was prepared with autologous porcine platelets. Sixty milliliters of whole blood was drawn from each pig after the induction of general anesthesia for surgery and collected in a syringe containing 10% by volume acid-citrate dextrose. The blood was centrifuged for 6 min at 150 g (GH 3.8 rotor, Beckman GS-6 Centrifuge, Fullerton, CA) and the supernatant was aspirated and collected as platelet-rich plasma in a 50-ml tube. Complete blood counts (CBCs) were performed on the whole blood and the platelet preparation.
Histology
At each designated time point, four ligaments from each age group were retrieved and fixed in neutral buffered formalin for 1 week, decalcified carefully, sectioned longitudinally in the sagittal plane of the ACL as large sections, dehydrated, and embedded in paraffin. Seven-micron sections were microtomed, placed onto pretreated large section glass slides and stored at 4°C. Representative sections from each ligament were stained with hematoxylin and eosin (H&E) staining, and immunohistochemical studies were carried out with the use of a mouse monoclonal antibody to α–smooth muscle actin (Sigma Chemical, St. Louis, MO) was performed for each sample. The healing ACL was divided into five evenly spaced areas for analysis: site 1, femoral stump; site 2, proximal ACL wound; site 3, central ACL wound; site 4, distal ACL wound; and site 5, tibial stump.
Cell number density and vascular density were analyzed with methods previously described.8,13 At each of the above noted five longitudinal locations, the number of cells within three 0.1-square-millimeter areas were measured by two independent, blinded, reviewers and the results averaged. At each location, the total number of cells were counted and divided by the area of analysis in order to yield the cell number density (#/mm3). For analysis of blood vessel density, the total number of blood vessels crossing the entire width of the section at each location was counted.
The cell size and shape were calculated by measuring the length of the major and minor axes of the cell for 15 fibroblasts in the central wound site of all ligament wounds. The area of the nucleus was calculated as the length of the major axis multiplied by that of the minor axis multiplied by pi and divided by 4.
Statistics
Fibroblast and blood vessel densities were each tested for the assumption of normality as a whole, and stratified by age category using the Shapiro-Wilk statistic. While there was a significantly positive skew in fibroblast counts overall, when stratified there was no statistically significant skew and so analyses proceeded without transformation. Blood vessel counts were also positively skewed, both overall and when stratified. The square root transformation maximally reduced the skewness of the overall distribution and the mean skewness across the age categories. Therefore, the square root of the number of blood vessels was analyzed.
Mixed linear models were used to compare the differences among age categories at different sites and after varying times since surgery. Random effects for sample, limb (sample), and site (sample) were included in a model with fixed effects for age category, weeks since surgery, site, and the two-way and the three-way interactions among these effects. The denominator degrees of freedom were determined using the Kenward-Roger procedure and the model was fit via residual estimation of maximum likelihood (REML). The simple effects describing differences between age categories at the 3 sites × 3 weeks since surgery were used as a priori omnibus tests for differences among groups without alpha adjustment. Follow-up pairwise comparisons within any significant effects were conducted using orthogonal contrasts and adjusting for multiplicity using the Holm test.
RESULTS
Surgical Outcomes
The systemic platelet count was not significantly different among the three age groups. The systemic platelet count (mean ± SD) in the Immature group was 412 ± 127 × 103/μL, in the Adolescent group was 318 ± 132 × 103/μL, and in the Adult group was 304 ± 102 × 103/μL (p > 0.05 for all comparisons). The platelet count in the PRP used in each group was also similar between groups. The PRP platelet count in the Immature group was 736 ± 236 × 103/μL, in the Adolescent group was 529 ± 210 × 103/μL, and in the Adult group was 615 ± 147 × 103/μL (p > 0.05 for all comparisons). The enrichment factor is the ratio between the platelet concentration in the platelet rich plasma and that in the systemic whole blood of the animal. These values resulted in similar enrichment factors of 1.8×, 1.8×, 2.1× for the Immature, Adolescent, and Adult groups, respectively (p > 0.05 for all comparisons).
All animals recovered uneventfully from surgery. No animals had any surgical complications or difficulty walking normally, redness, swelling, fever, or any signs of infection. All animals were ambulatory within a few hours of surgery, and ambulating normally by 1 week.
Cellular Repopulation of the Wound Site
The ACL wound site was found to be already completely populated with randomly oriented fibroblastic cells at the 1 week time point in all three groups of animals, and there were no statistically significant differences in cellular density of the ACL wound sites between the age groups at 1 week (Table 1). At 2 weeks following surgery, there was a significantly higher cell number in the Immature ligaments than in the Adult ligaments at the proximal ACL wound site, p < 0.01.
Table 1.
Fibroblast Count for Immature, Adolescent, and Adult Animals at the Femoral Insertion, Proximal, Central, Distal, and Tibial Insertion Sites of the ACL at Weeks 1, 2, and 4a
| Femoral Stump |
Proximal ACL |
Central ACL |
Distal ACL |
Tibial Stump |
|
|---|---|---|---|---|---|
| Site | Week 1 | ||||
| IMMATURE | 211 ± 35 | 572 ± 120 | 725 ± 68 | 691 ± 40 | 275 ± 120 |
| ADOLESCENT | 391 ± 189 | 728 ± 131 | 808 ± 308 | 671 ± 232 | 407 ± 35 |
| ADULT | 165 ± 57 | 591 ± 159 | 543 ± 130 | 618 ± 144 | 188 ± 37 |
|
Week 2 |
|||||
|---|---|---|---|---|---|
| IMMATURE | 648 ± 64 | 935 ± 251* | 1120 ± 161 | 823 ± 138 | 550 ± 73 |
| ADOLESCENT | 683 ± 80 | 816 ± 20 | 968 ± 324 | 644 ± 165 | 616 ± 147 |
| ADULT | 656 ± 162 | 551 ± 106* | 948 ± 91 | 721 ± 63 | 513 ± 91 |
|
Week 4 |
|||||
|---|---|---|---|---|---|
| IMMATURE | 846 ± 59 | 1121 ± 48† | 1660 ± 34‡# | 1036 ± 273 | 738 ± 68 |
| ADOLESCENT | 687 ± 167 | 1060 ± 188 | 1266 ± 197# | 843 ± 112 | 616 ± 14 |
| ADULT | 541 ± 71 | 747 ± 179† | 1075 ± 184‡ | 797 ± 197 | 527 ± 99 |
Values are expressed as mean ± SD, with n = 4, measured as fibroblast/mm2.
Indicates a significant difference between Immature and Adult at the proximal ACL site at week 2 (p < 0.01).
Indicates a significant difference between Immature and Adult at the proximal ACL site at week 4 (p < 0.01).
Indicates a significant difference between Immature and Adult at the central ACL site at week 4 (p < 0.01).
Indicates a significant difference between Immature and Adolescent at the central ACL site at week 4 (p < 0.001).
At 4 weeks following surgery, there were significantly higher cell numbers in the Immature ligaments at the proximal and central sections of the ACL wounds when compared with the Adult animals (p < 0.01 and p < 0.001, respectively). At 4 weeks following surgery, there was a significantly higher cell number at the central ACL wound site in the Adolescent ligaments when compared with the Adult ligaments (p < 0.01). The cellular density in the central ACL wound site in the Adult animals at 4 weeks was similar to that seen in the Immature animals at the same site at the 2-week time point (1,075 ± 184 vs. 1,120 ± 161) (Table 1).
Vascular Density within the Wound Site
At week 1, the Immature animals had a greater vessel density than the Adult animals at the central wound site (Table 2, p < 0.01). There was no significant difference in vascular density between the age groups at any other time point. At week 1, thin walled venules, as well as arterioles and capillaries, were noted throughout the ligament remnants. At week 2, venules and arterioles with multicellular wall layers were present in similar amounts across all age groups. Red blood cells were present within the capillary lumens. The walls of arterioles were noted to be thicker than in week 1, with a higher number of cell layers surrounding the lumen. Greater densities of arterioles were noted on the periphery of the scar region, in the epiligamentous region. Groups of arterioles and capillaries were seen within the original stump (recognized by definitive crimp).
Table 2.
Blood Vessel Density for Immature, Adolescent, and Adult Animals at the Femoral Stump, Proximal, Central, Distal, and Tibial Stump of the ACL at Weeks 1, 2, and 4a
| Femoral Stump |
Proximal ACL |
Central ACL |
Distal ACL |
Tibial Stump |
|
|---|---|---|---|---|---|
| Site | Week 1 | ||||
| IMMATURE | 66 ± 56 | 75 ± 34 | 138±71* | 97 ± 51 | 25 ± 18 |
| ADOLESCENT | 26 ± 27 | 27 ± 28 | 61 ± 42 | 70 ± 38 | 7 ± 1 |
| ADULT | 11 ± 5 | 47 ± 30 | 30 ± 14* | 30 ± 22 | 50 ± 05 |
|
Week 2 |
|||||
|---|---|---|---|---|---|
| IMMATURE | 66 ± 41 | 105 ± 42 | 198 ± 68 | 121 ± 42 | 47 ± 32 |
| ADOLESCENT | 31 ± 25 | 99 ± 104 | 140 ± 102 | 104 ± 76 | 21 ± 27 |
| ADULT | 21 ± 21 | 43 ± 19 | 115 ± 57 | 68 ± 25 | 15 ± 12 |
|
Week 4 |
|||||
|---|---|---|---|---|---|
| IMMATURE | 8.93 ± 3.23 | 13.31 ± 4.33 | 20.62 ± 7.66 | 12.37 ± 4.48 | 6.68 ± 2.96 |
| ADOLESCENT | 11.81 ± 7.69 | 10.87 ± 2.18 | 16.5 ± 4.72 | 14 ± 6.28 | 8.312 ± 6.14 |
| ADULT | 4.87 ± 0.95 | 8.87 ± 5.53 | 17.256.72 | 10.62 ± 2.93 | 3.5 ± 1.75 |
Values are expressed as mean ± SD, with n = 4, measured as vessels/mm2.
Indicates a significant difference between Immature and Adult at the central ACL site at week 1 (p < 0.01).
At the 4-week time point, the size of the arteriole walls remained large, with multicellular cell wall layers in all animals. The Immature animals showed a greater number of blood vessels at weeks 1 and 2 compared to Adolescent and Adult animals at all sites (Table 2). There was not much new organization of the blood vessels over the 4-week time point. Vessels continued to have relatively random orientation in all areas of the ligament. By 4 weeks, all animals showed capillaries in the central wound site having red blood cells within the lumen.
Cellular Orientation
At 1 week, the entire ACL was populated with randomly oriented fibroblastic cells in all age groups. There were no acellular areas noted within the scar mass. No necrotic areas were noted. By 2 weeks, the Immature and Adolescent ligament fibroblasts were still randomly oriented along the length of the ligament; however, the Adult ligament fibroblasts had more visible preferential orientation parallel to the long axis of the ligament. At 4 weeks, the cells were becoming oriented along the axis of the ligament in all groups.
Cell Size and Shape
The cells populating all wound sites at week 1 were ovoid and large (p > 0.05 for all comparisons). Immature and Adolescent animals showed an increased number of cells with multiple nucleoli within a definitive nucleus, while the Adult cells primarily had single nucleoli. By week 2, the size of the fibroblasts began to decline in all age groups with the Adult cells appearing to be the smallest (Table 3). The Immature cell nuclei remained ovoid while the Adolescent and Adult cells were primarily fusiform (14.3 ± 8.32, 6.55 ± 3.31, and 4.24 ± 2.86, respectively; mean ± SD, p < 0.01 for both comparisons at week 2). At week 4, all age groups exhibited a fusiform fibroblast shape, with Immature and Adult showing a significant difference between groups (p > 0.01).
Table 3.
Nuclear Area of Fibroblasts at the Central Wound Site of Immature, Adolescent, and Adult Fibroblasts at Weeks 1, 2, and 4a
| Week 1 | Week 2 | Week 4 | |
|---|---|---|---|
| IMMATURE | 31.41 ± 21.42*,† | 14.3 ± 8.32*,† | 9.41 ± 8.63† |
| ADOLESCENT | 14.95 ± 4.45* | 6.55 ± 3.31* | 6.35 ± 3.07 |
| ADULT | 13.56 ± 6.26† | 4.24 ± 2.86† | 2.5 ± 2.35† |
Values are expressed as mean ± SD, with measurements taken in μM.
Indicates a significant difference between Immature and Adolescent fibroblasts at weeks 1 and 2 (p < 0.01).
Indicates a significant difference between Immature and Adult fibroblasts at weeks 1, 2, and 4 (p < 0.01 for weeks 1 and 4, p < 0.001 for week 2).
Collagen Formation
At 2 weeks, in the Adult ligament wounds, there was randomly oriented crimp with a small waveform present at the central wound section which was not seen in the Immature or Adolescent animals. At 4 weeks, collagen formation was beginning to become more evident in all age groups with a greater degree of fibers noted in parallel with the fibroblasts orienting along the length of the axis of the healing ligament. Although disorganization still existed in the scar mass, the amount of organization seen at week 4 was more evident than at weeks 1 and 2 for all ages.
DISCUSSION
The data obtained here suggest that age has a significant effect on cellular repopulation of the ACL wound site treated with a collagen-platelet composite, even at only a few weeks after surgery. There was no significant effect of age on wound site vascular density in the first 4 weeks of ligament healing. Immature animals had higher cell densities within the wound sites compared to Adolescent and Adult animals, although the latter did catch up partially during the course of the experiment. In general, the observed processes were very similar to what has been reported previously as “ligamentization,” a term for the changes observed histologically when a tendinous ACL graft transforms into a ligamentous, ACL-like tissue.18–20 However, while the distinct phases of initial necrosis, proliferation, and remodeling/ligamentization can be identified in ACL grafts, we observed no necrosis in the healing ligaments studied here.
In all of the age groups, the central wound site was populated with large, ovoid fibroblasts at 1 week. These fibroblasts had characteristics of activated fibroblasts, suggesting they were actively participating in the invasion, population, and remodeling of the wound site. Over the 4 weeks of the experiment, the cell nuclei in all groups gradually became more fusiform; however, this occurred 2 weeks later in the Immature group than in the Adolescent or Adult groups. To the best of our knowledge, age-related differences in the processes of ligament healing are being reported for the first time. As all wound sites were repopulated by the 1-week time point, additional studies at even shorter time points may help to delineate if patient age significantly effects this initial invasion of the wound site.
We found cellular population to be very similar to the events previously described for incorporation of ACL grafts,21,22 healing of the medial collateral ligament,23 and the initial histological response of an ACL to injury.8 While there were generally large increases in cell numbers in all groups, the fibroblast density as a function of position remains the same regardless of the age of the animal, with the Immature group showing the highest numbers with significantly more cells than the Adult group. However, higher proliferative activity should not be confused with higher biosynthetic activity, i.e., a higher cell number is not necessarily a predictor of a better outcome. As a matter of fact, compared to the Adult animals, the faster proliferating Immature and Adolescent animals showed a less uniformly distributed matrix in our histological assessment. Similar patterns of rapidly proliferating cells, which eventually resulted in a poorly differentiated and mechanically inferior scar tissue, has been previously observed in an animal model of rotator cuff repair.24 However, at 4 weeks, we saw comparable levels of collagen organization in all groups.
In addition, it seems that the cellular repopulation originated from the center of the ACL stumps. Previous studies have shown that there is a large, mobile population of fibroblasts in the ACL stump and that these cells readily migrate into a collagenous biomaterial.25 It is possible that cells intrinsic to the ACL and located in the proximal and distal stump may participate in the populating of the provisional scaffold material, stimulated by the growth factors from the platelet concentrate. Pluripotential mesenchymal cells have been hypothesized to reside in the perivascular region of multiple tissues as pericytes.26,27 In this study, the hypertrophy of the cells in these perivascular areas, both between ruptured ligament fascicles (endoligament) and in the tissue covering the ligament (epiligament) may be one possible source of the cells invading the ACL wound site. High cell density within the wound site has been previously observed to occur before an increase in healing ligament strength,14 suggesting that the cellular invasion occurs, until some type of contact inhibition occurs at a high cell density. Once this occurs, there appears to be a shift toward collagen synthesis and improving regenerate tissue strength. This interpretation is supported by the observations in this article; namely, by the increasing cell numbers from either side, the presence of highly metabolically active fibroblasts, demonstrated by large nuclei, in the center of the implant, and the beginnings of an organized collagen structure several weeks later.
The increased cellular density in the younger animals is consistent with earlier in vitro studies of cellular proliferation and migration in our lab which show that the ACL cells from younger animals have greater proliferation and migration potential than those from Adolescent or Adult animals.17 Both the proliferation and migration of intrinsic ACL cells has been cited as a prerequisite for proper wound healing,28,29 and thus our findings might be a reason why Immature animals could be able to heal more quickly and efficiently than older age groups.
The second wound repair mechanism investigated in this study was revascularization of the ACL wound site. The small differences in vascular density noted at 1 week are more likely related to a higher vascular density in the intact Immature ACL given the location of the vessels in the proximal and distal ACL remnants. Other than this finding, there were no significant differences between the age groups in vascular density at any location or time point. This is likely due at least in part to the fact that the study was of relatively short duration. Recently, work in the Yorkshire pig model of the ACL healing, revascularization has been noted to occur between 6 and 9 weeks.14 During ligamentization of an ACL graft, revascularization occurs between 3 and 8 weeks in the rabbit model,30 and 6 and 20 weeks in the canine model,18 so the time points studied here may be too short to determine the full extent of vascular change that will be seen in this model. However, the observed increase in arterioles along the edges of the scar region in the epiligament is consistent with the findings during ligamentization20,31 and ACL remodeling.8 Additional studies at time points longer than 4 weeks are needed to determine if revascularization of the central wound site is significantly effected by patient age.
This study was a qualitative histologic analysis of healing ACL tissue. This type of study is limited in its ability to provide quantitative data, as can be done with biochemical assays on biopsies of the healing tissue or digests of the entire ligament. The numbers obtained in the histomorphometric analysis are also subject to the selection of the sites for analysis, a potential source of error which is only partly addressed by standardizing the locations of analysis and blinding the reviewers. However, there are also advantages of a histologic study when beginning to look at questions such as the effect of age on ligament healing. Entire sections of the ligament can be analyzed and regional variations determined. Features of the tissues, such as blood vessel location and overall organization and nonhomogeneity of the ligament can be appreciated in whole organ sections. Use of these studies as a first line analysis can guide future studies by providing a map for obtaining biopsies for biochemical or cell-based assays. For example, the regional variation seen in the cell density along the length of the ACL might suggest that the cranio-caudad location of a biopsy could influence the results and that future studies might wish to normalize the biopsy location for this reason.
The porcine model approximates the human knee in terms of biomechanics and wound healing attributes; however, the chief limitation of this model is the inability to restrain the animals postoperatively. The animals in this study were allowed to be weight-bearing as tolerated, and most animals were weight-bearing on the operative knees within a few hours of surgery. This may have placed excessive early stress on the repairs which would not be seen in a human knee when the patient was on crutches postoperatively. Future studies to evaluate the effect of rehabilitation on ACL healing are warranted in a model where rehabilitation may be better controlled such as the canine model.
In our gradual translation from the “bench” to the “bedside,” we have used various models, from 2D cell culture, to 3D hydrogels in vitro, to a central defect wound in vivo, and now to a complete transection. These models are all stepping stones with gradations of cost, complexity, and animal lives. As we optimize solutions in earlier models, we can then apply them to more complex models. In this article, we used ACL transection as a simplified model for ACL injury. In a true ACL injury, the ligament tissue likely has a broader zone of injury than that in the model used here. In addition, subchondral bone, articular cartilage, meniscus and capsular injury are also present. Future studies which add these degrees of complexity to the model will be warranted if we can demonstrate treatment efficacy in this simpler model.
In conclusion, our study showed that Immature animals repopulate a wound site more densely than Adolescent or Adult animals. A collagen-platelet composite is well incorporated and supports ACL cell migration, proliferation, and collagen production in the first few weeks of healing. Whether this increased cell response is beneficial for functional healing of the ligament requires further longer-term studies.
ACKNOWLEDGMENTS
This study was supported by NIH grant R01 AR054099.
REFERENCES
- 1.Myer GD, Ford KR, Hewett TE. Rationale and clinical techniques for anterior cruciate ligament injury prevention among female athletes. J Athl Train. 2004;39:352–364. [PMC free article] [PubMed] [Google Scholar]
- 2.Feagin JA, Jr, Curl WW. Isolated tear of the anterior cruciate ligament: 5-year follow-up study. Am J Sports Med. 1976;4:95–100. doi: 10.1177/036354657600400301. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan N, Wickiewicz TL, Warren RF. Primary surgical treatment of anterior cruciate ligament ruptures: a long-term follow-up study. Am J Sports Med. 1990;18:254–358. doi: 10.1177/036354659001800404. [DOI] [PubMed] [Google Scholar]
- 4.Sherman MF, Bonamo JR. Primary repair of the anterior cruciate ligament. Clin Sports Med. 1988;7:739–750. [PubMed] [Google Scholar]
- 5.Arnoczky SP. Anatomy of the anterior cruciate ligament. Clin Orthop Relat Res. 1983;172:19–25. [PubMed] [Google Scholar]
- 6.Krauspe R, Schmidt M, Schaible HG. Sensory innervation of the anterior cruciate ligament. An electrophysiological study of the response properties of single identified mechanoreceptors in the cat. J Bone Joint Surg [Am] 1992;74:390–397. [PubMed] [Google Scholar]
- 7.Von Porat A, Roos EM, Roos H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann Rheumatic Dis. 2004;63:269–273. doi: 10.1136/ard.2003.008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gill SS, Diduch DR. Outcomes after meniscal repair using the meniscus arrow in knees undergoing concurrent anterior cruciate ligament reconstruction. Arthroscopy. 2002;18:569–577. doi: 10.1053/jars.2002.29897. [DOI] [PubMed] [Google Scholar]
- 9.Murray MM, Martin SD, Spector M. Migration of cells from human anterior cruciate ligament explants into collagen-glycosaminoglycan scaffolds. J Orthop Res. 2000;18:557–564. doi: 10.1002/jor.1100180407. [DOI] [PubMed] [Google Scholar]
- 10.Spindler KP, Murray MM, Devin C, et al. The central ACL defect as a model for failure of intra-articular healing. J Orthop Res. 2006;24:401–406. doi: 10.1002/jor.20074. [DOI] [PubMed] [Google Scholar]
- 11.Murray MM, Forsythe B, Chen F, et al. The effect of thrombin on ACL fibroblast interactions with collagen hydrogels. J Orthop Res. 2006;24:508–515. doi: 10.1002/jor.20054. [DOI] [PubMed] [Google Scholar]
- 12.Murray MM, Spindler KP, Abreu E, et al. Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J Orthop Res. 2007;25:81–91. doi: 10.1002/jor.20282. [DOI] [PubMed] [Google Scholar]
- 13.Murray MM, Spindler KP, Ballard P, et al. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res. 2007;25:1007–1017. doi: 10.1002/jor.20367. [DOI] [PubMed] [Google Scholar]
- 14.Joshi S, Mastrangelo A, Magarian E, et al. Collagen-platelet composite enhances biomechanical and histologic healing of the porcine ACL. Am J Sports Med. 2009;37:2401–2410. doi: 10.1177/0363546509339915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dressler MR, Butler DL, Boivin GP. Age-related changes in the biomechanics of healing patellar tendon. J Biomech. 2005;39:2205–2212. doi: 10.1016/j.jbiomech.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Hefti FL, Kress A, Fasel J, et al. Healing of the transected anterior cruciate ligament in the rabbit. J Bone Joint Surg [Am] 1991;73:373–383. [PubMed] [Google Scholar]
- 17.Mastrangelo A, Magarian E, Palmer M, et al. The effect of skeletal maturity on the regenerative function of intrinsic ACL cells. J Orthop Res. 2009 doi: 10.1002/jor.21018. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnoczky SP, Tarvin GB, Marshall JL. Anterior cruciate ligament replacement using patellar tendon. An evaluation of graft revascularization in the dog. J Bone Joint Surg [Am] 1982;64:217–224. [PubMed] [Google Scholar]
- 19.Scheffler SU, Gonnermann J, Kamp J, et al. Remodeling of ACL allografts is inhibited by peracetic acid sterilization. Clin Orthop Relat Res. 2008;466:1810–1818. doi: 10.1007/s11999-008-0288-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen W, Unterhauser F, Pufe T, et al. The angiogenic peptide vascular endothelial growth factor (VEGF) is expressed during the remodeling of free tendon grafts in sheep. Arch Orthop Trauma Surg. 2003;123:168–174. doi: 10.1007/s00402-002-0462-z. [DOI] [PubMed] [Google Scholar]
- 21.Amiel D, Akeson WH, Renzoni S, et al. Nutrition of cruciate ligament reconstruction by diffusion. Collagen synthesis studied in rabbits. Acta Orthop Scand. 1986;57:201–203. doi: 10.3109/17453678608994375. [DOI] [PubMed] [Google Scholar]
- 22.Seitz H, Menth-Chiari WA, Lang S, et al. Histological evaluation of the healing potential of the anterior cruciate ligament by means of augmented and non-augmented repair: an in vivo animal study. Knee Surg Sports Traumatol Arthrosc. 2008;16:1087–1093. doi: 10.1007/s00167-008-0599-6. [DOI] [PubMed] [Google Scholar]
- 23.Gupte CM, Bull AM, Murray R, et al. Comparative anatomy of the meniscofemoral ligament in humans and some domestic mammals. Anat Histol Embryol. 2007;36:47–52. doi: 10.1111/j.1439-0264.2006.00718.x. [DOI] [PubMed] [Google Scholar]
- 24.Rodeo SA, Potter HG, Kawamura S, et al. Biologic augmentation of rotator cuff tendon-healing with use of a mixture of osteoinductive growth factors. J Bone Joint Surg. 2007;89:2485–2497. doi: 10.2106/JBJS.C.01627. [DOI] [PubMed] [Google Scholar]
- 25.Ahluwalia S, Fehm M, Murray MM, et al. Distribution of smooth muscle actin-containing cells in the human meniscus. J Orthop Res. 2001;19:659–664. doi: 10.1016/S0736-0266(00)00041-3. [DOI] [PubMed] [Google Scholar]
- 26.Caplan AI. All MSCs are pericytes? Cell Stem Cell. 2008;3:229–230. doi: 10.1016/j.stem.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Mogford JE, Tawil N, Chen A, et al. Effect of age and hypoxia on TGFbeta1 receptor expression and signal transduction in human dermal fibroblasts: impact on cell migration. J Cell Physiol. 2002;190:259–265. doi: 10.1002/jcp.10060. [DOI] [PubMed] [Google Scholar]
- 29.Raghow R. The role of extracellular matrix in post-inflammatory wound healing and fibrosis. FASEB J. 1994;8:823–831. doi: 10.1096/fasebj.8.11.8070631. [DOI] [PubMed] [Google Scholar]
- 30.Yoshikawa T, Tohyama H, Enomoto H, et al. Expression of vascular endothelial growth factor and angiogenesis in patellar tendon grafts in the early phase after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2006;14:804–810. doi: 10.1007/s00167-006-0051-8. [DOI] [PubMed] [Google Scholar]
- 31.Petersen L, Brittberg M, Lindahl A. Autologous chondrocyte transplantation of the ankle. Foot Ankle Clin. 2003;8:291–303. doi: 10.1016/s1083-7515(03)00045-7. [DOI] [PubMed] [Google Scholar]